Introduction
Vascular smooth muscle cells (VSMCs), the major
cellular component in the media layer of arteries, are highly
specialized cells that perform biosynthetic, proliferative, and
contractile functions both in health and disease (1). Apoptosis of VSMCs is closely
associated with physiological remodeling of the vasculature and
remodeling during disease, including in atherosclerotic plaque
rupture, restenosis following angioplasty and abdominal arterial
aneurysm (AAA) development (2–5).
Increased VSMC apoptosis has been observed in unstable
atherosclerotic plaques and is closely associated with plaque
vulnerability (6). Excessive
apoptosis of VSMCs in AAAs causes thinning and weakening of the
medial layer of the aortic wall, leading to loss of tensile forces
and aortic dilation (7,8). An incomplete understanding of the
molecular mechanisms that regulate VSMC apoptosis has limited the
development of diagnostic and therapeutic strategies in these
cardiovascular diseases. Therefore, exploring the mechanisms
underlying VSMC apoptosis may be useful for the prevention and
treatment of cardiovascular diseases. Tumor necrosis factor-α
(TNF-α) is an endogenous cytokine involved in the process of
inflammation under pathological conditions, including
atherosclerosis and vascular calcification (9–11).
Although TNF-α has been demonstrated to suppress proliferation and
induce apoptosis of VSMCs (12–14),
the specific pathways that regulate this process have not been
fully elucidated.
In recent years, microRNAs (miRs/miRNAs) have
emerged as a group of single-stranded, small, non-coding RNA
molecules that exert their biological effects at the
post-transcriptional level. They function through base pairing of
their seed region (position 2–8) with the 3′-untranslated region
(UTR) of target genes (15,16).
At present, >1,800 miRNAs (1,881 precursor and 2,581 mature
miRNAs) have been identified in humans (www.mirbase.org) and have been reported to be involved
in almost all aspects of human physiology and pathology, including
stem cell renewal, tumor formation, cardiovascular diseases,
metabolic disorders, genetic diseases and neurodegenerative
diseases (17–19). Previous studies have investigated
the role of miRNAs in regulating VSMC apoptosis under different
pathological conditions. miR-487b induces VSMC apoptosis and loss
of medial integrity during hypertension-induced remodeling of the
aorta via downregulation of insulin receptor substrate 1 (20). miR-138 acts as a negative regulator
of pulmonary aortic smooth muscle cell apoptosis during hypoxic
pulmonary vascular remodeling via downregulation of
serine/threonine kinase 4 (also termed Mst1) (21). miR-92a suppresses
H2O2-induced VSMC apoptosis by targeting the
mitogen-activated protein kinase kinase 4-c-Jun N-terminal kinase 1
pathway (22). A cluster of miRNAs
can regulate a gene cooperatively, and a single miRNA can have
binding sites in several target genes and exhibit different
biological effects in different cell types.
In the present study, miRNA expression in murine
VSMCs was analyzed by microarray analysis following TNF-α
stimulation for 24 h. The present results revealed that miR-494 was
one of the most significantly downregulated miRNAs, indicating its
potential role in regulating cell apoptosis. Although miR-494 has
been reported to regulate apoptosis in several tissues, the
specific role of miR-494 in TNF-α-induced VSMC apoptosis remains
unclear. The current study aimed to investigate whether miR-494 is
involved in regulating TNF-α-induced VSMC apoptosis and the
underlying mechanisms by which this miRNA exerts its modulatory
effect in vitro.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), TNF-α and
pentobarbital sodium were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Fetal bovine serum (FBS) was purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Lipofectamine® 2000 was purchased from Invitrogen
(Thermo Fisher Scientific, Inc.). Maxima SYBR-Green/ROX
quantitative polymerase chain reaction (qPCR) Master Mix (2X;
Thermo Fisher Scientific, Inc.) was used to investigate gene
expression by qPCR. A Cell Death Detection ELISA kit was purchased
from Roche Diagnostics (Basel, Switzerland; cat. no. 11920685001).
B-cell lymphoma-2-like 11 (BCL2L11) and β-actin antibodies,
horseradish peroxidase-conjugated goat-anti-mouse secondary
antibody and the electrochemiluminescence detection kit for western
Blotting was purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). miR-494 mimics and miR-494 inhibitors (2′-O-methyl
modified) and respective oligo controls were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Cell culture and transfection
The study was approved by The Ethics Review Board of
The Second Xiangya Hospital of Central South University (Changsha,
China). In total, eight C57BL/6 mice (age, 8 weeks; weight, 25–30
g; female to male ratio, 1:1) were purchased from The Animal
Multiplication Centre of Qinglong Mountain (Nanjing, China) and
housed in individual cages. The animals were provided with food and
water ad libitum, in a controlled environment (temperature
20–24°C; humidity 40–60%) and under a 12-h light/dark cycle. The
animals were euthanized using an intraperitoneal injection of 150
mg/kg pentobarbital sodium and primary murine VSMCs were isolated
from the abdominal aorta, following an enzymatic dissociation
procedure as previously described (23). VSMCs were cultured at 37°C and 5%
CO2 in DMEM supplemented with glutaMAX™ (Gibco; Thermo
Fisher Scientific, Inc.) and 15% FBS. Cells at passage 3–8 were
used for subsequent experiments. For induction of cell apoptosis,
VSMCs were cultured in serum-free DMEM for 12 h prior to treatment
with vehicle (dimethyl sulfoxide) or TNF-α (5–50 ng/ml) for 24 h at
37°C and 5% CO2.
For transient transfection of miR-494 mimic
(5′-UGAAACAUACACGGGAAACCUC-3′), miR-494 inhibitor
(5′-GGUUUCCCGUGUAUGUUUCAUU-3′) and their control oligonucleotides
(control miR mimic, 5′-UUCUCCGAACGUGUCACGU-3′; control miR
inhibitor 5′-ACGUGACACGUUCGGAGAA-3′), Lipofectamine®
2000 and 50 nM oligos were mixed according to the manufacturer's
instructions and added to the cells for 48 h prior to subsequent
experimentation.
Cell death detection
Murine VSMCs were seeded in a 12-well plate at a
density of 25,000 cells/well, incubated for 12 h at 37°C and 5%
CO2 in serum-free DMEM, and then stimulated with 5–50
ng/ml TNF-α for 24 h at 37°C and 5% CO2. The cell layers
were rinsed twice with PBS and incubated with the lysis buffer
provided in the kit for 30 m in at 4°C. Lysates were extracted and
centrifuged at 15,000 rpm and 4°C for 10 min. The Cell Death
Detection ELISA kit was used to quantify the amount of cytoplasmic
histone-associated DNA fragments in the cell lysates according to
the manufacturer's protocol. Absorbance was measured at 405 nm and
apoptotic cell death is expressed as ELISA absorbance units.
To investigate the effects of miR-494 on cell
apoptosis, cells were transfected with miR-494 mimics or miR-494
inhibitors for 48 h prior to TNF-α treatment. To determine whether
the effects of miR-494 on TNF-α induced VSMC apoptosis were
dependent on BCL2L11, VSMCs were cotransfected with scramble small
interfering RNA (siRNA) + control miR inhibitor, scramble siRNA +
miR-494 inhibitor, BCL2L11 siRNA + control miR inhibitor or BCL2L11
siRNA + miR-494 inhibitor for 48 h, and subsequently stimulated
with TNF-α (10 ng/ml) for 24 h at 37°C. The BCL2L11 siRNA and
scramble siRNA were purchased from Sigma-Aldrich (Merck KGaA).
BCL2L11 siRNA (5′-ACUUACAUCAGAAGGUUGC-3′) or scramble siRNA
(5′-UAAGGCUAUGAAGAGAUAC-3′) and used at a concentration of 10 nM,
and 50 nM miR-494 inhibitor, mimic or control oligos were
transfected into cells using Lipofectamine® 2000.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining
VSMCs were seeded at a density of 60,000 cells/well
in 6-well plates and incubated in serum-free DMEM with or without
10 ng/ml TNF-α for 24 h at 37°C and 5% CO2. To evaluate
the effect of TNF-α on VSMC apoptosis, a TUNEL assay was performed
using an in situ Cell Death Detection kit (Roche
Diagnostics, Indianapolis, IN, USA; cat. no. 11684795910), as
previously described (24). Cells
were fixed in freshly prepared paraformaldehyde (4% in PBS; pH 7.4)
for 1 h at 20°C. During the labeling reaction, cells were incubated
with 50 µl TUNEL reaction mixture at 37°C for 1 h in the dark. The
nuclei were counterstained with 10 ug/ml DAPI for 2 min at room
temperature. The coverslips were mounted on slides with antifade
mounting medium (50 mM Tris-PO4, 50 mM
NaH2PO4, 20% polyvinyl alcohol and 30%
glycerol). TUNEL-positive cells were visualized under an inverted
florescence microscope (Nikon Corporation, Tokyo, Japan;
magnification, ×100) equipped with a charge-coupled device digital
camera. Images were processed using Imaging Software NIS-Elements
BR version 3.0 (Nikon Corporation; magnification, ×100). In total,
10 randomly selected fields were analyzed, and TUNEL positive cells
and total cells were counted in each group. Cell death was
calculated and is expressed as percentage of apoptotic cells.
To investigate whether miR-494 relies on BCL2L11 to
regulate cell apoptosis, four groups of cells were transfected with
scramble siRNA + control miR inhibitors, scramble siRNA + miR-494
inhibitors, BCL2L11 siRNA + control miR inhibitors and BCL2L11
siRNA + miR-494 inhibitors for 48 h, and incubated with TNF-α (10
ng/ml) for 24 h at 37°C and 5% CO2.
miRNA microarray analysis
Murine VSMCs were pre-cultured in serum-free DMEM
for 12 h and then incubated with 10 ng/ml TNF-α for 24 h at 37°C
and 5% CO2. Total RNA was isolated using an RNA
isolation kit (Cells-to-CT Kit; Ambion; Thermo Fisher Scientific,
Inc.) and pooled from three control and three TNF-α-treated cell
groups and hybridized using a µParaflo® Microfluidic
Biochip Technology microarray platform (Chip ID miRhsa 12.0; LC
Sciences, Houston, TX, USA), as previously described (25). The microarray values were analyzed
following subtraction of the background, were profiled using the LC
Science miRNA expression profiling service, and normalized using
locally weighted scatterplot smoothing method (25). Each sample was repeated three times
and significant signal differences (P<0.01) between TNF-α and
control group were analyzed based on miRBase version 17.0
(http://www.mirbase.org/). Hierarchical clustering
of the log2 fold change value was performed using
GeneSpring GX software version 7.3 (Agilent Technologies, Inc.,
Santa Clara, CA, USA) and visualized using a heat map (Heatmap
Illustrator; version 1.0; http://hemi.biocuckoo.org/).
Reverse transcription (RT)-qPCR
Total RNA was extracted from VSMCs using the
mirVana™ miRNA Isolation kit (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. RNA (1 µg) was
reverse transcribed to cDNA using oligo(dT) primers provided in the
RT kit (Roche Diagnostics; cat. no. 04897030001) in each RT
reaction. qPCR was performed using the Maxima SYBR Green/ROX qPCR
Master Mix (2X). The primers used for qPCR are listed in Table I. Each reaction was prepared in a
total volume of 25 µl containing 12.5 µl Maxima SYBR-Green/ROX qPCR
Master Mix (Roche Diagnostics), 0.3 µM forward Primer, 0.3 µM
reverse Primer, template DNA and nuclease-free-water. The
thermocycling conditions were the following: Initial denaturation
at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, 60°C
for 30 sec and 72°C for 30 sec. Expression of miR-494 relative to
U6 and BCL2L11 relative to GAPDH were evaluated using the
2−ΔΔCq method (26).
Each sample was measured using three technical replicates.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| BCL2L11 |
F:5′-GGCTCAACTACCGCAGAGTC-3′ |
|
|
R:5′-GAGTTAAGTCTACCCGCCCG-3′ |
| GAPDH |
F:5′-AGGTCGGTGTGAACGGATTTG-3′ |
|
|
R:5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
| miRNA-494 |
F:5′-TGAAACATACACGGGAAACC-3′ |
|
|
R:5′-GTGCAGGGTCCGAGGT-3′ |
| U6 |
F:5′-CGCTTCGGCAGCACATATACTA-3′ |
|
|
R:5′-GCGAGCACAGAATTAATACGAC-3′ |
Western blot analysis
To detect protein expression levels of BCL2L11 and
β-actin, western blot analysis was performed as previously
described (24). Total protein was
extracted from VSMCs using radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Haimen, China), and the
concentration was determined by bicinchoninic acid assay. The
proteins (30 µg per lane) were separated by 12% SDS-PAGE. Proteins
were transferred to a polyvinylidene difluoride membrane, and
membrane was subsequently blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. The
primary antibodies, including anti-BCL2L11 (cat. no. sc-374358;
Santa Cruz Biotechnology, Inc.) and anti-β-actin (cat. no.
sc-47778; Santa Cruz Biotechnology, Inc.), were diluted at 1:1,000
and were incubated at 4°C overnight. The membranes were washed with
TBS for 15 min at room temperature three times. Subsequently, the
membrane was incubated with mouse Immunoglobulin Gκ binding protein
conjugated to horseradish peroxidase (Santa Cruz Biotechnology,
Inc.; cat. no. sc-516102-CM; 1:1,000) at 37°C for 1 h. The membrane
was washed with TBS at room temperature for 15 min three times.
Protein bands were visualized using an electrochemiluminescence
detection kit (Santa Cruz Biotechnology, Inc.). The optical density
was analyzed by AlphaEaseFC software (version 5.0; ProteinSimple,
San Jose, CA, USA).
miRNA target site prediction
In order to predict miRNA target sites, three online
software tools, including TargetScan version 7.2 (www.targetscan.org/), DIANA microT-CDS version 5.0
(http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=MicroT_CDS/index)
and PicTar (pictar.mdc-berlin.de/ update: March 26, 2007), were
used to search for base pairing of the miRNA seed sequence with the
3′-UTR region of target genes. The University of California Santa
Cruz Genome Browser (http://genome-euro.ucsc.edu/cgi-bin/hgGateway?redirect=manual&source=genome.ucsc.edu;
assembly date: December 2013) was used to determine sequence
conservation.
Dual luciferase reporter assay
The BCL2L11 luciferase reporter plasmid (Shanghai
Yeasen Biotechnology Co., Ltd., Shanghai, China) containing the
3′-UTR of wild-type BCL2L11 (WT-pGL3-BCL2L11) or mutant BCL2L11
(MUT-pGL3-BCL2L11), and miR-494 mimics or control miR mimic were
cotransfected into VSMCs with Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Luciferase activities
were measured by the Luciferase Assay System and compared with
Renilla luciferase activity (Promega Corporation, Madison,
WI, USA), 48 h after transfection.
To generate MUT-pGL3-BCL2L11, the QuickChange
Site-Directed Mutagenesis kit (Agilent Technologies, Inc.) was used
to induce two point mutations in the 3′-UTR region of WT BCL2L11.
The generation of the wild-type and mutant oligos was performed as
previously described (27). The
sequences of the wild-type and mutant primers are listed in
Table II.
 | Table II.Primers used for plasmid
construction. |
Table II.
Primers used for plasmid
construction.
| Gene | Primer sequence
(5′-3′) |
|---|
| WT BCL2L11 |
F:5′-TCTAGAGAGCCAAATGTCTGTGTGCAA-3′ |
|
|
R:5′-TCTAGAGAGTGGGAGACAGGGATGTTAAT-3′ |
| MUT BCL2L11 |
F:5′-TTTATTAGATTAGAAAGTCATTTATCACTCGTCAACTGAG-3′ |
|
|
R:5′-CTCAGTTGACGAGTGATAAATGACTTTCTAATCTAATAAA-3′ |
Statistical analysis
All experiments were repeated at least three times
and data are expressed as the means ± standard deviation.
Statistical analysis was performed using SPSS software version 15.0
(SPSS, Inc., Chicago, IL, USA). Statistical significance was
assessed using one-way analysis of variance followed by Tukey's
test for comparison between two groups. Multiple comparisons
between the groups were performed using the Dunnett's test or
Student-Newman-Keuls method. P<0.05 was considered to indicate a
statistically significant difference.
Results
TNF-α induces apoptosis of VSMCs
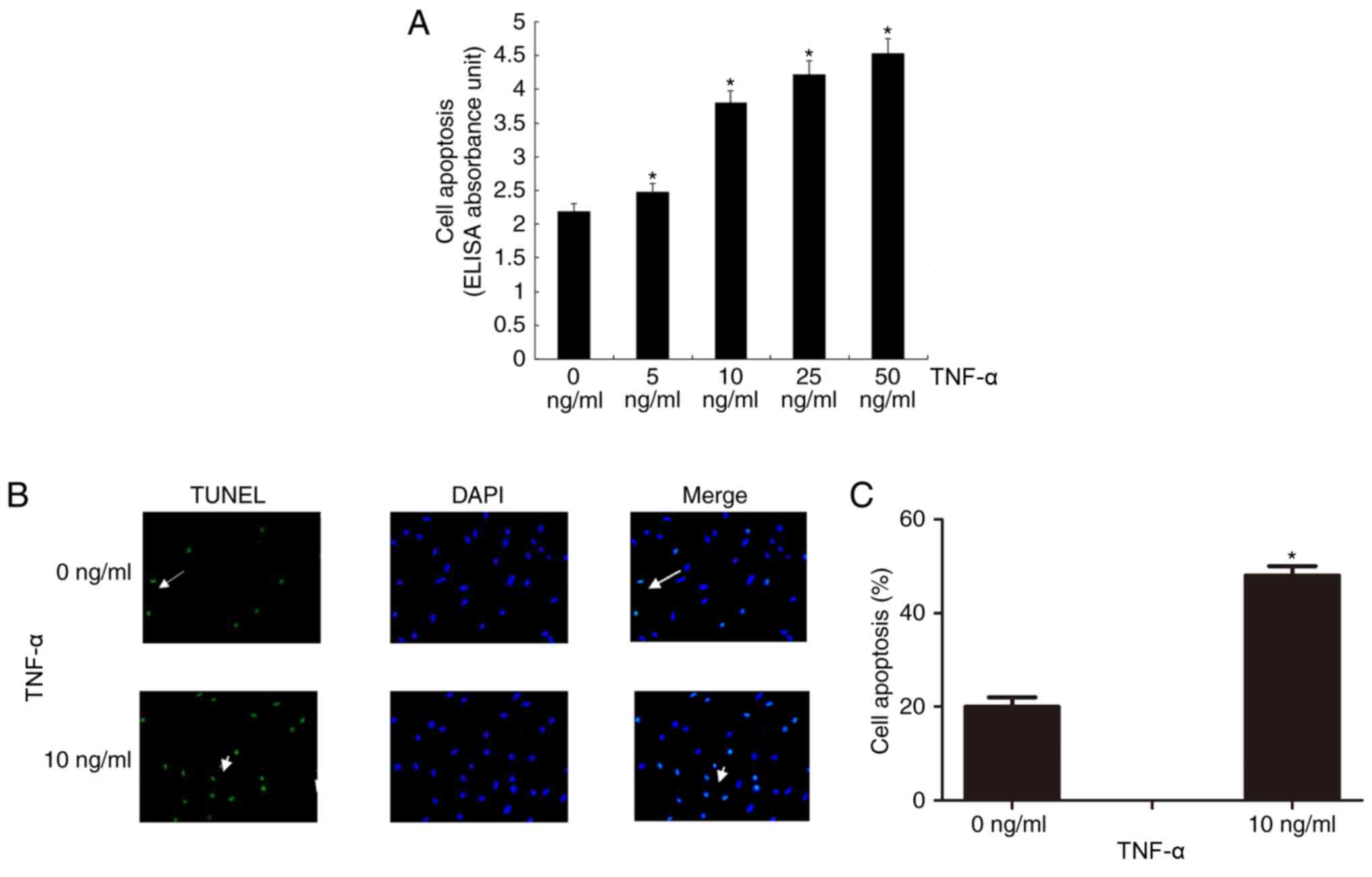
The Cell Death Detection ELISA assay revealed that
TNF-α induced VSMC apoptosis in a dose-dependent manner (Fig. 1A). Cell apoptosis was significantly
increased compared with the control treatment at all concentrations
of TNF-α (P<0.05).
In addition, TUNEL staining revealed that 10 ng/ml
TNF-α significantly induced VSMC apoptosis compared with the
control treatment (P<0.05; Fig. 1B
and C).
TNF-α downregulates miR-494 expression
in VSMCs
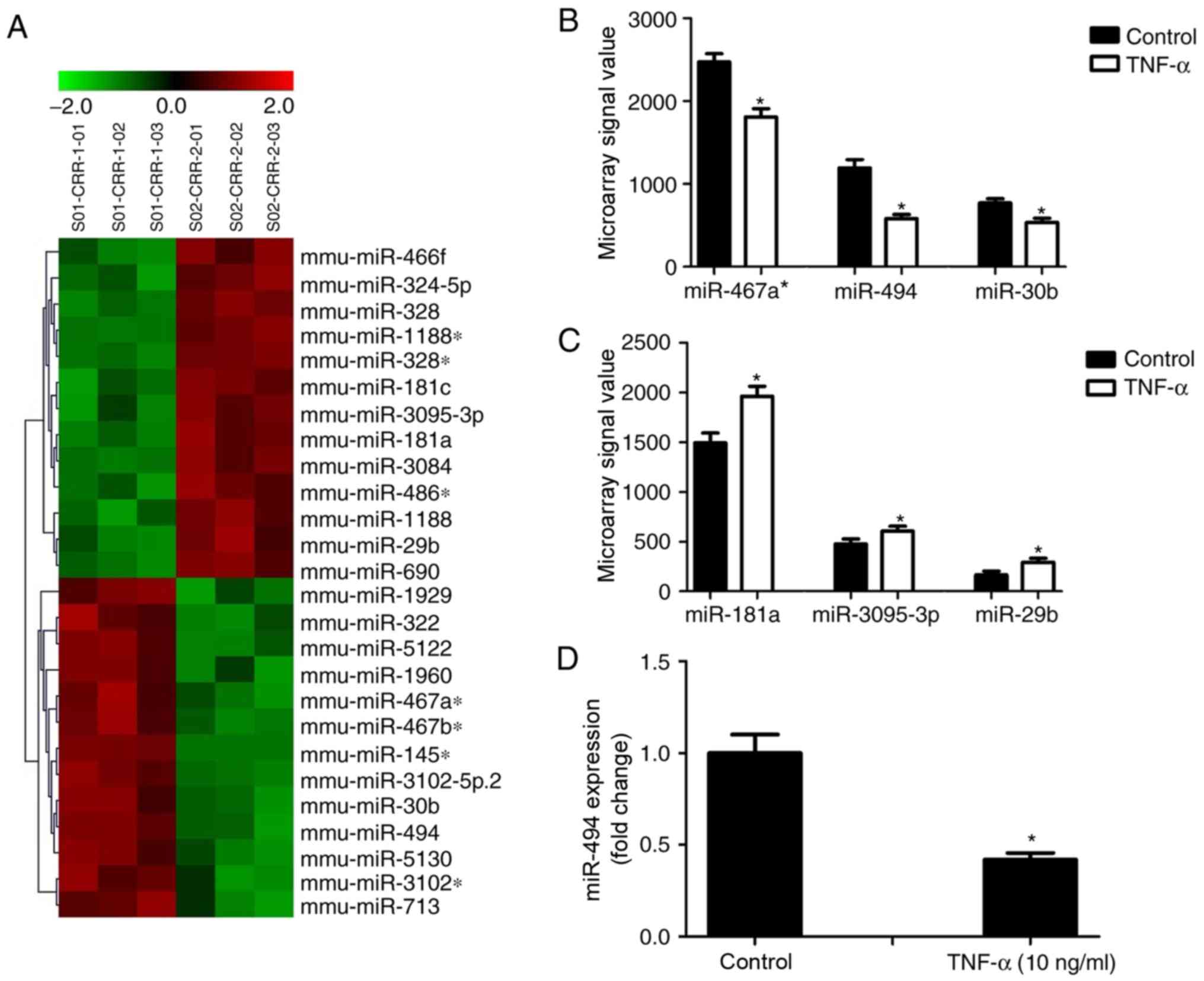
The miRNA expression profile of VSMCs following
TNF-α stimulation was evaluated by microarray analysis. The
analysis revealed that 13 miRNAs were significantly downregulated
following TNF-α treatment, including miR-467a-3p, miR-494 and
miR-30b; whereas 13 miRNAs were upregulated, including miR-181a,
miR-3095-3p and miR-29b (Fig. 2A,
Table III). These miRNAs were
among the most abundantly expressed miRNAs in VSMCs. The microarray
signal values of the three downregulated and three upregulated
miRNAs are shown in Fig. 2B and C.
Among the genes reported to be targets of miR-494, several have
been suggested to be involved in cell apoptosis (28–30).
Therefore, miR-494 was further investigated to determine its role
in apoptotic signaling pathways. The altered expression of miR-494
was verified by RT-qPCR, which revealed that its expression was
downregulated by >50% following treatment with 10 ng/ml TNF-α
compared with the control group (Fig.
2D).
 | Table III.Differentially expressed miRNAs in
VSMCs following treatment with 10 ng/ml TNF-α. P<0.01. |
Table III.
Differentially expressed miRNAs in
VSMCs following treatment with 10 ng/ml TNF-α. P<0.01.
| miRNA | Log2
fold change (TNF-α/control) |
|---|
|
mmu-miR-1188-5p | 1.92 |
| mmu-miR-486-3p | 1.69 |
| mmu-miR-3084 | 1.61 |
|
mmu-miR-1188-3p | 1.05 |
| mmu-miR-181c | 0.85 |
| mmu-miR-29b | 0.84 |
| mmu-miR-466f | 0.75 |
| mmu-miR-690 | 0.72 |
| mmu-miR-324-5p | 0.71 |
| mmu-miR-328-3p | 0.63 |
| mmu-miR-328-5p | 0.39 |
| mmu-miR-181a | 0.39 |
|
mmu-miR-3095-3p | 0.35 |
| mmu-miR-5122 | −0.37 |
|
mmu-miR-467a-3p | −0.45 |
| mmu-miR-30b | −0.52 |
|
mmu-miR-467b-3p | −0.53 |
| mmu-miR-1929 | −0.63 |
| mmu-miR-1960 | −0.65 |
| mmu-miR-5130 | −0.8 |
| mmu-miR-713 | −0.81 |
| mmu-miR-322 | −0.87 |
| mmu-miR-494 | −1.04 |
|
mmu-miR-3102-5p | −1.26 |
|
mmu-miR-3102-5p.2 | −1.85 |
| mmu-miR-145-3p | −11.71 |
TNF-α downregulates miR-494 and
upregulates BCL2L11 expression in VSMCs
To explore whether miR-494 is involved in VSMC
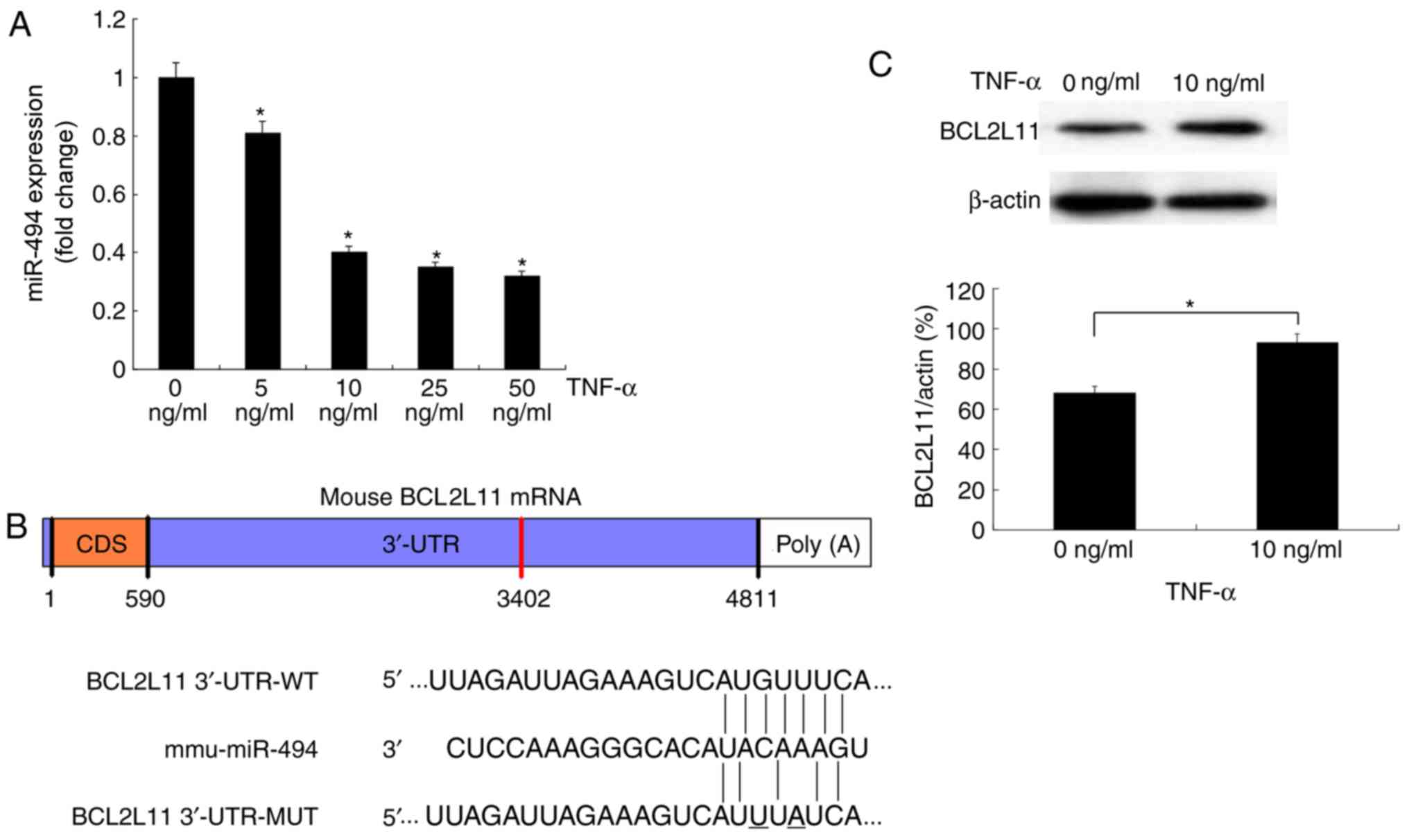
apoptosis, murine VSMCs were treated with TNF-α and total RNA was
extracted for RT-qPCR. The results demonstrated that the expression
levels of miR-494 were downregulated by treatment with TNF-α (5–50
ng/ml) in a dose-dependent manner (Fig. 3A).
Three publicly available tools (TargetScan, DIANA
microT-CDS and PicTar) were used to identify potential miR-494
target genes. The search results indicated that the 3′-UTR of
BCL2L11 contained a predicted miR-494 binding site (Fig. 3B), which was also previously
reported by Romano et al (31). To investigate whether BCL2L11 was
associated with TNF-α-mediated cell apoptosis, western blot
analysis was performed to evaluate the protein expression levels of
BCL2L11 following TNF-α treatment. The protein expression levels of
BCL2L11 were significantly increased following 24 h treatment with
TNF-α (Fig. 3C). Since the
expression levels of miR-494 were downregulated during VSMC
apoptosis, whereas BCL2L11 protein levels were upregulated, this
suggested that reduced miR-494 may result in increased BCL2L11
levels during TNF-α-mediated VSMC apoptosis.
miR-494 attenuates BCL2L11
expression
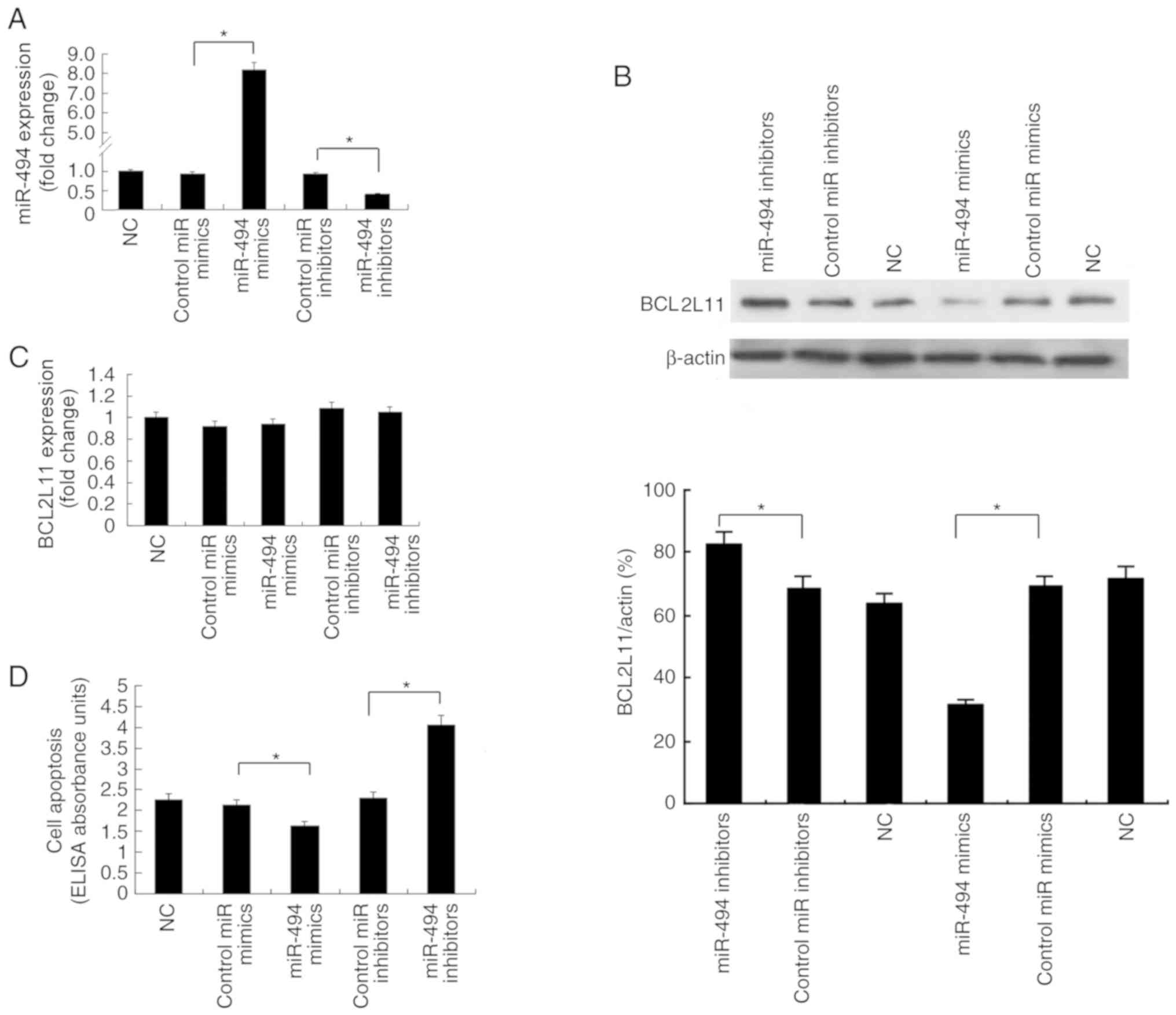
In order to investigate whether miR-494 regulates
BCL2L11 expression, VSMCs were transfected with miR-494 mimics or
inhibitors and their respective control oligos. RT-qPCR was used to
confirm the overexpression of miR-494 in VSMCs transfected with
miR-494 mimics and inhibition of miR-494 expression in cells
transfected with miR-494 inhibitors (Fig. 4A). Western blot analysis
demonstrated that transfection with miR-494 mimics resulted in
downregulated BCL2L11 protein levels, whereas transfection with
miR-494 inhibitors increased BCL2L11 protein expression (Fig. 4B). However, alteration of miR-494
levels had no obvious effect on BCL2L11 mRNA expression levels
(Fig. 4C), indicating that miR-494
attenuated the protein translation of BCL2L11 in VSMCs.
Transfection with miR-494 mimics decreased cell apoptosis, whereas
transfection with miR-494 inhibitors increased cell apoptosis
(Fig. 4D). Collectively, these
results indicated that miR-494 modulated VSMC apoptosis and
attenuated BCL2L11 expression via post-transcriptional
regulation.
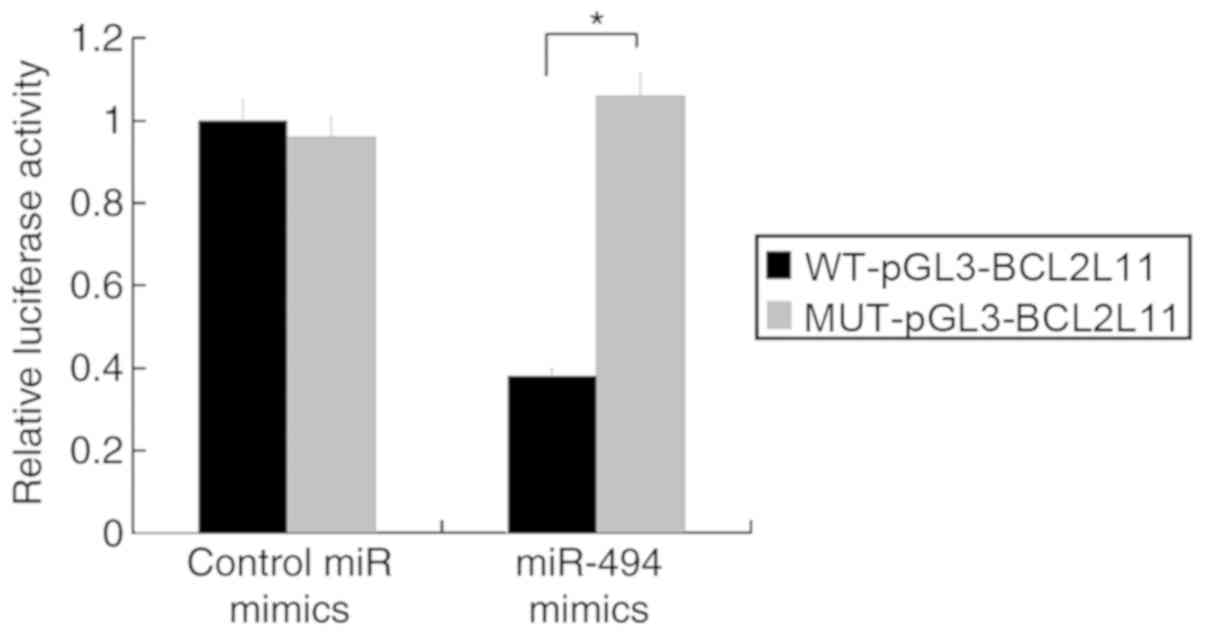
miR-494 directly targets BCL2L11 in
VSMCs
To determine whether miR-494 directly targets
BCL2L11 in VSMCs, luciferase reporter plasmids containing the
wild-type or mutant 3′-UTR sequences of BCL2L11 were constructed
and co-transfected with miR-494 mimics in murine VSMCs. The results
demonstrated that overexpression of miR-494 significantly
suppressed the luciferase activity of the WT-pGL3-BCL2L11 reporter
plasmid, but did not affect the MUT-pGL3-BCL2L11 reporter plasmid.
Control miR mimics did not have any effect on the luciferase
activity of the wild-type or mutant plasmids (Fig. 5). These results demonstrated that
the BCL2L11 3′-UTR was specifically targeted by miR-494.
miR-494 is dependent on BCL2L11 for
inhibition of TNF-α-induced apoptosis in VSMCs
As BCL2L11 was verified as an miR-494 target gene in
VSMCs, it was subsequently investigated whether the effect of
miR-494 on VSMC apoptosis was dependent on BCL2L11. The efficiency
of siRNA knockdown of BCL2L11 was verified by western blot analysis
(Fig. 6A). The impact of miR-494
on apoptosis of murine VSMCs with altered BCL2L11 expression levels
was detected by TUNEL staining (Fig.
6B and C) and cell death ELISA (Fig. 6D). Cell apoptosis was increased in
VSMCs transfected with miR-494 inhibitors and inhibited in cells
transfected with BCL2L11 siRNA compared with that of the control.
However, the effect of miR-494 inhibitors on cell apoptosis was
attenuated in cells co-transfected with BCL2L11 siRNA.
Collectively, these results demonstrated that miR-494 was dependent
on BCL2L11 to inhibit TNF-α-induced VSMC apoptosis in
vitro.
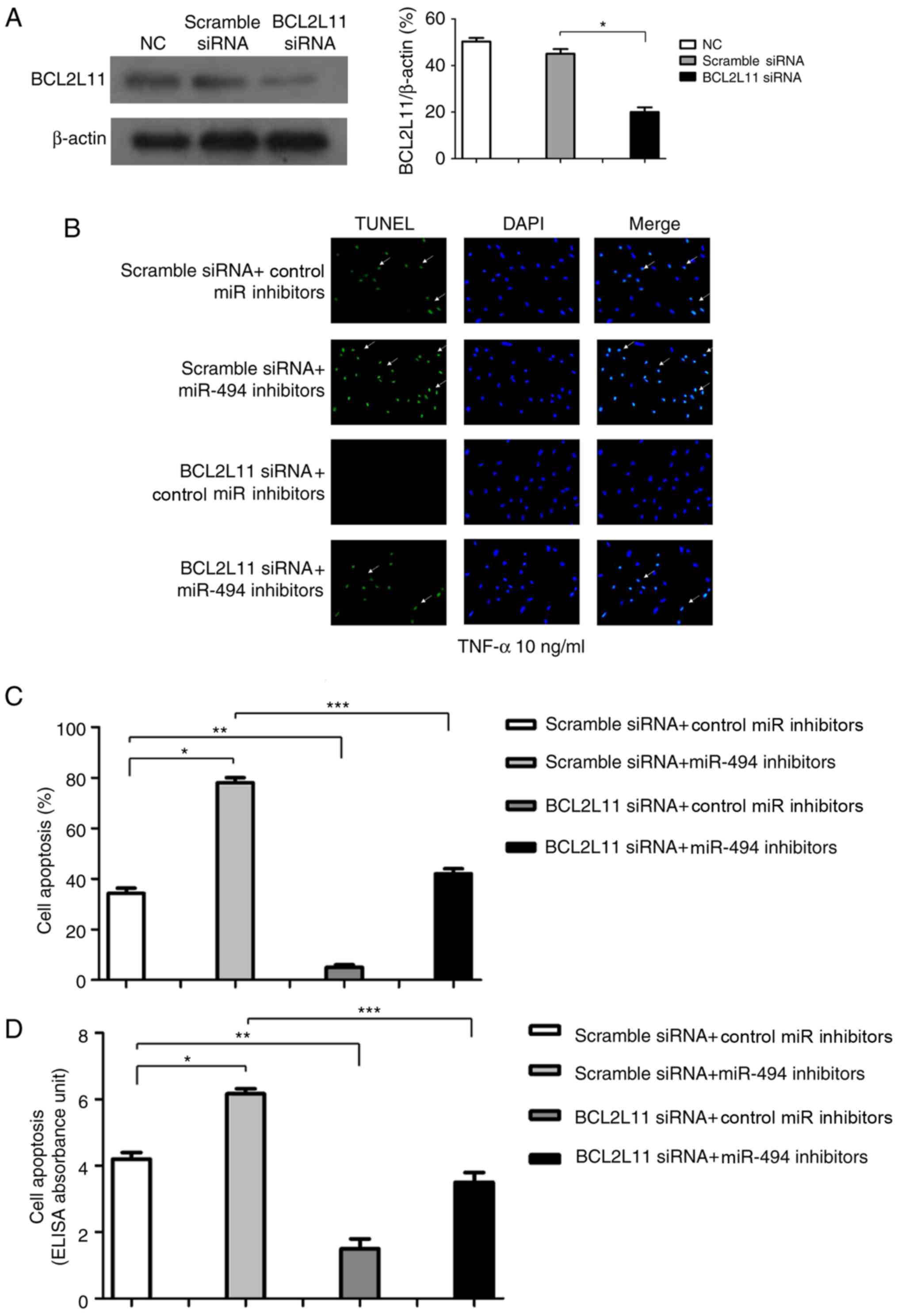 | Figure 6.miR-494 inhibits TNF-α induced VSMC
apoptosis via BCL2L11. (A) Efficiency of siRNA knockdown of BCL2L11
in murine VSMCs was determined by western blot analysis. Murine
VSMCs were transfected with scramble siRNA + control miR
inhibitors, scramble siRNA + miR-494 inhibitors, BCL2L11 siRNA +
control miR inhibitors and BCL2L11 siRNA + miR-494 inhibitor.
Subsequently, 48 h post-transfection, cells were incubated with 10
ng/ml TNF-α for 24 h. (B) Cell apoptosis was measured by TUNEL
assay (white arrows indicate apoptotic VSMCs; magnification, ×100).
(C) Quantitative analysis of TUNEL results. (D) Cell death ELISA
was performed and cell apoptosis is presented as ELISA absorbance
units. n=5. *P<0.05, **P<0.01, ***P<0.001. BCL2L11, B-cell
lymphoma-2-like 11; miR, microRNA; NC, negative control; siRNA,
small interfering RNA; TNF-α, tumor necrosis factor-α; TUNEL,
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling; VSMC, vascular smooth muscle cell. |
Discussion
Apoptosis is a form of programmed cell death that
can be induced in all cells. The different stages of this process
include blebbing, cell shrinkage, nuclear fragmentation and
chromosomal DNA fragmentation (32). Apoptosis of VSMCs, which occurs in
various vascular disorders, is an important feature involved in
vessel remodeling (33,34). Inhibition of VSMC apoptosis may be
a method to halt the initiation and progression of cardiovascular
disorders. TNF-α, a cytokine predominantly produced by activated
macrophages, is the major extrinsic mediator that induces cell
apoptosis. TNF-α binds to TNF receptor 1 and initiates a signaling
pathway that leads to caspase activation. The findings in the
present study demonstrated that TNF-α induced VSMC apoptosis in a
dose-dependent manner, in accordance with previous studies
(14).
Previous research on miRNAs expressed in VSMCs
demonstrated their fundamental roles in regulating various cell
functions, including proliferation, differentiation, calcification
and apoptosis (22,27,35,36).
However, the role of miRNAs in TNF-α-mediated VSMC apoptosis
remains unknown. The current study established an miRNA expression
profile, which may assist future research into the role of miRNAs
in vascular smooth muscle cells. The expression of miR-494 in
murine VSMCs was significantly downregulated during TNF-α-induced
apoptosis, indicating that miR-494 may have a role in VSMC
apoptosis.
To identify whether miR-494 is directly associated
with VSMC apoptosis, the expression of miR-494 was modulated prior
to TNF-α-induced apoptosis. Inhibition of miR-494 expression
promoted apoptosis of VSMCs, as demonstrated by the increased
release of cytoplasmic nucleosomes detected by the cell death ELISA
assay. Conversely, overexpression of miR-494 attenuated VSMC
apoptosis. These results suggested that miR-494 suppressed VSMC
apoptosis in vitro. However, these data contradicted the
study by Bai et al (37),
which reported that inhibition of miR-494 leads to overexpression
of secretagogin, leading to reduced cell apoptosis and increased
chemoresistance in small cell lung cancer. A possible explanation
for this phenomenon is that miR-494 may exert different effects in
different cell types, and under different stimuli.
Several target genes have been identified for
miR-494 in different cells. miR-494 acts as an anti-oncogene in
gastric carcinoma by targeting c-Myc (38). Another report demonstrated that
miR-494 upregulates hypoxia-inducible factor-1α expression and
protects against hypoxia-induced apoptosis in L02 human liver cells
(28). miR-494 exerts its
cardioprotective effects against ischemia/reperfusion-induced
injury by targeting pro-apoptotic genes [Rho-associated protein
kinase 1, phosphatase and tensin homolog (PTEN), and
calcium/calmodulin dependent protein kinase II δ] and
anti-apoptotic genes (leukemia inhibitory factor and fibroblast
growth factor receptor 2), and causes activation of the AKT
serine/threonine kinase 1 (AKT)-mitochondrial signaling pathway
(39). In pancreatic β-cells,
miR-494 promotes cell proliferation and inhibits cell apoptosis by
targeting PTEN (29). In a rat
spinal cord injury model, overexpression of miR-494 was
demonstrated to inhibit apoptosis and activate AKT/mechanistic
target of rapamycin kinase (mTOR) signaling via inhibition of PTEN
(30). miR-494 also inhibits
TNF-related apoptosis-inducing ligand-induced apoptosis (40) in non-small-cell lung cancer via
downregulation of BCL2L11 (31).
Among these target genes identified for miR-494, c-Myc oncogene
contributes to the genesis and process of various types of cancer
(41,42); PTEN is a dual-specificity
phosphatase whose inhibition activates different downstream
pathways, including AKT/mTOR signaling that serves a vital role in
cell survival and resisting apoptosis, as well as cell regeneration
(43–45); BCL2L11 (also known as BIM) is a
member of the BH3-only death activator family and is regulated by
its interaction with dynein light chain 1. BCL2L11 is one of the
most important apoptosis regulators; it induces apoptosis by
activating apoptotic proteins (BCL2 associated X apoptosis
regulator and BCL2 homologous antagonist/killer) and inactivating
anti-apoptotic BCL-2 proteins (46). Under normal physiological
conditions, BCL2L11 is sequestered by dynein light chain 1 to form
complexes on microtubules. BCL2L11 is released from dynein light
chain 1 following phosphorylation in response to a series of
apoptotic stimuli, including deprivation of growth cytokines,
ionizing radiation and cytotoxic peptides (47–50).
BCL2L11 promotes apoptosis of many tumor cell types, including lung
cancer, breast cancer, osteosarcoma and melanoma (51). Additionally, increased BCL2L11
protein expression leads to apoptosis in pulmonary arterial smooth
muscle cells (52), the critical
cells participating in pulmonary arterial hypertension. Therefore,
the present study investigated whether BCL2L11 is an miR-494 target
in vascular smooth muscle cells in regulating apoptosis. BCL2L11
siRNA inhibited VSMC apoptosis, and miR-494 mimics inhibited VSMC
apoptosis by suppressing BCL2L11 protein expression. The current
study supported the hypothesis that BCL2L11 is an important direct
target of miR-494 in VSMCs. It was confirmed that miR-494 targeted
the BCL2L11 3′-UTR through base pairing of the miRNA seed sequence,
suggesting miR-494 directly modulated BCL2L11 expression.
Overexpression of miR-494 decreased BCL2L11 protein levels, but not
mRNA levels, suggesting the effects were mediated by
post-transcriptional modulation. Additionally, transfection with
miR-494 mimics suppressed the luciferase activity of the
WT-pGL3-BCL2L11 reporter plasmid, but not that of the mutant
reporter; and finally, BCL2L11 siRNA abolished the VSMC apoptosis
that was promoted by miR-494 inhibitors. Taken together, these
findings demonstrated that miR-494 inhibited VSMC apoptosis via
post-transcriptional modulation of BCL2L11 mRNA.
In conclusion, the present study identified miR-494
as an important inhibitor of VSMC apoptosis, and demonstrated that
its effects were mediated by suppression of the target gene
BCL2L11. This may be a novel mechanism involved in regulating VSMC
apoptosis. Further investigation into miRNA-mediated modulation of
VSMC apoptosis may increase our understanding of in vivo
apoptosis mechanisms and provide novel strategies for prevention
and treatment of cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
National Basic Research Program of China (973 Program; grant no.
2014CB942903) and the National Natural Science Foundation of China
(grant nos. 81000313, 81370973 and 81770881).
Availability of data and materials
The datasets generated and analyzed during the
current study are available in the GEO repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE127016.
Authors' contributions
LY and RC conceived the study and designed the
experiments. RC, SY, JZ, LL, SL, XL and LY performed the
experiments. RC analyzed and interpreted the data, and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The care of animals conformed to the Guide for the
Care and Use of Laboratory Animals by the United States National
Institutes of Health. The study was approved by The Ethics Review
Board of The Second Xiangya Hospital of Central South University
(Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rzucidlo EM, Martin KA and Powell RJ:
Regulation of vascular smooth muscle cell differentiation. J Vasc
Surg. 45 (Suppl A):A25–A32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennett MR: Apoptosis of vascular smooth
muscle cells in vascular remodelling and atherosclerotic plaque
rupture. Cardiovasc Res. 41:361–368. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bennett MR, Sinha S and Owens GK: Vascular
smooth muscle cells in atherosclerosis. Circ Res. 118:692–702.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Durand E, Mallat Z, Addad F, Vilde F,
Desnos M, Guérot C, Tedgui A and Lafont A: Time courses of
apoptosis and cell proliferation and their relationship to arterial
remodeling and restenosis after angioplasty in an atherosclerotic
rabbit model. J Am Coll Cardiol. 39:1680–1685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Henderson EL, Geng YJ, Sukhova GK,
Whittemore AD, Knox J and Libby P: Death of smooth muscle cells and
expression of mediators of apoptosis by T lymphocytes in human
abdominal aortic aneurysms. Circulation. 99:96–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke MC, Figg N, Maguire JJ, Davenport
AP, Goddard M, Littlewood TD and Bennett MR: Apoptosis of vascular
smooth muscle cells induces features of plaque vulnerability in
atherosclerosis. Nat Med. 12:1075–1080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stehbens WE: Pathology and pathogenesis of
degenerative atherosclerotic aneurysms. In: Development of
Aneurysms. Keen RR and Dobrin PB: R.G, Landes Co.; Austin, TX: pp.
84–125. 2000
|
|
8
|
Tang PC, Coady MA, Lovoulos C, Dardik A,
Aslan M, Elefteriades JA and Tellides G: Hyperplastic cellular
remodeling of the media in ascending thoracic aortic aneurysms.
Circulation. 112:1098–1105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aghagolzadeh P, Bachtler M, Bijarnia R,
Jackson C, Smith ER, Odermatt A, Radpour R and Pasch A:
Calcification of vascular smooth muscle cells is induced by
secondary calciprotein particles and enhanced by tumor necrosis
factor-α. Atherosclerosis. 251:404–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tay C, Liu YH, Hosseini H, Kanellakis P,
Cao A, Peter K, Tipping P, Bobik A, Toh BH and Kyaw T:
B-cell-specific depletion of tumour necrosis factor alpha inhibits
atherosclerosis development and plaque vulnerability to rupture by
reducing cell death and inflammation. Cardiovasc Res. 111:385–397.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y,
Zhu J, Ma L, Guo J, Shi H, et al: Exosomes derived from mature
dendritic cells increase endothelial inflammation and
atherosclerosis via membrane TNF-α mediated NF-κB pathway. J Cell
Mol Med. 20:2318–2327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clarke M and Bennett M: The emerging role
of vascular smooth muscle cell apoptosis in atherosclerosis and
plaque stability. Am J Nephrol. 26:531–535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Cheng Y, Simoncini T and Xu S:
17β-Estradiol inhibits TNF-α-induced proliferation and migration of
vascular smooth muscle cells via suppression of TRAIL. Gynecol
Endocrinol. 32:581–586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HH and Kim K: Enhancement of
TNF-alpha-mediated cell death in vascular smooth muscle cells
through cytochrome c-independent pathway by the proteasome
inhibitor. FEBS Lett. 535:190–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Small EM and Olson EN: Pervasive roles of
microRNAs in cardiovascular biology. Nature. 469:336–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He Z, Jiang J, Kokkinaki M, Tang L, Zeng
W, Gallicano I, Dobrinski I and Dym M: MiRNA-20 and mirna-106a
regulate spermatogonial stem cell renewal at the
post-transcriptional level via targeting STAT3 and Ccnd1. Stem
Cells. 31:2205–2217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takasaki S: Roles of microRNAs in cancers
and development. Methods Mol Biol. 1218:375–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gargalionis AN and Basdra EK: Insights in
microRNAs biology. Curr Top Med Chem. 13:1493–1502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nossent AY, Eskildsen TV, Andersen LB, Bie
P, Brønnum H, Schneider M, Andersen DC, Welten SM, Jeppesen PL,
Hamming JF, et al: The 14q32 microRNA-487b targets the
antiapoptotic insulin receptor substrate 1 in hypertension-induced
remodeling of the aorta. Ann Surg. 258:743–751 752–743. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Ran Y, Zhang D, Chen J, Li S and Zhu
D: MicroRNA-138 plays a role in hypoxic pulmonary vascular
remodelling by targeting Mst1. Biochem J. 452:281–291. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Zhou M, Wang Y, Huang W, Qin G,
Weintraub NL and Tang Y: miR-92a inhibits vascular smooth muscle
cell apoptosis: Role of the MKK4-JNK pathway. Apoptosis.
19:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ellmark SH, Dusting GJ, Fui MN,
Guzzo-Pernell N and Drummond GR: The contribution of Nox4 to NADPH
oxidase activity in mouse vascular smooth muscle. Cardiovasc Res.
65:495–504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui RR, Mao DA, Yi L, Wang C, Zhang XX,
Xie H, Wu XP, Liao XB, Zhou H, Meng JC, et al: Apelin suppresses
apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt
signaling pathways. Amino Acids. 39:1193–1200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruan W, Xu JM, Li SB, Yuan LQ and Dai RP:
Effects of down-regulation of microRNA-23a on TNF-α-induced
endothelial cell apoptosis through caspase-dependent pathways.
Cardiovasc Res. 93:623–632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui RR, Li SJ, Liu LJ, Yi L, Liang QH, Zhu
X, Liu GY, Liu Y, Wu SS, Liao XB, et al: MicroRNA-204 regulates
vascular smooth muscle cell calcification in vitro and in vivo.
Cardiovasc Res. 96:320–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun G, Zhou Y, Li H, Guo Y, Shan J, Xia M,
Li Y, Li S, Long D and Feng L: Over-expression of microRNA-494
upregulates hypoxia-inducible factor-1 alpha expression via
PI3K/Akt pathway and protects against hypoxia-induced apoptosis. J
Biomed Sci. 20:1002013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He Y, Bai J, Liu P, Dong J, Tang Y, Zhou
J, Han P, Xing J, Chen Y and Yu X: miR-494 protects pancreatic
β-cell function by targeting PTEN in gestational diabetes mellitus.
EXCLI J. 16:1297–1307. 2017.PubMed/NCBI
|
|
30
|
Zhu H, Xie R, Liu X, Shou J, Gu W, Gu S
and Che X: MicroRNA-494 improves functional recovery and inhibits
apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal
cord injury. Biomed Pharmacother. 92:879–887. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Romano G, Acunzo M, Garofalo M, Di Leva G,
Cascione L, Zanca C, Bolon B, Condorelli G and Croce CM: MiR-494 is
regulated by ERK1/2 and modulates TRAIL-induced apoptosis in
non-small-cell lung cancer through BIM down-regulation. Proc Natl
Acad Sci USA. 109:16570–16575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Walsh K, Smith RC and Kim HS: Vascular
cell apoptosis in remodeling, restenosis, and plaque rupture. Circ
Res. 87:184–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clarke M and Bennett M: Defining the role
of vascular smooth muscle cell apoptosis in atherosclerosis. Cell
Cycle. 5:2329–2331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stein JJ, Iwuchukwu C, Maier KG and Gahtan
V: Thrombospondin-1-induced vascular smooth muscle cell migration
and proliferation are functionally dependent on microRNA-21.
Surgery. 155:228–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li P, Zhu N, Yi B, Wang N, Chen M, You X,
Zhao X, Solomides CC, Qin Y and Sun J: MicroRNA-663 regulates human
vascular smooth muscle cell phenotypic switch and vascular
neointimal formation. Circ Res. 113:1117–1127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bai Y, Sun Y, Peng J, Liao H, Gao H, Guo Y
and Guo L: Overexpression of secretagogin inhibits cell apoptosis
and induces chemoresistance in small cell lung cancer under the
regulation of miR-494. Oncotarget. 5:7760–7775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He W, Li Y, Chen X, Lu L, Tang B, Wang Z,
Pan Y, Cai S, He Y and Ke Z: miR-494 acts as an anti-oncogene in
gastric carcinoma by targeting c-myc. J Gastroenterol Hepatol.
29:1427–1434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Zhang X, Ren XP, Chen J, Liu H,
Yang J, Medvedovic M, Hu Z and Fan GC: MicroRNA-494 targeting both
proapoptotic and antiapoptotic proteins protects against
ischemia/reperfusion-induced cardiac injury. Circulation.
122:1308–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Li M, Chen D, Nie J, Xi Y, Yang X,
Chen Y and Yang Z: Expression of C-myc and β-catenin and their
correlation in triple negative breast cancer. Minerva Med.
108:513–517. 2017.PubMed/NCBI
|
|
42
|
Sadeghi S, Hojati Z and Tabatabaeian H:
Cooverexpression of EpCAM and c-myc genes in malignant breast
tumours. J Genet. 96:109–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park KK, Liu K, Hu Y, Kanter JL and He Z:
PTEN/mTOR and axon regeneration. Exp Neurol. 223:45–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park KK, Liu K, Hu Y, Smith PD, Wang C,
Cai B, Xu B, Connolly L, Kramvis I, Sahin M and He Z: Promoting
axon regeneration in the adult CNS by modulation of the PTEN/mTOR
pathway. Science. 322:963–966. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo S and Rubinsztein DC: BCL2L11/BIM: A
novel molecular link between autophagy and apoptosis. Autophagy.
9:104–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Biswas SC, Liu DX and Greene LA: Bim is a
direct target of a neuronal E2F-dependent apoptotic pathway. J
Neurosci. 25:8349–8358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Essafi A, Fernandez de Mattos S, Hassen
YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH and Lam EW: Direct
transcriptional regulation of Bim by FoxO3a mediates STI571-induced
apoptosis in Bcr-Abl-expressing cells. Oncogene. 24:2317–2329.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mestre-Escorihuela C, Rubio-Moscardo F,
Richter JA, Siebert R, Climent J, Fresquet V, Beltran E, Agirre X,
Marugan I, Marín M, et al: Homozygous deletions localize novel
tumor suppressor genes in B-cell lymphomas. Blood. 109:271–280.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang JY, Xia W and Hu MC: Ionizing
radiation activates expression of FOXO3a, Fas ligand, and Bim, and
induces cell apoptosis. Int J Oncol. 29:643–648. 2006.PubMed/NCBI
|
|
51
|
Akiyama T, Dass CR and Choong PF:
Bim-targeted cancer therapy: A link between drug action and
underlying molecular changes. Mol Cancer Ther. 8:3173–3180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kudryashova TV, Goncharov DA, Pena A,
Kelly N, Vanderpool R, Baust J, Kobir A, Shufesky W, Mora AL,
Morelli AE, et al: HIPPO-integrin-linked kinase cross-talk controls
self-sustaining proliferation and survival in pulmonary
hypertension. Am J Respir Crit Care Med. 194:866–877. 2016.
View Article : Google Scholar : PubMed/NCBI
|