Introduction
Glaucoma is a progressive neurodegenerative eye
disease and is a leading cause of irreversible blindness globally
(1–3). Increased intraocular pressure and
retinal ganglion cell (RGC) death are the most common symptoms of
glaucoma. Strategies to alleviate intraocular pressure, such as
topical drugs, laser treatment and surgery, cannot completely
suppress the progression of visual field defects in some patients
(1). Although some neuroprotective
strategies have been investigated, to date, there are no convincing
data to support an effective therapy for glaucoma (4–6).
Therefore, developing novel neuroprotective therapies to protect or
regenerate RGCs in glaucoma is necessary.
Although the precise mechanism underlying glaucoma
remains unclear, it is becoming increasingly clear that glial cell
activation and neuro-inflammation exacerbate the loss of RGCs in
the retina after optic nerve injury (7–9).
Therefore, anti-inflammatory agents may be a potential effective
neuro-protective therapy to protect RGCs in the retina of patients
with glaucoma.
Recently, various natural and potentially
therapeutic compounds have attracted the attention of researchers
(10–12). Caffeic acid phenethyl ester (CAPE),
an active phenolic component in the propolis of the honeybee hive,
has been reported to be a potent antioxidant and suppressor of
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)
(13). CAPE has a multitude of
beneficial biological properties, including antioxidant,
anti-inflammatory, antiviral, anti-proliferative, neuroprotective,
hepatoprotective and cardioprotective capacities (13–18).
Some studies have described the anti-apoptosis effect of CAPE in
neurons induced by ischemia-reperfusion or low potassium levels by
blocking the production of reactive oxygen species and inhibiting
caspase activity (19,20). However, there have been no
published studies investigating the role of CAPE in protecting
against RGC death using the optic nerve crush (ONC) model for
glaucoma.
In the present study, the survival and apoptosis of
RGCs after ONC injury with and without CAPE treatment were
compared. The protective role of CAPE in ONC-induced RGC apoptosis
and neuro-inflammation was demonstrated. The expression of
cytokines in the retina was suppressed by CAPE, as well as the
activation of NF-κB in astrocytes. The present study provides
detailed evidence supporting the neuroprotective effect of CAPE on
RGCs.
Materials and methods
Animals and the ONC model
All 72 Sprague-Dawley rats (8–12 weeks of age, male;
weight, 170–200 g) used in this study were provided by the Shanghai
Laboratory Animal Center of the Chinese Academy of Sciences
(Shanghai, China). Animals were housed in a temperature-controlled
room (22±3°C) with a 12-h light/dark cycle under specific-pathogen
free conditions and provided with free access to water and food.
All experimental animal protocols were approved by the
Institutional Animal Care and Use Committee of Wenzhou Medical
University (Wenzhou, Zhejiang, China). The ONC protocol was adapted
from previous studies (10,21).
Briefly, rats were anesthetized using pentobarbital sodium (40
mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A small
incision was then made in the superior and lateral conjunctiva, and
gentle dissection was performed to expose the optic nerve, and to
avoid tissue damage and infraorbital trauma. The optic nerve was
crushed for 10 sec using a vascular clip at the site 2 mm posterior
to the globe. The integrity of the retinal blood supply was
verified, and rats with severely reduced perfusion were excluded.
Before the application of the coverslip, the eye was moisturized
using sodium hyaluronate 1 mg/ml (Hylo-COMOD, Ursapharm, Germany).
For each experiment, ONC was performed on the left eye.
CAPE treatment
Rats were randomly assigned to one of four groups
(n=18 each): the control (Con); the Con + CAPE; the ONC; and the
ONC + CAPE. No surgery was performed in the control rats. Rats were
injected with 10 µmol/kg CAPE (Sigma-Aldrich; Merck KGaA)
intraperitoneally 10 min after the surgery according to a method
described in a previous study (22). Equal amounts of vehicle (i.e.,
instead of CAPE) were administered to the rats in the control and
the ONC groups.
Brn3a-labeled flat-mounted
retinas
Fourteen days after surgery, the rats were
euthanized with carbon dioxide, and the eyeballs were enucleated
and fixed using 4% paraformaldehyde at 4°C overnight. Retina flat
mounts were carefully prepared and incubated with blocking buffer
(0.3% Triton X-100 and 2% donkey serum) for 1 h at room temperature
after washing with phosphate-buffered saline (PBS). The retina flat
mounts were then incubated with rabbit monoclonal antibody for
brain-specific homeobox/POU domain protein 3A (Brn-3a) (dilution
1:100; cat. no. ab232480; Abcam, Cambridge, MA, USA) overnight at
4°C, followed by anti-rabbit Alexa Fluor 594 (dilution 1:500; cat.
no. R37117; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2
h at room temperature. The slides were then examined using a
confocal microscope (TCS SP5; Leica Microsystems GmbH, Wetzlar,
Germany; magnification, ×200), and 10 microphotographs were
captured for each retina.
TUNEL apoptosis assay
Terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) staining was performed using a commercially
available kit (One-Step TUNEL Apoptosis Assay kit; cat. no. C1086;
Beyotime Institute of Biotechnology, Haimen, China). Frozen tissue
sections were rinsed with PBS for 10 min and incubated with 0.5%
Triton X-100 for 5 min at room temperature. The TUNEL reaction
mixture (50 µl for each section) was added and stained for 60 min
at 37°C. DAPI was also used to visualize nuclei. TUNEL-positive
cells were examined using a confocal microscope, three random
fields for each sample was captured.
Immunofluorescent staining
Retinas were harvested on day 14 after ONC, and
frozen sections (8 µm thick) were prepared using a microtome (Leica
Microsystems GmbH). Sections were blocked with PBS containing 0.3%
Triton X-100, 1% BSA and donkey serum for 2 h to avoid non-specific
staining. After blocking, the sections were stained with primary
antibodies for mouse monoclonal anti-glial fibrillary acidic
protein (GFAP) (dilution 1:200; cat. no. ab10062) and rabbit
polyclonal anti-NF-κB-P65 (dilution 1:100; cat. no. ab16502) (both
from Abcam) at 4°C overnight, and further stained with fluorescein
conjugated secondary antibodies (anti-mouse Alexa Fluor 488
(dilution 1:500; cat. no. R37120; Thermo Fisher Scientific, Inc.)
and anti-rabbit Alexa Fluor 594). Sections were sealed and examined
using a scanning confocal microscope (Leica Microsystems GmbH).
Rat primary astrocyte culture and cell
treatment
Primary rat astrocytes were prepared according to
methods described in previous studies (23,24).
Briefly, mixed-glia cultures were prepared from the dissected
brains of newborn Sprague-Dawley rats (1–3 days) and cultured in
Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10%
fetal bovine serum (FBS). The culture medium was changed on days 3
and 6. For purification, oligodendrocytes and microglia were
removed by shaking the flasks at 200 rpm for 2 h at 37°C (days
10–12). The isolated astrocytes were confirmed by immunostaining
for CD11b and GFAP; astrocyte purity was >95%. The second
passage of astrocytes were used in this study.
Astrocyte migration assay
Astrocytes were seeded in 24-well plates. Astrocyte
monolayers were gently and perpendicularly scratched using a
sterile pipette tip across the well when the cells reached 70–80%
confluence. The wells were washed twice with medium to remove the
detached cells and treated with either CAPE or together with
lipopolysaccharide (LPS) for 16 h before being examined by inverted
phase contrast microscope. Photomicrographs of five randomly chosen
fields were captured, and the gap distance was quantitatively
evaluated using ImageJ v1.8.0 software (NIH; National Institutes of
Health, Bethesda, MD, USA). The migration rate was obtained by
comparing the migration distance of the experimental groups with
the control group.
Cell viability assay
Astrocyte viability was measured using a
commercially available Cell Counting Kit-8 (CCK-8; Dojindo
Laboratories, Kumamoto, Japan). Astrocytes were plated in 96-well
plates (5×103 cells/well) and treated with different
concentrations of CAPE (0, 1, 5, 10, 20 or 40 µM) or LPS (1 µg/ml)
for 48 h. To measure astrocyte viability, 10 µl of CCK-8 solution
was added to each well in the dark. After a 1-h incubation at 37°C,
absorbance was read using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA) at 450 nm.
Quantitative polymerase chain reaction
(PCR)
RNA from retinas and astrocytes was isolated using a
commercially available kit (miRNeasy Mini kit; Qiagen, Duesseldorf,
Germany) and complementary DNA was synthesized using
PrimeScript™ RT reagent kit with gDNA Eraser (Takara
Biotechnology Co., Ltd., Dalian, China). Real-time quantitative PCR
was performed using SYBR Premix Ex Taq™ II kit (Takara
Biotechnology Co., Ltd.). Messenger RNA (mRNA) expression of
interleukin (IL)-8, IL-6, inducible nitric oxide synthase (iNOS),
cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α) and C-C
motif ligand-2 (CCL-2) in retinas were normalized to β-actin using
the 2−ΔΔCq method (25).
Western blot analysis
Retinas were carefully separated from the eye balls,
placed in lysis buffer (1X RIPA Buffer, cat. no. 9806S; Cell
Signaling Technology, Danvers, MA, USA) and ultrasonicated on ice.
Cell lysates were centrifuged at 12,000 × g for 10 min at 4°C to
collect the supernatant protein. Protein concentrations were
determined using a Pierce BCA Protein Assay Kit (cat. no. 23225;
Thermo Fisher Scientific, Inc.) and 30 µg of each sample was
blotted with 10% SDS-polyacrylamide gel electrophoresis and
transferred on to polyvinylidene difluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% nonfat
milk at room temperature (20–25°C) for 2 h, the membranes were
incubated with monoclonal anti-GFAP (dilution 1:1,000; cat. no.
ab10062), polyclonal anti-NF-κB-P65 (dilution 1:1,000; cat. no.
ab16502) or monoclonal anti-β-actin (dilution 1:1,000; cat. no.
ab8226) (all from Abcam) for 18 h at 4°C. Protein expression was
determined using horseradish peroxidase-conjugated secondary
antibodies (dilution 1:2,000; cat. nos. 7074 and 7076; Cell
Signaling Technology) and visualized using SuperSignal™
West Femto Substrate Trial kit (Thermo Fisher Scientific, Inc.).
ImageJ v1.8.0 (National Institutes of Health) was used for
densitometry.
Statistical analysis
All data in the present study are expressed as mean
± standard deviation (SD) and analyzed using GraphPad Prism 6.0
(GraphPad Software Inc., La Jolla, CA, USA). Two-way analysis of
variance (ANOVA), followed by Turkey's multiple comparison test,
was used to compare the different groups; P<0.05 was considered
to indicate a statistically significant result.
Results
CAPE suppresses the loss of RGCs after
ONC
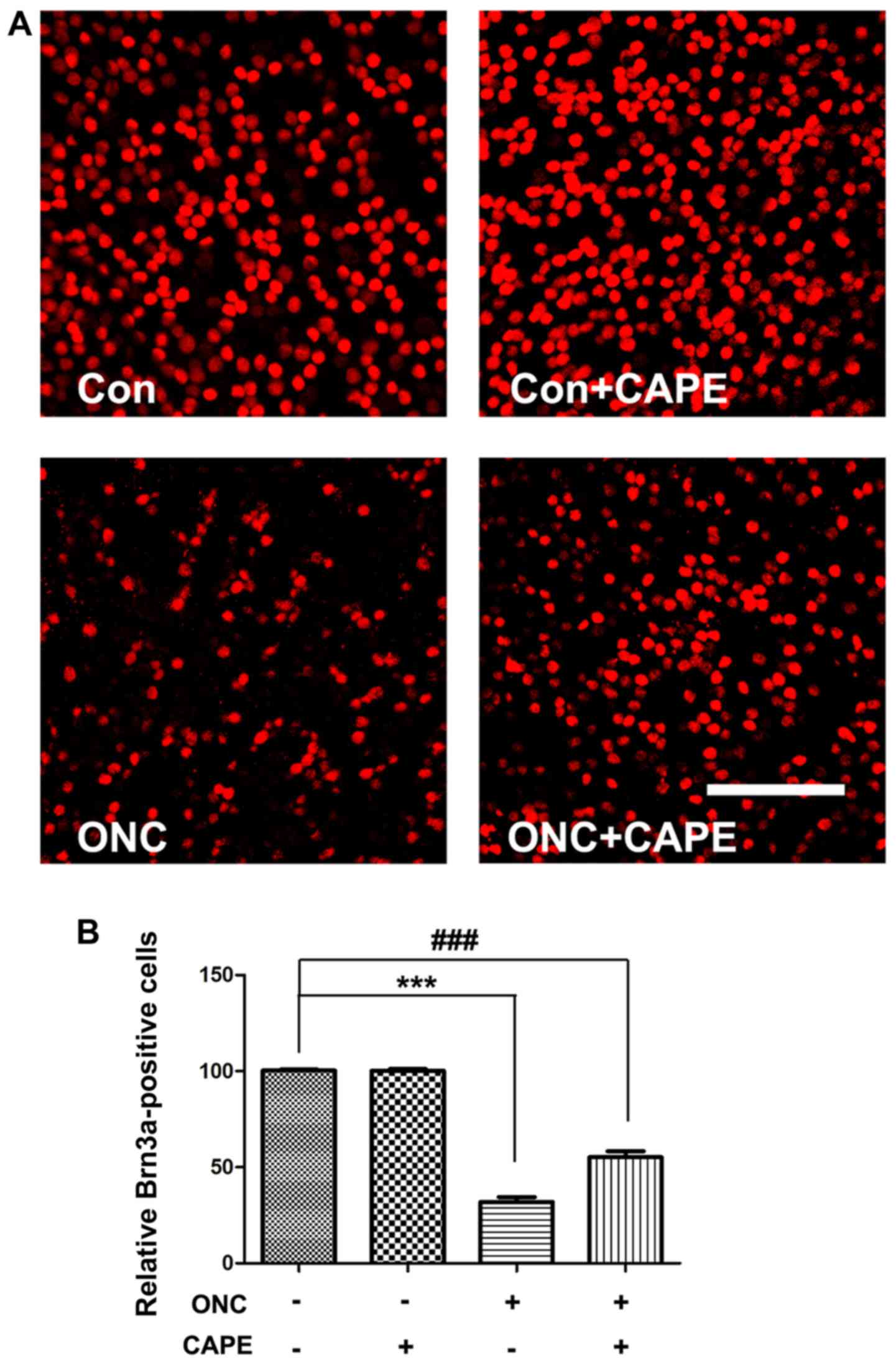
To investigate the protective effects of CAPE
against ONC-induced retinal damage, Brn3a immunofluorescence
staining was performed on the retinal flat mounts 14 days after ONC
injury. Representative retinal images are shown in Fig. 1A. Brn3a-labeled RGCs were counted
at the same distance from the optic nerve head. As expected, the
number of Brn3a-stained RGCs decreased in the vehicle-treated ONC
group (P<0.001) compared with the control group. Notably, CAPE
significantly suppressed the loss of RGCs compared with the
vehicle-treated ONC group (Fig.
1B), but demonstrated no effect on RGCs in intact retinas.
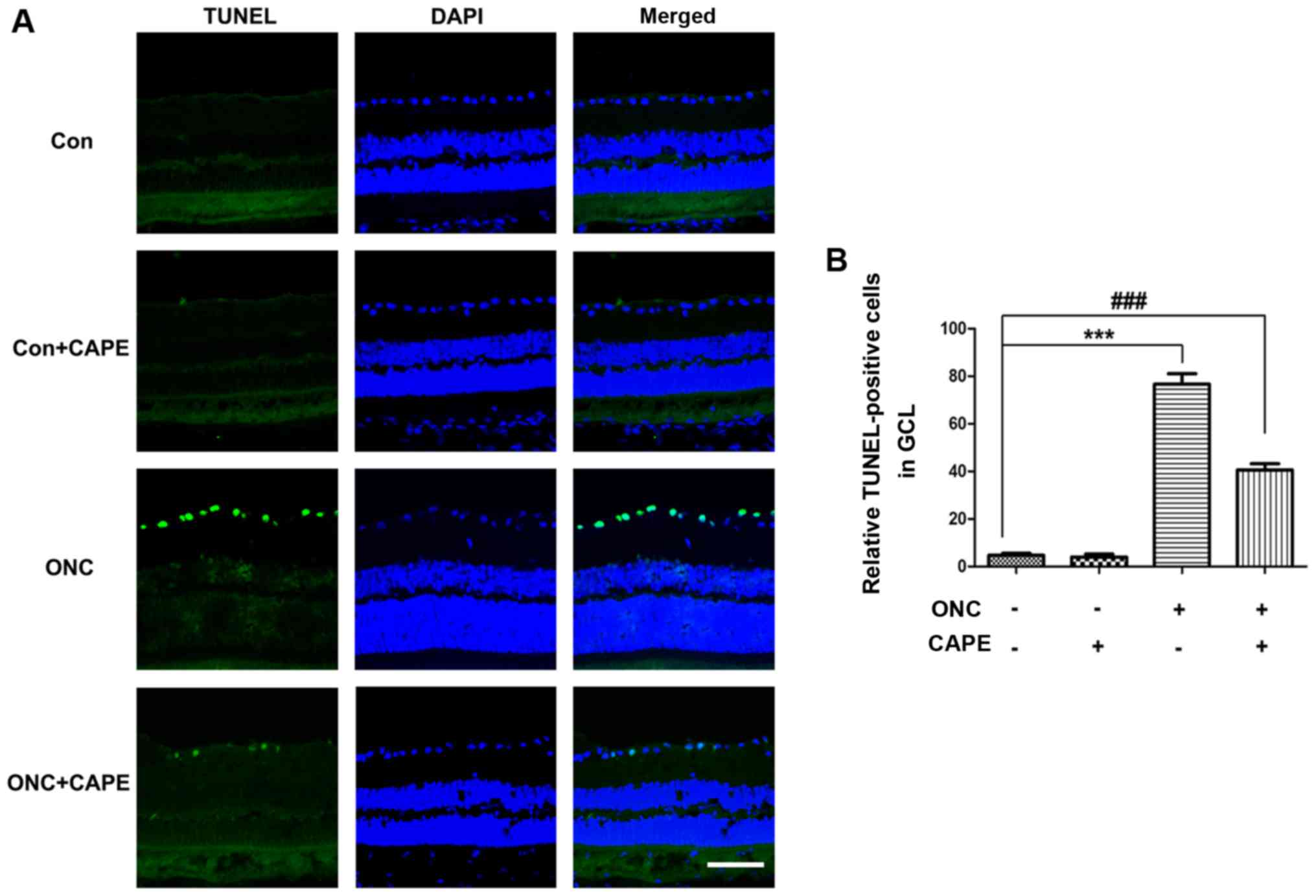
CAPE attenuates the apoptosis of RGCs
in retinas after ONC
To further examine the protective role of CAPE in
the retina after ONC, TUNEL-positive apoptotic cells in retina
frozen sections were stained at day 7. Few apoptotic cells in the
retinas were observed in the Con group and Con+CAPE group (Fig. 2A), and a large number of
TUNEL-positive cells were found in the retinas after ONC. However,
fewer TUNEL-stained cells were found in the ONC+CAPE group compared
with the ONC group (Fig. 2B).
These results confirmed the protective effect of CAPE in the
retina, and CAPE significantly attenuated RGC apoptosis induced by
ONC.
CAPE inhibits cytokine expression in
retinas after ONC
To determine whether the inflammatory response
caused by ONC injury is affected by CAPE treatment, cytokine
expression in retinas from the different groups was assessed using
real-time PCR. Compared with the control group, mRNA levels of IL-8
(Fig. 3A), IL-6 (Fig. 3B), iNOS (Fig. 3C), COX-2 (Fig. 3D), TNF-α (Fig. 3E) and CCL-2 (Fig. 3F), were significantly upregulated
in the ONC group. Expression of these pro-inflammatory cytokines
were suppressed in rats that received CAPE after ONC injury
(Fig. 3A-F). These results suggest
that CAPE could effectively inhibit inflammation after ONC.
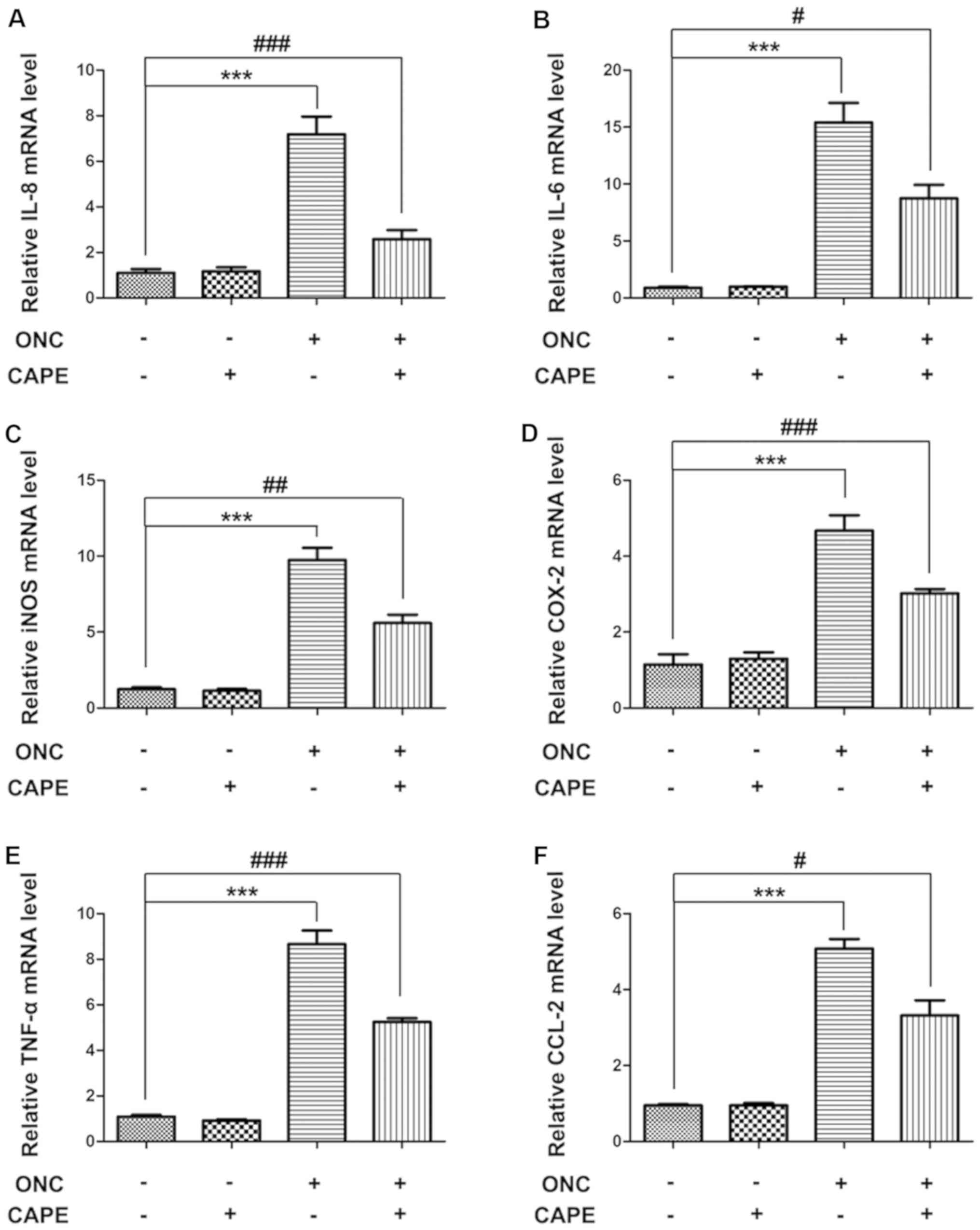 | Figure 3.Influence of CAPE on pro-inflammatory
cytokines following ONC. Relative mRNA levels of (A) IL-8, (B)
IL-6, (C) iNOS, (D) COX-2, (E) TNF-α and (F) CCL-2 in retinas 7
days after ONC were determined by quantitative PCR. (mean ± SD,
n=6, ***P<0.001 compared with the control group;
#P<0.05, ##P<0.01,
###P<0.001 compared with the ONC group). CAPE,
caffeic acid phenethyl ester; ONC, optic nerve crush; IL-8,
interleukin (IL)-8; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxygenase-2; TNF-α, tumor necrosis factor-α; CCL-2, C-C motif
ligand. |
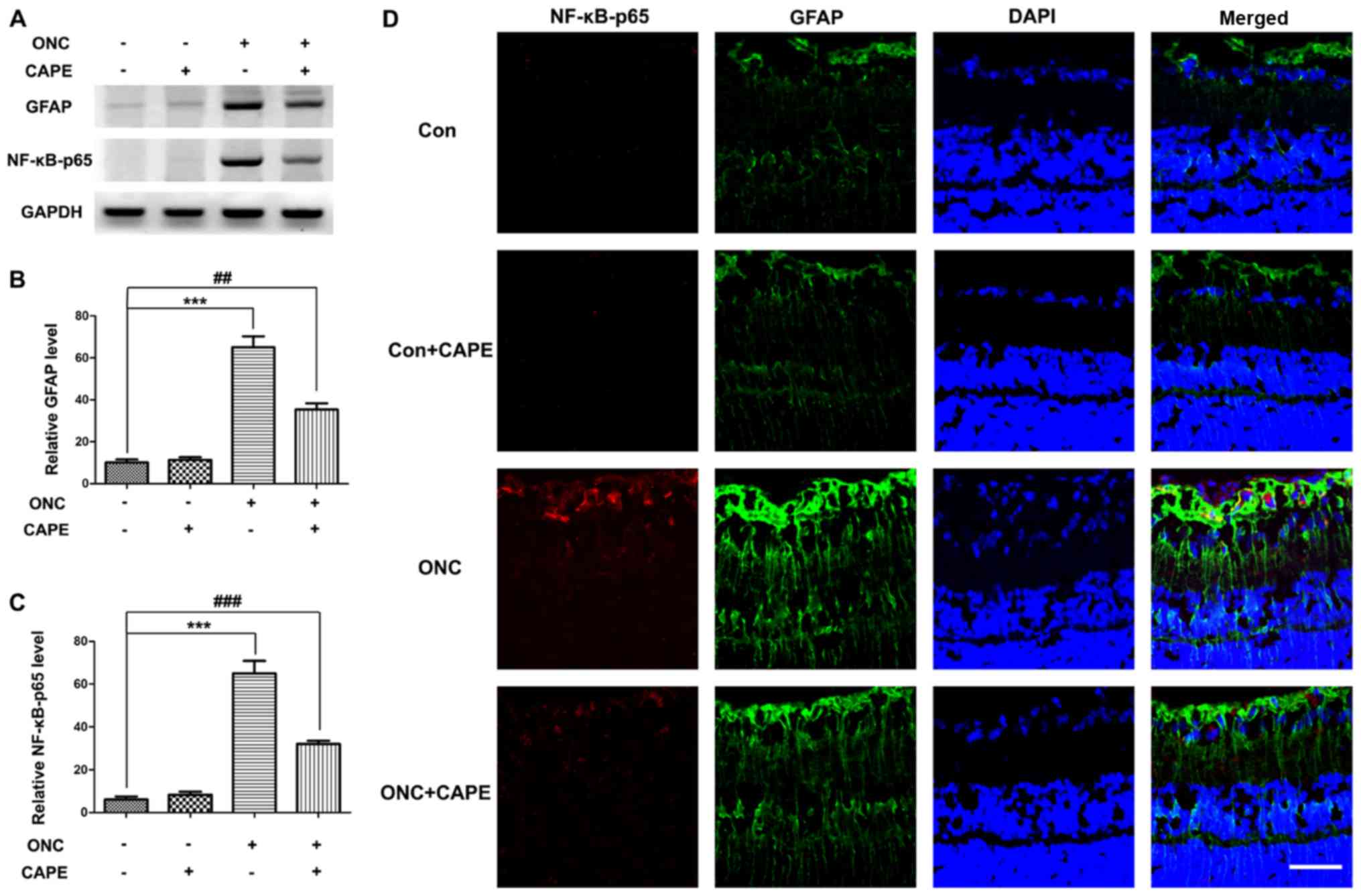
CAPE prevents gliosis caused by ONC
injury by inhibiting NF-κB activation
It is well established that astrocyte hypertrophy
and Müller cells (gliosis) are induced by ONC (26). To examine the contribution of
activated astrocytes to the protective role of CAPE after ONC
injury, the expression of GFAP by western blot analysis and
immunostaining of retinal sections was examined. As expected, the
expression of GFAP was increased on day 7 after ONC (Fig. 4A and B) and mainly localized in
astrocytes (Fig. 4D). The protein
level of GFAP was lower in the retinas of rats treated with CAPE
than that in the ONC group (Fig. 4A
and B). Additionally, the increase in NF-κB-p65 in the retina
caused by ONC injury was suppressed by CAPE (Fig. 4A and C). Importantly, double
immunostaining revealed that NF-κB-p65 was mainly expressed in
GFAP-positive cells (Fig. 4D),
suggesting that CAPE may attenuate the gliosis response by
inhibiting NF-κB activation.
CAPE suppresses the proliferation and
migration of astrocytes induced by LPS
To explore the effect of CAPE on astrocytes, rat
primary astrocytes were prepared and treated with different
concentrations of CAPE in vitro. Low doses of CAPE (0, 1, 5,
10 or 20 µM) demonstrated no effect on the viability of astrocytes;
however, 40 µM CAPE decreased the viability of astrocytes after a
48-h treatment (Fig. 5A). LPS
stimulated the proliferation of astrocytes, indicated by the
increase in cell viability (Fig.
5B). Notably, CAPE (5, 10 or 20 µM) inhibited the proliferation
of astrocytes induced by LPS, reflected by the significant decrease
in cell viability (Fig. 5B).
Furthermore, CAPE suppressed the migration of astrocytes induced by
LPS (Fig. 5C and D). Together,
these results suggested that the protective role of CAPE in ONC may
be associated with its effect on astrocyte proliferation and
migration.
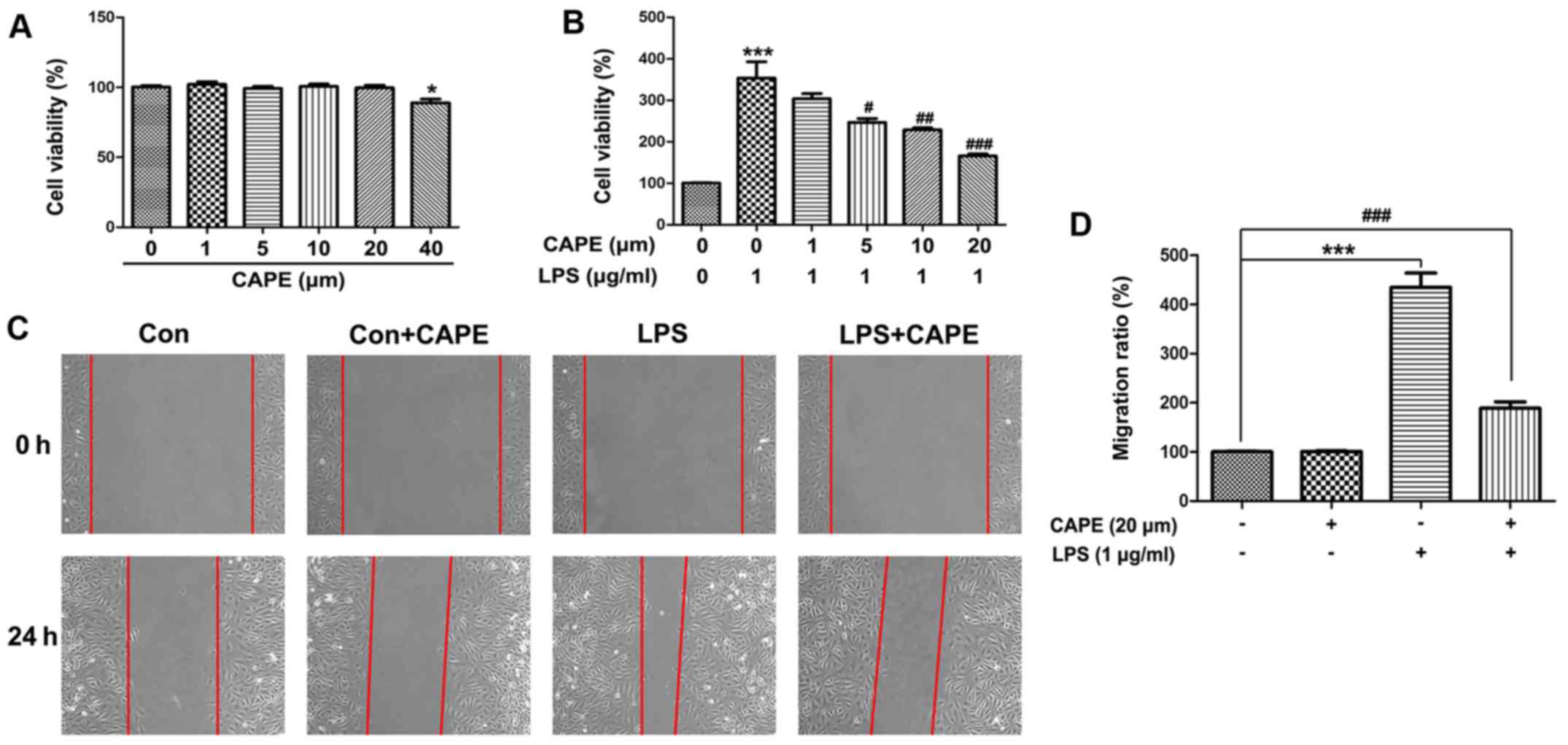 | Figure 5.Effect of CAPE on the viability and
migration of primary astrocytes. (A) Cell viability of astrocytes
exposed to different concentrations of CAPE (0, 1, 5, 10, 20 or 40
µM) for 48 h were detected with CCK-8. (B) The effect of different
doses of CAPE (0, 1, 5, 10 or 20 µM) on the cell viability of
LPS-treated astrocytes. (C) Migration of astrocytes treated with
LPS or CAPE were examined by scratch assay. No difference was found
between the Con and Con+CAPE groups 24 h after placing the scratch.
An obvious increase in astrocytes in the LPS group was found to
have migrated into the denuded space when compared to the Con
group. CAPE significantly suppressed the migration of the
astrocytes treated with LPS. (D) Migration ratio (%) of astrocytes.
(n=5, * P<0.05, ***P<0.001 compared with the Con group;
#P<0.05, ##P<0.01,
###P<0.001 compared with the LPS group). CAPE,
caffeic acid phenethyl ester; CCK-8, Cell Counting Kit-8; LPS,
lipopolysaccharide. |
CAPE suppresses the expression of
pro-inflammatory cytokines and the activation of NF-κB in
astrocytes
To investigate whether the effect of CAPE on
astrocytes was relevant to its protective function in ONC, the
expression of pro-inflammatory cytokines and the activation of
NF-κB in primary astrocytes were examined. As expected, the
increase in IL-8, IL-6, iNOS, COX-2, TNF-α and CCL-2 (Fig. 6A-F) in astrocytes were highly
suppressed by CAPE treatment. Similarly, the activation of NF-κB
was significantly suppressed by CAPE, demonstrated by protein
expression of GFAP and NF-κB-p65 (Fig.
6G-I). These data were consistent with the results in the ONC
model animals.
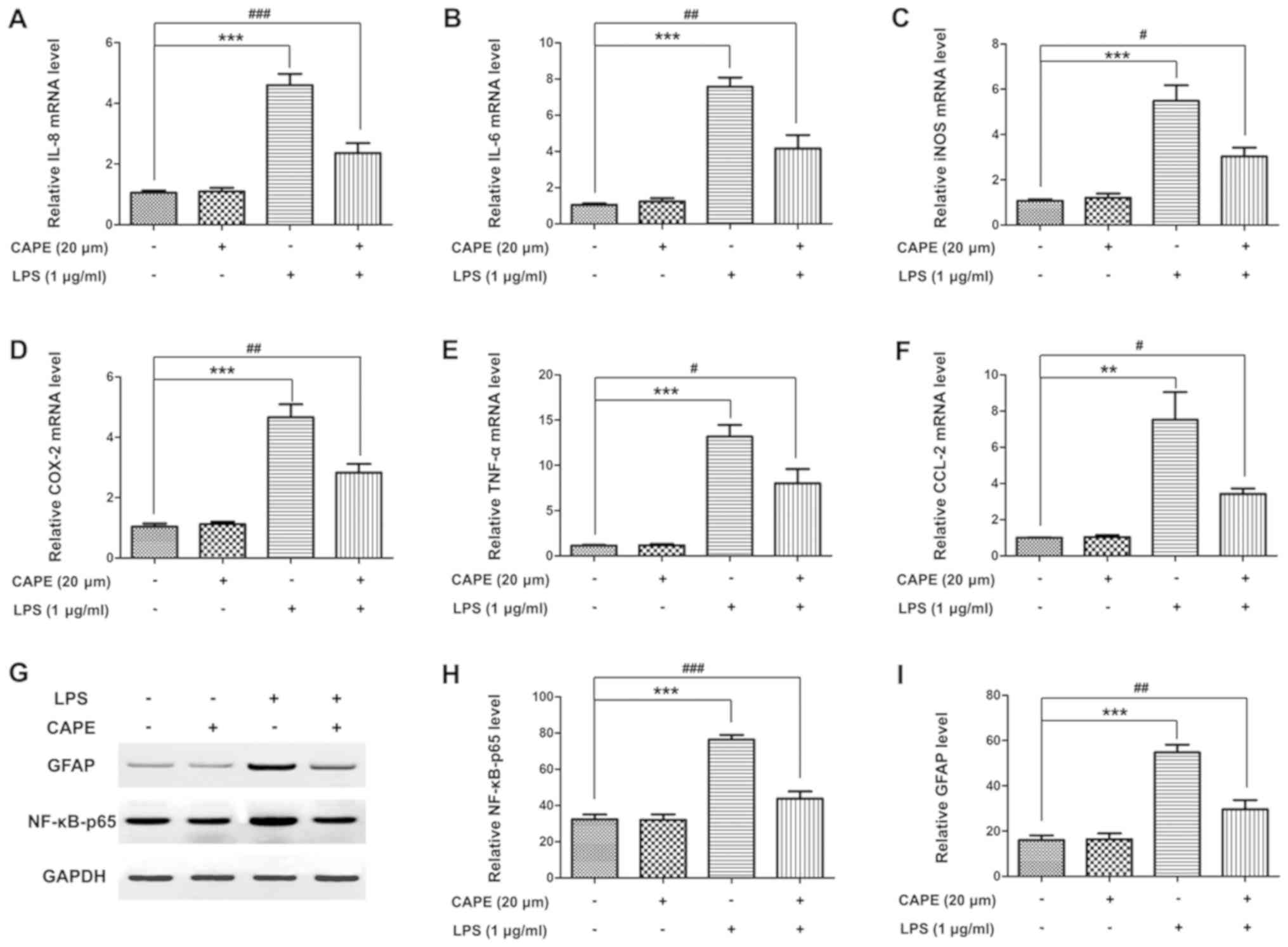 | Figure 6.CAPE suppresses the activation of
astrocytes. Astrocytes were exposed to CAPE (20 µM) or LPS (1
µg/ml) for 12 h. Total RNA of astrocytes from the different groups
was extracted and the relative mRNA levels of (A) IL-8, (B) IL-6,
(C) iNOS, (D) COX-2, (E) TNF-α and (F) CCL-2 in astrocytes were
examined quantitatively using real-time quantitative PCR. (G)
Protein expression of GFAP and NF-κB-p65 in astrocytes treated with
or without CAPE and LPS in vitro was assessed by western
blot analysis. The protein level of (H) GFAP and (I) NF-κB-p65 in
the different groups was evaluated by densitometric analysis. (mean
± SD, n=3, **P<0.01, ***P<0.001 compared with the control
group; #P<0.05, ##P<0.01,
###P<0.001 compared with the LPS group). CAPE,
caffeic acid phenethyl ester; LPS, lipopolysaccharide; IL-8,
interleukin (IL)-8; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxygenase-2; TNF-α, tumor necrosis factor-α; CCL-2, C-C motif
ligand. |
Discussion
Several animal models have been developed to study
glaucoma (27–29) among which optic nerve crush (ONC)
is widely used to evaluate the survival of injured retinal ganglion
cells (RGCs) and inflammation of glia. ONC is an acute injury that
kills the majority of RGCs within the first 2 weeks and triggers
atypical inflammatory response within the injured eye (30–33).
In the present study, using a rat ONC model, we have demonstrated
for the first time that caffeic acid phenethyl ester (CAPE),
exerted a neuroprotective role and attenuated inflammatory
responses by inhibiting nuclear factor kappa light-chain-enhancer
of activated B cells (NF-κB) activation.
RGC death is an important feature of glaucoma, which
can lead to irreversible vision loss. CAPE markedly attenuated the
symptoms of ONC, indicated by the increase in Brn3a-labeled RGCs
and the decrease in TUNNEL-positive apoptotic RGCs in the retinas
of rats that received CAPE treatment 10 min after surgery. In
agreement with these data, Shi and colleagues reported that CAPE
inhibited the apoptosis of retinal cells after ischemia-reperfusion
injury (22).
NF-κB signaling is always activated in response to
elevated intraocular pressure, vascular diseases and oxidative
stress. Oxidative stress has been reported to be largely
responsible for the loss of RGCs, molecular damage and cellular
dysfunction in glaucoma. NF-κB activation is a common pathological
pathway in many diseases characterized by inflammation, such as
rheumatoid arthritis, type I diabetes and glaucoma (34–37).
NF-κB activation was reported in both human glaucoma optic nerve
head astrocytes and in experimental animal models (38,39).
In this study, activation of NF-κB in astrocytes was also induced
in a rat ONC model and was suppressed in CAPE-treated animals.
Activated NF-κB enters the nucleus to induce transcription of
downstream inflammatory cytokines that exacerbate oxidative stress.
We found that CAPE also reduced the expression of cytokines
interleukin (IL)-8, IL-6, inducible nitric oxide synthase (iNOS),
cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α) and C-C
motif ligand-2 (CCL-2), which was consistent with previous studies
in experimental ulcerative colitis (40) and acute spinal cord injury
(41). These data suggest that the
protective effects of CAPE in ONC may be related to the alleviation
of neuro-inflammation in the retina.
Considering the decisive role of gliosis in the
pathological course of RGC damage (42,43)
we analyzed the expression of GFAP using western blot analysis and
immunohistochemical staining. As expected, GFAP in the retina was
upregulated after ONC injury. It was interesting to find that CAPE
alleviated gliosis caused by ONC, as evidenced by the significant
downregulation of GFAP and NF-κB compared with the ONC group. The
effect of CAPE in astrocytes was further confirmed in in
vitro experiments. CAPE suppressed reactive astrogliosis
induced by LPS in vitro, reflected by the inhibition of
proliferation and migration of primary cultured astrocytes, as well
as the expression of GFAP and NF-κB.
Data from the present study indicated that the
protective role of CAPE in experimental glaucoma may be mediated by
the anti-inflammatory function of CAPE in astrocytes through
inhibition of NF-κB activation. This may not be the only protective
role of CAPE; other pathological changes in glaucoma, including
mitochondrial damage, endothelial dysregulation and hypoxia may
also be influenced by CAPE treatment. We speculate that CAPE
possibly functions by regulating intracellular or extracellular
signals, such as reactive oxygen species, which are massively
generated in glaucoma-related oxidative stress and lead to the
activation of NF-κB (37,44). Further studies are necessary to
explore the molecular mechanisms by which CAPE inhibits activation
of NF-κB. Whether CAPE can block the translocation of NF-κB to the
nucleus and inhibit NF-κB binding to the target sequence warrants
further investigation.
In conclusion, our findings suggest that systemic
administration of CAPE appears to afford neuroprotection in a rat
model of ONC injury, and controls retinal gliosis and the release
of pro-inflammatory mediators by suppressing NF-κB activation. CAPE
may be a promising candidate for the treatment of glaucoma.
However, CAPE may act differently in mice, rats or humans;
therefore, more in-depth, systematic research is needed to
determine the molecular mechanisms, optimal concentrations, and
possible side effects of CAPE before its clinical application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ supervised the study; SJ and GL acquired the data
and wrote a draft of the manuscript; YJ, CC, GL and YX prepared the
experimental materials and performed the animal experiments and
in vitro assays; CC, XS and LH interpreted data, performed
the statistical analysis and analyzed the resul the ts; YJ and SJ
revised and approved the final version of the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experimental animal protocols were approved by
the Institutional Animal Care and Use Committee of Wenzhou Medical
University (Wenzhou, Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jonas JB, Aung T, Bourne RR, Bron AM,
Ritch R and Panda-Jonas S: Glaucoma. Lancet. 390:2183–2193. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bourne RR, Stevens GA, White RA, Smith JL,
Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, et
al: Causes of vision loss worldwide, 1990–2010: A systematic
analysis. Lancet Glob Health. 1:e339–e349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stevens GA, White RA, Flaxman SR, Price H,
Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Resnikoff S,
et al: Global prevalence of vision impairment and blindness:
Magnitude and temporal trends, 1990–2010. Ophthalmology.
120:2377–2384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doozandeh A and Yazdani S: Neuroprotection
in glaucoma. J Ophthalmic Vis Res. 11:209–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian K, Shibata-Germanos S, Pahlitzsch M
and Cordeiro MF: Current perspective of neuroprotection and
glaucoma. Clin Ophthalmol. 9:2109–2118. 2015.PubMed/NCBI
|
|
6
|
Calkins DJ, Pekny M, Cooper ML and
Benowitz L; Lasker/IRRF Initiative on Astrocytes and Glaucomatous
Neurodegeneration Participants, : The challenge of regenerative
therapies for the optic nerve in glaucoma. Exp Eye Res. 157:28–33.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bond WS and Rex TS: Evidence that
erythropoietin modulates neuroinflammation through differential
action on neurons, astrocytes and microglia. Front Immunol.
5:5232014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez VL and Caspi RR: Immune mechanisms
in inflammatory and degenerative eye disease. Trends Immunol.
36:354–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cueva Vargas JL, Belforte N and Di Polo A:
The glial cell modulator ibudilast attenuates neuroinflammation and
enhances retinal ganglion cell viability in glaucoma through
protein kinase A signaling. Neurobiol Dis. 93:156–171. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu Y, Yang B, Hu Y, Lu L, Lu X, Wang J, Xu
F, Yu S, Huang J and Liang X: Wogonin prevents TLR4-NF-κB-medicated
neuro-inflammation and improves retinal ganglion cells survival in
retina after optic nerve crush. Oncotarget. 7:72503–72517. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lindsey JD, Duong-Polk KX, Hammond D,
Leung CK and Weinreb RN: Protection of injured retinal ganglion
cell dendrites and unfolded protein response resolution after
long-term dietary resveratrol. Neurobiol Aging. 36:1969–1981. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akyol S, Ugurcu V, Balci M, Gurel A, Erden
G, Cakmak O and Akyol O: Caffeic acid phenethyl ester: Its
protective role against certain major eye diseases. J Ocul
Pharmacol Ther. 30:700–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Natarajan K, Singh S, Burke TR Jr,
Grunberger D and Aggarwal BB: Caffeic acid phenethyl ester is a
potent and specific inhibitor of activation of nuclear
transcription factor NF-kappa B. Proc Natl Acad Sci USA.
93:9090–9095. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parlakpinar H, Ozer MK, Ucar M, Gaffaroglu
M, Vardi N, Koc M and Acet A: Protective effects of caffeic acid
phenethyl ester (CAPE) on amikacin-induced nephrotoxicity in rats.
Cell Biochem Funct. 24:363–367. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kujumgiev A, Tsvetkova I, Serkedjieva Y,
Bankova V, Christov R and Popov S: Antibacterial, antifungal and
antiviral activity of propolis of different geographic origin. J
Ethnopharmacol. 64:235–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren J, Zhang N, Liao H, Chen S, Xu L, Li
J, Yang Z, Deng W and Tang Q: Caffeic acid phenethyl ester
attenuates pathological cardiac hypertrophy by regulation of
MEK/ERK signaling pathway in vivo and vitro. Life Sci. 181:53–61.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russo A, Cardile V, Sanchez F, Troncoso N,
Vanella A and Garbarino JA: Chilean propolis: Antioxidant activity
and antiproliferative action in human tumor cell lines. Life Sci.
76:545–558. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tolba MF, Omar HA, Azab SS, Khalifa AE,
Abdel-Naim AB and Abdel-Rahman SZ: Caffeic acid phenethyl ester: A
review of its antioxidant activity, protective effects against
ischemia-reperfusion injury and drug adverse reactions. Crit Rev
Food Sci Nutr. 56:2183–2190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irmak MK, Fadillioglu E, Sogut S, Erdogan
H, Gulec M, Ozer M, Yagmurca M and Gozukara ME: Effects of caffeic
acid phenethyl ester and alpha-tocopherol on reperfusion injury in
rat brain. Cell Biochem Funct. 21:283–289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amodio R, De Ruvo C, Sacchetti A, Di Santo
A, Martelli N, Di Matteo V, Lorenzet R, Poggi A, Rotilio D, Cacchio
M and Esposito E: Caffeic acid phenethyl ester blocks apoptosis
induced by low potassium in cerebellar granule cells. Int J Dev
Neurosci. 21:379–389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong H, Cui L, Xu F, Chen L, Jiang L,
Huang H, Xu J, Zhao X, Li L, Zeng S and Li M: Up-regulation of Wip1
involves in neuroinflammation of retinal astrocytes after optic
nerve crush via NF-κB signaling pathway. Inflamm Res. 65:709–715.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi Y, Wu X, Gong Y, Qiu Y, Zhang H, Huang
Z and Su K: Protective effects of caffeic acid phenethyl ester on
retinal ischemia/reperfusion injury in rats. Curr Eye Res.
35:930–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valapala M, Hose S, Gongora C, Dong L,
Wawrousek EF, Samuel Zigler J Jr and Sinha D: Impaired
endolysosomal function disrupts Notch signalling in optic nerve
astrocytes. Nat Commun. 4:16292013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mi H and Barres BA: Purification and
characterization of astrocyte precursor cells in the developing rat
optic nerve. J Neurosci. 19:1049–1061. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu J and Jakobs TC: The time course of
gene expression during reactive gliosis in the optic nerve. PLoS
One. 8:e670942013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKinnon SJ, Schlamp CL and Nickells RW:
Mouse models of retinal ganglion cell death and glaucoma. Exp Eye
Res. 88:816–824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schaub JA, Kimball EC, Steinhart MR,
Nguyen C, Pease ME, Oglesby EN, Jefferys JL and Quigley HA:
Regional retinal ganglion cell axon loss in a murine glaucoma
model. Invest Ophthalmol Vis Sci. 58:2765–2773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wisely CE, Sayed JA, Tamez H, Zelinka C,
Abdel-Rahman MH, Fischer AJ and Cebulla CM: The chick eye in vision
research: An excellent model for the study of ocular disease. Prog
Retin Eye Res. 61:72–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rathnasamy G, Foulds WS, Ling EA and Kaur
C: Glutamate inhibits the pro-survival effects of insulin-like
growth factor-1 on retinal ganglion cells in hypoxic neonatal rat
retina. Mol Neurobiol. 54:3453–3464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobrado-Calvo P, Vidal-Sanz M and
Villegas-Pérez MP: Rat retinal microglial cells under normal
conditions, after optic nerve section, and after optic nerve
section and intravitreal injection of trophic factors or macrophage
inhibitory factor. J Comp Neurol. 501:866–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bodeutsch N, Siebert H, Dermon C and
Thanos S: Unilateral injury to the adult rat optic nerve causes
multiple cellular responses in the contralateral site. J Neurobiol.
38:116–128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rovere G, Nadal-Nicolás FM, Sobrado-Calvo
P, García-Bernal D, Villegas-Pérez MP, Vidal-Sanz M and
Agudo-Barriuso M: Topical treatment with bromfenac reduces retinal
gliosis and inflammation after optic nerve crush. Invest Ophthalmol
Vis Sci. 57:6098–6106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Long J, He T, Belshaw R and Scott J:
Integrated genomic approaches identify major pathways and upstream
regulators in late onset Alzheimer's disease. Sci Rep. 5:123932015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Wang LM, Xu JZ, Tian K, Gu CX and Li
ZF: Gastrodia elata attenuates inflammatory response by
inhibiting the NF-κB pathway in rheumatoid arthritis
fibroblast-like synoviocytes. Biomed Pharmacother. 85:177–181.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oguiza A, Recio C, Lazaro I, Mallavia B,
Blanco J, Egido J and Gomez-Guerrero C: Peptide-based inhibition of
IκB kinase/nuclear factor-κB pathway protects against
diabetes-associated nephropathy and atherosclerosis in a mouse
model of type 1 diabetes. Diabetologia. 58:1656–1667. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saccà SC, Gandolfi S, Bagnis A, Manni G,
Damonte G, Traverso CE and Izzotti A6: From DNA damage to
functional changes of the trabecular meshwork in aging and
glaucoma. Ageing Res Rev. 29:26–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Agapova OA, Kaufman PL and Hernandez MR:
Androgen receptor and NFκB expression in human normal and
glaucomatous optic nerve head astrocytes in vitro and in
experimental glaucoma. Exp Eye Res. 82:1053–1059. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Erb C: Importance of the nuclear factor
kappaB for the primary open angle glaucoma-a hypothesis. Klin Monbl
Augenheilkd. 227:120–127. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khan MN, Lane ME, McCarron PA and
Tambuwala MM: Caffeic acid phenethyl ester is protective in
experimental ulcerative colitis via reduction in levels of
pro-inflammatory mediators and enhancement of epithelial barrier
function. Inflammopharmacology. 26:561–569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ak H, Gülşen İ, Karaaslan T, Alaca İ,
Candan A, Koçak H, Atalay T, Çelikbilek A, Demir İ and Yılmaz T:
The effects of caffeic acid phenethyl ester on inflammatory
cytokines after acute spinal cord injury. Ulus Travma Acil Cerrahi
Derg. 21:96–101. 2015.PubMed/NCBI
|
|
42
|
Spaide RF: Retinal vascular cystoid
macular edema: Review and new theory. Retina. 36:1823–1842. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tura A, Schuettauf F, Monnier PP,
Bartz-Schmidt KU and Henke-Fahle S: Efficacy of Rho-kinase
inhibition in promoting cell survival and reducing reactive gliosis
in the rodent retina. Invest Ophthalmol Vis Sci. 50:452–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chrysostomou V, Rezania F, Trounce IA and
Crowston JG: Oxidative stress and mitochondrial dysfunction in
glaucoma. Curr Opin Pharmacol. 13:12–15. 2013. View Article : Google Scholar : PubMed/NCBI
|