Introduction
Osteoarthritis (OA) is a type of degenerative joint
disease that seriously affects the health of the elderly, and is
characterized by articular cartilage degradation and joint
inflammation (1–3). The main clinical manifestations of OA
include recurrent joint pain and gradually increased joint movement
disorder. OA seriously endangers the physical health of middle-aged
and elderly people, and greatly affects patient quality of life,
causing a heavy burden for individuals, families and even society
(4). Articular cartilage
chondrocytes and extracellular matrix (ECM) are the two components
of articular cartilage. As the only cell component in articular
cartilage, the main function of chondrocytes is to maintain the
metabolic homeostasis of the ECM (5,6).
Apoptosis of chondrocytes is one of the main pathological features
of OA (7,8). Clinically, the primary treatment
methods of OA are to relieve joint pain, maintain or improve the
physiological function of the joint, and protect the tissue
structure of the joint; however, the current effects are
unsatisfactory. Therefore, identifying a safe, reliable and
effective OA treatment is an urgently required, long-term task.
Currently, the pathogenesis of OA remains largely unclear; the
study of cartilage damage is important to elucidate the
pathogenesis of OA, providing a theoretical basis for OA prevention
and intervention.
MicroRNAs (miRNAs/miRs), a family of small
non-coding RNAs, 19–25 nucleotides in length, regulate gene
expression by binding to the 3′ untranslated region (3′UTR) of the
target genes (9,10). miRNA is involved in the regulation
of many survival-associated activities such as organ development,
cell proliferation, cell differentiation and apoptosis (9,11,12).
Recently, an increasing body of research has shown that miRNAs
serve an important role in cartilage formation and OA development
(13,14). miR-195-5p, a member of the
miR-15a/b/16/195/497 family, is located on chromosome 17p13.1
(15). In recent years, the role
of miR-195-5p in tumors has been extensively studied (16–19).
However, the function of miR-195-5p in OA is largely unknown.
The purpose of the present study was to investigate
the differential expression level of miR-195-5p in normal and OA
cartilage tissues, and to study the biological effects and
mechanisms of miR-195-5p on LPS induced chondrocyte injury. We hope
to explore novel therapeutic targets and provide new theoretical
basis for the treatment of OA.
Materials and methods
Clinical samples
A total of 30 paired articular cartilage tissue
samples (n=60 samples in total) from 30 OA patients (male, 17;
female, 13; age 59.4±4.2 years) and 30 non-OA patients (no history
of OA or rheumatoid arthritis; male, 16; female, 14; age 59.1±6.2
years) were collected at the Sixth Hospital of Wuhan (Hubei, China)
from March 2016 to March 2017. The present study was approved by
the Ethics Committee of the Sixth Hospital of Wuhan, and all
patients provided written informed consent.
Cell culture and OA cell model
establishment
The murine chondrogenic cell line ATDC5 was obtained
from American Type Culture Collection (Manassas, VA, USA). ATDC5
cells were grown in Dulbecco's modified Eagle's medium/Ham's
Nutrient Mixture F-12 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and 2 mM Glutamine (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 37°C with 5% CO2.
To establish the cell model of OA, ATDC5 cells were
treated with 5 µg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck
KGaA) at 37°C for 5 h. Cells without any treatments were used as
the control.
Cell transfection
Inhibitor control, miR-195-5p inhibitor,
control-small interfering (si)-RNA and the REGγ-siRNA were
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
ATDC5 cells were seeded into a 6-well plate (1×106
cells/well) and cultured at 37°C for 24 h. Then, ATDC5 cells were
transfected with 100 nM miR-195-5p inhibitor
(5′-GCCAAUAUUUCUGUGCUGCUA-3′), 100 nM inhibitor control
(5′-CAGUACUUUUGUGUAGUACAA-3′), 2 µl control-siRNA
[5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and
5′-ACGUGACACGUUCGGAGAATT-3′ (antisense)], 2 µl REGγ-siRNA
[5′-CAGAAGACUUGGUGGCAAATT-3′ (sense) and
5′-UUUGCCACCAAGUCUUCUGTT-3′ (antisense)] or 100 nM miR-195-5p
inhibitor+2 µl REGγ-siRNA using Lipofectamine 3000®
regent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. At 24 h post-cell transfection,
transfection efficiency was detected via reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
MTT assay
ATDC5 cells (5×103 cells/well) were
cultured in 96-well plates. At 24 h post-transfection with either
miR-195-5p inhibitor, inhibitor control or miR-195-5p
inhibitor+REGγ-siRNA at 37°C, the cells were treated with LPS for 5
h at 37°C. Cells without LPS treatment were used as the control
group. Following stimulation, MTT solution (20 µl/well) was added
into the culture medium. Then the plates were incubated for 4 h at
37°C in humidified 95% air and 5% CO2. Then dimethyl
sulfoxide (150 µl) was used to dissolve the purple formazan. Cell
proliferation ability was assessed by measuring the absorbance at
490 nm using a Microplate Reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Apoptosis assay
To analyze cell apoptosis, flow cytometry was
performed with the Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis Detection kit (cat. no.
70-AP101-100; Hangzhou MultiSciences Biotech, Co., Ltd., Hangzhou,
China). Briefly, following the specific treatments, ATDC5 cells
(1×105 cells/well) were collected and washed with cold
PBS. Then the cells were stained with FITC-conjugated Annexin V and
propidium iodide without light according to the manufacturer's
instructions. Finally, the number of apoptotic cells was determined
using a flow cytometer (BD Biosciences; Becton-Dickinson and
Company, Franklin Lakes, NJ, USA), and FlowJo software version
7.6.1 (FlowJo LLC, Ashland, OR, USA) was used for data
analysis.
Enzyme-linked immunosorbent assay
(ELISA)
To detect the levels of IL-1β, IL-6 and TNF-α in
cell supernatants, ELISA was performed. Briefly, the cell culture
supernatant was collected by centrifugation (1,000 × g at 4°C for
10 min), then the concentrations of the inflammatory cytokines
(IL-1β, cat. no. ab100704; IL-6, cat. no. ab100712 and TNF-α, cat.
no. ab208348) were determined by ELISA (Abcam, Cambridge, UK)
according to the manufacturer's instructions of each kit.
RT-qPCR
The total RNA was extracted from tissues and cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
as per the manufacturer's instructions. Then the TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to perform the RT experiment.
Amplification conditions for RT experiment were 50°C for 15 min and
85°C for 2 min. Finally, the synthesized cDNAs were analyzed using
TaqMan Universal Master Mix II (Applied Biosystems; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocols.
Amplification conditions were: 10 min at 95°C, followed by 35
cycles of 15 sec at 95°C and 40 sec at 55°C. GAPDH and U6 were used
in the present study for normalizing mRNA and miRNA levels. Primer
sequences were as following: GAPDH forward,
5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; TNF-α forward,
5′-GAACTGGCAGAAGAGGCACT-3′ and reverse, 5′-GGTCTGGGCCATAGAACTGA-3′;
IL-1β forward, 5′-TGTGAAATGCCACCTTTTGA-3′ and reverse,
5′-TGAGTGATACTGCCTGCCTG-3′; IL-6 forward,
5′-CCGGAGAGGAGACTTCACAG-3′ and reverse, 5′-CAGAATTGCCATTGCACA-3′;
miR-195-5p forward, 5′-GGGGTAGCAGCACAGAAAT-3′ and reverse,
5′-TCCAGTGCGTGTCGTGGA-3′; REGγ forward,
5′-AAGGTTGATTCTTTCAGGGAGC-3′ and reverse,
5′-AGTGGATCTGAGTTAGGTCATGG-3′. Relative gene expressions were
calculated by the 2−ΔΔCq method (20).
Dual luciferase activity assay
The targets of miR-195-5p were predicted using
TargetScan bioinformatics software 7.1 (www.targetscan.org/vert_71), and REGγ was revealed to
be a potential target of miR-195-5p. To verify this prediction, the
wild-type (REGγ-WT) and mutant (REGγ-MUT) 3′UTR of REGγ were cloned
into a pmiR-RB-Report™ dual luciferase reporter gene
plasmid vector (Guangzhou RiboBio Co., Ltd.). ATDC5 cells
(5×104 cells/well) were co-transfected with 100 ng
REGγ-WT or 100 ng REGγ-MUT and 50 nM miR-195-5p mimic
(5′-UAGCAGCACAGAAAUAUUGGC-3′) or 50 nM mimic control
(5′-UUCUCCGAACGUGUCACGUTT-3′) using Lipofectamine 3000®
regent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. At 48 h post-transfection, the
dual-luciferase assay system (Promega Corporation, Madison, WI,
USA) was used to determine luciferase activity, which was
normalized to Renilla luciferase activity.
Western blot assay
Whole cell proteins were extracted from cells
(5×105 cells/well) using Radioimmunoprecipitation Assay
Lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China)
according to the manufacturer's protocols. The protein samples were
quantified using the BCA™ Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Western blotting was performed using a Bio-Rad
Bis-Tris Gel system (Bio-Rad Laboratories, Inc.) following the
manufacturer's instructions. Briefly, protein samples (30 µg/lane)
were separated by 12% SDS-PAGE and then transferred onto
polyvinylidene difluoride membranes. Then the membranes were
blocked with 5% non-fat milk at room temperature for 1.5 h,
incubated with primary antibodies at 4°C overnight, and
subsequently incubated with a secondary antibody at room
temperature for 4 h. The primary antibodies used in this study were
REGγ (cat. no. ab157157; 1:1,000; Abcam), Bcl-2 (cat. no. 4223;
1:1,000), Bax (cat. no. 14796; 1:1,000), β-catenin (cat. no. 25362;
1:1,000), c-Myc (cat. no. 5605; 1:1,000), Cyclin D1 (cat. no. 2978;
1:1,000), p-p65 (cat. no. 3033; 1:1,000) and β-actin (cat. no.
4970; 1:1,000) (all from Cell Signaling Technology, Inc., Danvers,
MA, USA). The horseradish peroxidase-conjugated secondary antibody
was obtained from Cell Signaling Technology, Inc. (cat. no. 7074;
1:2,000). At the end of this experiment, protein bands were
visualized using the enhanced chemiluminescence detection system
(Super Signal West Dura Extended Duration Substrate; Pierce; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Gel-Pro Analyzer densitometry software (version 6.3;
Media Cybernetics, Inc., Rockville, MD, USA) was used for band
density quantification.
Statistical analysis
All experiments were repeated three times. The data
were presented as the mean ± standard deviation. Statistical
analyses were carried out using SPSS 17.0 statistical software
(SPSS Inc., Chicago, IL, USA). Comparisons between two groups were
evaluated by Student's t-test, and comparisons between multiple
groups were analyzed using one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered statistically
significant.
Results
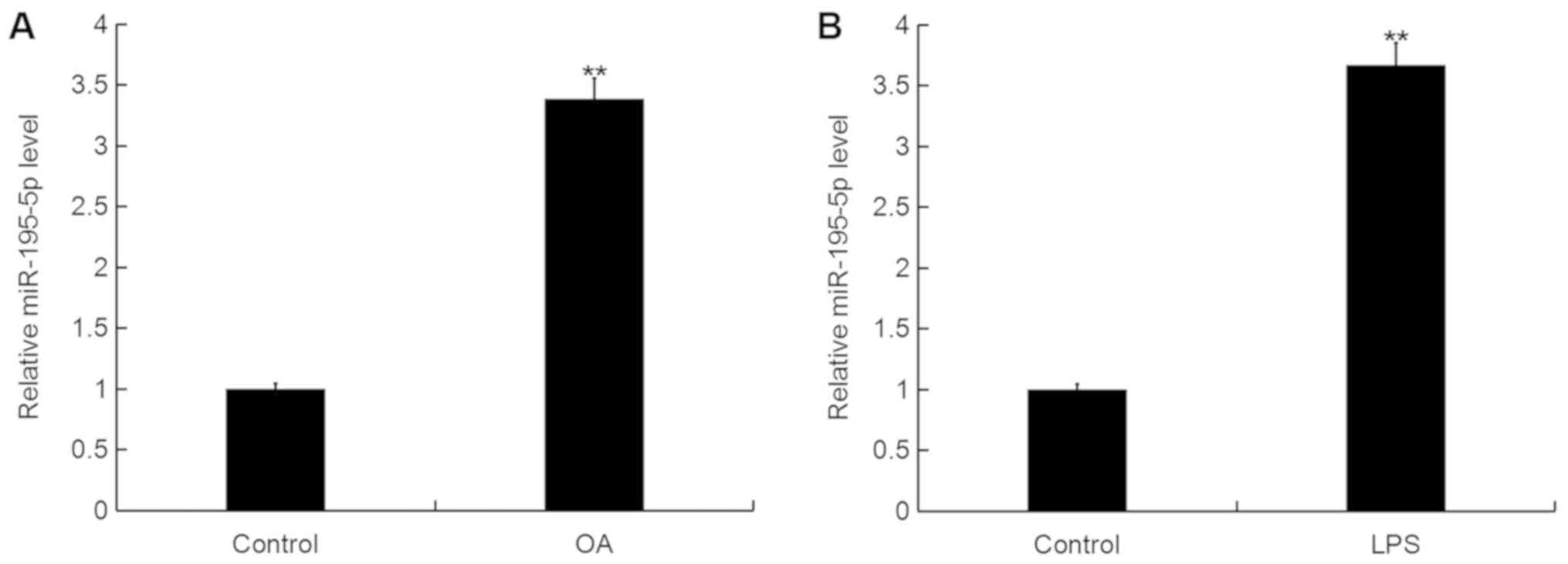
miR-195-5p is upregulated in OA
To determine the miR-195-5p expression level in OA,
RT-qPCR was performed. As shown in Fig. 1A, when compared with the control
group, the level of miR-195-5p was significantly upregulated in the
articular cartilage tissues of patients with OA. In addition, the
level of miR-195-5p in the murine chondrogenic cell line ATDC5 was
significantly increased following LPS treatment for 5 h in
comparison with the control group (Fig. 1B).
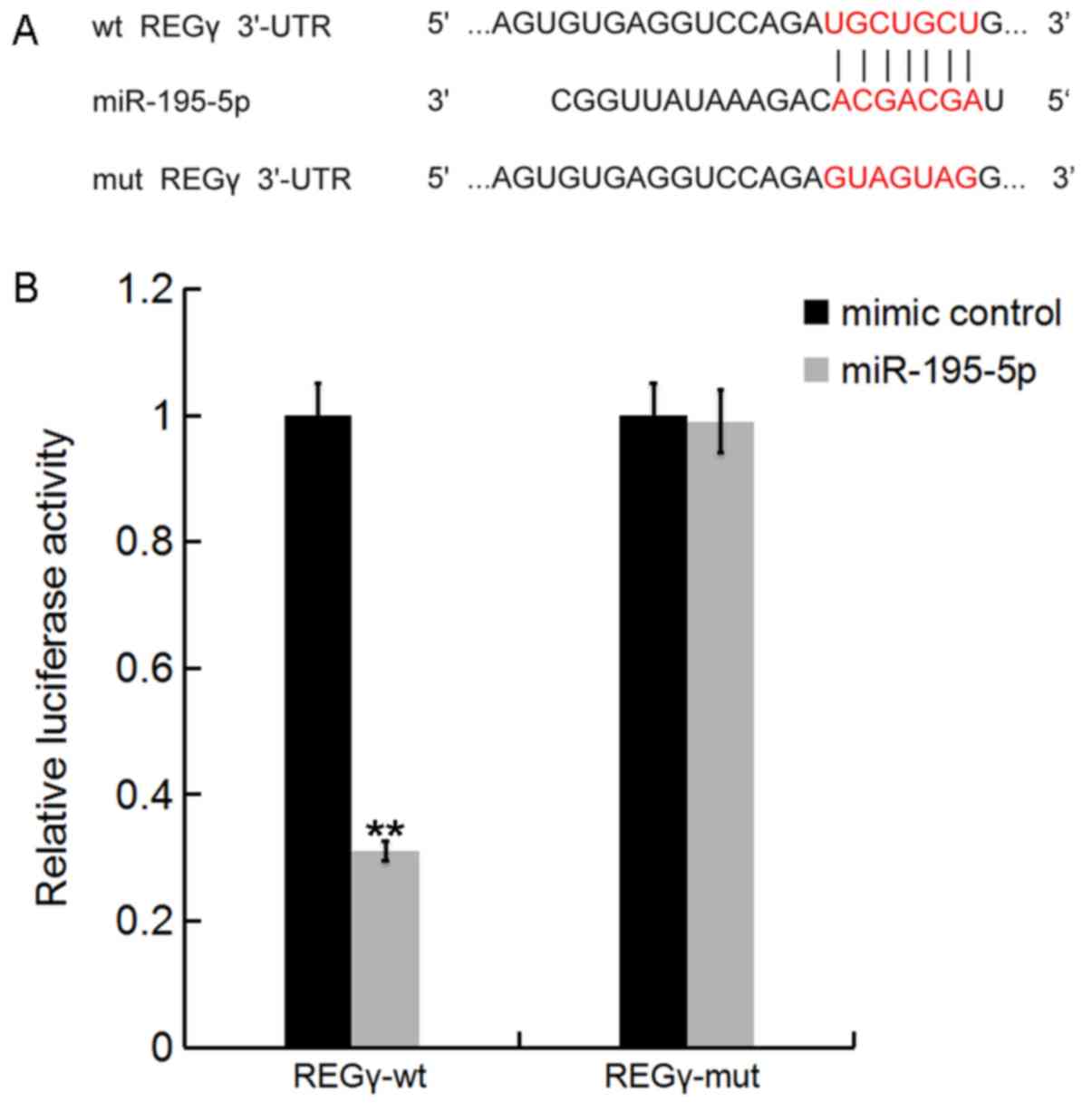
REGγ is a target of miR-195-5p
Bioinformatics tools on TargetScan (www.targetscan.org) were used to predict the potential
targets of miR-195-5p. The results revealed that REGγ was a
potential target for miR-195-5p (Fig.
2A). To determine whether miR-195-5p directly modulated REGγ
expression via interactions with potential binding sites, a
luciferase reporter assay was performed. As shown in Fig. 2B, compared with co-transfection
with REGγ-MUT and miR-195-5p mimic, luciferase activity was
markedly decreased by co-transfection with REGγ-WT and miR-195-5p
mimic. The results indicated that miR-195-5p directly targeted
REGγ.
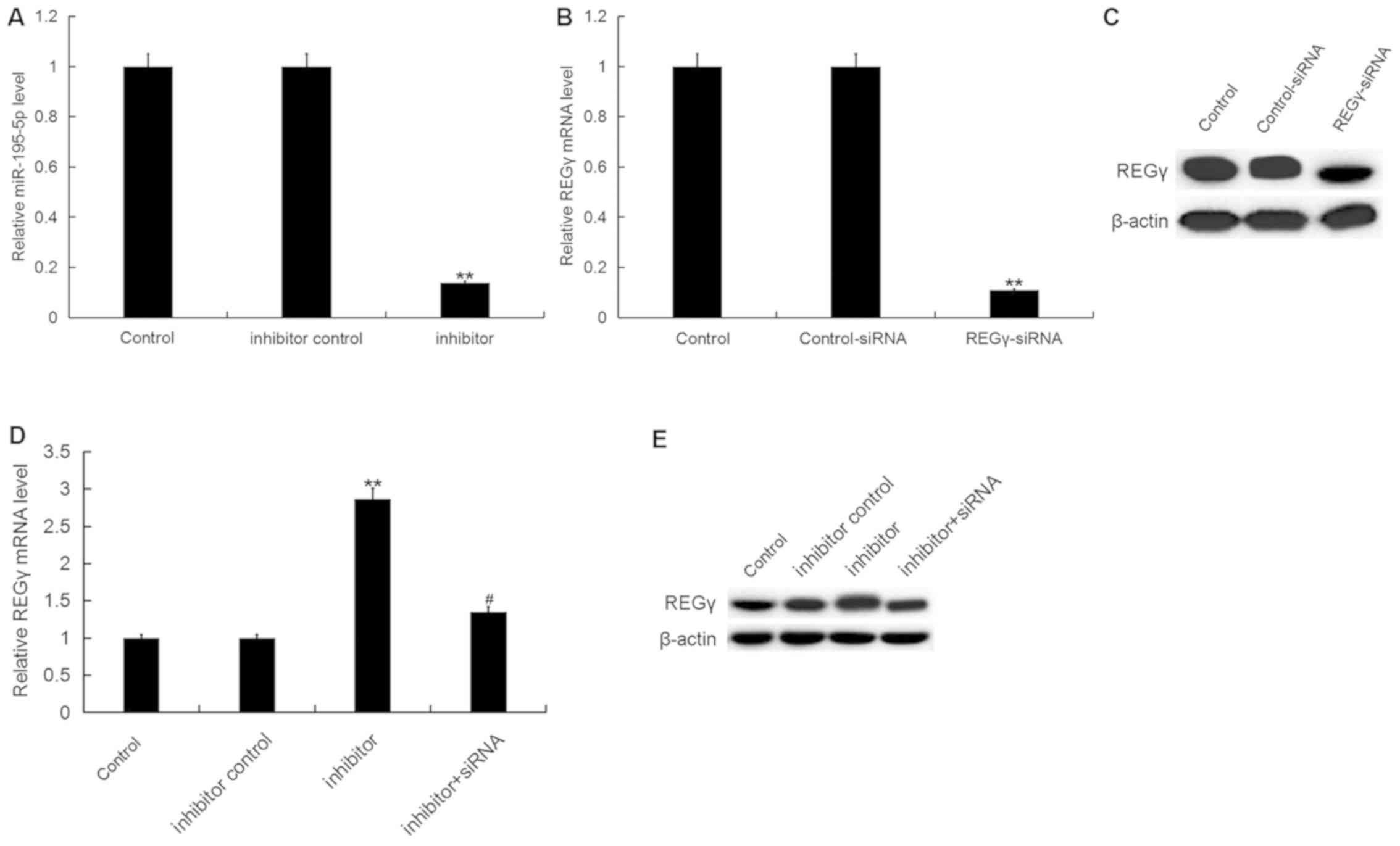
miR-195-5p inhibitor enhances the
ATDC5 cell proliferation ability inhibited by LPS
To investigate the effect of miR-195-5p on OA, the
present study firstly transfected ATDC5 cells with miR-195-5p
inhibitor, inhibitor control, control-siRNA, REGγ-siRNA or
miR-195-5p inhibitor+REGγ-siRNA respectively, and the transfection
efficiency was detected 24 h following cell transfection. As shown
in Fig. 3A, compared with the
control group, miR-195-5p inhibitor significantly downregulated the
miR-195-5p level in ATDC5 cells. REGγ-siRNA significantly reduced
the protein and mRNA levels of REGγ in ATDC5 cells (Fig. 3B and C). In addition, the
miR-195-5p inhibitor significantly upregulated the protein and mRNA
levels of REGγ, and this upregulation was attenuated by REGγ-siRNA
(Fig. 3D and E).
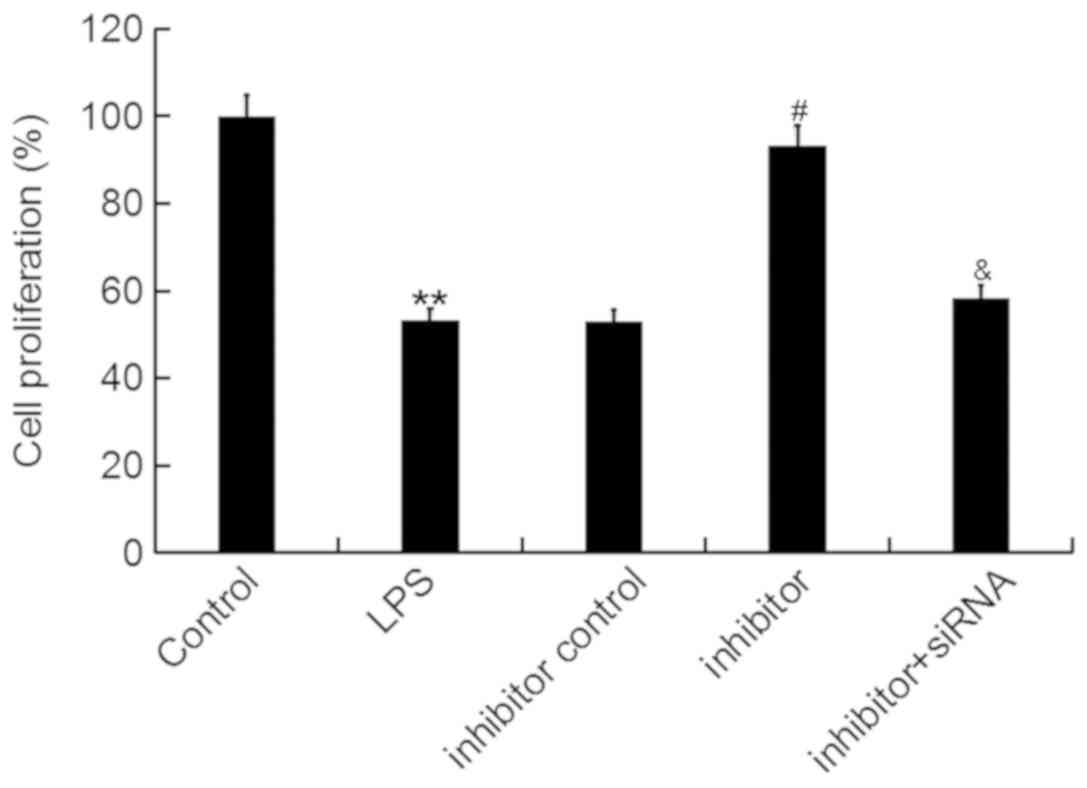
Then, an MTT assay was conducted to determine the
effect of miR-195-5p on ATDC5 cell proliferation ability. The data
showed that the miR-195-5p inhibitor significantly enhanced the
proliferation ability of ATDC5 cells, which was inhibited by LPS
treatment, and this enhancement was markedly reversed by REGγ-siRNA
(Fig. 4).
miR-195-5p inhibitor reduces the ATDC5
cell apoptosis induced by LPS
The present study further investigated the effect of
miR-195-5p on ATDC5 cell apoptosis via flow cytometry. The results
indicated that LPS treatment significantly induced ATDC5 cell
apoptosis, but this increase in cell apoptosis was inhibited by the
miR-195-5p inhibitor. Notably, the results also indicated that
REGγ-siRNA significantly eliminated the effect of the miR-195-5p
inhibitor on ATDC5 cell apoptosis (Fig. 5A and B). In addition, we the levels
of increased Bax and decreased Bcl-2 levels induced by LPS
treatment were reversed by the miR-195-5p inhibitor. Furthermore,
REGγ-siRNA significantly eliminated the effect of the miR-195-5p
inhibitor on the expression of Bcl-2 and Bax in LPS treated ATDC5
cells (Fig. 5C).
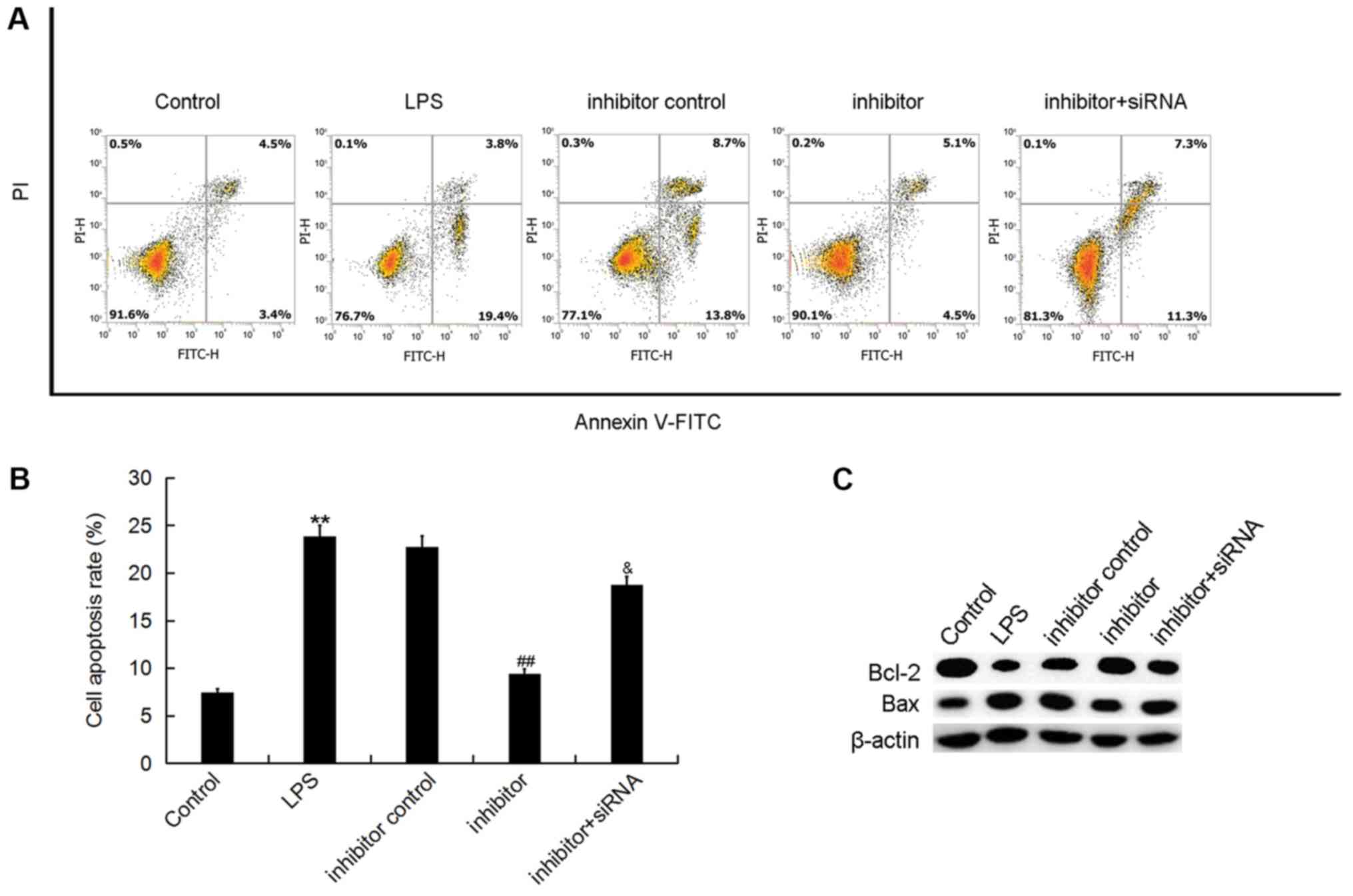 | Figure 5.Effect of miR-195-5p inhibitor on
ATDC5 cell apoptosis. At 24 h post-transfection with either the
miR-195-5p inhibitor, inhibitor control, or miR-195-5p
inhibitor+REGγ-siRNA at 37°C, the cells were treated with LPS for 5
h. Cells without any treatments were used as the control group.
Then, (A) ATDC5 cell apoptosis was assessed by using flow cytometry
and (B) the cell apoptosis rate was calculated. (C) The protein
levels of Bax and Bcl-2 were determined by western blotting. Data
were displayed as the mean ± standard deviation. **P<0.01 vs.
control group; ##P<0.01 vs. LPS group;
&P<0.05 vs. inhibitor group. miR, microRNA;
siRNA, small interfering RNA; LPS, lipopolysaccharide; Bcl-2,
B-cell lymphoma-2; Bax, Bcl-2-associated X; FITC, fluorescein
isocyanate; PI, propidium iodide. |
miR-195-5p inhibitor prevents the
inflammatory response in ATDC5 cells induced by LPS
The present study then investigated whether
miR-195-5p has an effect on the inflammatory response during OA
development. The results demonstrated that the mRNA and protein
levels of IL-1β, IL-6 and TNF-α were significantly enhanced by LPS
stimulation. The miR-195-5p inhibitor significantly reduced the
expression levels of IL-1β, IL-6 and TNF-α, and these reductions
were reversed by REGγ downregulation (Fig. 6). These results indicated that the
miR-195-5p inhibitor prevented the inflammatory response in ATDC5
cells induced by LPS.
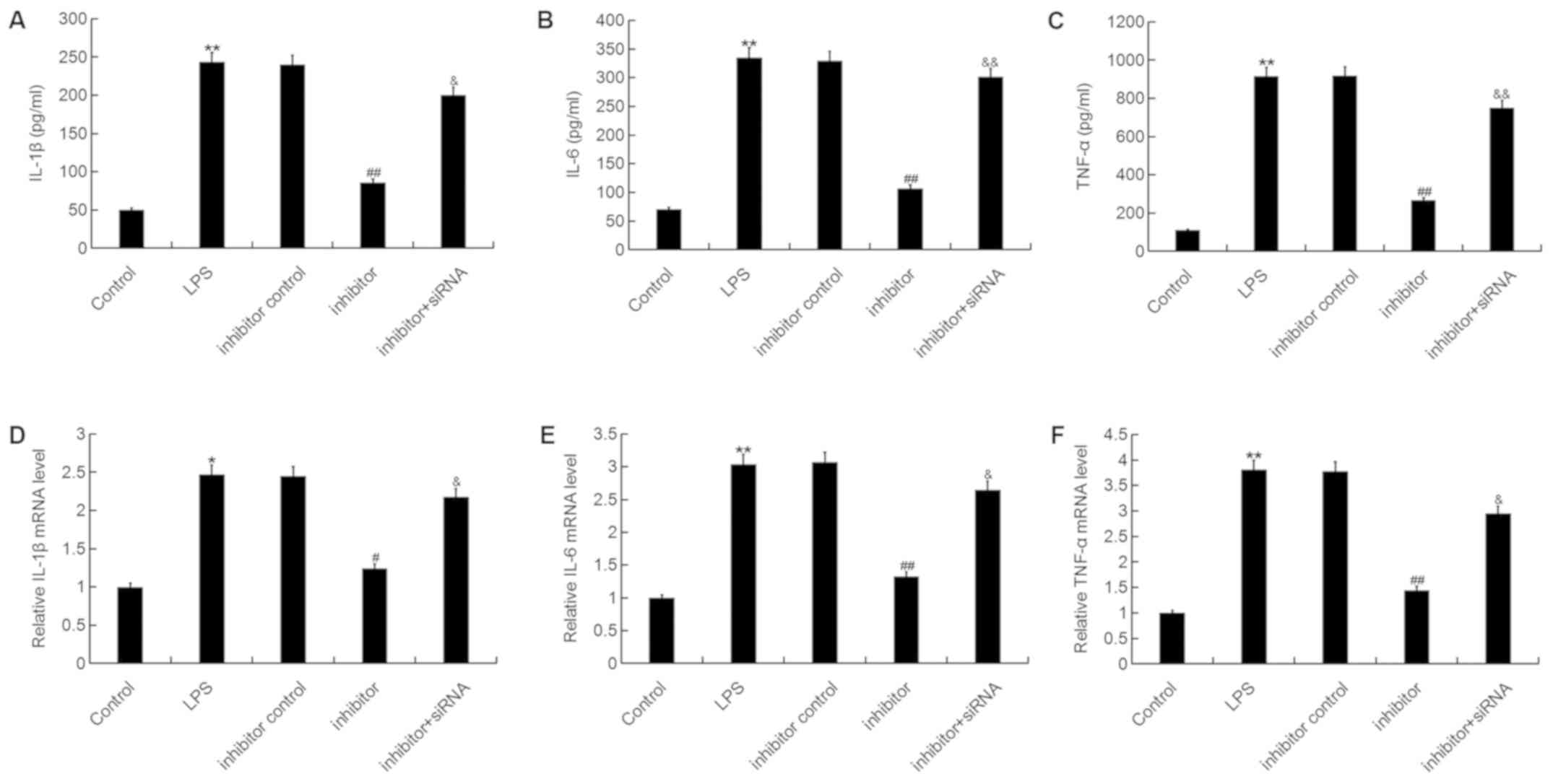 | Figure 6.Effect of the miR-195-5p inhibitor on
inflammatory factor expression in ATDC5 cells. At 24 h
post-transfection with either the miR-195-5p inhibitor, inhibitor
control, or miR-195-5p inhibitor+REGγ-siRNA at 37°C, the cells were
treated with LPS for 5 h. Cells without any treatment were used as
the control group. Then, the (A-C) protein and (D-F) mRNA levels of
(A and D) IL-1β, (B and E) IL-6 and (C and F) TNF-α in ATDC5 cells
were detected by reverse transcription-quantitative polymerase
chain reaction and ELISA assay. Data were presented as the mean ±
standard deviation. *P<0.05 and **P<0.01 vs. control group;
#P<0.05 and ##P<0.01 vs. LPS group;
&P<0.05 and &&P<0.01 vs.
inhibitor group. miR, microRNA; siRNA, small interfering RNA; LPS,
lipopolysaccharide; IL, interleukin; TNF, tumor necrosis
factor. |
miR-195-5p inhibitor represses
LPS-induced Wnt/β-catenin and nuclear factor (NF)-κB signaling
pathway activation in ATDC5 cells
Finally, to explore the molecular mechanism
underlying the effects of the miR-195-5p inhibitor on ATDC5 cells,
the Wnt/β-catenin and NF-κB signaling pathways were analyzed. As
shown in Fig. 7, LPS stimulation
significantly decreased the protein expression of REGγ, β-catenin,
c-Myc and Cyclin D1, and enhanced the phosphorylation of NF-κB
[phosphorylated (p)-p65] in ATDC5 cells, while the miR-195-5p
inhibitor markedly increased REGγ, β-catenin, c-Myc and Cyclin D1
protein expression and reduced p-NF-κB (p-p65) protein expression.
As expected, REGγ-siRNA significantly attenuated the effect of the
miR-195-5p inhibitor on REGγ, β-catenin, c-Myc, Cyclin D1 and p-p65
expression in ATDC5 cells.
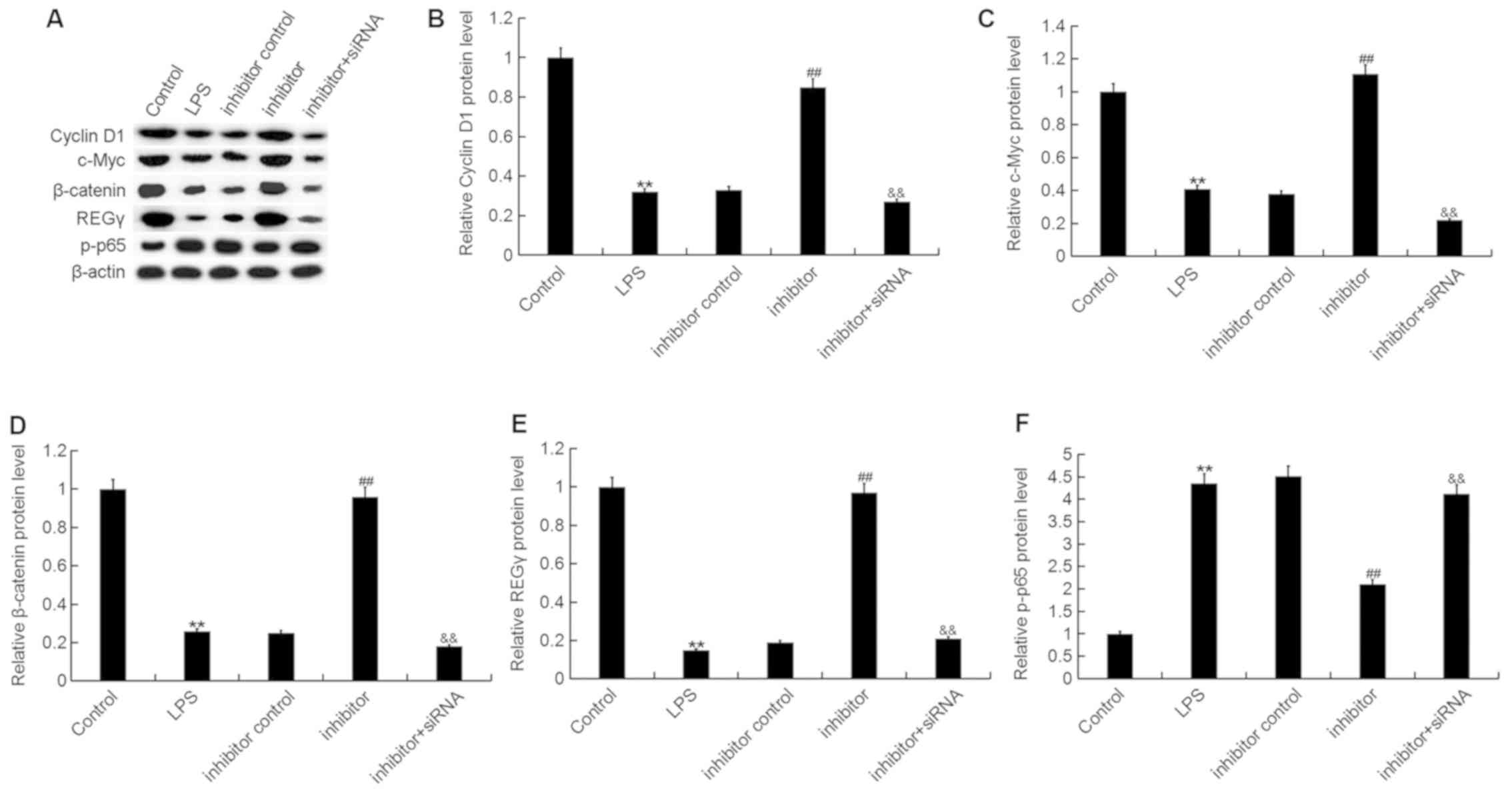 | Figure 7.Effect of the miR-195-5p inhibitor on
Wnt/β-catenin and NF-κB signaling pathway activation in ATDC5
cells. At 24 h post-transfection with either the miR-195-5p
inhibitor, inhibitor control, or miR-195-5p inhibitor+REGγ-siRNA at
37°C, the cells were treated with LPS for 5 h. Cells without any
treatment were used as the control group. (A) Then, the protein
levels of (B) Cyclin D1, (C) c-Myc, (D) β-catenin, (E) REGγ and (F)
p-p65 were detected using western blot assay and Gel-Pro Analyzer
densitometry software was used for band density quantification.
Data were displayed as the mean ± standard deviation. **P<0.01
vs. control group; ##P<0.01 vs. LPS group;
&&P<0.01 vs. inhibitor group. miR, microRNA;
siRNA, small interfering RNA; LPS, lipopolysaccharide; p-,
phosphorylated. |
Discussion
In the present study, miR-195-5p expression was
significantly increased in patients with OA and the OA cell model.
miR-195-5p downregulation repressed the effects of LPS on reduced
cell proliferation ability, enhanced cell apoptosis and increased
inflammation-associated factor expression by targeting REGγ. In
addition, the results suggested that miR-195-5p downregulation
inhibited LPS-induced Wnt/β-catenin signaling pathway repression
and activation of the NF-κB signaling pathway in ATDC5 cells. The
results of the present study indicated that the miR-195-5p
inhibitor protected chondrocytes from LPS-induced injury, therefore
it may be a promising therapeutic target for OA treatment.
OA is an orthopedic multiple degenerative
inflammatory disease. The main pathological manifestation of OA is
the destruction of the structural and functional integrity of
articular cartilage and the inflammatory response (21). The onset of OA is a complex process
involving multiple factors. Currently, the etiology and
pathogenesis of OA are not fully understood (22). Chondrocytes serve a very important
role in the pathogenesis of OA (23). Therefore, studies on chondrocyte
injury have potential significance for identifying effective
methods for treating OA.
Recently, an increasing body of evidence has
revealed that miRNAs serve an important role in cartilage formation
and OA development (13,14). miR-195-5p, a member of the
miR-15a/b/16/195/497 family, is currently extensively studied in
the development of tumors (16–19).
However, the role of miR-195-5p in OA is unknown. Therefore, we
conducted the present study.
The present study firstly detected the expression
level of miR-195-5p in OA, and the results revealed that miR-195-5p
was significantly upregulated in the articular cartilage tissues of
patients with OA and in the LPS-induced OA cell model, indicating
the potential role of miR-195-5p in OA. Then, it was identified
that REGγ, a proteasome activator, was a direct target of
miR-195-5p.
During the progression of OA, areas with severe
matrix degradation and destruction are often associated with the
excessive apoptosis of chondrocytes (24). The excessive apoptosis of
chondrocytes is considered to be one of the key factors in the
pathogenesis of OA (25). The
present study demonstrated that the inhibited cell proliferation
ability, and increased cell apoptosis of chondrocytes induced by
LPS treatment were inhibited by miR-195-5p downregulation. Another
important discovery was that REGγ knockdown significantly
eliminated the effect of miR-195-5p on chondrocyte apoptosis.
Chondrocytes can promote cartilage degradation
through the secretion of inflammatory factors (26). A large number of cytokines,
especially inflammatory factors, are involved in the regulation and
maintain the dynamic balance of articular cartilage. Inflammatory
factors can stimulate cascades in many ways, continuously
stimulating chondrocytes to secrete cytokines, which in turn
triggers OA (27–29). In the present study, LPS
significantly enhanced the levels of IL-1β, IL-6 and TNF-α, while
miR-195-5p inhibitor reversed these enhancements. It is worth
mentioning that the effect of the miR-195-5p inhibitor on the
expression of inflammatory factors in chondrocytes was eliminated
by REGγ silencing.
Finally, to explore the underlying mechanism of the
effects of the miR-195-5p inhibitor on LPS-induced chondrocyte
injury, the Wnt/β-catenin and NF-κB signaling pathways were
analyzed. The results revealed that the miR-195-5p inhibitor
inhibited the LPS-induced Wnt/β-catenin signaling pathway
repression and NF-κB signaling pathway activation in chondrocytes,
and this inhibition was reversed by REGγ knockdown.
In conclusion, to the best of our knowledge the
present study has reported for the first time that miR-195-5p was
abnormally high in OA, and its inhibition could inhibit chondrocyte
apoptosis and the inflammatory response in an OA cell model by
targeting REGγ. miR-195-5p may be a novel and promising therapeutic
target for the treatment of OA. However, this is a preliminary
study of the role of miR-195-5p in OA, and in order to make the
role of miR-195-5p in OA more convincing, a lot of further research
is still required. For example, the effect of miR-195-5p
overexpression on the OA cell model still requires investigation.
In addition, the expression of REGγ in the articular cartilage
tissues of patients with OA and the LPS-induced cell model should
be revealed. Furthermore, the in vitro study of OA is
significantly different from the in vivo OA in humans, and
some in vivo and clinical studies of miR-195-5p in OA are
required to confirm the role of miR-195-5p in OA. In the future, we
will perform in-depth research on these issues.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS contributed to study design and data collection;
JL contributed to statistical analysis; WG contributed to data
interpretation; WY contributed to manuscript preparation and
statistical analysis.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Sixth Hospital of Wuhan, and all patients provided
written informed consent.
Patient consent for publication
Informed patient consent was obtained to
publish.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnson VL and Hunter DJ: The epidemiology
of osteoarthritis. Best Pract Res Clin Rheumatol. 28:5–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burr DB and Gallant MA: Bone remodelling
in osteoarthritis. Nat Rev Rheumatol. 8:665–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salmon JH, Rat AC, Sellam J, Michel M,
Eschard JP, Guillemin F, Jolly D and Fautrel B: Economic impact of
lower-limb osteoarthritis worldwide: A systematic review of
costof-illness studies. Osteoarthritis Cartilage. 24:1500–1508.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin J, Shang L, Ping AS, Li J, Li XJ, Yu
H, Magdalou J, Chen LB and Wang H: Response to ‘TNF/TNFR signal
transduction pathway-mediated anti-apoptosis and anti-inflammatory
effects of sodium ferulate on IL-1β-induced rat osteoarthritis
chondrocytes in vitro’-authors' reply. Arthritis Res Ther.
15:4092013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HA, Lee YJ, Seong SC, Choe KW and Song
YW: Apoptotic chondrocyte death in human osteoarthritis. J
Rheumatol. 27:455–462. 2000.PubMed/NCBI
|
|
8
|
Yan S, Wang M, Zhao J, Zhang H, Zhou C,
Jin L, Zhang Y, Qiu X, Ma B and Fan Q: MicroRNA-34a affects
chondrocyte apoptosis and proliferation by targeting the SIRT1/p53
signaling pathway during the pathogenesis of osteoarthritis. Int J
Mol Med. 38:201–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim J, Yao F, Xiao Z, Sun Y and Ma L:
MicroRNAs and metastasis: Small RNAs play big roles. Cancer
Metastasis Rev. 37:5–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujii T, Shimada K, Nakai T and Ohbayashi
C: MicroRNAs in smoking-related carcinogenesis: Biomarkers,
functions, and therapy. J Clin Med. 7:E982018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu C, Tian B, Qu X, Liu F, Tang T, Qin A,
Zhu Z and Dai K: MicroRNAs play a role in chondrogenesis and
osteoarthritis (Review). Int J Mol Med. 34:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trachana V, Ntoumou E, Anastasopoulou L
and Tsezou A: Studying microRNAs in osteoarthritis: Critical
overview of different analytical approaches. Mech Ageing Dev.
171:15–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He JF, Luo YM, Wan XH and Jiang D:
Biogenesis of miRNA-195 and its role in biogenesis, the cell cycle,
and apoptosis. J Biochem Mol Toxicol. 25:404–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Zhang X, Zou C, Kung HF, Lin MC,
Dress A, Wardle F, Jiang BH and Lai L: miR-195 inhibits tumor
growth and angiogenesis through modulating IRS1 in breast cancer.
Biomed Pharmacother. 80:95–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo J, Wang M and Liu X: MicroRNA-195
suppresses tumor cell proliferation and metastasis by directly
targeting BCOX1 in prostate carcinoma. J Exp Clin Cancer Res.
34:912015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M, Zhang J, Tong L, Ma X and Qiu X:
miR-195 is a key negative regulator of hepatocellular carcinoma
metastasis by targeting FGF2 and VEGFA. Int J Clin Exp Pathol.
8:14110–14120. 2015.PubMed/NCBI
|
|
19
|
Chen S, Wang L, Yao X, Chen H, Xu C, Tong
L, Shah A, Huang T, Chen G, Chen J, et al: miR-195-5p is critical
in REGγ-mediated regulation of wnt/β-catenin pathway in renal cell
carcinoma. Oncotarget. 8:63986–64000. 2017.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taruc-Uy RL and Lynch SA: Diagnosis and
treatment of osteoarthritis. Prim Care. 40821–836. (vii)2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Creamer P and Hochberg MC: Osteoarthritis.
Lancet. 350:503–508. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanded LJ and Aigner T: Articular
cartilage and changes in arthritis. An introduction: Cell biology
of osteoarthritis. Arthritis Res. 3:107–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Héraud F, Héraud A and Harmand M:
Apoptosis in normal and human articular cartilage. Ann Rheum Dis.
59:959–965. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawaguchi H: Endochondral ossification
signals in cartilage degradation osteoarthritis progression in
experimental mouse models. Mol cells. 25:1–6. 2008.PubMed/NCBI
|
|
26
|
Henrotin YE, De Groote DD, Labasse AH,
Gaspar SE, Zheng SX, Geenen VG and Reginster JY: Effects of
exogenous IL-1 beta, TNF alpha, IL-6, IL-8 and LIF on cytokine
production by human articular chondrocytes. Osteoarthritis
Cartilage. 4:163–173. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marks PH and Donaldson ML: Inflammatory
cytokine profiles associated with damage in the anterior cruciate
ligament-deficient knee. Arthroscopy. 21:1342–1347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stannus OP, Jones G, Blizzard L, Cicuttini
FM and Ding C: Associations between serum levels of inflammatory
markers and change in knee pain over 5 years in older adults: A
prospective cohort study. Ann Rheum Dis. 72:535–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu G, Zhao X, Wang C, Geng Y, Zhao J, Xu
J, Zuo B, Zhao C, Wang C and Zhang X: MicroRNA-145 attenuates
TNF-α-driven cartilage matrix degradation in osteoarthritis via
direct suppression of MKK4. Cell Death Dis. 8:e31402017. View Article : Google Scholar : PubMed/NCBI
|