Introduction
The placenta is an organ that connects the
developing fetus to the uterine wall for nutrient uptake,
excretion, antibody transport and gas exchange via the maternal
blood supply. The placenta begins to develop upon implantation of
the blastocyst into the maternal endometrium (1). The outer layer of the blastocyst then
develops into the trophoblast, which forms the placenta (1). This layer is further divided into two
layers, including the underlying cytotrophoblast layer and
overlying syncytiotrophoblast layer. Cytotrophoblatic cells serve
an important role in the implantation of a newly fertilized egg in
the uterus (2). The
syncytiotrophoblast is the epithelial covering of the highly
vascular embryonic placental villi, which invades the uterine wall
to establish nutrient circulation between the embryo and mother
(2).
Maternal endocrine adaptations to pregnancy involve
the hypothalamus, pituitary, parathyroid, thyroid, adrenal glands
and ovaries, and are linked to fetal-placental-maternal unit
interactions. Maternal physiology during pregnancy is significantly
affected by placental hormones such as estrogen, progesterone (P4),
human chorionic gonadotropin (hCG), prolactin, parathyroid hormone,
adrenal hormones and human placental lactogen (3). Female sex steroid hormones such as
estradiol (E2) and P4 also have critical function during pregnancy.
E2 and P4 have many functions associated with fetal development
(4). In the case of estrogen,
there are three major naturally occurring forms in women, including
estrone (E1), E2 and estriol (E3) (4). Among these, E2 is the most potent and
prevalent type of endogenous estrogen, although other metabolites
of E2 also have estrogenic hormone activity and circulate at high
levels at certain phases in the menstrual cycle and during
pregnancy (5). Increasing levels
of E2 during the follicular phase promote endometrial
proliferation, which is further regulated by stimulating the
secretion of prolactin, which also promotes breast growth and milk
production (6). P4 prevents
uterine contractions by promoting smooth muscle relaxation in the
myometrium. During pregnancy, P4 causes developmental changes in
the endometrium that are necessary for formation of the maternal
portion of the placenta (3,7).
Placental synthesis of E2 via conversion of steroid
precursors requires the enzymes 3β-hydroxysteroid dehydrogenase
type 1 (HSD3B1), aromatase (CYP19A1), and 17β-hydroxysteroid
dehydrogenase type 3 (HSD17B3) (8). In addition, key enzymes involved in
the production of progesterone include side-chain cleavage enzyme
(CYP11A1) and 17α hydroxylase (CYP17A1). Cholesterol is converted
into PG by CYP11A1, which can be converted into P4 depending on
HSD3B1. HSD17B3 is for androgen synthesis, producing testosterone
from the precursor hormone androstenedione. CYP17A1 is a key enzyme
in the steroidogenic pathway that produces P4, mineralocorticoids,
glucocorticoids, androgen and E2 (9,10).
Previous studies have determined the steroidogenic
capacity and mechanism of the placenta during pregnancy in rats,
mice, cats, ewes and other species (11,12).
However, depending on the animal species there are several placenta
types, including discoid, zonary, cotyledonary and diffuse
placentas. The mature human placenta is a discoid organ with a
diameter of 20–25 cm and thickness of 3 cm, that weighs 400–600 g
(2). Since human placenta has a
discoid shape, which has a different structure and physiological
features from other species, physiological and functional studies
on the placenta should be separately conducted in humans (13). In the present study, the expression
levels of steroidogenic enzymes in human placenta was examined,
according to the gestational age. In addition, the levels of
steroid hormones in the serum and placenta were analyzed.
Materials and methods
Tissue and blood sample collection and
grouping
The present study was approved by the Institutional
Review Board of the Pusan National University Hospital Clinical
Trials Center (approval no. H-1302-005-015). Placental tissue
samples were received from Pusan National University Hospital and
collected after informed consent was obtained. Placenta samples
were from pregnant women (Age, 31.6±3.58 years; collection dates
between July 2012 and February 2014) who met the following
inclusion criteria: i) Singleton pregnancy; ii) normal pregnancy at
time of sample collection; and iii) healthy women with no
preexisting clinical conditions, including diabetes, hypertension,
or autoimmune disease. The normal placenta samples were divided
into early preterm (22–29 week gestation; n=10), late preterm
(30–36 week gestation; n=18), and term (37–40 week gestation; n=20)
groups following labor onset. The placental tissues were previously
confirmed by testing expression of tissue specific marker,
corticotropin-releasing hormone (14).
Human placental-derived BeWo cell
culture
BeWo human choriocarcinoma-derived cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
1% streptomycin/penicillin at 37°C in a humidified atmosphere of 5%
CO2, and allowed to attach during 24 h of incubation,
following which the medium was removed and replaced with phenol-red
free experimental medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) treated with charcoal-dextran FBS (Gibco; Thermo Fisher
Scientific, Inc.) for 24 h before treatment. steroid hormones were
dissolved in ethanol, diluted with experimental medium, and added
to wells for 24 h at room temperature. Cells were exposed a
physiological concentrations of E2 (100 nM), P4 (10 µM) or ethanol
as a vehicle control (15,16).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cells and tissues were extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. Total RNA concentration was measured using a
spectrophotometer. DNA (cDNA) was prepared from total RNA (1 µg) by
RT at 37°C for 60 min using M-MLV reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) and random primers
(9-mers; Takara Bio, Inc., Otsu, Japan). qPCR was performed using
cDNA template (2 µl) and SYBR Green (6 µl; Toyobo Life Science,
Osaka, Japan) with specific primers. The primer sequences for
CYP11A1, HSD3B1, CYP17A1, HSD17B3, CYP19A1 and β-actin are shown in
Table I. qPCR was carried out for
40 cycles using the following parameters: denaturation at 95°C for
15 sec, annealing at 55°C for 15 sec, and extension at 72°C for 45
sec. Fluorescence intensity was measured at the end of each
extension phase. The threshold value for the fluorescence intensity
of all samples was set manually. The reaction cycle at which PCR
products exceeded this fluorescence intensity threshold during the
exponential phase of PCR amplification was considered to be the
cycle of threshold (CT). Expression of the target gene was
quantified relative to that of β-actin, a housekeeping gene, based
on comparison of CTs at constant fluorescence intensity (17).
 | Table I.Primer sequences for quantitative
polymerase chain reaction analysis. |
Table I.
Primer sequences for quantitative
polymerase chain reaction analysis.
| Gene name | Primer | Sequence (5′-3′) | Fragment size
(bp) |
|---|
| β-actin | Forward |
GGACTTCGAGCAAGAGATGG | 234 |
|
| Reverse |
AGCACTGTGTTGGCGTACAG |
|
| CYP11A1 | Forward |
GCAACGTGGAGTCGGTTTAT | 229 |
|
| Reverse |
AGGGGCAAAAAGTTCTTGGT |
|
| HSD3B1 | Forward |
AGAGGCCTGTGTCCAAGCTA | 152 |
|
| Reverse |
TTTTGCTGTGTGGGTATGGA |
|
| CYP17A1 | Forward |
CTGATGCAAGCCAAGATGAA | 222 |
|
| Reverse |
GCTGAAACCCACATTCTGGT |
|
| HSD17B3 | Forward |
ATCCAGAGCCTCATCCATTG | 164 |
|
| Reverse |
AACGCCTTGGAAGCTGAGTA |
|
| CYP19A1 | Forward |
CCAGTGAAAAAGGGGACAAA | 175 |
|
| Reverse |
CCATGGCGATGTACTTTCCT |
|
Western blotting analysis
Protein samples were extracted from placental tissue
with PRO-PREP™ solution (iNtRON Biotechnology, Seoul, Korea) and
from BeWo cells with protein extraction reagent (Thermo Fisher
Scientific, Inc.). A total of 25 µg of protein per lane was
separated by 10–12% SDS-PAGE and transferred onto nitrocellulose
membranes. The membranes were blocked for 2 h at room temperature
with 5% skimmed milk or 5% donkey serum (Sigma-Aldrich; Merck KGaA)
in PBS with 0.05% Tween-20 (PBS-T). Next, membranes were incubated
with antibodies specific for CYP11A1 (1:1,000; cat. no. 14217, Cell
Signaling Technology, Inc., Dallas, TX, USA), HSD3B1 (1:2,000;
ab55268, Abcam, Cambridge, MA, USA), CYP17A1 (1:500; sc-66850,
Santa Cruz Biotechnology, Inc., CA, USA), HSD17B3 (1:2,000;
ab126228, Abcam), CYP19A1 (1:500; sc-14245, Santa Cruz
Biotechnology, Inc.) and β-actin (1:3,000; cat. no. 4970, Cell
Signaling Technology, Inc.) overnight at 4°C. Following this,
membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit (1:2,000; sc-2030, Santa Cruz Biotechnology, Inc.),
anti-mouse (1:2,000; cat. no. 7076, Cell Signaling Technology,
Inc.) and anti-goat (1:2,000; sc-2020, Santa Cruz Biotechnology,
Inc.) secondary antibodies in blocking solution at room temperature
for 1 h. Luminol reagent (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used to visualize antibody binding. Each blot was scanned
using Gel Doc 1000, version 1.5 (Bio Rad Laboratories, Inc.), and
band intensities were normalized to β-actin.
Histological analysis
Placental tissues were fixed with 10% formalin
overnight, embedded in paraffin wax, routinely processed and
sectioned into 4 µm thick slices. The sections were deparaffinized
with xylene, rehydrated with ethanol at graded decreasing
concentrations of 100–70%, and finally washed with distilled water.
The slides with placental sections were stained with hematoxylin
and eosin (Sigma-Aldrich; Merck KGaA) and washed with distilled
water. For immunohistochemistry (IHC), endogenous peroxidase
activity was quenched using a 10 min incubation step with 3%
hydrogen peroxide (H2O2) in methanol.
Non-specific binding was blocked by incubation with 10% bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA) and the slides were
incubated with primary antibody solution (CYP11A1 (1:200; ab175408,
Abcam), HSD3B1 (1:2,000; ab55268, Abcam), CYP17A1 (1:500; sc-66850,
Santa Cruz Biotechnology, Inc.) and CYP19A1 (1:500; sc-14245, Santa
Cruz Biotechnology, Inc.) overnight at 4°C. The slides were then
washed three times for 2 min each in PBS while slowly warming to
room temperature, followed by incubation with secondary antibody
(horseradish peroxidase-conjugated anti-rabbit (1:100; sc-2030,
Santa Cruz Biotechnology, Inc.), anti-mouse (1:1,000; cat. no.
7076, Cell Signaling Technology, Inc.) for 10 min at room
temperature. Primary antibody detection was performed with a
Polink-2 Plus HRP DAB kit (GBI Labs, Bothell, WA, USA). Images of
tissue were captured at ×40 using a model BX50F-3 optical
microscope (Olympus, Tokyo, Japan) and examined visually.
ELISA
Following E2 treatments, BeWo cell-cultured media
were centrifuged at 1,300 × g for 15 min at 4°C and stored at
−80°C. P4 accumulation in media was measured using a competitive
enzyme immunoassay kit (582601; Cayman Chemical Company, Ann Arbor,
MI, USA) following the manufacturer's protocol.
Blood was collected in plastic tubes under aseptic
conditions with ethylene diamine tetra-acetic acid as an
anti-coagulant, followed by centrifugation in order to separate
plasma. Serum and human placenta proteins were collected from each
volunteer and stored at −80°C, following which the sample was
slowly thawed at room temperature. Pregnenolone (PG),
dihydroepiandrosterone (DHEA), E2, P4, and testosterone (T)
concentrations were measured using an ELISA kit following the
manufacturer's protocol. ELISA kits for PG and DHEA were from Alpco
Diagnostics (11-PREHU-E01; Salem, NH, USA) and Enzo Life Science
(ADI-900-093; Ann Arbor, USA). E2, P4, and T ELISA kits were
purchased from Cayman Chemical (E2, 582251; P4, 582601; T,
582701).
Statistical analysis
Results are presented as the mean ± standard
deviation). Data were analyzed using a post hoc Tukey's test
following one-way analysis of variance (Sigma Plot version 10.0;
Systat Software, Inc., San Jose, CA, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
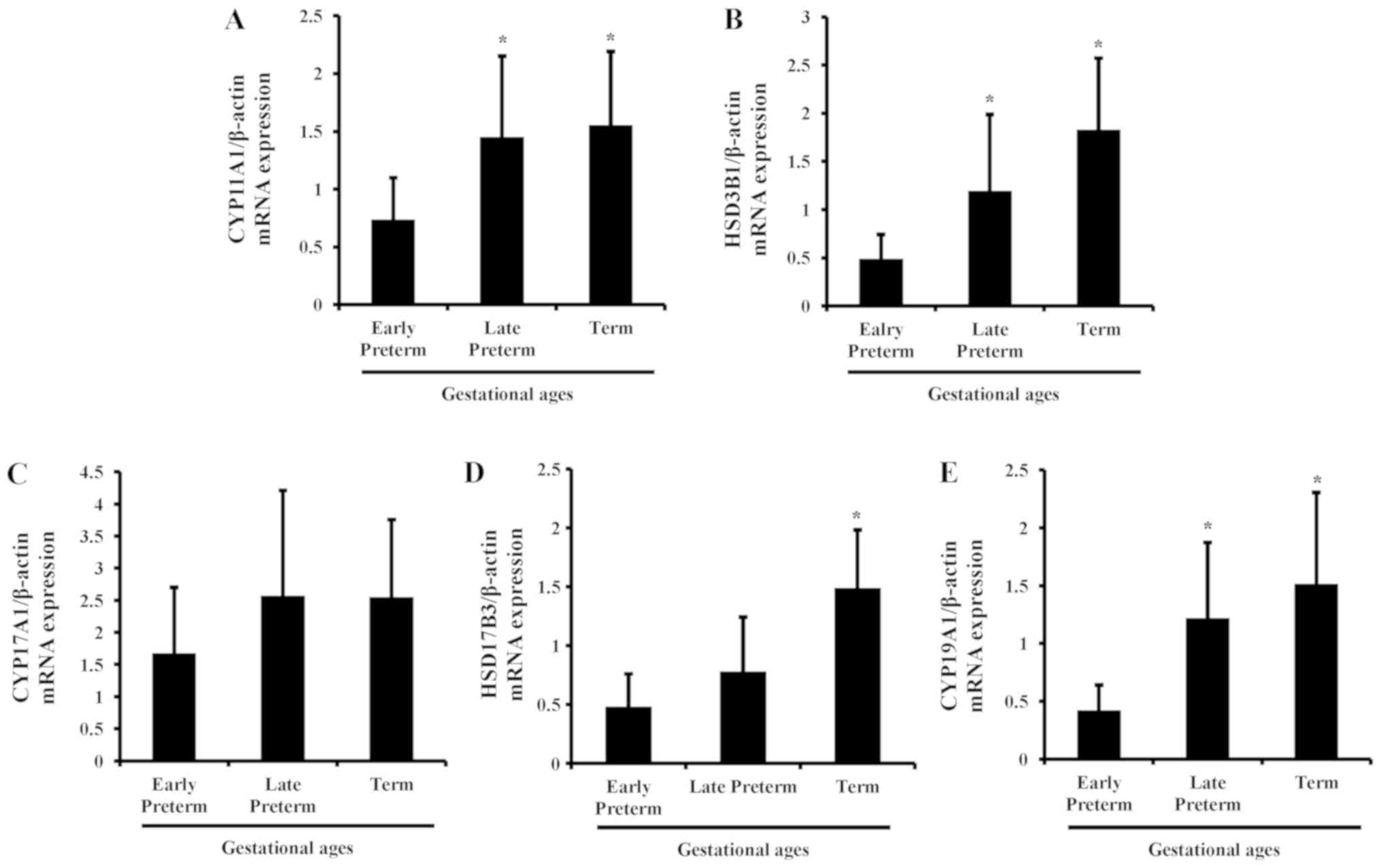
mRNA levels of steroidogenic enzymes
in the placenta during gestation
To evaluate the mRNA expression of steroidogenic
enzymes in human placenta according to gestational age, qPCR was
carried out in tissues of early preterm, late preterm and term
placentas. As gestational age increased, the mRNA expression of
steroidogenic enzymes was elevated in human placenta and maximized
in the term period. Among the genes, CYP11A1, HSD3B1, HSD17B3 and
CYP19A1 were significantly increased, compared with the early
preterm group; whereas the changes in CYP17A1 expression were not
significant (Fig. 1).
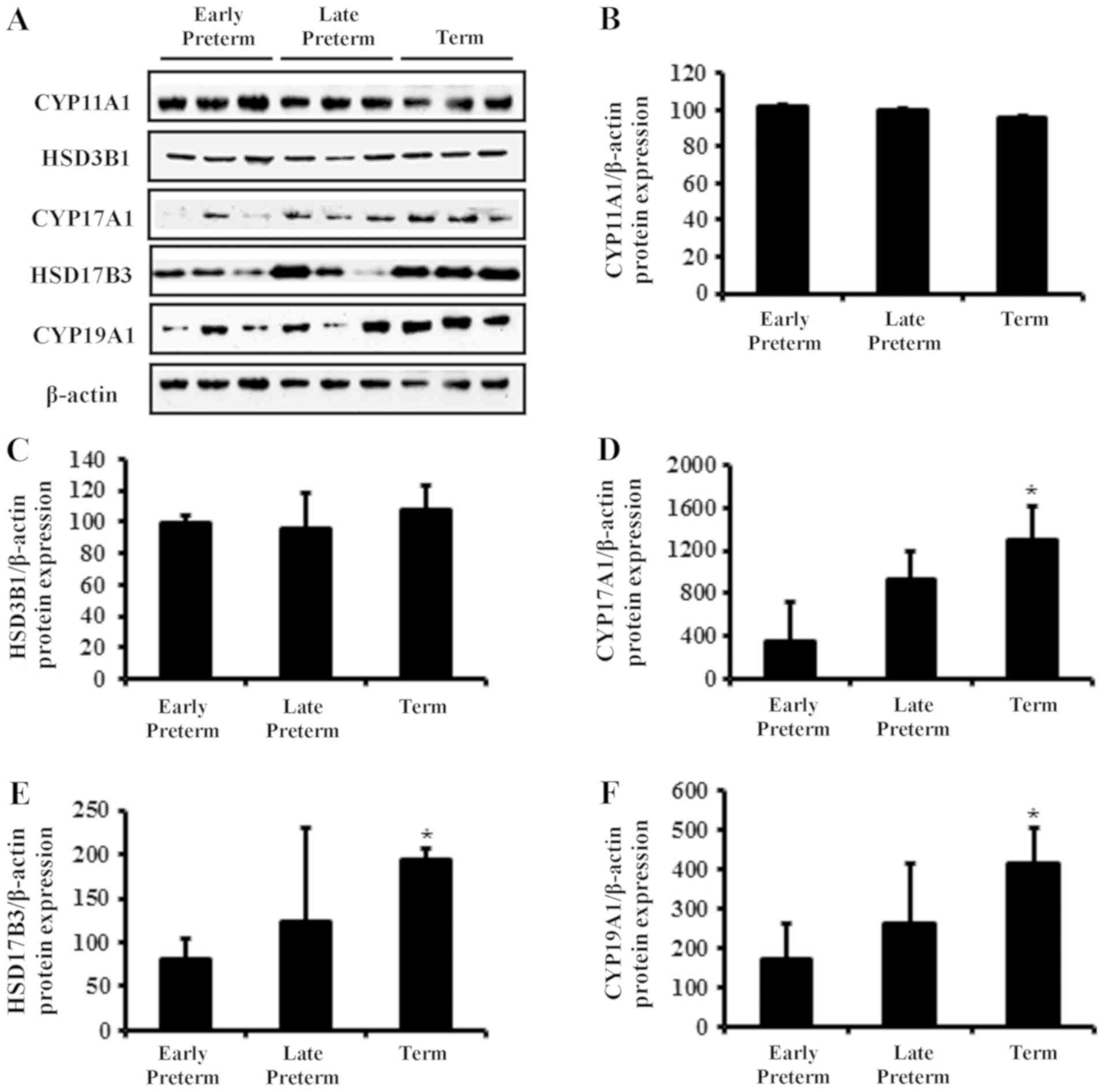
Protein levels of steroidogenic
enzymes in placenta during gestation
Protein expression levels of
steroidogenesis-associated enzymes in human placenta according to
gestational age were also investigated by western blotting
(Fig. 2A). The protein expression
of CYP11A1 and HSD3B1 was not significantly altered (Fig. 2B and C). However, similar to the
mRNA expression results, the protein levels of CYP17A1, HSD17B3 and
CYP19A1 were elevated in term placenta, compared with the early
preterm group (Fig. 2D-F).
Serum levels of steroid hormones
according to gestational age
As steroid hormone biosynthesis-related enzymes in
placenta was differentially regulated according to gestational age,
the concentration of steroid hormones in human serum and placenta
was determined with ELISAs. Concentrations of DHEA and P4 were
higher in serum than placenta, whereas the concentration of E2 was
higher in placenta than serum during all periods of gestation
(Table II). PG levels were stable
and similar between the serum and placenta. For gestational stage,
serum levels of DHEA, P4, T and E2 were significantly elevated at
term compared with the early preterm group. Concentrations of
steroid hormones including DHEA and E2 in placental tissue showed
similar patterns to those in serum, by elevating at term stage.
Notably, the level of T in placenta was reduced in term placenta,
which was opposite from serum concentration. The level of P4 was
also different from serum, which was not changed according to
gestational age.
 | Table II.Concentration of steroid hormones in
serum and placenta. |
Table II.
Concentration of steroid hormones in
serum and placenta.
|
| Serum | Placenta |
|---|
|
|
|
|
|---|
| Steroid hormone | Early preterm | Late preterm | Term | Early preterm | Late preterm | Term |
|---|
| PG (ng/ml) |
7.0±0.0 |
7.0±0.0 |
7.0±0.1 |
7.0±0.0 | 7.0±0.0 | 7.1±0.0 |
| DHEA (ng/ml) | 10.3±1.1 | 12.1±0.7a | 12.6±0.6a |
8.4±0.7 |
9.9±0.6a |
11.0±0.8a |
| P4 (ng/ml) |
4.4±0.1 |
5.3±0.2 |
5.7±0.2a |
2.8±0.1 | 2.8±0.1 | 2.8±0.3 |
| T (ng/ml) |
2.6±0.3 |
3.0±0.3 |
3.1±0.2a |
3.3±0.2 | 3.1±0.2 |
2.9±0.1a |
| E2 (ng/ml) | – | – | – | 11.8±2.2 |
14.6±2.4a |
18.5±2.1a |
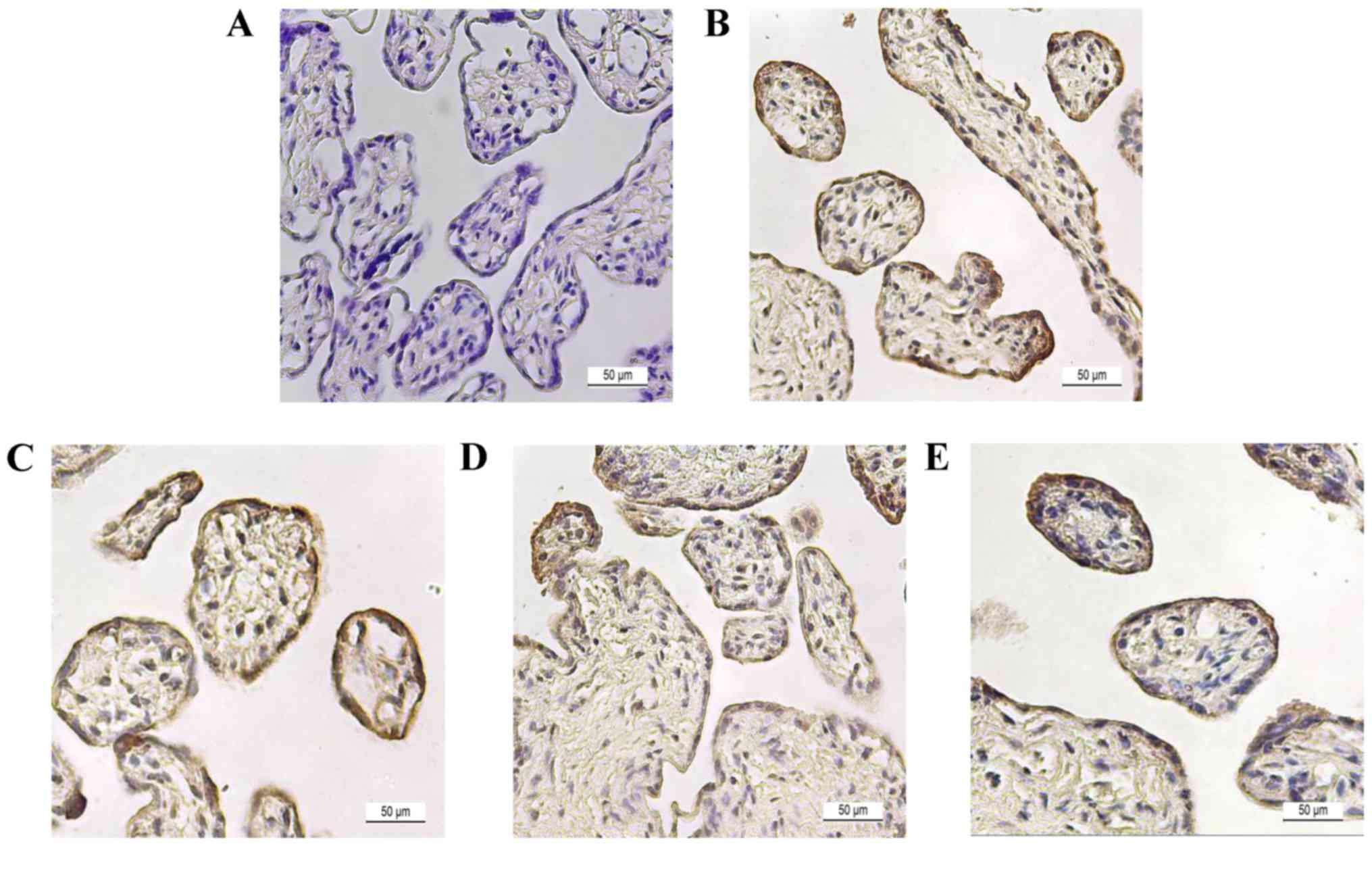
Histological analysis of steroidogenic
enzymes in placental tissue
To determine steroidogenic enzyme localization,
immunostaining was performed in late preterm placenta. Placental
tissue was stained with hematoxylin & eosin to confirm the
morphology of placental tissue (Fig.
3A). Tissue sections were immunostained with specific
antibodies of proteins and counter-stained with H&E to show the
spatial localizations of CYP11A1 (Fig.
3B), HSD3B1 (Fig. 3C), CYP17A1
(Fig. 3D), and CYP19A1 (Fig. 3E). In the image, the
cytotrophoblast section was surrounded with a layer of
syncytiotrophoblasts, which is a general morphological feature of
placenta. Steroidogenic enzymes, including HSD3B1, CYP17A1 and
CYP19A1, were localized to both cyto- and syncytiotrophoblast
cells, and signals were more dominant in syncytiotrophoblast cells.
However, expression of CYP11A1 was negligible in placenta.
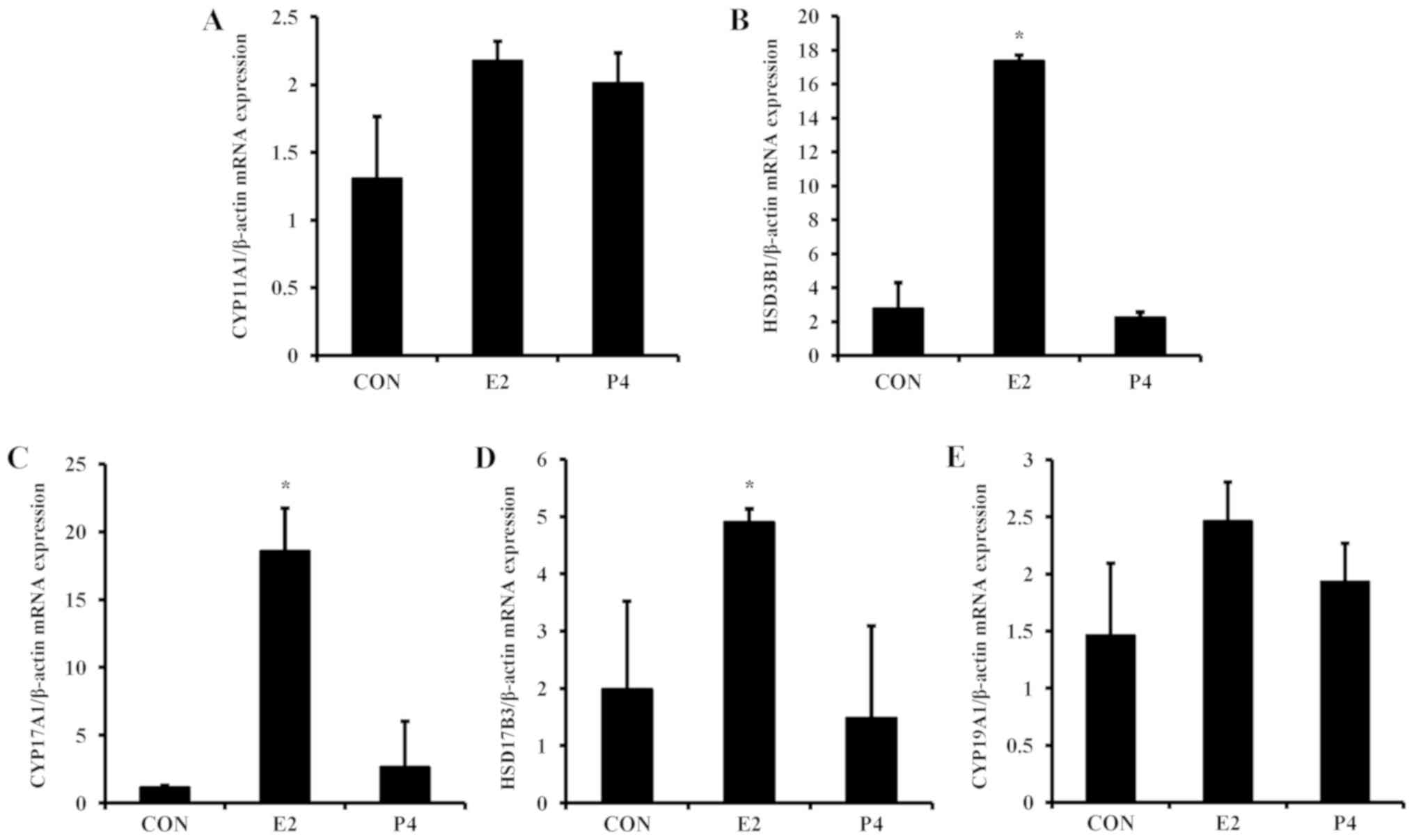
Regulation of steroidogenic enzymes by
E2 and P4
For the next experiment, the expression levels of
steroidogenic enzymes in placental-derived BeWo cells was examined.
To evaluate the mechanism of steroidogenic enzyme regulation, BeWo
cells were treated with E2 and P4, which are critical hormones
during pregnancy (2), for 24 h.
The mRNA expression of HSD3B1, CYP17A1, and HSD17B3 was
significantly elevated by E2 in BeWo cells (Fig. 4), whereas P4 did not alter mRNA
expression of these steroidogenic enzymes. Similar to the mRNA
results, E2 increased protein expression of
steroidogenesis-associated enzymes (Fig. 5A). Translation levels of HSD3B1,
CYP17A1, and HSD17B3 were elevated 2- to 3-fold in response to E2
treatment, whereas levels of CYP19A1 were not significantly altered
(Fig. 5B-E).
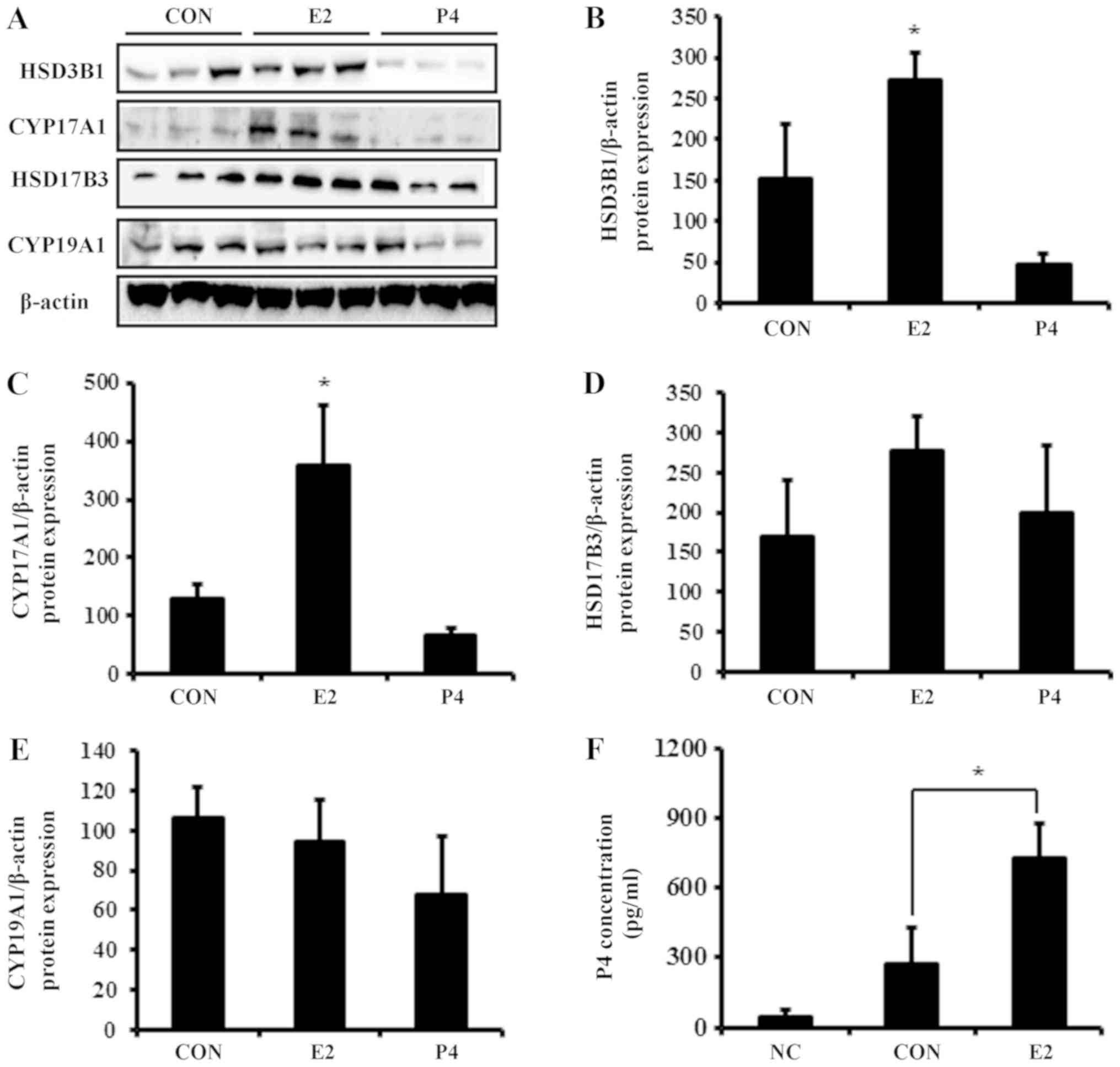 | Figure 5.Protein expression of steroidogenic
enzymes and P4 secretion in response to E2 and P4 treatment in
placental-derived BeWo cells. (A) Total protein was harvested from
BeWo cells for western blot analysis. Expression of (B) HSD3B1, (C)
CYP17A1, (D) HSD17B3 and (E) CYP19A1 was quantified and normalized
to β-actin. (F) Following treatment of BeWo cells with E2, medium
was harvested and P4 concentration was analyzed with ELISAs. Data
are expressed as the mean ± standard deviation. *P<0.05 vs.
control group. NC, culture media without cells; CON, culture media
with BeWo cells treated with vehicle; HSD3B1, 3β-hydroxysteroid
dehydrogenase type 1; CYP17A1, 17α hydroxylase; HSD17B3,
17β-hydroxysteroid dehydrogenase type 3; CYP19A1, aromatase; E2,
estradiol; P4, progesterone. |
Analysis of P4 secretion after E2
treatment
As the expression of steroidogenic enzymes was
elevated by E2, the modulation of P4 secretion was further
investigate following E2 treatment in BeWo cell-cultured media. P4
expression in conditioned cultured media (control group) collected
from BeWo cells was 0.27 ng/ml (Fig.
5F). E2 increased production of P4 by 3-fold compared with the
control, which was consistent with the expression results of
P4-metabolizing enzymes.
Discussion
Steroid hormones are mainly produced by
steroidogenesis, which is the biological process by which steroids
are generated from cholesterol or transformed into other steroids.
Humans and mammals predominantly synthesize active steroid hormones
from cholesterol mainly in the reproductive glands and placenta
(9). During normal pregnancy,
women experience numerous adjustments in their endocrine system
(18). Levels of E2 and P4
dramatically increase throughout pregnancy, suppressing the
hypothalamic axis and subsequently the menstrual cycle (19). Due to their various functions, E2
and P4 are associated with various physiological disorders during
pregnancy such as pre-eclampsia and premature birth (20).
In the current study, the expression of
steroidogenic enzymes were examined in human placenta according to
gestational age. The mRNA and protein expression of steroidogenic
enzymes was upregulated with gestational age in placenta tissue. To
analyze the association between serum and placental steroid
hormones, the concentrations of steroid hormones in human serum and
placenta were examined. In the results, serum DHEA, P4 and T was
elevated in the term stage of pregnancy, whereas PG levels were not
significantly altered according to gestational age. In our previous
study, serum E2 levels were shown to be enhanced in the late stage
of gestation (20). In addition to
serum, steroid hormones from placental tissue, including PG, DHEA,
P4, T and E2 were examined. Similar to the serum results, DHEA and
E2 levels were elevated in the term stage of pregnancy, whereas PG
and P4 levels were not altered. Unlike the serum results, placental
T levels were reduced in the term stage of pregnancy. It is
possible that serum T levels are elevated by the fetal
steroidogenic system, which stimulates negative feedback inhibition
of the hormone in maternal placenta (21). These results indicated that
expression of steroidogenic enzymes in placental tissue was
enhanced with gestational age, resulting in elevated serum and
placenta levels of E2 and DHEA in the term stage of gestation.
However, some steroid hormones such as P4 and T were differently
regulated in the placenta and serum. In addition, the basal levels
of the hormones including DHEA, P4, and T in serum at each duration
were occasionally higher than those of placenta. These results
suggested that not all steroid hormones originated from placenta,
indicating the involvement of other organs, including maternal
ovary and adrenal glands, as well as the fetal endocrine system. To
further examine the endocrine regulatory mechanism in the placenta,
placental-derived BeWo cells were treated with E2 and P4. E2
treatment upregulated the expression of steroidogenesis-associated
enzymes that were not significantly regulated by P4. Since
steroidogenic enzymes were significantly modulated by E2 only, P4
secretion in BeWo cell-cultured media following E2 treatment was
additionally examined. As expected, BeWo cells showed higher
secretion of P4 compared to the control group, indicating that the
steroidogenic process was enhanced by E2 via a positive feedback
mechanism.
The results of the present study show that E2 is
more important than P4 in human placenta in terms of its effects on
steroidogenesis during pregnancy, which is different from previous
studies on other species (11,12).
In cats, placental P4 concentrations appear to be independent on
gestational age (11). In bovines,
the maternal placenta contributes significantly to steroidogenesis
in early pregnancy, particularly for the synthesis of P4 (12). These controversial results may be
due to the different physiological features of placenta depending
on the species. In addition, different steroidal circumstances may
contribute to different interactions and communication between
mother, placenta and fetus in humans (7,19).
In summary, the levels of steroid hormones in human
placenta and serum were investigated according to gestational age.
As the gestational age increased, expression levels of
steroidogenic enzymes were stimulated in the placenta, which may
constitute the elevation of serum DHEA and E2. The in vitro
study suggested that this regulation of steroidogenic enzyme
expression was attributed to increased E2 levels via a positive
feedback mechanism in the late stage of gestation. The critical
roles of the regulated steroid hormones during gestation should be
investigated in future studies in order to understand physiological
ramifications of pregnancy.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Korea
Health Technology R&D Project through the Korea Health Industry
Development Institute (KHIDI), funded by the Ministry of Health
& Welfare, Republic of Korea (grant no. HI16C0313).
Availability of data and materials
The biospecimens and data used for this study were
provided by the Biobank of Pusan National University Hospital, a
member of the Korea Biobank Network. The datasets used and/or
analyzed during the current study are available from the
corresponding author on reasonable request.
Authors' contributions
SHH, MNP, JSJ, ONB and SS performed the experiments.
SCK, YJL and HSY collected human samples. SHH, KSL and BSA wrote
the article. KSL and BSA designed the project. SCK, SYY, YJL, ONB,
HSY, SS and KSL contributed to the discussion. SYY also provided
experimental materials.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Pusan National University Hospital Clinical
Trials Center (approval no. H-1302-005-015). Placental tissue
samples were received from Pusan National University Hospital and
collected after informed consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pijnenborg R, Bland JM, Robertson WB,
Dixon G and Brosens I: The pattern of interstitial trophoblastic
invasion of the myometrium in early human pregnancy. Placenta.
2:303–316. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gude NM, Roberts CY, Kalionis B and King
RG: Growth and function of the normal human placenta. Thromb Res.
114:397–407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gailly-Fabre E, Kerlan V and
Chirstin-Maitre S: Pregnancy-associated hormones and fetal-maternal
relations. Ann Endocrinol (Paris). 76:S39–S50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albrecht ED and Pepe GJ: Estrogen
regulation of placental angiogenesis and fetal ovarian development
during primate pregnancy. Int J Dev Biol. 54:397–408. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gruber CJ, Tschugguel W, Schneeberger C
and Huber JC: Production and actions of estrogens. N Engl J Med.
346:340–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu BT and Conney AH: Functional role of
estrogen metabolism in target cells: Review and perspectives.
Carcinogenesis. 19:1–27. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy VE, Smith R, Giles WB and Clifton
VL: Endocrine regulation of human fetal growth: The role of the
mother, placenta, and fetus. Endocr Rev. 27:141–169. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pepe GJ and Albrecht ED: Actions of
placental and fetal adrenal steroid hormones in primate pregnancy.
Endocr Rev. 16:608–648. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Payen AH and Hales DB: Overview of
steroidogenic enzymes in the pathyway from cholesterol to active
steroid hormones. Endocr Rev. 25:947–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller WL and Auchus RJ: The molecular
biology, biochemistry, and physiology of human steroidogenesis and
its disorders. Endocr Rev. 32:81–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siemieniuch MJ, Jursza E, Szostek AZ,
Skarzynski DJ, Boos A and Kowalewski MP: Steroidogenic capacity of
the placenta as a supplemental source of progesterone during
pregnancy in domestic cats. Reprod Biol Endocrinol. 10:892012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verduzco A, Fecteau G, Lefebvre R, Smith
LC and Murphy BD: Expression of steroidogenic proteins in bovine
placenta during the first half of gestation. Reprod Fertil Dev.
24:392–404. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furukawa S, Kuroda Y and Sugiyama A: A
comparison of the histological structure of the placenta in
experimental animals. J Toxicol Pathol. 27:11–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SC, Park MN, Lee YJ, Joo JK and An BS:
Interaction of steroid receptor coactivators and estrogen receptors
in the human placenta. J Mol Endocrinol. 56:239–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gambino YP, Perez Perez A, Duenas JL,
Calvo JC, Sanchez-Margalet V and Varone CL: Regulation of leptin
expression by 17beta-estradiol in human placental cells involves
membrane associated estrogen receptor alpha. Biochim Biophys Acta.
1823:900–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frazier K, Wallace K and LaMarca B:
Progesterone inhibits trophoblast TNF alpha production. The FASEB
J. 24:2010.
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Costantine MM: Physiologic and
pharmacokinetic changes in pregnancy. Front Pharmacol. 5:652014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurnar P and Magon N: Hormones in
pregnancy. Niger Med J. 53:179–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robboy SJ and Hoda RS: Pathology of the
human placenta. Int J Gynecol Pathol. 19:4012000. View Article : Google Scholar
|
|
21
|
Rodeck CH, Gill D, Rosenberg DA and
Collins WP: Testosterone levels in midtrimester maternal and fetal
plasma and amniotic fluid. Prenat Diagn. 5:175–181. 1985.
View Article : Google Scholar : PubMed/NCBI
|