Introduction
Inflammatory bowel disease (IBD) primarily comprises
two principal conditions, Crohn's disease and ulcerative colitis
(UC), characterized by chronic gastrointestinal inflammation with
alternating periods of relapse and remission (1). A variety of inflammatory mediators
are involved in the pathogenesis of IBD, including tumor necrosis
factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and intercellular
adhesion molecule-1 (ICAM-1) (2).
Excessive inflammatory mediators may lead to edema, ulceration and
carcinogenesis of colon tissue (2,3).
Therefore, effective regulation of the secretion of inflammatory
factors is essential for IBD treatment.
The etiology of IBD is not yet completely
understood; it is believed that a complex interaction among
genetic, immunological, metabolic, vascular, microbial and social
factors leads to dysregulated and persistent inflammation (4). The current therapies for IBD rely
highly on the use of immune suppressive drugs, including
5-aminosalicylic acid and corticosteroids (5). However, a number of patients either
do not respond to these agents or demonstrate significant adverse
effects (5). There is an urgent
need to develop novel and effective anti-inflammatory substances
with minimal side effects. Dietary flavonoids have been
demonstrated to effectively and mildly regulate expression of
inflammatory cytokines, alleviate the necrosis of colon tissue and
relieve clinical symptoms in patients with IBD (6,7).
Kaempferol
(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), a
flavonoid widely distributed in fruits, vegetables and plant-based
foods, has been reported to possess anti-inflammatory,
anti-diabetic, anti-hypertensive, anti-depressant and
anti-ulcerative properties by acting on various cellular pathways
(8–10). A previous study demonstrated that
kaempferol had the most effective anti-inflammatory activities
among eight polyphenols in a lipopolysaccharide (LPS)-induced Raw
264.7 cell model (11). In
vivo studies additionally suggested that kaempferol exerts a
distinct anti-inflammatory effect on dextran sulfate sodium-induced
UC in mice (12). However, to the
best of our knowledge, there are no studies at present that have
focused on the role keampferol serves in LPS-induced intestinal
microvascular endothelial cells. Intestinal microvascular
endothelial cells, the primary components of the intestinal
capillaries, have been demonstrated to be one of the most important
secretory and immune cells in the process of inflammation, which is
closely associated with the occurrence and progression of IBD
(12,13).
In order to further investigate the beneficial
effect of kaempferol and the possible mechanisms involved in the
intestinal inflammation process, LPS-stimulated rat intestinal
microvascular endothelial cells (RIMVECs) were used to establish a
cell model of IBD. It was demonstrated that kaempferol may
alleviate LPS-induced inflammatory mediators, including TNF-α,
IL-1β, IL-6, ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1),
by suppressing the activation of toll-like receptor 4 (TLR4),
signal transducer and activator of transcription (STAT) and nuclear
factor-κB (NF-κB).
Materials and methods
Reagents and antibodies
Kaempferol (purity ≥98%) was purchased from the
National Institutes for Food and Drug Control (Beijing, China).
Kaempferol was dissolved in dimethyl sulfoxide (DMSO); the final
concentration of DMSO was <0.1% (v/v) when kaempferol was added
to the experimental cells. Cell culture reagents, namely Dulbecco's
modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were
obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Endothelial cell growth supplement (ECGS) was purchased from
EMD Millipore (Billerica, MA, USA). LPS (Escherichia coli 055:B5)
was provided by Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). Rat TNF-α (cat. no. DY510),
IL-1β (cat. no. DY501), IL-6 (cat. no. DY506), ICAM-1 (cat. no.
DY583) and VCAM-1 (cat. no. DY809) ELISA kits were obtained from
R&D Systems, Inc. (Minneapolis, MN, USA). Antibodies for TLR4
(cat. no. ab22048), phosphorylated (p)-inhibitor of κB (I-κB) (cat.
no. ab133462) and p-NF-κB p65 (cat. no. ab28856) were purchased
from Abcam (Cambridge, UK). Antibodies for p-p38 (cat. no. 4511)
mitogen-activated protein kinase (MAPK) and p-STAT (cat. no. 9145)
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA). The antibody against β-actin (cat. no. AC006) was purchased
from ABclonal Biotech Co., Ltd. (Wuhan, China).
Cell culture and treatment
RIMVECs were isolated and cultured, as described
previously (14,15). A total of six neonatal rats at
1-day-old (gender undetermined; weight, 5–8 g) were purchased from
the Academy of the Military Medical Sciences (Beijing, China). The
present study was approved by the China Agriculture University
Institutional Animal Care and Use Committee (approval no.
CAU20160031201). RIMVECs were maintained with DMEM containing 15%
(v/v) FBS, 0.5% (w/v) ECGS, and 1% (v/v) penicillin-streptomycin
mixed solution. All cells were incubated at 37°C in a humidified
incubator with 5% CO2. Cells were starved in serum-free
medium for 12 h prior to each experiment.
Cell viability assay
Cell viability was measured with a CCK-8 assay.
RIMVECs were seeded at a density of 1×104 cells/well in
96-well plates for 24 h. Subsequently, the cells were treated with
100 µl of kaempferol at different concentrations (200, 100, 50, 25,
12.5 and 6.25 µM) for 12 h. Following treatment, the medium was
removed, and cells were cultured in 100 µl fresh DMEM containing
10% CCK-8 solution at 37°C for 2 h. Cell viability was determined
by measuring the absorbance with a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 450 nm.
Measurement of cytokines and adhesion
protein
TNF-α, IL-1β, IL-6, ICAM-1, and VCAM-1 protein
expression levels in culture supernatant were measured by ELISA,
according to the manufacturer's protocol. RIMVECs were seeded in
12-well plates (1×105 cells/well). At 90% confluency,
the cells were washed with PBS prior to the addition of medium with
different concentrations of kaempferol (50, 25 and 12.5 µM). After
3 h, cells were washed once with PBS and subsequently stimulated
with 10 µg/ml LPS (1 ml/well) for 6 h (TNF-α, IL-1β and IL-6) or 12
h (ICAM-1 and VCAM-1) prior to the collection of the supernatant
and measurement of inflammatory factors.
Western blot analysis
At the end of the incubation period, cells were
lysed in radioimmunoprecipitation lysis buffer (2% SDS; 10%
glycerol; 62.5 mM Tris-HCl buffer; pH 6.8) on ice. Protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China).
Samples with equal quantities of total protein (20 µg) were loaded
and separated by 10% SDS-PAGE and transferred to nitrocellulose
membranes (Pierce; Thermo Fisher Scientific, Inc.). The membranes
were blocked in 5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature, and were
immunoblotted with the specific primary antibodies against TLR4
(1:800), p-NF-κB p65 (1:1,000), p-I-κB (1:1,000), p-p38 (1:800),
p-STAT (1:800) and β-actin (1:2,000) overnight at 4°C. Membranes
were subsequently incubated with horseradish peroxidase-conjugated
secondary antibody (1:10,000; Cell Signaling Technology, Inc.; cat.
no. SC3901) for 1 h at room temperature. Subsequently, the blots
were visualized with an enhanced chemiluminescence immunoblotting
detection system (Beyotime Institute of Biotechnology) and
quantified using ImageJ (v1.51; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Data are expressed as mean ± standard deviation, and
analyzed by one-way analysis of variance followed by
Student-Newman-Keuls test for multiple comparisons. All analyses
were performed by GraphPad Prism 7 software (GraphPad Software
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Viability of RIMVECs treated with
kaempferol
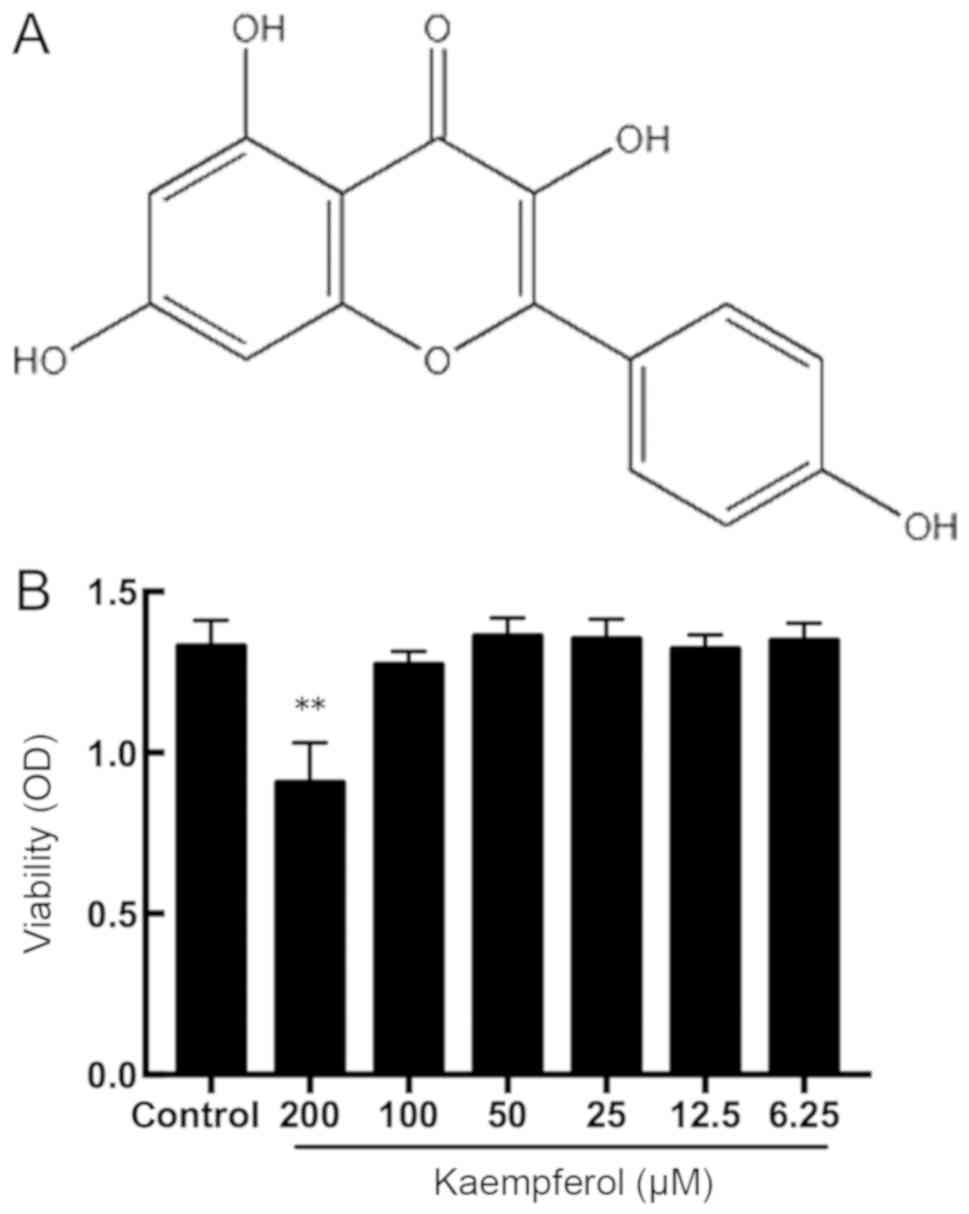
The chemical structure of kaempferol is presented in
Fig. 1A. The cytotoxicity of
kaempferol was assessed using the CCK-8 assay. As demonstrated in
Fig. 1B, a high concentration of
kaempferol (200 µM) significantly decreased cell viability
(P<0.01); whereas, treatment with concentrations between 6.25
and 100 µM kaempferol exhibited no effect on cell viability. These
results suggest that kaempferol is non-cytotoxic to RIMVECs within
the concentrations of 3.125 and 100 µM. According to the results,
concentrations of 12.5, 25 and 50 µM kaempferol were selected for
subsequent experimentation.
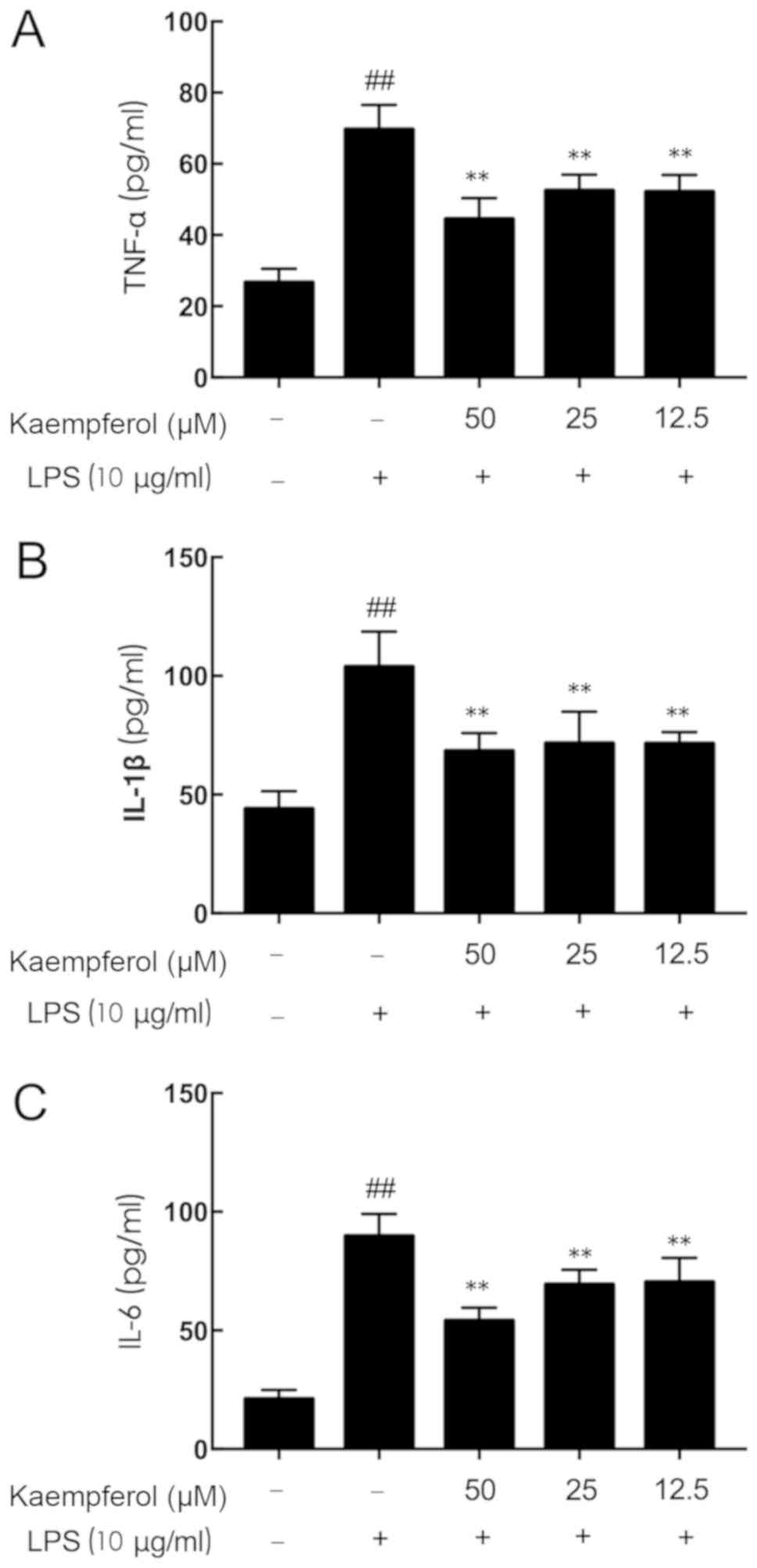
Kaempferol decreases TNF-α, IL-1β and
IL-6 upregulation induced by LPS
Pretreatment for 3 h with kaempferol at different
concentrations (12.5, 25 and 50 µM) was followed by treatment with
LPS (10 µg/ml) stimulation for 6 h. As presented in Fig. 2, LPS stimulation significantly
increased the secretion of inflammatory mediators above basal
expression levels (Fig. 2A, TNF-α,
2.61-fold; Fig. 2B, IL-1β,
2.36-fold; Fig. 2C, IL-6,
4.25-fold; P<0.01). Pretreatment with kaempferol at all
concentrations resulted in significant decreases in the
concentrations of TNF-α, IL-1β and IL-6 compared with the LPS-model
group (P<0.01).
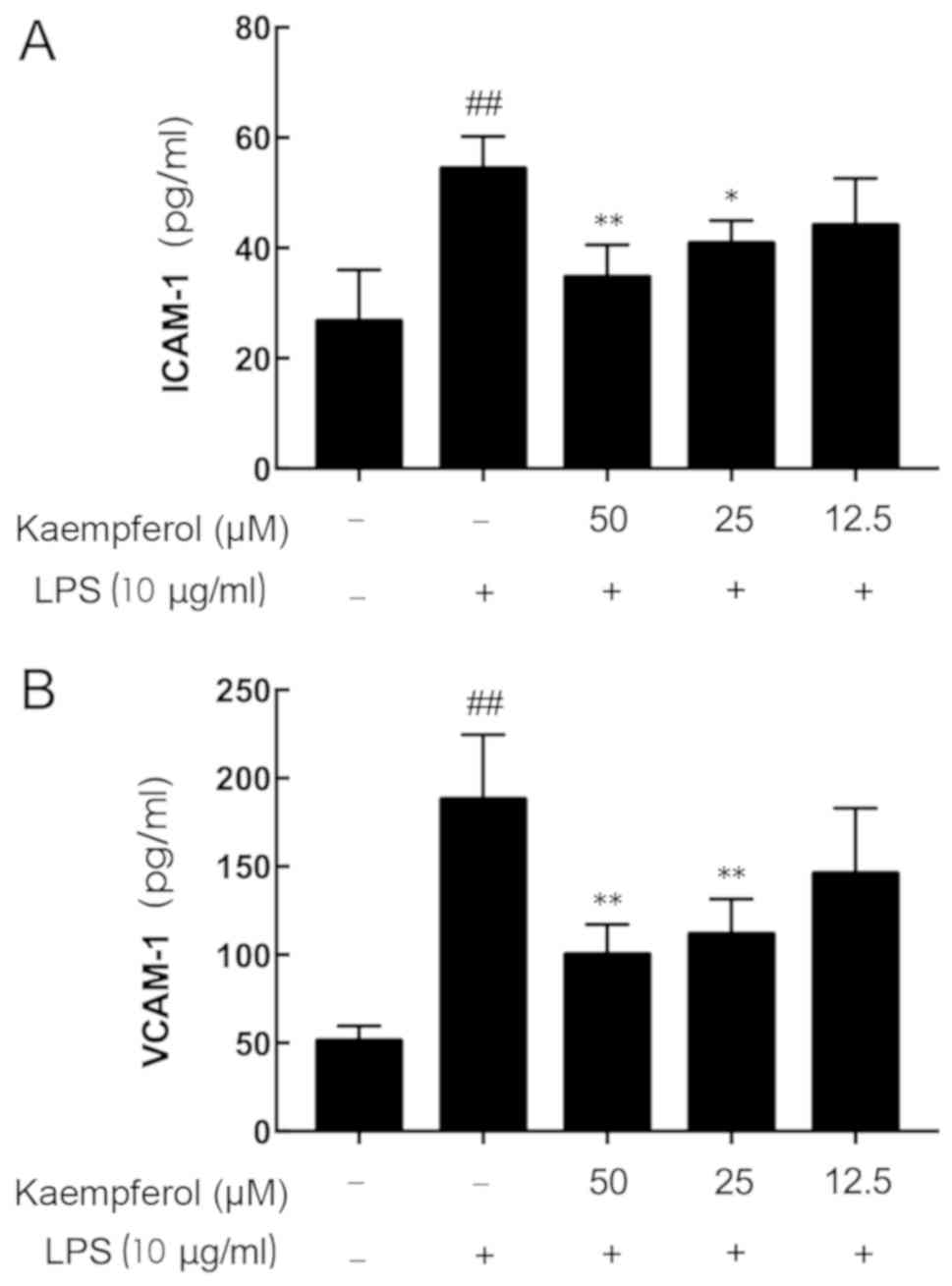
Kaempferol partially suppresses
LPS-induced ICAM-1 and VCAM-1 overproduction
As presented in Fig.
3, LPS stimulation significantly increased the secretion of
inflammatory mediators above the basal expression levels (Fig. 3A, ICAM-1, 2.04-fold; Fig. 3B, VCAM-1, 3.67-fold; P<0.01).
Although no significant reduction in ICAM-1 and VCAM-1 secretion
was observed in the 12.5 µM kaempferol group, significant decreases
in ICAM-1 and VCAM-1 concentration were observed in the 50 and 25
µM kaempferol groups compared with the LPS-treated group
(P<0.05).
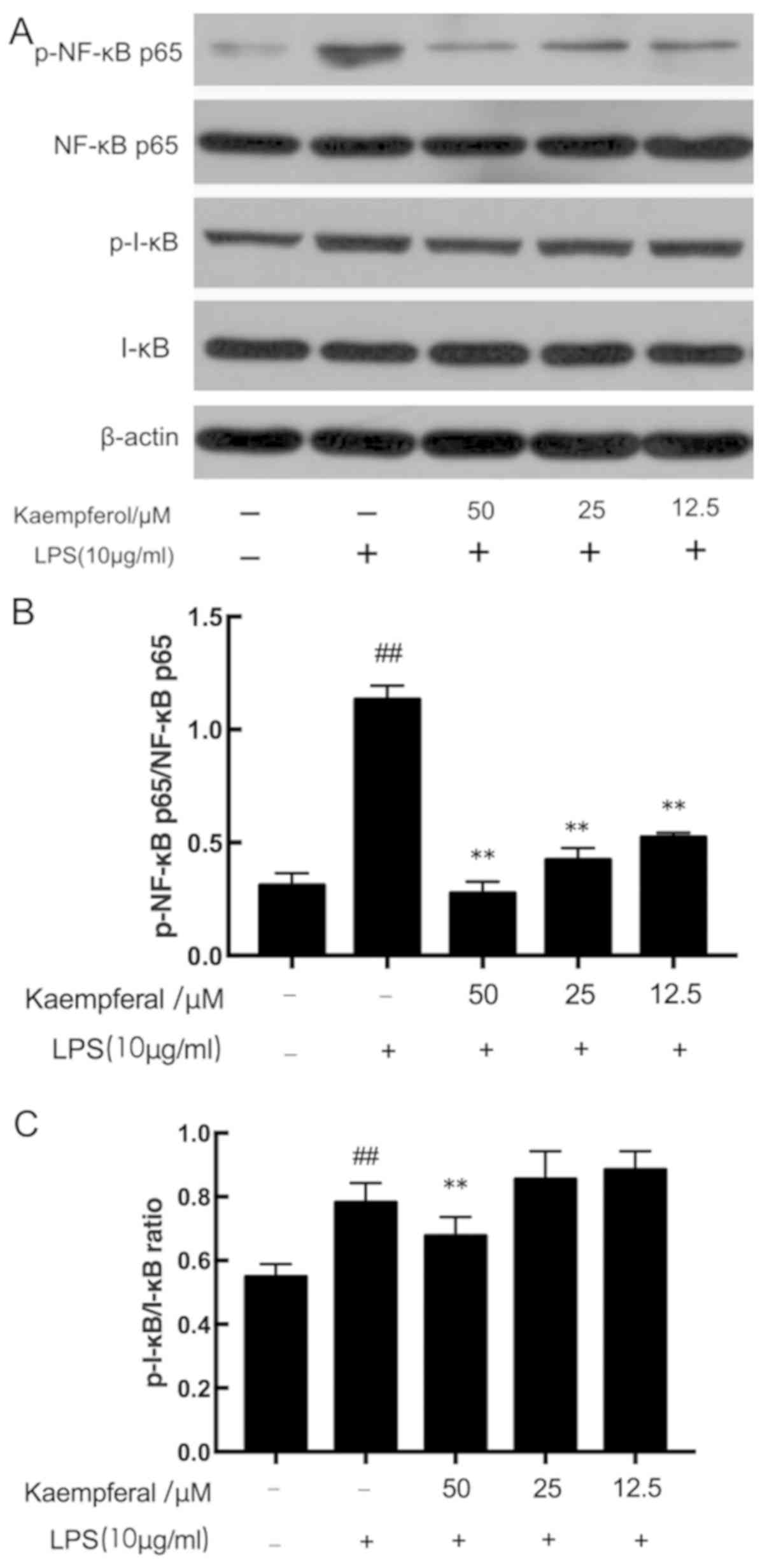
Kaempferol inhibits LPS-induced NF-κB
p65 and I-κB phosphorylation
The NF-κB pathway serves a critical role in the
secretion of cytokines (10). The
anti-inflammatory effect of kaempferol may be associated with the
prevention of NF-κB activation. As presented in Fig. 4, there was a significant increase
in the phosphorylation of the p65 subunit (p-NF-κB p65/NF-κB p65
ratio) and I-κB (p-I-κB/I-κB) in the model group compared with the
control group (P<0.01). However, pretreatment with three
different concentrations of kaempferol significantly decreased the
LPS-induced increased p-NF-κB p65/β-actin ratio (P<0.01). As for
I-κB, although 12.5 and 25 µM kaempferol was not able to decrease
the phosphorylation of I-κB induced by LPS, treatment with 50 µM
kaempferol significantly decreased the p-I-κB level
(P<0.01).
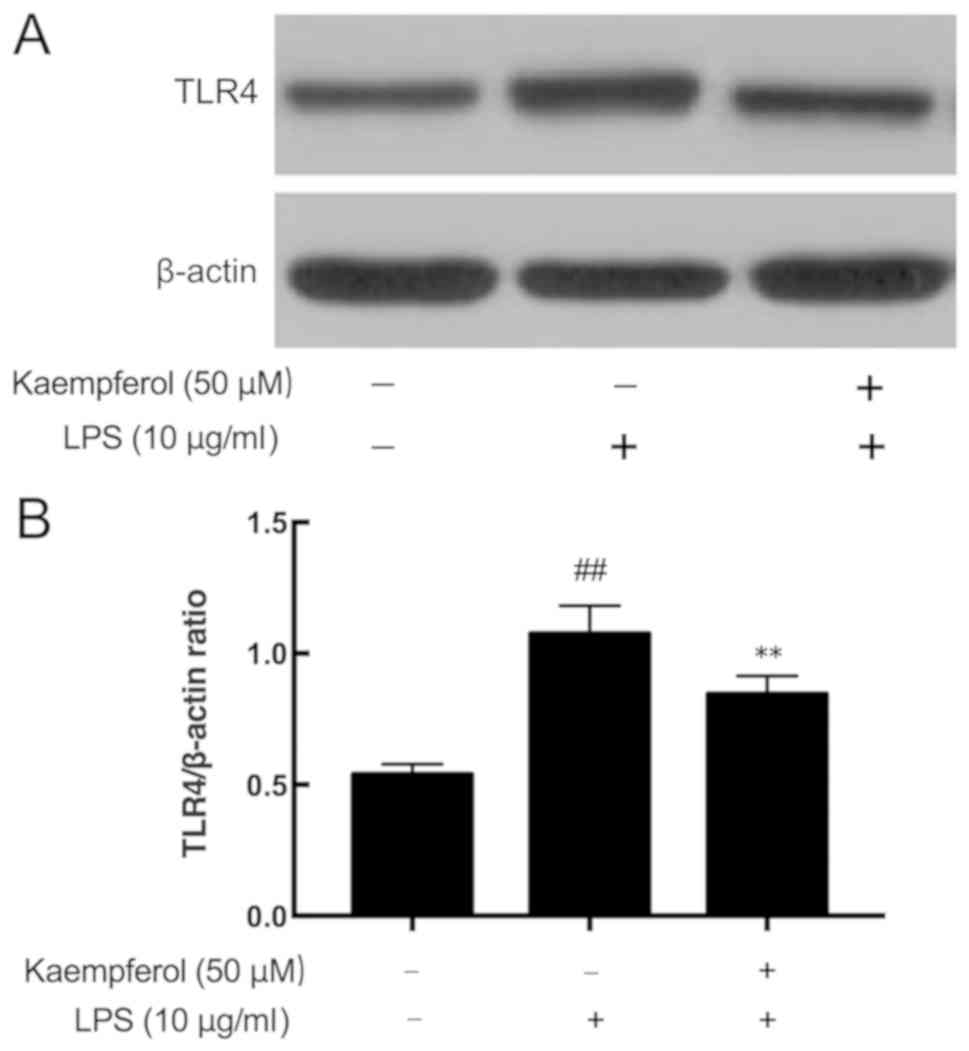
Kaempferol inhibits LPS-induced TLR4
overexpression
Activation of the TLR4 signaling pathway may
directly lead to the activation of NF-κB to regulate the production
of cytokines (9,10). The present results demonstrated
that LPS significantly upregulated TLR4 expression (Fig. 5; P<0.01). Pretreatment with
different concentrations of kaempferol significantly inhibited the
LPS-induced overexpression of TLR4 (Fig. 5; P<0.01).
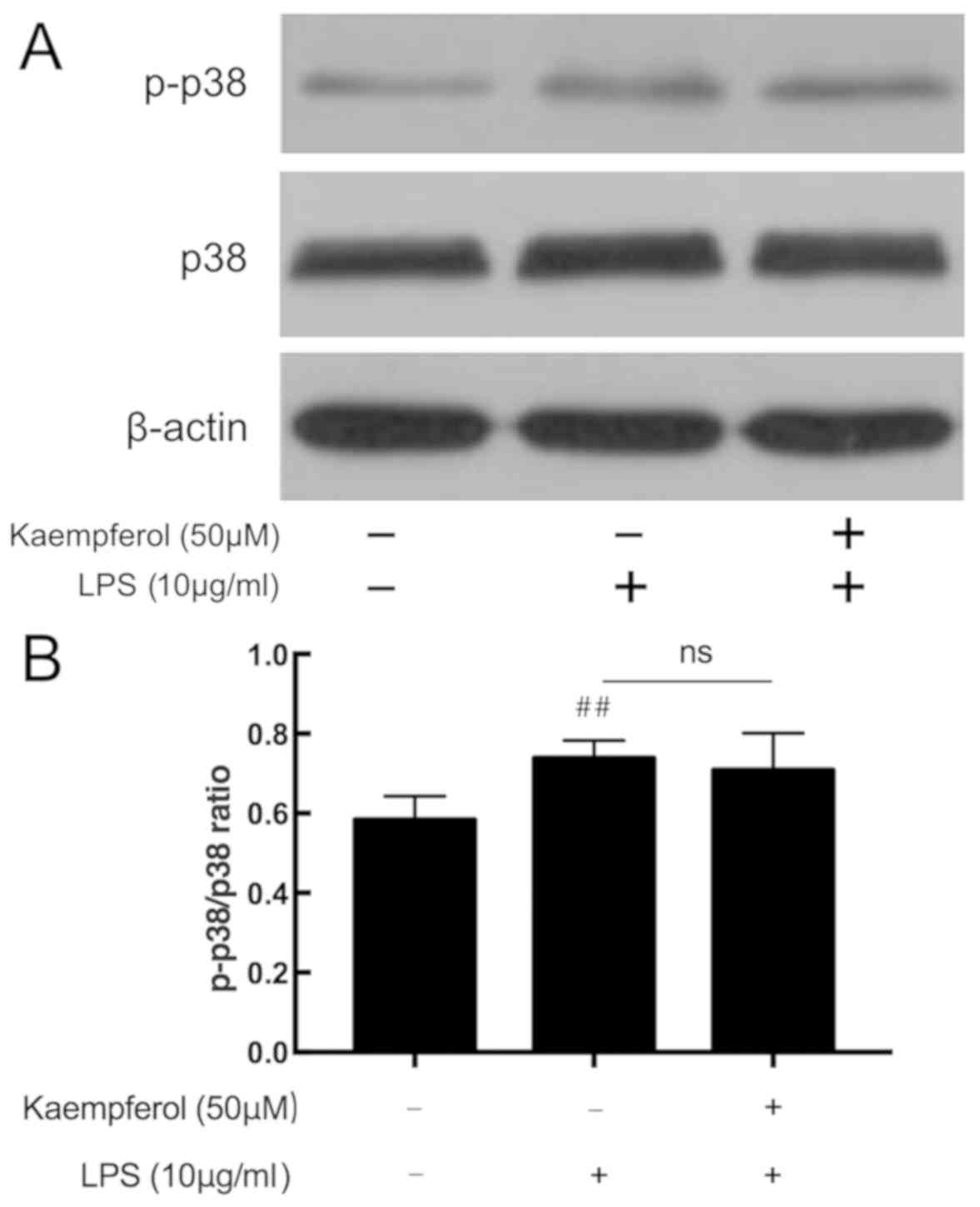
Kaempferol does not affect LPS-induced
p38 MAPK phosphorylation
The p38 MAPK signaling pathway additionally serves
an important role in inflammatory response. In response to LPS, the
level of p-p38 was significantly upregulated in the model group
compared with the control group (Fig.
6; P<0.01). Compared with the model group, kaempferol at 50
µM did not affect the expression level of p-p38.
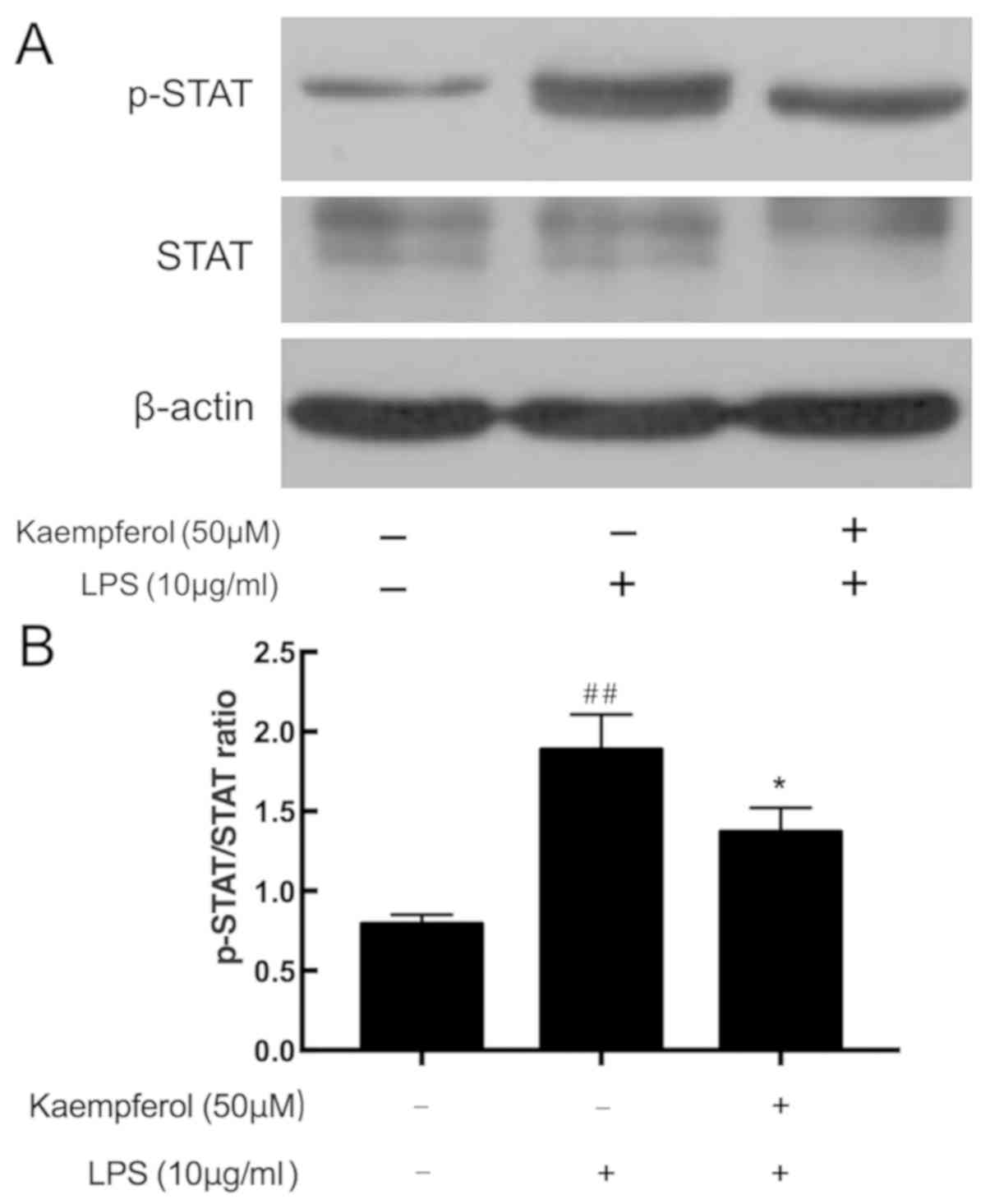
Kaempferol inhibits LPS-induced STAT
phosphorylation
The STAT pathway is another important inflammatory
signaling pathway. As demonstrated in Fig. 7, cells subjected to LPS exhibited
significantly increased STAT phosphorylation compared with the
control group (P<0.01). By contrast, significant downregulation
STAT phosphorylation was observed in the 50 µM kaempferol treatment
group compared with the model group (P<0.01).
Discussion
IBD is a chronic inflammatory disease of intestinal
mucosa and submucosa, characterized by disruption of the intestinal
epithelial barrier, increased production of inflammatory mediators
and excessive tissue injury (16).
Intestinal microvascular endothelial cells, the primary components
of intestinal capillaries, have been demonstrated to be one of the
most important secretory and immune cells in IBD (13). Intestinal microvascular endothelial
cells produce and respond to inflammatory mediators (17,18).
As demonstrated in previous studies, in response to LPS
stimulation, RIMVECs express the inflammatory cytokines, TNF-α,
IL-1β and IL-6, the chemokine IL-8, the adhesion molecule ICAM-1
and other inflammatory mediators, including nitric oxide and
endothelin-1 (14,19,20).
Considering that LPS is a principal pro-inflammatory factor in the
progression of IBD (21),
LPS-stimulated RIMVECs were selected as a cellular model of
intestinal inflammation.
Inflammatory mediators, including cytokines,
adhesion proteins and chemokines, have an important role in the
pathogenesis of IBD. TNF-α is the earliest and primary endogenous
mediator in the inflammatory process. It has a pro-inflammatory
effect through an increased production of IL-1β, IL-6, adhesion
molecules and pro-coagulant factors (17). Furthermore, TNF-α is an initiation
factor of acute immune responses, cytotoxicity and apoptosis, but
can also inhibit apoptosis (2,22).
In the present study, it was demonstrated that kaempferol was able
to protect RIMVECs from LPS-induced overexpression of three
inflammatory mediators, TNF-α, IL-1β and IL-6. Therefore, the
results of the present suggested that the inhibition of
inflammatory cytokines may contribute to the protective effects of
kaempferol in IBD.
In IBD, activation of microvascular endothelial
cells and adhesion of circulating immune cells is required for the
initiation and maintenance of inflammation (23). Pro-inflammatory cytokines,
including TNF-α and IL-1β, induce inflammatory responses in the
vascular endothelium, which results in enhanced expression of cell
adhesion molecules, including ICAM-1 and VCAM-1 (2). Overexpression of adhesion molecules
in the intestinal endothelium may promote recruitment and adhesion
of leukocytes, promoting the process of inflammatory response
(9,24). In the present study, the beneficial
effect of kaempferol was demonstrated in the altered production of
ICAM-1 and VCAM-1. The overexpression of ICAM-1 and VCAM-1 is
involved in inflammatory processes; however, are additionally
closely associated with intestinal carcinogenesis, secondary to IBD
(17,25). Therefore, the present results
suggest that kaempferol may effectively reduce the expression of
inflammatory mediators, against intestinal inflammation, and may
reduce the risk of carcinogenesis in patients with IBD.
NF-κB has an essential role in regulating the
expression of inflammatory cytokines and adhesion molecules
(26). Due to the pivotal role of
NF-κB in inflammation, the blockade of NF-κB activity is considered
to be associated with inhibition of the inflammatory signaling
cascade (27,28). Inhibition of NF-κB activation is an
effective strategy for preventing the progression of IBD in
experimental animal models and preventing inflammatory cytokine
production in patients with IBD (29). Under basal conditions, NF-κB is
sequestered in the cytoplasm by a family of inhibitory proteins
known as inhibitors of NF-κB (I-κBs). Following stimulation with
LPS, NF-κB is activated, triggering expression of a series of
inflammatory cytokine (28). In
the present study, LPS increased the phosphorylation of NF-κB p65
and I-κB, which effectively indicates the activation level of
NF-κB. However, kaempferol significantly reduced the LPS-induced
activation of NF-κB, suggesting that the anti-inflammatory
activities of kaempferol were due to the inhibitory effect on
NF-κB. The present study suggested a direct role of kaempferol in
the prevention of LPS-stimulated NF-κB activation in RIMVECs.
TLRs are pattern recognition receptors that ‘sense’
microbes and alert the immune system through activation of
transcription factors (including NF-κB) and the secretion of
inflammatory mediators (9). TLR4
is the specific ligand of LPS, serving an important role in
inflammatory disease, including IBD. Activation of TLR4 may lead to
phosphorylation of MAPKs, IκB kinases and NF-κB, eventually
contributing to the expression of pro-inflammatory cytokines
(30,31). Compared with the significantly
increased expression of TLR4 in LPS-treated RIMVECs, the
administration of kaempferol significantly reduces the TLR4
expression in LPS-treated cells. This suggests that kaempferol may
represent a novel strategy to limit the TLR4-mediated inflammatory
process.
The MAPK signaling pathways, including extracellular
signal-regulated kinase 1 and 2, c-jun N-terminal kinase and p38,
are closely associated with the expression of inflammatory
mediators and oxidative damage (32). Previous studies demonstrated that
MAPKs are highly activated and mediate pro-inflammatory cytokines,
including TNF-α, IL-1β and IL-6 in the colon tissue of an IBD mice
model and patients with IBD (33,34).
Kaempferol was demonstrated to suppress the expression of the
LPS-induced MAPK pathway in the human monocytic cell line THP-1
(35), in addition to suppressing
the production of cyclooxygenase-2 (COX-2), prostaglandin E2 and
matrix metalloproteinases (MMPs) via MAPK in IL-1β-induced
rheumatoid arthritis synovial fibroblasts (36). Additionally, kaempferol treatment
of the human synovial sarcoma cell line, SW982 cells, significantly
inhibited IL-1β-induced p38 MAPK phosphorylation, which were
involved in the production of IL-6, IL-8, MMP and COX-2 (37). However, the role of kaempferol in
IBD-associated cells has rarely been studied. In the present study,
it was identified that kaempferol did not demonstrate any
detectable influence on the phosphorylation of p38 in
LPS-stimulated RIMVECs. The present results suggest that the
anti-inflammatory effect of kaempferol is not dependent on the p38
MAPK pathway.
The STAT pathway is another crucial signaling
cascade involved in IBD. It is well documented that the STAT
pathway is not only associated with the inflammatory process in
IBD; however, is additionally associated with further serious
conditions, including colorectal cancer development (38,39).
Recent studies demonstrated that luteolin, a naturally occurring
flavonoid, inhibited the Janus kinase (JAK)/STAT pathway in
cytokine-induced HT-29 cells, a human epithelial cell line
(39). Curcumin, a natural phenol,
suppressed inflammation of TNBS-induced colitis in mice via STAT
signaling (40). Although it was
demonstrated that kaempferol attenuates inflammation by suppressing
the STAT pathway in human airway epithelial cells (41), to the best of our knowledge, no
study has focused on the effect of kaempferol on the JAK/STAT
pathway in IBD-associated cells. The results of the present suggest
that STAT may be associated with the suppressive effects of
kaempferol against the production of inflammatory mediators in
LPS-stimulated RIMVECs.
In conclusion, it was demonstrated that kaempferol
significantly inhibits the LPS-induced expression of the
inflammatory mediators TNF-α, IL-1β, IL-6, ICAM-1 and VCAM-1 by
inhibiting TLR4, NF-κB and STAT signaling, but not by activating
p38 MAPK signaling in RIMVECs. The present results provide novel
insight for the inflammatory effect of kaempferol in endothelial
cells. It suggests that kaempferol may be an effective therapeutic
agent for treatment of IBD, and that fruits and herbs rich in
kaempferol may be incorporated into potential health care products
for patients with IBD.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 31472228).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YB designed and participated in all of the
experiments, and was a major contributor in writing the manuscript.
PL, JZ and YH were in charge of culturing the primary cells. YF and
SZ performed the western blotting. ZL revised the manuscript, and
was a major contributor in designing the experiments. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the China
Agriculture University Institutional Animal Care and Use Committee
(approval no. CAU20160031201).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vezza T, Rodríguez-Nogales A, Algieri F,
Utrilla MP, Rodriguez-Cabezas ME and Galvez J: Flavonoids in
inflammatory bowel disease: A review. Nutrients. 8:2112016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moldoveanu AC, Diculescu M and Braticevici
CF: Cytokines in inflammatory bowel disease. Rom J Intern Med.
53:118–127. 2015.PubMed/NCBI
|
|
3
|
Klampfer L: Cytokines, inflammation and
colon cancer. Curr Cancer Drug Targets. 11:451–466. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu TC and Stappenbeck TS: Genetics and
pathogenesis of inflammatory bowel disease. Annu Rev Pathol.
11:127–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Boer NK, van Bodegraven AA, Jharap B,
de Graaf P and Mulder CJ: Drug Insight: Pharmacology and toxicity
of thiopurine therapy in patients with IBD. Nat Clin Pract
Gastroenterol Hepatol. 4:686–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dodda D, Chhajed R and Mishra J:
Protective effect of quercetin against acetic acid induced
inflammatory bowel disease (IBD) like symptoms in rats: Possible
morphological and biochemical alterations. Pharmacol Rep.
66:169–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park MY, Ji GE and Sung MK: Dietary
kaempferol suppresses inflammation of dextran sulfate
sodium-induced colitis in mice. Dig Dis Sci. 57:355–363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao L, Yao J, Han C, Yang J, Chaudhry MT,
Wang S, Liu H and Yin Y: Quercetin, Inflammation and Immunity.
Nutrients. 8:1672016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhaskar S, Sudhakaran PR and Helen A:
Quercetin attenuates atherosclerotic inflammation and adhesion
molecule expression by modulating TLR-NF-κB signaling pathway. Cell
Immunol. 310:131–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok
SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, et al: Quercetin
suppresses proinflammatory cytokines production through MAP kinases
andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage.
Mol Cell Biochem. 243:153–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park MY, Kwon HJ and Sung MK: Evaluation
of aloin and aloe-emodin as anti-inflammatory agents in aloe by
using murine macrophages. Biosci Biotechnol Biochem. 73:828–832.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Binion DG, West GA, Ina K, Ziats NP,
Emancipator SN and Fiocchi C: Enhanced leukocyte binding by
intestinal microvascular endothelial cells in inflammatory bowel
disease. Gastroenterology. 112:1895–1907. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swerlick RA and Lawley TJ: Role of
microvascular endothelial cells in inflammation. J Invest Dermatol.
100 Suppl:111S–115S. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan H, Zhang Y, Xu J, Qiao J, Suo Z, Hu G
and Mu X: Effect of anemonin on NO, ET-1 and ICAM-1 production in
rat intestinal microvascular endothelial cells. J Ethnopharmacol.
104:362–366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suo Z, Mu X, Xu J, Wang Z, Wang X, Duan H,
Hu G, Yang Z and Huang H: In vitro culture of rat intestinal mucous
microvascular endothelial cells. Acta Anatomica Sinica. 36:214–217.
2005.(In Chinese).
|
|
16
|
Kasper JY, Hermanns MI, Cavelius C,
Kraegeloh A, Jung T, Danzebrink R, Unger RE and Kirkpatrick CJ: The
role of the intestinal microvasculature in inflammatory bowel
disease: Studies with a modified Caco-2 model including endothelial
cells resembling the intestinal barrier in vitro. Int J
Nanomedicine. 11:6353–6364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Francescone R, Hou V and Grivennikov SI:
Cytokines, IBD, and colitis-associated cancer. Inflam Bowel Dis.
21:409–418. 2015. View Article : Google Scholar
|
|
18
|
Lu JT, Xu AT, Shen J and Ran ZH: Crosstalk
between intestinal epithelial cell and adaptive immune cell in
intestinal mucosal immunity. J Gastroenterol Hepatol. 32:975–980.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu YS, Lin RY, Bian YF, Fan K and Liu ZJ:
Protective effect of kushenlu on lipopolysaccharide-induced small
intestinal inflammation in rats. Int J Pharmacol. 13:473–480. 2017.
View Article : Google Scholar
|
|
20
|
Liu J, Xue J, Zhu Z, Hu G and Ren X:
Lactic acid inhibits NF-κB activation by lipopolysaccharide in rat
intestinal mucosa microvascular endothelial cells. Agr Sci China.
10:954–959. 2011. View Article : Google Scholar
|
|
21
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xanthoulea S, Pasparakis M, Kousteni S,
Brakebusch C, Wallach D, Bauer J, Lassmann H and Kollias G: Tumor
Necrosis Factor (TNF) receptor shedding controls thresholds of
innate immune activation that balance opposing TNF functions in
infectious and inflammatory diseases. J Exp Med. 200:367–376. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Binion DG and Rafiee P: Is inflammatory
bowel disease a vascular disease? Targeting angiogenesis improves
chronic inflammation in inflammatory bowel disease.
Gastroenterology. 136:400–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Medda R, Lyros O, Schmidt JL, Jovanovic N,
Nie L, Link BJ, Otterson MF, Stoner GD, Shaker R and Rafiee P: Anti
inflammatory and anti angiogenic effect of black raspberry extract
on human esophageal and intestinal microvascular endothelial cells.
Microvasc Res. 97:167–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dhawan A, Friedrichs J, Bonin MV,
Bejestani EP, Werner C, Wobus M, Chavakis T and Bornhäuser M:
Breast cancer cells compete with hematopoietic stem and progenitor
cells for intercellular adhesion molecule 1-mediated binding to the
bone marrow microenvironment. Carcinogenesis. 37:759–767. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Yang X, Liu T, Guan M, Feng X,
Dong W, Chu X, Liu J, Tian X, Ci X, et al: Kaempferol regulates
MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute
lung injury in mice. Int Immunopharmacol. 14:209–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Comalada M, Camuesco D, Sierra S,
Ballester I, Xaus J, Gálvez J and Zarzuelo A: In vivo quercitrin
anti-inflammatory effect involves release of quercetin, which
inhibits inflammation through down-regulation of the NF-kappaB
pathway. Eur J Immunol. 35:584–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui L, Feng L, Zhang ZH and Jia XB: The
anti-inflammation effect of baicalin on experimental colitis
through inhibiting TLR4/NF-κB pathway activation. Int
Immunopharmacol. 23:294–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Zhang DK, Yi WQ, Ouyang Q, Chen YQ
and Gan HT: NF-kappaB p65 antisense oligonucleotides may serve as a
novel molecular approach for the treatment of patients with
ulcerative colitis. Arch Med Res. 39:729–734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lucas K and Maes M: Role of the toll like
receptor (TLR) radical cycle in chronic inflammation: Possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akira S, Hoshino K and Kaisho T: The role
of Toll-like receptors and MyD88 in innate immune responses. J
Endotoxin Res. 6:383–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen C, Chen YH and Lin WW: Involvement of
p38 mitogen-activated protein kinase in lipopolysaccharide-induced
iNOS and COX-2 expression in J774 macrophages. Immunology.
97:124–129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu W, Liu Y, Wang Z, Yu T, Lu Q and Chen
H: Suppression of MAPK and NF-κB pathways by schisandrin B
contributes to attenuation of DSS-induced mice model of
inflammatory bowel disease. Pharmazie. 70:598–603. 2015.PubMed/NCBI
|
|
34
|
Amiot A and Peyrin-Biroulet L: Current,
new and future biological agents on the horizon for the treatment
of inflammatory bowel diseases. Ther Adv Gastroenter. 8:66–82.
2015. View Article : Google Scholar
|
|
35
|
Huang CH, Jan RL, Kuo CH, Chu YT, Wang WL,
Lee MS, Chen HN and Hung CH: Natural flavone kaempferol suppresses
chemokines expression in human monocyte THP-1 cells through MAPK
pathways. J Food Sci. 75:H254–H259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoon HY, Lee EG, Lee H, Cho IJ, Choi YJ,
Sung MS, Yoo HG and Yoo WH: Kaempferol inhibits IL-1β-induced
proliferation of rheumatoid arthritis synovial fibroblasts and the
production of COX-2, PGE2 and MMPs. Int J Mol Med. 32:971–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lian JJ, Cheng BF, Gao YX, Xue H, Wang L,
Wang M, Yang HJ and Feng ZW: Protective effect of kaempferol, a
flavonoid widely present in varieties of edible plants, on
IL-1β-induced inflammatory response via inhibiting MAPK, Akt, and
NF-κB signalling in SW982 cells. J Funct Foods. 27:214–222. 2016.
View Article : Google Scholar
|
|
38
|
Slattery ML, Lundgreen A, Kadlubar SA,
Bondurant KL and Wolff RK: JAK/STAT/SOCS-signaling pathway and
colon and rectal cancer. Mol Carcinog. 52:155–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nunes C, Almeida L, Barbosa RM and
Laranjinha J: Luteolin suppresses the JAK/STAT pathway in a
cellular model of intestinal inflammation. Food Funct. 8:387–396.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao HM, Xu R, Huang XY, Cheng SM, Huang
MF, Yue HY, Wang X, Zou Y, Lu AP and Liu DY: Curcumin suppressed
activation of dendritic cells via JAK/STAT/SOCS signal in mice with
experimental colitis. Front Pharmacol. 7:4552016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gong JH and Kang YH: Kaempferol inhibits
chemokine expression and attenuates airway inflammation by
suppressing STAT/JAK and NF-kB activity in human airway epithelial
cells. FASEB J. 25 Suppl 1:lb1–1130.6. 2011.
|