Introduction
Cervical cancer is the fourth most common type of
cancer diagnosed among women worldwide (1). A combined treatment regimen of
screening, surgery and radiotherapy has improved the prognosis of
early stage cervical cancer; however, it remains difficult to
prevent metastasis and to treat recurring cancer at the advanced
stages, which are the main causes of mortality in patients with
cervical cancer (2). The prominent
cause of cervical cancer has been reported to be human
papillomavirus (HPV) infection; the most prevalent carcinogenic HPV
strains are HPV-16 and HPV-18 (3).
The present study aimed to investigate the molecular mechanism
underlying the invasion and metastasis of cervical cancer, which
may aid developments in its prognosis and improve the curative rate
of patients with cervical cancer.
Nuclear factor κ-light-chain-enhancer of activated B
cells (NF-κB) is a protein complex that controls DNA transcription
and affects cell survival. All proteins in the NF-κB family contain
an N-terminal Rel homology domain (RHD), which possesses a
nuclear-localization sequence (NLS), and some RHDs also contain an
additional C-terminal transactivation domain (4). NF-κB signaling serves important roles
in transmitting signals from the cell membrane to the nucleus. As a
transcription factor, NF-κB can be rapidly activated and responds
to stimuli in the early stage of cervical cancer, including stress,
cytokines, and bacterial and viral antigens (5). Notably, the abnormal regulation of
NF-κB has been reported to lead to improper development of the
immune system, the development of inflammatory and autoimmune
diseases, and cancer (6,7).
Transgelin 2 (TAGLN2) is a member of the actin- and
calmodulin-binding protein family (8,9),
which regulates cell morphology and motility (10). TAGLN2 has been proposed as a tumor
suppressor in cancer metastasis (11). The TAGLN family has been reported
to interfere in extracellular signal-regulated kinase (ERK)1 and
ERK2 signaling, which is dependent on the calponin protein domain
of the TAGLN family (12).
Additionally, members of the TAGLN family can decrease the
expression of matrix metalloproteinase (MMP)-9 to inhibit cancer
metastasis (13); however,
research has suggested that TAGLN2 serves as an oncogene in
numerous types of cancer, including head and neck squamous cell
carcinoma (14), renal cell
carcinoma (15) and bladder cancer
(16). Therefore, the function of
TAGLN2 is controversial and it is of importance to investigate the
role of TAGLN2 in cervical cancer.
The present study aimed to determine the role of
TAGLN2 in cervical cancer and its underlying mechanism. The
findings of the present study may not only improve current
understanding of the progression of cervical cancer, but may lead
to the identification of a potential therapeutic target for the
treatment of cervical cancer.
Materials and methods
Patients and tissues
Human cervical tissues were collected from 56 female
patients with cervical cancer (age, 36–71 years) and 56 female
patients without cervical cancer who underwent hysterectomy for
uterine fibroids (normal cervical tissue, control) at The First
Affiliated Hospital of Zhejiang Chinese Medical University
(Hangzhou, China) between June 2015 and June 2017. According to the
International Federation of Gynecology and Obstetrics (FIGO)
criteria (17), 25 of the 56
patients with cervical cancer were of the I/II stages, whereas the
remaining 31 cases were of the III/IV stages. None of the 56
patients with cervical cancer received radiotherapy or chemotherapy
prior to surgery. All sample collections were approved by an
institutional review board of the Ethics Committee of The First
Affiliated Hospital of Zhejiang Chinese Medical University, and
informed consent was obtained from each patient. The tissues
samples were preserved in liquid nitrogen for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis. Patients were divided into higher and lower
TAGLN2 expression groups, using mean TAGLN2 expression as the
cut-off value.
Cell culture
Human cervical cancer cell lines, including HeLa
(HPV-18-positive), SiHa (HPV-16-positive) and C-33A (HPV-negative),
were purchased from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). Human normal cervical
epithelial cells (HcerEpic) (cat. no. BNCC340374) were purchased
from Bena Culture Collection (Beijing, China). Cells were cultured
in high-glucose Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
in a cell incubator containing 5% CO2 and 50% humidity
at 37°C. The medium was replaced with fresh cell culture medium
every 2 days and cells in the logarithmic phase were employed for
subsequent analysis. RT-qPCR and western blotting were conducted to
detect and compare the expression levels of TAGLN2 among the
various cell lines.
Cell transfection
The TAGLN2 sequence was inserted into a
pcDNA3.1-Flag plasmid (Invitrogen; Thermo Fisher Scientific, Inc.).
HeLa cells were seeded in 12-well plates at an initial
concentration of 6×104 cells/well. The following day,
cells were transfected with 1 µg TAGLN2 overexpression recombinant
plasmid or 1 µg empty plasmid vector [overexpression and negative
control (NC) groups, respectively] using the transfection reagent
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 6 h. Untreated cells were considered
the control group. Prior to conducting functional analyses, cells
were cultured in normal complete medium for 48 h post-transfection.
Cell transfection efficiency was assessed by analyzing the
expression levels of TAGLN2 via RT-qPCR and western blotting.
Cell viability assay
Cell Counting kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Nantong, China) was used to investigate cell
viability post-transfection. Cells were seeded in 96-well plates at
an initial density of 5×103 cells/well and were
incubated for 12, 24 and 48 h at 37°C. After incubating with 20 µl
CCK-8 reagent for 1 h at 37°C, a microplate reader was used for
analysis (BioTek Instruments, Inc., Winooski, VT, USA). Optical
density was measured at 450 nm.
Wound healing assay
The cell migratory abilities of the TAGLN2
overexpression group were determined using a wound healing assay,
and were compared with the abilities of the control and NC groups.
Cells were seeded in 12-well plates at an initial concentration of
1×105 cells/well. Following incubation at 37°C for 24 h,
the confluent monolayer cells were scratched gently using a 200-µl
pipette tip to obtain a cell-free area. Subsequently, cells were
cultured for a further 24 h at 37°C. Finally, the area of the
cell-free zone was observed under an optical microscope (Olympus
Corporation, Tokyo, Japan) and the rate of cell migration was
calculated relative to the control group.
Cell invasion assay
The cell invasive abilities of the TAGLN2
overexpression group were examined and compared with the abilities
of the control and NC groups via a Transwell invasion assay.
Specifically, each Transwell chamber (Corning Incorporation,
Corning, NY, USA) was coated with 100 µl melted Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) and the chambers were
maintained for 30 min at 37°C to ensure solidification of the
Matrigel. Subsequently, 100 µl serum-free medium-diluted cell
suspensions (1×106/ml) were added into the upper
chambers, whereas 600 µl complete culture medium containing 10% FBS
was added to the bottom chambers. Following incubation for 24 h at
37°C, the invaded cells were fixed with methanol for 15 min at room
temperature and were stained with 0.1% crystal violet for 30 min at
room temperature. Subsequently, five random high-power fields were
observed under an optical microscope (Olympus Corporation). The
rate of cell invasion was calculated relative to the control
group.
RT-qPCR
mRNA expression levels were determined via RT-qPCR
analysis. Total RNA was extracted from frozen tissues or the
various cell groups using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). cDNA was reverse transcribed from
1 µg RNA using PrimeScript RT Master Mix (Takara Biotechnology Co.,
Ltd., Dalian, Japan), according to the manufacturer's protocol. The
primer sequences for TAGLN2, E-cadherin, C-X-C chemokine receptor
type 4 (CXCR4), MMP-2, MMP-9, p50, transcription factor p65 (RelA),
inhibitor of NF-κB (IκB) and β-actin were designed and synthesized
by Invitrogen (Thermo Fisher Scientific, Inc.); the sequences are
presented in Table I. β-actin
served as an internal control. qPCR amplification was then
conducted using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.)
on an ABI 7500 thermocycler system (Applied Biosystems; Thermo
Fisher Scientific, Inc.), under the following conditions:
Pre-denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec, and annealing/extension at 60°C for
35 sec, and a final step at 95°C for 15 min. Data were analyzed
using the 7500 Fast System Sequence Detection Software v1.3.1
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative
gene expression levels were calculated using the 2−ΔΔCq
method (18).
 | Table I.Primer sequences applied in the
present study. |
Table I.
Primer sequences applied in the
present study.
| Gene | Direction | Sequence (5′-3′) |
|---|
| β-actin | Forward |
GTGGACATCCGCAAAGAC |
|
| Reverse |
GAAAGGGTGTAACGCAACT |
| TAGLN2 | Forward |
AGTGACATTCCCAGAGAGCC |
|
| Reverse |
GGCCCCTAAATTTTGGTCCC |
| E-cadherin | Forward |
ACGCATTGCCACATACACTC |
|
| Reverse |
GGTGTTCACATCATCGTCCG |
| MMP-2 | Forward |
CAGCCCTGCAAGTTTCCATT |
|
| Reverse |
GTTGCCCAGGAAAGTGAAGG |
| MMP-9 | Forward |
GAGACTCTACACCCAGGACG |
|
| Reverse |
GAAAGTGAAGGGGAAGACGC |
| CXCR4 | Forward |
TGTCATCACGCTTCCCTTCT |
|
| Reverse |
TTCCTTGGCCTCTGACTGT |
| p50 | Forward |
GGTGACAGGAGACGTGAAGA |
|
| Reverse |
TCCACCACATCTTCCTGCTT |
| RelA | Forward |
CTACACAGGACCAGGGACAG |
|
| Reverse |
GGAAGGGGTTGTTGTTGGTC |
| IκB | Forward |
TATCACGGAGACCCAGGAGA |
|
| Reverse |
GCTTGTGAATCTGCTCCTCG |
Western blotting
Protein was extracted from frozen tissues and cells
using the Tissue or Cell Total Protein Extraction kit (Sangon
Biotech Co., Ltd., Shanghai, China). Total protein concentration
was determined using a Bicinchoninic Acid assay (Beyotime Institute
of Biotechnology). Proteins (20 µg) were then separated by 10%
SDS-PAGE and were then transferred to polyvinylidene fluoride
(PVDF) membranes, which were used to conduct immunoblotting. The
PVDF membranes were blocked with 5% nonfat milk for 1 h at room
temperature. Subsequently, PVDF membranes were probed with specific
primary antibodies at 4°C overnight, and were then incubated with
appropriate horseradish peroxidase-conjugated secondary antibodies
(goat anti-rabbit; 1:2,000; cat. no. ab205718 and goat anti-mouse;
1:5,000; cat. no. ab205719; both Abcam, Cambridge, MA, USA) at 37°C
for 1 h. Subsequently, proteins were exposed to X-ray film, and
immunoreactive bands were detected using enhanced chemiluminescence
detection reagents (GE Healthcare, Chicago, IL, USA). β-actin was
used as a loading control. Protein quantities were determined by
Lab Works Image Acquisition and Analysis Software 4.0 (UVP, LLC,
Phoenix, AZ, USA). The primary antibodies employed were as follows:
Rabbit anti-TAGLN2 (1:500, cat. no. sc-166697; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-E-cadherin (1:1,000,
cat. no. ab133597; Abcam), anti-CXCR4 (1:1,000, cat. no. ab181020;
Abcam), anti-MMP-2 (1:1,000, cat. no. ab92536; Abcam), anti-MMP-9
(1:1,000, cat. no. ab38898; Abcam), anti-p50 (1:1,000, cat. no.
ab32360; Abcam), anti-RelA (1:1,000, cat. no. ab16502; Abcam),
anti-IκB (1:1,000, cat. no. ab32518; Abcam) and anti-β-actin
(1:2,000, cat. no. ab8227; Abcam).
Statistical analysis
Statistical analysis was conducted using SPSS 22.0
(IBM Corp., Armonk, NY, USA). Each experiment was repeated three
times. Data are presented as the means ± standard deviation.
One-way analysis of variance followed by Tukey's test was used to
calculate statistical significance. A χ2 test was used
to analyze categorical variables. Survival was investigated via
Kaplan-Meier analysis and log-rank test. P<0.05 was considered
to indicate a statistically significant difference. P<0.01 was
considered to indicate a highly statistically significant
difference.
Results
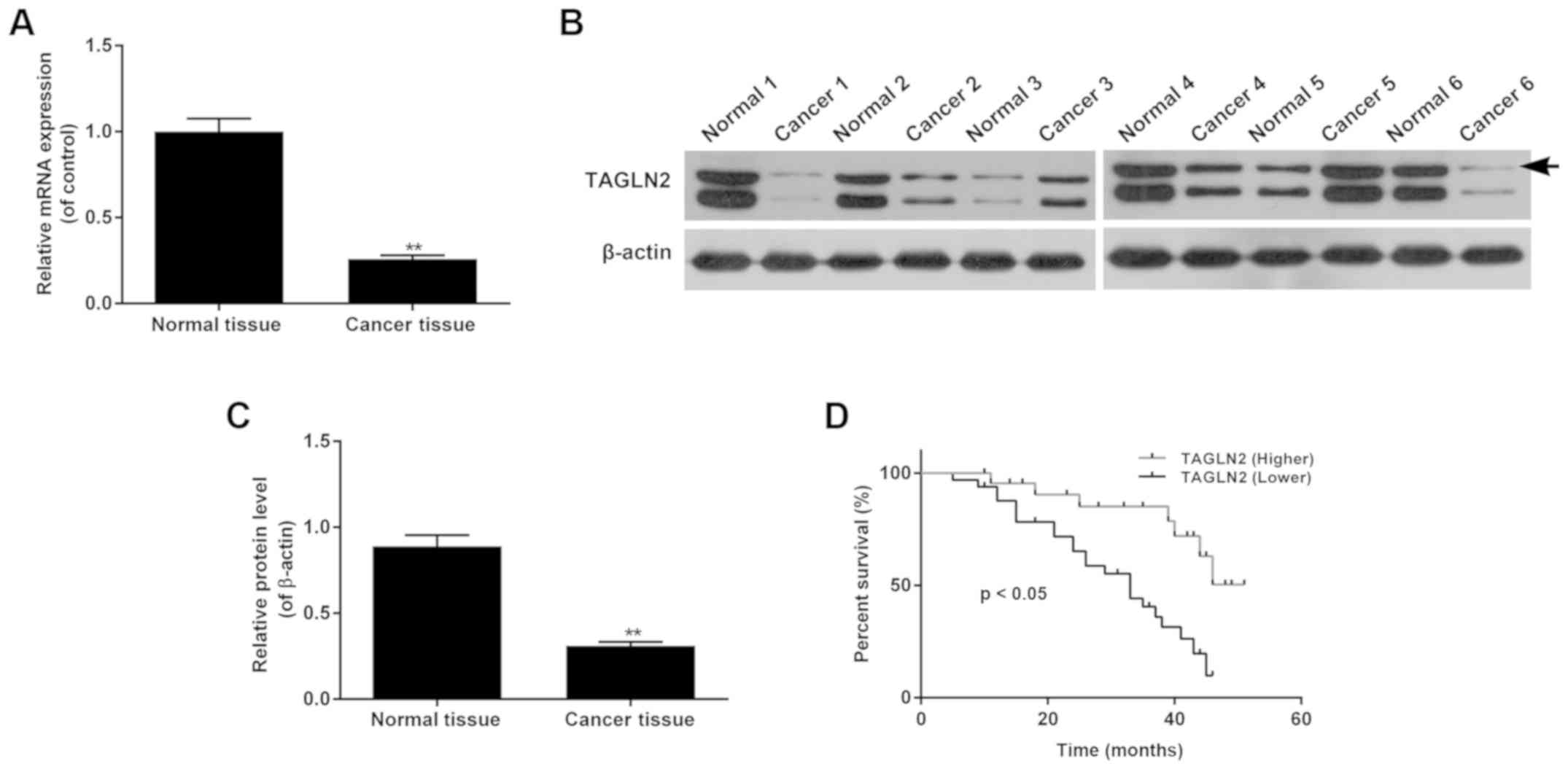
Expression levels of TAGLN2 are
associated with the severity of cervical carcinoma
To understand the biological function of TAGLN2 in
cervical cancer, the expression levels of TAGLN2 in cervical cancer
tissues were determined by RT-qPCR and western blotting. The
results revealed that the mRNA expression levels of TAGLN2 were
significantly lower in cervical cancer tissues than in normal
cervical tissues (Fig. 1A;
P<0.05). The representative western blot bands are presented in
Fig. 1B; the protein expression
levels of TAGLN2 were significantly lower in cervical cancer
tissues compared with in normal tissues (Fig. 1C). The clinical pathological
features were also evaluated, which indicated that independent of
age, the expression levels of TAGLN2 were associated with FIGO
stages and the histological grades of cervical cancer, and the
advanced stages of cancer were associated with higher incidences of
low TAGLN2 expression (Table II).
Survival analysis demonstrated that patients with cervical cancer
and higher expression levels of TAGLN2 survived for longer than
those with lower levels of TAGLN2 (Fig. 1D; P<0.05).
 | Table II.Analysis of TAGLN2 expression in
cervical carcinoma. |
Table II.
Analysis of TAGLN2 expression in
cervical carcinoma.
|
|
| TAGLN2 expression
levels |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Factor (n) | Low | High | P-value |
|---|
| Age (years) | <50 | 16 | 12 | 0.786 |
|
|
| ≥7 | 17 | 11 |
| FIGO stages | I and II (25) | 6 | 19 | 0.001a |
|
|
| III and IV
(31) | 27 | 4 |
| Histological
grade | Low
differentiation | 9 | 16 | 0.002a |
|
|
| High
differentiation | 24 | 7 |
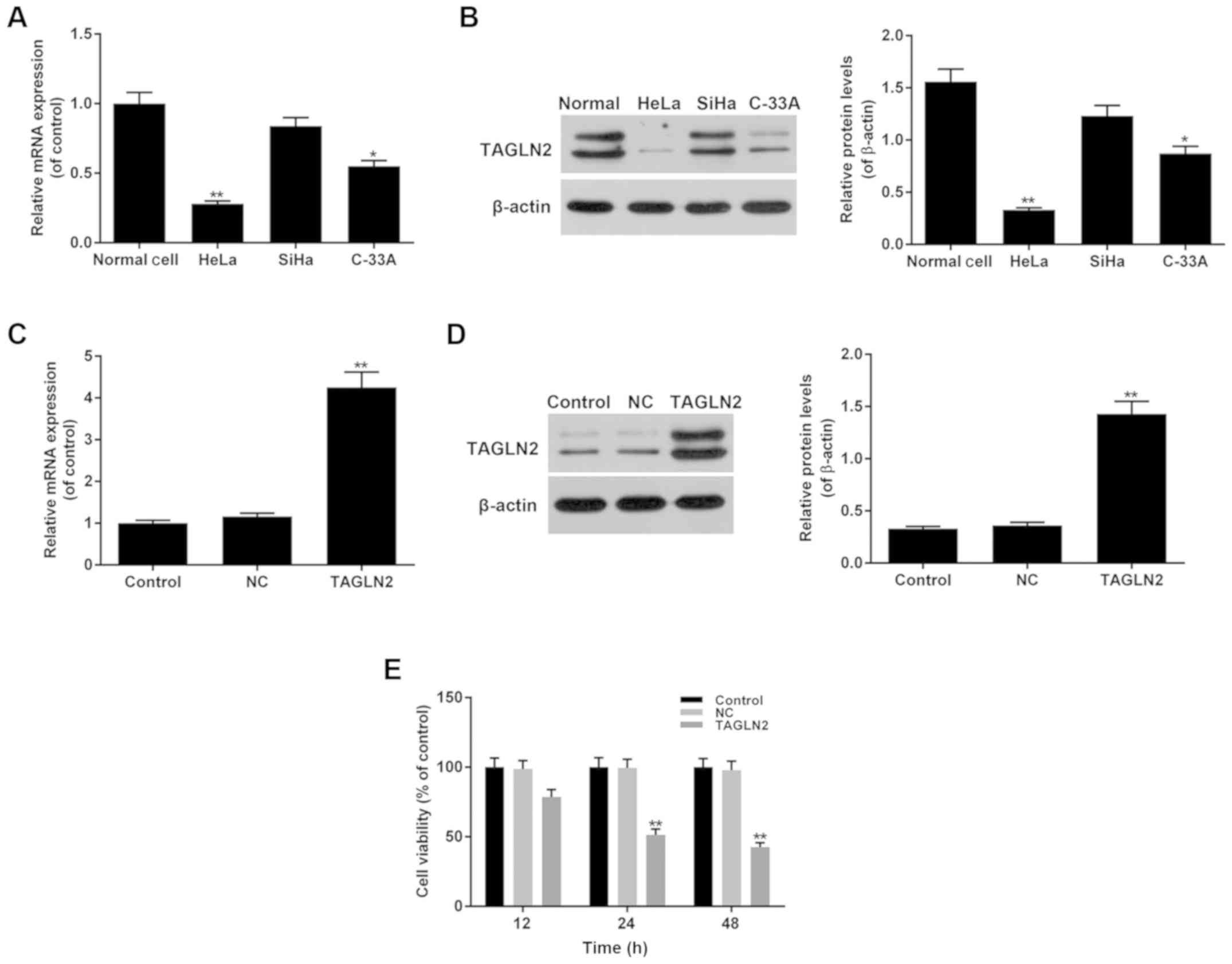
Effects of TAGLN2 on the viability of
cervical cancer cells
The expression levels of TAGLN2 were determined in
three cervical cancer cell lines, including HeLa (HPV-18-positive),
SiHa (HPV-16-positive) and C-33A (HPV-negative). The results
demonstrated that the expression levels of TAGLN2 were
significantly lower in HeLa and C-33A cells, and were markedly
decreased in SiHa cells compared with in normal cervical cells
(Fig. 2A and B; P<0.05). To
investigate the function of TAGLN2 in cervical cancer, a TAGLN2
overexpression model was generated using HeLa cells, which
exhibited the lowest levels of TAGLN2 expression compared with in
SiHa and C-33A cells.
The mRNA and protein expression levels of TAGLN2
were determined following the overexpression of TAGLN2. The data
revealed significantly higher expression levels of TAGLN2 in the
TAGLN2 overexpression group compared with in the NC and control
groups (Fig. 2C and D; P<0.05).
The cell viability of the TAGLN2 overexpression group was evaluated
using a CCK-8 assay; TAGLN2 was revealed to significantly inhibit
cell viability in a time-dependent manner (12, 24 and 48 h),
compared with in the NC and control groups (Fig. 2E; P<0.05).
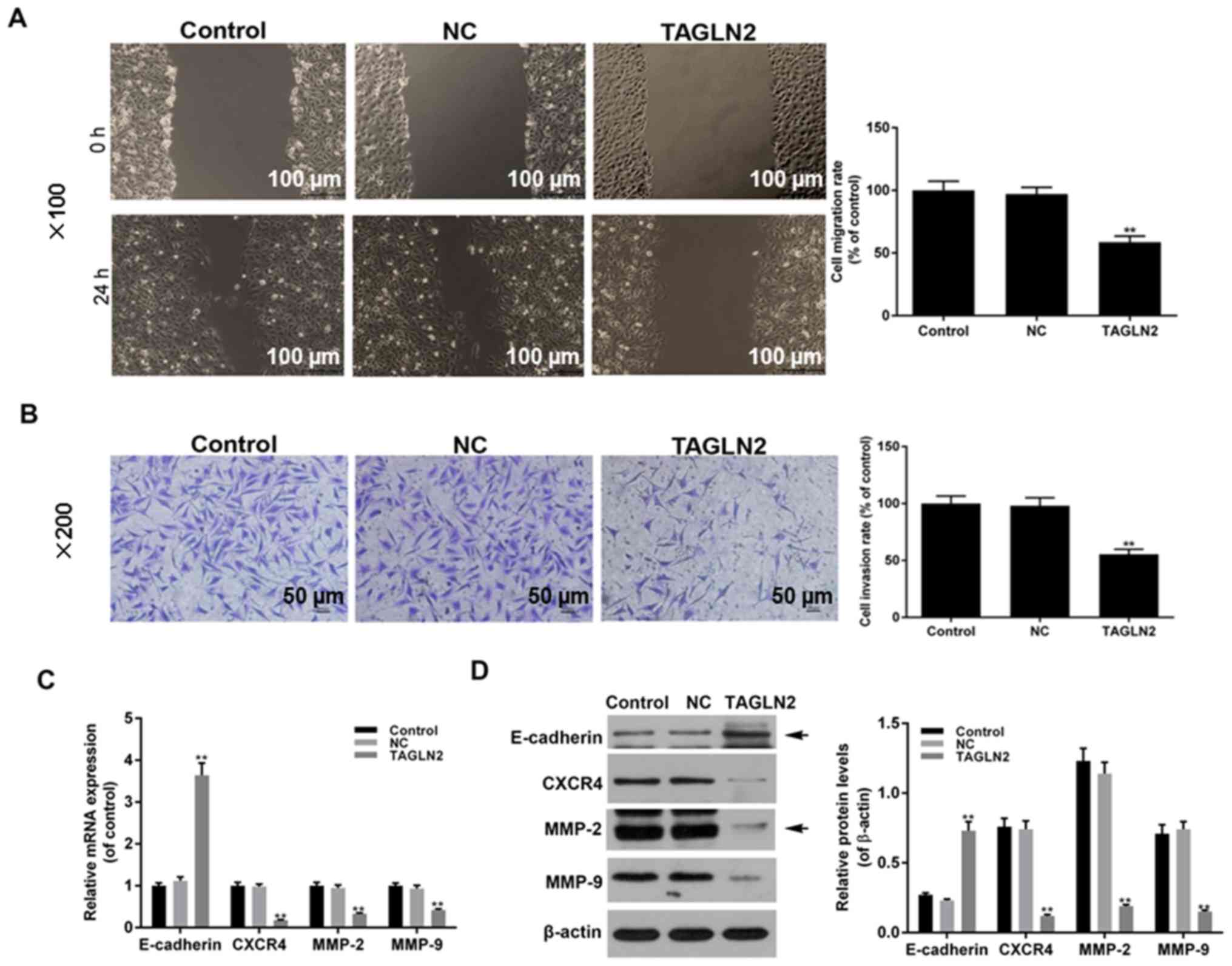
Effects of TAGLN2 on the migratory and
invasive abilities of cervical cancer cells
To further investigate the biological function of
TAGLN2 in cervical cancer cells, cell migratory and invasive
abilities were analyzed following TAGLN2 overexpression via wound
healing and Transwell invasion assays, respectively. The results
demonstrated that cell migration and invasion were significantly
suppressed in the TAGLN2 overexpression group compared with in the
NC and control groups (Fig. 3A and
B; P<0.05). In addition, RT-qPCR and western blotting
demonstrated that the mRNA and protein expression levels of
E-cadherin were significantly increased, whereas those of CXCR4,
MMP-2 and MMP-9 were significantly decreased in the TAGLN2
overexpression group compared with in the NC and control groups
(Fig. 3C and D; P<0.05). These
findings indicated that TAGLN2 overexpression may inhibit cell
migratory and invasive abilities via regulating the expression of
associated factors in HeLa cells.
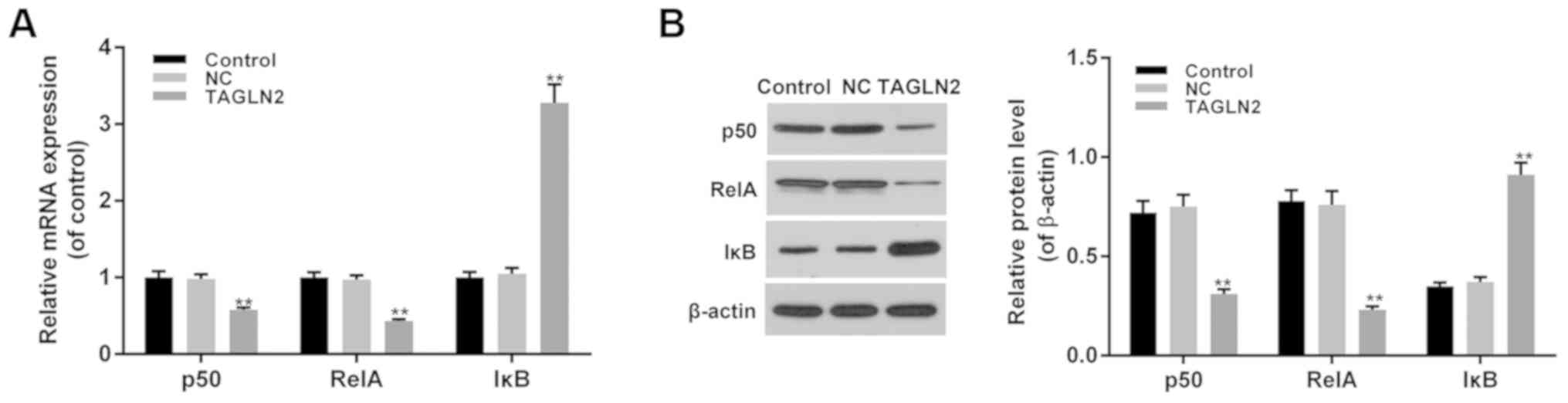
Effects of TAGLN2 on the NF-κB
signaling pathway in cervical cancer cells
In addition to migration- and invasion-associated
factors, the function of TAGLN2 in the NF-κB signaling pathway was
investigated. The results demonstrated that the expression levels
of p50 and RelA were significantly decreased, whereas those of IκB
were significantly increased in the TAGLN2 overexpression group
compared with in the NC and control groups (Fig. 4A and B; P<0.05). These findings
suggested that the inhibitory effects of TAGLN2 on cell metastasis
and invasion of HeLa cervical cancer cells may be associated with
the expression of factors associated with the NF-κB signaling
pathway.
Discussion
Cervical cancer is a malignancy that threatens
female health. The main issue affecting cervical cancer treatment
is that it is difficult to treat metastasizing or recurring
cervical cancer in patients in the advanced stages (19,20).
As a tumor suppressor associated with cancer metastasis (11), the present study proposed that
TAGLN2 may exert tumor-suppressing functions in cervical cancer. A
total of 56 cervical cancer tissue samples, as well as 56
corresponding normal tissues from healthy patients were collected
in the present study. In addition, HeLa cervical cancer cells were
employed to successfully construct a TAGLN2 overexpression cell
model to determine the function of TAGLN2 and its underlying
molecular mechanism.
In the present study, the expression levels of
TAGLN2 were decreased in cervical cancer tissues, and the
expression of TAGLN2 was associated with the clinical features of
cervical cancer. Higher degrees of differentiation and FIGO grades
of cervical cancer were associated with low expression levels of
TAGLN2, which were independent of age. Additionally, patients with
cervical cancer and higher levels of TAGLN2 expression exhibited
longer periods of survival than those with lower TAGLN2 expression
levels. These findings indicated that TAGLN2 may act as a
suppressing factor in cervical cancer.
It has been reported that HPV infection,
particularly with HPV-16 or HPV-18, is a prominent cause of
cervical cancer (21). Therefore,
three representative cervical cancer cell lines: HeLa
(HPV-18-positive), SiHa (HPV-16-positive) and C-33A cells
(HPV-negative), were employed to determine the expression levels of
TAGLN2 in the present study. It was revealed that the expression of
TAGLN2 was decreased in these three cell lines, and that the
expression of TAGLN2 was markedly lower in HeLa and C-33A cells
than in SiHa cells. Therefore, the expression of TAGLN2 may be
partially independent of HPV infection; however, further
investigation is required. To determine the biological mechanism
underlying the suppressive effects of TAGLN2 on cervical cancer, a
TAGLN2 overexpression model was generated in HeLa cells to evaluate
the function of TAGLN2 in cervical cell viability, and migration
and invasion. The results of the present study revealed that
overexpression of TAGLN2 may inhibit the viability of HeLa cells,
and cell migratory and invasive abilities were also suppressed by
TAGLN2. Notably, the reduced migration and invasion of HeLa cells
may be partly explained by reduced cell viability.
Epithelial-mesenchymal transition (EMT) is a
critical mechanism of metastasis and invasion in epithelial cells
(22,23). During EMT, cells lose polarity and
the expression of E-cadherin is reduced. MMPs belong to the family
of calcium-dependent zinc-containing endopeptidases, which serve
roles in degradation of the extracellular matrix, and induce tumor
metastasis and invasion (24,25).
MMP-2 and MMP-9 can induce the degradation of basement membranes by
degrading the main component, type IV collagen, facilitating cell
invasion and tumor metastasis (26). CXCR4 serves critical functions in
the metastasis and invasion of malignant tumors, and is involved in
the process of embryo generation (27,28).
A previous study reported that overexpression of TAGLN2 inhibits
the expression of MMP-9 and suppresses fibrosarcoma cell invasion
(13). These observations are
consistent with those of the present study, in which overexpression
of TAGLN2 was associated with the inhibited migration and invasion
of cervical cancer cells. In addition, overexpression of TAGLN2 was
suggested to promote the expression of E-cadherin, whereas the
expression levels of MMP-2, MMP-9 and CXCR4 were reduced.
The NF-κB signaling pathway is critical for the
regulation of tumor proliferation and metastasis (29,30).
Under normal conditions, IκB binds to NF-κB via its C-terminal
specific ankyrin repeat motif to obstruct the NLS of NF-κB and
suppress its nuclear translocation (31). Classical activation of the NF-κB
signaling pathway involves IκB kinase activation and IκB
phosphorylation, thereby inducing the expression of downstream
genes associated with this particular pathway (32,33).
The most common NF-κB dimers are composed of p50 and RelA (34,35).
In addition, the NF-κB signaling pathway has been reported to
contribute to the progression of cervical cancer (36,37).
In the present study, the NF-κB signaling pathway was activated in
cervical cancer cells; however, overexpression of TAGLN2 inhibited
the expression of RelA and p50, but promoted the expression of IκB,
thus indicating that TAGLN2 suppressed the NF-κB signaling
pathway.
In the present study, the tumor-suppressing role of
TAGLN2 was proposed in cervical cancer cells. Consistently, the
cancer-inhibiting function of TAGLN2 has been reported (11); however, in another study,
downregulation of TAGLN2 inhibited the migration and invasion in
breast cancer cells (38).
Additionally, a previous study revealed that in cervical cancer,
suppression of TAGLN2 inhibited cell migration, MMP secretion and
cell growth in vitro, and reduced tumor size in vivo
(39); variations in observations
may be due to differing cellular conditions. Furthermore, the small
sample size of the current study may affect the accuracy of the
data. Therefore, the role of TAGLN2 requires further in vivo
and in vitro investigation, as well as the use of larger
sample sizes. In addition, a limitation of the present study was
that only TAGLN2 overexpression was performed to explore the role
of TAGLN2 in cervical cancer. Therefore, experiments using TAGLN2
knockdown may also provide important insight.
In conclusion, the expression levels of TAGLN2 were
lower in cervical cancer tissues, which was associated with the
clinical features of patients with CRC. In HeLa cervical cancer
cells (HPV-18-positive), TAGLN2 was revealed to inhibit cell
viability, migration and invasion in the present study. The
biological mechanism underlying the effects of TAGLN2 may be
associated with the EMT process and the regulation of
EMT-associated factors, as well as the NF-κB signaling pathway. The
present study proposed a novel target gene for the diagnosis,
treatment and prognosis of cervical cancer; however, future
investigations regarding the regulation of TAGLN2 in cervical
cancer in vivo are required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and XD designed the study. QZ, XJ and WY
performed the experiments. QZ, XJ and WY performed data analysis.
QZ wrote the manuscript. QZ and XD contributed to manuscript
revisions, and all authors reviewed the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All sample collections were approved by an
institutional review board of the Ethics Committee of The First
Affiliated Hospital of Zhejiang Chinese Medical University
(Hangzhou, China), and informed consent from each patient was
obtained.
Patient consent for publication
Informed consent was obtained from all participants
for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang J and Yue X: Role and importance of
the expression of transcription factor FOXC2 in cervical cancer.
Oncol Lett. 14:6627–6631. 2017.PubMed/NCBI
|
|
2
|
Tsakogiannis D, Moschonas GD, Bella E,
Kyriakopoulou Z, Amoutzias GD, Dimitriou TG, Kottaridi C and
Markoulatos P: Association of p16 (CDKN2A) polymorphisms with the
development of HPV16-related precancerous lesions and cervical
cancer in the Greek population. J Med Virol. 90:965–971. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senapati R, Nayak B, Kar SK and Dwibedi B:
HPV genotypes co-infections associated with cervical carcinoma:
Special focus on phylogenetically related and non-vaccine targeted
genotypes. PLoS One. 12:e01878442017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilmore TD: Introduction to NF-kappaB:
players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Epinat JC and Gilmore TD: Diverse agents
act at multiple levels to inhibit the Rel/NF-kappaB signal
transduction pathway. Oncogene. 18:6896–6909. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gilmore TD: The Rel/NF-kappaB signal
transduction pathway: Introduction. Oncogene. 18:6842–6844. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Assinder SJ, Stanton JA and Prasad PD:
Transgelin: An actin-binding protein and tumour suppressor. Int J
Biochem Cell Biol. 41:482–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi K and Nadal-Ginard B: Molecular
cloning and sequence analysis of smooth muscle calponin. J Biol
Chem. 266:13284–13288. 1991.PubMed/NCBI
|
|
10
|
Prasad PD, Stanton JA and Assinder SJ:
Expression of the actin-associated protein transgelin (SM22) is
decreased in prostate cancer. Cell Tissue Res. 339:337–347. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leung WK, Ching AK, Chan AW, Poon TC, Mian
H, Wong AS, To KF and Wong N: A novel interplay between oncogenic
PFTK1 protein kinase and tumor suppressor TAGLN2 in the control of
liver cancer cell motility. Oncogene. 30:4464–4475. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leinweber BD, Leavis PC, Grabarek Z, Wang
CL and Morgan KG: Extracellular regulated kinase (ERK) interaction
with actin and the calponin homology (CH) domain of actin-binding
proteins. Biochem J. 344:117–123. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nair RR, Solway J and Boyd DD: Expression
cloning identifies transgelin (SM22) as a novel repressor of 92-kDa
type IV collagenase (MMP-9) expression. J Biol Chem.
281:26424–26436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nohata N, Sone Y, Hanazawa T, Fuse M,
Kikkawa N, Yoshino H, Chiyomaru T, Kawakami K, Enokida H, Nakagawa
M, et al: miR-1 as a tumor suppressive microRNA targeting TAGLN2 in
head and neck squamous cell carcinoma. Oncotarget. 2:29–42. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawakami K, Enokida H, Chiyomaru T,
Tatarano S, Yoshino H, Kagara I, Gotanda T, Tachiwada T, Nishiyama
K, Nohata N, et al: The functional significance of miR-1 and
miR-133a in renal cell carcinoma. Eur J Cancer. 48:827–836. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhodes LV, Nitschke AM, Segar HC, Martin
EC, Driver JL, Elliott S, Nam SY, Li M, Nephew KP, Burow ME and
Collins-Burow BM: The histone deacetylase inhibitor trichostatin A
alters microRNA expression profiles in apoptosis-resistant breast
cancer cells. Oncol Rep. 27:10–16. 2012.PubMed/NCBI
|
|
17
|
American College of Obstetricians and
Gynecologists, . ACOG practice bulletin. Diagnosis and treatment of
cervical carcinomas. Number 35, May 2002. American College of
Obstetricians and Gynecologists. Int J Gynecol Obstet. 78:79–91.
2002.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stehman FB, Rose PG, Greer BE, Roy M,
Plante M, Penalver M, Jhingran A, Eifel P, Montz F and Wharton JT:
Innovations in the treatment of invasive cervical cancer. Cancer.
98:2052–2063. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Munagala R, Donà MG, Rai SN, Jenson AB,
Bala N, Ghim SJ and Gupta RC: Significance of multiple HPV
infection in cervical cancer patients and its impact on treatment
response. Int J Oncol. 34:263–271. 2009.PubMed/NCBI
|
|
22
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Radisky DC: Epithelial-mesenchymal
transition. Cancer Res. 118:4325–4326. 2005.
|
|
24
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang TL, Lee LY, Wang CC, Liang Y, Huang
SF and Wu CM: Claudin-4 expression is associated with tumor
invasion, MMP-2 and MMP-9 expression in gastric cancer. Exp Ther
Med. 1:789–797. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith MC, Luker KE, Garbow JR, Prior JL,
Jackson E, Piwnica-Worms D and Luker GD: CXCR4 regulates growth of
both primary and metastatic breast cancer. Cancer Res.
64:8604–8612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tachibana K, Hirota S, Iizasa H, Yoshida
H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N,
Nishikawa S, et al: The chemokine receptor CXCR4 is essential for
vascularization of the gastrointestinal tract. Nature. 393:591–594.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lenardo MJ and Baltimore D: NF-kappaB: A
pleiotropic mediator of inducible and tissue-specific gene control.
Cell. 58:227–229. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thompson JE, Phillips RJ,
Erdjument-Bromage H, Tempst P and Ghosh S: I kappa B-beta regulates
the persistent response in a biphasic activation of NF-kappa B.
Cell. 80:573–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
DiDonato JA, Hayakawa M, Rothwarf DM,
Zandi E and Karin M: A cytokine-responsive IkappaB kinase that
activates the transcription factor NF-kappaB. Nature. 388:548–554.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Greten FR, Arkan MC, Bollrath J, Hsu LC,
Goode J, Miething C, Göktuna SI, Neuenhahn M, Fierer J, Paxian S,
et al: NF-kappaB is a negative regulator of IL-1beta secretion as
revealed by genetic and pharmacological inhibition of IKKbeta.
Cell. 130:918–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rahighi S, Ikeda F, Kawasaki M, Akutsu M,
Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D, et al:
Specific recognition of linear ubiquitin chains by NEMO is
important for NF-kappaB activation. Cell. 136:1098–1109. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ramdass B, Maliekal TT, Lakshmi S, Rehman
M, Rema P, Nair P, Mukherjee G, Reddy BK, Krishna S and
Radhakrishna Pillai M: Coexpression of Notch1 and NF-kappaB
signaling pathway components in human cervical cancer progression.
Gynecol Oncol. 104:352–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma XF, Zhang J, Shuai HL, Guan BZ, Luo X
and Yan RL: IKKβ/NF-κB mediated the low doses of bisphenol A
induced migration of cervical cancer cells. Arch Biochem Biophys.
573:52–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng X, Chen S, Yang Q, Cai J, Zhang W,
You H, Xing J and Dong Y: Salvianolic acid A reverses the
paclitaxel resistance and inhibits the migration and invasion
abilities of human breast cancer cells by inactivating transgelin
2. Cancer Biol Ther. 16:1407–1414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yakabe K, Murakami A, Kajimura T,
Nishimoto Y, Sueoka K, Sato S, Nawata S and Sugino N: Functional
significance of transgelin-2 in uterine cervical squamous cell
carcinoma. J Obstet Gynaecol Res. 42:566–572. 2016. View Article : Google Scholar : PubMed/NCBI
|