Introduction
Diabetes, characterized by hyperglycemia, is one of
the most common chronic diseases (1). Statistical analyses have suggested
that 415 million people worldwide suffer from diabetes, and the
number of patients will increase to 642 million by 2040 (2,3). A
total of >90% of all diabetics are diagnosed with type 2
diabetes (4). Type 2 diabetes is
characterized by inadequate insulin secretion from dysfunctional β
cells and insulin resistance (IR) (4,5).
Previous evidence has revealed that diabetes is a predominant risk
factor for cardiovascular disease (CVD) (6). Type 2 diabetes is one of the most
prevalent diseases in developing and developed countries and is
more susceptible to the occurrence of CVD than type 1 diabetes
(7,8). Metabolic syndrome, defined as the
aggregation of three or more metabolic disorders including obesity,
dyslipidemia, hyperglycemia and hypertension, may also increase the
risk of type 2 diabetes and CVD (9,10).
Currently, the discovery of novel therapeutic agents is still of
primary concern for the treatment of type 2 diabetes.
Apelin, an endogenous ligand for angiotensin II
protein J (APJ), was discovered in bovine stomach tissue by
Tatemoto et al in 1998 (11). Apelin, a 77-amino acid
prepropeptide, can be cleaved into active formsincluding Apelin-12,
−13, −17 and −36 (12). Apelin is
expressed in human plasma, kidney, heart, liver, brain, adipose
tissue, gastrointestinal tract and endothelium (13). Apelin/APJ is associated with
multiple physiological processes in the cardiovascular system,
including enhancement of cardiac contractility, relaxation of blood
vessels, and regulation of blood pressure and insulin sensitivity
(14,15). Metformin is one of the most widely
used drugs in the treatment of type 2 diabetes (16). Atorvastatin has been reported to
improve endothelial dysfunction (17). However, whether Apelin-13 has
protective effects in high-fat diet (HFD)-induced type 2 diabetes
in Goto-Kakizaki (GK) rats remains unclear.
The present study investigated the effects of
Apelin-13 administration on cardiac function, hyperglycemia, IR,
dyslipidemia, endothelial function, inflammation and glucose
metabolism in type 2 diabetic GK rats.
Materials and methods
Animals and grouping
A total of 32 specific-pathogen-free (SPF), male,
Goto-Kakizaki (GK) rats (12-weeks-old; 240–280 g) and a total of 8
non-diabetic, male, Wistar rats (12-weeks-old; 240–280 g) were
purchased from Shanghai SLAC Laboratory Animal (Shanghai, China).
The experiments were performed in accordance with the Guide for the
Care and Use of Laboratory Animals and were approved by the Animal
Care and Use Committee of China Medical University (Shenyang,
China). Ethical clearance was obtained from the Institutional
Animal Care and Use Committee (approval no. 2015052R). The animals
were maintained under SPF conditions (a 12-h light/dark cycle;
temperature, 21±2°C; humidity, 60±10%) with access to food and
water ad libitum.
Following an adaptive feeding for 1 week, the
animals were divided into 5 groups (n=8 rats/group): i) Control,
ii) GK-HFD, iii) Metformin, iv) Atorvastatin and v) Apelin-13.
Non-diabetic Wistar rats fed with a standard chow and treated with
distilled water by gavage were used as the control rats. The GK
rats in the GK-HFD, Metformin, Atorvastatin or Apelin-13 group were
fed with a high-fat diet (66.5% standard chow, 10% lard, 20%
sucrose, 2.5% cholesterol and 1% pig bile salt) and given distilled
water, metformin (350 mg/kg/d, by gavage; Sino-American Shanghai
Squibb Pharmaceuticals Ltd., Shanghai, China), atorvastatin (50
mg/kg/d, by gavage; Beijing Jialin Pharmaceutical Co., Ltd.,
Beijing, China) or Apelin-13 (200 µg/kg/d, intraperitoneal
injection; Anaspec Inc., Fremont, CA, USA) once daily for 4 weeks
simultaneously.
Hemodynamic parameters
Following 4 weeks of treatment, the rats underwent a
12 h starvation period. Then, the rats were anesthetized with 3%
pentobarbital sodium (35 mg/kg; Sinopharm Group Co., Ltd.,
Shanghai, China) and hemodynamic parameters were monitored using
RM6240BD multi-channel physiological signal monitor (Chengdu
Instrument Factory, Chengdu, China), including heart rate, left
ventricular end diastolic pressure (LVEDP), the maximum rate of
left ventricular pressure fall (-dP/dtmax) and maximum
rate of left ventricular pressure rise (+dP/dtmax).
Assessment of biochemical parameters
in serum
Fasting venous blood samples were obtained from each
rat and serum was obtainedby centrifugation at 1,550 × g for 10 min
at 4°C. Subsequently, serum levels of fasting insulin (FINS; cat.
no. F01PZA), endothelin-1 (ET-1; cat. no. D11PZA) and leptin (cat.
no. C16PDA) were measured using commercial kits obtained from
Beijing North Institute of Biological Technology (Beijing, China).
Tumor necrosis factor-α (TNF-α; cat. no. XFFM1870) level in serum
was examined using a commercial kit from Shanghai Xinfan
Biotechnology Co., Ltd. (Shanghai, China). Nitric oxide (NO; cat.
no. A012-1) levels and the activity of constitutive nitric oxide
synthase (cNOS; cat. no. A014-1-1) were measured using kits
obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). All procedures using commercial kits were conducted
according to the manufacturer's protocol. Total cholesterol (TC),
triglyceride (TG), high density lipoprotein-cholesterol (HDL-C),
low density lipoprotein-cholesterol (LDL-C) levels were determined
using a Beckman 700 automatic biochemical analyzer (Beckman
Coulter, Inc., Brea, CA, USA).
Determination of fasting plasma
glucose (FPG)
The animals were deprived of food for 12 h and blood
samples were obtained from the tail vein. The levels of FPG were
measured using the ACCU-CHEK Active Glucose Monitoring System
(Roche Diagnostics GmbH, Mannheim, Germany) once weekly.
Measurement of homeostasis model
assessment for insulin resistance (HOMA-IR)
Homeostasis model assessment for insulin resistance
(HOMA-IR) was calculated using the following formula:
FPGxFINS/22.5.
Apelin-12 expression by ELISA
Apelin-12 expression levels in serum, myocardial
tissues and aortic tissues were examined by ELISA according to the
manufacturer's protocol (cat. no. EK-057-23; Beijing Shengke Boyuan
Biotechnology Co., Ltd., Beijing, China).
Reverse
transcription-semi-quantitative polymerase chain reaction
(RT-sqPCR) analysis
Myocardial or aortic tissues were lysed with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and liquid nitrogen. Total RNAs were
isolated from the tissues and RNA concentration was determined by
measuring optical density260. Subsequently, total RNAs
were reverse-transcribed into cDNAs and PCR was performed in a
final 25 µl volume using a RT-PCR kit (Takara Biotechnology Co.,
Ltd., Dalian, China). The PCR conditions were: 95°C for 5 min; 35
cycles at 94°C for 30 sec, 59°C for 30 sec and 72°C for 40 sec,
with a final extension at 72°C for 5 min. The PCR products were run
on 1.2% agarose gels and stained with 0.5 µg/ml ethidium bromide
(Amresco, LLC, Solon, OH, USA). The optical densities of the bands
were quantified using a Gel Documentation system (GDS-8000; UVP,
LLC, Phoenix, AZ, USA). β-actin was used as an internal control.
The relative gene expression level was normalized to β-actin
levels. All primers were synthesized by Beijing SBS Genetech Co.,
Ltd (Beijing, China) and the primer sequences were as follows:
Forward, 5′-TGCTCTGGCTCTCCTTGACT-3′ and reverse,
5′-ATGGGTCCCTTATGGGAGAG-3′ for Apelin-12; forward,
5′-ATCTGGACCCACACCTTC-3′ and reverse, 5′-AGCCAGGTCCAGACGCA-3′ for
β-actin.
Western blotting
Myocardial tissues were lysed using
radioimmunoprecipitation assay lysis buffer, and homogenized using
an ultrasonic homogenizer UP200S (Hielscher, Teltow, Germany).
Following incubation on ice for 20 min, the tissue homogenates were
centrifuged at 4°C at 20,000 × g for 10 min and the supernatant was
harvested. Protein concentration was determined using the
bicinchoninic acid assay method. Proteins were then separated by
10% SDS-PAGE and transferred onto nitrocellulose membranes (GE
Healthcare, Chicago, IL, USA). Following blocking with 5% non-fat
milk at room temperature for 1 h, the membranes were incubated at
4°C overnight with primary antibodies against Apelin-12 (1:1,000;
cat. no. orb364270; Biorbyt LLC, San Francisco, CA, USA), glucose
transporter (GLUT) 4 (1:2,000; cat. no. PA5-19621; Invitrogen;
Thermo Fisher Scientific, Inc.) and phosphorylated (p)-5′adenosine
monophosphate-activated protein kinase (AMPK; 1:1,000; cat. no.
18167-1-AP; Wuhan Sanying Biotechnology, Wuhan, China), followed by
incubation with horseradish peroxidase-conjugated secondary
antibody (1:3,000; cat. no. bs-40296G-HRP; BIOSS, Beijing, China)
at room temperature for 1–2 h. Subsequently, protein bands were
developed using Super ECL Plus Detection Reagent (cat. no.
C05-07004; BIOSS) and quantified using Scion Image software version
1.6.3 (Scion Corporation, Frederick, MD, USA). β-actin (1:5,000;
cat. no. bsm-33036M; BIOSS) served as an internal control.
Statistical analysis
Data were expressed as the means ± standard
deviation. Statistical analyses were performed using GraphPad Prism
version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA) with
one-way analysis of variance followed by Newman-keuls post-hoc
test. P<0.05 was considered to indicate astatistically
significant difference.
Results
Treatment with metformin, atorvastatin
and Apelin-13 improves cardiac function
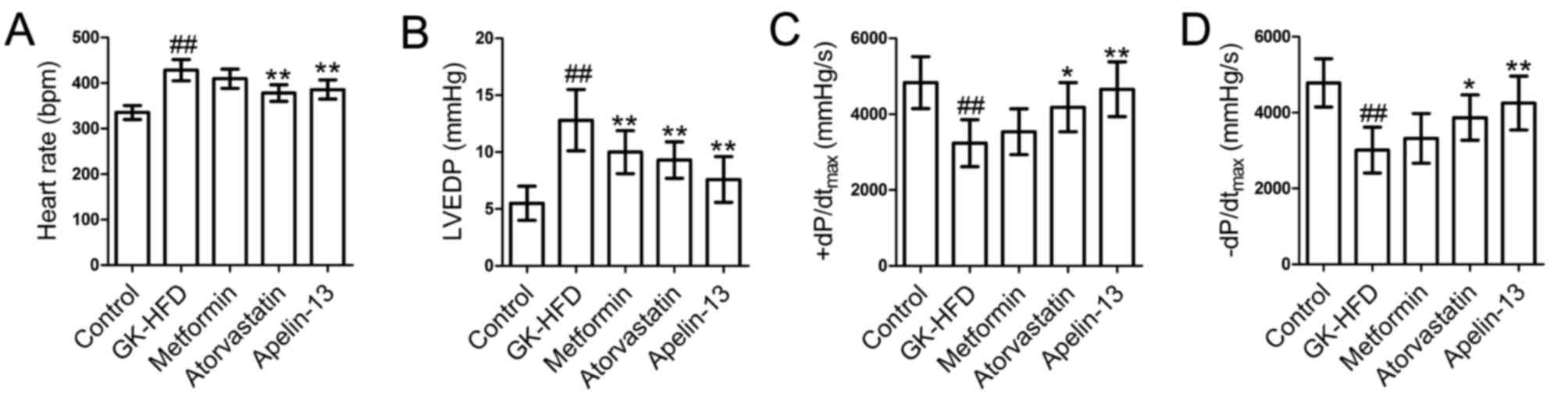
The results demonstrated that heart rate (Fig. 1A) and LVEDP (Fig. 1B) in the GK-HFD group were
significantly increased compared with control group, whereas
+dP/dtmax (Fig. 1C) and
-dP/dtmax (Fig. 1D)
were decreased compared with control. There was a significant
decrease in heart rate and LVEDP in the Atorvastatin and Apelin-13
groups compared with the GK-HFD group, and an increase in
+dP/dtmax and -dP/dtmax. Treatment with
metformin significantly decreased LVEDP compared with the GK-HFD
group, whereas no statistically significant differences were
observed for heart rate and ±dP/dtmax.
Treatment with metformin, atorvastatin
and Apelin-13 improves insulin resistance
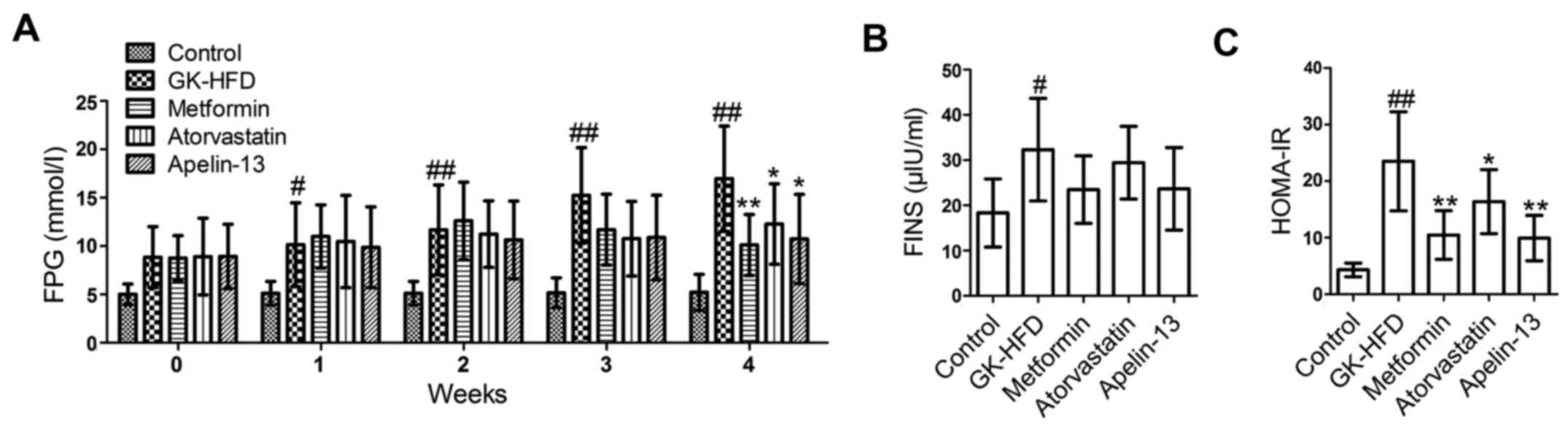
Compared with the control group, the rats in the
GK-HFD group had significantly increased levels of FPG (Fig. 2A), FINS (Fig. 2B) and HOMA-IR (Fig. 2C). However, metformin, atorvastatin
and Apelin-13 treatment lowered the levels of FPG, FINS and HOMA-IR
compared with the GK-HFD group.
Treatment with metformin, atorvastatin
and Apelin-13 improves lipid metabolism
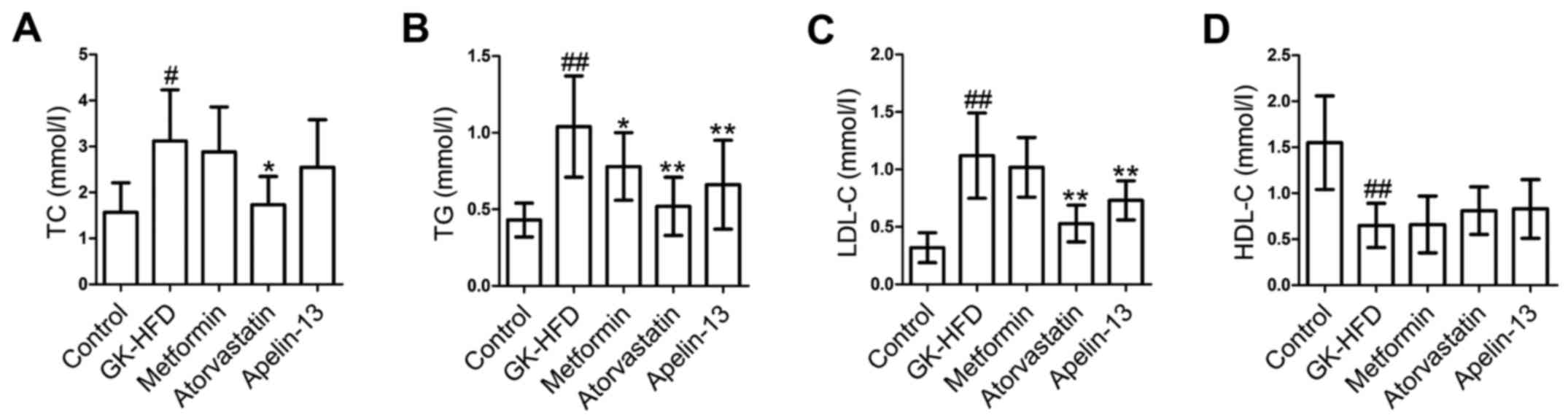
The present study then evaluated the effect of
metformin, atorvastatin and Apelin-13 on serum levels of TC, TG,
LDL-C and HDL-C in rats. The GK-HFD group demonstrated markedly
increased levels of TC (Fig. 3A),
TG (Fig. 3B) and LDL-C in serum
(Fig. 3C) and significantly
decreased HDL-C (Fig. 3D) compared
with the control group. However, treatment with metformin,
atorvastatin and Apelin-13 decreased serum levels of TC, TG and
LDL-C and increased HDL-C in GK-HFD rats.
Effect of metformin, atorvastatin and
Apelin-13 treatment on ET-1, NO and cNOS
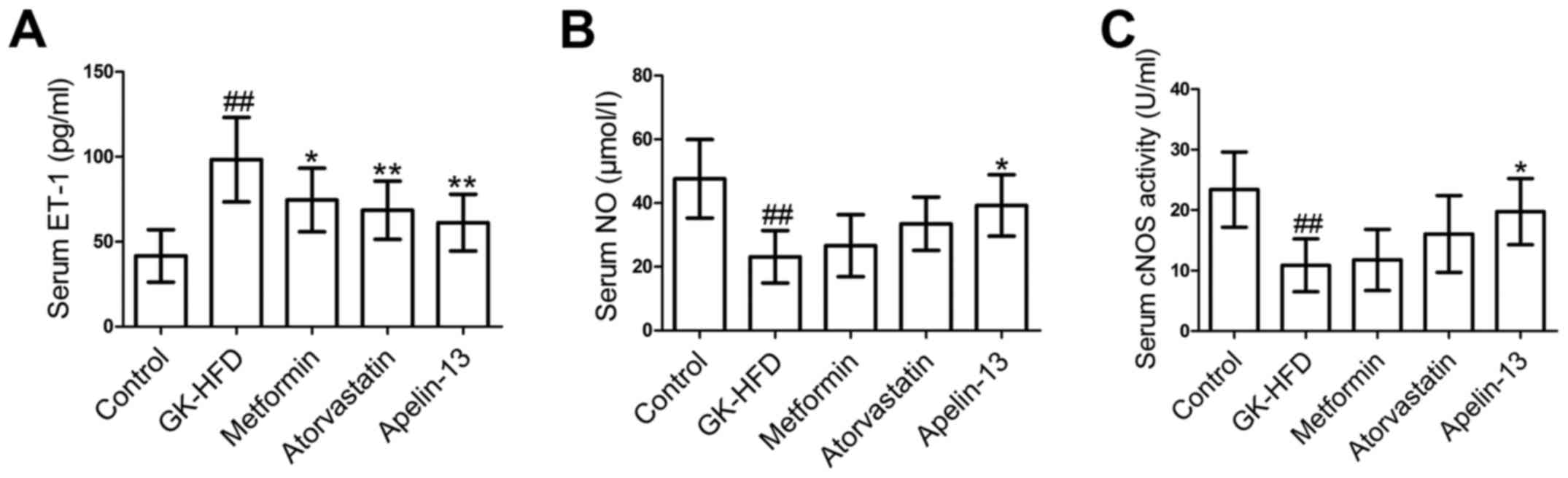
The ET-1 level in serum (Fig. 4A) was significantly increased in
the GK-HFD group compared with the control group, however was
significantly decreased in the Metformin, Atorvastatin and
Apelin-13 groups, compared with GK-HFD group. In addition, the
GK-HFD group exhibited a significant decrease in serum NO level
(Fig. 4B) and a reduction in cNOS
activity (Fig. 4C). However,
metformin, atorvastatin and Apelin-13 administration elevated NO
level and cNOS activity.
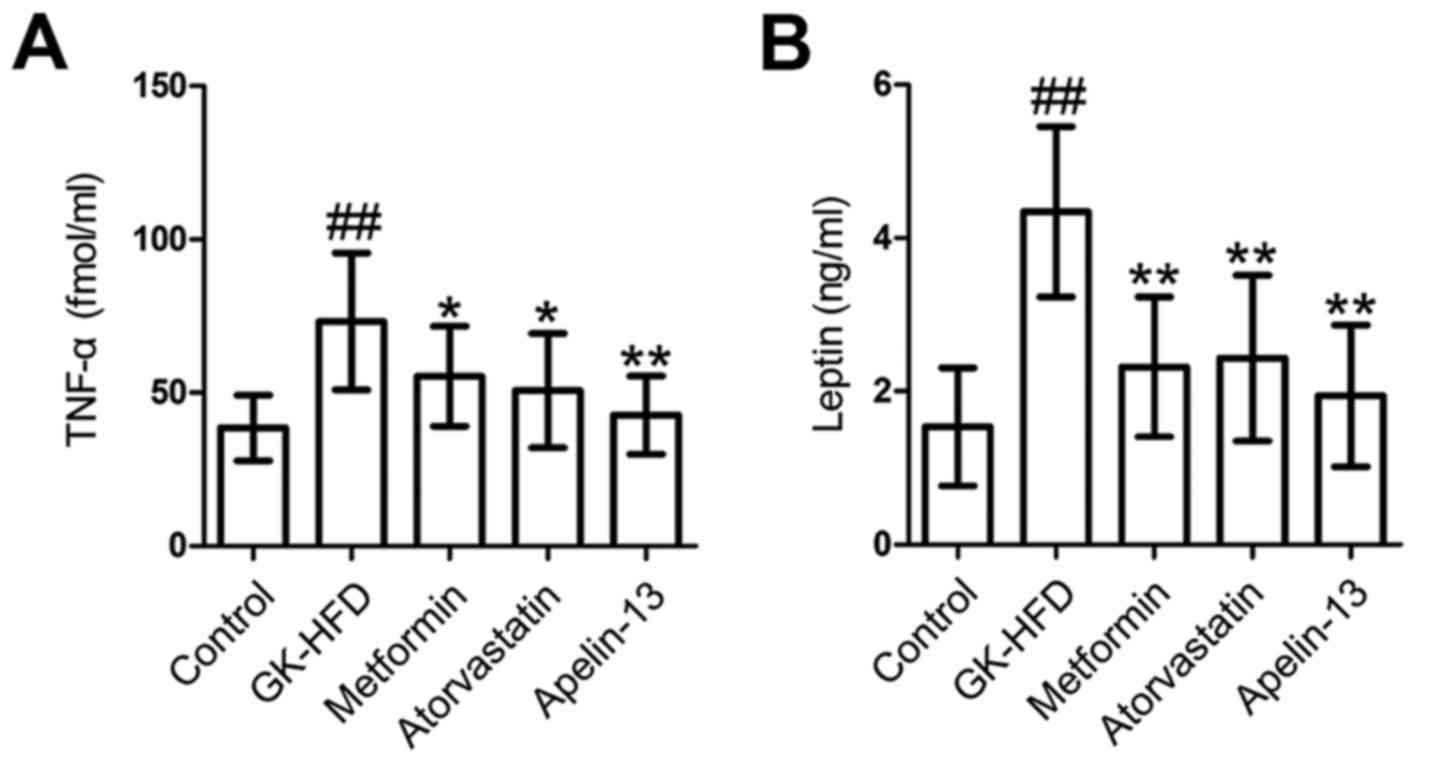
Effect of metformin, atorvastatin and
Apelin-13 treatment on serum TNF-α and leptin
Compared with the control rats, GK-HFD rats
exhibited a significant increase in TNF-α (Fig. 5A) and leptin (Fig. 5B) in serum. However, the serum
levels of TNF-α and leptin in the Metformin, Atorvastatin and
Apelin-13 groups were significantly decreased, compared with the
GK-HFD group.
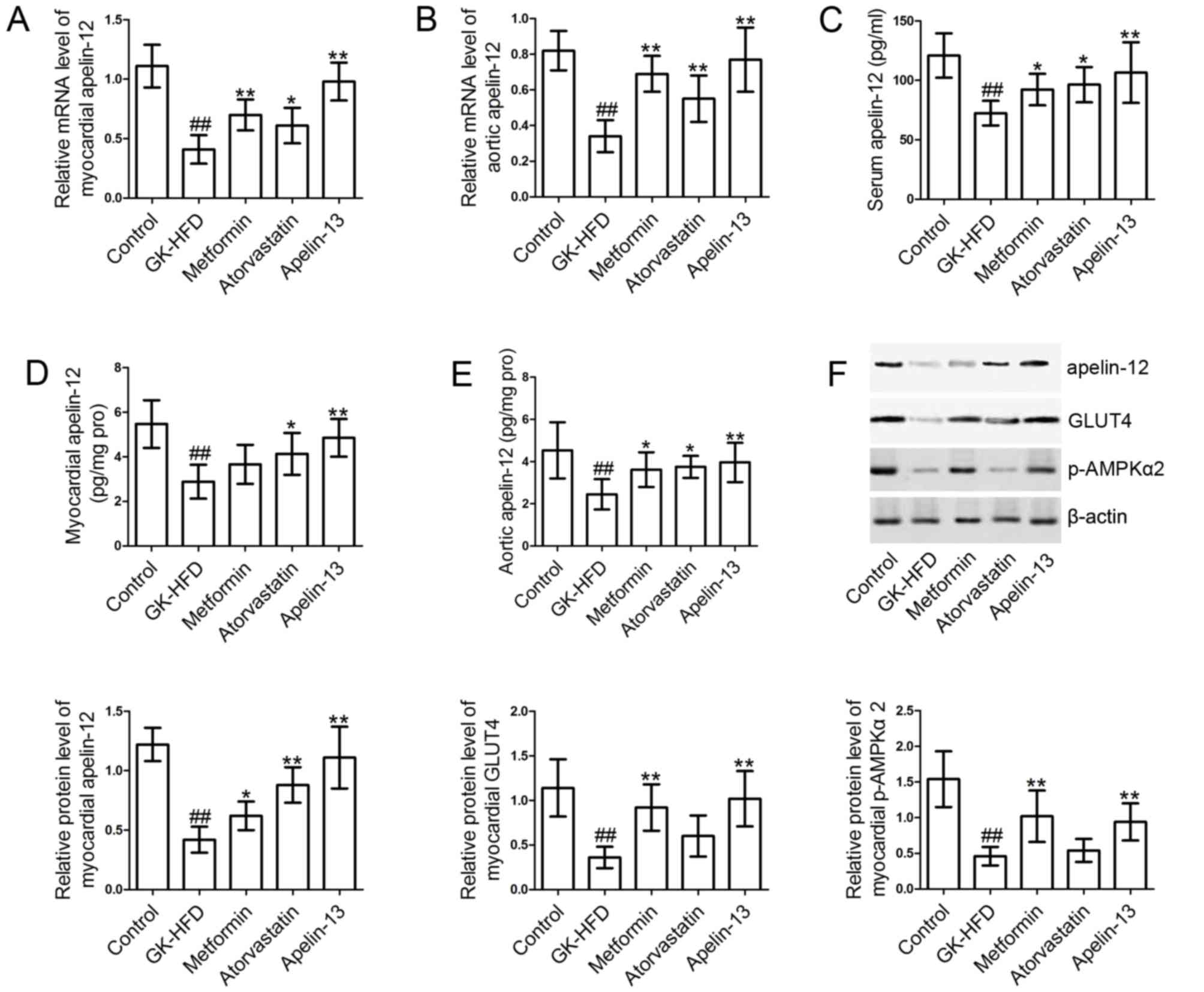
Effect of metformin, atorvastatin and
Apelin-13 treatment on Apelin-12 expression
Following this, the expression levels of Apelin-12
in serum, myocardial tissues and aortic tissues were measured. As
presented in Fig. 6, the levels of
Apelin-12 in myocardial tissues (Fig.
6A, D and F), aortic tissues (Fig.
6B and E) and serum (Fig. 6C)
in the GK-HFD group were significantly decreased compared with the
control group. However, treatment with metformin, atorvastatin and
Apelin-13 significantly induced the expression of Apelin-12 in
serum, myocardial tissues and aortic tissues, compared with the
GK-HFD group.
Effect of metformin, atorvastatin and
Apelin-13 treatment GLUT4 and p-AMPKα2
The levels of GLUT4 and p-AMPKα2 (Fig. 6F) in the myocardial tissues of
GK-HFD rats were significantly decreased compared with control
group. However, metformin and Apelin-13 injections markedly
elevated GLUT4 and p-AMPKα2 levels, compared with the GK-HFD group.
Atorvastatin treatment resulted in slight increases in GLUT4 and
p-AMPKα2 levels; however, the differences were not statistically
significant.
Discussion
The GK rat may be used as a genetic animal model of
type 2 diabetes (18). In the
present study, type 2 diabetes was induced in GK rats. The effects
of metformin, atorvastatin and Apelin-13 on cardiac function,
hyperglycemia, IR, dyslipidemia, endothelial function, inflammation
and glucose metabolism in GK-HFD rats were investigated.
Hemodynamic indices, including HR, LVEDP,
+dP/dtmax and -dP/dtmax, are often used to
evaluate cardiac function (19).
+dP/dtmax indicates systolic cardiac function, whereas
LVEDP and -dP/dtmax indicate diastolic cardiac function
(20). The results demonstrated
that Apelin-13 decreased heart rate and LVEDP, and increased
+dP/dtmax and -dP/dtmax, indicating the
improvement of LV systolic and diastolic function in GK rats fed
with HFD.
IR and high FPG are important risk factors for type
2 diabetes (21,22). HOMA-IR, calculated from FPG and
FINS, is commonly used as a primary index for IR evaluation in the
prevention of diabetes and screening of high-risk groups (23). The HOMA-IR was used to determine IR
on the basis of fasted insulin and glucose levels. Elevated HOMA-IR
has a positive impact on the development of type 2 diabetes in
patients with impaired insulin secretion (24). In the present study, it was
demonstrated that Apelin-13 significantly reduced the elevated FPG,
FINS and HOMA-IR. The results suggested that Apelin-13 improved IR
in the rat model of type 2 diabetes.
Dyslipidemia, characterized by increases in TC, TG
and LDL-C, and decreases in HDL-C, is a common disorder in type 2
diabetics (25,26). TC and TG, two predominant types of
lipids in plasma, are predictors of the balance of lipid metabolism
(27). Elevated plasma HDL-C
results in a cardioprotective effect, whereas higher LDL-C levels
are considered an atherogenic factor (28). In the present study, it was
demonstrated that Apelin-13 decreased TC, TG and LDL-C, and
increased HDL-C concentration in serum, which indicated that
Apelin-13 improved dyslipidemia in a rat model of type 2
diabetes.
Endothelial dysfunction has a vital role in the
progression of diabetic vasculopathy and hypertension (29). It has been reported that ET-1 is
overproduced in animal models of diabetes and patients (30). ET-1 is primarily produced in the
endothelium, cardiomyocytes, vascular smooth muscle cells,
fibroblasts, leukocytes and macrophages (31). ET-1, which is a potent peptide
vasoconstrictor with proinflammatory and profibrotic properties,
participates in the development of diabetic vasculopathy via
regulation of vascular homeostasis (30,32,33).
NO is important in various physiological processes. cNOS produces a
small amount of NO and has been reported to be associated with
β-cell dysfunction during the development of type 2 diabetes
(34). In addition, a previous
study revealed that overproduction of ET-1 may contribute to
endothelial dysfunction by inhibiting NO secretion (31). The results of the present study
demonstrated that Apelin-13 injection resulted in decreased levels
of ET-1 and increased NO serum levels and cNOS activity. These
results suggested that Apelin-13 alleviated endothelial dysfunction
by regulating the imbalance of ET-1 secretion and NO
production.
Diabetes is an inflammatory disease in which the
levels of pro-inflammatory cytokines, including TNF-α, are elevated
in the serum of patients (35,36).
A previous study indicated that high levels of TNF-α is a crucial
risk factor for diabetes (37).
Furthermore, TNF-α is an important indicator of insulin resistance
in obesity and may serve as a target for improving obesity-induced
insulin resistance in patients with type 2 diabetes (38). Leptin, first discovered by Zhang
et al in 1994, is a hormone that is secreted from adipose
tissues and circulates in the blood (39,40).
Leptin controls body weight and adipose tissue mass via regulation
of energy homeostasis (41).
Patients with obesity and type 2 diabetes usually have an increased
plasma level of leptin and leptin resistance results in a failure
to improve hyperglycemia (42). In
addition, elevated leptin levels in plasma are correlated with IR,
independent of insulin sensitivity and obesity (41). It was demonstrated that Apelin-13
injection reduced the increased levels of TNF-α and leptin induced
by diabetes. These results suggested that Apelin-13 may alleviate
diabetic disorders via inhibition of TNF-α and leptin
secretion.
A previous study suggested that the circulating
levels of Apelin are decreased in patients with type 2 diabetes
(43). Apelin, associated with
glucose uptake and IR, may promote the translocation of GLUT4 from
the cytoplasm to the plasma membrane (44). GLUT4 is primarily present in
cardiac, adipose and skeletal tissues, and is an insulin-regulated
glucose transporter (45). Glucose
is transported across the cell membrane via glucose transporters
(GLUTs). GLUT4 is reported to be decreased in diabetic patients,
which leads to a decrease in the uptake/utilization of glucose;
whereas, cardiac contractility and metabolism are improved when
GLUT4 is upregulated (46). AMPK,
a conserved serine/threonine protein kinase, acts as a target for
metabolic syndrome prevention (47). In the present study, it was
demonstrated that Apelin-13 elevated Apelin-12 expression in serum,
myocardial tissues and aortic tissues and resulted in increases in
myocardial GLUT4 and p-AMPKα2 levels. These results indicated that
Apelin-13 may enhance glucose metabolism and activate the AMPK
signaling pathway.
In conclusion, the results of the present study
demonstrated that Apelin-13 exerted beneficial effects on cardiac
function, hyperglycemia, IR, dyslipidemia, endothelial function,
inflammation and glucose metabolism, via upregulation of Apelin-12
and activation of the AMPK signaling pathway in type 2 diabetes.
This protective effect of Apelin was comparable to that of
metformin or atorvastatin. These findings indicate that Apelin-13
may be a potential therapeutic agent for the treatment of type 2
diabetes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ML and JH conceived the study and designed the
experiments. ML and HJF performed the experiments and analyzed the
data. ML and HJF provided reagents, materials and analysis tools.
ML and JH wrote and revised the manuscript.
Ethics approval and consent to
participate
The experiments were performed in accordance with
the Guide for the Care and Use of Laboratory Animals and were
approved by the Animal Care and Use Committee of China Medical
University (Shenyang, China). Ethical clearance was obtained from
the Institutional Animal Care and Use Committee (approval no.
2015052R).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yakaryilmaz FD and Ozturk ZA: Treatment of
type 2 diabetes mellitus in the elderly. World J Diabetes.
8:278–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koch A, Grammatikos G, Trautmann S,
Schreiber Y, Thomas D, Bruns F, Pfeilschifter J, Badenhoop K and
Penna-Martinez M: Vitamin D supplementation enhances
c18(dihydro)ceramide levels in type 2 diabetes patients. Int J Mol
Sci. 18:E15322017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bilir B, Bilir Ekiz B, Yilmaz I, Atile
Soysal N, Yildirim T, Kara SP, Gumustas SA, Orhan AE and Aydin M:
Association of apelin, endoglin and endocan with diabetic
peripheral neuropathy in type 2 diabetic patients. Eur Rev Med
Pharmacol Sci. 20:892–898. 2016.PubMed/NCBI
|
|
4
|
Peiro C, Lorenzo O, Carraro R and
Sanchez-Ferrer CF: IL-1β inhibition in cardiovascular complications
associated to diabetes mellitus. Front Pharmacol. 8:3632017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dunn JS, Mlynarski WM, Pezzolesi MG,
Borowiec M, Powers C, Krolewski AS and Doria A: Examination of
PPP1R3B as a candidate gene for the type 2 diabetes and MODY loci
on chromosome 8p23. Ann Hum Genet. 70:587–593. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tun NN, Arunagirinathan G, Munshi SK and
Pappachan JM: Diabetes mellitus and stroke: A clinical update.
World J Diabetes. 8:235–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shukla SK, Liu W, Sikder K, Addya S,
Sarkar A, Wei Y and Rafiq K: HMGCS2 is a key ketogenic enzyme
potentially involved in type 1 diabetes with high cardiovascular
risk. Sci Rep. 7:45902017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naqvi S, Naveed S, Ali Z, Ahmad SM, Khan
Asadullah R, Raj H, Shariff S, Rupareliya C, Zahra F and Khan S:
Correlation between glycated hemoglobin and triglyceride level in
type 2 diabetes mellitus. Cureus. 9:e13472017.PubMed/NCBI
|
|
9
|
Kupelian V, Hayes FJ, Link CL, Rosen R and
McKinlay JB: Inverse association of testosterone and the metabolic
syndrome in men is consistent across race and ethnic groups. J Clin
Endocrinol Metab. 93:3403–3410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saibandith B, Spencer JPE, Rowland IR and
Commane DM: Olive polyphenols and the metabolic syndrome.
Molecules. 22:E10822017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cirillo P, Ziviello F, Pellegrino G, Conte
S, Cimmino G, Giaquinto A, Pacifico F, Leonardi A, Golino P and
Trimarco B: The adipokine apelin-13 induces expression of
prothrombotic tissue factor. Thromb Haemost. 113:363–372. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folino A, Montarolo PG, Samaja M and
Rastaldo R: Effects of apelin on the cardiovascular system. Heart
Fail Rev. 20:505–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Q, Cao J and Chen L: Apelin/APJ
system: A novel therapeutic target for oxidative stress-related
inflammatory diseases (Review). Int J Mol Med. 37:1159–1169. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang S, Chen L, Lu L and Li L: The
apelin-APJ axis: A novel potential therapeutic target for organ
fibrosis. Clin Chim Acta. 456:81–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YW, He SJ, Feng X, Cheng J, Luo YT,
Tian L and Huang Q: Metformin: A review of its potential
indications. Drug Des Devel Ther. 11:2421–2429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sena CM, Matafome P, Louro T, Nunes E and
Seica RM: Effects of atorvastatin and insulin in vascular
dysfunction associated with type 2 diabetes. Physiol Res.
63:189–197. 2014.PubMed/NCBI
|
|
18
|
Howarth FC, Qureshi MA, Sobhy ZH, Parekh
K, Yammahi SR, Adrian TE and Adeghate E: Structural lesions and
changing pattern of expression of genes encoding cardiac muscle
proteins are associated with ventricular myocyte dysfunction in
type 2 diabetic goto-kakizaki rats fed a high-fat diet. Exp
Physiol. 96:765–777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Qi H, Wang Y, Wu M, Cao Y, Huang W,
Li L, Ji Z and Sun H: Allicin protects against myocardial apoptosis
and fibrosis in streptozotocin-induced diabetic rats.
Phytomedicine. 19:693–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wichi R, Malfitano C, Rosa K, De Souza SB,
Salemi V, Mostarda C, De Angelis K and Irigoyen MC: Noninvasive and
invasive evaluation of cardiac dysfunction in experimental diabetes
in rodents. Cardiovasc Diabetol. 6:142007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun
YC and Ko YL: Triglyceride glucose-body mass index is a simple and
clinically useful surrogate marker for insulin resistance in
nondiabetic individuals. PLoS One. 11:e01497312016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Relimpio F: ‘The relative contributions of
insulin resistance and beta-cell dysfunction to the pathophysiology
of Type 2 diabetes’ by kahn se. Diabetologia. 46:17072003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Q, Li X, Song P and Xu L: Optimal
cut-off values for the homeostasis model assessment of insulin
resistance (HOMA-IR) and pre-diabetes screening: Developments in
research and prospects for the future. Drug Discov Ther. 9:380–385.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morimoto A, Tatsumi Y, Soyano F, Miyamatsu
N, Sonoda N, Godai K, Ohno Y, Noda M and Deura K: Increase in
homeostasis model assessment of insulin resistance (HOMA-IR) had a
strong impact on the development of type 2 diabetes in Japanese
individuals with impaired insulin secretion: The Saku study. PLoS
One. 9:e1058272014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu XW, Deng FY and Lei SF: Meta-analysis
of atherogenic index of plasma and other lipid parameters in
relation to risk of type 2 diabetes mellitus. Prim Care Diabetes.
9:60–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mooradian AD: Dyslipidemia in type 2
diabetes mellitus. Nat Clin Pract Endocrinol Metab. 5:150–159.
2009.PubMed/NCBI
|
|
27
|
Tian L and Fu M: The relationship between
high density lipoprotein subclass profile and plasma lipids
concentrations. Lipids Health Dis. 9:1182010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian L, Liu Y, Qin Y, Long S, Xu Y and Fu
M: Association of the low-density lipoprotein
cholesterol/high-density lipoprotein cholesterol ratio and
concentrations of plasma lipids with high-density lipoprotein
subclass distribution in the Chinese population. Lipids Health Dis.
9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong WT, Wong SL, Tian XY and Huang Y:
Endothelial dysfunction: The common consequence in diabetes and
hypertension. J Cardiovasc Pharmacol. 55:300–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsumoto T, Lopes RA, Taguchi K,
Kobayashi T and Tostes RC: Linking the beneficial effects of
current therapeutic approaches in diabetes to the vascular
endothelin system. Life Sci. 118:129–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kalani M: The importance of endothelin-1
for microvascular dysfunction in diabetes. Vasc Health Risk Manag.
4:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Finch J and Conklin DJ: Air
pollution-induced vascular dysfunction: Potential role of
endothelin-1 (ET-1) system. Cardiovasc Toxicol. 16:260–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ergul A: Endothelin-1 and diabetic
complications: Focus on the vasculature. Pharmacol Res. 63:477–482.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaneko Kurohane Y and Ishikawa T: Dual
role of nitric oxide in pancreatic β-cells. J Pharmacol Sci.
123:295–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie W and Du L: Diabetes is an
inflammatory disease: Evidence from traditional chinese medicines.
Diabetes Obes Metab. 13:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ziamajidi N, Nasiri A, Abbasalipourkabir R
and Moheb Sadeghi S: Effects of garlic extract on TNF-α expression
and oxidative stress status in the kidneys of rats with STZ +
nicotinamide-induced diabetes. Pharm Biol. 55:526–531. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao L, Sun T and Wang L: Chitosan
oligosaccharide improves the therapeutic efficacy of sitagliptin
for the therapy of chinese elderly patients with type 2 diabetes
mellitus. Ther Clin Risk Manag. 13:739–750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Wang X, Zhao Y, Zhao P, Wang L,
Zhai Q, Zhang X, Tian W, Xiang X and Li T: Upregulation of tumor
necrosis factor-α-induced protein 8-like 2 mrna is negatively
correlated with serum concentrations of tumor necrosis factor-α and
interleukin 6 in type 2 diabetes mellitus. J Diabetes Res.
2017:48023192017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hosoi T and Ozawa K: Possible
pharmacological approach targeting endoplasmic reticulum stress to
ameliorate leptin resistance in obesity. Front Endocrinol
(Lausanne). 7:592016.PubMed/NCBI
|
|
41
|
Jacobo-Cejudo MG, Valdes-Ramos R,
Guadarrama-Lopez AL, Pardo-Morales RV, Martinez-Carrillo BE and
Harbige LS: Effect of n-3 polyunsaturated fatty acid
supplementation on metabolic and inflammatory biomarkers in type 2
diabetes mellitus patients. Nutrients. 9:E5732017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Catalan V, Gomez-Ambrosi J, Rodriguez A,
Salvador J and Fruhbeck G: Adipokines in the treatment of diabetes
mellitus and obesity. Expert Opin Pharmacother. 10:239–254. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Erdem G, Dogru T, Tasci I, Sonmez A and
Tapan S: Low plasma apelin levels in newly diagnosed type 2
diabetes mellitus. Exp Clin Endocrinol Diabetes. 116:289–292. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu S, Sun F, Li W, Cao Y, Wang C, Wang Y,
Liang D, Zhang R, Zhang S, Wang H and Cao F: Apelin stimulates
glucose uptake through the PI3K/Akt pathway and improves insulin
resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 353:305–313.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shen X, Zhou N, Mi L, Hu Z, Wang L, Liu X
and Zhang S: Phloretin exerts hypoglycemic effect in
streptozotocin-induced diabetic rats and improves insulin
resistance in vitro. Drug Des Devel Ther. 11:313–324. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Williams LJ, Nye BG and Wende AR:
Diabetes-related cardiac dysfunction. Endocrinol Metab (Seoul).
32:171–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kang OH, Shon MY, Kong R, Seo YS, Zhou T,
Kim DY, Kim YS and Kwon DY: Anti-diabetic effect of black ginseng
extract by augmentation of AMPK protein activity and upregulation
of GLUT2 and GLUT4 expression in db/db mice. BMC Complement Altern
Med. 17:3412017. View Article : Google Scholar : PubMed/NCBI
|