Introduction
Sepsis is a leading cause of mortality in hospitals
in the USA, which is characterized by a systemic inflammatory
response to life-threatening infection and results in widespread
tissue injury. Macrophages mediate the innate and adaptive immune
response, by producing inflammatory cytokines and cell scavenging
(1,2). Macrophages and their monocyte
precursors are distributed in every type of tissue in the body.
Upon tissue damage or infection, monocytes are rapidly recruited to
the lesion site, where they differentiate into tissue macrophages
(2–4). Severe sepsis has been demonstrated to
induce macrophage dysfunction (2–4). A
previous study demonstrated that the endotoxin lipopolysaccharide
(LPS) is a ligand of toll-like receptor 4 (TLR4) (5), and the expression of TLR4 is a key
determinant of LPS response intensity and susceptibility in a mouse
model of infection (6).
Furthermore, myeloid differentiation factor 2 (MD-2) is able to
bind to TLR4 to form the TLR4/MD-2 complex (7). Following LPS binding to TLR-4/MD-2,
toll/interleukin 1 receptor/resistance protein (TIR)
domain-containing adaptors are recruited to activate intracellular
signaling pathways, including the translocation of nuclear factor
(NF)-κB to the nucleus and production of pro-inflammatory
cytokines, including interleukin (IL)-6 and tumor necrosis factor
(TNF)-α (8–10). Therefore, TLR4 and its downstream
signaling pathways serve a critical role in the regulation of
sepsis and sepsis-associated disorders (11,12).
As targeting individual inflammatory cytokines in septic states is
not a successful strategy, TLR4 is a potential therapeutic target
for alleviation of sepsis-induced inflammatory response (13). In addition, sphingosine kinase
1/sphingosine-1-phosphate (SPHK1/S1P) is upregulated in phagocytes
and peritoneal phagocytes from patients with severe sepsis
(14), suggesting their
involvement in sepsis development. LPS may activate the SPHK1/S1P
signaling axis in a number of cell types, including macrophages, to
trigger the translocation of SPHK1 to the plasma membrane where it
converts its substrate sphingosine to the bioactive sphingolipid
S1P (15). SPHK1 is increasingly
recognized as an important mediator of the inflammatory response
elicited by various inflammatory stimuli, including LPS, TNF-α and
IL-1β, and involves TLR signaling (16–20).
Therefore, further study of the role of these mediators in sepsis
development and response may aid the development of novel
strategies to effectively control sepsis.
Quinolones (QNs) are synthetic, broad-spectrum
antimicrobial agents that are clinically used against Gram-positive
and Gram-negative bacteria (21).
These antimicrobial agents have been demonstrated to modify immune
and inflammatory responses in vivo and in vitro
(21). In this regard,
anti-infection treatment should not only consider the bacterial
sensitivity and antibiotic potency; however additionally, the
association between the in vivo efficacy and
immunoregulatory effects of antibiotics. Moxifloxacin (MXF) is a
fluoroquinolone that is effective against Gram-positive and
Gram-negative bacteria (22). Its
bidirectional effects on the activation and inhibition of the
immune response were demonstrated by its effects on the production
of a number of cytokines (including TNF-α and IL6) in human and
murine leukocytes (23). Similar
immunomodulatory effects of fluoroquinolones in inflammatory states
and infection have additionally been demonstrated in various
previous in vitro studies; for example, clinically relevant
concentrations of MXF were demonstrated to inhibit the synthesis of
inflammatory mediators, including IL-1, TNF-α, IL-6 and IL-8, in
human peripheral blood mononuclear cells and endothelial cells
following LPS stimulation (24).
However, whether MXF affects the LPS-stimulated
macrophage inflammatory reaction and whether the regulatory pathway
involves TLR4 and SPHK1 remains to be elucidated. In the present
study, an in vitro sepsis inflammatory reaction model was
initially established in LPS-stimulated mouse peritoneal
macrophages. The effects of MXF on pro-inflammatory factor
secretion and the underlying mechanisms were subsequently
investigated. To assess the effect of MXF on the inflammatory
response in LPS-stimulated macrophages, TLR4, SPHK1 and
pro-inflammatory factor expression levels were determined by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), western blotting and ELISAs. The present study
demonstrated that the TLR4 and SPHK1 pathways mediated the
inhibitory effect of MXF on pro-inflammatory factor expression.
Materials and methods
Isolation and purification of mouse
peritoneal macrophages
The present study was approved by the Institutional
Care and Use Committee, Experimental Animal Centre of Jinzhou
Medical University (Jinzhou, China). A total of 200 male C57BL/6J
mice (6–8 weeks old; 20–25 g weight) were obtained from our animal
center and housed in standard Plexiglas cages under a controlled
temperature (21–25°C) and 50% humidity with food and water
available ad libitum under a 12 h light/dark cycle with
lights on at 6:00 a.m. Food supply was restricted 3 days prior to
the experiments to achieve a target weight of 85% their expected
weight under conditions of unrestricted food access.
To isolate and purify mouse peritoneal macrophages,
the mice were euthanized and 70% ethanol was subsequently sprayed
onto the abdomen. The outer layer of the peritoneum was incised
using scissors, and ice-cold RPMI-1640 (cat. no. SH30809.01;
HyClone; Thermo Fisher Scientific, Inc., Logan, UT, USA) was
subsequently injected into the peritoneal cavity using a 5 ml
syringe. Subsequent to gently massaging the peritoneum to dislodge
any attached cells into the RPMI-1640 medium, the fluid from the
peritoneum was collected into a tube using a 5 ml syringe, kept on
ice, and centrifuged at 250 × g at 4°C for 5 min. The supernatant
was discarded prior to resuspension of cells in RPMI-1640
supplemented with 10% fetal bovine serum (FBS; cat. no. FSP500;
ExCell Bio, Shanghai, China), 100 U/ml penicillin and 3.7 g/l
NaHCO3, and counted using a hemocytometer. Cells were
then added into 6-well tissue culture plates at a density of
1×106 cells/well and cultured for 2 h at 37°C to ensure
their adherence to the substrate; non-adherent cells were removed
by gently washing with warm PBS three times. In total, 90% pure
macrophages were collected for the experiments following a previous
study (25).
Macrophage treatment
Macrophages were seeded in 6-well plates at a
density of 5×105 cells/well or 12-well plates at a
density of 2×105 cells/well, cultured overnight and
subsequently exposed to different conditions of external
stimulations and cultured for 24 h at 37°C in a cell culture
incubator with 95% air and 5% CO2: i) Control group
(normal peritoneal macrophages without any treatment); ii) LPS
group (normal peritoneal macrophages treated with 500 ng/ml LPS);
iii) 8MXF/LPS group (normal peritoneal macrophages treated with 8
µg/ml MXF and 500 ng/ml LPS for 2 h); iv) 16MXF/LPS group (normal
peritoneal macrophages treated with 16 µg/ml MXF and 500 ng/ml LPS
for 2 h); v) 32MXF/LPS group (normal peritoneal macrophages treated
with 32 µg/ml MXF and 500 ng/ml LPS for 2 h; and vi) 64MXF/LPS
group (normal peritoneal macrophages treated with 64 µg/ml MXF and
500 ng/ml LPS for 2 h). Subsequently, total cellular RNA and
protein were extracted from these treated macrophages for RT-qPCR
and western blotting, or cells were fixed in 4% formaldehyde at the
room temperature for 20 min prior to immunostaining.
Cell viability MTT assay
Macrophages were seeded in 96-well culture plates at
a density of 5×103 cells/well and cultured at 37°C for
24 h, prior to the addition of various doses of MXF (0, 50, 100,
200, 400, 800 and 1,600 µg/ml) for 24 h. MTT solution was added to
each well to reach a final concentration of 5 mg/ml. After a 2-h
incubation at 37°C, the culture medium was replaced with 200 µl
dimethyl sulfoxide to solubilize the formazan crystals produced by
MTT. The absorbance was measured at 490 nm with a spectrophotometer
and the percentage of viable cells was calculated. The experiment
was set to five replicates and repeated at least three times. The
growth inhibition was calculated by the equation: % cell
viability=(ODc-ODt)/(ODc-ODblank) ×100; where ODt and
ODc are the optical densities in treated cultures and control
cultures, respectively.
RT-qPCR
Total RNA was isolated from the treated macrophages
using an RNeasy Mini kit (BioTeke Corporation, Beijing,
China) and reverse transcribed into cDNA with the M-MuLV Reverse
Transcriptase kit (BioTeke Corporation) and incubated at 42°C for
50 min according to the manufacturer's protocols. qPCR was
performed in an Exicycler™ 96 Detection system (Bioneer
Corporation, Daejeon, Korea) with 10 µl reaction mixture,
containing 5 µl SYBR green Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 0.5 µl (10 µM) of each
forward and reverse primer, and 4 µl cDNA template with the
FastStart SYBR Green Reagents kit (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) according to the manufacturer's protocol. The
qPCR conditions were 95°C for 10 min and 40 cycles of 95°C for 10
sec, 60°C for 20 sec, 72°C for 30 sec and held at 4°C. The primers
were synthesized by Invitrogen (Thermo Fisher Scientific, Inc.) and
the sequences were as follows: SPHK1 forward,
5′-ACGAGCAGGTGACTAATGAAGA-3′ and reverse,
5′-GTGCCCACTGTGAAACGAA-3′; NF-κB forward,
5′-AGCATTAACCTCCTGGAGACG-3′ and reverse,
5′-TTGGGAGCACTGCTTTGGAT-3′; TLR4 forward,
5′-GTAGAGATGAATACCTCCTTAGTGT-3′ and reverse,
5′-TTTTACAGCGACCAATAAGTATCAG-3′; β-actin forward,
5′-CTGTGCCCATCTACGAGGGCTAT-3′ and reverse,
5′-TTTGATGTCACGCACGATTTCC-3′. The relative expression level of mRNA
was analyzed using the 2−∆∆Cq method, where
ΔΔCq=ΔCqtreated-ΔCqcontrol according to a
previous study (26).
Protein extraction and western
blotting
Treated peritoneal macrophages were washed with
ice-cold PBS twice, lysed in ice-cold radioimmunoprecipitation
assay buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.1%
sodium dodecyl sulfate, 0.5% sodium deoxycholate, 2 mM
ethylenediaminetetraacetic acid, 50 mM NaF, 10 µg/ml leupeptin, 2
mM Na3VO4, 15 µg/ml aprotinin and 1 mM
phenylmethane sulfonyl fluoride) on ice for 30 min, homogenized
using a vortex and centrifuged 13,000 × g at 4°C for 15 min. The
supernatant was transferred into a fresh tube and kept on ice, and
the protein concentration was determined with a Bradford assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts of
protein samples (30 µg/lane) were loaded and separated by SDS-PAGE
(10% gels), and electrically transferred onto polyvinylidene
fluoride membranes at 30 V for 1 h. Following a rinse in tap water,
the membranes were blocked in 5% (w/v) fat-free milk at room
temperature for 1 h and incubated overnight with primary antibodies
against TLR4 (19811-1-AP; Proteintech, Rosemont, IL, USA) at a
dilution of 1:500, SPHK1 (10670-1-AP; Proteintech) at a dilution of
1:1,000 and β-actin (CAB340MI22; Cloud-Clone Corp, Atlanta, GA,
USA) at a dilution of 1:500, NF-κB (10745-1-AP, Proteintech) at a
dilution of 1:500, or PKA (55388-1-AP, Proteintech) at a dilution
of 1:500, at 4°C overnight. The membranes were subsequently
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit immunoglobulin G secondary antibodies (ZB-2305; OriGene
Technologies, Inc., Beijing, China) at a dilution of 1:2,000 at
room temperature for 90 min. Following three washes with Tris-based
saline-0.1%Tween 20 (T8220; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China), the blots were visualized
with enhanced chemiluminescence (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol, and the images were
captured using the Kodak Image Station 4000R scanner (Kodak,
Rochester, NY, USA). The band intensity of the target proteins was
quantified using ImageJ software version 1.5.0 (National Institutes
of Health, Bethesda, MD, USA).
Immunofluorescence
Cultured macrophages were fixed in 4%
paraformaldehyde at room temperature for 30 min and subsequently
incubated with 1% Triton X-100 for 15 min followed by 1 h of
blocking in 5% goat serum (Beyotime Biotechnology, Shanghai, China)
at the room temperature. Subsequently, the macrophages from
different treatment groups were incubated with specific primary
antibodies, including mouse anti-TLR4 (1:100; 19811-1-AP;
Proteintech), anti-SPHK1 (1:1,000; 10670-1-AP; Proteintech) and
anti-NF-κB p65 (1:1,000; 10745-1-AP; Proteintech) at 4°C overnight.
Subsequently, macrophages were incubated with Cy3-conjugated
secondary anti-mouse antibody (1:2,000; SA00009-1; Proteintech) at
them room temperature for 1 h and the macrophages were mounted onto
glass slides with mounting medium containing DAPI. Images were
captured at 400× magnifications using a Nikon epifluorescence
microscope (Nikon Eclipse E800; Nikon Corporation, Tokyo, Japan).
Analysis was performed for 30–50 cells from each sample using the
Image Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
and a total of 150–500 cells per treatment group were statistically
analyzed.
ELISA
Macrophages were seeded into 24-well culture plates
at a density of 1×105 cells/well and subsequently
treated as detailed above. The supernatant was collected through
centrifugation at 1,000 × g at 4°C for 10 min to assess the
expression levels of IL-6 (at a dilution of 1:4, cat. no. SEA079Mu)
and TNF-α (cat. no. SEA133Mu) using these ELISA kits according to
the manufacturer's protocols (OriGene Technologies, Inc.).
Statistical analysis
The data were expressed as the mean ± standard error
of three repeated experiments and analyzed using one-way analysis
of variance followed by Tukey's post-hoc test. All statistical
analyses were performed by using Graphpad Prism 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Assessment of MXF half maximal
inhibitory concentration (IC50) in mouse
macrophages
In the present study, a cell viability MTT assay was
initially performed to determine cell viability (cytotoxicity)
following macrophage treatment with different concentrations of MXF
in the presence or absence of 500 ng/ml LPS for 24 h. Higher MXF
doses decreased the cell viability and increased the cell
inhibition rate (Table I). The
IC50 of MXF was 294.8 µg/ml, whereas, IC50 of
MXF plus LSP was 281.82 µg/ml (Tables
I and II).
 | Table I.Viability of mouse peritoneal
macrophages following treatment with MXF for 24 h. |
Table I.
Viability of mouse peritoneal
macrophages following treatment with MXF for 24 h.
| MXF, µ/ml | Mean | SD | Cell inhibition,
% |
|---|
| 0 | 0.535 | 0.032 | – |
| 50 | 0.436 | 0.025 | 18.44 |
| 100 | 0.420 | 0.013 | 21.50 |
| 200 | 0.370 | 0.023 | 30.89 |
| 400 | 0.181 | 0.015 | 66.23 |
| 800 | 0.121 | 0.005 | 77.45 |
| 1,600 | 0.108 | 0.011 | 79.88 |
 | Table II.Viability of mouse peritoneal
macrophages following treatment with LPS and MXF for 24 h. |
Table II.
Viability of mouse peritoneal
macrophages following treatment with LPS and MXF for 24 h.
| Treatment | Mean | SD | Cell inhibition,
% |
|---|
| 0 mg/ml | 0.557 | 0.032 | 0.00 |
| 0 mg/l MXF + 500
ng/ml LPS | 0.549 | 0.018 | 1.44 |
| 50 mg/l MXF + 500
ng/ml LPS | 0.445 | 0.030 | 20.14 |
| 100 mg/l MXF + 500
ng/ml LPS | 0.421 | 0.011 | 24.49 |
| 200 mg/l MXF + 500
ng/ml LPS | 0.367 | 0.025 | 34.17 |
| 400 mg/l MXF + 500
ng/ml LPS | 0.182 | 0.018 | 67.25 |
| 800 mg/l MXF + 500
ng/ml LPS | 0.119 | 0.004 | 78.55 |
| 1,600 mg/l MXF +
500 ng/ml LPS | 0.114 | 0.015 | 79.61 |
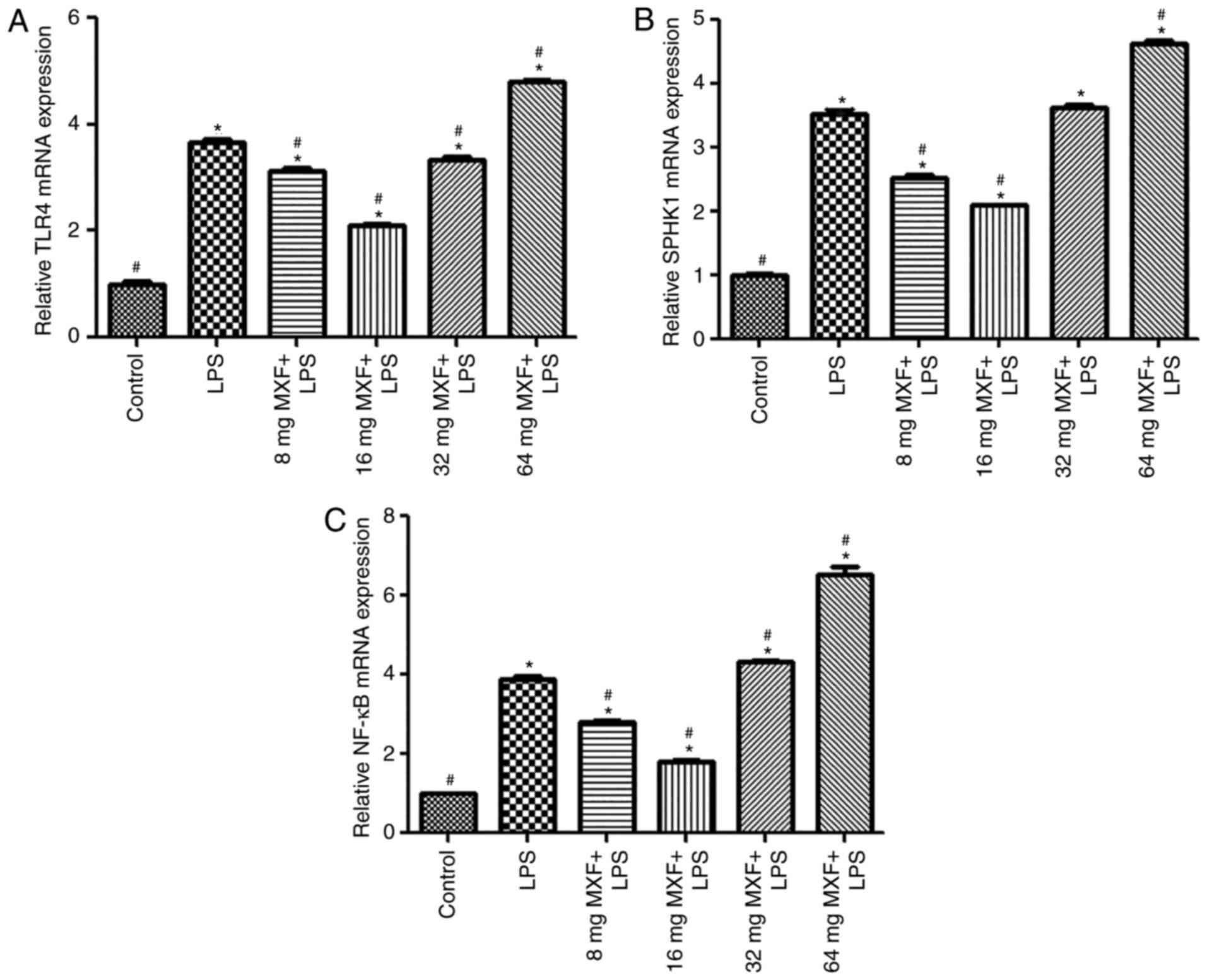
Bidirectional effects of MXF on the
expression of TLR4, SPHK1 and NF-κB mRNA following treatment with
LPS
To assess the effects of MXF on the inflammatory
response of macrophages, LPS was used to induce the inflammatory
response. It was observed that LPS significantly induced the
expression of TLR4 (P<0.05; Fig.
1A), SPHK1 (P<0.05; Fig.
1B) and NF-κB (P<0.05; Fig.
1C) mRNA, compared with the control group. At doses of 8 and 16
µg/ml, MXF significantly decreased the expression levels of TLR4,
SPHK1 and NF-κB mRNA compared with the LPS group (P<0.05),
suggesting that MXF had an inhibitory effect on the inflammatory
reaction at lower doses. In contrast, the higher MXF doses (32 and
64 µg/ml) increased the expression levels of TLR4 (32 µg/ml,
P<0.05; 64 µg/ml, P<0.05), SPHK1 (64 µg/ml; P<0.05) and
NF-κB (32 µg/ml, P<0.05; 64 µg/ml, P<0.05) mRNA compared with
LPS treatment alone, suggesting that high doses of MXF promoted the
inflammatory response, although 32 µg/ml MXF had no significant
effect on SPHK1 mRNA expression (P>0.05).
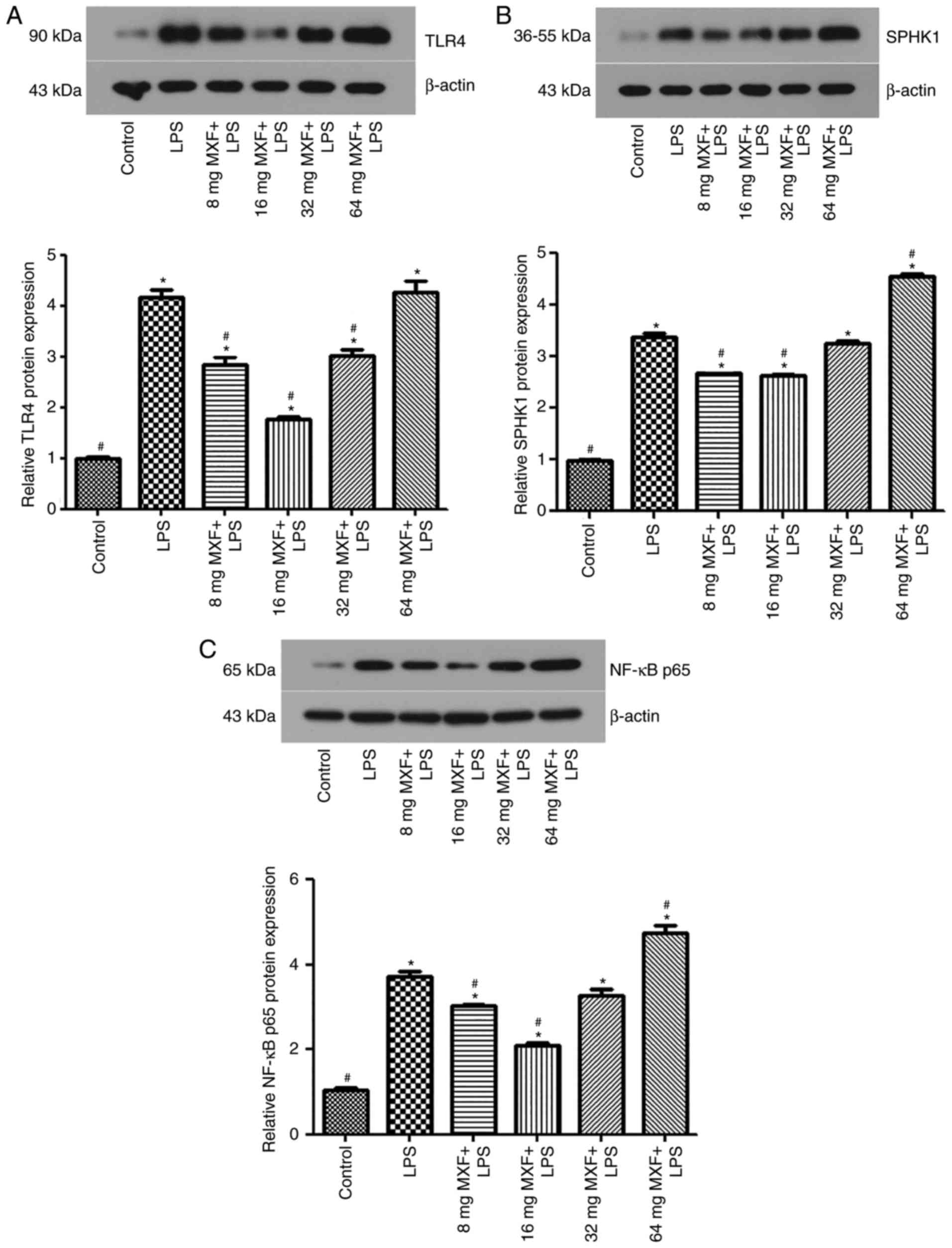
Bidirectional effects of MXF on the
protein expression of TLR4, SPHK1 and NF-κB p65 following LPS
treatment
Western blotting (Fig.
2) and immunofluorescence analysis (Fig. 3) was performed to detect
alterations in TLR4 (Figs. 2A,
3A and D), SPHK1 (Figs. 2B, 3B
and E) and NF-κB p65 (Figs.
2C, 3C and F) following
treatment with LPS. Western blotting demonstrated that the protein
expression levels of TLR4, SPHK1 and NF-κB p65 increased following
treatment with LPS, compared with the control group (P<0.05).
MXF treatment at 8 and 16 µg/ml downregulated the expression of
TLR4 (P<0.05), SPHK1 (P<0.05) and NF-κB p65 (P<0.05),
compared with the LPS alone group. These results suggested that low
MXF doses prevented the effects of LPS on the expression of these
proteins in intestinal macrophages. However, at doses of 32 µg/ml,
MXF had no significant effect on SPHK1 (P>0.05) and NF-κB p65
(P>0.05) protein expression; however, increased TLR4 expression
(P<0.05) compared with the LPS alone group. At 64 µg/ml, MXF
increased NF-κB p65 (P<0.05) expression; however, had no effect
on TLR4 (P>0.05) and SPHK1 (P>0.05) expression, compared with
the LPS alone group. These results suggested that MXF promoted the
inflammatory response at higher doses, whereas MXF suppressed the
inflammatory response at lower doses.
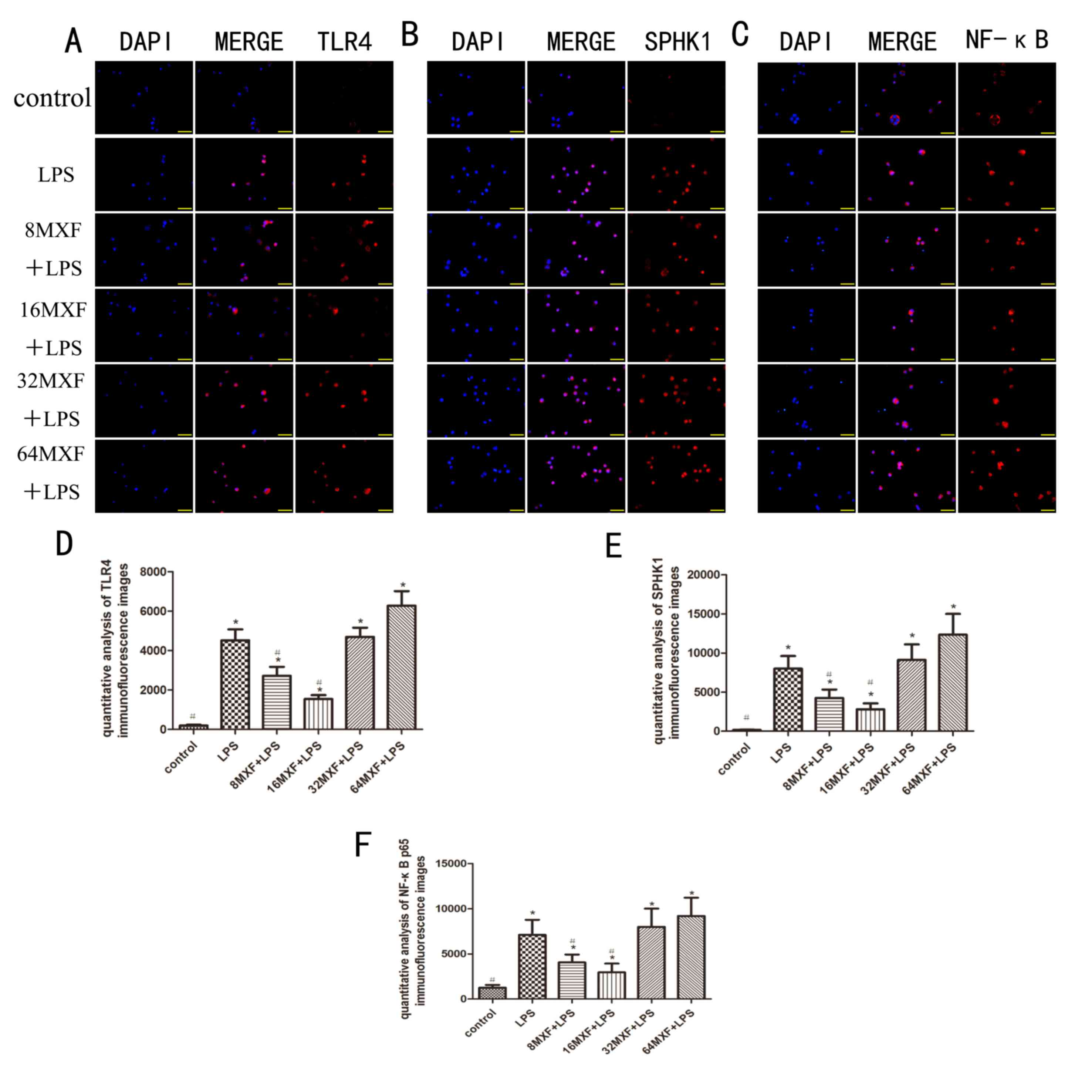 | Figure 3.Effects of MXF on the protein
expression of TLR4, SPHK1, and NF-κB in LPS-stimulated macrophages
by immunofluorescence. Treated macrophages were assessed by
immunofluorescence analysis of (A) TLR4, (B) SPHK1 and (C) NF-κB.
(D) TLR4, (E) SPHK1 and (F) NF-κB immunofluorescence was
quantitatively analyzed. Blue indicates DAPI staining and red
indicates (A) TLR4, (B) SPHK1 and (C) NF-κB. The data are expressed
as the mean ± standard error of the mean. *P<0.05 vs. the
control group; #P<0.05 vs. the LPS group. MXF,
moxifloxacin; TLR4, toll-like receptor 4; SPHK1, sphingosine kinase
1; NF-κB, nuclear factor- κB; LPS, lipopolysaccharide. |
The immunofluorescence experiments demonstrated that
the expression of TLR4, SPHK1 and NF-κB p65 was significantly
increased by treatment with LPS compared with the control group
(P<0.05; Fig. 3). Treatment
with MXF at doses of 8 and 16 µg/ml decreased the expression of
TLR4 (P<0.05), SPHK1 (P<0.05) and NF-κB p65 (P<0.05)
compared with the LPS alone group. However, at doses of 32 and 64
µg/ml, MXF had no significant effects on the expression of TLR4
(P>0.05), SPHK1 (P>0.05) or NF-κB p65 (P>0.05). These
results suggested that lower MXF doses inhibited the effects of LPS
on the expression of these proteins in intestinal macrophages.
Effects of MXF on IL-6 and TNF-α
production following LPS stimulation
After 24 h of treatment with LPS, the ELISA data
demonstrated treatment with LPS resulted in a significant increase
of IL-6 and TNF-α expression (P<0.05; Fig. 4) compared with the control group.
At doses of 8 and 16 µg/ml, MXF decreased the expression of IL-6
(both P<0.05) and TNF-α (P<0.05 at 8 µg/ml and P<0.05 at
16 µg/ml), compared with the LPS alone group. MXF at 32 µg/ml did
not affect the production of IL-6 and TNF-α (P>0.05), whereas,
MXF at 64 µg/ml significantly increased the expression levels of
IL-6 and TNF-α in macrophages, compared with the LPS alone group
(P<0.05). These results supported the effects of MXF on the
production of IL-6 and TNF-α by LPS-stimulated intestinal
macrophages.
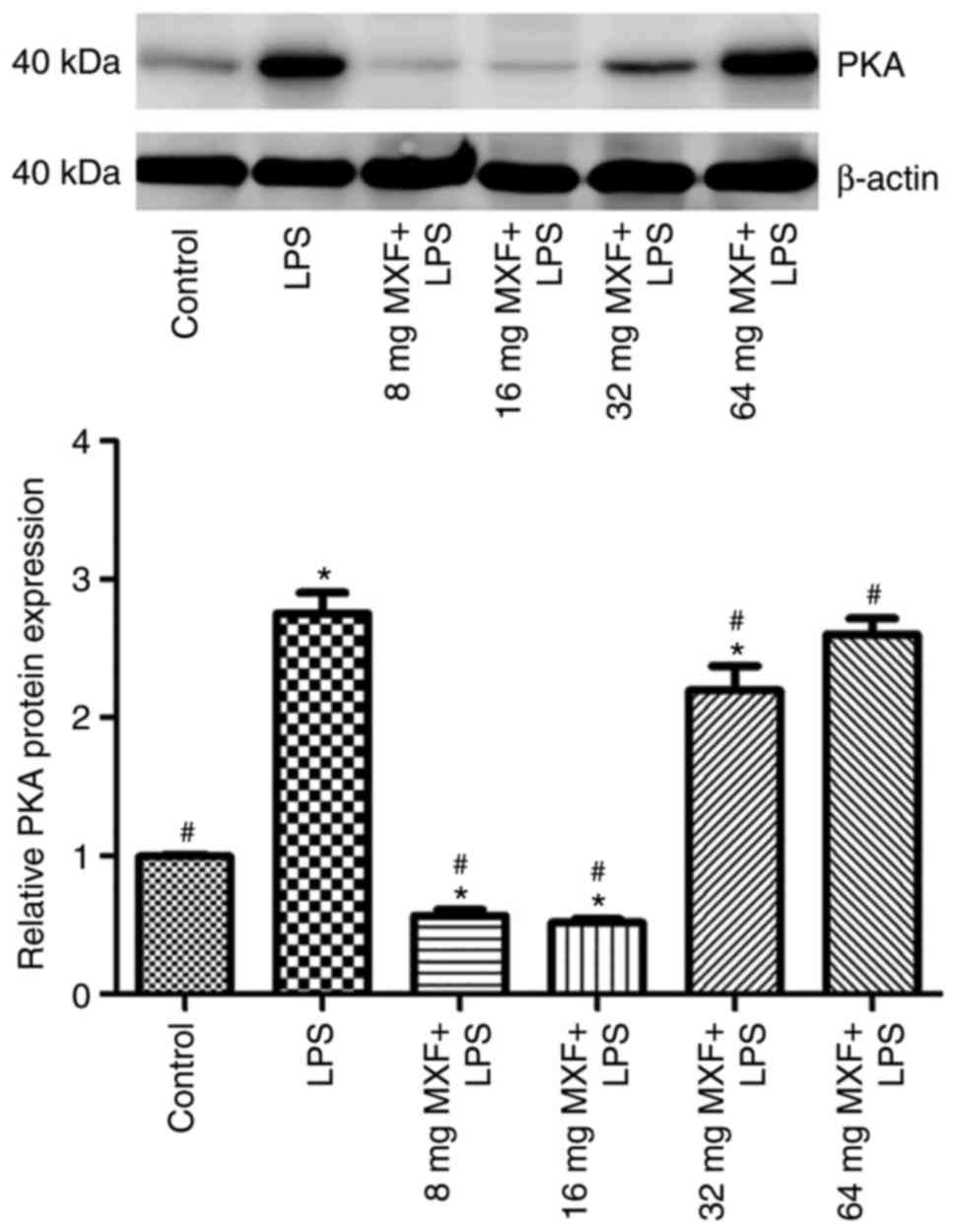
Effects of MXF on the expression of
protein kinase A (PKA) in LPS-stimulated macrophages
As PKA may mediate the effect of MXF on the
regulation of synthesis and secretion of these cytokines (27), PKA protein expression in LPS and
MXF-treated macrophages was determined (Fig. 5). It was demonstrated that LPS
induced PKA expression, whereas low MXF doses prevented the effects
of LPS on PKA expression. Higher MXF doses did not exert inhibitory
effects, suggesting that the effects of MXF on the synthesis and
secretion of the investigated cytokines may be through PKA
suppression in macrophages.
Discussion
In the present study, it was demonstrated that
treatment of macrophages with 16 µg/ml MXF had the most optimum
inhibitory effect on LPS-stimulated expression of NF-κB, TLR4,
SPHK1, IL-6 and TNF-α in mouse peritoneal macrophages. However,
this inhibitory effect was attenuated at higher doses of MXF (32
µg/ml) and MXF at 64 µg/ml exerted opposing effects on the
expression of these proteins in LPS-treated macrophages. These
results suggested that low doses MXF had an inhibitory effect on
the inflammatory response, whereas MXF at high doses promoted
inflammation. These data were consistent with those reported in a
previous study that demonstrated the bidirectional effects of MXF
on inflammation (28).
Macrophages are responsible for the clearance of
pathogens and additionally instruct other immune cells, and thus
have a central role in protecting the host. However, they may
additionally contribute to the pathogenesis of inflammatory and
degenerative diseases (29). In
the present study, mouse peritoneal macrophages were isolated and
cultured to further investigate the inflammation-induced molecular
mechanisms. The prototypical LPS was used as the endotoxin, due to
its ability to bind to the CD14/TLR4/MD-2 receptor complex in
macrophages and other cell types, to induce the secretion of
pro-inflammatory cytokines, including NF-κB and transcription
factor AP-1 (30,31). Activation of NF-κB stimulates a
number of inflammation-associated transcription factors to
subsequently induce the expression of various cytokines, including
TNF-α and IL-1/6/8, in addition to adhesins, which may induce the
inflammatory response (32,33).
Dysregulation of inflammation causes upregulation of cytokines and
adhesion expression, which is involved in numerous inflammatory
disorders, including endotoxemia and sepsis (34,35).
IL-6 and TNF-α are the two most notable pro-inflammatory cytokines
secreted by macrophages, and hypersecretion of these cytokines
induces widespread tissue damage in the body (36,37).
In the present study, LPS was utilized as an agent to induce a
pro-inflammatory state. It was demonstrated that the expression of
TLR4 and NF-κB, and the secretion of IL-6 and TNF-α was
significantly induced by treatment with LPS, which was consistent
with the LPS-induced pro-inflammatory states demonstrated in
previous studies (38,39).
Purswani et al (40) demonstrated that MXF was able to
regulate the inflammatory reaction in alveolar macrophages and
peripheral blood monocytes by decreasing TNF and IL-12 expression,
in addition to increasing IL-10 expression. In a previous in
vitro study, MXF was demonstrated to prevent the LPS-induced
increase in TNF-α, IL-1 and IL-6 expression in THP-1 cells,
cultured from human peripheral blood monocytes (41). The inhibitory effects of MXF on the
synthesis and secretion of these cytokines may be associated
intracellular signal transduction mechanisms, including the cyclic
adenosine monophosphate (cAMP) and PKA pathways (1).
In the present study, MXF doses between 8 and 64
µg/ml were used, which mimicked human clinical doses; MXF is
typically administered at 400 mg twice a day in adults, and the
half-life of MXF is 11.5–15.6 h after a single oral dose in human
volunteers (42–44). One hour after taking MXF, the peak
plasma concentration is ~4.1 mg/l after 1 h, and can reach a plasma
concentration of 13.5±0.42 mg/l following a single oral dose of 400
mg MXF in a volunteer subject (42–44).
The present data demonstrated that the IC50 of MXF was
294.8 mg/l, and the maximum concentration of MXF used was 64 mg/l,
which was far below the IC50 of 294.8 mg/l, although it
was theoretically a very high dose compared with the clinical
dosage. The inhibitory effect of QNs on the synthesis of TNF-α may
be mediated via a decrease in cAMP degradation, induced by the
inhibition of phosphodiesterase (45). There is a close association between
the decreased synthesis of intracellular TNF-α and cAMP, since cAMP
is additionally a key second messenger (45). MXF may manipulate the function of
topoisomerase II and IV, which influence multiple transcription
factors and enzymes to interfere with DNA replication,
transcription, repair and recombination during cell proliferation
and repair (46). Ceramide and
sphingosine are phosphorylated by SPHK1 to produce S1P, which
inhibits cell apoptosis and promotes cell proliferation through a
number of mechanisms, whereas QNs inhibit cell proliferation via an
opposite mechanism (47,48). Therefore, MXF may inhibit cell
proliferation and NF-κB activity, potentially via inhibition of
SPHK1 and topoisomerases. This is a potential mechanism for the
observed effects of MXF on inflammation (27).
QNs may affect the transcription factors via direct
regulation of cell-membrane receptor activities and various
intracellular kinase pathways. However, there is little evidence to
suggest that the drug directly binds to the corresponding receptors
(including TLR4) or kinases. In the present study, it was
demonstrated that low and higher (8 vs. 16 µg/ml) doses of MXF
resulted in the same directional alterations in TLR4 and SHPHK1
expression and cytokine secretion in LPS-stimulated macrophages,
which suggested that the QNs, receptors and kinases together
influence or respond to the inflammation reaction, although the
underlying mechanism of the pro-inflammatory effects of MXF at high
doses (64 µg/ml) remain to be elucidated. It was hypothesized that
this observed phenomenon may be due to the functional integrity
impairment of the macrophages.
Moxifloxacin is a fourth-generation QN that has a
strong antibacterial activity in Gram-positive and Gram-negative
bacteria, and thus has wide clinical uses. In recent years, a
number of studies have demonstrated the immunomodulatory effects of
MXF (49–52). Anti-infection therapies should not
only consider the sensitivity and potency of antibacterial agents;
however, the association between antibacterial in vivo
efficacy and immunoregulation additionally requires consideration.
The application of antibacterial agents is not limited to the
treatment of infections; however, may additionally be developed as
treatment for diseases of the immune system. Therefore, future
studies investigating the immunoregulatory effects of MXF may lead
to future clinical applications and further clarification of the
underlying mechanisms.
To the best of the authors' knowledge, the present
study was the first to investigate the effects of MXF on TLR4 and
SPHK1 expression in macrophages, and demonstrated a bidirectional
influence of MXF, which may be an important mechanism of the effect
of MXF on inflammation. The exact underlying mechanisms of the
effect of MXF on TLR4 and SPHK1 expression require further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by the
Liaoning Province Natural Science Foundation of China (grant no.
2015020364).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZQ and BC designed the study. ZQ, HY and NL
collected and analyzed the data. ZQ, HY, NL, XY, XH and FS
contributed samples collection and intellectual input. ZQ drafted
and wrote the manuscript. ZQ and BC revised the manuscript
critically for intellectual content. All authors gave intellectual
input to the study and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The animal study was approved by the Institutional
Care and Use Committee, Experimental Animal Centre of Jinzhou
Medical University (Jinzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Underhill DM and Ozinsky A: Phagocytosis
of microbes: Complexity in action. Annu Rev Immunol. 20:825–852.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang X, Venet F, Wang YL, Lepape A, Yuan
Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS and Ayala A:
PD-1 expression by macrophages plays a pathologic role in altering
microbial clearance and the innate inflammatory response to sepsis.
Proc Natl Acad Sci USA. 106:6303–6308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munoz C, Carlet J, Fitting C, Misset B,
Blériot JP and Cavaillon JM: Dysregulation of in vitro cytokine
production by monocytes during sepsis. J Clin Invest. 88:1747–1754.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ayala A and Chaudry IH: Immune dysfunction
in murine polymicrobial sepsis: Mediators, macrophages, lymphocytes
and apoptosis. Shock. 6 Suppl 1:S27–S38. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wittebole X, Coyle SM, Kumar A, Goshima M,
Lowry SF and Calvano SE: Expression of tumour necrosis factor
receptor and Toll-like receptor 2 and 4 on peripheral blood
leucocytes of human volunteers after endotoxin challenge: A
comparison of flow cytometric light scatter and immunofluorescence
gating. Clin Exp Immunol. 141:99–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalis C, Kanzler B, Lembo A, Poltorak A,
Galanos C and Freudenberg MA: Toll-like receptor 4 expression
levels determine the degree of LPS-susceptibility in mice. Eur J
Immunol. 33:798–805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fitzgerald KA, Rowe DC and Golenbock DT:
Endotoxin recognition and signal transduction by the
TLR4/MD2-complex. Microbes Infect. 6:1361–1367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the drosophila toll protein
signals activation of adaptive immunity. Nature. 388:394–397. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poltorak A, He X, Smirnova I, Liu MY, Van
Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al:
Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations
in Tlr4 gene. Science. 282:2085–2088. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoshino K, Takeuchi O, Kawai T, Sanjo H,
Ogawa T, Takeda Y, Takeda K and Akira S: Cutting edge: Toll-like
receptor 4 (TLR4)-deficient mice are hyporesponsive to
lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J
Immunol. 162:3749–3752. 1999.PubMed/NCBI
|
|
11
|
Xu H, Su Z, Wu J, Yang M, Penninger JM,
Martin CM, Kvietys PR and Rui T: The alarmin cytokine, high
mobility group box 1, is produced by viable cardiomyocytes and
mediates the lipopolysaccharide-induced myocardial dysfunction via
a TLR4/phosphatidylinositol 3-kinase gamma pathway. J Immunol.
184:1492–1498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brubaker SW, Bonham KS, Zanoni I and Kagan
JC: Innate immune pattern recognition: A cell biological
perspective. Annu Rev Immunol. 33:257–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng Y, Gao J, Cui Y, Li M, Li R, Cui C
and Cui J: Neuroprotective effects of resatorvid against traumatic
brain injury in rat: Involvement of neuronal autophagy and TLR4
signaling pathway. Cell Mol Neurobiol. 37:155–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spiegel S and Milstien S: The outs and the
ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol.
11:403–415. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vyas V, Ashby CR Jr, Olgun NS, Sundaram S,
Salami O, Munnangi S, Pekson R, Mahajan P and Reznik SE: Inhibition
of sphingosine kinase prevents lipopolysaccharide-induced preterm
birth and suppresses proinflammatory responses in a murine model.
Am J Pathol. 185:862–869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matloubian M, Lo CG, Cinamon G, Lesneski
MJ, Xu Y, Brinkmann V, Allende ML, Proia RL and Cyster JG:
Lymphocyte egress from thymus and peripheral lymphoid organs is
dependent on S1P receptor 1. Nature. 427:355–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tarrasón G, Aulí M, Mustafa S, Dolgachev
V, Domènech MT, Prats N, Domínguez M, López R, Aguilar N, Calbet M,
et al: The sphingosine-1-phosphate receptor-1 antagonist, W146,
causes early and short-lasting peripheral blood lymphopenia in
mice. Int Immunopharmacol. 11:1773–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuhara S, Simmons S, Kawamura S, Inoue
A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, et
al: The sphingosine-1-phosphate transporter Spns2 expressed on
endothelial cells regulates lymphocyte trafficking in mice. J Clin
Invest. 122:1416–1426. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Camaré C, Trayssac M, Garmy-Susini B,
Mucher E, Sabbadini R, Salvayre R and Negre-Salvayre A: Oxidized
LDL-induced angiogenesis involves sphingosine 1-phosphate:
Prevention by anti-S1P antibody. Br J Pharmacol. 172:106–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vettorazzi S, Bode C, Dejager L, Frappart
L, Shelest E, Klaßen C, Tasdogan A, Reichardt HM, Libert C,
Schneider M, et al: Glucocorticoids limit acute lung inflammation
in concert with inflammatory stimuli by induction of SphK1. Nat
Commun. 6:77962015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haruki T, Miyazaki D, Matsuura K, Terasaka
Y, Noguchi Y, Inoue Y and Yamagami S: Comparison of toxicities of
moxifloxacin, cefuroxime, and levofloxacin to corneal endothelial
cells in vitro. J Cataract Refract Surg. 40:1872–1878. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miravitlles M and Anzueto A: Moxifloxacin:
A respiratory fluoroquinolone. Expert Opin Pharmacother.
9:1755–1772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shukla P, Verma AK, Dwivedi P, Yadav A,
Gupta PK, Rath SK and Mishra PR: Moxifloxacin-loaded nanoemulsions
having tocopheryl succinate as the integral component improves
pharmacokinetics and enhances survival in E. coli-induced
complicated intra-abdominal infection. Mol Pharm. 11:4314–4326.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blau H, Klein K, Shalit I, Halperin D and
Fabian I: Moxifloxacin but not ciprofloxacin or azithromycin
selectively inhibits IL-8, IL-6, ERK1/2, JNK, and NF-kappaB
activation in a cystic fibrosis epithelial cell line. Am J Physiol
Lung Cell Mol Physiol. 292:L343–L352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan Q, Yin MZ and Li SP: Separation
cultivation and identification of rat peritoneal macrophagee. J
Wuhan Univ Technol. 31:40–42. 2009.
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weiss T, Shalit I, Blau H, Werber S,
Halperin D, Levitov A and Fabian I: Anti-inflammatory effects of
moxifloxacin on activated human monocytic cells: Inhibition of
NF-kappaB and mitogen-activated protein kinase activation and of
synthesis of proinflammatory cytokines. Antimicrob Agents
Chemother. 48:1974–1982. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim A, Lim KS, Lee H, Chung H, Yoon SH, Yu
KS, Cho JY, Jang IJ and Chung JY: A thorough QT study to evaluate
the QTc prolongation potential of two neuropsychiatric drugs,
quetiapine and escitalopram, in healthy volunteers. Int Clin
Psychopharmacol. 31:210–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Neill LA, Kishton RJ and Rathmell J: A
guide to immunometabolism for immunologists. Nat Rev Immunol.
16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kopp F, Kupsch S and Schromm AB:
Lipopolysaccharide-binding protein is bound and internalized by
host cells and colocalizes with LPS in the cytoplasm: Implications
for a role of LBP in intracellular LPS-signaling. Biochim Biophys
Acta. 1863:660–672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pahwa R, Devaraj S and Jialal I: The
effect of the accessory proteins, soluble CD14 and
lipopolysaccharide-binding protein on Toll-like receptor 4 activity
in human monocytes and adipocytes. Int J Obes (Lond). 40:907–911.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cardenas H, Arango D, Nicholas C, Duarte
S, Nuovo GJ, He W, Voss OH, Gonzalez-Mejia ME, Guttridge DC,
Grotewold E and Doseff AI: Dietary apigenin exerts
immune-regulatory activity in vivo by reducing NF-κB activity,
halting leukocyte infiltration and restoring normal metabolic
function. Int J Mol Sci. 17:3232016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee AS, Jung YJ, Thanh TN, Lee S, Kim W,
Kang KP and Park SK: Paricalcitol attenuates
lipopolysaccharide-induced myocardial inflammation by regulating
the NF-κB signaling pathway. Int J Mol Med. 37:1023–1029. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frieri M, Kumar K and Boutin A: Review:
Immunology of sinusitis, trauma, asthma, and sepsis. Allergy Rhinol
(Providence). 6:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao H, Zheng Q, Hu X, Shen H and Li F:
Betulin attenuates kidney injury in septic rats through inhibiting
TLR4/NF-κB signaling pathway. Life Sci. 144:185–193. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim YS, Hwang JW, Jang JH, Son S, Seo IB,
Jeong JH, Kim EH, Moon SH, Jeon BT and Park PJ: Trapa japonica
pericarp extract reduces LPS-induced inflammation in macrophages
and acute lung injury in mice. Molecules. 21:3922016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sugiyama K, Muroi M, Kinoshita M, Hamada
O, Minai Y, Sugita-Konishi Y, Kamata Y and Tanamoto K: NF-κB
activation via MyD88-dependent Toll-like receptor signaling is
inhibited by trichothecene mycotoxin deoxynivalenol. J Toxicol Sci.
41:273–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van der Mark VA, Ghiboub M, Marsman C,
Zhao J, van Dijk R, Hiralall JK, Ho-Mok KS, Castricum Z, de Jonge
WJ, Oude Elferink RP and Paulusma CC: Phospholipid flippases
attenuate LPS-induced TLR4 signaling by mediating endocytic
retrieval of Toll-like receptor 4. Cell Mol Life Sci. 74:715–730.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jurga AM, Rojewska E, Makuch W and Mika J:
Lipopolysaccharide from Rhodobacter sphaeroides (TLR4 antagonist)
attenuates hypersensitivity and modulates nociceptive factors.
Pharm Biol. 56:275–286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Purswani MU, Eckert SJ, Arora HK and Noel
GJ: Effect of ciprofloxacin on lethal and sublethal challenge with
endotoxin and on early cytokine responses in a murine in vivo
model. J Antimicrob Chemother. 50:51–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shalit I, Halperin D, Haite D, Levitov A,
Romano J, Osherov N and Fabian I: Anti-inflammatory effects of
moxifloxacin on IL-8, IL-1beta and TNF-alpha secretion and NFkappaB
and MAP-kinase activation in human monocytes stimulated with
Aspergillus fumigatus. J Antimicrob Chemother. 57:230–235. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang W, Su DH, Zhong HY and Jiang YS:
Blood drug concentration and pharmacokinetic analysis of
moxifloxacin hydrochloride tablets. Fujian Medical Journal.
3:68–71. 2013.
|
|
43
|
Wise R, Andrews JM, Marshall G and Hartman
G: Pharmacokinetics and inflammatory-fluid penetration of
moxifloxacin following oral or intravenous administration.
Antimicrob Agents Chemother. 43:1508–1510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lubasch A, Keller I, Borner K, Koeppe P
and Lode H: Comparative pharmacokinetics of ciprofloxacin,
gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and
moxifloxacin after single oral administration in healthy
volunteers. Antimicrob Agents Chemother. 44:2600–2603. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bailly S, Fay M, Roche Y and
Gougerot-Pocidalo MA: Effects of quinolones on tumor necrosis
factor production by human monocytes. Int J Immunopharmacol.
12:31–36. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Houssaye S, Gutmann L and Varon E:
Topoisomerase mutations associated with in vitro selection of
resistance to moxifloxacin in Streptococcus pneumoniae. Antimicrob
Agents Chemother. 46:2712–2715. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hait NC, Oskeritzian CA, Paugh SW,
Milstien S and Spiegel S: Sphingosine kinases, sphingosine
1-phosphate, apoptosis and diseases. Biochim Biophys Acta.
1758:2016–2026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xiong H, Wang J, Guan H, Wu J, Xu R, Wang
M, Rong X, Huang K, Huang J, Liao Q, et al: SphK1 confers
resistance to apoptosis in gastric cancer cells by downregulating
Bim via stimulating Akt/FoxO3a signaling. Oncol Rep. 32:1369–1373.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huckle AW, Fairclough LC and Todd I:
Prophylactic antibiotic use in COPD and the potential
anti-inflammatory activities of antibiotics. Respir Care.
63:609–619. 2008. View Article : Google Scholar
|
|
50
|
Beisswenger C, Honecker A, Kamyschnikow A,
Bischoff M, Tschernig T and Bals R: Moxifloxacin modulates
inflammation during murine pneumonia. Respir Res.
15:822014.PubMed/NCBI
|
|
51
|
Chen TC and Chang SW: Moxifloxacin
modulated TGF-β1-related interleukin-12 secretion in corneal
fibroblasts. Invest Ophthalmol Vis Sci. 58:5692–5702. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Uehara H, Das SK, Cho YK, Archer B and
Ambati BK: Comparison of the anti-angiogenic and anti-inflammatory
effects of two antibiotics: Clarithromycin versus moxifloxacin.
Curr Eye Res. 41:474–484. 2016.PubMed/NCBI
|