Introduction
Obesity is a chronic disease induced by nutritional
and metabolic disorders, and is a global health problem; 5–10% of
cases are due to genetic factors, whereas 90–95% of cases arise
from excessive diets and a lack of exercise (1). Obesity involves the accumulation of
adipose tissue in the body and disruption of normal metabolic
activities, resulting in tissue damage (2). Obesity leads to the development of a
variety of conditions and disorders, including hypertension,
insulin resistance, diabetes, inflammation, and obesity-induced
heart and kidney injury, thereby markedly impairing human health
(3–7).
An increasing number of clinical and animal studies
have demonstrated the involvement of the innate immune system and
inflammatory responses in the development of obesity-induced heart
and kidney injury (8). Previous
studies have characterized obesity-associated complications as
low-grade chronic inflammatory diseases (9,10).
Numerous anti-inflammatory compounds have been used in the
treatment of obesity-induced tissue injury, including curcumin,
berberine and resveratrol, indicating the importance of inhibiting
inflammation in treating obesity-associated complications (11–13).
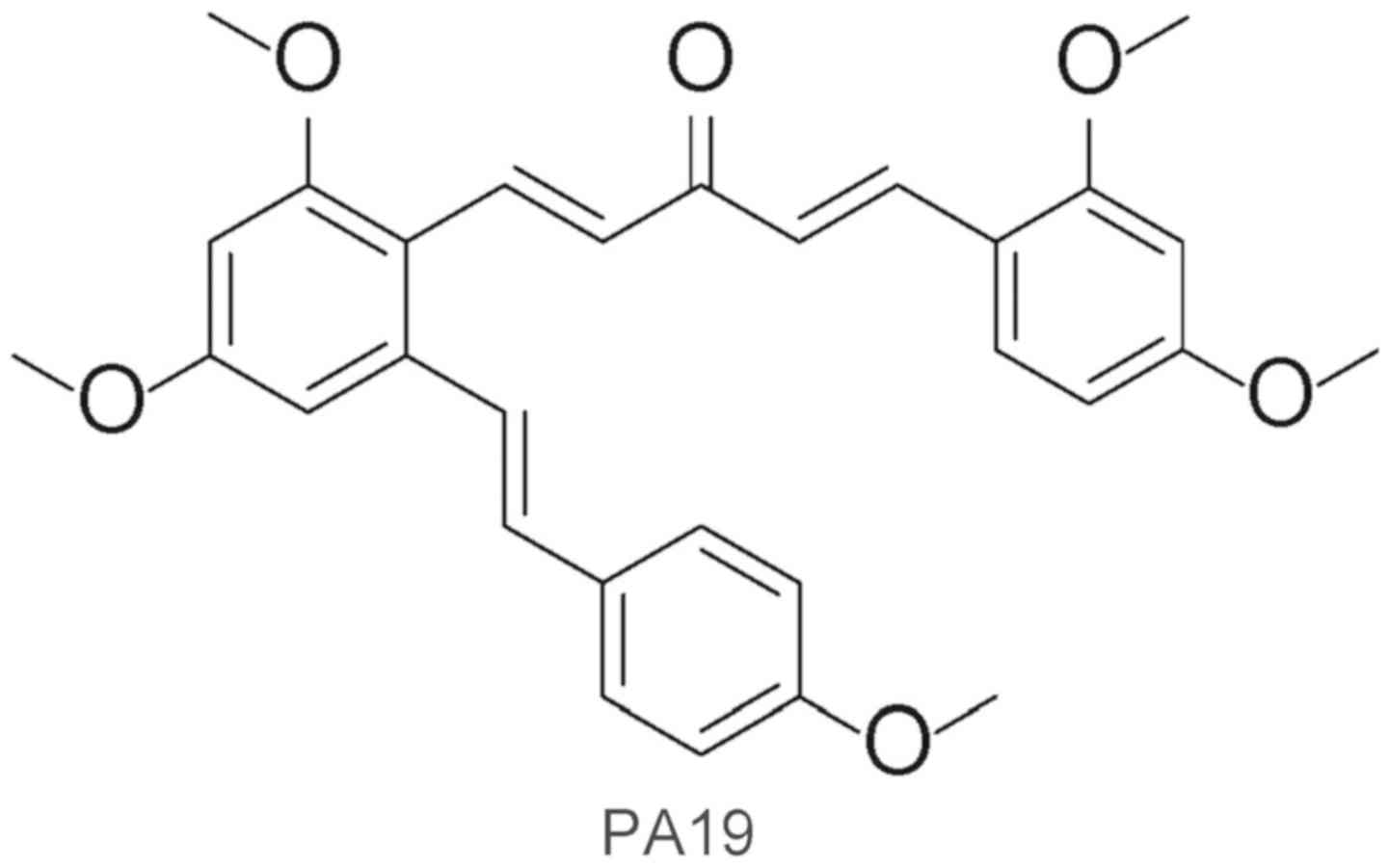
(1E,4E)-1-{2,4-Dimethoxy-6-[(E)-4-methoxystyryl]phenyl}-5-(2,4-dimethoxyphenyl)penta-1,4-dien-3-one
(PA19) is an analog of resveratrol that was synthesized by the
present research group (Fig. 1)
(14). It was reported to exhibit
more potent effects than resveratrol in suppressing
lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNF-α)
and interleukin (IL)-6 production in mouse peritoneal macrophages
(15,16). In the present study, the protective
effects of PA19 on obesity-induced heart and kidney injury, and
inflammation were investigated in a mouse model of obesity in
vivo.
Materials and methods
Chemicals
PA19 was prepared at a purity of 99.2% as previously
described (12), and dissolved in
0.5% sodium carboxylmethylcellulose (CMC-Na; Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) for in vivo
experiments.
Animals
A total of 21 male C57BL/6 mice (8–10 weeks old,
18–22 g) and a total of 20 male 18–20 g ICR mice (8–10 weeks old)
were obtained from the Animal Center of Wenzhou Medical University
(Wenzhou, China). All animal care and experimental procedures
complied with the Ordinance in Experimental Animal Management
(order no. 1998-02; Ministry of Science and Technology, Beijing,
China) and were approved by the Wenzhou Medical University Animal
Policy and Welfare Committee (Wenzhou, China). Mice were housed at
22°C, with 55±15% humidity and maintained under a 12-h light/dark
cycle in an SPF environment. All animals were provided with free
access to water. Following an acclimatization period of 1 week,
mice were randomly assigned into three weight-matched groups for 20
weeks (n=7/group): Mice were fed a standard rodent diet (CON) or a
high-fat diet (HFD), or fed a HFD and received intragastric
administration of PA19 (10 mg/kg/day) via oral gavage on alternate
days for the final 12 weeks of the study (HFD + PA19). The mice in
the CON and HFD groups received vehicle treatment (0.5% CMC-Na
solution) as a control. The body weights of the mice were monitored
weekly during the 20-week period of the study. The mice were
subsequently sacrificed under anesthesia, heart and kidney tissues
were fixed in 4% paraformaldehyde at room temperature for 48 h for
histological, and gene and protein expression analyses.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA); the concentration of RNA was determined using a
SpectraMax M5 microplate reader (Molecular Devices, LLC, Sunnyvale,
CA, USA) and the purity of the samples was estimated from the
optical density ratio (A260/A280, 1.8–2.2). RT was performed using
an M-MLV Platinum RT-qPCR kit (Invitrogen; Thermo Fisher
Scientific, Inc.) with the reaction parameters of incubation at
65°C for 5 min, 37°C for 52 min and 70°C for 15 min. qPCR was
conducted with a total reaction volume of 20 µl using iQ™
SYBR®-Green Supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and a MasterCycler Realplex4
(Eppendorf, Hamburg, Germany). A total of 3 parallel experiments of
one sample were conducted. A total of 60 cycles of qPCR were
performed. The thermocycling conditions were as follows:
Denaturation at 95°C for 2 min; annealing at 95°C for 15 sec and
extension at 60°C for 30 sec. Primers were obtained from Invitrogen
(Thermo Fisher Scientific, Inc.); the sequences are presented in
Table I. The relative expression
of each gene normalized to β-actin was determined using the
2−ΔΔCq method (17).
mRNA expression levels were calculated as percentages of the
expression in the median tissue from the HFD group.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Species | Forward primer | Reverse primer |
|---|
| IL-1β | Mouse |
5′-ACTCCTTAGTCCTCGGCCA-3′ |
5′-CCATCAGAGGCAAGGAGGAA-3′ |
| IL-6 | Mouse |
5′-GAGGATACCACTCCCAACAGACC-3′ |
5′-AAGTGCATCATCGTTGTTCATACA-3′ |
| VCAM-1 | Mouse |
5′-TGCCGAGCTAAATTACACATTG-3′ |
5′-CCTTGTGGAGGGATGTACAGA-3′ |
| ICAM-1 | Mouse |
5′-GCCTTGGTAGAGGTGACTGAG-3′ |
5′-GACCGGAGCTGAAAAGTTGTA-3′ |
| β-actin | Mouse |
5′-CCGTGAAAAGATGACCCAGA-3′ |
5′-TACGACCAGAGGCATACAG-3′ |
Western blotting
Heart and kidney tissues from each mouse were
homogenized in radioimmunoprecipitation assay (RIPA) buffer
(P0013B; Beyotime Institute of Biotechnology, Haimen, China;) with
protease inhibitor. The Bradford assay (Bio-Rad Laboratories, Inc.)
was performed to determine the concentration of protein. Following
boiling in loading buffer for 10 min, total protein samples (60 µg)
were separated via 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc.). Membranes were
blocked with 5% milk for 1.5 h at room temperature and then
incubated with primary antibodies overnight at 4°C. The following
primary antibodies were used: Vascular cell adhesion molecule 1
(VCAM-1; 1:1,000; ab134047; Abcam, Cambridge, UK), intercellular
adhesion molecule 1 (ICAM-1; 1:1,000; ab171123, Abcam), monocyte
chemoattractant protein 1 (MCP-1; 1:1,000; sc-52701, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), collagen 1 (Col-1; 1:1,000;
ab34710, Abcam) and β-actin (1:1,000; ab8226, Abcam). The membranes
were subsequently washed in TBST (TBS solution containing 0.2%
Tween-20) and incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:3,000; 7074; Cell
Signaling Technology, Inc., Danvers, MA, USA) for 1 h at room
temperature. Blots were visualized using an enhanced
chemiluminescence reagent (Bio-Rad Laboratories, Inc.) and protein
expression was quantified using ImageJ software (version 1.42q;
National Institutes of Health, Bethesda, MD, USA).
ELISA
Heart and kidney tissues from each mouse were
homogenized in RIPA buffer with protease inhibitor, and the
Bradford assay was performed to determine the concentration of
protein. Following adjustment to equal concentrations, ELISAs were
performed on the samples using mouse IL-6 (85-88-7064-76;
eBioscience; Thermo Fisher Scientific, Inc.) and mouse IL-1β ELISA
kits (70-EK201B/3-96; MultiSciences, Hangzhou, China) according to
the manufacturer's protocols.
Heart and kidney function tests
Blood samples were collected through the eyeball
after sacrifice of the animals and centrifuged at 3,000 × g for 15
min at 4°C to isolate the serum. Heart and kidney injury and
failure were determined by measuring the serum expression levels of
various biochemical markers, including blood urea nitrogen (BUN),
serum creatinine (Cr), creatine kinase (CK), CK-muscle/brain
(CK-MB), total cholesterol (TCH), alkaline phosphatase (AKP), low
density lipoprotein (LDL) and aspartate transaminase (AST) using
respective commercial kits (Jiancheng Bioengineering Institute,
Nanjing, China).
Heart and kidney histopathology
Kidney and heart tissues were fixed in 4%
paraformaldehyde for 48 h at room temperature and embedded in
paraffin. Samples were sectioned (5-µm thickness) and mounted on
slides. Then, sections were stained with H&E and Sirius Red
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) according to the manufacturer's protocols. To analyze the
extent of damage, 5 fields of one specimen were observed under a
light microscope (magnification, ×200; Nikon Corporation, Tokyo,
Japan). The area of fibrosis in the heart and kidney tissues was
determined using ImageJ software (version 1.42q, National
Institutes of Health).
Immunohistochemistry
Heart and kidney tissues were sectioned as
aforementioned, deparaffinized in xylene and rehydrated in a graded
alcohol series. Antigen retrieval was performed in 10 mM sodium
citrate buffer (pH 6.5) by microwaving (10 min). Endogenous
peroxidase activity was blocked by incubation in 3% hydrogen
peroxide for 30 min at room temperature. Sections were then blocked
for 30 min at 37°C in 1% bovine serum albumin (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) dissolved in phosphate buffer solution,
prior to incubation with primary anti-epidermal growth factor-like
module-containing mucin-like hormone receptor-like 1 (F4/80)
antibody (1:200; sc-377009; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. Then, sections were incubated with HRP-conjugated
secondary antibodies (1:200; 33201ES60; Yeasen, Shanghai, China) at
37°C for 30 min, prior to immersion in 3′3-diaminobenzidine
solution (OriGene Technologies, Inc., Beijing, China) for 1 min.
Finally, sections were stained with hematoxylin for 5 min at room
temperature, dehydrated and mounted, and images were captured using
a light microscope (5 fields of one specimen were randomly
selected; magnification, ×200, Nikon Corporation).
Acute toxicity assay
The 20 male 18–20 g ICR mice were randomly divided
into two experimental groups: CON group and PA19 group. CON and PA
group mice were received 0.5% CMC-Na and 500 mg/kg PA,
respectively. The treatments were administered once by oral gavage.
Animals were closely observed every 12 h following administration
on general behavior; mortality and body weights were also
measured.
Statistical analysis
All data were obtained from three independent
experiments. The results were presented as the mean ± standard
error of the mean. All data were analyzed using GraphPad Pro Prism
5.01 (GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis
of variance followed by a Dunnett's post-hoc test was employed to
analyze the differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
PA19 reduces the expression of
obesity-induced kidney and heart biochemical markers in the
serum
Heart and kidney tissues and serum were collected
from mice following CON or HFD feeding for 20 weeks. As presented
in Table II, animals fed with the
HFD exhibited significantly increased serum expression levels of
lipid accumulation markers (TCH and LDL), renal function markers
(BUN and Cr) and cardiac function markers (CK, CK-MB, AKP and AST)
compared with the CON group; however, treatment with PA19 (10
mg/kg) suppressed the increased expression of these markers. An
acute toxicity assay was conducted to determine the effects of
chronic PA19 treatment in vivo; it was demonstrated that
intragastric administration of PA19 (500 mg/kg) did not
significantly alter body weight (Fig.
S1) or the serum expression levels of the aforementioned
biochemical markers (Table SI)
compared with treatment with 0.5% CMC-Na. The results indicated
that PA19 treatment at the selected dose was safe for mice and
attenuated the increased serum expression levels of functional
biomarkers following exposure to an HFD.
 | Table II.Blood biochemical data following 20
weeks on the CON, or the HFD with or without PA19 treatment. |
Table II.
Blood biochemical data following 20
weeks on the CON, or the HFD with or without PA19 treatment.
| Factor | CON | HFD | PA19 + HFD |
|---|
| TCH (mmol/l) | 97.57±5.66 |
254.30±6.64b |
201.70±9.95c |
| LDL (mmol/l) | 0.53±0.06 |
1.40±0.07b |
0.98±0.07c |
| BUN (mmol/l) | 8.16±1.01 |
18.61±0.80b |
14.91±0.48c |
| Cr (mmol/l) | 9.27±1.82 |
57.20±5.79b |
36.21±5.22c |
| CK-MB (U/l) | 748.6±146.4 |
1,324.0±56.51a | 1,288.0±54.60 |
| CK (U/l) | 0.17±0.11 |
0.75±0.08b |
0.47±0.07c |
| AKP (U/l) | 0.033±0.01 |
0.550±0.03b | 0.440±0.02 |
| AST (U/l) | 0.044±0.01 |
0.087±0.01a |
0.049±0.01c |
PA19 reduces obesity-induced heart and
kidney injury and fibrosis
The cardioprotective effects of PA19 were
investigated by determining the effects of PA19 treatment on
obesity-induced alterations in the morphology of cardiac tissue.
H&E staining revealed that the heart tissue of mice in the HFD
group presented structural abnormalities that were not observed in
the PA19-treated group, including broken fibers and irregular
cellular structures (Fig. 2A).
Additionally, Sirius Red staining revealed that PA19 treatment
attenuated HFD-induced collagen deposition in heart tissues and
significantly reduced fibrosis compared with the control (Fig. 2B and C). It was demonstrated that
feeding with the HFD induced significant increases in the levels of
Col-1 mRNA and protein expression in heart tissue compared with the
CON group, but were suppressed by PA19 treatment (Fig. 2D, E and S2A).
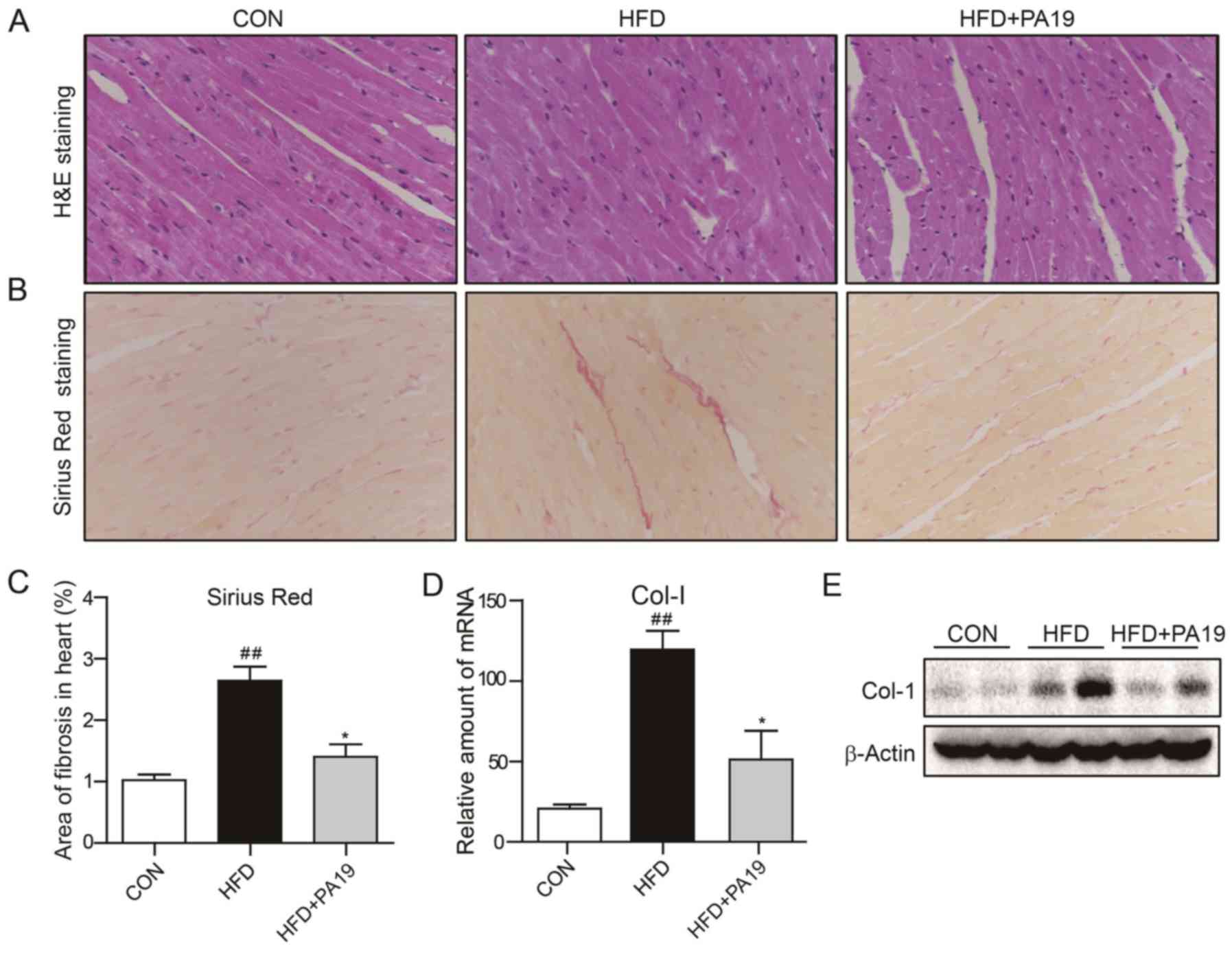 | Figure 2.PA19 reduces HFD-induced cardiac
injury and fibrosis. Obesity was induced over 20 weeks in male
C57BL/6 mice with an HFD, plus oral gavage of PA19 (10 mg/kg) or
vehicle (0.5% sodium carboxymethylcellulose) once every 2 days
during the final 12 weeks. Representative images of (A) H&E and
(B) Sirius Red staining of heart tissues (magnification, ×200). (C)
Relative area of fibrosis in cardiac tissues. (D) mRNA expression
of Col-1 in heart tissues as determined by reverse
transcription-quantitative polymerase chain reaction. (E) Protein
expression of Col-1 in cardiac tissues as determined by western
blotting. N=7/group. Data are presented as the mean ± standard
error of the mean. *P<0.05 vs. HFD group; ##P<0.01
vs. CON group. Col-1, collagen 1; CON, control diet; HFD, high-fat
diet; PA19,
(1E,4E)-1-{2,4-dimethoxy-6-[(E)-4-methoxystyryl]phenyl}-5-(2,4-dimethoxyphenyl)penta-1,4-dien-3-one. |
H&E staining of kidney tissue revealed that the
HFD group exhibited glomerular shrinkage and expansion of the
mesangial matrix compared with the CON group (Fig. 3A). Conversely, treatment with PA19
markedly improved obesity-induced renal injury. The extent of
fibrosis in the kidneys induced by HFD was also evaluated. Sirius
Red staining revealed notable collagen deposition in the kidney
tissues of HFD mice (Fig. 3B and
C), in addition to significant increases in the expression of
Col-1 at the mRNA and protein levels compared with the CON group
(Fig. 3D, E and S2B). PA19 treatment significantly
reduced the extent of HFD-induced fibrosis and decreased the
expression of Col-1 in kidney tissue. The results suggested that
treatment with PA19 protects heart and kidney tissue from
obesity-induced injury and fibrosis.
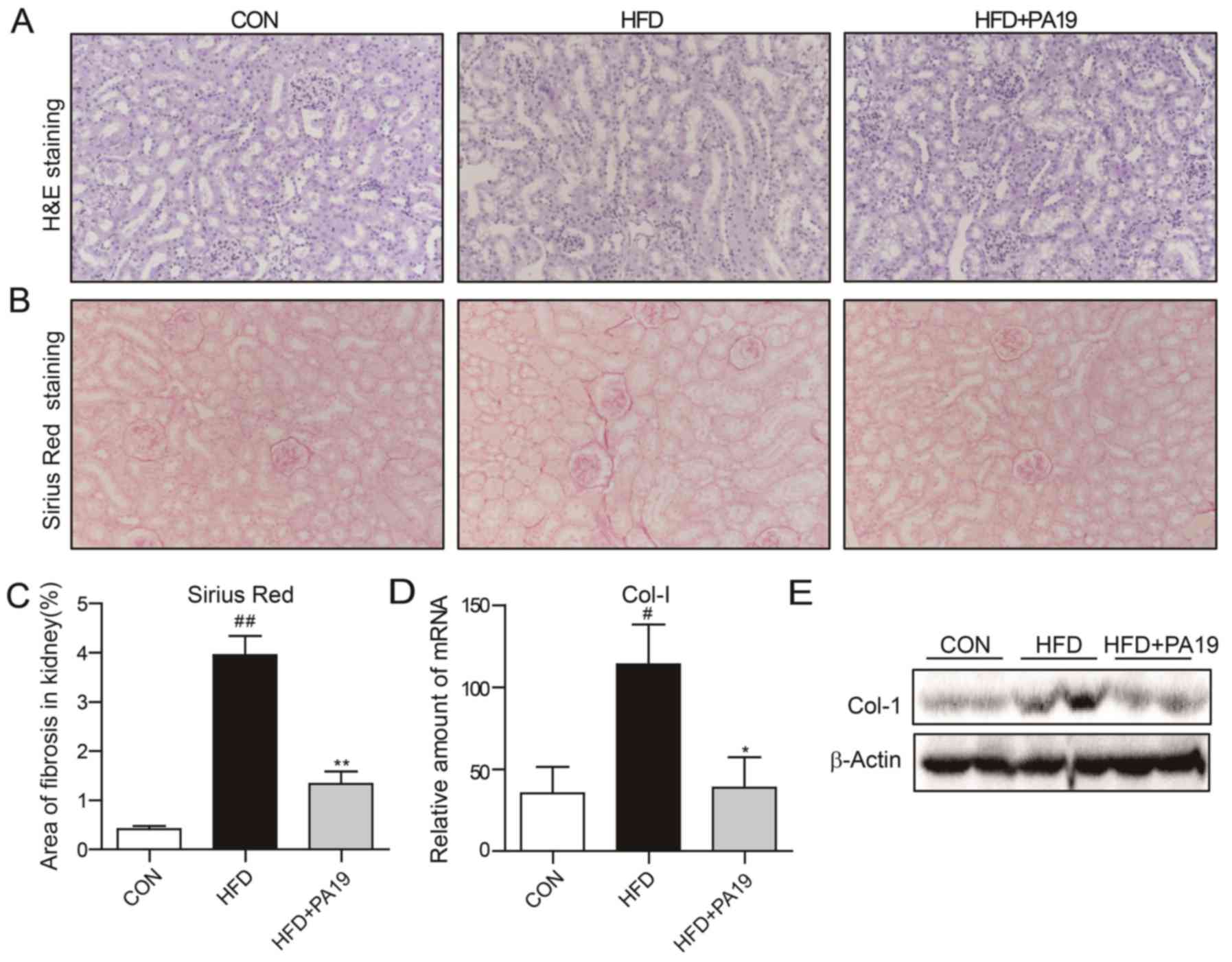 | Figure 3.PA19 reduces HFD-induced renal injury
and fibrosis. Representative images of (A) H&E and (B) Sirius
Red staining of kidney tissues (magnification, ×200). (C) Relative
area of fibrosis in renal tissues. (D) mRNA expression of Col-1 in
kidney tissues as determined by reverse transcription-quantitative
polymerase chain reaction. (E) Protein expression of Col-1 in renal
tissues as determined by western blotting. N=7/group. Data are
presented as the mean ± standard error of the mean. *P<0.05,
**P<0.01 vs. HFD group; #P<0.05,
##P<0.05 vs. CON group. Col-1, collagen 1; CON,
control diet; HFD, high-fat diet; PA19,
(1E,4E)-1-{2,4-dimethoxy-6-[(E)-4-methoxystyryl]phenyl}-5-(2,4-dimethoxyphenyl)penta-1,4-dien-3-one. |
PA19 reduces obesity-induced
myocardial and renal inflammation
The association between the potential cardio- and
renoprotective effects of PA19 against obesity-induced damage and
its anti-inflammatory properties was investigated.
Immunohistochemical staining of F4/80 expression revealed that
there was marked HFD-induced inflammatory cell infiltration of
cardiac tissue; however, the percentage of F4/80+ cells
in tissue samples from the HFD + PA19 group was significantly lower
compared with in the HFD group (Fig.
4A and B). Additionally, RT-qPCR, ELISA and western blot
analyses demonstrated that HFD exposure significantly increased the
mRNA and protein expression levels of inflammatory cytokines (IL-6
and IL-1β), chemokines (MCP-1) and adhesion molecules (VCAM-1 and
ICAM-1) in heart tissue compared with in the CON group (Fig. 4C-J). Conversely, PA19 treatment
significantly suppressed the HFD-induced upregulation of these
markers.
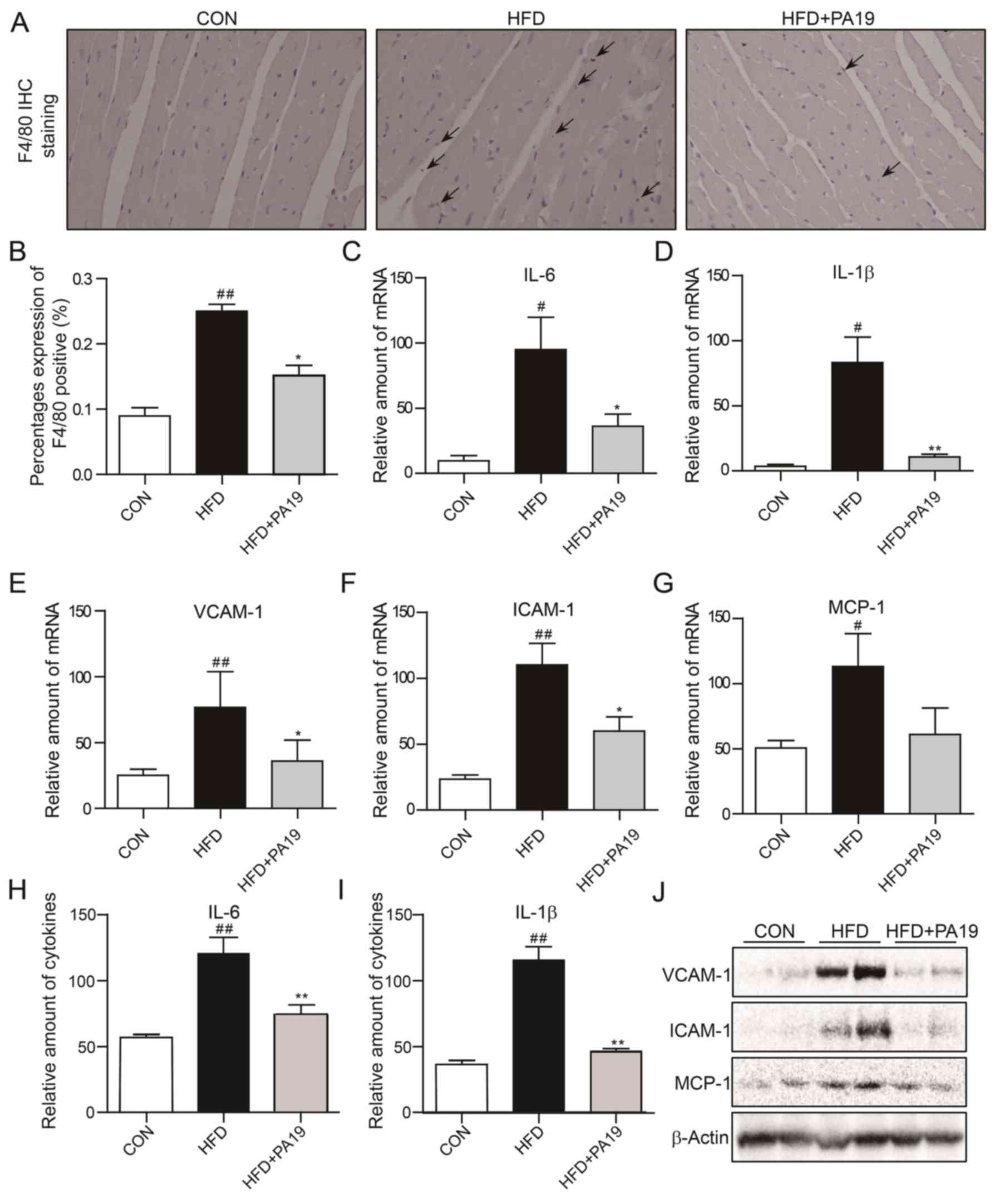 | Figure 4.PA19 reduces HFD-induced cardiac
inflammation. Representative images of (A) anti-F4/80 staining of
heart tissues (magnification, ×200). (B) Quantification of F4/80
expression in cardiac tissue sections. mRNA expression of (C) IL-6,
(D) IL-1β, (E) VCAM-1, (F) ICAM-1 and (G) MCP-1 in heart tissues as
determined by reverse transcription-quantitative polymerase chain
reaction. Protein expression of (H) IL-6 and (I) IL-1β as
determined by ELISA. (J) Protein expression of VCAM-1, ICAM-1 and
MCP-1 in cardiac tissues as determined by western blotting.
N=7/group. Data are presented as the mean ± standard error of the
mean. *P<0.05, **P<0.01 vs. HFD group; #P<0.05,
##P<0.01 vs. CON group. CON, control diet; F4/80,
epidermal growth factor-like module-containing mucin-like hormone
receptor-like 1; HFD, high-fat diet; ICAM-1, intercellular adhesion
molecule 1; IL, interleukin; MCP-1, monocyte chemoattractant
protein 1; PA19,
(1E,4E)-1-{2,4-dimethoxy-6-[(E)-4-methoxystyryl]phenyl}-5-(2,4-dimethoxyphenyl)penta-1,4-dien-3-one;
VCAM-1, vascular cell adhesion molecule 1. |
As presented in Fig. 5A
and B, feeding with the HFD induced a marked increase in the
number of F4/80+ inflammatory cells in the renal
sections; however, inflammatory cell infiltration was significantly
attenuated by treatment with PA19. As presented in Fig. 5C-J, the expression levels of
inflammatory cytokines, chemokines and adhesion molecules were
significantly increased in kidney tissues from HFD mice compared
with the CON group, but were suppressed by PA19 treatment. Of note,
the protein expression of IL-1β was not significantly reduced
following PA19 administration. Collectively, these results
indicated that PA19 exhibits anti-inflammatory properties in
cardiac and renal tissues in vivo.
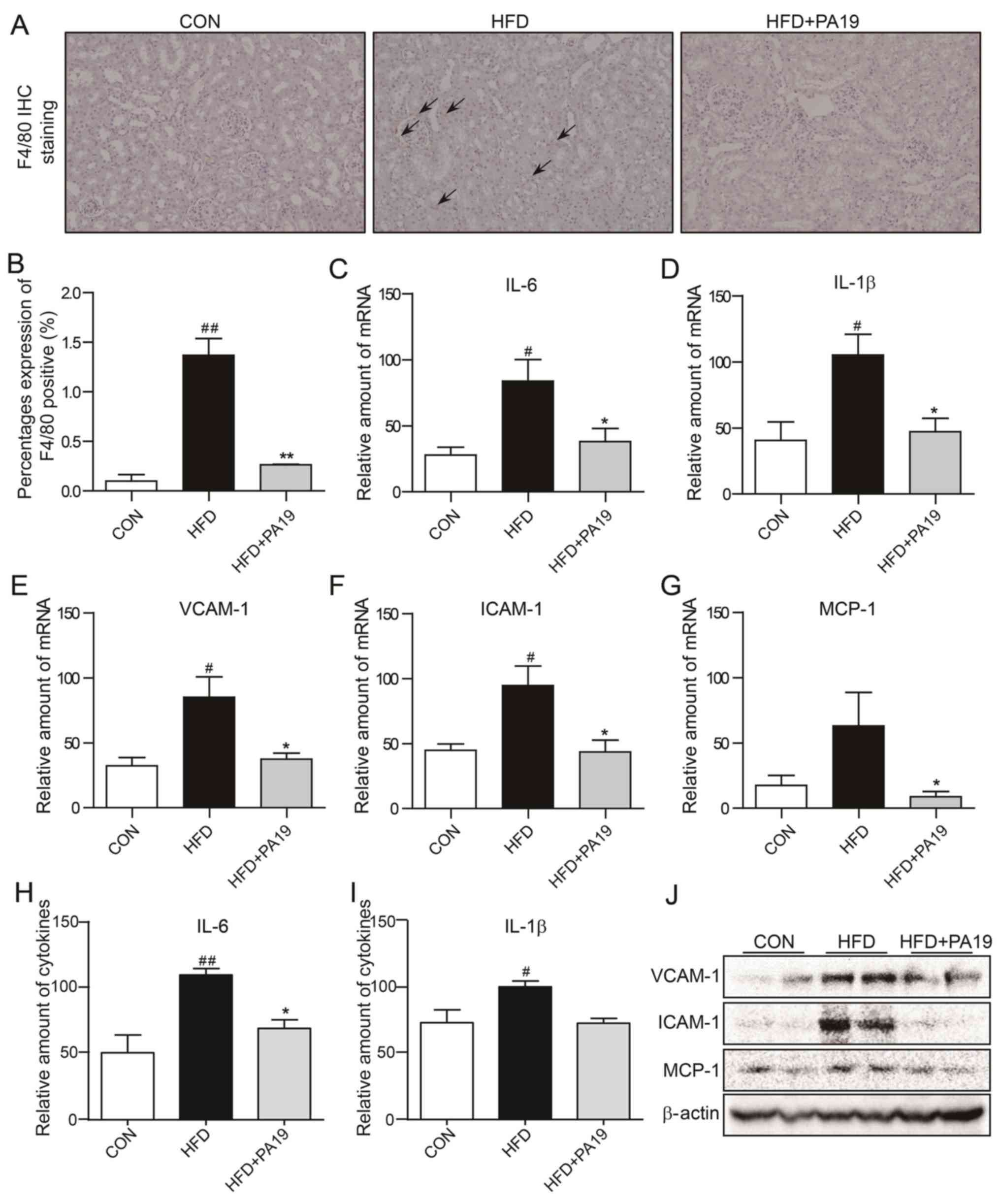 | Figure 5.PA19 reduces HFD-induced renal
inflammation. Representative images of (A) anti-F4/80 staining of
kidney tissues (magnification, ×200). (B) Quantification of F4/80
expression in renal tissue sections. mRNA expression of (C) IL-6,
(D) IL-1β, (E) VCAM-1, (F) ICAM-1 and (G) MCP-1 in kidney tissues
as determined by reverse transcription-quantitative polymerase
chain reaction. Protein expression of (H) IL-6 and (I) IL-1β as
determined by ELISA. (J) Protein expression of VCAM-1, ICAM-1 and
MCP-1 in renal tissues as determined by western blotting.
N=7/group. Data are presented as the mean ± standard error of the
mean. *P<0.05, **P<0.01 vs. HFD group; #P<0.05,
##P<0.01 vs. CON group. CON, control diet; F4/80,
epidermal growth factor-like module-containing mucin-like hormone
receptor-like 1; HFD, high-fat diet; ICAM-1, intercellular adhesion
molecule 1; IL, interleukin; MCP-1, monocyte chemoattractant
protein 1; PA19,
(1E,4E)-1-{2,4-dimethoxy-6-[(E)-4-methoxystyryl]phenyl}-5-(2,4-dimethoxyphenyl)penta-1,4-dien-3-one;
VCAM-1, vascular cell adhesion molecule 1. |
Discussion
Obesity is a global health issue that may lead to
numerous associated disorders, including insulin resistance,
diabetes and inflammation (18);
therefore, it is important to identify novel approaches to treat
obesity-associated complications. In the present study, the
protective effects of PA19, a resveratrol analog, were investigated
in an established mouse model of obesity. It was revealed that PA19
attenuated obesity-induced lipid accumulation in the serum, injury,
collagen deposition, inflammatory cytokine expression and
inflammatory cell infiltration in the heart and kidneys. These
findings suggested that PA19 exhibits cardio- and renoprotective
effects against obesity-induced injury via the inhibition of
inflammatory responses.
Long-term over-nutrition commonly leads to obesity
and associated complications, such as heart and kidney injury
(19). Increased fat accumulation
progressively impairs arterial function and thickening of the blood
vessel wall reduces blood flow; thus, systemic blood circulation is
reduced, and the risk of thrombosis and burden on the heart are
increased (20,21). Thickening of the walls of renal
blood vessels increases renal blood pressure and burden on the
kidney. Consequentially, glomerular hypertension and
hyperfiltration can occur, commonly leading to the development of
glomerulosclerosis (22,23). The results of the present study
revealed that mice fed an HFD exhibited lipid accumulation in the
serum, with heart and kidney injury, whereas treatment with PA19
attenuated these effects.
Fibrosis is an important pathological process in the
development of obesity-associated complications. Renal fibrosis is
the histological manifestation of a progressive and usually
irreversible process resulting in end-stage kidney disease
(24). Cardiac fibrosis is
associated with metabolic dysfunction, and may contribute to the
increased incidence of heart failure associated with obesity
(25,26). Col-1 is a major contributor to
fibrosis; it has been reported that excessive cardiac Col-1
synthesis and deposition may be involved in the progression of
myocardial fibrosis that accompanies the development of heart
failure in patients with hypertensive heart disease (27). The expression of Col-1 has been
reported to be markedly increased in various types of renal
fibrosis (28,29). Sirius Red staining, and RT-qPCR and
western blot analyses revealed that PA19 treatment suppressed the
collagen deposition and upregulation of Col-1 in heart and kidney
tissues in HFD mice. These findings suggested that PA19 effectively
inhibits obesity-induced tissue injury and fibrosis.
Obesity is characterized as a low-grade chronic
inflammatory disease (9,10). This inflammatory state is reflected
by the increased production of inflammatory cytokines and the
infiltration of inflammatory cells into tissues (9). Cells that infiltrate tissues are
typically highly activated, producing proinflammatory cytokines and
chemoattractants (26,30), consequentially attracting
additional inflammatory cells that secrete more proinflammatory
cytokines. In the present study, staining of heart and kidney
tissues from HFD mice revealed the infiltration of
F4/80+ cells, and increased expression levels of
inflammatory cytokines, chemokines and adhesion molecules compared
with in tissues from CON mice; the effects were attenuated by PA19
treatment.
Resveratrol is a natural product originating from
grapes and other plants (31).
Resveratrol has received increasing attention as a potential
therapy in the prevention and treatment of atherosclerosis,
cardiovascular and cerebrovascular diseases, due to its
anti-inflammatory properties and low toxicity (32–34).
Furthermore, various studies have reported that resveratrol may
attenuate obesity and associated complications via a number of
mechanisms (35,36); however, the biological activity of
resveratrol is limited by its photosensitivity and metabolic
instability (37). In our previous
study, a series of resveratrol-curcumin hybrids were synthesized,
and their anti-inflammatory properties were evaluated in
vitro and in vivo (14). It was demonstrated that one
compound, PA19, was more effective than resveratrol in suppressing
the LPS-induced production of TNF-α and IL-6 (14). In the present study, it was
revealed that PA19 attenuated obesity-induced heart and kidney
injury in HFD-fed mice, potentially via the inhibition of
inflammation and cellular infiltration.
In conclusion, it was demonstrated that mice fed
with an HFD exhibited lipid accumulation, and injury, fibrosis,
inflammation and inflammatory cell infiltration in heart and kidney
tissues; these effects were attenuated by treatment with PA19. The
findings of the present study indicated the contribution of
inflammatory processes in the development of obesity-associated
cardiac and renal damage, and suggested that the anti-inflammatory
compound PA19 may be a potential therapeutic agent in the treatment
of obesity-associated complications.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Funding of China (grant nos. 81770850 to
XS, 81872918 to YZ, and 81503123 to YZ) and Zhejiang Provincial
Natural Science Foundation of China (grant nos. LY18H310011 to YZ,
and LY17H050007 to XS).
Availability of data and materials
The datasets from current study are available from
the corresponding author on reasonable request.
Authors' contributions
WZ, HC and YZ designed the study. WZ, HC, CS and BW
performed the experiments. BB and HL conducted the statistical
analyses. XS and GL participated in the analyzing of results. WZ
and YZ wrote and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal care and experimental procedures complied
with the Ordinance in Experimental Animal Management and were
approved by the Wenzhou Medical University Animal Policy and
Welfare Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Long JR, Shu XQ, Cai Q, Wen W, Kataoka N,
Gao YT and Zheng W: CYP19A1 genetic polymorphisms may be associated
with obesity-related phenotypes in Chinese women. Int J Obes
(Lond). 31:418–423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Polyzos SA, Kountouras J and Mantzoros CS:
Adipose tissue, obesity and non-alcoholic fatty liver disease.
Minerva Endocrinol. 42:92–108. 2017.PubMed/NCBI
|
|
3
|
Roberts CK, Hevener AL and Barnard RJ:
Metabolic syndrome and insulin resistance: Underlying causes and
modification by exercise training. Compr Physiol. 3:1–58.
2013.PubMed/NCBI
|
|
4
|
Turer CB, Brady TM and de Ferranti SD:
Obesity, hypertension, and dyslipidemia in childhood are key
modifiable antecedents of adult cardiovascular disease: A call to
action. Circulation. 137:1256–1259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abderrahmani A, Yengo L, Caiazzo R,
Canouil M, Cauchi S, Raverdy V, Plaisance V, Pawlowski V, Lobbens
S, Maillet J, et al: Increased hepatic PDGF-AA signaling mediates
liver insulin resistance in obesity associated type 2 diabetes.
Diabetes. 67:1310–1321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kenchaiah S, Evans JC, Levy D, Wilson PW,
Benjamin EJ, Larson MG, Kannel WB and Vasan RS: Obesity and the
risk of heart failure. N Engl J Med. 347:305–313. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wahba IM and Mak RH: Obesity and
obesity-initiated metabolic syndrome: Mechanistic links to chronic
kidney disease. Clin J Am Soc Nephrol. 2:550–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Heredia FP, Gómez-Martínez S and Marcos
A: Obesity, inflammation and the immune system. Proc Nutr Soc.
71:332–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cancello R and Clement K: Is obesity an
inflammatory illness? Role of low-grade inflammation and macrophage
infiltration in human white adipose tissue. BJOG. 113:1141–1147.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graziani F, Cialdella P, Liuzzo G, Basile
E, Brugaletta S, Pedicino D, Leccesi L, Guidone C, Iaconelli A,
Mingrone G, et al: Cardiovascular risk in obesity: Different
activation of inflammation and immune system between obese and
morbidly obese subjects. Eur J Intern Med. 22:418–423. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vors C, Couillard C, Paradis M, Gigleux I,
Marin J, Vohl M, Couture P and Lamarche B: Supplementation with
resveratrol and curcumin does not affect the inflammatory response
to a high-fat meal in older adults with abdominal obesity: A
randomized, placebo-controlled crossover trial. J Nutr.
148:379–388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS,
Lee MR, Oh GT, Park HS, Lee KU, Lane MD and Kim JB: Berberine
improves lipid dysregulation in obesity by controlling central and
peripheral AMPK activity. Am J Physiol Endocrinol Metab.
296:E812–E819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rossi EL, Khatib SA, Doerstling SS, Bowers
LW, Pruski M, Ford NA, Glickman RD, Niu M, Yang P, Cui Z, et al:
Resveratrol inhibits obesity-associated adipose tissue dysfunction
and tumor growth in a mouse model of postmenopausal claudin-low
breast cancer. Mol Carcinog. 57:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan J, Xu T, Xu F, Zhang Y, Liu Z, Chen W,
Fu W, Dai Y, Zhao Y, Feng J and Liang G: Development of
resveratrol-curcumin hybrids as potential therapeutic agents for
inflammatory lung diseases. Eur J Med Chem. 125:478–491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zong Y, Sun L, Liu B, Deng YS, Zhan D,
Chen YL, He Y, Liu J, Zhang ZJ, Sun J and Lu D: Resveratrol
inhibits LPS-induced MAPKs activation via activation of the
phosphatidylinositol 3-kinase pathway in murine RAW 264.7
macrophage cells. PLoS One. 7:e441072012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SM, Zhang Y, Tsuchiya H, Smalling R,
Jetten AM and Wang L: Small heterodimer partner/neuronal PAS domain
protein 2 axis regulates the oscillation of liver lipid metabolism.
Hepatology. 61:497–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Latifi SM, Karandish M, Shahbazian H, Taha
JM, Cheraghian B and Moradi M: Prevalence of Metabolically Healthy
Obesity (MHO) and its relation with incidence of metabolic
syndrome, hypertension and type 2 diabetes amongst individuals aged
over 20 years in Ahvaz: A 5 year cohort study (2009–2014). Diabetes
Metab Syndr. 11 (Suppl 2):S1037–S1040. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siddeek B, Li N, Mauduit C, Chehade H,
Rigal E, Tolsa J, Armengaud J, Yzydorczyk C, Benahmed M, Vergely C
and Simeoni U: Transient postnatal over nutrition induces long-term
alterations in cardiac NLRP3-inflammasome pathway. Nutr Metab
Cardiovasc Dis. 28:944–951. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blokhin IO and Lentz SR: Mechanisms of
thrombosis in obesity. Curr Opin Hematol. 20:437–444. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Köchli S, Endes K, Infanger D, Zahner L
and Hanssen H: Obesity, blood pressure, and retinal vessels: A
meta-analysis. Pediatrics. 141:e201740902018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis S, Nehus E, Inge T, Zhang W,
Setchell K and Mitsnefes M: Effect of bariatric surgery on urinary
sphingolipids in adolescents with severe obesity. Surg Obes Relat
Dis. 14:446–451. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hall JE, Henegar JR, Dwyer TM, Liu J, Da
Silva AA, Kuo J and Tallam L: Is obesity a major cause of chronic
kidney disease? Adv Ren Replace Ther. 11:41–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sowers JR: Metabolic risk factors and
renal disease. Kidney Int. 71:719–720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cavalera M, Wang J and Frangogiannis NG:
Obesity, metabolic dysfunction, and cardiac fibrosis:
Pathophysiological pathways, molecular mechanisms, and therapeutic
opportunities. Transl Res. 164:323–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang Q, Wang J, Zhang Y, Wang L, Li W, Han
J, Huang W, Liang G and Wang Y: Inhibition of myeloid
differentiation factor-2 attenuates obesity-induced cardiomyopathy
and fibrosis. Biochim Biophys Acta Mol Basis Dis. 1864:252–262.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Querejeta R, López B, González A, Sánchez
E, Larman M, Martinez Ubago JL and Díez J: Increased collagen type
I synthesis in patients with heart failure of hypertensive origin:
Relation to myocardial fibrosis. Circulation. 110:1263–1268. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohashi K, Iwatani H, Kihara S, Nakagawa Y,
Komura N, Fujita K, Maeda N, Nishida M, Katsube F, Shimomura I, et
al: Exacerbation of albuminuria and renal fibrosis in subtotal
renal ablation model of adiponectin-knockout mice. Arterioscler
Thromb Vasc Biol. 27:1910–1917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stokes MB, Holler S, Cui Y, Hudkins KL,
Eitner F, Fogo A and Alpers CE: Expression of decorin, biglycan,
and collagen type I in human renal fibrosing disease. Kidney Int.
57:487–498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aleksandrova K, Mozaffarian D and Pischon
T: Addressing the perfect storm: Biomarkers in obesity and
pathophysiology of cardiometabolic risk. Clin Chem. 64:142–153.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jung HJ, Seu YB and Lee DG: Candicidal
action of resveratrol isolated from grapes on human pathogenic
yeast C. albicans. J Microbiol Biotechnol. 17:1324–1329.
2007.PubMed/NCBI
|
|
32
|
Malhotra A, Bath S and Elbarbry F: An
organ system approach to explore the antioxidative,
anti-inflammatory, and cytoprotective actions of resveratrol. Oxid
Med Cell Longev. 2015:8039712015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cucciolla V, Borriello A, Oliva A,
Galletti P, Zappia V and Della Ragione F: Resveratrol: From basic
science to the clinic. Cell Cycle. 6:2495–2510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gambini J, Inglés M, Olaso G, Lopez-Grueso
R, Bonet-Costa V, Gimeno-Mallench L, Mas-Bargues C, Abdelaziz KM,
Gomez-Cabrera MC, Vina J and Borras C: Properties of resveratrol:
In vitro and in vivo studies about metabolism,
bioavailability, and biological effects in animal models and
humans. Oxid Med Cell Longev. 2015:8370422015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Modi S, Yaluri N and Kokkola T:
Strigolactone GR24 and pinosylvin attenuate adipogenesis and
inflammation of white adipocytes. Biochem Biophys Res Commun.
499:164–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aguirre L, Fernández-Quintela A, Arias N
and Portillo MP: Resveratrol: Anti-obesity mechanisms of action.
Molecules. 19:18632–18655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:4932006. View Article : Google Scholar : PubMed/NCBI
|