Introduction
Osteoarthritis (OA) is a multifactorial articular
disease characterized by cartilage degeneration, subchondral
sclerosis and osteophyte formation (1–3).
Normal function of articular cartilage is highly dependent on the
homeostasis of the extracellular matrix (ECM), which serves as the
mechanical structure and is involved in signal transduction in
chondrocytes (4–7). Previous studies have demonstrated the
roles of the interaction between anabolic factors, including
transforming growth factor β, and catabolic factors, including
matrix metalloproteinases (MMPs) and aggrecanases, in the
maintenance and regeneration of ECM in chondrocytes (8–10).
However, the exact molecular mechanisms involved in OA remain
largely unclear. Although progress in OA therapy has been
incremental, the majority of treatments only improve clinical
symptoms, as opposed to restoring the damaged ECM (11). In addition, inhibition of OA by the
regulation of specific genes has been an unsuccessful strategy
(12,13).
Stromal cell-derived factor 1 (SDF-1) is a cytokine
that is associated with inflammation, and is identified in the
synovial membranes adjacent to articular cartilage (14–16).
Binding of SDF-1 and its ligand C-X-C chemokine receptor type 4
(CXCR4), a G protein-coupled receptor located in the surface of
chondrocytes, induces the release of MMPs from the ECM, thereby
exacerbating OA (16,17). As a CXCR4 inhibitor, the compound
AMD3100 blocks the SDF-1/CXCR4 axis and has been effectively
utilized in the treatment of OA (18–20).
However, observed side effects and the unstable nature of AMD3100
limit its clinical application (20–22).
TN14003 was designed based on T140, a 14-residue peptide that
possesses a high level of anti-human immunodeficiency virus (HIV)
activity and antagonism of T cell line-tropic HIV-1 entry among all
the antagonists of CXCR4 (23).
TN14003 was generated by amidating the COOH-terminal of T140 and by
substituting basic residues with non-basic polar amino acids to
decrease the total-positive charges of the molecule, and is far
less cytotoxic and more stable in serum compared with T140
(23,24).
MicroRNA (miRNA) belong to a class of small
noncoding RNA encoded by endogenous genes, and dysregulation of
miRNAs results in numerous diseases that occur in various
physiological and pathological processes (25,26).
Accumulating studies investigating the roles of miRNAs in bone and
cartilage have identified a number of miRNAs that serve important
roles in the pathogenesis of OA (27–30).
Therefore, the identification of abnormally expressed miRNAs and
the associated biological consequences of their targets is
essential to determining the potential molecular mechanisms in the
OA pathological process. Unfortunately, the changes in miRNA
expression in chondrocytes as a result of inhibiting the
SDF-1/CXCR4 axis by drugs including TN14003 remains largely
unclear.
Using a series of bioinformatic approaches, the
present study aimed to systematically evaluate the aberrant miRNA
expression levels in OA chondrocytes treated with TN14003. The key
miRNA miR-146a-5p was also confirmed as a differentially expressed
miRNA, and the expression levels of its targets involved in the
process of SDF-1/CXCR4 axis inhibition were measured, following
molecular manipulation of the expression of miR-146a-5p in
chondrocytes.
Materials and methods
Cartilage tissue collection and cell
cultivation
OA cartilage was obtained from the weight-bearing
surface of the femoral condyle and tibial plateau of 4 female and 1
male patients diagnosed with OA (using the American College of
Rheumatology classification criteria), aged between 57 and 69 years
old with an average age of 63.4±2.42, and undergoing total knee
arthroplasty between October 2017 to March 2018 in the Department
of Sports Medicine of the First Affiliated Hospital of Kunming
Medical University (Kunming, China) (31,32).
Written informed consent was obtained from all patients, and the
present study was approved by the Ethics Committee at the First
Affiliated Hospital of Kunming Medical University (Kunming,
China).
Chondrocytes were digested with 0.15% collagenase
and cultured in high glucose Dulbecco's Modified Eagle Medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 100 U/ml penicillin and 100 µg/ml
streptomycin. Culture medium was filtered to remove bacteria using
a 0.22 µm microfilter. The first generation chondrocytes were used
and divided into two groups: Treatment and control. To mimic the
osteoarthritic environment of the knee joint, each group was
treated with 100 ng/ml SDF-1 (PeproTech, Rocky Hill, NJ, USA). The
treatment group was pretreated with 1 µM TN14003 (Scilight
Biotechnology, LLC, Beijing, China) for 2 h prior to the addition
of SDF-1. Each group of chondrocytes was incubated at 37°C and 5%
CO2 for 2 days.
miRNA extraction and reverse
transcription
For miRNA screening, total RNA was isolated from
cartilage tissues with or without TN14003 treatment, purified and
prepared using the Qiagen RNeasy Mini kit (Qiagen, Hilden, Germany;
cat. no. 74106) according to manufacturer's protocol. The integrity
and quantity of samples were determined via Nanodrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.) and Agilent 2100
Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). miRNAs
were isolated from total RNA using the All-in-One™ miRNA reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Detection kit (GeneCopoeia, Inc., Rockville, MD, USA), according to
the manufacturer's protocol.
miRNA RT-qPCR and verification
To calibrate the initial results, differentially
expressed miRNAs were identified using miProfile™ human
inflammatory miRNA qPCR Arrays (GC08017K18014P; GeneCopoeia, Inc.).
The chondrocyte samples were isolated from 5 patients with OA and
the validation was performed using samples with or without TN14003
treatment. Each well contained a forward primer for the mature
miRNA sequence, and a universal adaptor reverse primer cross-linked
to the 96-well plate. The primers for measuring miRNA expression
were designed as summarized in Table
I, and the qPCR was performed using 20 µl reaction volumes
containing 1 µl reverse transcription product and SYBR Green Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
amplification conditions were as follows: Pre-incubation at 50°C
for 2 min, enzyme activation at 95°C for 10 min, then 40 cycles of
denaturation at 95°C for 10 sec, annealing at 55°C for 30 sec and
extension at 72°C for 30 sec. Detection was performed using an ABI
7500 instrument (Applied Biosystems; Thermo Fisher Scientific,
Inc.), and 2−ΔΔCq was used to calculate the relative
expression of miRNAs, as previously described (33). Alterations with a fold-change >2
and P<0.05 were considered to be differentially expressed.
 | Table I.Sequences of polymerase chain
reaction primers used for the detection of miRNA expression. |
Table I.
Sequences of polymerase chain
reaction primers used for the detection of miRNA expression.
| miRNA | Primer sequence,
5′-3′ | Annealing
temperature, °C |
|---|
| miR-126-3p | Forward:
TCGTACCGTGAGTAATAATGCG | 60 |
|
| Reverse:
GCTGTCAACGATACGCTACGTAAC |
|
| miR-124-3p | Forward:
TAAGGCACGCGGTGAATGCC | 60 |
|
| Reverse:
GCTGTCAACGATACGCTACGTAAC |
|
| miR-130a-3p | Forward:
CAGTGCAATGTTAAAAGGGCAT | 60 |
|
| Reverse:
GCTGTCAACGATACGCTACGTAAC |
|
| miR-185-5p | Forward:
TGGAGAGAAAGGCAGTTCCTGA | 60 |
|
| Reverse:
GCTGTCAACGATACGCTACGTAAC |
|
| miR-146a-5p | Forward:
TGAGAACTGAATTCCATGGGTT | 60 |
|
| Reverse:
GCTGTCAACGATACGCTACGTAAC |
|
| miR-155-5p | Forward:
TTAATGCTAATCGTGATAGGGGT | 60 |
|
| Reverse:
GCTGTCAACGATACGCTACGTAAC |
|
| miR-221-3p | Forward:
AGCTACATTGTCTGCTGGGTTTC | 60 |
|
| Reverse:
GCTGTCAACGATACGCTACGTAAC |
|
| U6 | Forward:
CTCGCTTCGGCAGCACA | 60 |
|
| Reverse:
AACGCTTCACGAATTTGCGT |
|
| Oligo dT
Adaptor |
GCTGTCAACGATACGCTACGTAACGGCATG |
|
|
ACAGTGTTTTTTTTTTTTTTTTTV |
|
Bioinformatic analysis
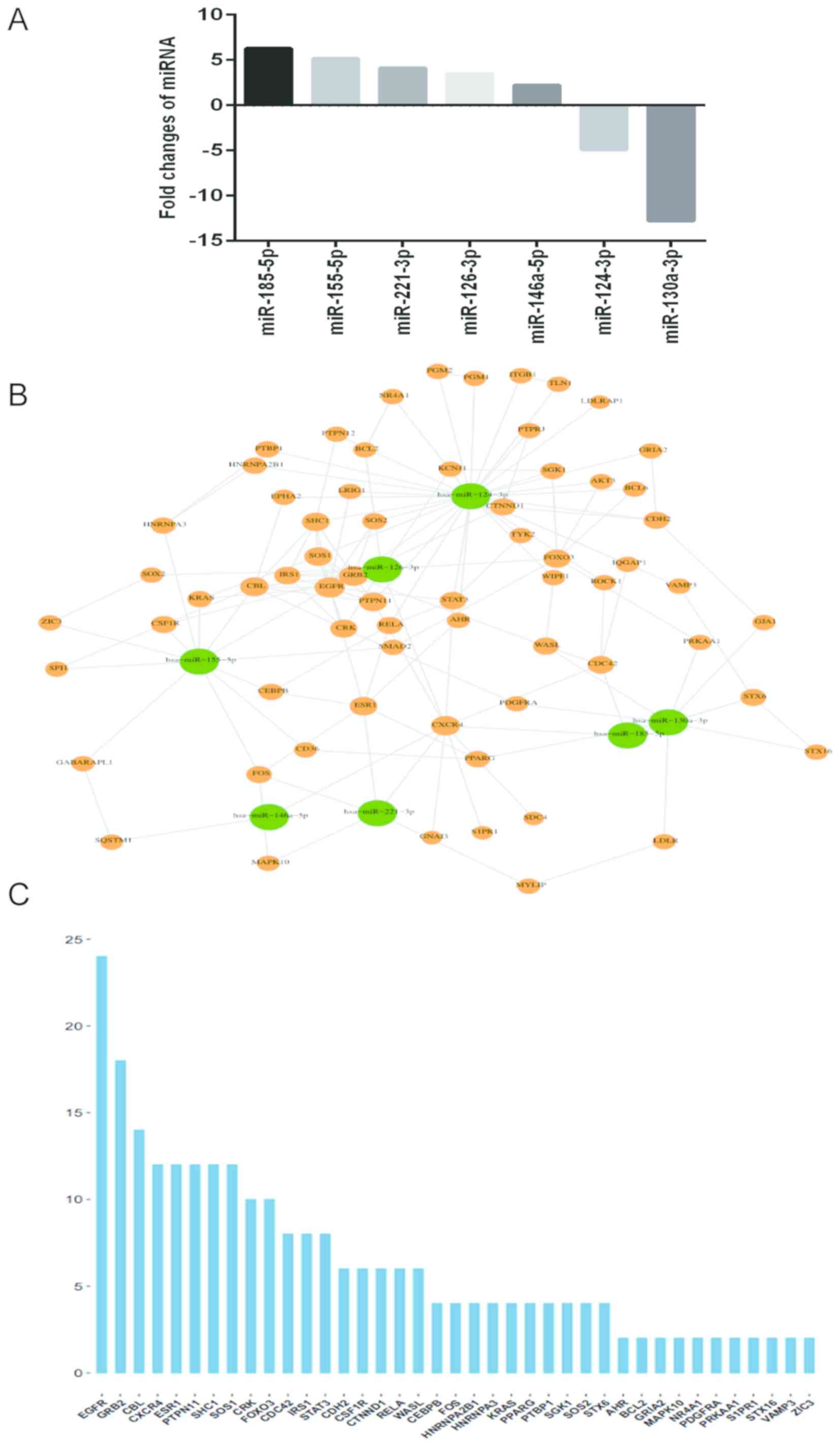
A total of 7 differentially expressed miRNAs
(miR-146a-5p, miR-221-3p, miR-126-3p, miR-185-5p, miR-155-5p,
miR-124-3p and miR-130a-3p) were identified (Fig. 1). The targets of these miRNAs were
predicted using miRDB (34)
(http://mirdb.org/miRDB/), miRTarBase (35) (http://mirtarbase.mbc.nctu.edu.tw/), miRWalk (36) (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/)
and TargetScan (37) (http://www.targetscan.org/). Genes targeted by miRNAs
were analyzed by Gene Ontology (GO) analysis (38) (http://geneontology.org/page/go-enrichment-analysis)
and the Database for Annotation, Visualization and Integrated
Discovery pathway analysis (39)
(http://www.genome.jp/) to compile gene annotation
terms and involved signaling pathways. Finally, miRNA-target
networks were constructed to visually enrich the gene dataset. The
enrichment result was visualized by Enrichment Map as a plugin of
Cytoscape version 3.2.0 (40). The
revised P-value was characterized by the false discovery rate (FDR)
to avoid false positivity, and an FDR<0.05 was used as the
cut-off value.
Cell transfection
Chondrocytes were seeded in 6-well plates at
5×104 cells per well and incubated at 37°C for 24 h.
Chondrocytes were separately transfected with miR-146a-5p mimics,
miR-146a-5p inhibitors and negative controls (NC; GeneCopoeia,
Inc.) at a 100 nM concentration for 48 h using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc, MA, USA), according to the protocol of the
manufacturer. The sequences for miR-146a-5p mimics, miR-146a-5p
inhibitors and the corresponding NC were as follows: miR-146a-5p
mimic, 5′-UGAGAACUGAAUUCCAUGGGUU-3′ (sense) and
5′-CCCAUGGAAUUCAGUUCUCAUU-3′ (antisense); mimics NC,
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGGAATT-3′
(antisense); miR-146a-5p inhibitor, 5′-AACCCAUGGAAUUCAGUUCUCA-3′;
inhibitor NC, 5′-CAGUACUUUUGUGUAGUACAA-3′. The chondrocytes was
cultured for 48 h after transfection and then used for subsequent
experiments.
RT-qPCR assay for mRNA expression
Total RNA was extracted from cartilage tissues using
TRIzol® regent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA (1 µg) was
transcribed into cDNA using the RevertAid™ First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc). cDNA (40 µg/µl) was
used as a template for amplification of CXCR4, type II collagen
(Col II), aggrecan (ACAN) and MMP-3 genes, and β-actin served as an
internal reference. SYBR Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used for RT-qPCR analysis. The
amplification conditions were as follows: Pre-incubation at 50°C
for 2 min, enzyme activation at 95°C for 10 min, then 40 cycles of
denaturation at 95°C for 10 sec, annealing at 55°C for 30 sec and
extension at 72°C for 30 sec. All primers (Table II) were obtained from the NCBI
database and designed by Premiers Express Software v1.0 (BioTools
Incorporated, Edmonton, AB, Canada). Reactions for each sample were
performed in at least three independent experiments. Cycle
threshold values were measured and data were calculated the by
2−ΔΔCq method (33).
 | Table II.Sequences of polymerase chain
reaction primers used for detection mRNA expression. |
Table II.
Sequences of polymerase chain
reaction primers used for detection mRNA expression.
| Target | Forward primer
5′-3′ | Reverse primer
5′-3′ |
|---|
| CXCR4 |
TCAGTGGCTGACCTCCTCTT |
CTTGGCCTTTGACTGTTGGT |
| Col II |
ATGCACCTTGGATGCCATGA |
ATGCACCTTGGATGCCATGA |
| ACAN |
ACATCTCAGCAGCATCATCACC |
CATCACCACGCAGTCCTCAC |
| MMP-3 |
GGACAAAGGATACAACAGGGAC |
TCATCTTGAGACAGGCGAAA |
| β-actin |
CCACCATGTACCCAGGCATT |
ACTCCTGCTTGCTGATCCAC |
Western blot analysis
Total protein was extracted from cells following
lysis with radioimmunoprecipitation assay buffer and quantified by
the BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). Total
protein (30 µg) was separated via electrophoresis on 10% SDS-PAGE
gels prior to transfer to polyvinylidene fluoride membranes.
Membranes were probed with primary antibodies in TBS with 5%
non-fat milk. The antibodies included were anti-CXCR4 (LifeSpan
BioSciences, Inc., Seattle, WA, USA; 1:1,000 dilution; cat. no.
LS-B2160-0.05), anti-Col II (Abcam, Cambridge, UK; 1:1,000
dilution; cat. no. ab188570) and anti-β-actin (Abcam, Cambridge,
UK; 1:5,000 dilution; cat. no. ab8227), and horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (Abcam,
Cambridge, UK; 1:10,000 dilution; cat. no. ab97051) was used as the
secondary antibody. Proteins of interest were visualized using
enhanced chemiluminescent reagent (Thermo Fisher Scientific, Inc.).
The band intensities were quantified by densitometry using ImageJ
1.46r software (National Institutes of Health, Bethesda, MD, USA)
(41). Experiments were repeated
at least 3 times.
Statistical analysis
All quantitative data were analyzed with SPSS 18.0
(SPSS, Inc. Chicago, IL, USA) and presented as mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance with the Least-Significant Difference
correction to determine differences between groups. P<0.05 were
considered to indicate a statistically significant difference.
Results
Identification of candidate miRNAs
whose alterations are in response to TN14003 treatment in
SDF-1-stimulated chondrocytes
To evaluate the effects of TN14003 on chondrocytes,
OA patients-derived chondrocytes were treated with SDF-1 alone or
SDF-1 + TN14003 for 48 h. Cells were harvested to investigate the
alteration of miRNA profile upon the inhibition of CXCR4/SDF-1 axis
by TN14003. There were 7 differentially expressed miRNAs identified
in cartilage samples via microarray analysis (Table III). Among these miRNAs, 5 miRNAs
(miR-146a-5p, miR-221-3p, miR-126-3p, miR-185-5p and miR-155-5p)
were significantly upregulated, and 2 miRNAs (miR-124-3p and
miR-130a-3p) were significantly downregulated in the treatment
group compared with the control group (Fig. 1A). In order to understand the
mechanism of these miRNA alterations and consequently their
involvement in OA treatments, the miRWalk database and Cytoscape
software was used to analyze the miRNA-mRNA interactions through
their visualization as a network (Fig.
1B). While the interactions between miRNAs and potential
targets were built, a sequence diagram was performed to reveal the
importance of targets. The importance of targets was determined
according to the number of connections between each gene and miRNA
and other genes in the miRNA-target network (Fig. 1C). As demonstrated in the network
map, the miRNAs hsa-miR-146a-5p, hsa-miR-221-3p, hsa-miR-126-3p and
hsa-miR-185-5p exhibited direct interaction with the mRNA CXCR4
(Fig. 1B). Among all the potential
targets, EGFR, GRB2, CBL, CXCR4, ESR1, PTPN11, SHC1 and SOS1 were
the targeted mRNAs with highest importance scores (Fig. 1C).
 | Table III.Expression levels of 7 targeted
miRNAs in cartilage samples with and without TN14003 treatment via
microarray analysis. |
Table III.
Expression levels of 7 targeted
miRNAs in cartilage samples with and without TN14003 treatment via
microarray analysis.
| miRNA name | Fold change
Treatment group/control group | P-value |
|---|
| miR-146a-5p | 2.12 | 0.00043 |
| miR-221-3p | 4.41 | 0.00024 |
| miR-126-3p | 3.49 | 0.00007 |
| miR-185-5p | 6.29 | 0.00003 |
| miR-155-5p | 5.15 | 0.00019 |
| miR-124-3p | −4.91 | 0.00017 |
| miR-130a-3p | −12.78 | 0.00022 |
Verification of candidate miRNAs, GO
terms assignment and pathways analysis of miR-146a-5p and its
targets
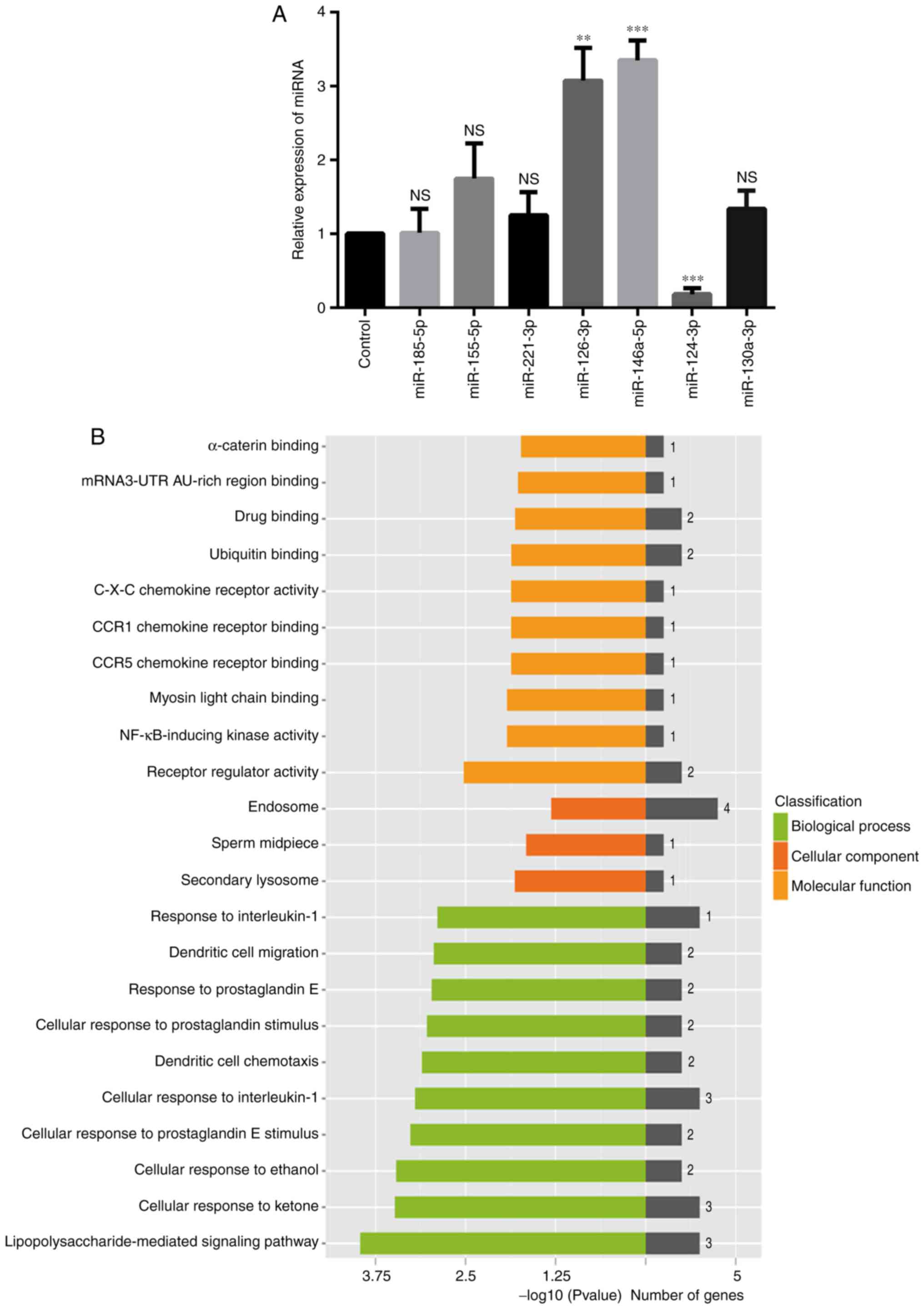
To validate the differentiated expression of the 7
miRNAs identified from the initial screening, the levels of these
miRNAs in control-treated and TN14003-treated chondrocytes were
measured by RT-qPCR assays. Compared with the initial microarray
results, the expression levels of miR-146a-5p, miR-126-3p and
miR-124-3p were validated by the RT-qPCR approach: As indicated in
Fig. 1A, the expression of both
miR-146a-5p and miR-126-3p was upregulated, while the expression of
miR-124-3p was downregulated; the results of RT-qPCR analysis
presented in Fig. 2A confirmed
this observation. However, other miRNAs that did not exhibit the
same changes, or exhibited no statistical differences in expression
were excluded from subsequent analyses (Fig. 2A). Notably, miR-146a-5p was
upregulated >3-fold. A previous study suggested an association
between miR-146a-5p and OA, as evidenced by its >2-fold
increased expression compared with healthy controls (42). Besides, accumulating data indicate
that miR-146a-5p is a representative miRNA known to be associated
with OA (43,44). Therefore, the present study focused
on miR-146a-5p and its targets to delineate their associations with
OA treatments by TN14003.
GO analysis indicated that miR-146a-5p and its
targets were primarily grouped into ‘receptor regulator activity’
or ‘nuclear factor-kappa-light-chain-enhancer of activated B cells
(NF-κB)-inducing kinase (NIK) activity’ in the Molecular Functions
category, into ‘lipopolysaccharide-mediated signaling pathways’ or
‘cellular response to ketones’ in the Biological Processes
category, and into ‘secondary lysosome’, ‘sperm midpiece’ or
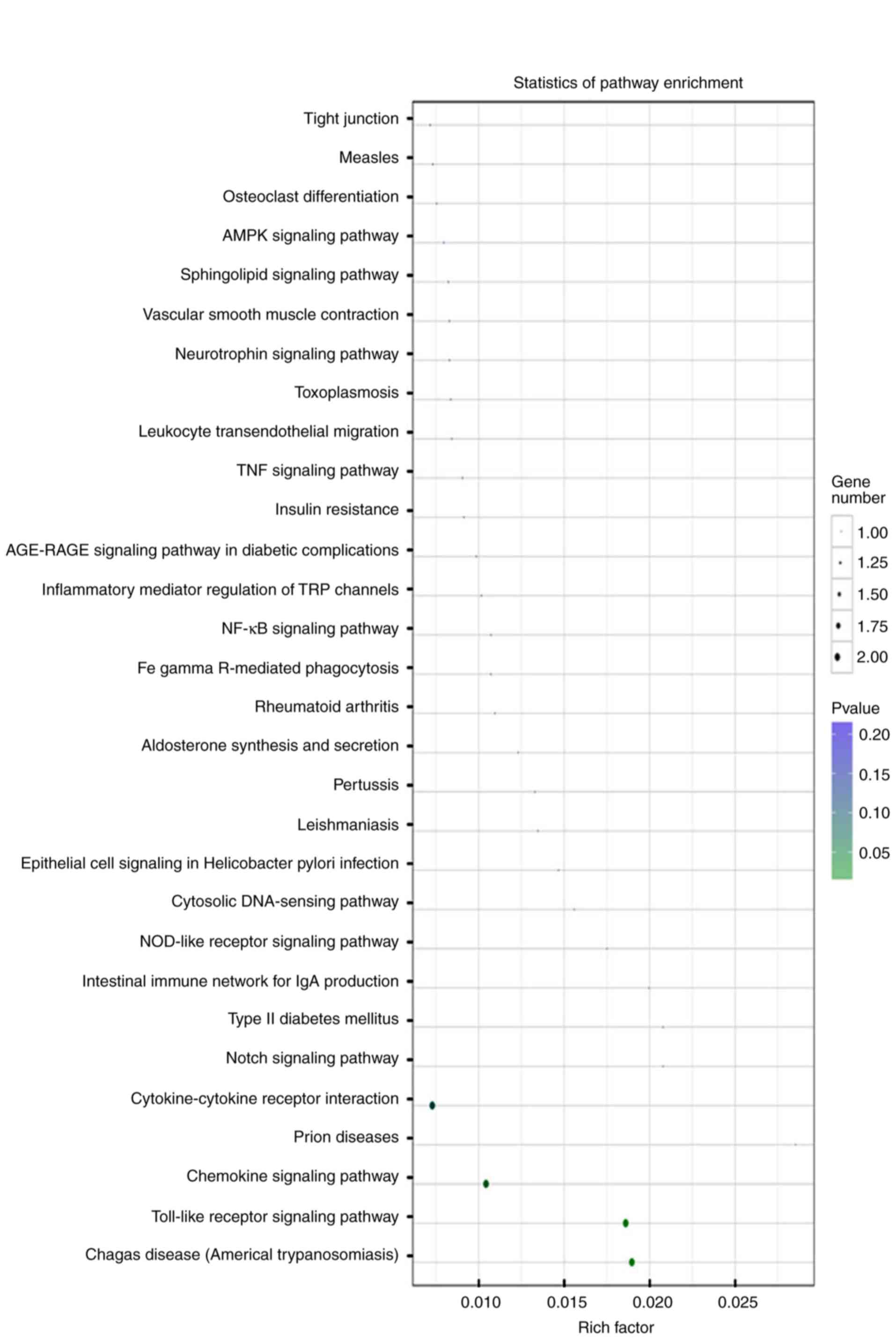
‘endosomes’ in the Cellular Components category (Fig. 2B). Enriched pathways of miR-146a-5p
and its targets were primarily involved in the ‘chemokine signaling
pathway’ or the ‘Toll-like receptor signaling pathway’ (Fig. 3). These results indicated that
these identified inflammation-associated GO terms and pathways are
important to the roles of the SDF-1/CXCR4 axis in the pathogenesis
of OA.
Molecular manipulation of miR-146a-5p
expression in chondrocytes transfected with the mimic or inhibitor
of miR-146a-5p
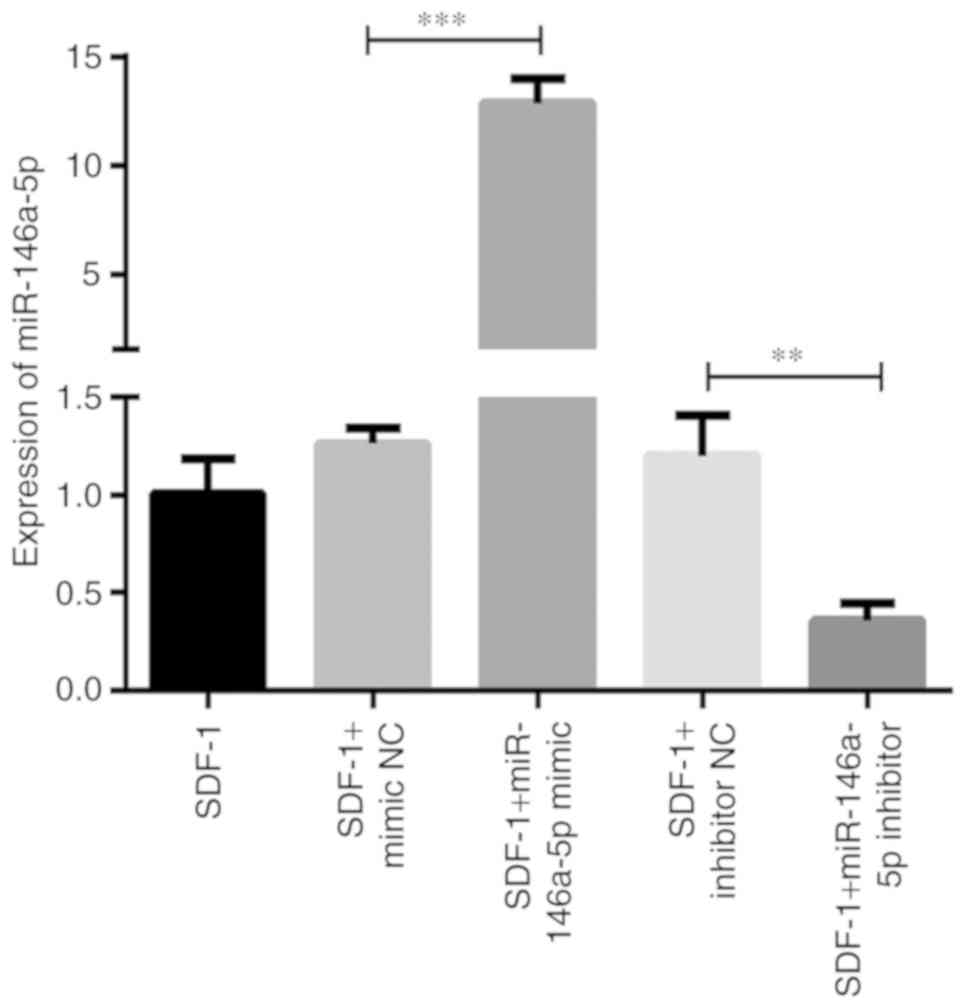
To additionally reveal the roles of miR-146a-5p in
the process of OA development, the mimic and inhibitor of
miR-146a-5p were designed to examine the effects of molecular
manipulation of miR-146a-5p on OA-associated molecules. First, an
RT-qPCR assay was performed to verify the effect of cell
transfection and successful upregulation and downregulation of
miR-146a-5p expression. As demonstrated in Fig. 4, the level of miR-146a-5p was
significantly increased ~12-fold compared with the mimic control in
chondrocytes transfected with the miR-146a-5p mimic, and markedly
downregulated to ~20% of the inhibitor control when the miR-146a-5p
inhibitor was transfected. As hypothesized, there were no
significant changes in miR-146a-5p expression between chondrocytes
that were treated with SDF-1 only and transfected with negative
controls (Fig. 4).
Association between miR-146a-5p
expression and the mRNA levels of Col II, ACAN, CXCR4 and
MMP-3
Next, RT-qPCR was performed to measure the
expression of cartilage degeneration-associated factors (including
Col II, ACAN, CXCR4 and MMP-3) following transfection of the
chondrocytes with the mimic and inhibitor of miR-146a-5p, and
corresponding NCs. As indicated in Fig. 5A and B, in the chondrocytes
transfected with the miR-146a-5p mimic, the expression levels of
Col II and ACAN were significantly increased. However, there was no
significant difference in Col II expression between chondrocytes
transfected with the inhibitor NC and the miR-146a-5p inhibitor.
The expression levels of ACAN decreased markedly in the
chondrocytes transfected with the miR-146a-5p inhibitor. In
contrast, as indicated in Fig. 5C and
D, the mRNA expression levels of CXCR4 and MMP-3 decreased in
the chondrocytes following transfection with the miR-146a-5p mimic,
and increased following transfection with the miR-146a-5p
inhibitor. These results demonstrate a positive association between
miR-146a-5p expression and the mRNA expression levels of Col II and
ACAN, and a negative association between miR-146a-5p expression and
the mRNA expression of CXCR4 and MMP-3.
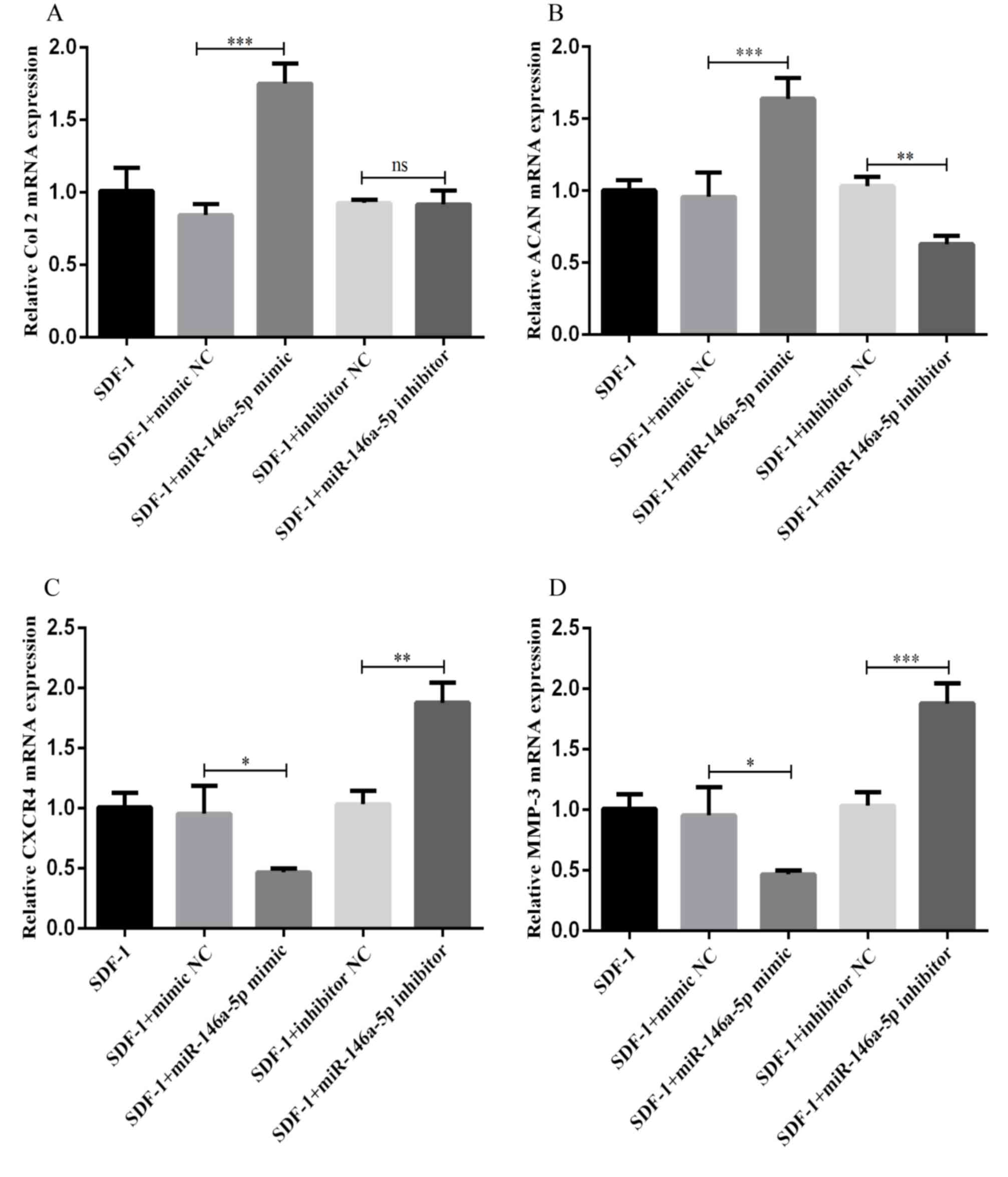 | Figure 5.Association between miR-146a-5p
expression and the mRNA levels of Col II, ACAN, CXCR4 and MMP-3.
(A-D) Chondrocytes were separately transfected with miR-146a-5p
mimics, miR-146a-5p inhibitors and NC for 48 h using
Lipofectamine® 3000. Following stimulation with 100
ng/ml SDF-1 for 2 days, cells were harvested to evaluate the levels
of (A) Col II, (B) ACAN, (C) CXCR4 and (D) MMP-3 by reverse
transcription quantitative polymerase chain reaction. Data are
summarized from 3 independent experiments with similar results. n=3
for each group. *P<0.05, **P<0.01 and ***P<0.001 vs. the
corresponding control group. miRNA, miRNA; Col II, type II
collagen; ACAN, aggrecan; CXCR4, C-X-C chemokine receptor type 4;
MMP-3, matrix metalloproteinase-3; SDF-1, stromal cell-derived
factor 1; NC, negative control; NS, not significant. |
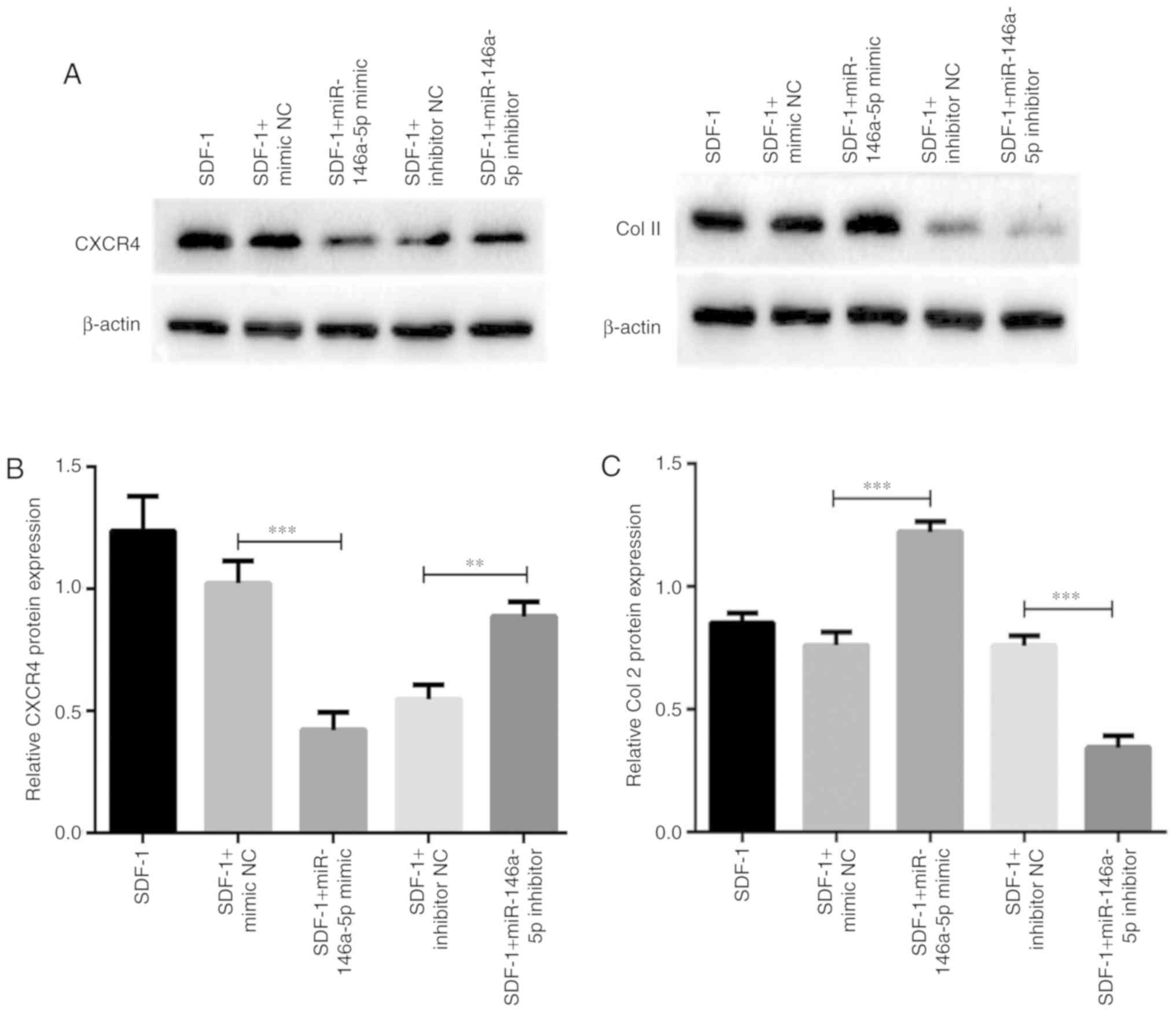
Association between miR-146a-5p
expression and the protein levels of CXCR4 and Col II
As CXCR4 was confirmed to be a target of
miR-146a-5p, and Col II is the principal component of cartilage
ECM, effects of miR-146a-5p expression on the protein levels of
these 2 factors were additionally assessed. Consistent with the
alteration of mRNA expression, expression of the CXCR4 protein was
downregulated as a result of miR-146a-5p mimic transfection
(Fig. 6A and B). Concomitantly,
the protein level of CXCR4 was significantly upregulated following
miR-146a-5p inhibitor transfection. As hypothesized, the opposite
pattern of expression was observed for Col II protein in
chondrocytes transfected with the mimic or inhibitor of miR-146a-5p
(Fig. 6A and C). Taken together,
these results highlight the critical roles of miR-146a-5p on
regulating the expression of cartilage degeneration-associated
factors.
Discussion
Antagonists of CXCR4 (AMD3100, T140 and TN14003)
have been demonstrated to successfully inhibit the SDF-1/CXCR4 axis
by competing with CXCR4 for its ligand SDF-1 (45). These antagonists have been utilized
in the treatment of HIV infection and various types of cancer
(46,47). AMD3100 and T140 were indicated to
be efficient in the management of OA in previous studies (48,49).
However, TN14003 is recommended above AMD3100 and T140, as AMD3100
is a weak partial antagonist, and T140 possesses unstable
properties, limiting their clinical applications (50,51).
In addition, previous studies have demonstrated that TN14003 is a
more effective in inhibiting MMP-3, MMP-9 and MMP-13 release, and
in Col-II and ACAN degradation, when compared to AMD3100 and T140
(52,53). The present study investigated
whether TN14003 therapy may elicit an alteration in miRNA profile
in chondrocytes derived from patients with OA, and also identified
that miR-146a-5p was a CXCR4/SDF-1 axis inhibitor that induced
differentially expressed miRNA, which regulated the expression of
cartilage degeneration-associated factors, including CXCR4, Col II,
ACAN and MMP-3.
It has been established that miRNAs serve key roles
in the pathological processes of OA (54,55).
Kopańska et al (42)
identified 4 miRNAs (miR-138-5p, miR-146a-5p, miR-335-5p and
miR-9-5p) in OA cartilage that were upregulated >2-fold compared
with healthy controls, indicating an association between miRNA and
OA. Zheng et al (56)
demonstrated that miR-221-3p was significantly downregulated in OA
compared with normal controls, and that upregulating miR-221-3p may
inhibit interleukin 1β (IL-1β)-induced cartilage degradation via
targeting of the SDF-1/CXCR4 axis. The present study indicated that
84 miRNAs were differentially expressed in OA chondrocytes, and
miR-146a-5p, miR-126-3p and miR-124-3p were validated, suggesting
that these miRNAs may exert their effects via inhibition of
SDF-1/CXCR4 with TN14003 treatment.
miR-146a-5p is a representative miRNA known to be
associated with OA (43,44). In addition to the data from
Kopańska et al (42),
Genemaras et al (57)
suggested that following stimulation with IL-1β and tumor necrosis
factor-α (TNF-α), miR-146a was significantly upregulated in pig
chondrocytes, indicating an interaction between miR-146a and
inflammatory cytokines in the promotion of OA. In addition,
Spinello et al (58)
detected a parallel effect between miR-146a and the CXCR4
antagonist. The present study determined that CXCR4 protein
expression was decreased following AMD3100 treatment. The
sensitivity of leukemic blast cells to cytotoxic drugs was
demonstrated to be increased, and this effect was augmented with
the overexpression of miR-146a. However, unlike miR-146-5p, which
has been extensively studied, few studies have explored the role of
miR-126-3p and miR-124-3p in the process of OA.
OA is an aseptic inflammatory disease (59,60).
Several miRNAs, including miR-146a-5p, have been demonstrated to be
genetic markers of inflammation, and to function as promoters of OA
(61,62). Notably, miR-146a-5p was upregulated
in the treatment group in the present study, indicating that it may
serve a parallel role with TN14003. Although a number of studies
have investigated the role of miR-146a-5p by comparing miRNA
profiles between OA and normal chondrocytes, few studies have
focused on miRNA expression profile following therapy with specific
inhibitors, including CXCR4 antagonists. Through a computational
approach to mine miR-146a-5p associated genes and pathways, the
present study revealed that the ‘receptor regulatory activity’ or
‘NIF activity’ (Molecular Functions), ‘cellular response to
interleukin-1’ (Biological Processes), ‘cytokine-cytokine receptor
interaction’, ‘NF-κB signaling pathway’ and ‘osteoclast
differentiation pathways’ were involved. Activation of the
SDF-1/CXCR4 signaling axis has been verified to be a process of
cytokine-to-receptor transmembrane transport, and this activity may
regulate disease progress via the NF-κB pathway (63). This indicated that miR-146a-5p may
be associated with the SDF-1/CXCR4 axis through the regulation of
the NF-κB pathway.
Numerous genes are negatively regulated by
complementary pairing with miRNAs, and dysregulation of genes may
affect OA (64). Additionally, OA
therapy based on miRNAs has been developed in previous years, and
may result in high-efficiency treatment with less biological
toxicity (65). Yang et al
(61) predicted that CXCR4 may
function as a direct target of miR-146a-5p, as verified by the fact
that CXCR4 expression was decreased and miR-146a-5p was upregulated
in endometrial tissue samples. In addition, Labbaye et al
(51) determined that two ‘seed’
regions of the 3′-untranslated region in CXCR4 mRNA directly
interacted with miR-146a, thereby demonstrating that CXCR4 mRNA
translation was inhibited by miR-146a. In the present study, CXCR4
was predicted to be a target of miR-146a-5p with high importance.
Then, RT-qPCR and western blot analysis were used to determine
whether several key factors in chondrocytes associated with the
SDF-1/CXCR4 axis were regulated by miR-146a-5p. It was identified
that the expression levels of Col II and ACAN were positively
associated with miR-146a-5p expression, and levels of CXCR4 and
MMP-3 were negatively associated with miR-146a-5p expression. The
results suggest that miR-146a-5p may serve a parallel and additive
role with TN14003 in blocking the SDF-1/CXCR4 axis and inhibiting
cartilage degeneration.
There are certain recognized limitations of the
present study that must be considered. The effect of miR-146a-5p on
cartilage degeneration was determined in the present study, but a
Cell Counting Kit-8 assay should be performed to evaluate the
effect of miR-146a-5p on chondrocyte survival. In addition, the
primary aim of the present study to investigate chondrocytes, and
may not capture the role of miR-146a-5p on the neighboring tissues
containing synovium and subchondral bone. Finally, in order to
fully demonstrate the function of miR-146a-5p in SDF-1-induced
cartilage degeneration by targeting CXCR4, an in vivo
investigation should be included in future studies.
In conclusion, the present study provided compelling
evidence for the critical roles of miRNAs in SDF-1-induced
cartilage degradation by miRNA microarray profiling in OA
chondrocytes following TN14003 treatment. miR-146a-5p was detected
to be differentially expressed and it most likely represents the
key miRNA that participates in the regulation of the SDF-1/CXCR4
axis through the inhibition of CXCR4. Although additional work
involving the biocompatibility of miR-146a-5p mimics in
vitro and in vivo may be required to fully delineate its
roles in OA pathogenesis, the present study offers a promising
framework through which diagnostic and therapeutic biomarkers of OA
may be determined. The combined use of TN14003 and miR-146a-5p
mimics may represent an approach for developing effective
OA-targeted therapies with decreased side effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos., 81460340 and
81760403).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YLL designed the study. DJ performed the research
and wrote the paper. RH and KW analyzed data. GFC and CH collated
the data and checked the results. LJY helped perform the research.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and the present study was approved by the Ethics Committee
at the First Affiliated Hospital of Kunming Medical University.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OA
|
osteoarthritis
|
|
SDF-1
|
stromal cell-derived factor 1
|
|
CXCR4
|
C-X-C chemokine receptor type 4
|
|
RT-qPCR
|
reverse transcription quantitative
polymerase chain reaction
|
|
GO
|
gene ontology
|
|
NF-κB
|
nuclear factor-κ-light-chain-enhancer
of activated B cells
|
|
ECM
|
extracellular matrix
|
|
MMPs
|
matrix metalloproteinase
|
|
mRNA
|
messenger RNA
|
|
IL-1β
|
interleukin 1β
|
|
TNF-α
|
tumor necrosis factor α
|
|
Col II
|
collagen type II
|
|
ACAN
|
aggrecan
|
|
MMP-3
|
matrix metalloproteinases 3
|
References
|
1
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li G, Yin J, Gao J, Cheng TS, Pavlos NJ,
Zhang C and Zheng MH: Subchondral bone in osteoarthritis: insight
into risk factors and microstructural changes. Arthritis Res Ther.
15:2232013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Man GS and Mologhianu G: Osteoarthritis
pathogenesis-a complex process that involves the entire joint. J
Med Life. 7:37–41. 2014.PubMed/NCBI
|
|
4
|
Gao Y, Liu S, Huang J, Guo W, Chen J,
Zhang L, Zhao B, Peng J, Wang A, Wang Y, et al: The ECM-cell
interaction of cartilage extracellular matrix on chondrocytes.
Biomed Res Int. 2014:6484592014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maldonado M and Nam J: The role of changes
in extracellular matrix of cartilage in the presence of
inflammation on the pathology of osteoarthritis. Biomed Res Int.
2013:2848732013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haslauer CM, Elsaid KA, Fleming BC,
Proffen BL, Johnson VM and Murray MM: Loss of extracellular matrix
from articular cartilage is mediated by the synovium and ligament
after anterior cruciate ligament injury. Osteoarthritis Cartilage.
21:1950–1957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sophia Fox AJ, Bedi A and Rodeo SA: The
basic science of articular cartilage: Structure, composition, and
function. Sports Health. 1:461–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aigner T, Soeder S and Haag J: IL-1beta
and BMPs-interactive players of cartilage matrix degradation and
regeneration. Eur Cell Mater. 12:49–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mariani E, Pulsatelli L and Facchini A:
Signaling pathways in cartilage repair. Int J Mol Sci.
15:8667–8698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fosang AJ and Beier F: Emerging frontiers
in cartilage and chondrocyte biology. Best Pract Res Clin
Rheumatol. 25:751–766. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldring MB and Marcu KB: Epigenomic and
microRNA-mediated regulation in cartilage development, homeostasis,
and osteoarthritis. Trends Mol Med. 18:109–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hart LE: In knee OA, intraarticular
triamcinolone increased cartilage loss and did not differ from
saline for knee pain. Ann Intern Med. 167:JC272017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McAlindon TE, LaValley MP, Harvey WF,
Price LL, Driban JB, Zhang M and Ward RJ: Effect of intra-articular
triamcinolone vs saline on knee cartilage volume and pain in
patients with knee osteoarthritis: A randomized clinical trial.
JAMA. 317:1967–1975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomas NP, Wu WJ, Fleming BC, Wei F, Chen
Q and Wei L: Synovial inflammation plays a greater role in
post-traumatic osteoarthritis compared to idiopathic osteoarthritis
in the Hartley guinea pig knee. BMC Musculoskelet Disord.
18:5562017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scanzello CR: Chemokines and inflammation
in osteoarthritis: Insights from patients and animal models. J
Orthop Res. 35:735–739. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yellowley C: CXCL12/CXCR4 signaling and
other recruitment and homing pathways in fracture repair. Bonekey
Rep. 2:3002013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanbe K, Takagishi K and Chen Q:
Stimulation of matrix metalloprotease 3 release from human
chondrocytes by the interaction of stromal cell-derived factor 1
and CXC chemokine receptor 4. Arthritis Rheum. 46:130–137. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomas NP, Li P, Fleming BC, Chen Q, Wei
X, Xiao-Hua P, Li G and Wei L: Attenuation of cartilage
pathogenesis in post-traumatic osteoarthritis (PTOA) in mice by
blocking the stromal derived factor 1 receptor (CXCR4) with the
specific inhibitor, AMD3100. J Orthop Res. 33:1071–1078. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang XY, Chen Y, Tang XJ, Jiang LH and Ji
P: AMD3100 attenuates matrix metalloprotease-3 and −9 expressions
and prevents cartilage degradation in a monosodium
iodo-acetate-induced rat model of temporomandibular osteoarthritis.
J Oral Maxillofac Surg. 74:927.e1–927.e13. 2016. View Article : Google Scholar
|
|
20
|
Hendrix CW, Flexner C, MacFarland RT,
Giandomenico C, Fuchs EJ, Redpath E, Bridger G and Henson GW:
Pharmacokinetics and safety of AMD-3100, a novel antagonist of the
CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents
Chemother. 44:1667–1673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stamatopoulos B, Meuleman N, De Bruyn C,
Pieters K, Mineur P, Le Roy C, Saint-Georges S, Varin-Blank N,
Cymbalista F, Bron D and Lagneaux L: AMD3100 disrupts the
cross-talk between chronic lymphocytic leukemia cells and a
mesenchymal stromal or nurse-like cell-based microenvironment:
Pre-clinical evidence for its association with chronic lymphocytic
leukemia treatments. Haematologica. 97:608–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liles WC, Broxmeyer HE, Rodger E, Wood B,
Hübel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G and
Dale DC: Mobilization of hematopoietic progenitor cells in healthy
volunteers by AMD3100, a CXCR4 antagonist. Blood. 102:2728–2730.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamamura H, Omagari A, Hiramatsu K, Gotoh
K, Kanamoto T, Xu Y, Kodama E, Matsuoka M, Hattori T, Yamamoto N,
et al: Development of specific CXCR4 inhibitors possessing high
selectivity indexes as well as complete stability in serum based on
an anti-HIV peptide T140. Bioorg Med Chem Lett. 11:1897–1902. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamamura H, Fujisawa M, Hiramatsu K,
Mizumoto M, Nakashima H, Yamamoto N, Otaka A and Fujii N:
Identification of a CXCR4 antagonist, a T140 analog, as an
anti-rheumatoid arthritis agent. FEBS Lett. 569:99–104. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hernando E: microRNAs and cancer: Role in
tumorigenesis, patient classification and therapy. Clin Transl
Oncol. 9:155–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sondag GR and Haqqi TM: The role of
microRNAs and their targets in osteoarthritis. Curr Rheumatol Rep.
18:562016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu XM, Meng HY, Yuan XL, Wang Y, Guo QY,
Peng J, Wang AY and Lu SB: MicroRNAs' involvement in osteoarthritis
and the prospects for treatments. Evid Based Complement Alternat
Med. 2015:2361792015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jingsheng S, Yibing W, Jun X, Siqun W,
Jianguo W, Feiyan C, Gangyong H and Jie C: MicroRNAs are potential
prognostic and therapeutic targets in diabetic osteoarthritis. J
Bone Miner Metab. 33:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu C, Tian B, Qu X, Liu F, Tang T, Qin A,
Zhu Z and Dai K: MicroRNAs play a role in chondrogenesis and
osteoarthritis (review). Int J Mol Med. 34:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Castell MV, van der Pas S, Otero A,
Siviero P, Dennison E, Denkinger M, Pedersen N, Sanchez-Martinez M,
Queipo R, van Schoor N, et al: Osteoarthritis and frailty in
elderly individuals across six European countries: Results from the
European Project on OSteoArthritis (EPOSA). BMC Musculoskelet
Disord. 16:3592015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Damen J, van Rijn RM, Emans PJ, Hilberdink
WKHA, Wesseling J, Oei EHG and Bierma-Zeinstra SMA: Prevalence and
development of hip and knee osteoarthritis according to American
College of Rheumatology criteria in the CHECK cohort. Arthritis Res
Ther. 1:42019. View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:Database Issue. D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: A resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46(D1): D296–D302.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gene Ontology Consortium: Gene ontology
consortium: Going forward. Nucleic Acids Res. 43:Database Issue.
D1049–D1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Isserlin R, Merico D, Voisin V and Bader
GD: Enrichment Map-a Cytoscape app to visualize and explore OMICs
pathway enrichment results. F1000Res. 3:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kopańska M, Szala D, Czech J, Gabło N,
Gargasz K, Trzeciak M, Zawlik I and Snela S: MiRNA expression in
the cartilage of patients with osteoarthritis. J Orthop Surg Res.
12:512017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamasaki K, Nakasa T, Miyaki S, Ishikawa
M, Deie M, Adachi N, Yasunaga Y, Asahara H and Ochi M: Expression
of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum.
60:1035–1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhong JH, Li J, Liu CF, Liu N, Bian RX,
Zhao SM, Yan SY and Zhang YB: Effects of microRNA-146a on the
proliferation and apoptosis of human osteoarthritis chondrocytes by
targeting TRAF6 through the NF-κB signalling pathway. Biosci Rep.
37(pii): BSR201605782017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Burger JA, Stewart DJ, Wald O and Peled A:
Potential of CXCR4 antagonists for the treatment of metastatic lung
cancer. Expert Rev Anticancer Ther. 4:621–30. 2011. View Article : Google Scholar
|
|
46
|
Tamamura H, Omagari A, Hiramatsu K,
Kanamoto T, Gotoh K, Kanbara K, Yamamoto N, Nakashima H, Otaka A
and Fujii N: Synthesis and evaluation of bifunctional anti-HIV
agents based on specific CXCR4 antagonists-AZT conjugation. Bioorg
Med Chem. 9:2179–2187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Green MM, Chao N, Chhabra S, Corbet K,
Gasparetto C, Horwitz A, Li Z, Venkata JK, Long G, Mims A, et al:
Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic
hematopoietic stem cell transplantation enhances hematopoietic
recovery. J Hematol Oncol. 9:712016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li P, Deng J, Wei X, Jayasuriya CT, Zhou
J, Chen Q, Zhang J, Wei L and Wei F: Blockade of hypoxia-induced
CXCR4 with AMD3100 inhibits production of OA-associated catabolic
mediators IL-1β and MMP-13. Mol Med Rep. 2:1475–1482. 2016.
View Article : Google Scholar
|
|
49
|
Wang K, Li Y, Han R, Cai G, He C, Wang G
and Jia D: T140 blocks the SDF-1/CXCR4 signaling pathway and
prevents cartilage degeneration in an osteoarthritis disease model.
PLoS One. 12:e01760482017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang WB, Navenot JM, Haribabu B, Tamamura
H, Hiramatu K, Omagari A, Pei G, Manfredi JP, Fujii N, Broach JR
and Peiper SC: A point mutation that confers constitutive activity
to CXCR4 reveals that T140 is an inverse agonist and that AMD3100
and ALX40-4C are weak partial agonists. J Biol Chem.
277:24515–24521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Labbaye C, Spinello I, Quaranta MT, Pelosi
E, Pasquini L, Petrucci E, Biffoni M, Nuzzolo ER, Billi M, Foà R,
et al: A three-step pathway comprising PLZF/miR-146a/CXCR4 controls
megakaryopoiesis. Nat Cell Biol. 10:788–801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu T, Wu Y, Helman JI, Wen Y, Wang C and
Li L: CXCR4 promotes oral squamous cell carcinoma migration and
invasion through inducing expression of MMP-9 and MMP-13 via the
ERK signaling pathway. Mol Cancer Res. 9:161–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Villalvilla A, Gomez R, Roman-Blas JA,
Largo R and Herrero-Beaumont G: SDF-1 signaling: A promising target
in rheumatic diseases. Expert Opin Ther Targets. 18:1077–1087.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vicente R, Noël D, Pers YM, Apparailly F
and Jorgensen C: Deregulation and therapeutic potential of
microRNAs in arthritic diseases. Nat Rev Rheumatol. 12:211–220.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang S, Zhou C, Zheng H, Zhang Z, Mei Y
and Martin JA: Chondrogenic progenitor cells promote vascular
endothelial growth factor expression through stromal-derived
factor-1. Osteoarthritis Cartilage. 25:742–749. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zheng X, Zhao FC, Pang Y, Li DY, Yao SC,
Sun SS and Guo KJ: Downregulation of miR-221-3p contributes to
IL-1β-induced cartilage degradation by directly targeting the
SDF1/CXCR4 signaling pathway. J Mol Med (Berl). 6:615–627. 2017.
View Article : Google Scholar
|
|
57
|
Genemaras AA, Ennis H, Kaplan L and Huang
CY: Inflammatory cytokines induce specific time- and
concentration-dependent MicroRNA release by chondrocytes,
synoviocytes, and meniscus cells. J Orthop Res. 34:779–790. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Spinello I, Quaranta MT, Riccioni R, Riti
V, Pasquini L, Boe A, Pelosi E, Vitale A, Foà R, Testa U and
Labbaye C: MicroRNA-146a and AMD3100, two ways to control CXCR4
expression in acute myeloid leukemias. Blood Cancer J. 1:e262011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang ZY, Stabler T, Pei FX and Kraus VB:
Both systemic and local lipopolysaccharide (LPS) burden are
associated with knee OA severity and inflammation. Osteoarthritis
Cartilage. 24:1769–1775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Choi YS, Park JK, Kang EH, Lee YK, Kim TK,
Chung JH, Zimmerer JM, Carson WE, Song YW and Lee YJ: Cytokine
signaling-1 suppressor is inducible by IL-1beta and inhibits the
catabolic effects of IL-1beta in chondrocytes: Its implication in
the paradoxical joint-protective role of IL-1beta. Arthritis Res
Ther. 15:R1912013. View
Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang RQ, Teng H, Xu XH, Liu SY, Wang YH,
Guo FJ and Liu XJ: Microarray analysis of microRNA deregulation and
angiogenesis-related proteins in endometriosis. Genet Mol Res.
15:2016.
|
|
62
|
Lu Y, Cao DL, Jiang BC, Yang T and Gao YJ:
MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6
signaling in the spinal cord. Brain Behav Immun. 49:119–129. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu Z, Ma C, Shen J, Wang D, Hao J and Hu
Z: SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus
pulposus cells via the NF-κB pathway. Mol Med Rep. 14:783–789.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang H, Song B and Pan Z: Downregulation
of microRNA-9 increases matrix metalloproteinase-13 expression
levels and facilitates osteoarthritis onset. Mol Med Rep.
17:3708–3714. 2018.PubMed/NCBI
|
|
65
|
Si HB, Zeng Y, Liu SY, Zhou ZK, Chen YN,
Cheng JQ, Lu YR and Shen B: Intra-articular injection of
microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression
by modulating extracellular matrix (ECM) homeostasis in rats.
Osteoarthritis Cartilage. 25:1698–1707. 2017. View Article : Google Scholar : PubMed/NCBI
|