Introduction
Acute pancreatitis (AP) is a serious disease
associated with the release of digestive enzymes into the systemic
circulation and pancreatic interstitium, which has notable
morbidity and mortality (1–4). It
is generally known that cytokines are involved in the pathogenic
mechanism of AP (5). Cytokines not
only increase pancreatic damage, but also cause subsequent systemic
inflammatory response syndrome (SIRS) (5,6). The
role of oxidative stress has been a concern in the pathophysiology
of acute pancreatitis (6,7). In the initial stage of AP,
inflammatory cells (neutrophils and macrophages) infiltrate into
the pancreatic tissues (8). Such
infiltrating inflammatory cells (particularly neutrophils) produce
reactive oxygen species (ROS), which subsequently destroy lipid
membranes through lipid peroxidation and induce the development of
widespread inflammation (2,9,10).
Increasing evidence indicates that the interaction between
oxidative stress and cytokines is closely associated with the
development of AP, leading to the amplification of uncontrolled
inflammatory cascades and multiple organ dysfunction syndrome
(MODS) (8,11). Organs that commonly fail in AP
include the lungs and liver, increasing the severity of AP and
leading to a poor prognosis (2,12).
Therefore, the implementation of effective measures to control
oxidative stress and cytokines would conduce to impede the
development of AP.
Carbon monoxide (CO) is one of the products produced
by heme degradation by the rate-limiting enzyme heme oxygenase-1 in
mammals. It is known to be a poisonous gaseous molecule, due to its
capacity to combine with hemoglobin (13). There is increasing evidence
indicating that CO is a cytoprotective and homeostatic molecule
that has crucial signaling capabilities under physiological and
pathophysiological conditions (14–17).
Transition metal carbonyl compounds, known as CO-releasing
molecules (CORMs), have been used in biological systems for
releasing CO in a controlled way without markedly altering
carboxy-hemoglobin (CO-Hb) levels (18). Lipid-soluble metal carbonyl complex
tricarbonyldichlororuthenium (II) dimer ([Ru(CO)3Cl2]2), also
termed CORM-2, was the first compound to make this technology
feasible (19). CORM-2 is able to
spontaneously transfer CO and serve an important role in
CO-mediated pharmacology (20).
Studies have revealed that CORM-2 is able to inhibit inflammatory
responses in various experimental models (21–24).
However, to the best of our knowledge, there is no relevant
research on the effect of CORM-2 on AP.
Caerulein is a cholecystokinin analog that is
commonly used to induce either acute or chronic pancreatitis. It is
recognized that large doses of caerulein cause acute interstitial
(edematous) pancreatitis characterized by massive disruption of the
acinar cells (25–27). Caerulein-induced acute pancreatitis
is currently recognized as an in vivo study model for AP
(25–27). In the present study, caerulein was
used to induce AP in a mouse model, and it was hypothesized that
CORM-2 may have a protective effect on AP mice induced by
caerulein. The potential molecular mechanism was also examined.
Materials and methods
Ethics statement
All experiments were strictly in accordance with the
Guidelines for the Care and Use of Laboratory Animals published by
the National Institutes of Health (Bethesda, MD, USA; NIH
Publication No. 85-23, revised in 1996). The present study was
approved by the Animal Ethics Committee of Jiangsu University
(Zhenjiang, China).
Materials
Tricarbonyldichlororuthenium (II) dimer (CORM-2),
and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). CORM-2 was dissolved in DMSO to
acquire a 40 mM stock solution. Inactive (i)CORM-2 was used as the
negative control, and it was prepared as previously described
(28). The primary antibodies of
nuclear factor (NF)-κB, phosphorylated inhibitor of NF-κB subunit α
(p-IκB-α), intercellular adhesion molecule 1 (ICAM-1) and vascular
cell adhesion molecule 1 (VCAM-1) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The nuclear protein
extraction buffer kit was purchased from Vazyme (Piscataway, NJ,
USA). Tumor necrosis factor-α (TNF-α; JER-06), interleukin-6 (IL-6;
JER-04) and IL-1β (JER-01) ELISA kits were obtained from Joyee
Biotechnics Co., Ltd. (Shanghai, China). Caerulein (purity ≥97% by
high-performance liquid chromatography) was obtained from
Sigma-Aldrich (Merck KGaA).
AP model and experimental
protocol
C57BL/6 mice (n=180; male, 6–8 weeks old, 20±2 g)
were obtained from the Experimental Animal Center of Jiangsu
University, Zhenjiang, Jiangsu, China. The mice were housed in
standard wire-topped cages and in temperature-controlled units
(18–23°C with 40–60% humidity and 12-h light/dark cycle). Food and
water were supplied ad libitum.
Caerulein was used to induce AP in the mice. The
mice were randomly divided into four groups (n=30 mice/group): i)
The control group, in which mice were treated hourly (×10) with
normal saline (0.9% NaCl) (equal in volume to caerulein)
intraperitoneally (i.p.); ii) the AP group, in which mice were
treated hourly (×10) with caerulein (50 µg/kg, suspended in normal
saline, i.p.); iii) the AP+CORM-2 group, in which mice received
CORM-2 [8 mg/kg, intravenously (i.v.)] treatment 30 min after the
induction of pancreatitis (first caerulein injection), and
thereafter received hourly caerulein i.p. (×10); and iv) the
AP+iCORM-2 group, in which mice received iCORM-2 (8 mg/kg, i.v.)
treatment 30 min after the induction of pancreatitis (first
caerulein injection), and thereafter received hourly caerulein i.p.
(×10). The dosage of CORM-2 used in the present study was based on
our previous studies (29,30).
In another set of experiments, mice were randomly
divided into four groups (n=15 mice/group), and were monitored for
5 days to observe their survival rate.
Tissue harvesting
A total of 12 h after the induction of pancreatitis,
the mice were sacrificed by overdose of anesthesia. Blood samples
were collected by cardiac puncture and serum was immediately
obtained by centrifugation 3,000 × g for 5 min at 4°C. The
separated serum was used for the subsequent determination of
lipase, amylases, aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) levels. Meanwhile, pancreas, lung and liver
tissues were harvested and immediately frozen in liquid nitrogen or
fixed with 10% formalin at room temperature overnight for following
analysis.
Biochemical measurement
The activity of serum amylase and lipase was
detected to assess pancreatic damage using a commercial kit
(SNM144-BOU, Biolebo Technology Co., Ltd., Beijing, China),
according to the manufacturers' protocol. Liver injury was assessed
by measuring the enzymatic activities of ALT and AST in serum
samples using a set of commercial kits (Roche Diagnostics GmbH,
Mannheim, Germany), according to the manufacturer's protocol.
Morphological examination
Samples of 10% formalin-fixed pancreas, lung and
liver tissues were embedded in paraffin and segmented to 4 µm for
routine histology. The fixed tissues were stained with hematoxylin
and eosin (H&E), and examined by two experienced morphologists
who were unaware of the sample identity. A total of 10 randomly
selected microscope fields (magnification, ×200) were tested for
each pancreas tissue sample. The severity of pancreatic injury was
scored in accordance with the previously described 0–4 (normal to
severe) scale (31).
Apoptotic cell determination
A terminal deoxynucleotidyl-transferase-mediated
dUTP nick end labelling (TUNEL) kit (Roche Diagnostics,
Indianapolis, IN, USA) was used to measure apoptosis in pancreatic
cells, according to the manufacturers' protocol. Tissue was fixed
in 10% neutral formalin overnight, dehydrated and embedded in
paraffin wax. Paraffin-embedded pancreas sections (4 µm) were
incubated for 20 min at 60°C prior to being deparaffinized and
rehydrated. Following digestion with 20 µg/ml proteinase K at room
temperature for 20 min, the sections were washed with PBS three
times, and incubated in 25 mM cobalt chloride according to the
manufacturer's protocol. Tissue sections were mounted using
Aqua-Poly/Mount mounting medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Finally, apoptotic cells were observed under a
microscope, and 10 microscope fields (magnification, ×200) were
randomly selected for analysis in each viewed sample. Finally,
apoptotic cells were observed under a microscope, and 10 microscope
fields (magnification, ×200) were randomly selected for analysis in
each viewed sample.
Measurement of lung edema
A previous study included a detailed description of
the measurement of whole lung wet/dry ratio, an index of lung edema
(32). Lung tissues were dissected
from the heart and large blood vessels, the trachea was separated
at the carina, external liquid was removed by blotting, and the
lungs were placed on a pre-weighed pan to obtain the wet weight.
Subsequently, the lungs were incubated overnight in a dry
atmosphere at 85°C and were re-weighed to obtain the dry
weight.
Immunohistochemical staining. Pancreatic tissues
were fixed in 10% formalin and 3–4 µm slices were prepared from
paraffin-embedded tissues. Following paraffin removal and
rehydration, slices were subjected to heat-mediated antigen repair
in sodium citrate buffer (10 mM sodium citrate, pH 6.0) and blocked
in 10% normal goat serum (Santa Cruz Biotechnology, Inc.) for 2 h
at room temperature. The samples were subsequently incubated with
the primary antibodies against ICAM-1 (1:200; sc-1511; Santa Cruz
Biotechnology, Inc.) and VCAM-1 (1:200; sc1504; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. Following washing three
times with PBS, samples were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibody (1:100;
sc-2004; Santa Cruz Biotechnology, Inc.) at room temperature for 20
min. Subsequently, when the samples had again been washed three
times with TBS, they were stained with freshly prepared
diaminobenzidine chromogen (brown) for 3–5 min at room temperature,
washed with distilled water, and counterstained with hematoxylin
for 10 sec at room temperature. The samples were dehydrated with a
gradient of ethanol. Finally, the slides were mounted with mounting
medium, labelled and viewed under the microscope (IX51-A12PH;
Olympus Corporation, Tokyo, Japan). Among the pancreas samples, 10
microscope fields (magnification, ×200) were randomly selected for
analysis in each tissue sample. The average optical density of
ICAM-1 and VCAM-1 was evaluated using Image Pro-Plus software 6.0
(Media Cybernetics, Inc., Rockville, MD, USA).
Myeloperoxidase (MPO) activity
detection
MPO activity was determined in tissues from the
pancreas, lung and liver, according to a previous study (33). Tissue samples were homogenized in
50 mM potassium phosphate buffer (PB, pH 6.0) and centrifuged at
10,000 × g for 10 min at room temperature. The precipitate was
collected and the pellet was suspended in 50 mM PB containing 0.5%
hexadecyltrimethylammonium bromide. The samples were sonicated (30
times, and each time was 5–10 s at room temperature at 20 kHz and
200 W) and centrifuged again at 10,000 × g for 10 min at room
temperature. Aliquots of 0.3 ml were added to 2.3 ml reaction
mixture containing 50 mM PB, o-dianisidine, and 20 mM
H2O2 solution. One unit of enzyme activity
was regarded as the MPO content which resulted in a change in
absorbance detection at 460 nm for 3 min. MPO activity is expressed
as U/g tissue.
Malondialdehyde (MDA) activity
detection
The MDA levels in pancreatic, lung and liver tissue
samples were also assessed. The tissue samples were homogenized
with a 1.15% KCl solution. An aliquot (100 µl) of the homogenate
was added to a reaction mixture, which included 200 µl 8.1% SDS,
1,500 µl 20% acetic acid (pH 3.5), 1,500 µl 0.8% thiobarbituric
acid and 700 µl distilled water. The samples were boiled for 1 h at
95°C and centrifuged for 10 min at 3,000 × g at room temperature.
The absorbance at 650 nm was determined by spectrophotometry.
TNF-α, IL-6 and IL-1β level
determination
To determine the levels of TNF-α, IL-1β and IL-6 in
the serum and tissue homogenates of the pancreas, lung and liver,
ELISA kits were used, according to the manufacturer's instructions
of each kit.
Western blotting
A nuclear protein extraction buffer kit (Vazyme) was
used for nucleic protein extraction. BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.) was used to evaluate the protein
concentrations. Samples (10 µg of protein) were separated on 10%
SDS-PAGE gels and transferred to PVDF membranes. The membranes were
blocked with 5% non-fat milk at room temperature for 1.5 h and
subsequently incubated with anti-mouse NF-κB-specific polyclonal
antibody (1:1,000; cat. no. sc-514451) or anti-mouse
p-IκB-α-specific polyclonal antibody (1:1,000; cat. no. sc-7977) at
4°C overnight and secondary HRP-conjugated goat anti-mouse IgG
antibody at the correct concentration (1:10,000; cat. no. sc-2031;
all from Santa Cruz Biotechnology, Inc.) at room temperature for 2
h. Enhanced chemiluminescence was used to visualize the bands using
FluorChem FC3 (ProteinSimple, San Jose, CA, USA), and AlphaView
3.4.0 software (ProteinSimple) was used for quantitative
analysis.
Statistical analysis
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for the statistical analyses. All experiments
were repeated at least three times and the data are presented as
the mean ± standard deviation. Comparisons between groups were
analyzed using one-way factorial analysis of variance followed by
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of CORM-2 on the function of
the pancreas in caerulein-induced AP
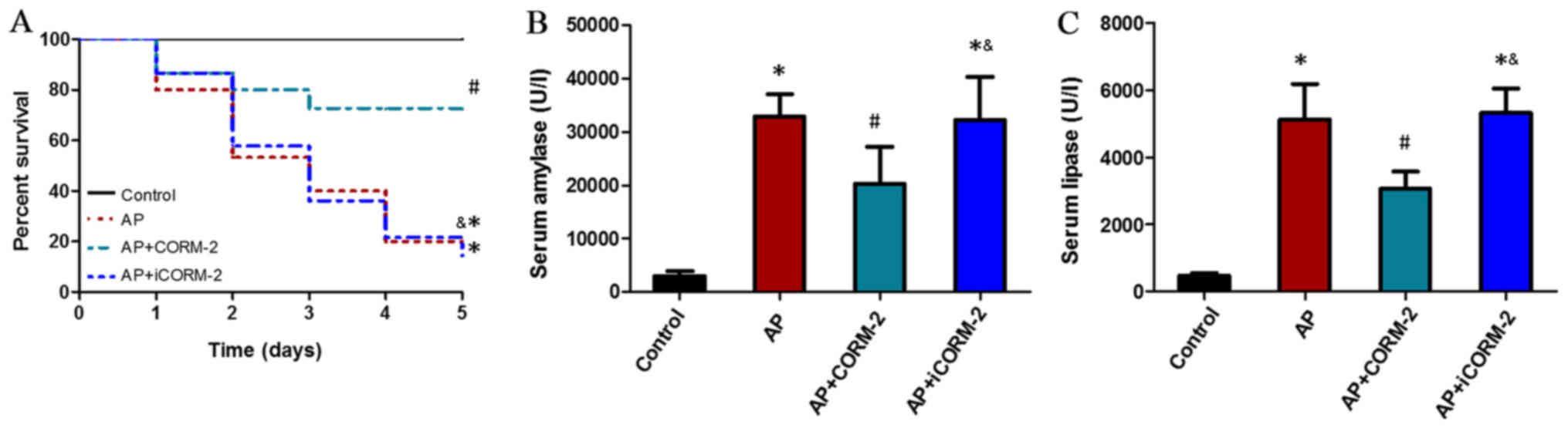
To confirm the therapeutic effects of CORM-2,
caerulein hyperstimulation animal models of AP were used. The
survival rate was calculated to be 100% (15/15) in the control
group, 20% (3/15) in the AP group, 73.3% (11/15) in the CORM-2
group, and 20% (3/15) in the iCORM-2 group at 5 days (Fig. 1A). This suggested that the
administration of CORM-2 led to significantly lower mortality
compared with the AP group and protected against the lethality of
AP, and that treatment with iCORM-2 did not serve a protective role
against mortality (Fig. 1A). The
levels of serum amylase and lipase were examined, as these reflect
the degree of pancreatic injury. The levels of serum amylase and
lipase were increased at 12 h post-AP induction, and were reduced
by the administration of CORM-2 (Fig.
1B and C). The AP animals treated with iCORM-2 exhibited
unaltered serum amylase and lipase activity compared with the AP
group (Fig. 1B and C).
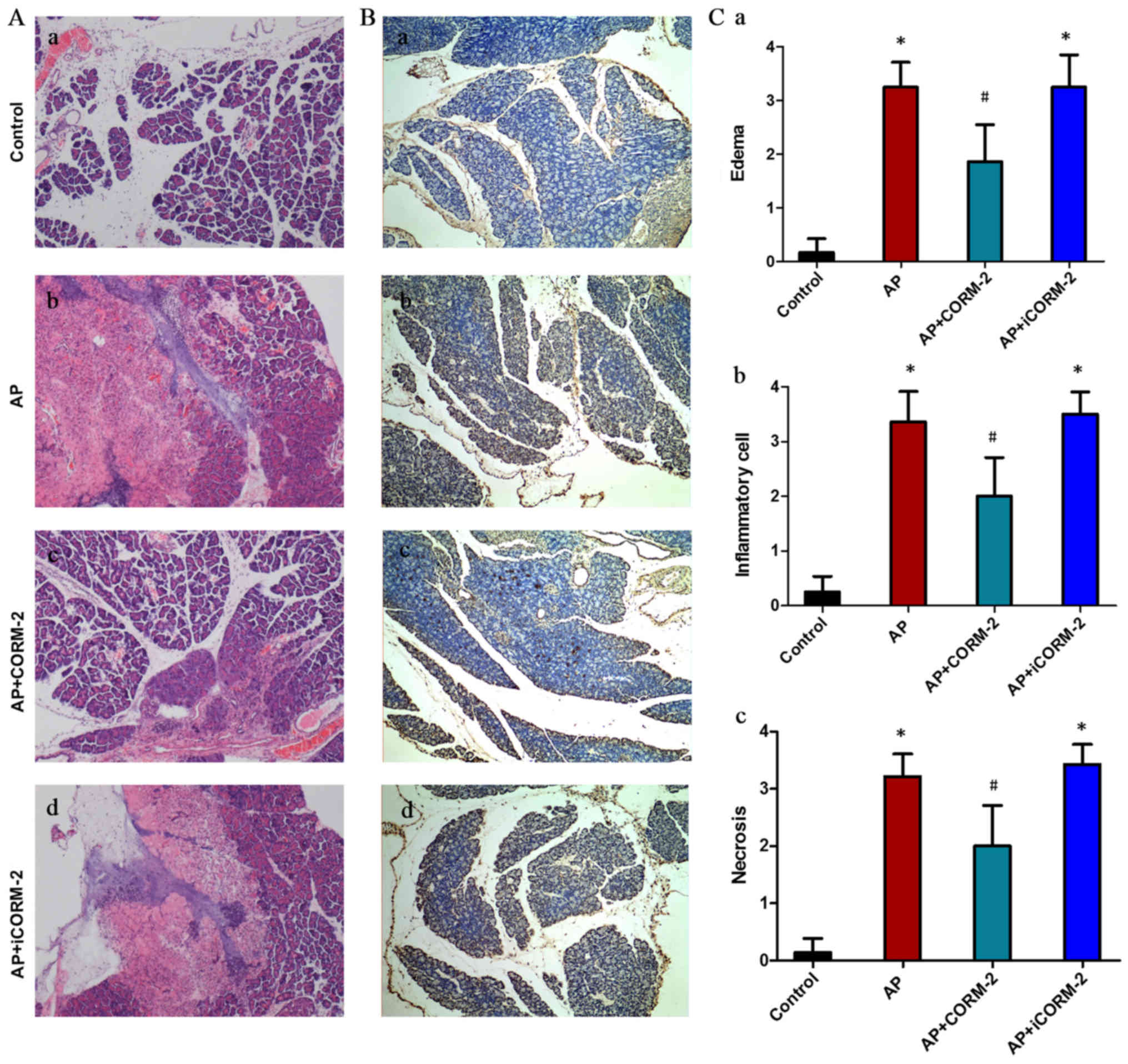
The severity of AP was also assessed by histological
examination (Fig. 2). The
pancreatic architecture was normal in control group mice (Fig. 2Aa), whereas the pancreatic tissues
in AP group mice exhibited severe pathological damage at 12 h
post-AP induction (Fig. 2Ab).
Treatment with CORM-2 improved the pancreatic damage induced by
caerulein (Fig. 2Ac), while
iCORM-2 treatment had no marked alleviating effect on injury in the
pancreas (Fig. 2Ad).
Histopathological scoring of the pancreatic injury indicated that
caerulein induction induced edema, inflammatory cell infiltration,
and necrosis of the acinar cells compared with the control group
(Fig. 2C). This damage was
markedly alleviated by treating the AP mice with CORM-2 (Fig. 2C). However, iCORM-2 treatment
failed to obviously improve the histological score (Fig. 2C). Previous clinical and
experimental studies have reported that apoptosis is observed
during the course of AP (34,35).
In order to further investigate the effect of CORM-2 on pancreatic
cell apoptosis in caerulein-induced AP, a TUNEL assay was performed
to detect pancreatic cell apoptosis. As presented in Fig. 2B, a mass of apoptotic cells
(including pancreatic acinar cells, intercalated ducts cells and
certain types of islet cells) with brown nuclei was observed in the
pancreases from mice of the AP group (Fig. 2Bb), although not in mice of the
control group (Fig. 2Ba). The
number of apoptotic cells with brown nuclei was significantly
reduced by treatment with CORM-2 (Fig.
2Bc). No significant alterations in staining were seen in the
iCORM-2 treatment group compared with the AP group (Fig. 2Bd).
Effect of CORM-2 on organ function in
lung and liver tissues during caerulein-induced AP
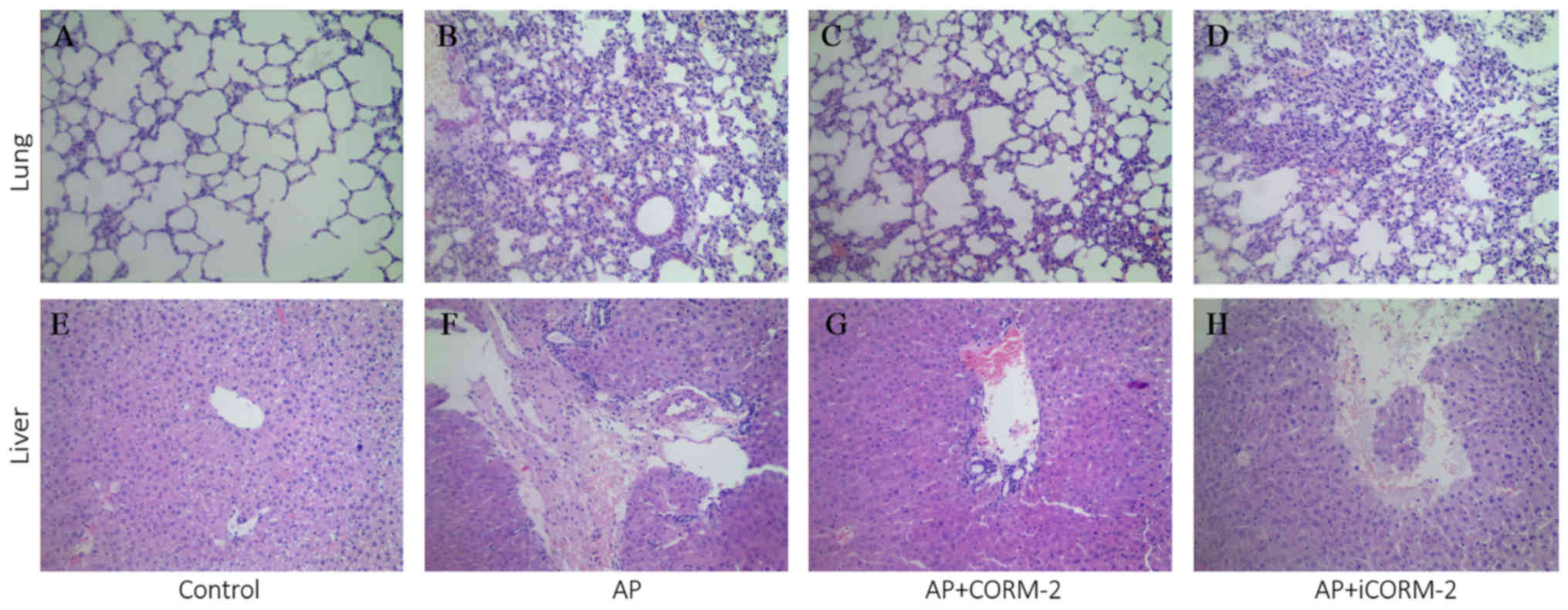
In addition to pancreas injury, caerulein injections
may also induce injury to the lung and liver in wild-type mice, as
previously reported (2,12,25,26).
The severity of lung and liver injury was evaluated by histological
analysis in the current study (Fig.
3). H&E staining demonstrated the normal structure of the
sections of lung and liver in the control group (Fig. 3A and E). In caerulein-induced AP
mice, increased numbers of alveolar epithelial cells and red blood
cells with inflammatory cell infiltration were observed in the lung
(Fig. 3B). Furthermore, marked
degeneration and necrosis of liver tissues, hyperemia in the
central veins of the liver and infiltration of the liver by
inflammatory cells was also observed (Fig. 3F). These morphological alterations
caused systemic inflammation and organ damage in the lung and
liver, and were also observed in the iCORM-2-treated AP group
(Fig. 3D and H). Following in
vivo CORM-2 treatment, the pathological alterations in the lung
and liver were decreased (Fig. 3C and
G), indicating that CORM-2 had a protective effect on vital
organs in AP.
Additionally, lung injury induced by caerulein
(Fig. 4) was also characterized by
marked pulmonary edema (Fig. 4F),
sequestration of lung neutrophils (increase in MPO content;
Fig. 4B), lipid peroxidation
(increase in MDA content; Fig. 4C)
and an increase in pro-inflammatory cytokine levels (TNF-α, IL-1β
and IL-6; Fig. 4D). The
manifestation of the liver injury induced by caerulein also
included an increase in the serum concentrations of ALT and AST
(Fig. 4A), while CORM-2 treatment
effectively relieved the injury to the lung and liver, and this was
not the case with iCORM-2 (Fig.
4).
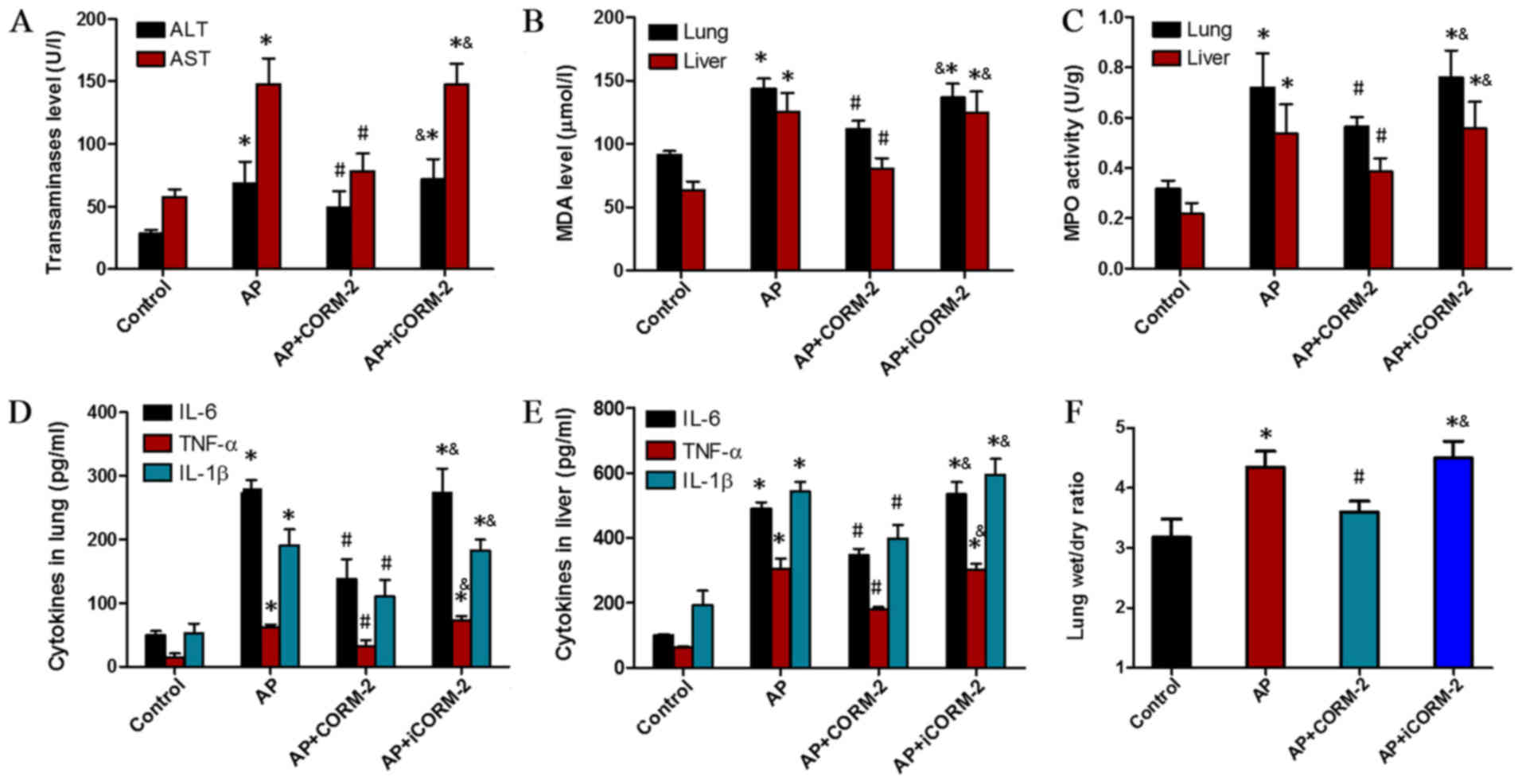 | Figure 4.Effects of CORM-2 on organ function,
MPO levels, MDA activity and cytokine levels in liver and lung
tissues. (A) Serum ALT and AST levels, (B) MDA levels, (C) MPO
activity, levels of TNF-α, IL-6 and IL-1β in (D) the lungs and (E)
liver, and (F) the lung wet/dry weight ratio were observed to be
significantly elevated in the AP group compared with the control
group. In contrast, the corresponding values in the AP+CORM-2 group
were significantly lower than those in the AP and AP+iCORM-2
groups. These data are expressed as the mean ± standard deviation,
n=5 for each group. *P<0.05 vs. control group;
#P<0.05 vs. AP group; &P<0.05 vs.
AP+CORM-2. ALT, alanine aminotransferase; AST, aspartate
aminotransferase; IL, interleukin; TNF-α, tumor necrosis factor-α;
MPO, myeloperoxidase; MDA, malondialdehyde; AP, acute pancreatitis;
CORM-2, CO-releasing molecule 2; iCORM-2, inactive CO-releasing
molecule 2. |
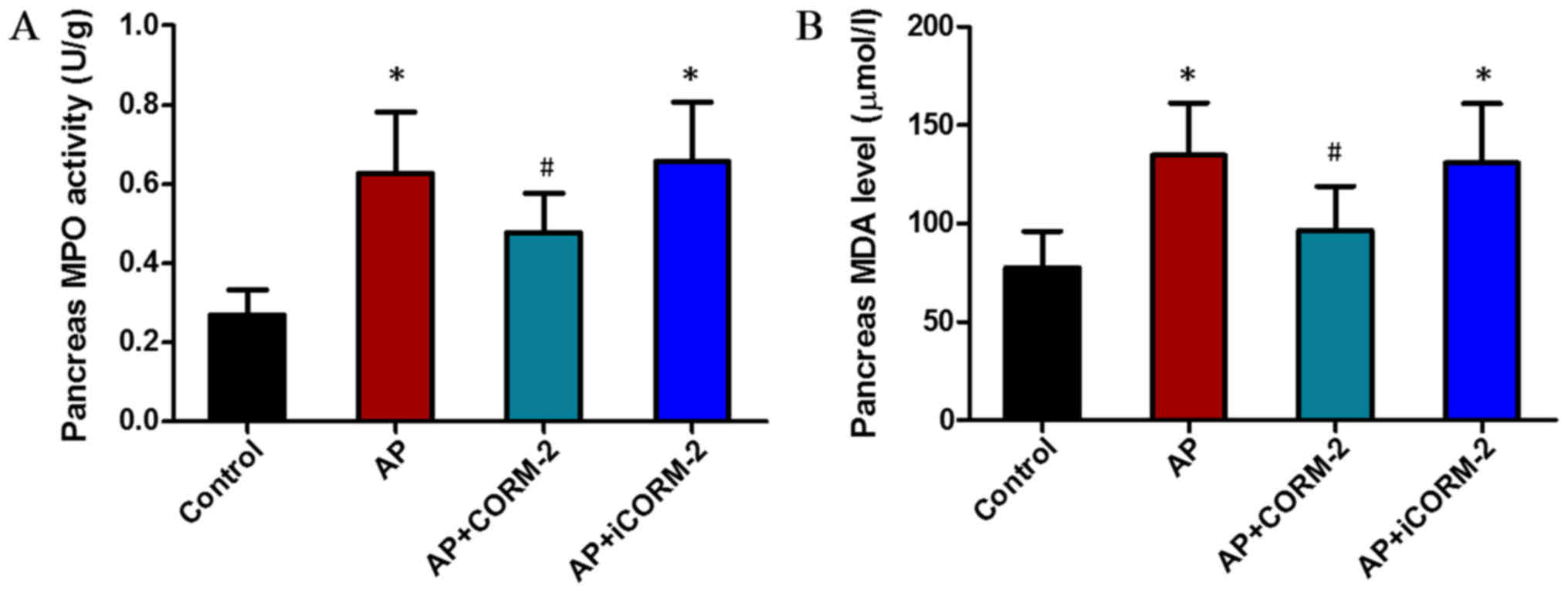
Effect of CORM-2 on MPO and MDA
activity in the pancreas of caerulein-induced AP animals
The levels of MPO and MDA that were increased by
caerulein injection were reduced by treatment with CORM-2. The
results for MPO levels were also in accordance with the
histological results. In the AP group, significant areas of
inflammatory cell infiltration were evident (Fig. 5A) and the histological score of
inflammatory cell infiltration was significantly increased
(Fig. 2Cb). Treatment with CORM-2
reduced neutrophil infiltration and the extent of lipid
peroxidation in the pancreas (Fig. 5A
and B). There was no significant difference between the AP
group and the AP+iCORM-2 group (Fig.
5A and B).
Effect of CORM-2 on the expression of
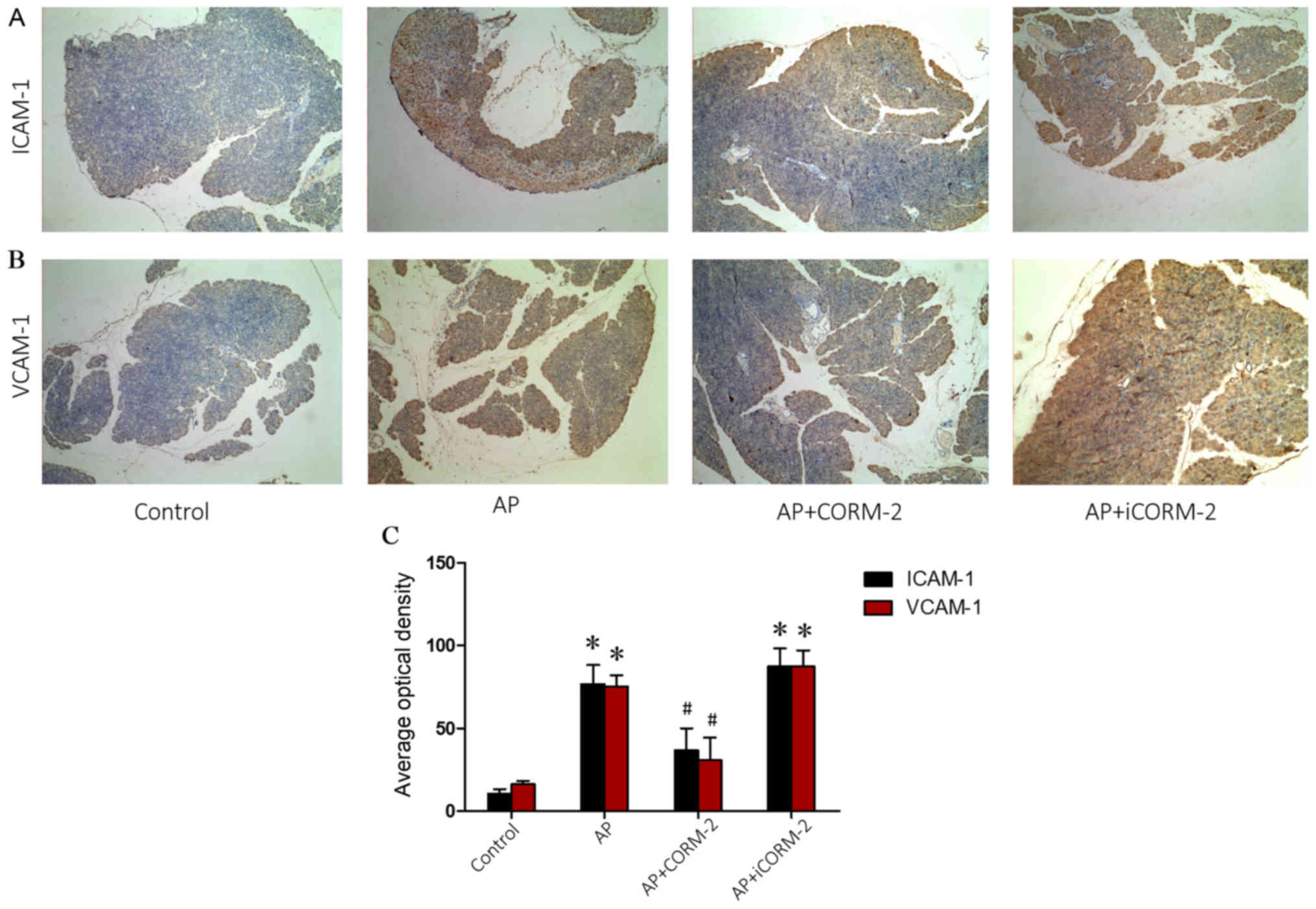
ICAM-1 and VCMP-1 in the pancreas of caerulein-induced AP
animals
Increased adherence of neutrophils to endothelial
cells leads to the accumulation of neutrophils in tissues, which is
primarily mediated by adhesion molecules (36). Sequestered neutrophils in the
pancreas augment the injury to the tissue (37). Therefore, the expression of the
ICAM-1 and VCAM-1 adhesion molecules was assessed, as they
facilitate the attachment of neutrophils to the endothelium. No
positive staining for ICAM-1 and VCAM-1 was observed in the
pancreatic tissue sections that were obtained from mice in the
control group (Fig. 6A and B).
Sections obtained from AP mice 12 h post-caerulein induction
exhibited strong positive staining for ICAM-1 and VCAM-1 (Fig. 6A and B). The degree of pancreatic
staining for ICAM-1 and VCAM-1 was reduced in tissue sections
obtained from CORM-2-treated AP mice (Fig. 6A and B). No marked alterations in
staining were observed in the iCORM-2-treated AP group compared
with the AP group (Fig. 6A and B).
Quantitative analysis of the average optical density for ICAM-1 and
VCAM-1 is presented in Fig.
6C.
Effect of CORM-2 on the production of
pro-inflammatory cytokines in the pancreas of caerulein-induced AP
animals
For the purpose of investigating the regulation of
inflammation by CORM-2 treatment, ELISA was performed to detect the
levels of the pro-inflammatory cytokines IL-6, IL-1β and TNF-α in
the serum and pancreas of mice. The levels of IL-6, IL-1β and TNF-α
in the serum and pancreas of the mice markedly increased at 12 h
post-AP induction (Fig. 7A). In
vivo administration of CORM-2 significantly decreased the
caerulein-induced increase in the levels of IL-6, IL-1β and TNF-α
(Fig. 7). However, treatment with
iCORM-2 did not alter the levels of these cytokines compared with
the AP group (Fig. 7).
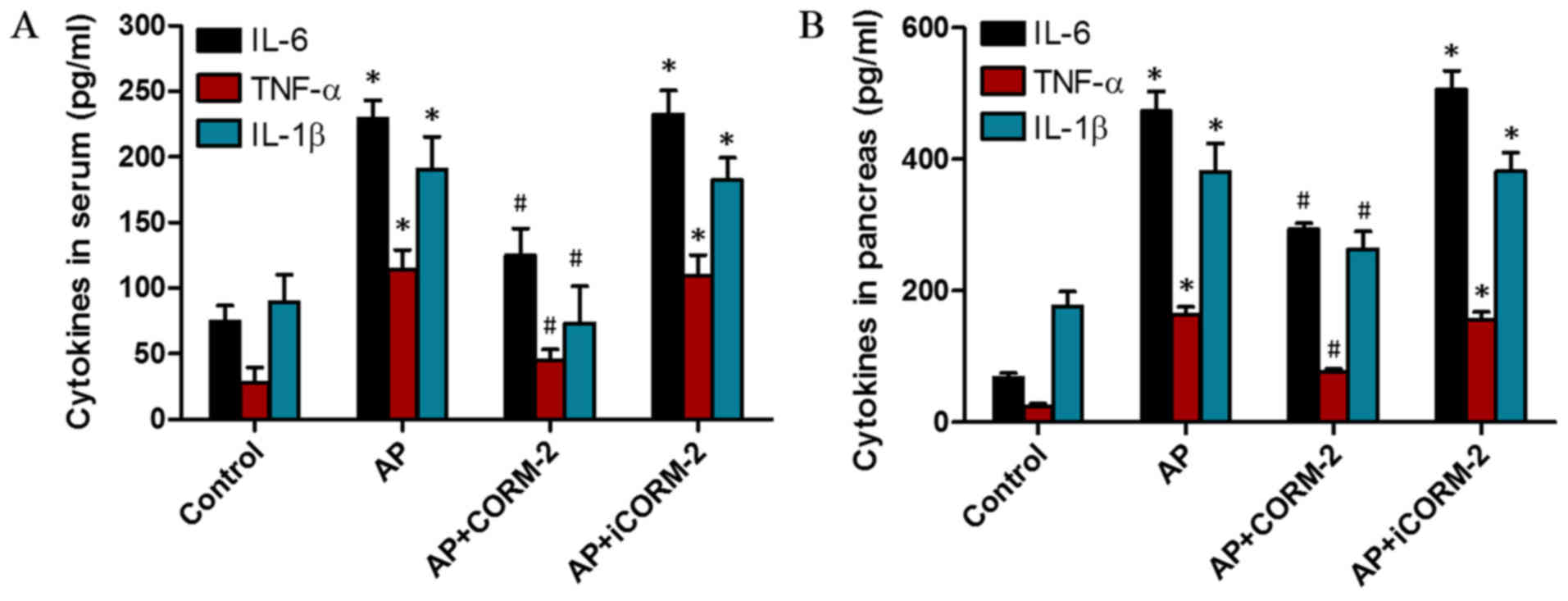 | Figure 7.Effects of CORM-2 on the cytokine
levels in the serum and pancreas of caerulein-induced AP mice. The
levels of IL-6, IL-1β and TNF-α in the serum and pancreas were
assessed following the induction of AP for 12 h. In the AP group,
the levels of the cytokines IL-6, IL-1β and TNF-α in (A) the serum
and (B) pancreatic homogenates were significantly increased
compared with the corresponding control groups. In the AP+CORM-2
group, the increased levels of those cytokines in the serum and
pancreatic homogenate were reduced compared with the corresponding
AP group. Treatment with iCORM-2 did not decrease the increased
levels of these cytokines. These data are presented as the mean ±
standard deviation, n=5 for each group. *P<0.05 vs. control
group; #P<0.05 vs. AP group. IL, interleukin; TNF-α,
tumor necrosis factor-α; AP, acute pancreatitis; CORM-2,
CO-releasing molecule 2; iCORM-2, inactive CO-releasing molecule
2. |
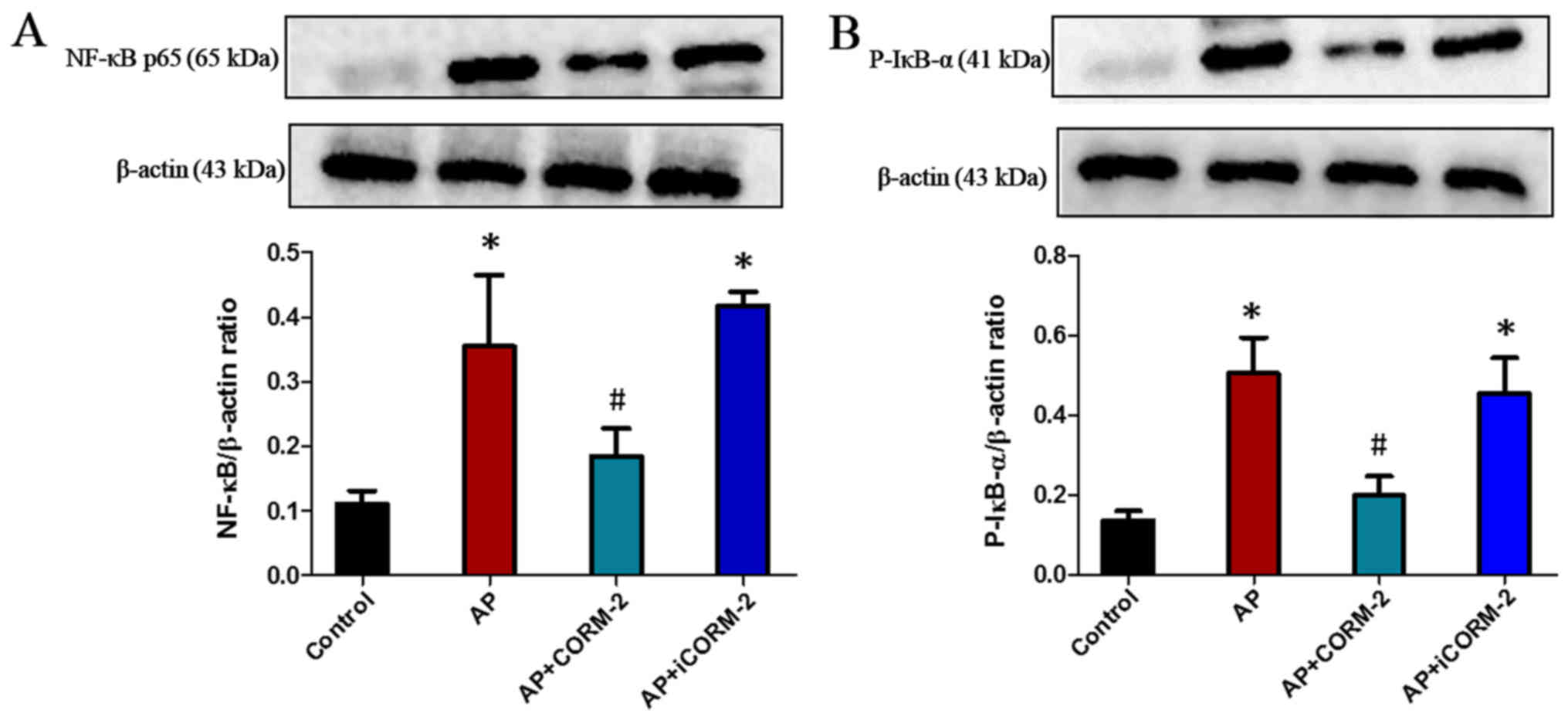
Effect of CORM-2 on NF-κB activation
in the pancreas of caerulein-induced AP animals
To examine whether the NF-κB pathway was involved in
the observed anti-inflammatory effects of CORM-2-derived CO,
western blotting was used to detect the expression of NF-κB (p65)
in the pancreas. The results revealed that AP mice exhibited a
significant increase in NF-κB expression compared with control
animals (Fig. 8A). This increase
was prevented by treatment with CORM-2, although not by iCORM-2
(Fig. 8A). Furthermore, the
phosphorylation of IκB-α (p-IκB-α), which is required for the
initiation of NF-κB activation, was also examined by western
blotting in the pancreas. There was a significant increase in the
level of p-IκB-α in the pancreas of AP mice, which was suppressed
by administration of CORM-2 (Fig.
8B). No significant difference in the p-IκB-α level in the
pancreas was observed following treatment with iCORM-2 compared
with the pancreas from AP mice (Fig.
8B).
Discussion
AP is an inflammatory disease accompanied by acinar
cell damage, and the rapid secretion and release of inflammatory
factors results in local pancreatic inflammation and systemic
complications (25,38,39).
It is generally known that cytokines and oxidative stress are
involved in the pathophysiology of AP (40,41).
Crosstalk between cytokines and oxidative stress helps to convert
local inflammatory processes into systemic inflammatory responses,
which may subsequently lead to the development of SIRS and the
occurrence of MODS and mortality (6). Despite the considerable morbidity and
mortality associated with AP, effective therapeutic measures have
not thus far been available.
Previous studies have indicated that exogenous CO
has particular and independent roles in the modulation of
inflammation (16,17,28).
In the present study, CORM-2 was used due to its ability to release
CO in biological systems in a controlled manner (18,39).
Studies have demonstrated that CORM-2 inhibits the production and
release of cytokines in sepsis induced by lipopolysaccharide (LPS)
and cecal ligation and puncture (42–44).
In addition, Xue and Habtezion (45) reported that CORM-2 reduced the
levels of systemic inflammatory cytokines and suppressed systemic
and pancreatic macrophage TNF-α secretion in a mouse model of AP.
However, the potential mechanisms of CO in AP were not completely
understood.
To study the therapeutic function of CO, an AP mouse
model was established using caerulein. In the present study, it was
demonstrated that caerulein induced significant mortality, highly
elevated levels of serum amylase and lipase, and markedly increased
apoptosis of the pancreas at 12 h post-induction of AP. Treatment
with CORM-2 decreased mortality, reduced serum amylase and lipase
levels and inhibited the apoptosis of the pancreas. Treatment of AP
mice with iCORM-2 exerted no notable effects on pancreatic damage
when compared with AP mice. Furthermore, mild histological
alterations in the pancreas were observed in the CORM-2 treatment
group compared with the AP group, whereas treatment with iCORM-2
failed to relieve pancreatic damage.
Severe AP leads to SIRS and multiple organ failure,
highlighting the severity of AP (11,40).
The present study assessed the injury to the lung and liver induced
by AP. Mice in the AP group had apparent lung and liver damage with
characteristics of considerable histological alterations, high
levels of MPO and MDA, and increased expression of TNF-α, IL-1β and
IL-6 in tissues, in addition to increased serum AST and ALT levels,
which were attenuated by treatment with CORM-2. No statistical
difference was determined between the AP group and the AP+iCORM-2
group.
MPO is an enzyme that is largely present in the
azurophilic granules of neutrophils, which is used as an indicator
of neutrophil accumulation (15,28).
MPO activity is associated with the number of histologically
determined polymorphonuclear neutrophils in tissues. Infiltration
of neutrophils in tissues produces ROS that subsequently damage
lipid membranes via peroxidation and give rise to a variety of
inflammatory processes (4,6). Oxidative stress and inflammation may
interact and occur simultaneously, and are associated with major
acinar cell damage (6). The
present study identified that pancreatic MPO and MDA activity
levels were increased following AP induction and evidently
attenuated by the administration of CORM-2. The results also
demonstrated that CORM-2 effectively prevented neutrophil
chemotaxis and infiltration in the pancreas following AP and
suppressed oxidative stress, and consequently decreased pancreatic
injury. In addition, oxidative stress is one of the principal
causes of DNA damage and death in acinar cells. Apoptosis was
examined by TUNEL in AP mice, and the apoptosis level in the
pancreas was observed to be significantly reduced following
treatment with CORM-2.
Adhesion molecules are important regulatory factors
of neutrophil flux, and they are able to regulate the processes of
neutrophil chemoattraction, adhesion and migration from the
vasculature to the tissues (4).
ICAM-1 and VCAM-1 are involved in neutrophil adhesion. It was
identified that treatment with caerulein increased the expression
of ICAM-1 and VCAM-1 in the pancreas of mice. By contrast, it was
demonstrated that the expression of ICAM-1 and VCAM-1 was decreased
in CORM-2-treated mice at 12 h after caerulein induction. No
significant differences in expression levels were determined in
iCORM-2-treated mice compared with AP mice.
The development of pancreatic injury results in a
local inflammatory response that leads to SIRS and distant organ
failure (39). The systemic
manifestations of the disease are mediated by the free radicals and
different cytokines released during the AP process. TNF-α and IL-1β
are the key cytokines involved in the progression of SIRS and the
subsequent organ failure in AP (46,47).
IL-6 is a foremost induction factor of the acute-phase protein
response (47). The serum level of
IL-6 is considered to be a marker of AP severity, although it does
not form the basis of the occurrence and spread of systemic
inflammatory responses (6). The
present study indicated that the levels of TNF-α, IL-1β and IL-6 in
the serum and pancreas of mice increased at 12 h post-AP induction,
and were attenuated by treatment with CORM-2. These data indicated
that CORM-2 exerted potent anti-inflammatory effects on AP.
The NF-κB family members, as a crucial factor in the
transmission of intracellular signals, are ubiquitous, and trigger
transcription factors that mediate immune and inflammatory
reactions by regulating the expression of cytokines (48,49).
In a number of experimental models of pancreatitis, it has been
reported that the pancreatic damage is associated with NF-κB dimer
p65/p50-mediated nuclear translocation of cytokine release
(2,39). Additionally, the phosphorylation of
IκB-α in the pancreas, which is required for the activation of
NF-κB, was assessed by western blotting. The results indicated that
NF-κB p65 and p-IκB-α were markedly increased at 12 h post-AP and
that this increase was suppressed by treatment with CORM-2.
Although exogenous CO has therapeutic and
anti-inflammatory effects in a variety of diseases and injury
models, its use in clinical disease remains limited. The principal
issue is the method of administration of CO. Although low-dose
inhaled CO has been demonstrated to have anti-inflammatory effects
on ventilator-induced lung injury, it is also associated with
reduced levels of TNF-α (50–52).
However, it is difficult to ensure a certain dose range of
therapeutic CO without increasing the level of CO-Hb (53). The concentrations of CORM-2 and
iCORM-2 used in this study have previously been demonstrated to be
non-toxic to mammalian cells in vitro and to mice in
vivo (20,54). However, the safety of intravenous
injection with CORM-2 in vivo has not been demonstrated
satisfactorily. Therefore, a novel method of administration of CO
requires further investigation.
In summary, it was demonstrated that the application
of CORM-2 attenuated mortality, pancreatic damage, lung and liver
injury in a mouse model of AP. CORM-2 suppressed neutrophil
infiltration and oxidative stress in the pancreas, lung and liver,
and decreased local and systemic inflammatory cytokines. The
mechanism by which CORM-2-derived CO inhibited pro-inflammatory
cytokine release involved NF-κB activation. However, the current
study is a preliminary study of the effect of CORM-2 on AP. The
current research had certain limitations: i) Mice from the
AP+CORM-2 group received treatment with CORM-2 (8 mg/kg, i.v.) 30
min after the induction of pancreatitis (first caerulein
injection), and in order to verify the effect of CORM-2 on AP,
investigation of different timings and dosages of CORM-2 therapy is
necessary; ii) there is a marked difference between the
caerulein-induced AP animal model and human AP; and iii) whether
NF-κB pathway activation is involved in the protective effects of
CORM-2 on AP mice requires further experiments to confirm. In the
future, in-depth research may be performed to address these
issues.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81272148 and 81471903) and
the Jiangsu Natural Science Foundation (grant no. BK2012703).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and XW conducted experiments and drafted the
manuscript. XX carried out the western blotting,
immunohistochemical staining and helped to revise the manuscript.
WQ participated in organ function assay and the data analysis. BS
conceived of the study, helped to draft the manuscript and
finalized the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Jiangsu University (Zhenjiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yadav D and Lowenfels AB: Trends in the
epidemiology of the first attack of acute pancreatitis: A
systematic review. Pancreas. 33:323–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohashi S, Nishio A, Nakamura H, Kido M,
Ueno S, Uza N, Inoue S, Kitamura H, Kiriya K, Asada M, et al:
Protective roles of redox-active protein thioredoxin-1 for severe
acute pancreatitis. Am J Physiol Gastrointest Liver Physiol.
290:G772–G781. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhatia M, Ramnath RD, Chevali L and
Guglielmotti A: Treatment with bindarit, a blocker of MCP-1
synthesis, protects mice against acute pancreatitis. Am J Physiol
Gastrointest Liver Physiol. 288:G1259–G1265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cuzzocrea S, Genovese T, Mazzon E, Di
Paola R, Muià C, Britti D and Salvemini D: Reduction in the
development of cerulein-induced acute pancreatitis by treatment
with M40401, a new selective superoxide dismutase mimetic. Shock.
22:254–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Escobar J, Pereda J, Arduini A, Sandoval
J, Sabater L, Aparisi L, López-Rodas G and Sastre J: Cross-talk
between oxidative stress and pro-inflammatory cytokines in acute
pancreatitis: A key role for protein phosphatases. Curr Pharm Des.
15:3027–3042. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou HX, Han B, Hou LM, An TT, Jia G,
Cheng ZX, Ma Y, Zhou YN, Kong R, Wang SJ, et al: Protective effects
of hydrogen gas on experimental acute pancreatitis. PLoS One.
11:e01544832016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pereda J, Escobar J, Sandoval J, Rodríguez
JL, Sabater L, Pallardó FV, Torres L, Franco L, Viña J, López-Rodas
G and Sastre J: Glutamate cysteine ligase up-regulation fails in
necrotizing pancreatitis. Free Radic Biol Med. 44:1599–1609. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gómez-Cambronero LG, Sabater L, Pereda J,
Cassinello N, Camps B, Viña J and Sastre J: Role of cytokines and
oxidative stress in the pathophysiology of acute pancreatitis:
therapeutical implications. Curr Drug Targets Inflamm Allergy.
1:393–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altavilla D, Famulari C, Passaniti M,
Galeano M, Macrì A, Seminara P, Minutoli L, Marini H, Calò M,
Venuti FS, et al: Attenuated cerulein-induced pancreatitis in
nuclear factor-kappaB-deficient mice. Lab Invest. 83:1723–1732.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu JH, Lim JW, Namkung W, Kim H and Kim
KH: Suppression of cerulein-induced cytokine expression by
antioxidants in pancreatic acinar cells. Lab Invest. 82:1359–1368.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pereda J, Sabater L, Aparisi L, Escobar J,
Sandoval J, Viña J, López-Rodas G and Sastre J: Interaction between
cytokines and oxidative stress in acute pancreatitis. Curr Med
Chem. 13:2775–2787. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frossard JL, Hadengue A, Spahr L, Morel P
and Pastor CM: Natural history of long-term lung injury in mouse
experimental pancreatitis. Crit Care Med. 30:1541–1546. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bak I, Szendrei L, Turoczi T, Papp G, Joo
F, Das DK, de Leiris J, Der P, Juhasz B, Varga E, et al: Heme
oxygenase-1-related carbon monoxide production and ventricular
fibrillation in isolated ischemic/reperfused mouse myocardium.
FASEB J. 17:2133–2135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song H, Zhao H, Qu Y, Sun Q, Zhang F, Du
Z, Liang W, Qi Y and Yang P: Carbon monoxide releasing molecule-3
inhibits concurrent tumor necrosis factor-α- and
interleukin-1β-induced expression of adhesion molecules on human
gingival fibroblasts. J Periodontal Res. 46:48–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patterson EK, Fraser DD, Capretta A,
Potter RF and Cepinskas G: Carbon monoxide-releasing molecule 3
inhibits myeloperoxidase (MPO) and protects against MPO-induced
vascular endothelial cell activation/dysfunction. Free Radic Biol
Med. 70:167–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng M, Zhang Q, Joe Y, Kim SK, Uddin MJ,
Rhew H, Kim T, Ryter SW and Chung HT: Carbon monoxide-releasing
molecules reverse leptin resistance induced by endoplasmic
reticulum stress. Am J Physiol Endocrinol Metab. 304:E780–E788.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee TS and Chau LY: Heme oxygenase-1
mediates the anti-inflammatory effect of interleukin-10 in mice.
Nat Med. 8:240–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motterlini R, Mann BE, Johnson TR, Clark
JE, Foresti R and Green CJ: Bioactivity and pharmacological actions
of carbon monoxide-releasing molecules. Curr Pharm Des.
9:2525–2539. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seixas JD, Santos MF, Mukhopadhyay A,
Coelho AC, Reis PM, Veiros LF, Marques AR, Penacho N, Gonçalves AM,
Romão MJ, et al: A contribution to the rational design of
Ru(CO)3Cl2L complexes for in vivo delivery of CO. Dalton Trans.
44:5058–5075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motterlini R, Clark JE, Foresti R,
Sarathchandra P, Mann BE and Green CJ: Carbon monoxide-releasing
molecules: Characterization of biochemical and vascular activities.
Circ Res. 90:E17–E24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chung SW, Liu X, Macias AA, Baron RM and
Perrella MA: Heme oxygenase-1-derived carbon monoxide enhances the
host defense response to microbial sepsis in mice. J Clin Invest.
118:239–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun B, Sun Z, Jin Q and Chen X:
CO-releasing molecules (CORM-2)-liberated CO attenuates leukocytes
infiltration in the renal tissue of thermally injured mice. Int J
Biol Sci. 4:176–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee S, Lee SJ, Coronata AA, Fredenburgh
LE, Chung SW, Perrella MA, Nakahira K, Ryter SW and Choi AM: Carbon
monoxide confers protection in sepsis by enhancing beclin
1-dependent autophagy and phagocytosis. Antioxid Redox Signal.
20:432–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Qin W, Qiu X, Cao J, Liu D and Sun
B: A novel role of exogenous carbon monoxide on protecting cardiac
function and improving survival against sepsis via mitochondrial
energetic metabolism pathway. Int J Biol Sci. 10:777–788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pastor CM, Pugin J, Kwak B, Chanson M,
Mach F, Hadengue A and Frossard JL: Role of Toll-like receptor 4 on
pancreatic and pulmonary injury in a mice model of acute
pancreatitis associated with endotoxemia. Crit Care Med.
32:1759–1763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhatia M, Slavin J, Cao Y, Basbaum AI and
Neoptolemos JP: Preprotachykinin-a gene deletion protects mice
against acute pancreatitis and associated lung injury. Am J Physiol
Gastrointest Liver Physiol. 284:G830–G836. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharif R, Dawra R, Wasiluk K, Phillips P,
Dudeja V, Kurt-Jones E, Finberg R and Saluja A: Impact of toll-like
receptor 4 on the severity of acute pancreatitis and
pancreatitis-associated lung injury in mice. Gut. 58:813–819. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen WC, Wang X, Qin WT, Qiu XF and Sun
BW: Exogenous carbon monoxide suppresses Escherichia coli vitality
and improves survival in an Escherichia coli-induced murine sepsis
model. Acta Pharmacol Sin. 35:1566–1576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu DM, Sun BW, Sun ZW, Jin Q, Sun Y and
Chen X: Suppression of inflammatory cytokine production and
oxidative stress by CO-releasing molecules-liberated CO in the
small intestine of thermally-injured mice. Acta Pharmacol Sin.
29:838–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun BW, Jin Q, Sun Y, Sun ZW, Chen X, Chen
ZY and Cepinskas G: Carbon liberated from CO-releasing molecules
attenuates leukocyte infiltration in the small intestine of
thermally injured mice. World J Gastroenterol. 13:6183–6190. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng W, Hui Y, Yu J, Wang W, Xu S, Chen C
and Xiong X: A new pathological scoring method for adrenal injury
in rats with severe acute pancreatitis. Pathol Res Pract.
210:1011–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barker PM, Nguyen MS, Gatzy JT, Grubb B,
Norman H, Hummler E, Rossier B, Boucher RC and Koller B: Role of
gammaENaC subunit in lung liquid clearance and electrolyte balance
in newborn mice. Insights into perinatal adaptation and
pseudohypoaldosteronism. J Clin Invest. 102:1634–1640. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hillegass LM, Griswold DE, Brickson B and
Albrightson-Winslow C: Assessment of myeloperoxidase activity in
whole rat kidney. J Pharmacol Methods. 24:285–295. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Chen G, Gong H, Huang W, Long D
and Tang W: Amelioration of experimental acute pancreatitis with
dachengqi decoction via regulation of necrosis-apoptosis switch in
the pancreatic acinar cell. PLoS One. 7:e401602012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bang S, Kang YH, Reynolds C and Kang M:
The pan-Bcl-2 family inhibitor ABT-737 synergizes with DNA damaging
agents by enhancing apoptosis in acute lymphoblastic leukemia
cells. Cancer Research. 69:2009.
|
|
36
|
Funaro A, Ortolan E, Ferranti B, Gargiulo
L, Notaro R, Luzzatto L and Malavasi F: CD157 is an important
mediator of neutrophil adhesion and migration. Blood.
104:4269–4278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dawra R, Ku YS, Sharif R, Dhaulakhandi D,
Phillips P, Dudeja V and Saluja AK: An improved method for
extracting myeloperoxidase and determining its activity in the
pancreas and lungs during pancreatitis. Pancreas. 37:62–68. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Armbruster C and Kriwanek S: Multicentre
audit of death from acute pancreatitis. Br J Surg. 81:16971994.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen P, Sun B, Chen H, Wang G, Pan S, Kong
R, Bai X and Wang S: Effects of carbon monoxide releasing
molecule-liberated CO on severe acute pancreatitis in rats.
Cytokine. 49:15–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rae D, Bowyer RC and Wharton RQ:
Inflammatory mediators in acute pancreatitis. Br J Surg.
82:8551995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Beaux AC, Goldie AS, Ross JA, Carter DC
and Fearon KC: Serum concentrations of inflammatory mediators
related to organ failure in patients with acute pancreatitis. Br J
Surg. 83:349–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Qin W, Song M, Zhang Y and Sun B:
Exogenous carbon monoxide inhibits neutrophil infiltration in
LPS-induced sepsis by interfering with FPR1 via p38 MAPK but not
GRK2. Oncotarget. 7:34250–34265. 2016.PubMed/NCBI
|
|
43
|
Sun BW, Zhang P, Zou XQ, Shi GS and Sun Y:
Inhibitive effect of exogenous carbon monoxide-releasing molecules
2 on the activation of Janus kinase/signal transducer and activator
of transcription pathway in sepsis. Zhonghua Shao Shang Za Zhi.
26:100–103. 2010.(In Chinese). PubMed/NCBI
|
|
44
|
Sun BW, Shi GS, Zhang P, Zou XQ and Chen
X: Inhibitive effect of exogenous carbon monoxide-releasing
molecules 2 on tissue factor expression in sepsis. Zhonghua Shao
Shang Za Zhi. 25:111–114. 2009.(In Chinese). PubMed/NCBI
|
|
45
|
Xue J and Habtezion A: Carbon
monoxide-based therapy ameliorates acute pancreatitis via TLR4
inhibition. J Clin Invest. 124:437–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim GY, Roh SI, Park SK, Ahn SC, Oh YH,
Lee JD and Park YM: Alleviation of experimental septic shock in
mice by acidic polysaccharide isolated from the medicinal mushroom
Phellinus linteus. Biol Pharm Bull. 26:1418–1423. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bettaieb A, Chahed S, Tabet G, Yang J,
Morisseau C, Griffey S, Hammock BD and Haj FG: Effects of soluble
epoxide hydrolase deficiency on acute pancreatitis in mice. PLoS
One. 9:e1130192014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin MJ, Yamamoto Y and Gaynor RB: The
anti-inflammatory agents aspirin and salicylate inhibit the
activity of I(kappa)B kinase-beta. Nature. 396:77–80. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun B, Zou X, Chen Y, Zhang P and Shi G:
Preconditioning of carbon monoxide releasing molecule-derived CO
attenuates LPS-induced activation of HUVEC. Int J Biol Sci.
4:270–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dolinay T, Szilasi M, Liu M and Choi AM:
Inhaled carbon monoxide confers antiinflammatory effects against
ventilator-induced lung injury. Am J Respir Crit Care Med.
170:613–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Motterlini R and Otterbein LE: The
therapeutic potential of carbon monoxide. Nat Rev Drug Discov.
9:728–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tzeng E: Carbon monoxide: Vascular
therapeutic for the future. Vascular. 17 (Suppl 1):S55–S62. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thom SR, Weaver LK and Hampson NB:
Therapeutic carbon monoxide may be toxic. Am J Respir Crit Care
Med. 171:13182005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Desmard M, Foresti R, Morin D, Dagouassat
M, Berdeaux A, Denamur E, Crook SH, Mann BE, Scapens D, Montravers
P, et al: Differential antibacterial activity against Pseudomonas
aeruginosa by carbon monoxide-releasing molecules. Antioxid Redox
Signal. 16:53–63. 2012. View Article : Google Scholar
|