Introduction
Parkinson's disease (PD) is the second most common
neurodegenerative disorder worldwide and affects 1% of the
population over the age of 60 (1).
This neurodegenerative disorder is characterized by the progressive
loss of substantia nigra dopaminergic neurons as well as striatal
projections, which causes the typical symptomatology, including
bradykinesia, tremor, muscle rigidity, and postural instability
(1). However, only 5–10% of PD
cases are considered to involve genetic factors (1). Although recent studies have found
that several factors play a key role in the neuronal pathogenesis
of PD (2), the etiology of PD
remains elusive. It is now considered that α-synuclein (SNCA), a
major component of Lewy bodies, is one of the morphological markers
of PD. Several site mutations in SNCA have been identified as the
cause for early onset PD in a dominant mode of inheritance
(3). Moreover, methylation of
intron 1 of human SNCA results in reduction of gene expression in
the brains of PD patients, suggesting that methylation of SNCA is
correlative with PD pathogenesis (3). Despite the lack of any differences in
the postmortem analysis of regional specific methylation at the
anterior cingulate or putamen of the brains of PD patients and
healthy individuals, methylation in the substantia nigra of PD
patients was significantly and specifically decreased (4). Furthermore, single CpG analysis
revealed a fluctuation in the methylation levels of various brain
regions and LBD stages, which is suggestive of a potential role for
DNA methylation of α-synuclein in the occurrence of PD (5).
One of the first-line antiepileptic drugs (AEDs) is
lithium, a drug that is also used in the treatment of bipolar
disorder (6). Similar to other
anticonvulsants, lithium inhibits some of the functions of sodium
and calcium channels (6). Despite
the need for deeper investigation into its in vivo target,
lithium treatment could induce a significant change in the activity
of histone deacetylase (HDAC) as well as glycogen synthase kinase
(GSK-3). Further studies have revealed that the inhibition of
GSK-3β activity induced by lithium mimics resulted in a reduction
of DNA methylation in neural stem cells (7). Similar to valproic acid (VPA),
lithium was recently demonstrated to be an effective regulator for
the expression of several PD-related miRNAs (6). In neuroblastoma cells, lithium
treatment attenuated the apoptosis induced by rotenone, an
inhibitor of mitochondrial complex 1 that induces PD-like
neurodegeneration in vivo (8). It has also been suggested that by
enhancing the autophagic pathway that is associated with the
degradation of aberrant accumulated α-synuclein protein in PD,
lithium could, act as a neuroprotective agent in rotenone-induced
SH-SY5Y cells as well as in MPTP-lesioned mice (6,8–10).
Other studies reported that lithium could increase the expression
of neurotrophins, which are involved in neural survival as well as
plasticity, for example the nerve growth factors (NGF),
brain-derived neurotrophic factor (BDNF), and the glial cell
line-derived neurotrophic factor (GDNF) (11,12).
Herein, in order to detect the effects of lithium on
the neurodegenerative symptoms using behavioral tests, a commonly
used PD-like mouse model was employed, and the animals were fed
chow with lithium carbonate for 5 weeks. Biochemical assays were
conducted to explore the molecular events associated with PD
pathogenesis and further elucidate the relationship between lithium
and the development and progression of this disease.
Materials and methods
Animals
A total of 80 male C57BL/6 mice (7–8 weeks, ~25 g)
were obtained from the Shanghai Experimental Animal Center of
Chinese Academy of Sciences (Shanghai, China). Each individual cage
contained 4 mice, with free access to water and food. All of the
animal rooms were maintained at a temperature of 21–23°C and
humidity of 40–60%, with a 12-h light-dark cycle. To develop the
MPTP PD model, male C57BL/6J mice were injected intraperitoneally
(i.p.) with 1-methyl-4-phenyl-1,2,3,6-tetrahydrapyridine (MPTP) at
20 mg/kg (body weight; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) twice a week, plus 250 mg/kg probenecid injected 0.5 h
before. Administration of the treatment was carried out for 5 weeks
with feeding of 0.2% lithium carbonate (Sigma-Aldrich; Merck KGaA)
by chow. On the 36th day, the mice were subjected to behavioral
tests. Upon completion, mice were anesthetized with 250 mg/kg
avertin intraperitoneally and perfused transcardially with sterile
saline. Brains were quickly harvested, and one hemibrain was
immediately frozen in liquid nitrogen for biochemical studies while
the other hemibrain was post-fixed in 4% paraformaldehyde for
immunohistochemical stains. All animal procedures complied with the
current ethical considerations of the Shanghai University of
Traditional Chinese Medicine's Animal Ethics Committee, which is in
accordance with the National Research Council criteria. All animal
experiments were reviewed and approved by the Institutional Animal
Care and Use Committee (IACUC) of Shanghai University of
Traditional Chinese Medicine and were performed in accordance with
the relevant guidelines and regulations as well as approved by the
guidelines on ethical standards for investigation of PD in
conscious animals. Animals were sedated or anesthetized using
carbon dioxide prior to cervical dislocation. Cervical dislocation
to euthanize mice was performed by trained research personnel after
the approval of the IACUC of Shanghai University of Traditional
Chinese Medicine's Animal Ethics Committee and the method was
performed in accordance with the American Veterinary Medical
Association (AVMA) Guidelines for the Euthanasia of Animals (2013
Edition).
Behavioral tests
Open-field test
The open field activity was assessed in a 27×27×38
cm chamber with 50 lux (lx) illumination. Fifteen minutes of free
movement was tracked by TruScan Open Field version 2.04 software
2.04 (Coulbourn Instruments, Holliston, MA, USA). Total movement
times and distances were scored to assess locomotor activity while
a percentage of movement time in the margin area (the area within
8.76 cm from the chamber wall) was calculated for scrutinizing the
autonomous activity (13).
Rotarod performance test
Rotarod training was performed concurrently for 10
min during 5 consecutive days: On the first day, the mice were
placed on the rotating rod at 1.5 × g for 5 min. Every 30 sec, the
rod speed was increased by 0.4 × g up to 5.5 × g, and then
maintained at this speed for 1 min. On the second day, a 1.5 × g
speed was used for 1.5 min. The rod speed was increased by 0.4 × g
every 30 sec until it reached 7.3 × g, where it was maintained for
10 min. On the third, fourth, and fifth days, the latencies of
falling off the rod with a linear increase in rod speed from 1.5 ×
g up to 15 × g for 5 min were measured 3 times and averaged. The
actual test protocol was the same for the last 3 days of the
training protocol.
Bisulfite conversion and
methylation-specific-PCR (MSP)
For the bisulfite modification, the EZ DNA
Methylation-Gold™ Kit (Zymo Research Corp., Irvine, CA, USA) was
used. In this technique, unmethylated cytosines are converted to
uracil while methylated cytosines remain unchanged in
bisulfite-treated DNA. Thus, the methylation status of the DNA
sample can be analyzed by PCR amplification, called ‘methylation
specific-PCR.’ DNA (~1 µg) was used for bisulfite conversion, and
then MSP was carried out using specific primers for both bisulfite
converted methylated and unmethylated DNA samples in a total 25 µl
mix containing 1X PCR buffer, 15 mM MgCl2, 200 µM of
each dNTP, 0.4 µM of each primer, <1 µg template DNA, and 2.5 U
HotStarTaq DNA Polymerase (Qiagen, Inc., Valencia, CA, USA). After
the initial denaturation step at 94°C for 15 min, 35 cycles were
performed for the regions of SNCA using the following conditions:
denaturation at 94°C for 1 min, at annealing temperature for each
PCR bisulfide conversion-specific primer pair for 1 min, extension
at 72°C for 1 min, and then final extension at 72°C for 10 min.
Bisulfite-treated DNA was amplified using primers SNCA-PromF,
5′-AAAATTTTGAAGATATTTGAATTAAAG-3′ and SNCA-PromR,
5′-CTAATCCTCCTCCTTCTCCTTCTC-3′; SNCA-IntF,
5′-GGAGTTTAAGGAAAGAGATTTGATT-3′ and SNCA-IntR,
5′-CAAACAACAAACCCAAATATAATAA-3′, specifically designed for the
bisulfite-treated DNA. The PCR products SNCA (−926/-483; intron 1)
were cloned into pMD 18-T Vector (Takara Biotechnology Co., Ltd.,
Dalian, China) following the manufacturer's instructions. Plasmid
DNA was isolated from at least 10 clones per region (Wizard Plus SV
Minipreps; Promega Corporation, Madison, WI, USA), sequenced using
vector-specific primers and the Big Dye Terminator v1.1 Cycle
Sequencing Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and analyzed on an ABI PRISM 310 Genetic
Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Quality control for DNA methylation was performed using BiQ
(software tool for DNA methylation analysis; http://biq-analyzer.bioinf.mpi-inf.mpg.de/).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA, including mRNA and miRNA, was isolated
from samples using an RNeasy Mini Kit (Qiagen, Inc.). Next, cDNA
was synthesized using a PrimeScript™ RT reagent kit (Takara
Biotechnology Co., Ltd.) and subsequently used as templates for
qPCR. The primers were designed using Primer 5.0 (Premier Biosoft
International, Palo Alto, CA, USA). Primers of real-time PCR were
SNCA F, 5′-GGACCAGTTGGGCAAGAATG-3′ and R,
5′-GGGCACATTGGAACTGAGCAC-3′; GAPDH F, 5′-CGGAGTCAACGGATTTGGTC-3′
and R, 5′-TTCTCCATGGTGGTGAAGAC-3′; miR-148a F,
5′-TCAGTGCACTACAGAACTTTGT-3′ and R, 5′-GCTGTCAACGATACGCTACG-3′; U6
F, 5′-CTTCGGCAGCACATATAC-3′ and R, 5′-GAACGCTTCACGAATTTGC-3′. qPCR
was performed in triplicate with SYBR-Green (Takara Biotechnology
Co., Ltd.) on an Applied Biosystems 7500 Fast Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction
parameters were set as follows: 95°C for 5 min, followed by 40
cycles of 10 sec for 95°C and 1 min for 60°C. For miRNA RT-qPCR, 1
mg of isolated RNA was reverse-transcribed with stem-loop primers
from a BioTNT miRNA qPCR Detection Primer Set (BioTNT
Biotechnologies Co, Ltd., Shanghai, China). All reactions were
repeated in triplicate. The relative mRNA and miRNA expression
levels were separately analyzed using the 2−∆∆Cq method
(14) with GADPH and U6 as the
endogenous controls.
miRNA expression microarrays
Total RNA (including miRNAs) was isolated from the
brain tissue of the substantia nigra using TRIzol®
(Thermo Fisher Scientific, Inc.) with slight modification.
Polyacryl carrier was added to improve RNA recovery. The global
miRNA expression profiles were obtained using the Agilent SurePrint
Mouse miRNA Microarrays (Agilent Technologies, Inc., Santa Clara,
CA, USA) containing 1,881 mouse miRNAs based on Sanger miRBase
release 12.0. RNA samples were processed, labeled, and hybridized
onto the microarrays according to the manufacturer's protocols.
Microarray slides were then scanned using the G3 High Resolution
Scanner, and microarray data were extracted by Feature Extraction
software version 10.7.3.1 (Agilent Technologies, Inc.).
Immunohistochemistry
Animals were anaesthetized with ether and perfused
through the heart with 4% paraformaldehyde in 0.1 mM phosphate
buffer pH 7.4. Brains were removed, post-fixed overnight in the
same fixative, and then washed in buffered 18% sucrose until they
sunk. Sections were cut with a freezing microtome at 30-µm
thickness, and then permeabilized for 20 min with
phosphate-buffered saline (PBS) containing 0.1% Triton X-100,
before a 20-min incubation in methanol containing 0.3%
H2O2 to quench endogenous peroxidase
activity. Subsequently, the sections were incubated for 30 min in
PBS containing 2% normal goat serum to block non-specific binding
sites. For immunohistochemical localization of tyrosine hydroxylase
(TH), the TH antibody was used at a dilution of 1:1,000 (cat. no.
sc-136100, Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Antibody exposure was performed overnight at 4°C, followed by a
90-min incubation at room temperature with a horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG)
secondary antibody (cat. no. A0216, Beyotime Institute of
Biotechnology, Haimen, China). The immunoreaction was visualized
using DAB Horseradish Peroxidase Color Development Kit (Beyotime
Institute of Biotechnology). Digital images were captured using a
Leica DM2000 Microscopic imaging system, and TH-positive neuronal
counts and the integrated optical density value (IOD) of
α-synuclein-positive fibers were estimated within the substantia
nigra by ImageJ software (NIH) as previously described (15).
Western blotting
The substantia nigra of wild-type, MPTP-treated, and
lithium-treated mice (n=3), were dissected, pooled, and homogenized
in lysis buffer (Beyotime Institute of Biotechnology) on ice plus
1:100 volume of phenylmethylsulfonyl fluoride (PMSF), before final
centrifugation at 14,000 × g for 5 min to remove debris. The BCA
kit (Beyotime Institute of Biotechnology) was used to assess the
protein concentrations. After heating at 100°C with loading buffer
for 4 min, the proteins (30 µg) were resolved by SDS-PAGE (12% for
a-synuclein, 6% for DNMT1) and transferred to nitrocellulose
membranes (Amersham; GE Healthcare, Chicago, IL, USA) at 200 mA for
40 min. Tris-buffered saline and Tween-20 (TBST) containing 5%
skimmed milk powder was used to block the membranes at room
temperature for 1 h, and then membranes were washed with TBST prior
to incubation with antibodies against α-synuclein (ab27766,
1:2,000; Abcam, Cambridge, MA, USA) and DNMT1 (ab19905, 1:2,000;
Abcam) overnight at 4°C. After washing thrice with TBST, the
membranes were incubated with horseradish peroxidase conjugated
anti-rabbit (cat. no. A0208) or anti-mouse IgG (cat. no. A0216,
Beyotime Institute of Biotechnology) at a dilution of 1:2,000 at
room temperature for 45 min. After washing for three further times
with TBST, the proteins were visualized using an enhanced
chemiluminescence kit (EMD Millipore, Billerica, MA, USA), and then
quantified using Quantity One software version 4.4.6 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), normalized to GAPDH.
Statistical analysis
The raw data were analyzed by Origin 8.5 software
(OriginLab, Northampton, MA, USA). Results of data analysis were
expressed as the means ± standard error of the mean (SEM) with the
number of experiments indicated in the figure legends. Behavioral
experiments were statistically analyzed by one-way ANOVA. Tukey's
test was employed to compare the differences between all the
groups. For all statistical tests, a value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Lithium improves behavioral
performance in an MPTP-induced Parkinson mouse model
To examine the effects lithium had on MPTP-induced
Parkinson mice, behavioral tasks were performed after 5 weeks of
lithium treatment. The results revealed a significant reduction in
the locomotor activity in the MPTP-treated mice and lithium
treatment relieved this effect satisfactorily. As revealed in
Fig. 1A and B, the average
movement distance was 534.23±48.01 cm and the average activity time
was 246.33±6.48 sec. Following impairment, the movement distance
and activity time values were reduced to 315.09±25.59 cm (MPTP
group vs. CTRL group, P<0.01) and 194.80±40.79 sec (MPTP group
vs. CTRL group, P<0.01), respectively, however, after lithium
administration for 5 weeks, those values rebounded to 423.40±33.21
(lithium group vs. MPTP group, P<0.05) cm and 242.75±8.05 sec
(lithium group vs. MPTP group, P<0.05), respectively.
Furthermore, in rotarod tests, the compound also prolonged the drop
time of MPTP-treated mice, resulting in an increase in the drop
time for MPTP-treated mice from 148.59±13.03 sec (MPTP group vs.
CTRL group, P<0.05) to 204.70±23.27 sec (lithium group vs. MPTP
group, P<0.05) bringing it close to that of the control group
results 228.62±15.79 sec (lithium group vs. CTRL group, P>0.05;
Fig. 1C).
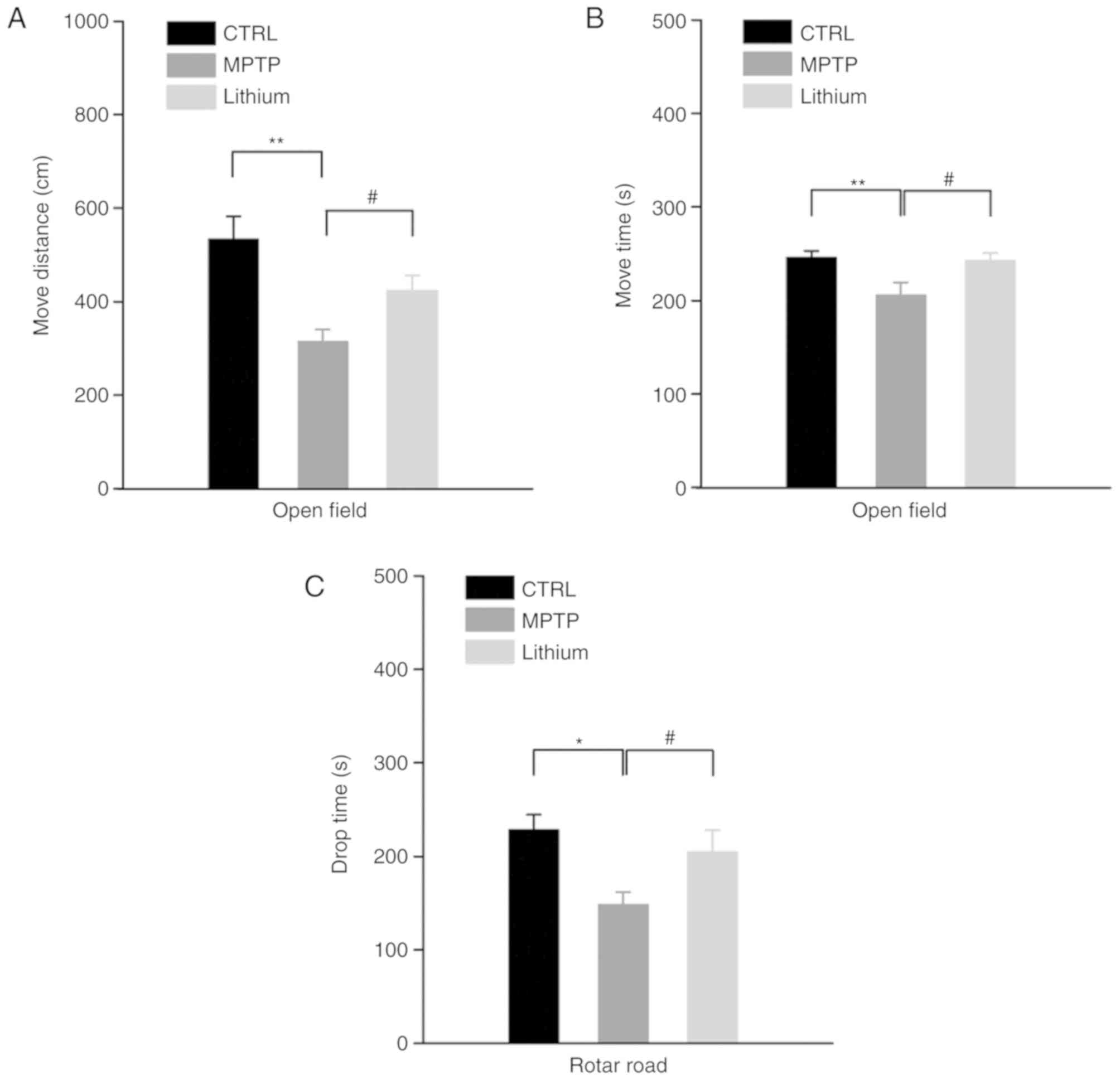 | Figure 1.Effects of lithium on behavioral
performance of an MPTP-induced PD model. (A and B) Results of
behavioral tests revealed that the locomotor activity of
MPTP-treated mice was significantly reduced (*P<0.05 and
**P<0.01, n=10) and lithium treatment satisfactorily relieved
this alteration (#P<0.05, n=10). (C) In rotarod
tests, the compound also prolonged the drop time of MPTP-treated
mice (*P<0.05, MPTP group vs. the control group, n=10;
#P<0.05, lithium group vs. the MPTP group, n=10).
MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD, Parkinson's
disease. |
Lithium alleviates typical
pathological alterations in the substantia nigra region of
MPTP-impaired mice
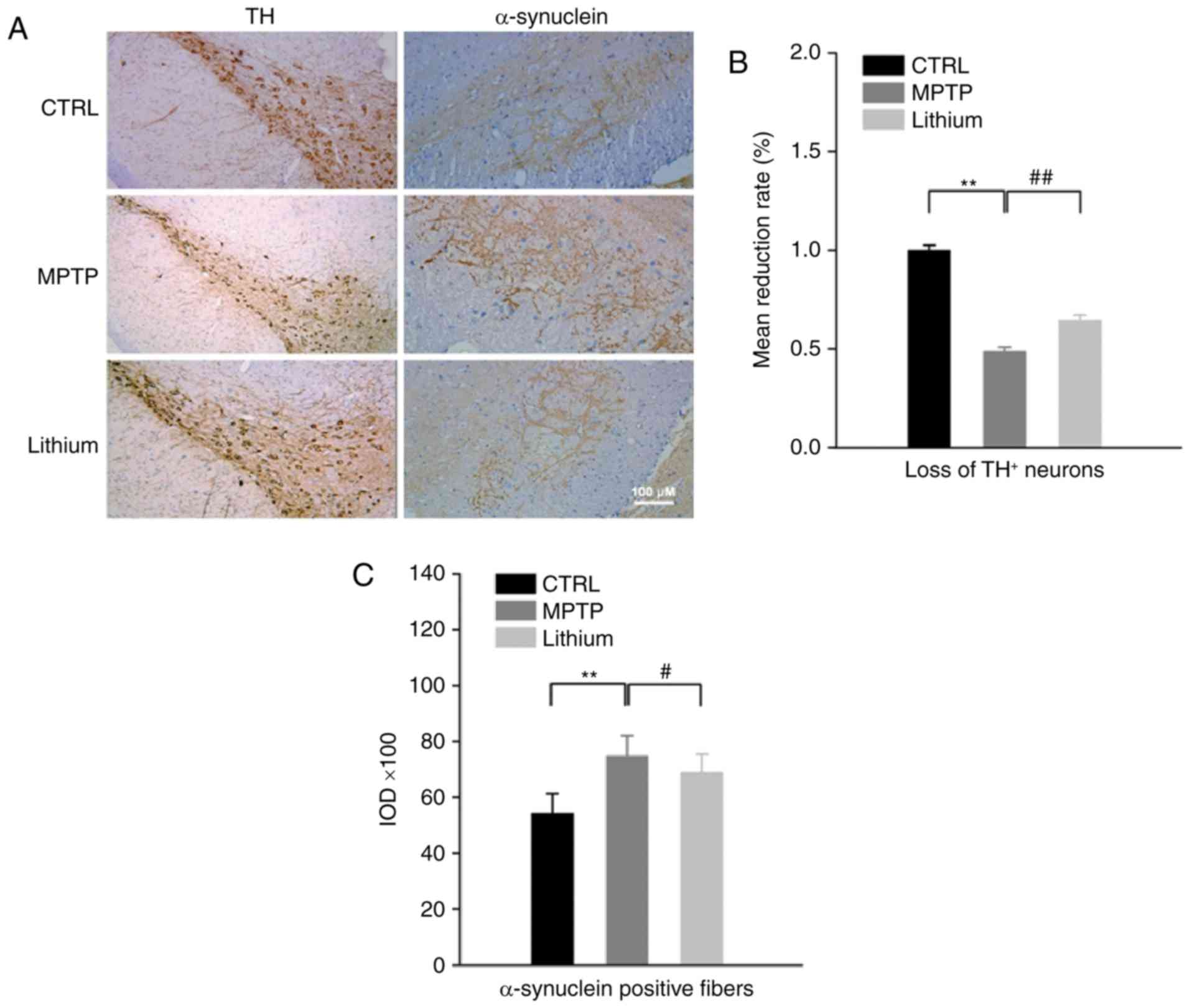
A reduction in TH-positive neurons and an increase
in expression of α-synuclein are both characteristic of PD
pathology. Corresponding pathological changes were also observed in
MPTP-impaired mice, and lithium treatment was able to mitigate
these alterations as well. Using immunohistochemical staining, a
significant loss of TH+ neurons in the substantia nigra
regions of MPTP-impaired mice at 6 weeks (48.60±0.022% reduction;
P<0.01 compared to control) was revealed. Conversely, the number
of TH+ neurons rebounded considerably after lithium
treatment (32.20±0.029% increased; P<0.01 compared to MPTP
group; Fig. 2). In addition, the
integrated optical density value (IOD) of α-synuclein-positive
fibers in MPTP-impaired mice increased from 54,370±699 to
74,790±736 (37.5% increased; P<0.01 compared to the control),
however, after lithium treatment it decreased to 68,958±654 (11.3%
increased; P<0.05 compared to the MPTP group; Fig. 2).
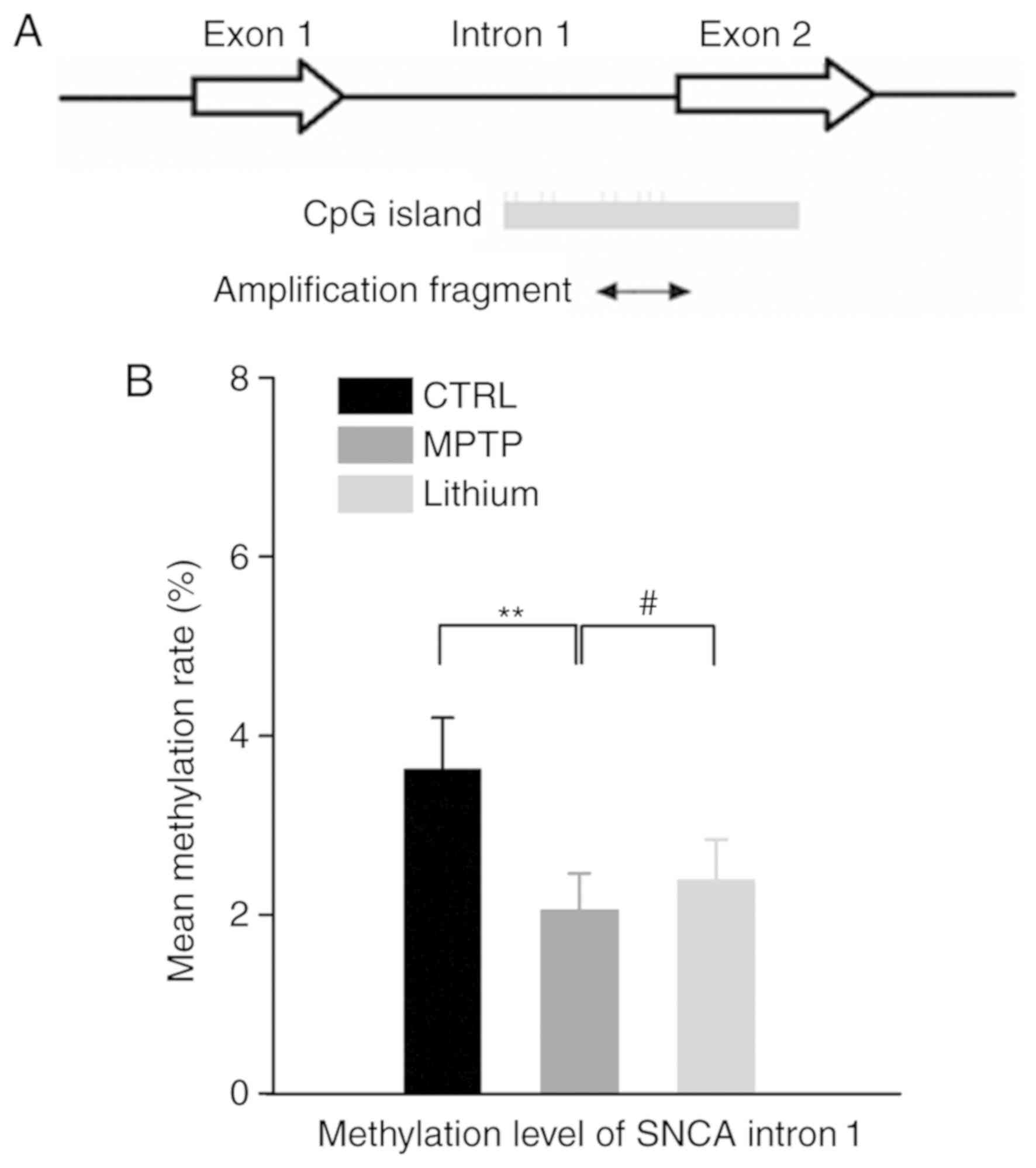
Lithium increases the methylation of
SNCA intron 1 in the substantia nigra of MPTP-impaired mice
To investigate whether epigenetic changes may
contribute to the dysregulation of SNCA expression in the
MPTP-induced PD animal model, DNA from the substantia nigra regions
of each group was analyzed. Significantly fewer methylated CpG
sites in the DNA of PD mice were revealed. There was a significant
decrease in the mean methylation rate of SNCA (−926/-483; intron 1)
in PD mouse substantia nigra (2.05±0.41%, P<0.01) when compared
with that determined in the control group (3.62±0.58%). Although
the methylation rate was lower (2.38±0.46%) in the lithium-treated
group when compared to the control group, the opposite was revealed
with comparison to the MPTP group, which resulted in a significant
increase in the methylation rate (P<0.05; Fig. 3).
Lithium alters the miRNA and protein
expression profiles in the substantia nigra of MPTP-impaired
mice
The role of miRNAs in the pathogenesis of
Parkinsonism has attracted increasing attention. Furthermore, the
recent discovery of miRNA expression and regulation has led to the
consideration of lithium as a novel mechanism of neuroprotection.
SurePrint Mouse miRNA Microarrays (Agilent Technologies, Inc.) were
utilized to profile changes in the miRNA expression of
MPTP-impaired mice. The results revealed alterations in the
expression levels of 39 miRNAs >1.5-fold in the substantia nigra
of PD mice (data not shown). The results also revealed an
upregulation in the expression of miR-148a in PD mice, an effect
which was reversed after lithium treatment, resulting in a
downregulation of this particular miRNA. RT-qPCR confirmed this
result (Fig. 4A). Previous
research has indicated that miR-148a could be considered as a
potential inhibitor of DNMT1, a DNA methylase, and thus play a role
in the establishment and regulation of tissue-specific patterns of
methylated cytosine residues. Further RT-qPCR results revealed
decreased expression of DNMT1 in the substantia nigra of PD mice
compared to the control, and a significant difference was recorded
when compared with the lithium-treated group (Fig. 4B). These results were also
confirmed by western blot analysis, which presented a significant
reduction in the expression of DNMT1 protein in PD mice compared
with the control group. Furthermore, lithium treatment resulted in
a significant increase in DNMT1 expression in PD mice (Fig. 4C).
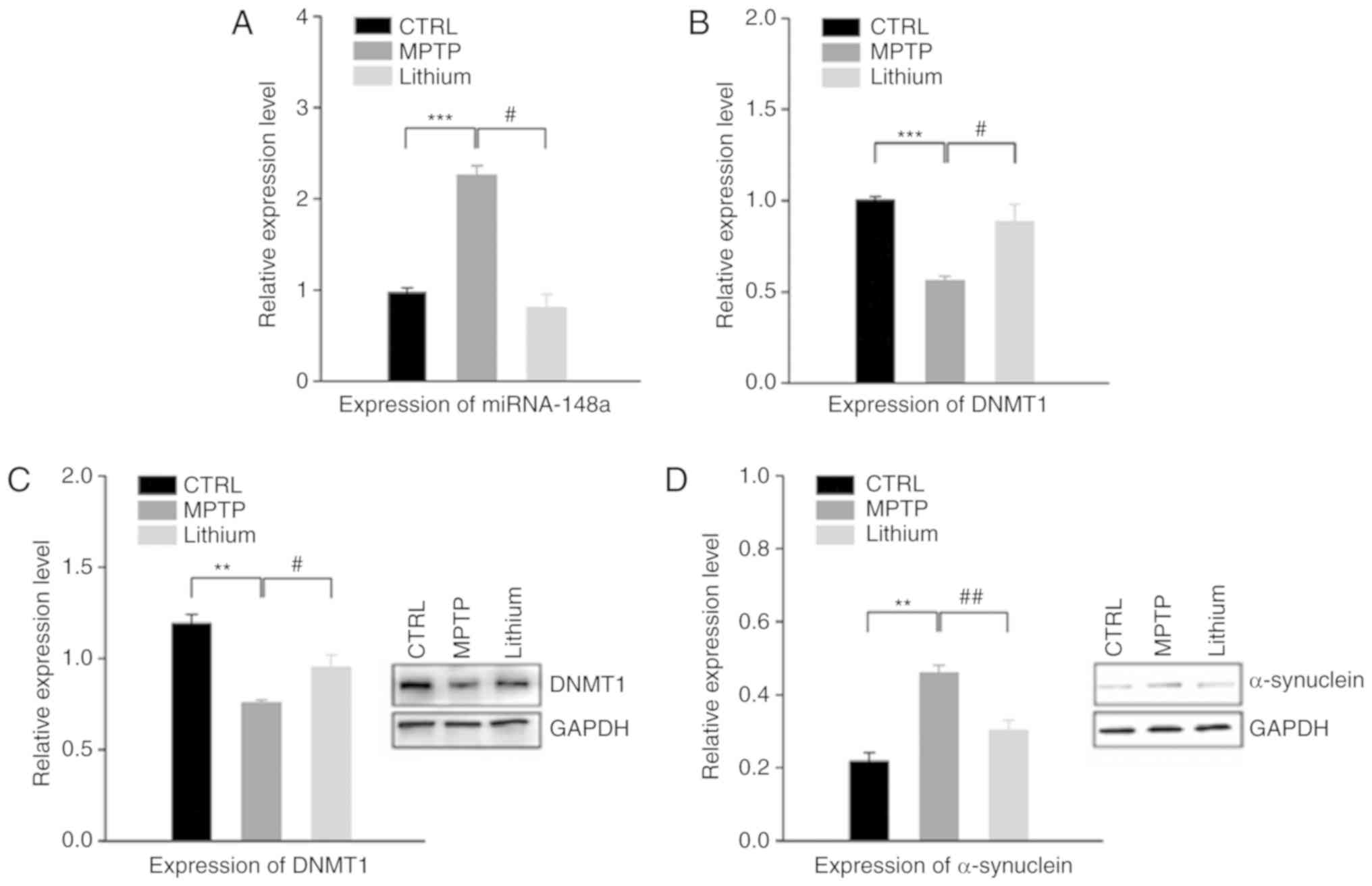 | Figure 4.Effects of lithium on the regulation
of the miRNA and protein expression profiles in substantia nigra.
(A) Results of RT-qPCR assays revealed an upregulation in the
expression level of miRNA-148a in the substantia nigra of PD mice
compared to that of the control group. However, miRNA-148a
expression was downregulated following lithium treatment. (B) The
expression level of DNMT1 decreased in the substantia nigra of PD
mice compared with the control group, and increased significantly
in the lithium-treated group (vs. MPTP group,
#P<0.05). (C) These results were further confirmed by
western blot analysis, which revealed a reduction in DNMT1 in the
substantia nigra of PD mice, and a significant difference between
the MPTP and lithium group. (D) The expression of α-synuclein
significantly increased in the substantia nigra of PD mice. This
effect was attenuated by oral administration of lithium.**P<0.01
and ***P<0.001, as indicated; #P<0.05 and
##P<0.01, as indicated. PD, Parkinson's disease;
MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. |
Aberrant aggregation of α-synuclein is considered to
be the major neuropathological characteristic of PD, and
α-synuclein has been detected in insoluble inclusions within Lewy
bodies and neurites. In the present study, a significant increase
was observed in the expression of α-synuclein in the substantia
nigra of mice. However, the increase of α-synuclein in PD mice
could be attenuated through administration of lithium (Fig. 4D).
Discussion
Lithium mood stabilizers have long been used to
treat epilepsy as well as bipolar disorder (BD), a mental illness
that causes deficits in cellular plasticity as well as resiliency
(8). Recent studies indicate that
lithium could also be effective as a treatment for
neurodegenerative diseases through diverse mechanisms. For example,
pretreatment with lithium resulted in an obvious decrease in the
size of quinolinic acid (QA)-induced lesions of striatum and loss
of striatal medium-sized neurons in a rat excitotoxic model
(16). In tauopathy mouse models,
chronic treatment with lithium decreased the phosphorylation of tau
as well as neuronal degeneration mediated by GSK-3 (17), findings which were corroborated
with the research that lithium could increase neurogenesis in the
rat hippocampus (18). An
increasing number of studies have illuminated the beneficial
effects of lithium on PD. Lithium chloride could promote
dopaminergic differentiation of human immortalized RenVm cells
(neuronal stem cell) by increasing expression of tyrosine
hydroxylase and β-catenin marker (19). Moreover, low-dose lithium treatment
could also prevent motor impairment, as demonstrated by the open
field test, pole test, and rearing behavior. In parkin mutant
transgenic mice, lithium prevented parkin-induced dopaminergic
striatal degeneration, striatal astrogliosis, and microglial
activation (20). The present
results revealed that lithium treatment significantly counteracted
the reduction in the movement distance as well as activity time of
MPTP-treated mice in open field tests. Additionally, it prolonged
the drop time of MPTP-treated mice in rotarod tests, which may be
relative to its neuroprotective effects on nigral neurons. Clinical
studies have revealed that low-dose lithium adjunct therapy may
reduce off-time in Parkinson's disease.
It is well known that α-synuclein is a key component
of Lewy bodies found in PD patients (21,22),
of which site mutations and multiplications induce familial
parkinsonian syndromes with high penetrance (23). Previous studies suggest that the
gene SNCA equally harbors significant risk haplotypes for sporadic
PD (24) and that MPTP treatment
significantly decreases the DNA methylation of SCNA CpGs. In the
present study, the effects of chronic lithium treatment were
investigated on the neurodegenerative phenotype of an MPTP-induced
mouse model of Parkinson's disease. In order to determine whether
or not lithium could counteract this alteration, bisulfite specific
cloning-based sequencing was performed to analyze the methylation
status of SCNA intron 1. Our findings indicated that lithium could
effectively prevent the decrease in DNA methylation. We then set
out to discriminate the upstream modulator elements of SCNA DNA
methylation.
Abundant in all multicellular organisms, miRNAs are
21–24 nucleotides long non-protein-coding RNAs. They are of
importance in translational repression as well as mRNA degradation
through binding either to the 3′-untranslated regions (3′-UTRs) of
mRNAs (25), predominantly, or to
coding regions (26). This
mechanism allows miRNA-regulating drugs to play a crucial role in
regulating the functions of the nervous system. For example,
muscarinic M1-receptor knockout mice exhibited mania-like
behavioral deficits (e.g., hypersensitivity to amphetamine-induced
hyperlocomotion), and lithium treatment normalized these behavioral
deficits in part through the enhancement of the M1-receptor-ERK
pathway signaling (27). To
further investigate whether or not lithium could act effectively by
this method in the MPTP-induced PD mouse model, SurePrint Mouse
miRNA Microarrays (Agilent Technologies, Inc.) were utilized to
profile changes in the miRNA expression of MPTP-impaired mice. It
was revealed that, in the substantia nigra of PD mice, 39 miRNAs
exhibited altered expression levels at greater than 1.5-fold (data
not shown). It was observed that miR-148a was upregulated in PD
mice, however lithium treatment could reduce this altered level of
expression. DNMT1, a maintenance DNA methylation enzyme, was
predicted as a potential target gene of miR-148a, which was
revealed to be correlated with DNA hypomethylation as well as
α-synuclein expression (28).
Based on these results, we could postulate that lithium achieved
its protective effect against PD-like neurodegenerative symptoms,
at least partially, by reducing the expression miR-148a and
alleviating the suppression of DNMT1, eventually resulting in a
reduction in the generation of neurotoxic α-synuclein. Further
experiments are required to determine whether or not miR-148a could
in fact bind directly to the 3′-UTR of DNMT1 in vivo.
Acknowledgements
The authors would like to thank Professor Dazheng Wu
(Putuo Hospital, Shanghai University of Traditional Chinese
Medicine) and Associate Professor Peihao Yin (Putuo Hospital,
Shanghai University of Traditional Chinese Medicine) for their
guidance on the experiments and manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (nos. 81202814 and 81603410),
the Shanghai Municipal Commission of Health and Family Planning
(20124Y116, 20184Y0086), and the Key Speciality Program (no.
2016102A) of Putuo Hospital, Shanghai University of Traditional
Chinese Medicine.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QZ and JT designed the study, performed part of the
experiments, interpreted the data and performed the data analysis.
JC, YZ, QX, and YB performed part of the experiments. HL, QZ, and
JT interpreted the data, drafted the manuscript and revised it
critically for intellectual content. All authors read and approved
the final version of the manuscript prior to submission.
Ethics approval and consent to
participate
All of the experimental animal protocols complied
with the current ethical considerations of Shanghai University of
Traditional Chinese Medicine's Animal Ethics Committee, which is in
accordance with the National Research Council criteria. All animal
experiments and procedures were reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of Shanghai
University of Traditional Chinese Medicine and were performed in
accordance with the relevant guidelines and regulations, as well as
approved by the guidelines on ethical standards for investigation
of experimental pain in conscious animals.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Tysnes OB and Storstein A: Epidemiology of
Parkinson's disease. J Neural Transm (Vienna). 124:901–905. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lotankar S, Prabhavalkar KS and Bhatt LK:
Biomarkers for parkinson's disease: Recent advancement. Neurosci
Bull. 33:585–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jowaed A, Schmitt I, Kaut O and Wullner U:
Methylation regulates alpha-synuclein expression and is decreased
in Parkinson's disease patients' brains. J Neurosci. 30:6355–6359.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumoto L, Takuma H, Tamaoka A, Kurisaki
H, Date H, Tsuji S and Iwata A: CpG demethylation enhances
alpha-synuclein expression and affects the pathogenesis of
Parkinson's disease. PLoS One. 5:e155222010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Boni L, Tierling S, Roeber S, Walter J,
Giese A and Kretzschmar HA: Next-generation sequencing reveals
regional differences of the alpha-synuclein methylation state
independent of Lewy body disease. Neuromolecular Med. 13:310–320.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiu CT, Wang Z, Hunsberger JG and Chuang
DM: Therapeutic potential of mood stabilizers lithium and valproic
acid: Beyond bipolar disorder. Pharmacol Rev. 65:105–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rao JS, Keleshian VL, Klein S and Rapoport
SI: Epigenetic modifications in frontal cortex from Alzheimer's
disease and bipolar disorder patients. Transl Psychiatry.
2:e1322012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou L, Xiong N, Liu L, Huang J, Han C,
Zhang G, Li J, Xu X, Lin Z and Wang T: Lithium protects
dopaminergic cells from rotenone toxicity via autophagy
enhancement. BMC Neurosci. 16:822015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiong N, Jia M, Chen C, Xiong J, Zhang Z,
Huang J, Hou L, Yang H, Cao X, Liang Z, et al: Potential autophagy
enhancers attenuate rotenone-induced toxicity in SH-SY5Y.
Neuroscience. 199:292–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moors TE, Hoozemans JJ, Ingrassia A,
Beccari T, Parnetti L, Chartier-Harlin MC and van de Berg WD:
Therapeutic potential of autophagy-enhancing agents in Parkinson's
disease. Mol Neurodegener. 12:112017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukumoto T, Morinobu S, Okamoto Y, Kagaya
A and Yamawaki S: Chronic lithium treatment increases the
expression of brain-derived neurotrophic factor in the rat brain.
Psychopharmacology (Berl). 158:100–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emamghoreishi M, Keshavarz M and Nekooeian
AA: Acute and chronic effects of lithium on BDNF and GDNF mRNA and
protein levels in rat primary neuronal, astroglial and
neuroastroglia cultures. Iran J Basic Med Sci. 18:240–246.
2015.PubMed/NCBI
|
|
13
|
Kovacsics CE, Gottesman II and Gould TD:
Lithium's antisuicidal efficacy: Elucidation of neurobiological
targets using endophenotype strategies. Annu Rev Pharmacol Toxicol.
49:175–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ribeiro D, Ellwanger K, Glagow D,
Theofilopoulos S, Corsini NS, Martin-Villalba A, Niehrs C and
Arenas E: Dkk1 regulates ventral midbrain dopaminergic
differentiation and morphogenesis. PLoS One. 6:e157862011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senatorov VV, Ren M, Kanai H, Wei H and
Chuang DM: Short-term lithium treatment promotes neuronal survival
and proliferation in rat striatum infused with quinolinic acid, an
excitotoxic model of Huntington's disease. Mol Psychiatry.
9:371–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noble W, Planel E, Zehr C, Olm V, Meyerson
J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, et al:
Inhibition of glycogen synthase kinase-3 by lithium correlates with
reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA.
102:6990–6995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen G, Masana MI and Manji HK: Lithium
regulates PKC-mediated intracellular cross-talk and gene expression
in the CNS in vivo. Bipolar Disord. 2:217–236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soleimani M and Ghasemi N: Lithium
chloride can induce differentiation of human immortalized RenVm
cells into dopaminergic neurons. Avicenna J Med Biotechnol.
9:176–180. 2017.PubMed/NCBI
|
|
20
|
Lieu CA, Dewey CM, Chinta SJ, Rane A,
Rajagopalan S, Batir S, Kim YH and Andersen JK: Lithium prevents
parkinsonian behavioral and striatal phenotypes in an aged parkin
mutant transgenic mouse model. Brain Res. 1591:111–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spillantini MG, Schmidt ML, Lee VM,
Trojanowski JQ, Jakes R and Goedert M: Alpha-synuclein in Lewy
bodies. Nature. 388:839–840. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Braak H, Del Tredici K, Rub U, de Vos RA,
Jansen Steur EN and Braak E: Staging of brain pathology related to
sporadic Parkinson's disease. Neurobiol Aging. 24:197–211. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singleton AB, Farrer M, Johnson J,
Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra
A, Nussbaum R, et al: alpha-Synuclein locus triplication causes
Parkinson's disease. Science. 302:8412003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mizuta I, Satake W, Nakabayashi Y, Ito C,
Suzuki S, Momose Y, Nagai Y, Oka A, Inoko H, Fukae J, et al:
Multiple candidate gene analysis identifies alpha-synuclein as a
susceptibility gene for sporadic Parkinson's disease. Hum Mol
Genet. 15:1151–1158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai EC: Micro RNAs are complementary to 3′
UTR sequence motifs that mediate negative post-transcriptional
regulation. Nat Genet. 30:363–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Forman JJ, Legesse-Miller A and Coller HA:
A search for conserved sequences in coding regions reveals that the
let-7 microRNA targets Dicer within its coding sequence. Proc Natl
Acad Sci USA. 105:14879–14884. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Creson TK, Austin DR, Shaltiel G, McCammon
J, Wess J, Manji HK and Chen G: Lithium treatment attenuates
muscarinic M(1) receptor dysfunction. Bipolar Disord. 13:238–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Desplats P, Spencer B, Coffee E, Patel P,
Michael S, Patrick C, Adame A, Rockenstein E and Masliah E:
Alpha-synuclein sequesters Dnmt1 from the nucleus: A novel
mechanism for epigenetic alterations in Lewy body diseases. J Biol
Chem. 286:9031–9037. 2011. View Article : Google Scholar : PubMed/NCBI
|