Introduction
Dry eye is a multifactorial ocular surface disease,
usually caused by insufficient secretion of lipid, mucus and aqua,
leading to abnormal quality, quantity and dynamics of the tear,
which induces fatigue, pain, dryness and a burning sensation in the
eye (1,2). Dry eye may additionally affect visual
function and may associate with ocular surface inflammation or
increased tear osmotic pressure (3). At present, dry eye has become one of
the most common ocular surface diseases. According to the
preliminary results of an epidemiological survey, the incidence
rate of dry eye is high, reaching 5–35% around the world (4). The incidence rate of females is
higher compared with males; the majority of females are in
perimenopause (5), of which the
incidence rate of females over 50 is twice as high as that of
males. At present, there exist numerous methods to treat dry eye,
of which artificial tears and lacrimal puncta occlusion are the
primary ones (6,7).
Compared with the materials commonly used in
lacrimal puncta occlusion, the amniotic membrane does not have any
nerves, blood vessels or lymphatic vessels, and has tough tenacity
and low antigenicity (8–10). In addition, it has certain
biological functions, including inhibiting conjunctival squamous
metaplasia and inflammation with a low degree of foreign body
sensation and irritation (11–14).
A previous study suggested that the amniotic membrane may reduce
the formation of novel blood vessels, inhibit fibrosis and scar
hyperplasia (15). The amniotic
lacrimal duct stent is a novel and ideal lacrimal duct, which uses
supporting material that is fabricated based on the amniotic
membrane. Compared with the currently used lacrimal duct stent, the
amniotic lacrimal duct stent has lower antigenicity and better
tissue compatibility, with no hormone-like side effects or patient
discomfort. It additionally possesses a certain biological
function, enabling it to retain residual tears and to treat
lacrimal duct obstruction (16).
The author hypothesized that following the implantation of an
amniotic lacrimal stent at the lacrimal duct, the dry eye symptom
may be alleviated by retaining residual tears (16). The present study aimed to examine
the therapeutic effect of amniotic lacrimal stent implantation in
perimenopausal female rabbits, which may provide experimental
evidence for treating dry eye in females in perimenopause.
Materials and methods
Materials
Amniotic membrane lacrimal stents were purchased
from Jiangxi Ruiji Biological Engineering Technology Co., Ltd.
(Nanchang, China). Schirmer I test (SIT) paper was obtained from
Bausch + Lomb (Rochester, NY, USA). Polyvinyl chloride filter paper
and plastic film were purchased from Shanghai Peninsula Industrial
Co., Ltd. (Shanghai, China).
Experimental animals
New Zealand white rabbits were purchased from the
Animal Experiment Department of Nanchang University School of
Medicine (Nanchang, China). In total, 48 New Zealand white rabbits
(age, 3 months; weight, 1.5–2.0 kg) were randomly selected (12–12 h
alternating light and dark, ambient temperature 20–25°C, artificial
feeding). The 48 selected female rabbits were evaluated by
ophthalmoscopy and slit lamp microscopy, and the result
demonstrated that there were no abnormalities in the rabbits. The
SIT was ≥10 mm/5 min. Ethics approval was obtained from the Medical
Ethics Committee of The First Affiliated Hospital of Nanchang
University (Nanchang, China).
Surgery and grouping
Incision and suture in the skin of the ovary area
were conducted in 12 female rabbits (group A, sham operation
group). In order to reduce experimental errors caused by surgical
operations, the other 36 female rabbits underwent bilateral
ovariectomy. The female rabbits were divided into groups B, C and D
(12 eyes each; all right eyes). The postoperative group B (negative
control group) was not treated and only observed for 6 weeks. On
the day of operation, the lacrimal duct stents were implanted into
the lacrimal passages of the female rabbits of group C for 5 sec
and subsequently removed (sham-implantation group). This step was
conducted to demonstrate that short-term implantation does not
serve a role in treatment. Only by implanting the stent into the
body for a long period of time, it is able to gradually function
and form a better contrast compared with the other groups. The
group D rabbits (amniotic lacrimal duct scaffold group) were
implanted with lacrimal duct stents for 6 weeks. Subsequent to the
completion of all operations for all the groups, SIT, corneal
fluorescein (FL) staining, optical coherence tomography angiography
(OCTA) and corneal confocal microscope scanning were performed
prior to and following implantation on the 14, 28th and 42nd day.
The rabbits were alive prior to conducting the SIT, corneal FL
staining, OCTA and corneal confocal microscopy, and measuring the
biomechanical properties of amniotic membrane. All of the
operations were conducted in the same test environment and by the
same operator.
SIT
SIT was performed with 5×35 mm scale test paper.
Subsequent to folding 8 mm, the folded end was placed at one-third
of the inferior conjunctiva sac of the female rabbits in each
group, and the rest of the test paper was suspended perpendicularly
to the skin surface. After 5 min, the wet length of the scale
filter paper was recorded. According to the standard (17), the moist test strip was longer
compared with the 10 mm considered normal. All of the operations
were conducted in the same test environment and by the same
operator.
Corneal FL score examination
In total, one drop of 1% fluorescein sodium eye
drops was applied to the eyes of the female rabbits in each group.
Subsequent to blinking, the corneal epithelial staining was
observed by using slit lamps, magnification, ×10. The score was
measured as previously described (18): i) No staining spots on corneal
epithelium recorded as zero points; ii) punctate staining and
<30 points in the corneal epithelium recorded as one point; iii)
corneal epithelial visible spot-like staining and >30 points,
with no diffusion recorded as two points; iv) diffused infiltration
of the corneal epithelium staining, with no plaque formation
recorded as three points; and v) visible corneal fluorescein plaque
recorded as four points.
Confocal microscopy
The confocal microscopy, magnification, ×10. was
performed by the same operator. Measurement of corneal epithelial
alterations in the eyes of the female rabbits in each group was
performed as previously described (19). The rabbit heads were held to ensure
that their eyes were straight, and 5 g.l−1 Alcaine eye
drops (Alcon, Fort Worth, TX, USA) were administered. The full
thickness of the central cornea was scanned, clear and effective
images were saved, and the density of corneal-activated stromal and
inflammatory cells was calculated using computer software Image J
V1.8.0 (National, Institutes of Health, Bethesda, MD, USA).
OCTA measurement
The OCTA was imaged with the AngioVue OCTA system
(Optovue, Inc., Fremont, CA, USA) retinal-imaging mode (AngioRetina
mode), and its split-spectrum amplitude-decorrelation angiography
algorithm was used for imaging. Scanning parameters were set as
follows: i) Light source: 840 nm; ii) beam width: 22 µm; iii)
lateral resolution: 15 µm; and iv) axial resolution: 5 µm. All
operations were repeated three times. The autofocus function was
turned off to measure corneal full thickness and corneal epithelial
thickness to create a chronic lateral 304×304 A-scan of 70,000
beats/second and capture subsequent cross-sectional scans (B-scan)
to obtain images.
Nerve density (mm/mm2)
The confocal was used to observe the nerve fibers.
The corneal plexus was observed with a depth of observation of
35–50 µm. The length of the subintimal nerve fibers was determined
using AUTOCAD 2016 software (Autodesk, Inc., San Rafeal, CA, USA).
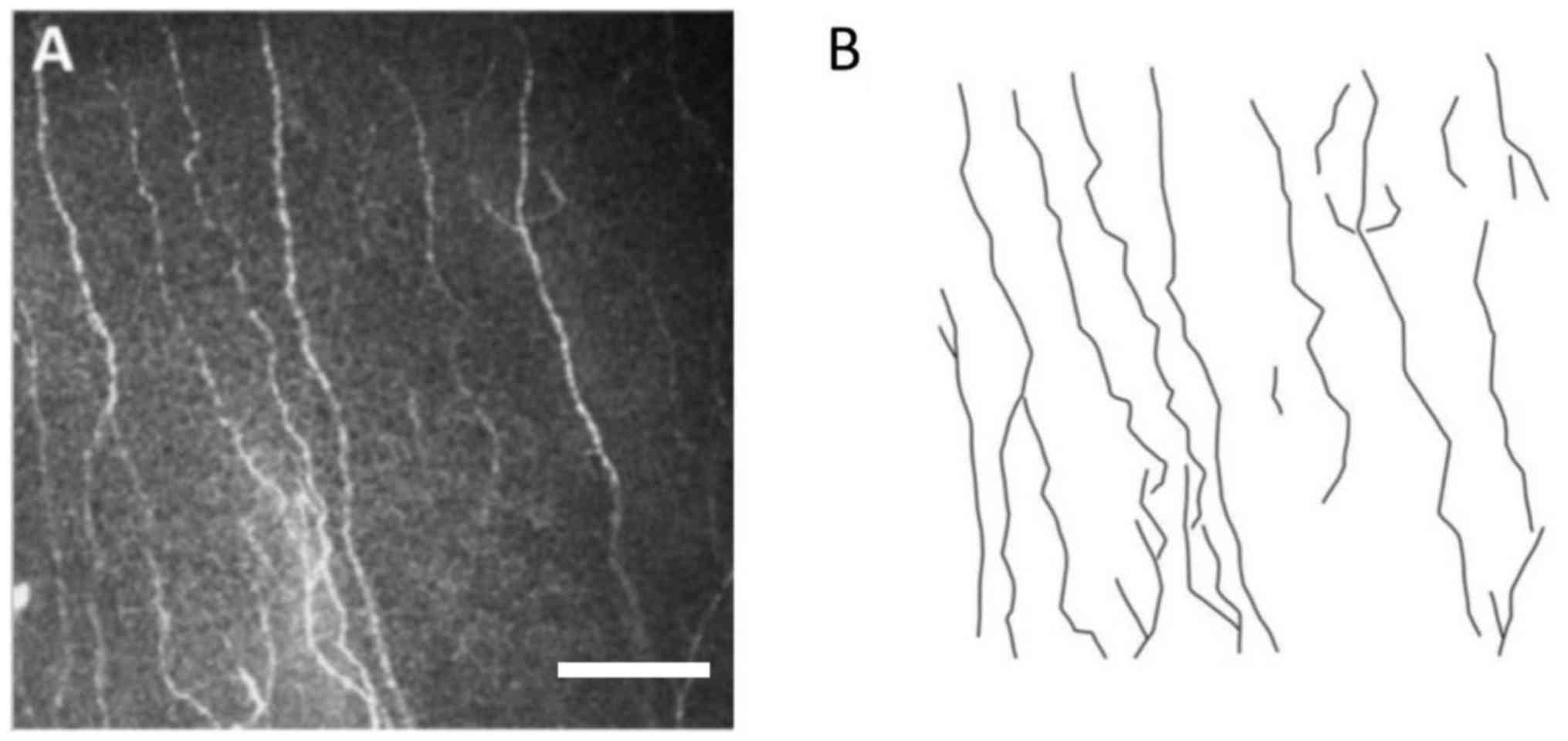
Each image was obtained according to the actual corneal area of
0.16 mm2 (400×400 µm) per frame, and the shape of nerve
fibers was outlined (Fig. 1). The
total length of the broken line was determined by the
characteristics of the broken line. The total length obtained was
divided by area (0.16 mm2) to obtain nerve fiber density
(mm/mm2). The ACCMetrics software was used to make
statistics of the neural quantity and morphological parameters of
the selected images. The density of the branch of the cornea nerve:
The number of branches of the nerve fibers in the image per square
millimeter, in strips/mm2.
Statistical analysis
GraphPad Prism 4.00 statistical software (GraphPad
Software, Inc., La Jolla, CA, USA) was used for the statistical
analysis. Data are presented as the mean ± standard deviation from
three independent experiments. The data were analyzed using the
χ2 test and the test level was α=0.05. Treatment effects
prior to and following treatment and group differences were
compared by one-way analysis of variance, followed by Dunnett's
post hoc test for comparison of multiple sets of data. Student's
t-test was used to compare two sets of data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Nerve fibres and polyline
analysis
Representative images of nerve fibers and polyline
analysis are presented in Fig.
1.
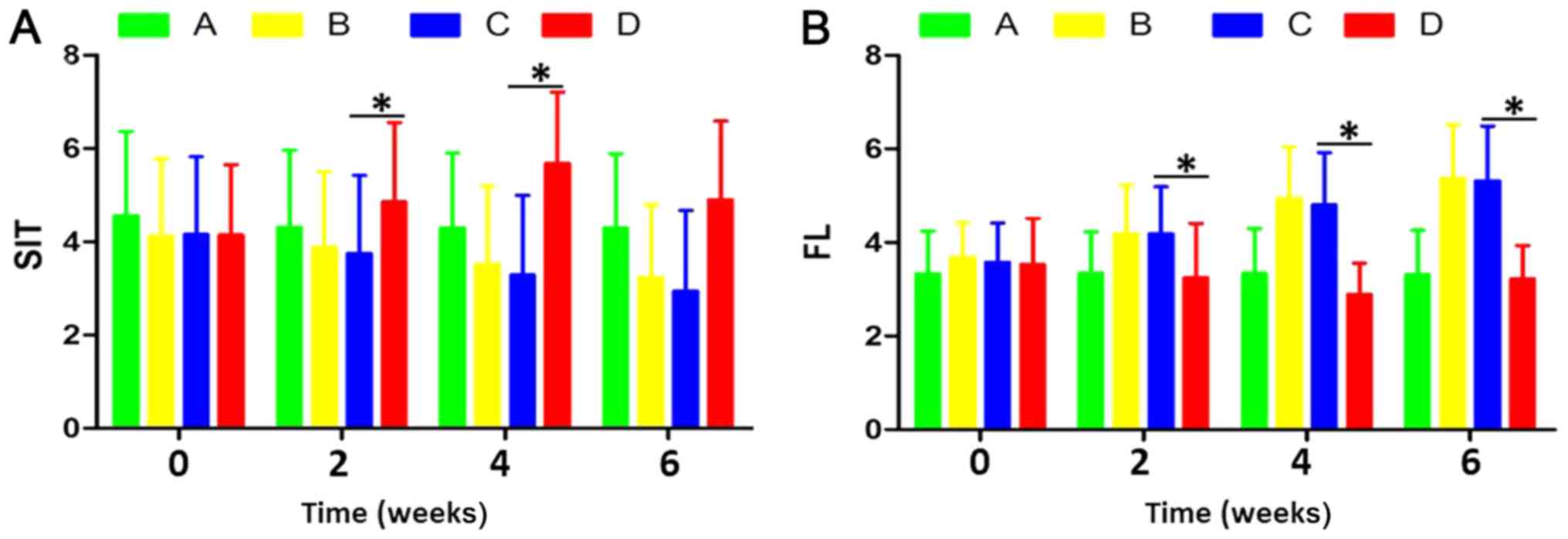
Comparison of SIT result between
groups prior to and following implantation
The tear secretion in each time period is presented
in Table I. Compared with
pretreatment, tear secretion volume in groups B, C and D was
significantly altered after 2 and 4 weeks (F=11.538; P=0.014), and
there was no significant difference between group A and
pre-experiment (F=0.572; P=0.124). Compared with group A, the
amount of lacrimal fluid secretion was not increased significantly
in group D (F=0.992; P=0.062). Compared with group C, the secretion
of lacrimal fluid in group D was significantly increased (F=10.543;
P=0.009). In the fourth week of the experiment, the SIT of the
rabbit was normal (16).
 | Table I.Comparison of each period of tear
secretion prior to and following implantation of the amniotic
lacrimal ducts (mm). |
Table I.
Comparison of each period of tear
secretion prior to and following implantation of the amniotic
lacrimal ducts (mm).
|
|
|
| Post-treatment,
weeks |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Group | N | Pretreatment | 2 | 4 | 6 | F | P |
|---|
| A | 12 | 4.49±1.55 |
4.31±1.55c |
4.29±1.51c |
4.31±1.49c | 0.572 | 0.124 |
| B | 12 | 4.12±1.49 |
3.88±1.52c |
3.52±1.56c |
3.23±1.46c | 11.538 | 0.014 |
| C | 12 | 4.08±1.56 |
3.75±1.57c |
3.29±1.61c |
2.95±1.61c | 13.437 | 0.009 |
| D | 12 | 4.15±1.41 |
4.86±1.62b,c |
5.72±1.42a–c |
4.96±1.58c | 7.643 | 0.032 |
FL score comparison between groups
prior to and following implantation
The results of corneal FL score in each period are
presented in Table II. Compared
with prior to intervention, the FL scores of groups B, C and D
significantly altered (F=8.894; P=0.017) and there was no
significant difference between group A and pre-experiment (F=0.719;
P=0.071). Compared with group A, the FL score of group D was not
significantly decreased (F=0.921; P=0.067). The FL score in group D
was significantly decreased compared with group C (F=10.543;
P=0.009; Fig. 2). In the fourth
week of the experiment, the FL staining of the rabbits was normal
(17).
 | Table II.Comparison of corneal fluorescein
score prior to and following implantation of amniotic lacrimal duct
scaffolds. |
Table II.
Comparison of corneal fluorescein
score prior to and following implantation of amniotic lacrimal duct
scaffolds.
|
|
|
| Following,
weeks |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Group | N | Prior | 2 | 4 | 6 | F | P |
|---|
| A | 12 | 3.33±0.85 |
3.35±0.79c |
3.32±0.78c |
3.34±0.86c | 0.719 | 0.071 |
| B | 12 | 3.68±0.69 |
4.19±0.96c |
4.94±1.06c |
5.38±1.08c | 8.894 | 0.017 |
| C | 12 | 3.58±0.79 |
4.18±0.93c |
4.81±1.07c |
5.34±1.11c | 10.153 | 0.015 |
| D | 12 | 3.58±0.89 |
3.21±1.06b,c |
2.85±0.62a–c |
3.24±0.67b,c | 15.654 | 0.039 |
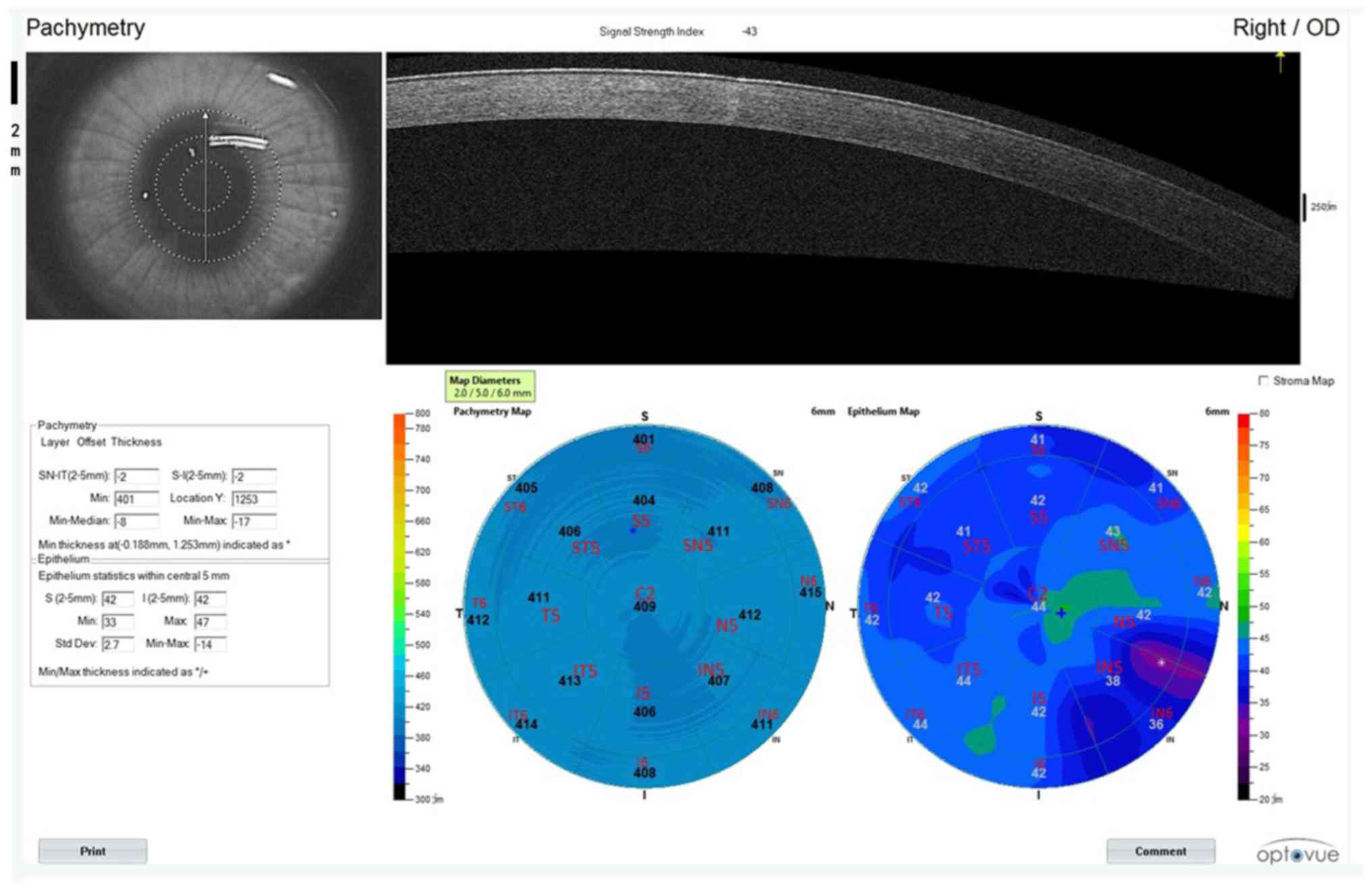
Thickness of corneal epithelium is
measured by OCTA
Taking the right eye as an example, the cornea was
divided into 17 regions, of which the central corneal diameter was
2 mm within the central ring, the middle and outer ring were 5 and
6 mm away from the corneal center, respectively. The middle ring
and the outer ring were equally divided into the following areas:
Inferior (I), inferior temporal (IT), superior (S), superior nasal
(SN), nasal (N), inferior nasal (IN) temporal (T), superior
temporal (ST). Corneal epithelial and full corneal thickness was
measured in all regions (Fig.
3).
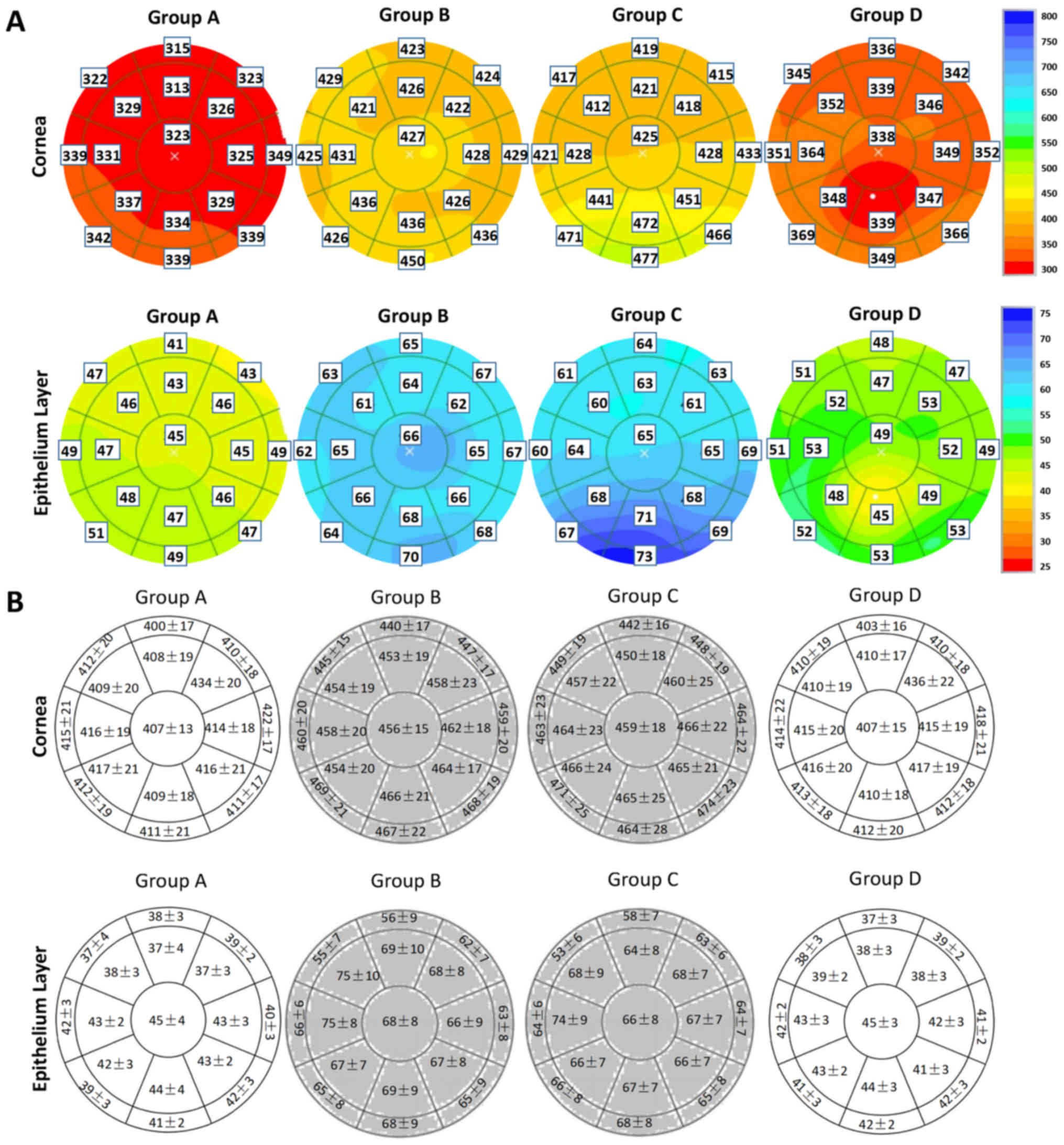
Representative images of the thickness of cornea and
corneal epithelium from four individuals in the four groups are
presented in Fig. 4A. The
thickness of the corneal epithelium in groups A and D was
significantly thinner compared with groups B and C (P<0.05). The
upper part of the corneal epithelium (ST5, S5, SN5, ST6, S6 and
SN6) was significantly thinner compared with the central cornea
epithelium (P<0.05). The corneal thickness of group D was
significantly thinner compared with groups B and C (P<0.05). The
corneas of rabbits in groups A, B, C and D were all thin at the
central region and thick at the periphery. In addition, the lower
part of the cornea (T5, IT5, IN5, N5, T6 and N6) in each group was
significantly thicker compared with the central cornea (P<0.05;
Fig. 4B).
Corneal confocal microscopy
results
Corneal confocal microscopy was conducted to assess
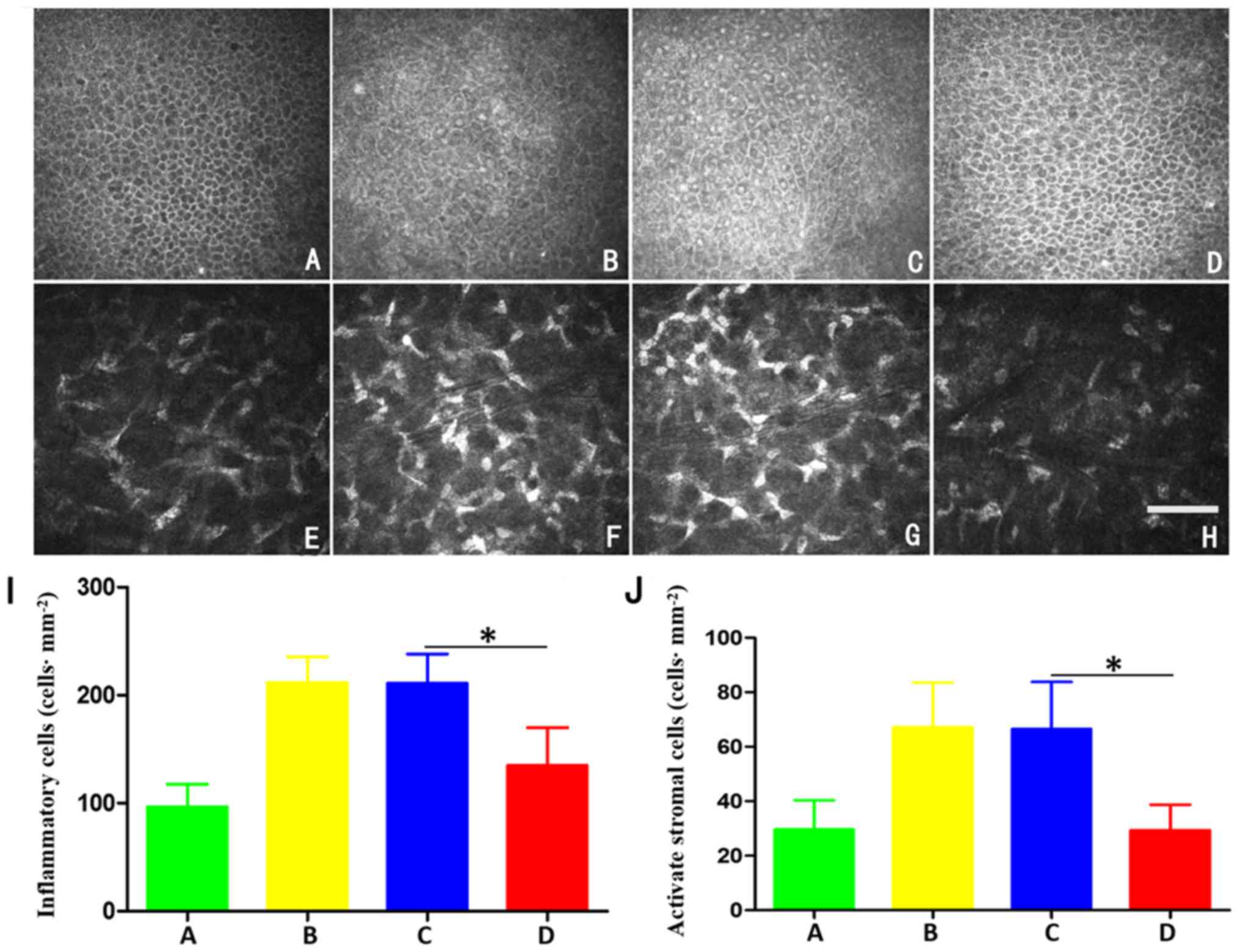
inflammatory cells and activated stromal cells (Fig. 5). In group A, the bright glowing
activated stromal cells with a narrow cell boundary were visible
(Fig. 5A). Compared with group A,
a larger number of bright inflammatory cells was observed in groups
B and C, and the number of activated interstitial cells was
significantly increased with more marked deformation (Fig. 5B, C and I; P<0.05). After 6
weeks implantation of amniotic lacrimal duct scaffold, the
epithelial basal cell number was slightly increased, compared with
group A. However, compared with groups B and C, epithelial basal
cells volume in group D was decreased, and the number of bright
inflammatory cells was decreased (Fig.
5D). The pictures of activated stromal cells of group A, B, C
and D are shown in Fig. 5E-H. The
number of activated stromal cells and inflammatory cells in group B
were 67±15 and 211±21 cells/mm2, respectively after 6
weeks of intervention. The density of activated stromal cells and
inflammatory cells in group C were 66±17 and 219±25
cells/mm2, respectively. The density of activated
stromal cells and inflammatory cells in group D were 29±8 and
135±32 cells/mm2, and the difference between group D and
groups B and C was statistically significant (both P<0.05).
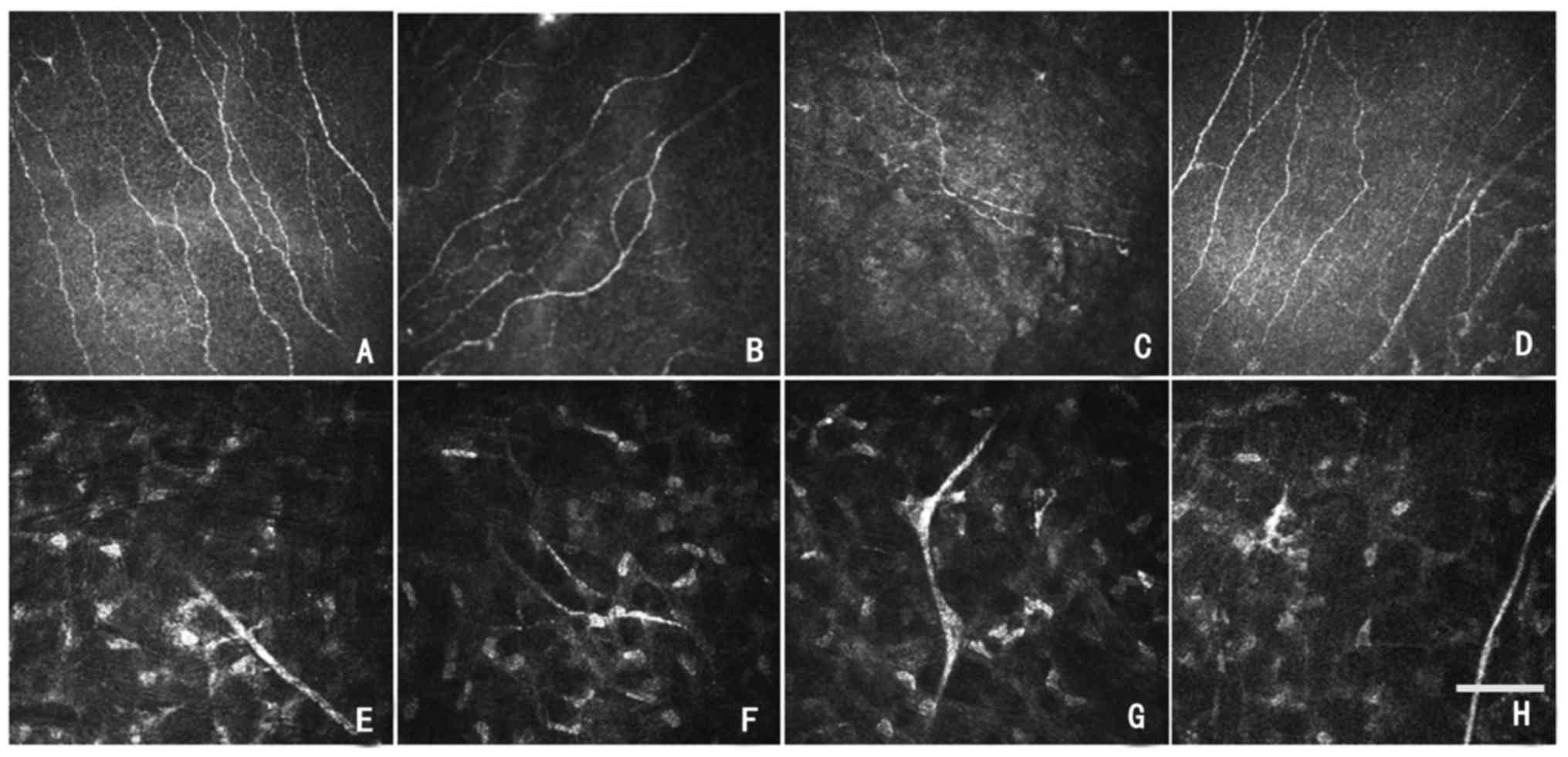
There were only a few luminescent cells in the
anterior corneal stroma in group A (Fig. 6A); whereas, a large number of
luminescent cells were detected in group B and group C, and the
numbers of luminescent cells increased significantly compared with
group A (Fig. 6B and C). Following
implantation of the amniotic lacrimal duct for 6 weeks, group D
demonstrated a slight increase in stromal luminescent compared with
group A; however, the numbers were decreased compared with group B
and group C (Fig. 6D).
A clear and straight corneal stroma nerve trunk was
visible in group A (Fig. 6E). In
total, 6 weeks after implantation of amniotic lacrimal duct
scaffolds, the nerve trunk of corneal stroma in group D was
slightly curved compared with group A; however, improved
significantly compared with group B and C (Fig. 6E-H).
Quantitative analysis of nerve prior
to and following the amniotic membrane lacrimal duct stent
implantation
Prior to amniotic lacrimal duct stent implantation,
there was no significant difference in the density, branch or
curvature of the corneal subcutaneous nerve in each group in
Fig. 6 (P>0.05). There was no
significant difference in the density, branch or curvature of
corneal epithelium in group A prior to and following implantation
(P>0.05). The density, branch or curvature of the corneal
subcutaneous nerve in groups B, C and D altered prior to and
following amniotic lacrimal stent implantation, and the alterations
in group B and group C were more marked, the difference was
statistically significant (P<0.05). Following amniotic lacrimal
duct stent implantation for 6 weeks, the density, branch or
curvature of corneal subcutaneous nerve in group D were
significantly improved compared with group C (P<0.05; Table III).
 | Table III.Comparison of nerve under corneal
endothelium in different groups. |
Table III.
Comparison of nerve under corneal
endothelium in different groups.
| Group | Case number | Nerve fiber
density, mm/mm2 | Nerve fibre
branches, branch number per image | Curvature
score |
|---|
| A | 12 |
20.12±4.21b |
10.95±2.04b |
3.32±0.67b |
| B | 12 |
15.32±2.96a |
7.21±1.87a |
1.91±0.42a |
| C | 12 |
16.21±2.75a |
7.53±1.92a |
1.82±0.51a |
| D | 12 |
19.11±3.53b |
9.81±1.74b |
3.23±0.78b |
Discussion
With aging and the wide application of electronic
equipment, dry eye has become one of the most common eye diseases.
Causes of dry eye syndrome are very complicated, including
ophthalmic surgery, inflammation, immune diseases and metabolic
diseases (20). However, due to
symptoms, including dryness, foreign body sensation, fatigue,
photophobia and a burning sensation in patients (1), clinical treatment must be actively
sought (21). Dry eye may be
induced if abnormality happens at any of the process of tear
formation, distribution, evaporation and removal. Therefore, when
designing the causative treatment therapy, the retention and use of
limited remaining tear and extrinsic artificial tears are
additionally important factors to consider. However, the additives
or preservatives and other ingredients in artificial tears
inevitably affect the ocular surface environment and cornea
(22). Frequent use of artificial
tear will additionally affect the composition of the tear film
distribution and accelerate tear evaporation (23). There are a number of effective
means of retention and use of tears, of which lacrimal duct
embolism is one of the most important treatments (24). Therefore, the present study
selected amniotic lacrimal stent treatment to examine the treatment
of lacrimal duct embolism.
Amniotic membrane, derived from the trophoblast
layer of transparent tissue, containing a large number of bioactive
ingredients with relatively soft texture, may nourish the corneal
nerve (14). As a lacrimal
support, the amniotic membrane is easy to use, safe and non-toxic.
It may function as the support itself and may additionally reduce
the damage to the eye tissue to a great extent, to improve the
current foreign body lacrimal support (25). Furthermore, the anatomy of the
amniotic lacrimal support lacrimal duct is similar to the native
lacrimal structure, which may adapt to the use of obstruction of
the canaliculus to retain residual tears, to avoid the loss of
immune proteins and ionic components, and to protect the eye tissue
and increase tear film stability, thus serving a therapeutic role
in the treatment of dry eye (26).
The experimental results demonstrated that compared
with groups B and C, following implantation of the amniotic
lacrimal duct stent, SIT and FL values of group D were
significantly improved; whereas, the SIT and FL values in groups B
and C deteriorated. The number of epithelial cells in group D was
decreased compared with groups B and C, and the density of
inflammatory cells was decreased. The density of corneal epithelial
cells in patients with dry eye decreased; whereas, the increase of
inflammatory cell density may cause apoptosis of epithelial cells,
and epithelial cell apoptosis may lead to the reduction of corneal
tear film stability. However, inflammation is not a single factor
in dry eye. At the same time, inflammation stimulates epithelial
cell edema leading to an increase in the thickness of the corneal
epithelium. Multiple mixing mechanisms may lead to squamous
epithelial hyperplasia or metaplasia of the corneal epithelium. The
implantation of an amniotic lacrimal duct scaffold effectively
retains tears, resulting in corneal nutrition, protection and
lubrication, and the improvement of dry eye symptoms (27).
As a novel technology with great potential, OCTA may
directly observe the range of the lesion and the alteration of the
length, caliber, area and other aspects of the corneal epithelium
with high sensitivity (28,29).
The experimental results demonstrated that following implantation
of amniotic lacrimal duct stents, the corneal epithelial
hyperplasia of group D was decreased; whereas, the corneal
thickness of group B and C increased significantly. Dry eye results
in the instability of tear film in female rabbit models, causing
corneal damage to a certain degree and increasing inflammatory
factors. With time, pro-inflammatory cytokines continue to be
secreted, and increase the damage to ocular surface cells. When the
corneal microenvironment stability is disrupted, corneal cell
proliferation and repair occur (30). Furthermore, the stability of the
corneal microenvironment is difficult to recover in dry eye, which
hinders the proliferation of corneal cells to a certain extent and
affects the corneal tissue repair and healing following injury,
leading to the increase of corneal thickness (31). The amniotic lacrimal duct stent
implantation retains tears to a certain extent, and maintains the
stability of the corneal microenvironment (30). Consequently, corneal damage may be
alleviated, and it was hypothesized that amniotic lacrimal support
for perimenopausal dry eye in rabbits has good efficacy, which
provides novel insight for future studies.
In conclusion, amniotic lacrimal duct stents may
serve a therapeutic effect for dry eye, which has specific clinical
value. However, the possible adverse reactions upon application
require further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660158, 81460092
and 81400372); Natural Science Key Project of Jiangxi Province
(grant no. 20161ACB21017); Youth Science Foundation of Jiangxi
Province (grant no. 20151BAB215016, 20161BAB215198); Key Research
Foundation of Jiangxi Province (grant no. 20151BBG70223 and
20181BBG70004); Education Department Key Project of Jiangxi
Province (grant no. GJJ160020); Teaching Reform of Degree and
Graduate Education Research Project of Jiangxi Province (grant no.
JXYJG-2018-013); Grassroots Health Appropriate Technology ‘Spark
Promotion Plan’ Project of Jiangxi Province (grant no. 20088003);
Health Development Planning Commission Science Foundation of
Jiangxi Province (grant no. 20175116); Health Development Planning
Commission Science TCM Foundation of Jiangxi Province (grant no.
20150823).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
MM contributed to the study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. QY contributed to the study design, data collection,
statistical analysis and data interpretation. LY contributed to the
statistical analysis and data interpretation. KL contributed to the
manuscript preparation, literature search and funds collection. LHY
contributed to the study design, data collection and funds
collection. YM contributed to the literature search, statistical
analysis and funds collection. NJ contributed to the literature
search, statistical analysis and funds collection. QL contributed
to the study design, statistical analysis and manuscript
preparation. WS contributed to the literature search, statistical
analysis and funds collection. XX contributed to the statistical
analysis and data interpretation. PZ contributed to the study
design, data collection and statistical analysis. YS contributed to
the study design, data collection, statistical analysis, data
interpretation, manuscript preparation and funds collection. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Medical Ethics
Committee of The First Affiliated Hospital of Nanchang
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cuevas M, González-García MJ, Castellanos
E, Quispaya R, Parra Pde L, Fernández I and Calonge M: Correlations
among symptoms, signs, and clinical tests in evaporative-type dry
eye disease caused by Meibomian gland dysfunction (MGD). Curr Eye
Res. 37:855–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uchino Y, Uchino M, Dogru M, Ward S, Yokoi
N and Tsubota K: Changes in dry eye diagnostic status following
implementation of revised Japanese dry eye diagnostic criteria. Jpn
J Ophthalmol. 56:8–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
The definition and classification of dry
eye disease: Report of the definition and classification
subcommittee of the international dry eye workshop (2007). Ocul
Surf. 5:75–92. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
The epidemiology of dry eye disease:
Report of the epidemiology subcommittee of the international Dry
Eye WorkShop (2007). Ocul Surf. 5:93–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wenderlein M and Mattes S: The ‘dry eye’
phenomenon and ovarian function. Study of 700 women pre-and
postmenopausal. Zentralbl Gynakol. 118:643–649. 1996.(In German).
PubMed/NCBI
|
|
6
|
Gayton JL: Etiology, prevalence and
treatment of dry eye disease. Clin Ophthalmol. 3:405–412. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schaumberg DA, Sullivan DA, Buring JE and
Dana MR: Prevalence of dry eye syndrome among US women. Am J
Ophthalmol. 136:318–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
John T and John OC: Ultrastructural
characteristics of four types of human amniotic membranes. Invest
Ophthalmol Vis Sci. 42:271–274. 2000.
|
|
9
|
Mamede AC, Carvalho MJ, Abrantes AM,
Laranjo M, Maia CJ and Botelho MF: Amniotic Membrane: From
structure and functions to clinical applications. Cell Tissue Res.
349:477–458. 2012. View Article : Google Scholar
|
|
10
|
Lceffelbein DJ, Eaumann C, Stoeckelhuber
M, Hasler R, Mücke T, Steinsträßer L, Drecoll E, Wolff KD and
Kesting MR: Amniotic membrabe as part of a skin substitute for
full-thickness wounds: An experimental evaluation in a porcine
model. J Biomed Mater Res B Appl Biomater. 100:1245–1256. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tseng SC, Espana EM, Kawakita T, Di
Pascuale MA, Li W, He H, Liu TS, Cho TH, Gao YY, Yeh LK and Liu CY:
How does amniotic membrane work? Ocul Surf. 2:177–187. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao Y, Zhou X, Yu Y, Pei CG, Zhou Q, Li
J, Yang L, Dong WJ and Yi JL: Novel sutureless transplantation for
primary pterygium associated with cysts. Int J Ophthalmol.
4:280–283. 2011.PubMed/NCBI
|
|
13
|
Shao Y, Yu Y, Liu QP, Li JM, Dong F, Huang
X, Pei CG, Tu P, Li HH and Gao GP: Effects of Honghua preserved
amnion membrane on scar healing in experimental glaucoma surgery.
Int J Ophthalmol. 7:226–231. 2014.PubMed/NCBI
|
|
14
|
Rauz S and Saw VP: Serum eye drops,
amniotic membrane and limbal epithelial stem cells-tools in the
treatment of ocular surface disease. Cell Tissue Bank. 11:13–27.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan Y, Qiu F, Qu YL, Li C, Shao Y, Xiao Q,
Liu Z and Li W: Amniotic membrane inhibits squamous metaplasia of
human conjunctival epithelium. Am J Physiol Cell Physiol.
301:C115–C125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li LP, Kang HH, Ma MY, Ye L, Li QhH, Lan
DY, Liu JX, Xu Qh, Yang YC, Yuan Q and Shao YI: Biological
characteristics of ECM amniotic membrane rod and its application in
rabbit dry eyes. Rec Adv Ophthalmol. 38:709–714. 2018.
|
|
17
|
Lemp MA: Report of the national eye
institute/industry workshop on clinical trials in dry eyes. CLAO J.
21:221–232. 1995.PubMed/NCBI
|
|
18
|
Pauly A, Brognole-baudouin F, Labbé A,
Liang H, Warnet JM and Baudouin C: New tools for the evaluation of
toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci.
48:5473–5483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ang M, Sim DA, Keane PA, Sng CC, Egan CA,
Tufail A and Wilkins MR: Optical coherence tomography angiography
for anterior segment vasculature imaging. Ophthalmology.
122:1740–1747. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michelle H and Esen KA: Dry eye: An
inflammatory ocular disease. J Ophthalmic Vis Res. 9:240–250.
2014.PubMed/NCBI
|
|
21
|
Fayet B, Koster H, Benabderrazik S,
Bernard JA and Pouliquen Y: Six canalicular stenoses after 34
punctal plugs. Eur J Ophthalmol. 1:154–155. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Javadi MA and Feizi S: Dry eye syndrome. J
Ophthalmic Vis Res. 6:192–198. 2011.PubMed/NCBI
|
|
23
|
Lemp MA: Management of dry eye disease. Am
J Manag Care. 14 (3 Suppl):S88–S101. 2008.PubMed/NCBI
|
|
24
|
Management and therapy of dry eye disease:
Report of the management and therapy subcommittee of the
international dry eye worksho (2007). Ocul Surf. 5:163–178. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allen CL, Clare G, Stewart EA, Branch MJ,
McIntosh OD, Dadhwal M, Dua HS and Hopkinson A: Augmented dried
versus cryopreserved amniotic membrane as an ocular surface
dressing. PLoS One. 8:e784412013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alfawaz AM, Algehedan S, Jastaneiah SS,
Al-Mansouri S, Mousa A and Al-Assiri A: Efficacy of punctal
occlusion in management of dry eyes after laser in situ
keratomileusis for myopia. Curr Eye Res. 39:257–262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang QC, Bao J, Li C, Tan G, Wu AH, Ye L,
Ye LH, Zhou Q and Shao Y: A murine model of dry eye induced by
topical administration of erlotinib eye drops. Int J Mol Med.
41:1427–1436. 2018.PubMed/NCBI
|
|
28
|
Ang M, Cai Y, Shahipasand S, Sim DA, Keane
PA, Sng CC, Egan CA, Tufail A and Wilkins MR: En face optical
coherence tomography angiography for corneal neovascularization. Br
J Ophthalmol. 100:616–621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ang M, Cai Y, MacPhee B, Sim DA, Keane PA,
Sng CC, Egan CA, Tufail A, Larkin DF and Wilkins MR: Optical
coherence tomography angiography and indocyanine green angiography
for corneal vascularisation. Br J Ophthalmol. 100:1557–1563. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin H and Yiu SC: Dry eye disease: A
review of diagnostic approaches and treatments. Saudi J Ophthalmol.
28:173–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ljubimov AV and Saghizadeh M: Progress in
corneal wound healing. Qrog Retin Eye Res. 49:17–45. 2015.
View Article : Google Scholar
|