Introduction
Malignant cancer poses a major threat to human life
and health. Current treatment methods, including surgery,
chemotherapy and radiotherapy, aim to directly kill tumor cells or
induce cell s apoptosis (1).
However, chemotherapy and radiotherapy have toxic side effects,
since they induce the death of a large number of bone marrow cells
and other normal dividing cells, in addition to tumor cells
(2). Therefore, these methods have
numerous limitations in clinical applications.
Angiogenesis is the process of new blood vessel
formation. Tumor growth depends on continuous and extensive
angiogenesis, which provides nutrients and oxygen to the tumor
tissues. Distant metastasis of tumor cells occurs via blood
vessels. Therefore, targeting tumor angiogenesis has become an
important alternative therapy for the control of tumor growth and
metastasis (3,4). Anti-angiogenesis therapies that
target the vascular endothelial system are widely used for cancer
treatment (5–7). However, existing anti-angiogenic
agents lack specificity, and thus impair angiogenesis in tumor and
normal tissues (8,9). Certain tumors have even been reported
to develop resistance to anti-angiogenesis drugs (8). An alternative non-drug therapy that
overcomes these limitations is necessary for anti-angiogenesis
therapy.
Recent advances in ultrasound technology have led to
a better understanding of the biological effects of ultrasound. The
high-intensity focused ultrasound (HIFU) technology has presented
promising results in the treatment of various cancer types, such as
pancreatic cancer (10). HIFU is a
non-invasive technique that delivers focused, high-intensity
ultrasound energy (≥3 W/cm2) to a specific area of the
body to instantly raise its temperature to approximately 65–70°C,
which results in irreversible cell death through coagulation
necrosis (11). These thermal
effects are considered to be the primary mechanism by which HIFU
ablates tumor cells (12,13). However, specific ablation of tumor
cells remains a challenge, given that repeated HIFU treatments have
been reported to cause damage to healthy tissues that are in the
path of the acoustic beam (14,15).
Low-intensity pulsed ultrasound (LIPUS) delivers
ultrasound waves at a much lower intensity (usually <300
mW/cm2) and at a lower frequency range of 20 kHz to 1
MHz compared with HIFU; therefore, it does not exhibit hyperthermal
effects. LIPUS has been widely used for fracture healing, wound
healing in different tissue types, inhibition of bacterial growth,
enhanced drug delivery to the brain and in vitro
thrombolysis (16–18). LIPUS induced selective apoptosis of
brain tumor cells in rat brain glioma (19), without damage to the surrounding
normal tissues, or any of the side effects that occur with
radiation therapy and chemotherapy (20). However, the therapeutic potential
of LIPUS for anti-angiogenesis therapy remains largely unknown.
In the present study, the effects of different doses
of LIPUS on the proliferation, migration, apoptosis and
angiogenesis of human endothelial cells, including human umbilical
vein endothelial cells (HUVECs) and human microvascular endothelial
cells (HMECs), were investigated. It was observed that a mean
intensity of 210 mW/cm2 significantly promoted apoptosis
and inhibited angiogenesis via increased phosphorylation of p38
mitogen-activated protein kinase (MAPK) and endoplasmic reticulum
(ER) stress signals. The study results suggested a potential role
of LIPUS in anti-angiogenesis therapy.
Materials and methods
Culture of HUVECs and HMECs
HUVECs were purchased from the American Type Culture
Collection (ATCC; cat. no. PCS-100-010; Manassas, VA, USA) and were
incubated in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), and 1%
penicillin (100 U/ml) and streptomycin (100 µg/ml). HMECs were
purchased from the ATCC (cat. no. CRL-3243) and cultured in
endothelial cell medium (cat. no. 1001; ScienCell Research
Laboratories, Inc., San Diego, CA, USA) containing 5% (cat. no.
0025; ScienCell Research Laboratories, Inc.), 1% endothelial cell
growth supplement (cat. no. 1052; ScienCell Research Laboratories,
Inc) and 1% penicillin/streptomycin solution. The cells were
maintained at 37°C in 5% CO2. At 80–90% confluence, the
cells were suspended at a concentration of 1×106 cells/ml and
plated on 6-cm culture dishes. The cell suspension was exposed to
LIPUS for 1 min and then subjected to various analyses. In
addition, the effect of culture for 24 h with a specific inhibitor
of p38 MAPK, SB203580 (4 µM; Selleck Chemicals, Houston, TX, USA)
subsequent to LIPUS exposure for 1 min was also examined.
LIPUS stimulation
LIPUS irradiation was performed using a set of
ultrasound devices that included a signal generator (Agilent
Technologies, Inc., Santa Clara, CA, USA), a wide-band power
amplifier (Electronics and Innovation Ltd, Rochester, NY, USA) and
a planar transducer (Chongqing Haifu Medical Technology Co., Ltd.,
Chongqing, China). The planar transducer was set at a frequency of
0.5 MHz, the voltage applied to the transducer was 44 V, the
ultrasonic intensity was 70–280 mW/cm2, the number of
cycles was 1,000–4,000 and the mean acoustic pressure was 0.5 MPa.
Briefly, a 6-cm dish seeded with 1×106 cells was placed on top of
the transducer (diameter size, 6 cm), with degassed water between
the dish and transducer. Subsequently, the cell suspension was
exposed to LIPUS for 1 min and cultured for 24 h. The control cell
suspension was treated identically to the experimental group, with
the exception of the LIPUS treatment. A temperature test paper
(TMCHallcrest, Glenview, IL, USA) was adhered to the inner surface
of the 6-cm dish to measure the temperature. Details of the
different doses of LIPUS used in the experiment are listed in
Table I.
 | Table I.List of acoustic parameters used in
the present study, all used at a frequency of 0.5 MHz. |
Table I.
List of acoustic parameters used in
the present study, all used at a frequency of 0.5 MHz.
| No. of cycles | Ultrasonic power
(W) | Ultrasonic
intensity (mW/cm2) | Temperature
(°C) |
|---|
| 1,000 | 1.42 | 70 | 30 |
| 2,000 | 2.80 | 140 | 35 |
| 3,000 | 4.16 | 210 | 35 |
| 4,000 | 5.54 | 280 | 38 |
Flow cytometry analysis of cell
apoptosis
The apoptosis rates of cells were analyzed by flow
cytometry using an Annexin V-Alexa Fluor® 647 detection
kit (cat. no. FMSAV647-100, FCMACS Biotech Co. Ltd., Nanjing,
China) according to the manufacturer's protocol. LIPUS-treated
cells and control cells were seeded in 12-well plates at a density
of 5×105 cells/well and harvested 24 h later. The cells
were then centrifuged at 300 × g for 5 min at 4°C. Following two
washes with PBS, the cells were resuspended in 100 µl of binding
buffer. Subsequently, cells were stained with 5 µl Annexin V-Alexa
Fluor® 647 and 10 µl propidium iodide (PI) for 15 min in
the dark prior to conducting the flow cytometry analysis (BD
Biosciences, San Jose, USA). Early apoptotic cells (Annexin
V-positive/PI-negative) were located in the lower right quadrant.
Late apoptotic or necrotic cells (positive for Annexin V and PI)
were located in the upper right quadrant. Live cells (negative for
Annexin V and PI) were located in the lower left quadrant. Dead
cells (Annexin V-negative/PI-positive) were located in the upper
left quadrant (Fig. 1A and B). The
early apoptosis rate was calculated based on the number of cells in
the lower right quadrant following the manufacturer's protocol.
Cell Counting Kit-8 (CCK-8) assay
The viability of HUVECs and HMECs was assessed using
a CCK-8 assay (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Briefly, cells were seeded at a concentration of
8×103 cells/well in 96-well plates for 24 h. Next, 10 µl
CCK-8 reagent was added to each well, and the cells were incubated
for an additional 2–4 h. The absorbance at 450 nm was then measured
using a microplate reader.
Real-time cell analysis (RTCA)
RTCA, a label-free dynamic technology, was used in
the current study to monitor the proliferation and migration of
HUVECs in real time. To evaluate cell proliferation, cell
suspensions (100 µl; 2,000-3,000 cells/well) were seeded in an
E-Plate assay plate (ACEA Biosciences, Inc.; Agilent Technologies,
Inc., Santa Clara, CA, USA), placed at room temperature on an
ultra-clean bench for 30 min and were monitored continuously for 48
h using the RTCA TP System (ACEA Biosciences, Inc.; Agilent
Technologies, Inc.).
In order to monitor cell migration, 100 µl
serum-free cell suspension (3,000 cells/well) was added to the
upper chamber of a CIM-Plate test plate (ACEA Biosciences, Inc.;
Agilent Technologies, Inc.), while serum-containing medium was
added to the lower chamber. The set-up was placed at room
temperature on an ultra-clean bench for 30 min and then placed on a
test bench to monitor cell migration for 24 h using the RTCA TP
System (ACEA Biosciences, Inc.; Agilent Technologies, Inc.).
Endothelial cell tube formation
assay
To examine the effect of LIPUS on angiogenesis in
vitro, a capillary-like tube formation assay was performed.
Matrigel matrix (cat. no. 354234; Corning Incorporated, Corning,
NY, USA) was pipetted into pre-chilled 96-well plates (50 µl
Matrigel/well) and polymerized at 37°C for 30–60 min. Next, HUVECs
(2×104 cells/well) or HMECs (2.5×104
cells/well) in complete media were seeded in Matrigel-coated
plates. After 8 h of incubation, images of the tubular structures
were captured.
Western blotting
Following treatment, HUVECs cells were lysed using
whole cell lysis buffer (cat. no. KGP250/KGP2100; Nanjing Keygen
Biotech Co., Ltd., Nanjing, China) containing 1% PMSF on ice for 30
min to extract total protein. The protein concentration was
determined using a Bicinchoninic Acid Protein Quantification kit
(cat. no. P0009; Beyotime Institute of Biotechnology, Beijing,
China). Equal amounts of protein (30 µg/lane) were separated via
8–15% SDS-PAGE. Proteins were then transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA) and blocked
with 5% bovine serum albumin (MP Biomedicals, LLC, Santa Ana, CA,
USA) for 2 h at room temperature. Subsequently, the membranes were
probed overnight at 4°C with primary antibodies recognizing the
following antigens: β-tubulin (cat. no. 2128), p38 (cat. no. 8690),
phosphorylated (p)-p38 (cat. no. 4511), extracellular
signal-regulated kinase (ERK; cat. no. 4695), p-ERK (cat. no.
4370), c-Jun N-terminal kinase (JNK; cat. no. 9252), p-JNK (cat.
no. 4668), B-cell lymphoma-2 (Bcl-2; cat. no. 2876), activating
transcription factor-4 (ATF-4; cat. no. 11815), phosphorylated
eukaryotic initiation factor 2α (p-eIF2α; cat. no. 3597),
Bcl-2-associated X protein (Bax; cat. no. 2772), cleaved Caspase-3
(cat. no. 9661) and light chain 3B (LC3B; cat. no. 2775). All
primary antibodies were purchased from Cell Signaling Technology,
Inc., (Danvers, MA, USA) and used at a dilution of 1:1,000.
Membranes were then washed and incubated with corresponding
horseradish peroxidase-labeled goat anti-rabbit (1:5,000, PV-9003;
ZSGB-Bio, China) or goat anti-mouse secondary antibodies (1:5,000,
ZB-2305; ZSGB-Bio, China) for 2 h at room temperature. Finally, the
target proteins were detected using an enhanced chemiluminescence
kit (cat. 34096; Thermo Fisher Scientific, Inc.) and exposed on a
ChemiDoc MP imager (Bio-Rad, California, USA). Bands were
normalized to β-tubulin, and protein levels were quantified by
ImageJ software (v.1.8.0; National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean from three independent experiments. Treatment group values
were compared with control values using GraphPad Prism software
(v.6.0; GraphPad Software, Inc., La Jolla, CA, USA). Comparisons
between two observations were assessed by unpaired Student's
t-test. One-way analysis of variance was used, followed by the
Bonferroni post-hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
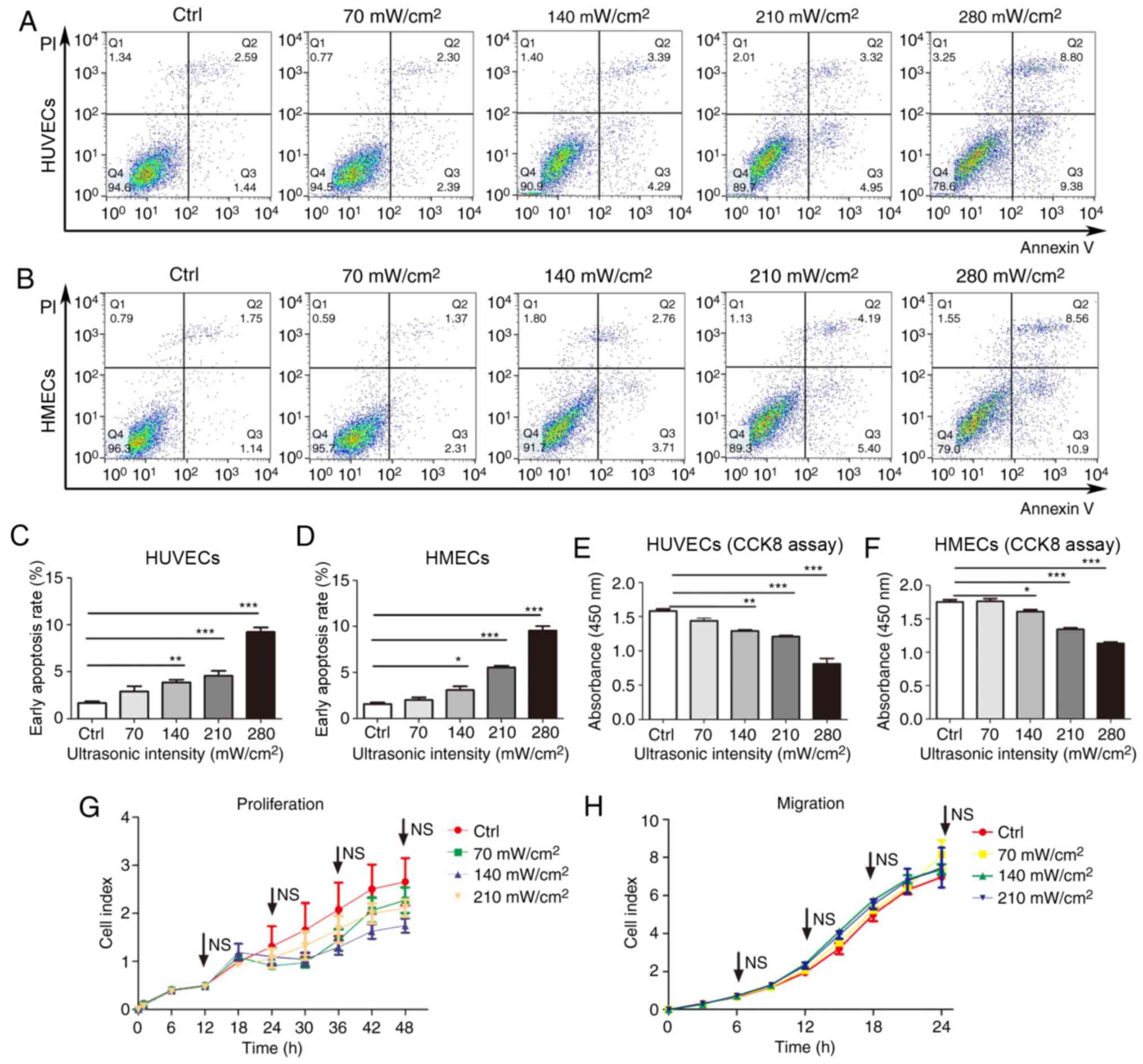
LIPUS promotes the apoptosis and
inhibits the viability of HUVECs and HMECs
To study the effects of LIPUS on the apoptosis of
HUVECs and HMECs, the cells were treated with different doses of
ultrasonic intensities (Table I).
Flow cytometry analysis demonstrated that a single treatment of
LIPUS for a duration of 1 min promoted early apoptosis in HUVECs
and HMECs in a dose-dependent manner (Fig. 1A-D). Ultrasound intensities as low
as 140 mW/cm2 promoted early apoptosis compared with the
control group. The early apoptosis rate was significantly higher at
the dose of 140, 210 and 280 mW/cm2 in LIPUS-treated
cells compared with that observed in control cells (Fig. 1C and D). Additionally, the rates of
late apoptosis and cell death were significantly increased when the
dose was increased to 280 mW/cm2 (data not shown).
To analyze the thermal effects of LIPUS, the present
study also measured the temperature of the cell suspension treated
with LIPUS at doses ranging between 70 and 280 mW/cm2.
The results revealed that the temperature increased from 30°C to
35°C at a LIPUS dose range of 70–210 mW/cm2, whereas the
temperature reached 38°C at a dosage intensity of 280
mW/cm2 (Table I). To
investigate the effects of LIPUS under normal physiological
temperature conditions (<37.5°C), a mean dose intensity of 210
mW/cm2 was selected for use in further experiments.
Next, the effects of LIPUS on HUVEC and HMEC
viability were assessed using a CCK-8 assay. It was demonstrated
that LIPUS treatment reduced the viability of these cells in a
dose-dependent manner, with the lowest viability observed at a
dosage intensity of 280 mW/cm2 (Fig. 1E and F). Furthermore, the effects
of LIPUS on the proliferation and migration of HUVECs were
analyzed. The cells were treated with different doses of LIPUS, and
then cell proliferation and migration were measured at 48 and 24 h,
respectively. The results revealed that dose intensities of 70–210
mW/cm2 did not markedly affect the proliferation and
migration of LIPUS-treated HUVECs as compared with untreated cells
(Fig. 1G and H).
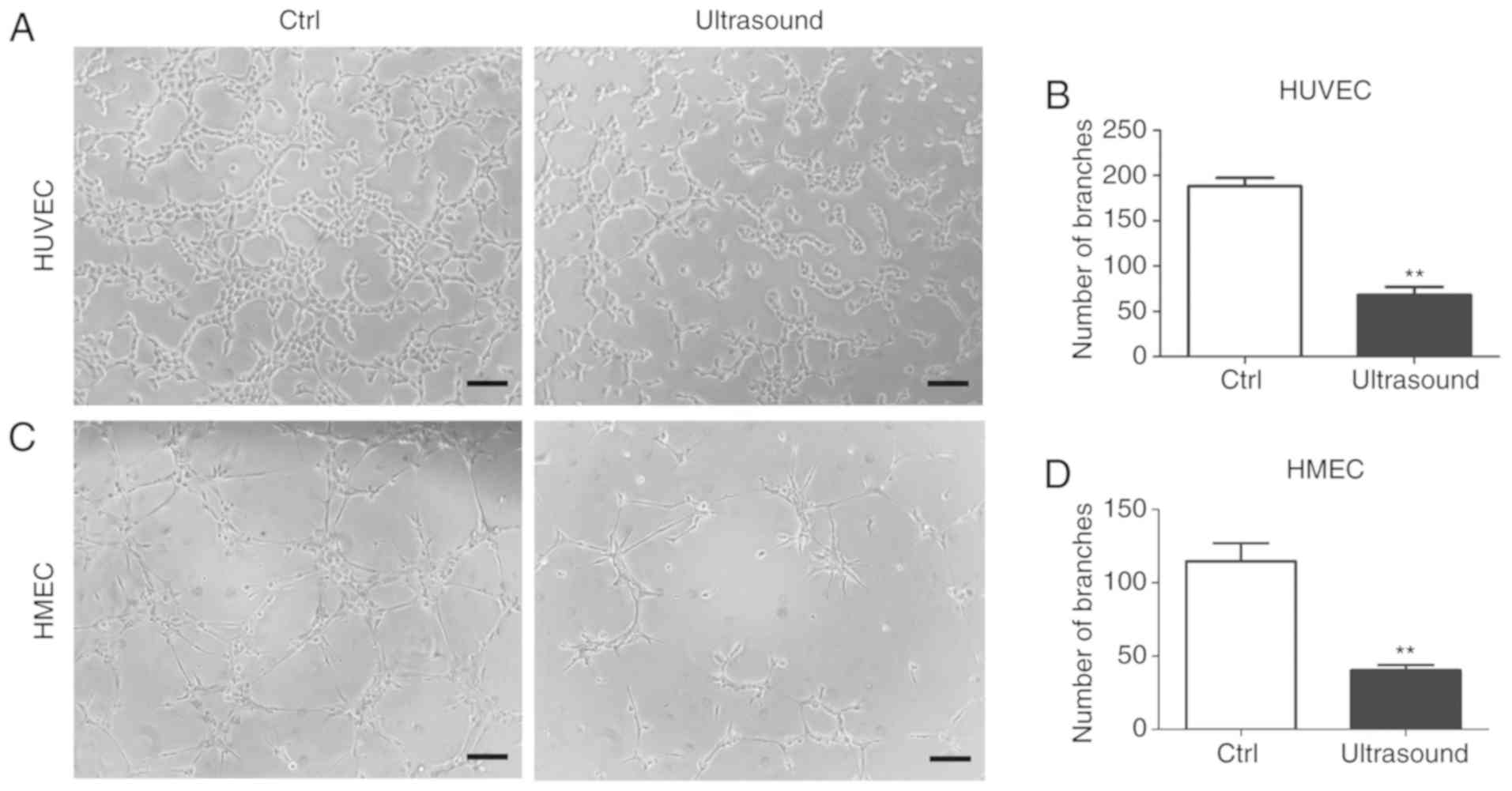
LIPUS inhibits angiogenesis in HUVECs
and HMECs
To determine the potential anti-angiogenic effects
of LIPUS in HUVECs and HMECs, an in vitro angiogenesis assay
was performed. After 8 h of incubation, LIPUS treatment was
observed to significantly inhibit tube formation in treated cells
as compared with that observed in untreated cells (Fig. 2A-D).
LIPUS regulates the expression of
apoptosis-associated proteins
To examine the mechanism underlying the
LIPUS-induced apoptosis, the levels of apoptosis marker proteins
were measured in LIPUS-treated HUVECs. The protein level of cleaved
Caspase-3 was significantly increased in cells treated with LIPUS
at a dose of 210 mW/cm2 as compared with that in control
cells. LIPUS-treated cells also exhibited a marked reduction in the
levels of the apoptosis inhibitor, Bcl-2, and a higher Bax/Bcl-2
ratio, which is usually used for the measurement of the apoptotic
potential of cells (21). In
addition, the protein levels of the two forms of the
autophagy-marker protein LC3B (namely LC3-I and-II) were also
measured, and it was observed that these proteins were not markedly
affected by LIPUS treatment (Fig. 3A
and B).
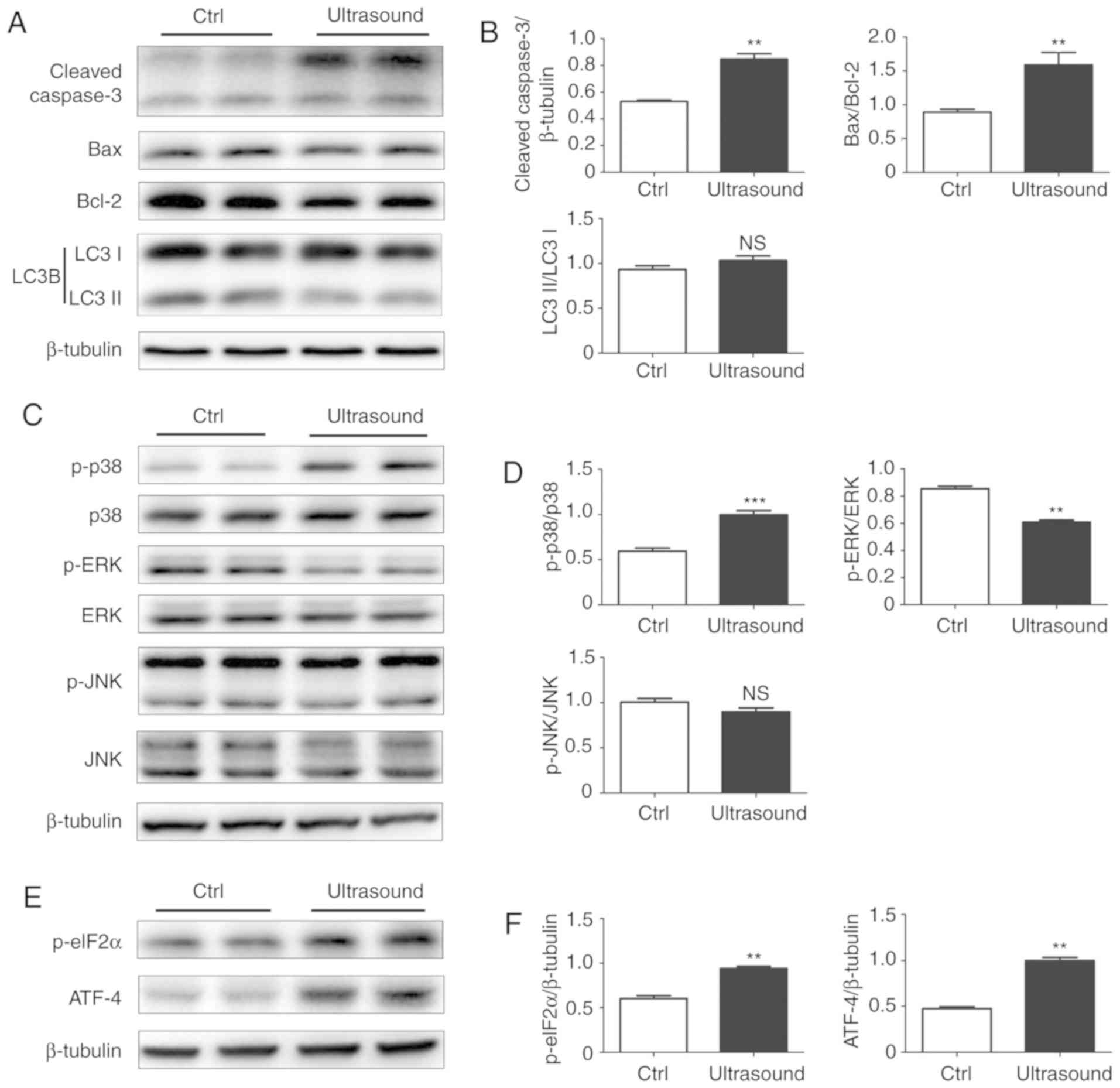 | Figure 3.Low-intensity pulsed ultrasound
regulated the expression of apoptosis-associated proteins. (A)
Cleaved Caspase-3, Bcl-2, Bax and LC3B levels, measured by western
blotting. (B) Quantification of the relative levels of cleaved
Caspase-3, Bax and LC3-II, which were respectively normalized to
β-tubulin, Bcl-2 and LC3-I levels. (C) Phosphorylation and total
protein levels of p38, ERK and JNK were assessed by western
blotting. (D) Quantification of p-p38, p-ERK and p-JNK protein
levels, normalized to their total protein levels. (E) Protein
levels of ATF-4 and p-eIF2α were assessed by western blotting. (F)
Quantification of ATF-4 and p-eIF2α protein levels, normalized to
β-tubulin. All values are expressed as the mean ± standard error of
three independent experiments. **P<0.01 and ***P<0.001, vs.
Ctrl group. HUVECs, human umbilical vein endothelial cells; HMECs,
human microvascular endothelial cells; Bcl-2, B-cell lymphoma-2;
Bax, Bcl-2-associated X protein; LC3, light chain 3; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; ATF-4, activating transcription factor-4; eIF2α, eukaryotic
initiation factor 2α; Ctrl, control; p-, phosphorylated. |
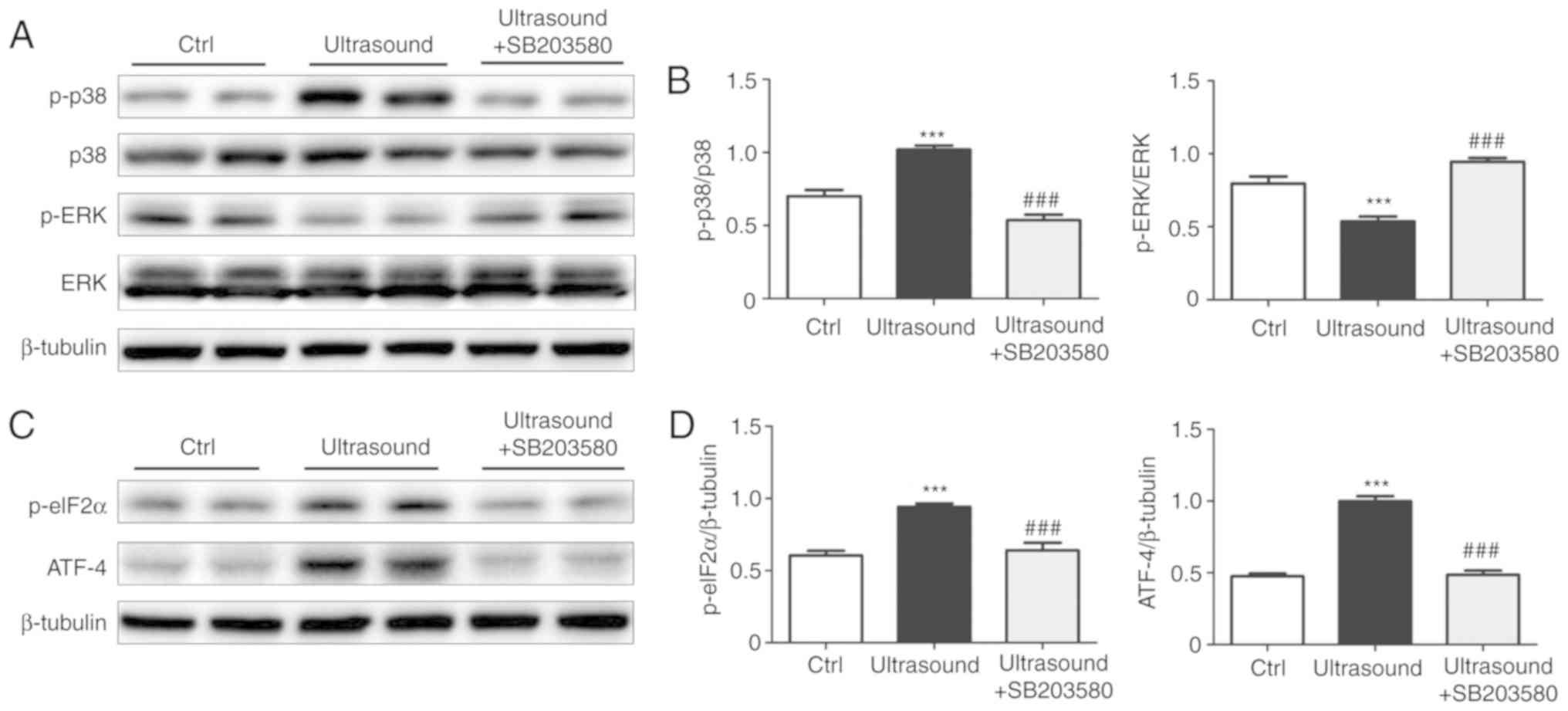
LIPUS increases p38 MAPK
phosphorylation and activates ER stress signaling
To investigate the molecular mechanisms of
LIPUS-induced apoptosis in HUVECs, the activated MAPK members were
analyzed using western blotting. It was observed that LIPUS-treated
cells exhibited significantly higher levels of p-p38 compared with
the control cells. By contrast, LIPUS treatment resulted in
decreased phosphorylation of ERK in these cells, while no
significant difference was observed in the phosphorylation levels
of JNK between treated and untreated cells (Fig. 3C and D).
ER stress signals are known to serve a major role in
cell apoptosis (22). To
investigate whether LIPUS-induced apoptosis in HUVECs was mediated
by the ER stress signaling pathway, the levels of key ER stress
proteins, including ATF-4 and p-eIF2α, were also measured. The data
indicated that the levels of ATF-4 and p-eIF2α were markedly
increased in LIPUS-treated cells when compared with those in
control cells (Fig. 3E and F).
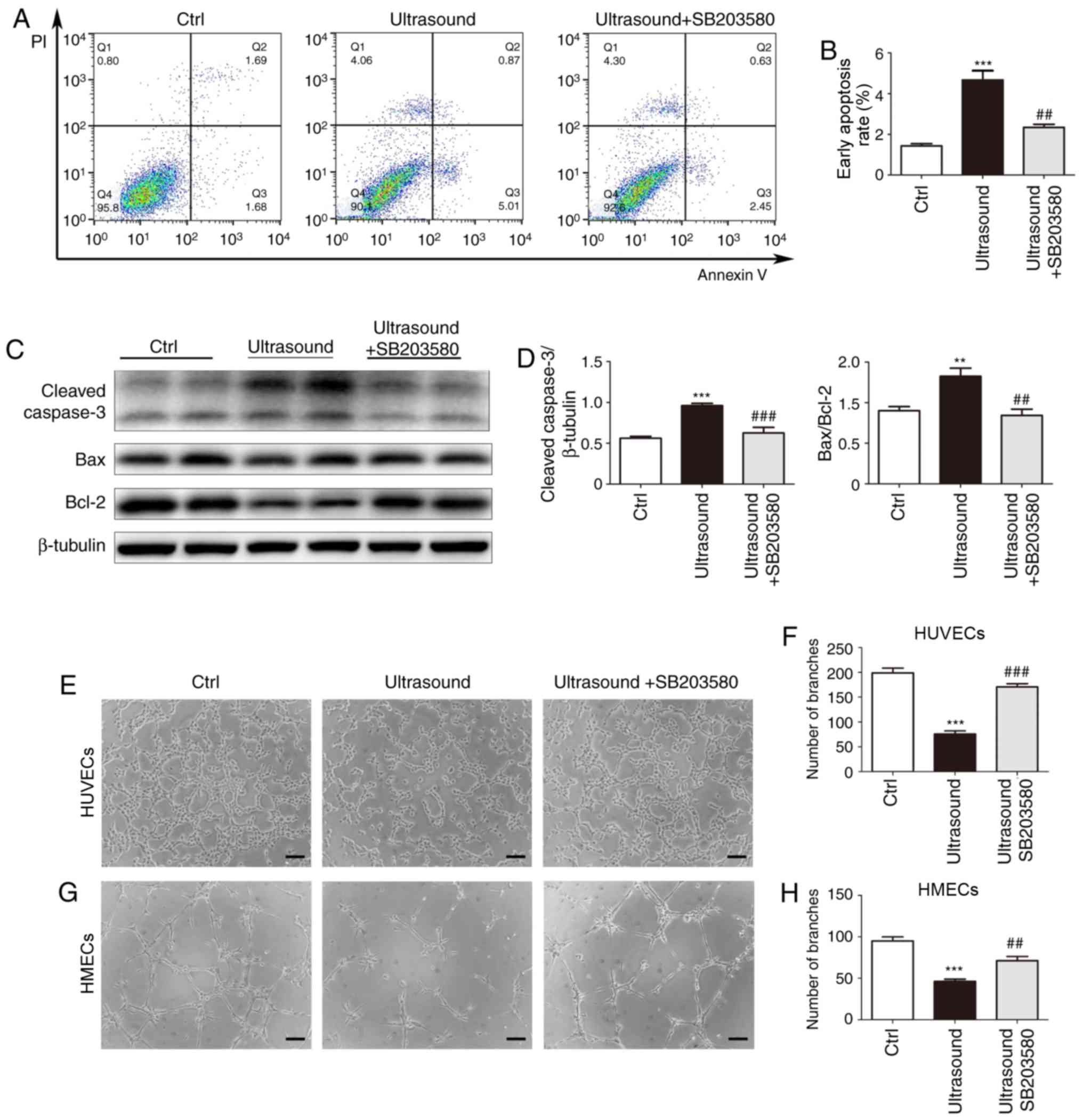
Inhibition of p38 phosphorylation
rescues the pro-apoptotic and anti-angiogenic effects of LIPUS
To determine whether the pro-apoptotic effect of
LIPUS on HUVECs was dependent on p38 phosphorylation, the specific
p38 MAPK inhibitor SB203580 was used to decrease p-p38 levels in
LIPUS-treated HUVECs. Flow cytometry analysis revealed that p38
inhibition in LIPUS-treated HUVECs resulted in reduced levels of
apoptosis compared with those in inhibitor-free, LIPUS-treated
HUVECs (Fig. 4A and B). Western
blotting also revealed that p38 inhibition reversed the
LIPUS-induced changes in the expression levels of Bcl-2 and cleaved
Caspase-3 in HUVECs (Fig. 4C and
D). The effect of p38 inhibition on angiogenesis subsequent to
LIPUS treatment was examined. HUVECs and HMECs treated with both
LIPUS and SB203580 exhibited recovered tube formation compared with
the LIPUS-treated cells alone (Fig.
4E-H). These findings suggested that p38 phosphorylation served
a key role in mediating the anti-angiogenic effect of LIPUS.
The effect of p38 inhibition on ERK and ER stress
proteins was also examined in LIPUS-treated HUVECs. Western
blotting revealed that p38 inhibition reversed the LIPUS-induced
effects, leading to a significant increase of p-ERK levels
(Fig. 5A and B), and marked
reduction of ATF-4 and p-eIF2α levels (Fig. 5C and D) as compared with the
LIPUS-treated cells alone.
Discussion
LIPUS is increasingly used for various therapeutic
purposes, including tumor ablation, bone repair, targeted drug
delivery and chemotherapy (23).
The mechanical signal of LIPUS is translated into a number of
cellular effects, including anti-angiogenesis, anti-inflammatory
responses and cytotoxicity (23–25).
Therefore, LIPUS is an attractive, non-invasive and non-toxic
option for standard oncology treatments, such as surgery,
radiotherapy, gene therapy and chemotherapy (10). HUVECs are derived from endothelial
vein tissues of the umbilical cord, while HMECs are derived from
dermal endothelial tissues. These two cell lines are useful in
vitro models for the study of endothelial functions, such as
angiogenesis. Studies have reported that low-intensity ultrasound
with microbubbles induces the evident microvascular damage in
tumors, mainly due to inertial cavitation, which can cause cell
necrosis or apoptosis (26,27).
Acoustic cavitation can lead to mechanical damage of small blood
vessels due to the expansion and collapse caused by oscillation of
the microbubbles (28). These
previous studies have tested different LIPUS parameters and
radiation times. In the present study, the effect of LIPUS
treatment on HUVECs and HMECs was examined at ultrasound dose
intensities of 70–280 mW/cm2 for the same radiation time
period, as previously reported (26–28).
The results revealed that a larger dose of LIPUS directly inhibited
cell viability and tube formation, and promoted apoptosis. The
pro-apoptotic effect of LIPUS observed in the current study may be
due to mechanical stimulation rather than inertial cavitation. As
the temperature of cells treated with LIPUS at a dosage intensity
of 210 mW/cm2 was 35°C (close to the normal
physiological temperature), possible thermal effects of LIPUS can
be ruled out. The present in vitro study has highlighted the
therapeutic potential of LIPUS in inhibiting angiogenesis and tumor
growth via vascular endothelial cell apoptosis. Further in
vivo studies are necessary to fully understand the effect of
LIPUS on tumor angiogenesis.
The two main signaling pathways that regulate cell
apoptosis are the extrinsic pathway, which is mediated by the
activation of cell-surface death receptors by external signals, and
the intrinsic pathway, which is activated by intracellular signals
that cause mitochondrial damage (29,30).
Numerous factors, such as death receptor-mediated signaling
molecules, anticancer drugs and growth factor inhibitors, can
damage mitochondrial function and induce apoptosis (21). Bcl-2 is part of the Bcl-2 family of
proteins that are well-known for their role in apoptosis
regulation; this protein is localized in the mitochondrial outer
membrane and serves a key role in cell survival by inhibiting
pro-apoptotic molecules (31). Bax
is a pro-apoptotic protein that is also a member of the Bcl-2
family and induces apoptosis via cytochrome C-mediated cleavage of
Caspase-3 (32). In the current
study, the effect of LIPUS treatment on the expression levels of
Bcl-2, Bax and cleaved Caspase-3 was assessed. Decreased expression
of Bcl-2 and increased expression of cleaved Caspase-3 were
observed in HUVECs in response to LIPUS treatment. Although no
significant differences were observed in Bax levels between
LIPUS-treated and control cells, the ratio of Bax to Bcl-2 was
significantly increased following LIPUS treatment, which indicated
high susceptibility of these cells to apoptosis. These results
suggested that the mitochondrial intrinsic pathway served an
important role in mediating LIPUS-induced apoptosis in HUVECs.
ERK, JNK and p38 are members of the MAPK family of
signaling proteins that are involved in the initiation of
apoptosis. These proteins are activated by extracellular stimuli,
including mitogens, ultraviolet irradiation, heat shock, osmotic
stress and cytokines, and exert various cellular effects, such as
differentiation, proliferation, autophagy and apoptosis (24,33–35).
The p38 pathway is known to regulate tumor cells in multiple ways;
however, despite the tumor-suppressive and anti-proliferative
properties of p38 in certain tissues, the p38 pathway also exerts
oncogenic effects by influencing cancer metabolism and
chemoresistance in cancer tissues (36). Several chemotherapeutic agents
require p38 activity for the induction of apoptosis (37). For instance, cyclophosphamide, a
commonly used chemotherapeutic drug for breast cancer, induces
apoptosis via activation of the p38 MAPK pathway (38). The present study demonstrated that
a pro-apoptotic dose of LIPUS led to the activation of p38 and the
inhibition of ERK, while inhibition of p38 phosphorylation reversed
the pro-apoptotic and anti-angiogenic effects of LIPUS. No
significant change in the levels of JNK was observed when cells
were exposed to LIPUS. Activation of JNK mainly occurs due to
endogenous chemical stimuli, such as DNA damage and inflammatory
cytokines (39). JNK can also be
activated by mechanical stimulation (40,41).
However, the present study data suggested that JNK may not be
involved in LIPUS-induced apoptosis in HUVECs. Our previously study
reported that a lower dose of LIPUS with an ultrasonic intensity of
109.4 mW/cm2 promoted apoptosis in rat preadipocytes via
activation of p38 (42). These
results suggested a universal role for p38 in mediating
LIPUS-induced apoptosis, independent of cell type.
Previous studies have demonstrated that p38
signaling controls the expression of several ER stress proteins by
regulating the activity of transcription factors, such as ATF-1,
ATF-2, C/EBP homologous protein (CHOP) and multiple cyclic AMP
response element-binding proteins (33,43).
CHOP is an ER-specific pro-apoptotic transcription factor that is
induced by growth arrest and DNA damage, and CHOP expression is
regulated by the eIF2α/ATF-4 pathway (22,44).
Regulation of the eIF2α/ATF-4 pathway by p38 has also been reported
to cause apoptosis via autophagy (43). The current study results
demonstrated that LIPUS treatment resulted in increased expression
of p-eIF2α and ATF-4, and that p38 inhibition reversed the
upregulation of p-eIF2α and ATF-4 levels. However, no change was
observed in the expression levels of LC3B, an autophagy protein, in
LIPUS-treated cells. These findings suggested that p38 served a key
role in LIPUS-induced apoptosis via activation of the ER stress
response without affecting autophagy. The detailed mechanism by
which p38 mediates eIF2α/ATF-4 upregulation is a subject for
further study.
However, there are certain limitations to the
current study, which should be taken into consideration. Firstly,
the experiments were performed in HUVECs and HMECs rather than
primary endothelial cells isolated from tumor tissues. Since
obtaining sufficient numbers of the primary tumor vascular
endothelial cells is almost impossible, it was speculated that the
artificially-induced endothelial cells differentiated from tumor
stem cells may act as an alternative approach. Furthermore, the
treatment strategy and indicated dose of ultrasound were produced
under in vitro conditions. Whether the dose of ultrasound is
applicable for in vivo anti-angiogenesis therapy in solid
tumors requires further investigation in animal tumor models.
In conclusion, the results of the present study
revealed that LIPUS promoted apoptosis in HUVECs via p38
MAPK-mediated activation of the ER stress response. The findings
suggested that LIPUS is an effective and low-risk option for
inhibiting endothelial cell function, and this technique can
potentially be used as an anti-angiogenic therapy in tumor
treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81627802 and 81570247),
and the Priority Academic Program Development of Jiangsu Higher
Education Institutions (grant no. PAPD 2014–2016).
Availability of data and materials
All data generated or analyzed during the present
study are included in the published article.
Authors' contributions
ZS, TX and WS designed the study and drafted the
manuscript. XG, JT and DZ made substantial contributions to the
study conception and design, data analysis and interpretation, and
drafting and revising of the manuscript. YS and WS assisted with
the molecular biology experiments. XK assisted with LIPUS
manipulation. YS, WS and XK revised the manuscript. ZS, YW and TX
performed the experiments and analyzed the data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LIPUS
|
low-intensity pulsed ultrasound
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
HMECs
|
human microvascular endothelial
cells
|
|
HIFU
|
high-intensity focused ultrasound
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
ATF-4
|
activating transcription factor-4
|
|
eIF2α
|
eukaryotic initiation factor 2α
|
|
p-
|
phosphorylated
|
|
ER
|
endoplasmic reticulum
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RTCA
|
real-time cell analysis
|
|
CHOP
|
C/EBP homologous protein
|
References
|
1
|
Stylianopoulos T: The solid mechanics of
cancer and strategies for improved therapy. J Biomech Eng.
139:2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukumura D and Jain RK: Tumor
microenvironment abnormalities: Causes, consequences, and
strategies to normalize. J Cell Biochem. 101:937–949. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin JD, Fukumura D, Duda DG, Boucher Y
and Jain RK: Reengineering the tumor microenvironment to alleviate
hypoxia and overcome cancer heterogeneity. Cold Spring Harb
Perspect Med. 6:2016. View Article : Google Scholar
|
|
5
|
Jain RK: Normalizing tumor
microenvironment to treat cancer: Bench to bedside to biomarkers. J
Clin Oncol. 31:2205–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vassilakopoulou M, Psyrri A and Argiris A:
Targeting angiogenesis in head and neck cancer. Oral Oncol.
51:409–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tas SW, Maracle CX, Balogh E and Szekanecz
Z: Targeting of proangiogenic signalling pathways in chronic
inflammation. Nat Rev Rheumatol. 12:111–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simon T, Gagliano T and Giamas G: Direct
effects of anti-angiogenic therapies on tumor cells: VEGF
signaling. Trends Mol Med. 23:282–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye W: The complexity of translating
anti-angiogenesis therapy from basic science to the clinic. Dev
Cell. 37:114–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Bataineh O, Jenne J and Huber P:
Clinical and future applications of high intensity focused
ultrasound in cancer. Cancer Treat Rev. 38:346–353. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kennedy JE: High-intensity focused
ultrasound in the treatment of solid tumours. Nat Rev Cancer.
5:321–327. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown MR, Farquhar-Smith P, Williams JE,
ter Haar G and deSouza NM: The use of high-intensity focused
ultrasound as a novel treatment for painful conditions-a
description and narrative review of the literature. Br J Anaesth.
115:520–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan H, Zhou W and Wang S: Pulsed focused
ultrasound stimulates the release of tumor biomarkers into the
blood circulation. Radiology. 285:1058–1060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hariharan P, Myers MR and Banerjee RK:
HIFU procedures at moderate intensities-effect of large blood
vessels. Phys Med Biol. 52:3493–3513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao Y, Yin H, Li Z and Wan M: Cavitation
distribution within large phantom vessel and mechanical damage
formed on surrounding vessel wall. Ultrason Sonochem. 20:1376–1383.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizrahi N, Zhou EH, Lenormand G, Krishnan
R, Weihs D, Butler JP, Weitz DA, Fredberg JJ and Kimmel E: Low
intensity ultrasound perturbs cytoskeleton dynamics. Soft matter.
8:2438–2443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rutten S, Nolte PA, Korstjens CM and
Klein-Nulend J: Low-intensity pulsed ultrasound affects RUNX2
immunopositive osteogenic cells in delayed clinical fracture
healing. Bone. 45:862–869. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hitchcock KE and Holland CK:
Ultrasound-assisted thrombolysis for stroke therapy: Better
thrombus break-up with bubbles. Stroke. 41:S50–S53. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Z, Chen J, Chen L, Yang X, Zhong H,
Qi X, Bi Y and Xu K: Low frequency and intensity ultrasound induces
apoptosis of brain glioma in rats mediated by caspase-3, Bcl-2, and
survivin. Brain Res. 1473:25–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou XY, Wu SY, Zhang ZC, Wang F, Yang YL,
Li M and Wei XZ: Low-intensity pulsed ultrasound promotes
endothelial cell-mediated osteogenesis in a conditioned medium
coculture system with osteoblasts. Medicine (Baltimore).
96:e83972017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marquez RT and Xu L: Bcl-2: Beclin 1
complex: Multiple, mechanisms regulating autophagy/apoptosis toggle
switch. Am J Cancer Res. 2:214–221. 2012.PubMed/NCBI
|
|
22
|
Rovetta F, Stacchiotti A, Consiglio A,
Cadei M, Grigolato PG, Lavazza A, Rezzani R and Aleo MF: ER
signaling regulation drives the switch between autophagy and
apoptosis in NRK-52E cells exposed to cisplatin. Exp Cell Res.
318:238–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furusawa Y, Zhao QL, Hassan MA, Tabuchi Y,
Takasaki I, Wada S and Kondo T: Ultrasound-induced apoptosis in the
presence of Sonazoid and associated alterations in gene expression
levels: A possible therapeutic application. Cancer Lett.
288:107–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Q, Walmsley AD, Cooper PR and Scheven
BA: Ultrasound stimulation of different dental stem cell
populations: Role of mitogen-activated protein kinase signaling. J
Endod. 42:425–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee GS, Park JH, Shin US and Kim HW:
Direct deposited porous scaffolds of calcium phosphate cement with
alginate for drug delivery and bone tissue engineering. Acta
Biomater. 7:3178–3186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mason TJ: Therapeutic ultrasound an
overview. Ultrason Sonochem. 18:847–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou R, Xu Y, Lu Q, Zhang Y and Hu B:
Effect of low-frequency low-intensity ultrasound with microbubbles
on prostate cancer hypoxia. Tumour Biol. 39:10104283177192752017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Brayman AA, Bailey MR and Matula
TJ: Blood vessel rupture by cavitation. Urol Res. 38:321–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y and Brandizzi F: IRE1: ER stress
sensor and cell fate executor. Trends Cell Biol. 23:547–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhutia SK, Dash R, Das SK, Azab B, Su ZZ,
Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, et al: Mechanism of
autophagy to apoptosis switch triggered in prostate cancer cells by
antitumor cytokine melanoma differentiation-associated gene
7/interleukin-24. Cancer Res. 70:3667–3676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu H, Che X, Zheng Q, Wu A, Pan K, Shao A,
Wu Q, Zhang J and Hong Y: Caspases: A molecular switch node in the
crosstalk between autophagy and apoptosis. Int J Biol Sci.
10:1072–1083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Obata T, Brown GE and Yaffe MB: MAP kinase
pathways activated by stress: The p38 MAPK pathway. Crit Care Med.
28:N67–N77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Whitmarsh AJ: A central role for p38 MAPK
in the early transcriptional response to stress. BMC Biol.
8:472010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trempolec N, Dave-Coll N and Nebreda AR:
SnapShot: p38 MAPK substrates. Cell. 152:924–924.e1. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grossi V, Peserico A, Tezil T and Simone
C: p38alpha MAPK pathway: A key factor in colorectal cancer therapy
and chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olson JM and Hallahan AR: p38 MAP kinase:
A convergence point in cancer therapy. Trends Mol Med. 10:125–129.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pang H, Cai L, Yang Y, Chen X, Sui G and
Zhao C: Knockdown of osteopontin chemosensitizes MDA-MB-231 cells
to cyclophosphamide by enhancing apoptosis through activating p38
MAPK pathway. Cancer Biother Radiopharm. 26:165–173. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsui H, Fukuno N, Kanda Y, Kantoh Y,
Chida T, Nagaura Y, Suzuki O, Nishitoh H, Takeda K, Ichijo H, et
al: The expression of Fn14 via mechanical stress-activated JNK
contributes to apoptosis induction in osteoblasts. J Biol Chem.
289:6438–6450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mu C, Lv T, Wang Z, Ma S, Ma J, Liu J, Yu
J and Mu J: Mechanical stress stimulates the osteo/odontoblastic
differentiation of human stem cells from apical papilla via erk 1/2
and JNK MAPK pathways. Biomed Res Int. 2014:4943782014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu T, Gu J, Li C, Guo X, Tu J, Zhang D,
Sun W and Kong X: Low-intensity pulsed ultrasound suppresses
proliferation and promotes apoptosis via p38 MAPK signaling in rat
visceral preadipocytes. Am J Transl Res. 10:948–956.
2018.PubMed/NCBI
|
|
43
|
Jiang Q, Li F, Shi K, Wu P, An J, Yang Y
and Xu C: Involvement of p38 in signal switching from autophagy to
apoptosis via the PERK/eIF2α/ATF-4 axis in selenite-treated NB4
cells. Cell Death Dis. 5:e12702014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rozpedek W, Pytel D, Mucha B, Leszczynska
H, Diehl JA and Majsterek I: The role of the PERK/eIF2α/ATF-4/CHOP
signaling pathway in tumor progression during endoplasmic reticulum
stress. Curr Mol Med. 16:533–544. 2016. View Article : Google Scholar : PubMed/NCBI
|