Introduction
Scars are traces of wound healing and are the end
result of tissue repair. Large scars, which may be caused by burns,
lacerations, surgery and vaccination, affect the patient's quality
of life both physically and psychologically (1). Excessive scarring may occur due to
pain, itching and contracture (1).
Severe trauma, inappropriate wound closure and occasionally
standard surgery may lead to the formation of atypical raised
scars, termed hypertrophic scars (HS) (2). HS is characterized by excessive
growth of dense fibrous tissues (3–5). At
present, there are many methods for treating HS, including surgery,
steroid injection and laser surgery, but the effects remain
unsatisfactory (6). Recently, the
molecular mechanisms of HS pathogenesis have been unraveled, thus
providing new promise for the use of gene therapy to treat HS. Many
genes that regulate extracellular matrix deposition and fibroblast
hyperplasia are involved in the development and progression of HS.
Increasing evidence has indicated that microRNAs (miRNAs/miRs) are
involved in the progression of HS (7–10).
miRNAs, a family of small (~22 nt), non-coding,
single stranded and highly conserved RNAs, negatively regulate the
expression of target genes during various cellular events,
including proliferation, apoptosis and differentiation, through
binding to the 3′untranslated region (UTR) of target genes
(11–14). Dysregulation of miRNA expression is
involved in various pathophysiological processes, including wound
healing, and is closely associated with the formation of HS
(7–10). MiR-486-5p, a well-studied miRNA in
cancer, has been reported as a tumor inhibitor in a variety of
cancer types, including breast, colorectal, lung and gastric cancer
(15–18). miR-486-5p serves an important role
in the regulation of cell growth (15,19),
and fibroblast hyperplasia is one of the main features of HS
formation (20). Therefore, it was
hypothesized that miR-486-5p may be involved in HS pathology. To
the best of our knowledge, the expression and functional role of
miR-486-5p in HS remains unknown. Thus, their relationship was
investigated in the current study.
A large number of studies have demonstrated a key
role for transforming growth factor-β (TGF-β) in HS formation
(21). TGF-β signaling involves
mothers against decapentaplegic homolog (Smad) proteins (22). During HS progression, Smad2
upregulation and increased TGF-β production often occur. Silencing
of Smad2 gene expression inhibits the TGF-β signaling pathway and
consequently reduces HS formation (23). In the present study, Smad2 was
predicted as a potential target gene of miR486-5p by bioinformatics
software, suggesting a role of the miR486-5p/Smad2 axis in HS
formation.
In the present study, the differential expression of
miR-486-5p in HS tissues and cells was determined. Smad2, one of
the important members of the TGF-β signaling pathway (24), was identified as a direct target of
miR486-5p and was upregulated in HS. Smad2 was negatively regulated
by miR-486-5p in human hypertrophic scar fibroblasts (hHSFs).
Although the relationship between miR-486-5p and Smad2 in pulmonary
fibrosis and lens epithelial cells has been studied (25,26),
its role in HS is unclear. The present study demonstrated that
miR-486-5p inhibited the proliferation, increased the apoptosis and
induced G1/S phase arrest of hHSFs by targeting Smad2. Hyperplasia
of fibroblasts is one of the main features of HS formation
(20). Therefore, the current
study indicated that miR-486-5p may be a promising therapeutic
target for HS management.
Materials and methods
Clinical samples
A total of 60 HS (during scar excision; 32–57 years
old; gender ratio, 1:1) and 60 normal control skin (NCS; during
auto-skin grafting; 29–54 years old; gender ratio, 1:1) tissues
were collected from the thigh during biopsies at The Eighth
People's Hospital of Shanghai between February 2015 and September
2017. All tissues were immediately stored in liquid nitrogen until
use. The present study was approved by the Ethics Committee of The
Eighth People's Hospital of Shanghai. Informed consent was obtained
from each patient.
Cell culture
hHSFs (27) were
obtained from Shanghai Guandao Biological Engineering Co., Ltd.
(cat no. C0618; Shanghai, China; sgdbio.chemdrug.com), and human embryonic skin
fibroblasts CCC-ESF-1 (; http://www.biomart.cn/infosupply/30393402.htm?from=search_1)
were purchased from Shanghai Zibo Biological Technology Co., Ltd.
(cat no. YB-ATCC-3084; Shanghai, China; shybio.biomart.cn). Both
cell lines were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum (both Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 10,000 units/ml penicillin, and 10,000
µg/ml streptomycin. Prior to cell transfection, cells were
incubated at 37°C with 5% CO2 for 24 h to reach 70–80%
confluence.
Cell transfection
miR-486-5p mimics (mimic; sense:
CGGGGCAGCUCAGUACAGGAUU; anti-sense: UCCUGUACUGAGCUGCCCCGAG) and
mimic-control (mimic-c; sense: UUCUCCGAACGUGUCACUUTT; anti-sense:
ACGUGACACGUUCGGAGAAATT) were obtained from Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China). control-plasmid (control-p; cat. no.
sc-108083) and Smad2-plasmid (plasmid; cat. no. sc-421525-ACT) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). To perform cell transfection, Lipofectamine® 3,000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used according to the manufacturer's instructions. Cells were
transfected with 50 nM mimic, 50 nM mimic-c, 50 nM mimic + 2 µl
control-p or 50 nM mimic + 2 µl plasmid. Untreated cells were used
as the control group (control). hHSFs cells were harvested for
subsequent experimentation 48 h after cell transfection.
Western blot analysis
Total protein from hHSFs was extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) 48 h after transfection. A
bicinchoninic acid protein assay was performed to determine the
quality of the protein samples. Equal amount of protein (30 µg per
lane) were separated by SDS-PAGE (12% gel) and transferred to
polyvinylidene difluoride membranes. Membranes were blocked with 5%
skimmed milk in Tris buffered saline with 0.1% Tween-20 at room
temperature for 1.5 h, followed by incubation with primary
antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA)
against Smad2 (cat. no. 5339; 1:1,000), cyclin-dependent kinase
(CDK)2 (cat. no. 2546; 1:1,000), CDK4 (cat. no. 12790; 1:1,000),
apoptosis regulator Bcl-2 (Bcl-2; cat. no. 4223; 1:1,000),
apoptosis regulator BAX (Bax; cat. no. 5023; 1:1,000) and β-actin
(no. 4970; 1:5,000) at 4°C overnight. Subsequently, the membranes
were incubated with anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibody (cat no. 7074; 1:2,000;
Cell Signaling Technology, Inc.) at room temperature for 2 h.
Finally, protein bands were visualized with an enhanced
chemiluminescence detection system (Applygen Technologies, Inc.,
Beijing, China). ImageJ 1.38X (National Institutes of Health,
Bethesda, MD, USA) was used to perform densitometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from cells and
tissues. Reverse transcription of RNA into cDNA was performed using
miScript Reverse Transcription kit (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's instructions. RT-qPCR was conducted
using the SYBR Premix Ex Taq™ II (TliRNaseH Plus) kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's protocol. U6 and
GAPDH were used as internal control for miRNA and mRNA,
respectively. Primer sequences for PCR were: GAPDH forward,
5′CTTTGGTATCGTGGAAGGACTC3′; reverse, 5′GTAGAGGCAGGGATGATGTTCT3′; U6
forward, 5′GCTTCGGCAGCACATATACTAAAAT3′; reverse,
5′CGCTTCACGAATTTGCGTGTCAT3′; miR-486-5p forward,
5′ACACTCCAGCTGGGTCCTGTACTGAGCTGCCC3′; reverse,
5′CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCCGAG3′; Smad2 forward,
5′CGTCCATCTTGCCATTCACG3′; reverse, 5′CTCAAGCTCATCTAATCGTCCTG3′.
Relative gene expression was analyzed using the 2−ΔΔCq
method (28).
MTT assay
hHSFs (5×103 cells/well) were seeded into
96-well plates and cultured at 37°C with 5% CO2. MTT
solution (20 µl) was added into each well 48 h after transfection,
and the plates were incubated at 37°C for another 4 h. DMSO was
used to dissolve the purple formazan. Next, optical density at 570
nm of each sample was detected using a microplate reader.
Cell apoptosis assay
Following transfection for 48 h, hHSFs were
subjected to a cell apoptosis assay. hHSFs (106) were
dyed with Annexin V/propidium iodide (PI) using an apoptosis
detection kit (cat. no. 556547; BD Biosciences, Franklin Lakes, NJ,
USA) for 15 min at room temperature in the dark, according to the
manufacturer's protocol. At last, cell apoptosis was analyzed by
flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA), and data
were analyzed using WinMDI software (version 2.5; Purdue University
Cytometry Laboratories; www.cyto.purdue.edu/flowcyt/software/Catalog.htm).
Cell cycle assay
Transfected hHSFs were seeded in six-well plates
(2×105 cells/well) and cultured for 24 h at 37°C.
Following treatment with 0.3 µM nocodazole (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 4°C for 24 h, the cells were
collected, washed with PBS solution, and fixed with cold 70%
ethanol overnight at −20°C. Subsequently, cells were incubated with
10 mg/ml RNase A, 400 mg/ml PI, and 0.1% Triton X at 37°C for 15
min. Finally, cell cycle distribution of hHSFs were analyzed by
flow cytometry, and the percentage of cells within each phase of
the cell cycle was determined using ModFit LT version 4.1 (Verity
Software House, Inc., Topsham, ME, USA).
Dual-luciferase reporter assay
TargetScanHuman 7.1 (www.targetscan.org/vert_71) was used to predict the
target genes of miR-486-5p, which indicated that Smad2 was
potential target of miR-486-5p. The dual-luciferase reporter vector
pmiR-RB-REPORT™ (Guangzhou RiboBio Co., Ltd., Guangzhou,
China) was used in current study. To confirm that miR-486-5p
directly bound to the 3′-UTR of Smad2, the vectors named
Smad2-3′-UTR-WT and Smad2-3′-UTR-MUT with the wild-type and mutated
3′-UTR of Smad2 mRNA were constructed. Then, hHSFs were
co-transfected with Smad2-3′UTR-WT or Smad2-3′UTR-MUT, and mimic or
mimic-c, using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C for 48 h. Luciferase activity was
subsequently determined using a dual luciferase reporter assay
system (Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocols, and was normalized to Renilla
luciferase activity.
Statistical analysis
Data were presented as the mean ± standard deviation
of at least three experimental repeats. SPSS software version 18.0
(IBM Corp., Armonk, NY, USA) was used to perform the statistical
analysis. For statistical comparisons, one-way analysis of variance
followed by Tukey's post-hoc test, or Student's t-test were used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-486-5p expression is decreased in
HS tissues and hHSFs
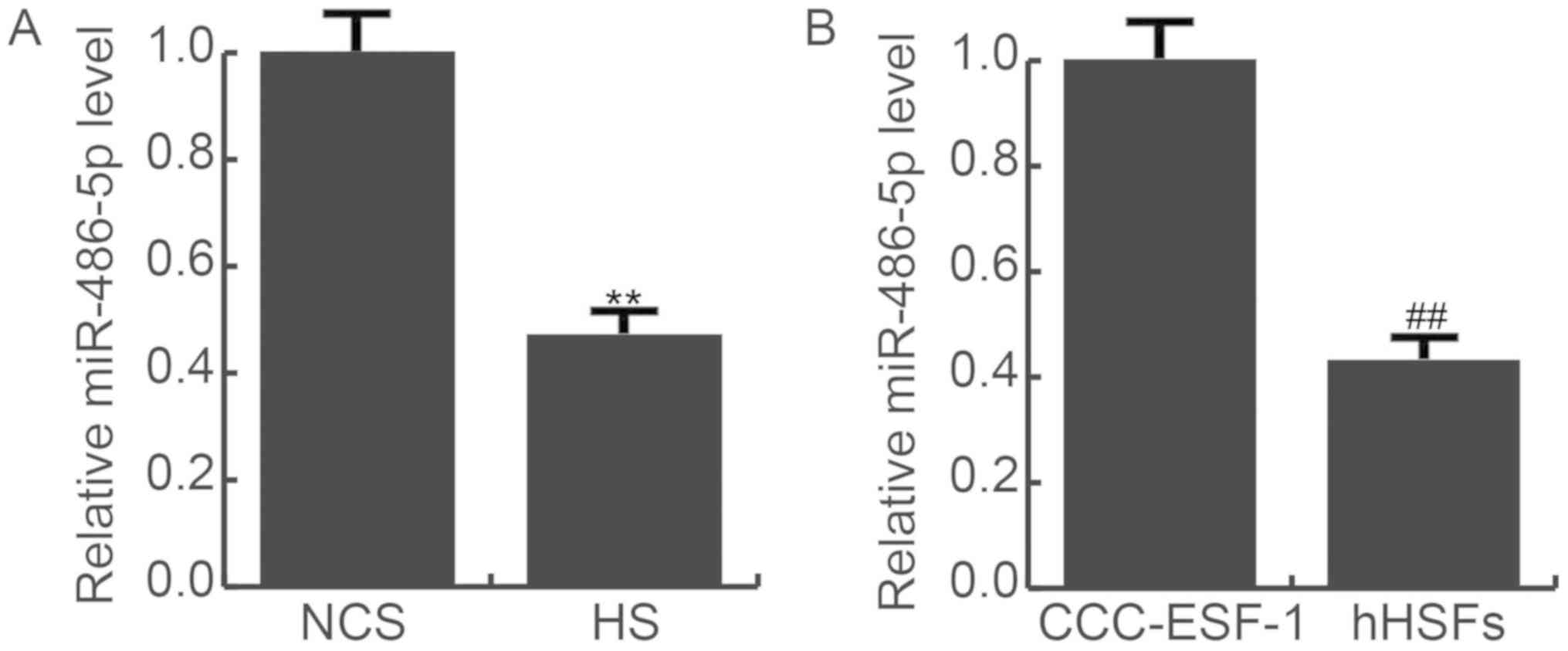
As presented in Fig.
1, it was shown that compared with the NCS tissues, the RNA
expression of miR-486-5p was significantly decreased in HS tissues
(Fig. 1A). miR-486-5p expression
was also detected in CCC-ESF-1 cells and hHSFs, which showed that
the RNA expression of miR-486-5p was significantly lower in hHSFs,
compared with CCC-ESF-1 cells (Fig.
1B).
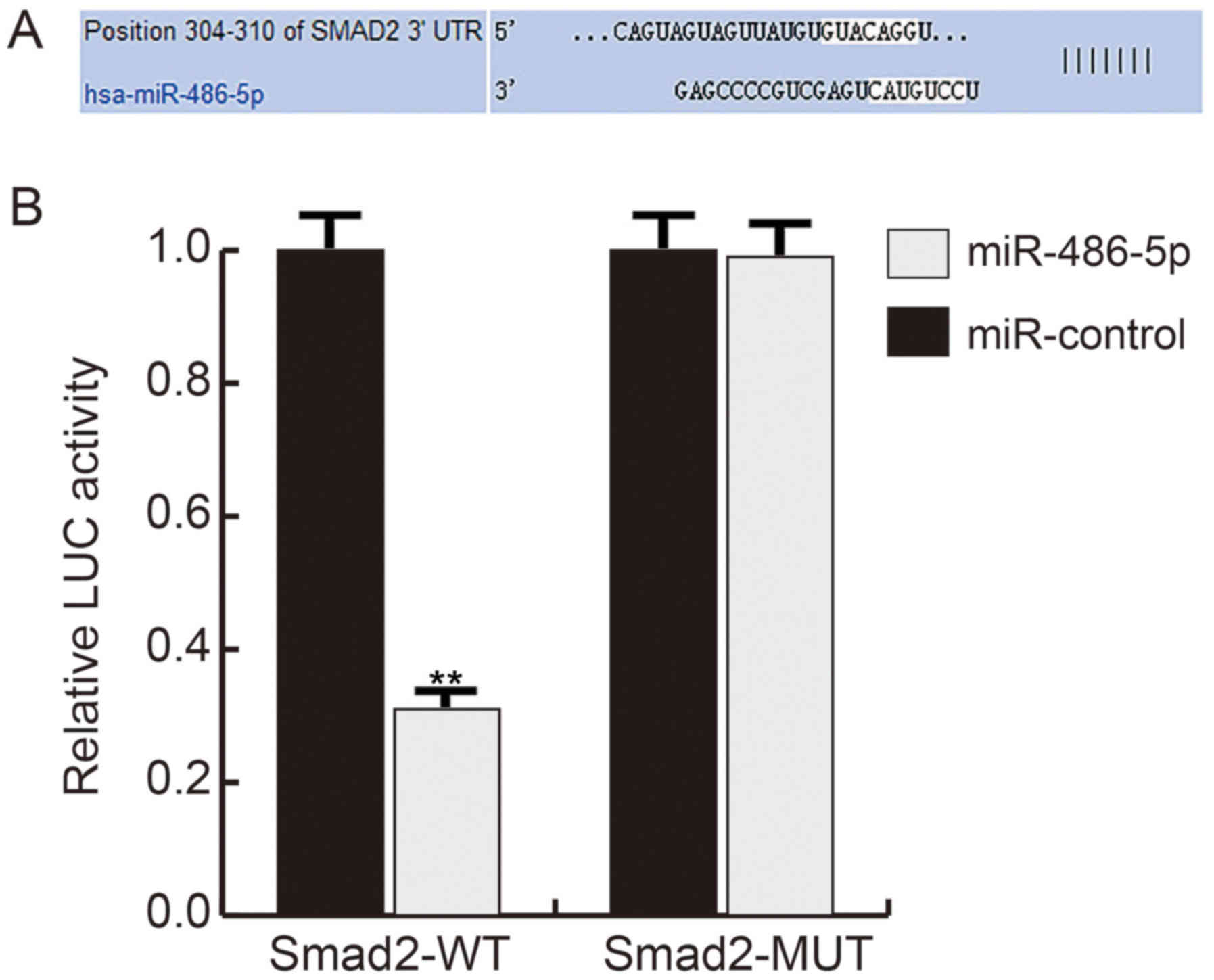
miR-486-5p directly targets Smad2
TargetScan software suggested that miR-486-5p may
bind to the 3′-UTR of Smad2 (Fig.
2A). To confirm the binding site, a dual luciferase reporter
assay was performed, which indicated that compared with the control
group, mimic transfection significantly reduced the luciferase
activity in hHSFs transfected with Smad2-WT, while no significant
difference was observed in cells co-transfected with Smad2-MUT and
mimic or mimic-c, demonstrating that miR-486-5p directly targets
Smad2 (Fig. 2B).
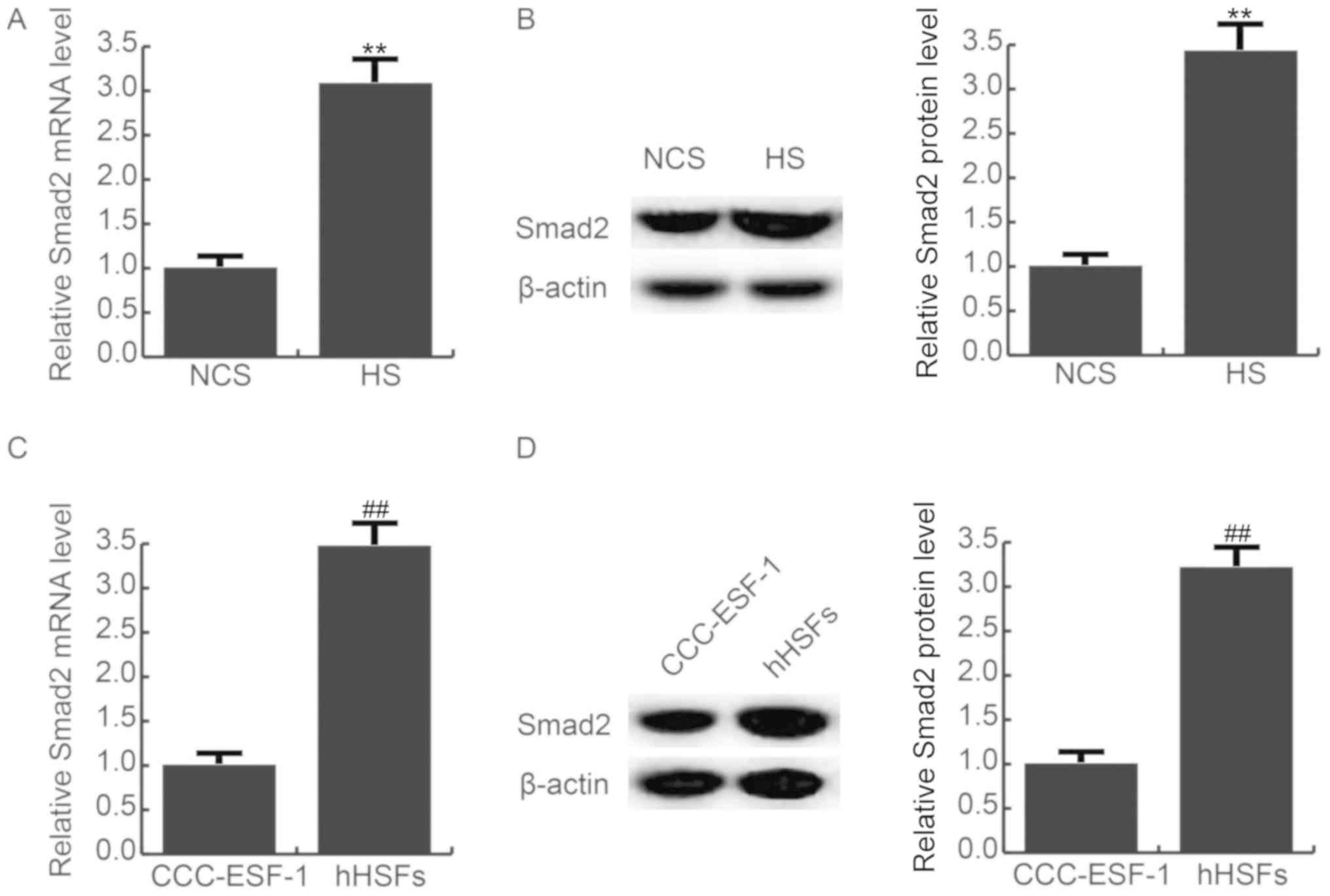
Smad2 expression is increased in HS
tissues and hHSFs
As presented in Fig.
3, it was found that compared with the NCS tissues, the mRNA
(Fig. 3A) and protein (Fig. 3B) expression of Smad2 was
significantly increased in HS tissues. The mRNA (Fig. 3C) and protein (Fig. 3D) expression l of Smad2 was also
significantly enhanced in hHSFs, compared with the CCC-ESF-1
cells.
miR-486-5p transfection inhibits cell
proliferation
To investigate the role of miR-486-5p in hHSFs,
miR-486-5p mimic, mimic-c, control-p, Smad2-plasmid or
mimic+plasmid were transfected into hHSFs. The transfection
efficiency was examined by RT-qPCR 48 h after transfection.
miR-486-5p expression was significantly increased in hHSFs
transfected with miR-486-5p mimic compared with the mimic-c
(Fig. 4A), and Smad2-plasmid
markedly enhanced Smad2 mRNA expression in hHSFs, compared with the
control-p group (Fig. 4B). In
addition, the protein (Fig. 4C)
and mRNA (Fig. 4D) expression of
Smad2 in each group was detected. Cell proliferation was measured
using a MTT assay, and the results revealed that mimic transfection
significantly inhibited the proliferation of hHSFs, compared with
the mimic-c group, and this inhibition was prevented by plasmid
transfection (Fig. 4E).
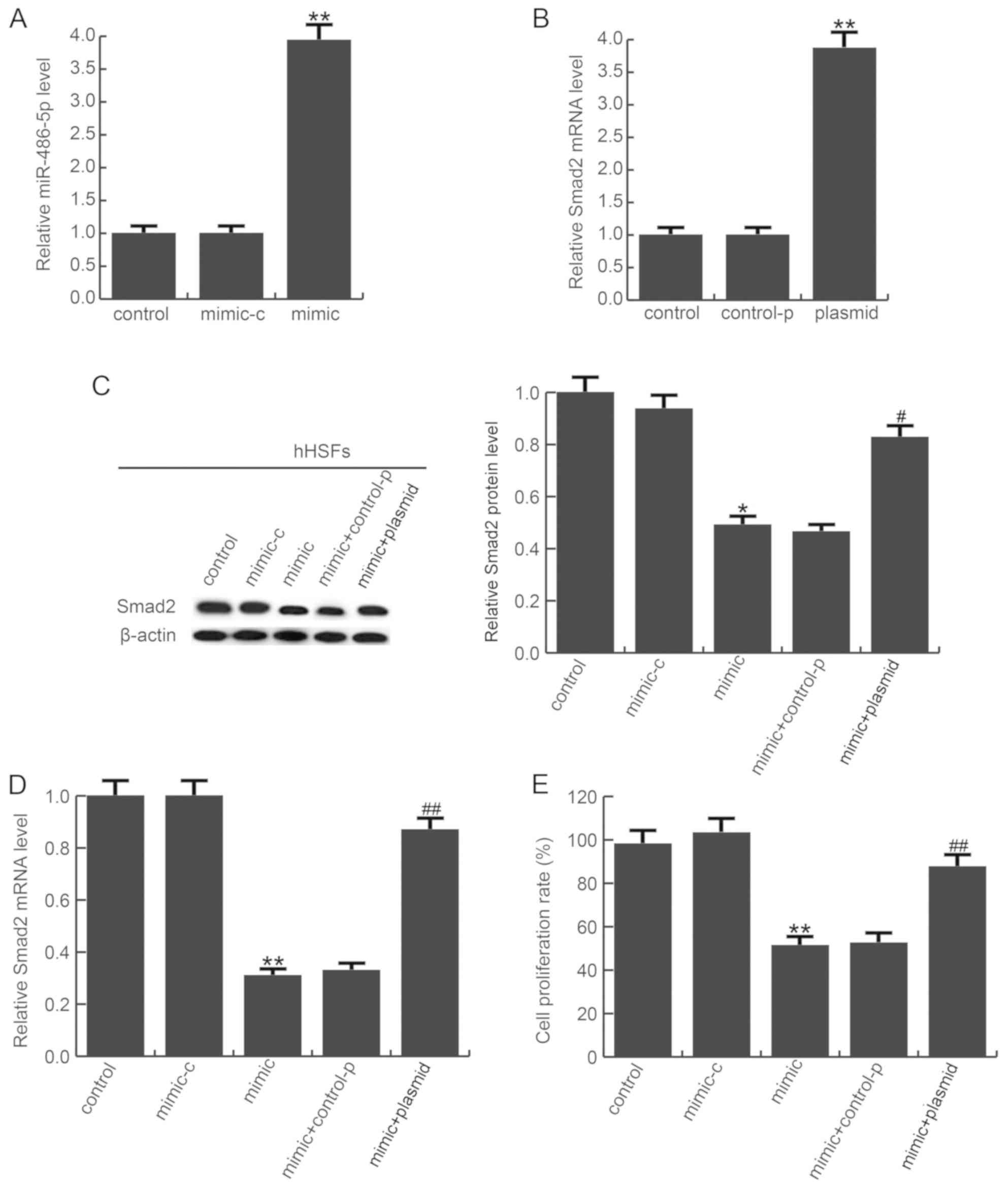 | Figure 4.miR-486-5p decreases hHSF
proliferation. Transfection efficiency of (A) miR-486-5p mimics and
(B) Smad2 plasmid was examined by RT-qPCR. (C) Smad protein and (D)
mRNA expression was determined in each group by western blotting
and RT-qPCR, respectively. (E) Cell proliferation was detected with
MTT assays. Data are presented as the mean ± standard deviation;
**P<0.01 vs. control; ##P<0.01 vs. mimic. OD,
optical density. Smad2, mothers against decapentaplegic homolog 2;
hHSFs, human hypertrophic scar fibroblasts; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; miR,
microRNA; control, untreated cells; mimic-c, cells transfected with
mimic-control; mimic, cells transfected with miR-486-5p mimics;
control-p: cells transfected with control-plasmid; plasmid, cells
transfected with Smad2-plasmid. |
miR-486-5p transfection induces cell
apoptosis and G1/S phase arrest in hHSFs
Flow cytometry analysis revealed that the number of
apoptotic cells increased in hHSFs transfected with miR-486-5p
mimic, compared with the control group, and this increase was
reduced by plasmid co-transfection (Fig. 5A and B). Furthermore, the
expression of pro-apoptotic protein Bax and anti-apoptotic protein
Bcl-2 was measured by western blotting. As expected, miR-486-5p
mimic significantly increased Bax and decreased Bcl-2 protein
expression. These alterations were eliminated by plasmid
overexpression (Fig. 5C).
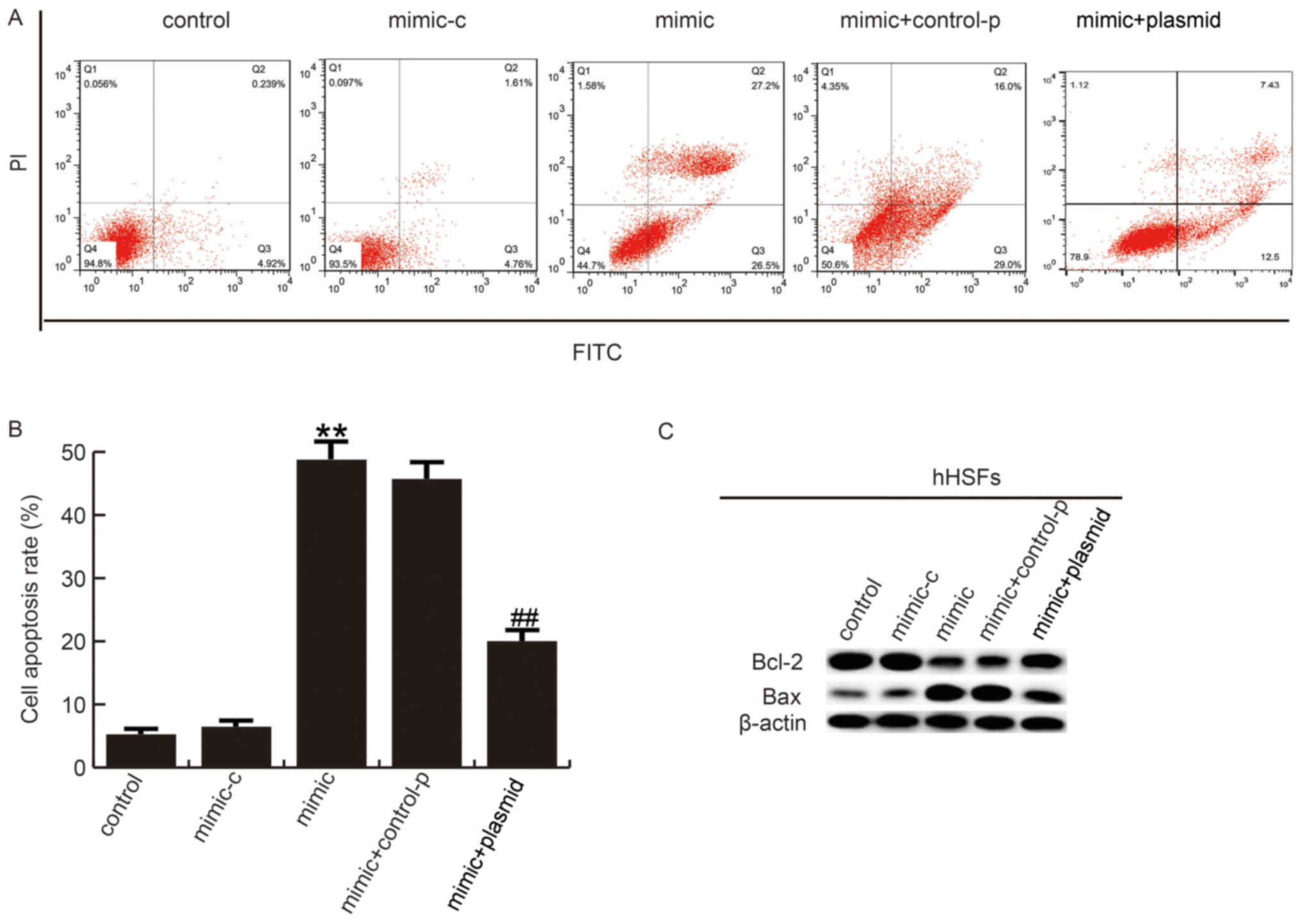 | Figure 5.miR-486-5p increases hHSF apoptosis.
(A) At 48 h post-transfection, flow cytometry was performed and (B)
the results were quantified to assess the effect of miR-486-5p on
hHSF apoptosis. (C) The effects of miR-486-5p on Bcl-2 and Bax
protein expression were analyzed by western blotting. Data are
presented as the mean ± standard deviation. **P<0.01 vs.
control; ##P<0.01 vs. mimic. miR, microRNA; hHSFs,
human hypertrophic scar fibroblasts; PI, propidium iodide; FITC,
fluorescein isothiocyanate; Bcl-2, apoptosis regulator Bcl-2; Bax,
apoptosis regulator BAX; control, untreated cells; mimic-c, cells
transfected with mimic-control; mimic, cells transfected with
miR-486-5p mimics; control-p: Cells transfected with
control-plasmid; plasmid, cells transfected with Smad2-plasmid. |
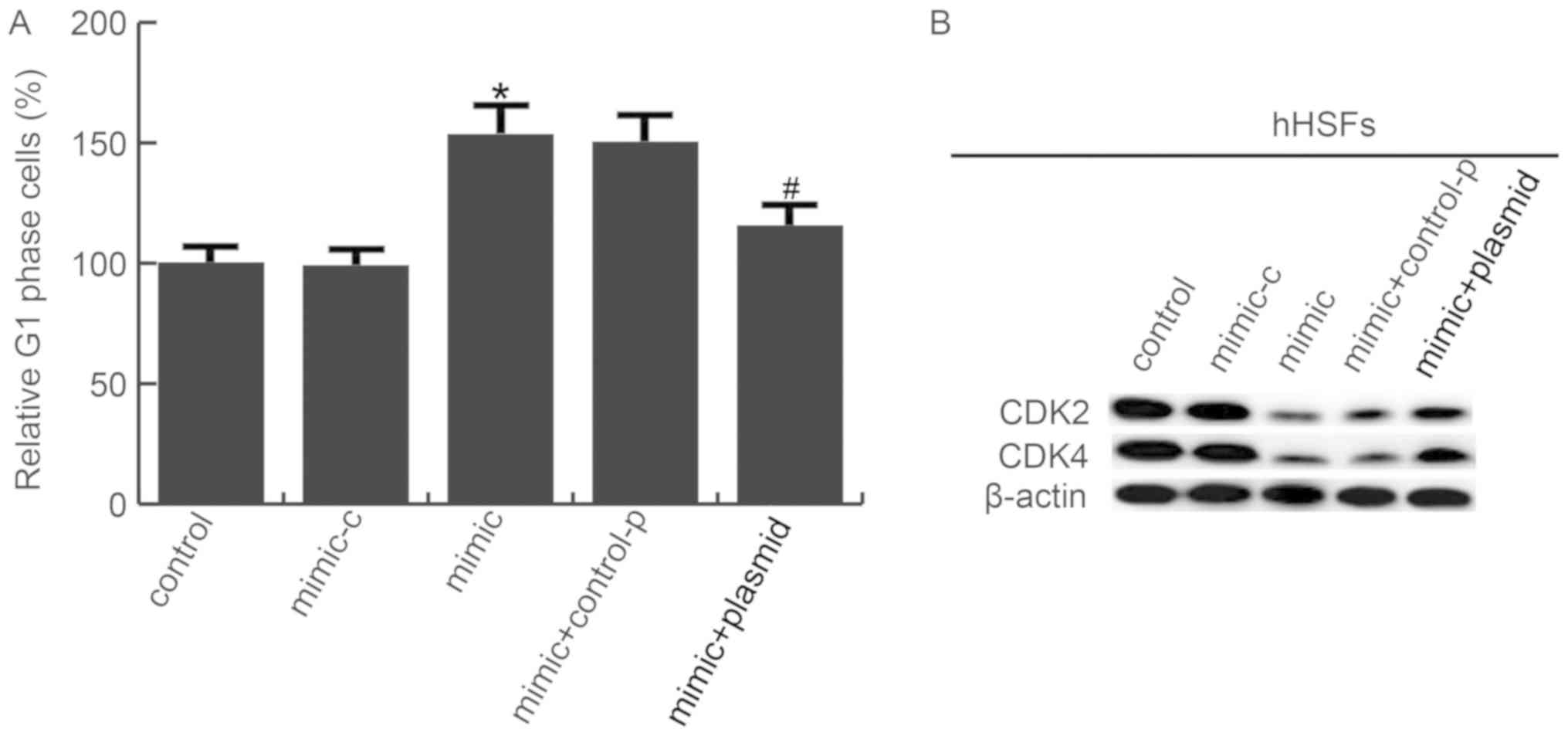
Next, it was determined whether miR-486-5p affected
the cell cycle distribution of hHSFs. As presented in Fig. 6A, a marked accumulation of hHSFs in
G1/S phase was observed in mimic transfected group, suggesting that
miR-486-5p induced G1/S phase arrest in hHSFs. In addition, cell
cycle-associated genes expression was determined. It was
demonstrated that CDK2 and CDK4 were significantly downregulated in
hHSFs transfected with miR-486-5p mimics, compared with the control
group; plasmid co-transfection reduced this decrease (Fig. 6B).
Discussion
In the present study, it was determined that
miR-486-5p inhibited hHSF proliferation, induced apoptosis and
increased G1/S phase arrest by repressing Smad2 expression. It was
revealed that the miR-486-5p/Smad2 axis may act as a potential
therapeutic target for the treatment of HS.
Normal and pathological wound healing processes are
complex (29,30). Histologically, HS is characterized
by excessive fibroblast and mast cell proliferation, accompanied by
excessive extracellular matrix accumulation (20). Unfortunately, the precise
pathogenesis of HS remains unclear and current treatments for HS
are limited (31). It has been
suggested that abnormal miRNA expression has critical function in
the progression of skin fibrosis (32). Several studies have demonstrated
the anti-cancer or tumor promoting effects of miR-486-5p in various
tumors: For example, miR-486-5p may be involved in prostate cancer
progression by negatively regulating several tumor suppressor
pathways (33). miR-486-5p may
also promote the development of hepatocellular carcinoma via
negative regulation of serine/threonine-protein kinase NEK2
expression (34). Youness et
al (35) reported that
miR-486-5p acts as a tumor suppressor in hepatocellular carcinoma
through the repression of essential proteins involved in
insulin-like growth factor (IGF) signaling, including IGF-1
receptor and its downstream mediators mammalian target of
rapamycin, signal transducer and activator of transcription (STAT)
3 and proto-oncogene c-Myc. Furthermore, transfer of miR-486-5p
from human endothelial colony forming cell-derived exosomes may
attenuate ischemic kidney injury (36). miR-486-5p may suppress
TGF-β2-induced proliferation of lens epithelial cells (26), as well as lung fibrosis (37,38).
These reports indicate that miR-486-5p may have a potential
therapeutic effect on HS. Therefore, the present study was
conducted.
First, the RNA expression of miR-486-5p was detected
in HS and NCS tissues, as well as in hHSFs and human embryonic skin
fibroblasts (CCC-ESF-1). The results confirmed that miR-486-5p
expression was significantly decreased in HS tissues and cells.
Then, it was determined that Smad2 was direct target of miR-486-5p
and was negatively regulated by miR-486-5p in hHSFs. It was also
demonstrated that Smad2 was significantly upregulated in HS tissues
and cells. Smad proteins are signal transducers and transcriptional
modulators that mediate multiple signaling pathways. Smad2 mediates
TGF-β signaling, thus regulating multiple cellular processes, such
as cell proliferation, apoptosis and differentiation (37,38).
The effects of miR-486-5p on hHSF proliferation was subsequently
examined, by transfecting hHSFs with miR-486-5p mimic. The findings
suggested that miR-486-5p overexpression inhibited hHSF
proliferation, induced apoptosis and increased G1/S phase arrest.
Furthermore, it was revealed that CDK2, CDK4 and Bcl-2 expression
was repressed, and Bax expression was enhanced by miR-486-5p mimic
in hHSFs. In addition, the effects of miR-486-5p on hHSFs were
eliminated by Smad2 overexpression.
To the best of the authors' knowledge, this was the
first study to reveal that miR-486-5p inhibited hHSF proliferation,
promoted apoptosis and induced G1/S phase arrest through the
regulation of cell apoptosis and cell cycle-associated genes via
Smad2. Thus, miR-486-5p may be a useful target for the treatment of
HS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data sets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YS designed the study. YS, LW and PY analyzed the
data. YL and WC analyzed the data and prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Eighth People's Hospital of Shanghai. Informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tyack ZF, Pegg S and Ziviani J: Postburn
dyspigmentation: Its assessment, management, and relationship to
scarring-a review of the literature. J Burn Care Rehabil.
18:435–440. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gauglitz GG, Korting HC, Pavicic T,
Ruzicka T and Jeschke MG: Hypertrophic scarring and keloids:
Pathomechanisms and current and emerging treatment strategies. Mol
Med. 17:113–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aarabi S, Bhatt K A, Shi Y, Paterno J,
Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H and Gurtner GC:
Mechanical load initiates hypertrophic scar formation through
decreased cellular apoptosis. FASEB J. 21:3250–3261. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Veer WM, Bloemen MC, Ulrich MM,
Molema G, van Zuijlen PP, Middelkoop E and Niessen FB: Potential
cellular and molecular causes of hypertrophic scar formation.
Burns. 35:15–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Younai S, Nichter LS, Wellisz T, Reinisch
J, Nimni ME and Tuan TL: Modulation of collagen synthesis by
transforming growth factor-beta in keloid and hypertrophic scar
fibroblasts. Ann Plast Surg. 33:148–154. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuccaro J, Ziolkowski N and Fish J: A
systematic review of the effectiveness of laser therapy for
hypertrophic burn scars. Clin Plast Surg. 44:767–779. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li P, He QY and Luo CQ: Overexpression of
miR-200b inhibits the cell proliferation and promotes apoptosis of
human hypertrophic scar fibroblasts in vitro. J Dermatol.
41:903–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao YY, Fan PJ, Lei SR, Qi M and Yang XH:
MiR-138/peroxisome proliferator-activated receptor β signaling
regulates human hypertrophic scar fibroblast proliferation and
movement in vitro. J Dermatol. 42:485–495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Zhang Y, Jiang BH, Zhang Q, Zhou
RP, Zhang L and Wang C: Study on the role of Hsa-miR-31-5p in
hypertrophic scar formation and the mechanism. Exp Cell Res.
361:201–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L and Li J, Li Q, Yan H, Zhou B, Gao
Y and Li J: Non-coding RNAs: The new insight on hypertrophic Scar.
J Cell Biochem. 118:1965–1968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2017. View Article : Google Scholar
|
|
13
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang G, Liu Z, Cui G, Wang X and Yang Z:
MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in
breast cancer cells. Tumour Biol. 35:11137–11145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu C, Li M, Hu Y, Shi N, Yu H, Liu H and
Lian H: miR-486-5p attenuates tumor growth and lymphangiogenesis by
targeting neuropilin-2 in colorectal carcinoma. Onco Targets Ther.
9:2865–2871. 2016.PubMed/NCBI
|
|
17
|
Peng Y, Dai Y, Hitchcock C, Yang X, Kassis
ES, Liu L, Luo Z, Sun HL, Cui R, Wei H, et al: Insulin growth
factor signaling is regulated by microRNA-486, an underexpressed
microRNA in lung cancer. Proc Natl Acad Sci USA. 110:15043–15048.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh HK, Tan AL, Das K, Ooi CH, Deng NT, Tan
IB, Beillard E, Lee J, Ramnarayanan K, Rha SY, et al: Genomic loss
of miR-486 regulates tumor progression and the OLFM4 antiapoptotic
factor in gastric cancer. Clin Cancer Res. 17:2657–2667. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma X, Wei J, Zhang L, Deng D, Liu L, Mei
X, He X and Tian J: miR-486-5p inhibits cell growth of papillary
thyroid carcinoma by targeting fibrillin-1. Biomed Pharmacother.
80:220–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tredget EE, Nedelec B, Scott PG and
Ghahary A: Hypertrophic scars, keloids, and contractures. The
cellular and molecular basis for therapy. Surg Clin North Am.
77:701–730. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie JL, Qi SH, Pan S, Xu YB, Li TZ, Liu XS
and Liu P: Expression of Smad protein by normal skin fibroblasts
and hypertrophic scar fibroblasts in response to transforming
growth factor beta1. Dermatol Surg. 34:1216–1225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang ZF, Zhang YG, Hu DH, Shi JH, Liu JQ,
Zhao ZT, Wang HT, Bai XZ, Cai WX, Zhu HY and Tang CW: Smad
interacting protein 1 as a regulator of skin fibrosis in
pathological scars. Burns. 37:665–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin L, Zhao X, Ji S, He C, Wang G, Tang C,
Gu S and Yin C: The use of gene activated matrix to mediate
effective SMAD2 gene silencing against hypertrophic scar.
Biomaterials. 35:2488–2498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji X, Wu B, Fan J, Han R, Luo C, Wang T,
Yang J, Han L, Zhu B, Wei D, et al: The anti-fibrotic effects and
mechanisms of MicroRNA-486-5p in pulmonary fibrosis. Sci Rep.
5:141312015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu B, Sun J, Lei X, Zhu Z, Pei C and Qin
L: MicroRNA-486-5p suppresses TGF-β2-induced proliferation,
invasion and epithelial-mesenchymal transition of lens epithelial
cells by targeting Smad2. J Biosci. 42:575–584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi J, Liu Y, Hu K, Zhang Y, Wu Y and Zhang
X: MicroRNA-26a inhibits hyperplastic scar formation by targeting
Smad2. Exp Ther Med. 15:4332–4338. 2018.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Armour A, Scott PG and Tredget EE:
Cellular and molecular pathology of HTS: Basis for treatment. Wound
Repair Regen. 15 (Suppl 1):S6–S17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schäfer M and Werner S: Transcriptional
control of wound repair. Annu Rev Cell Dev Biol. 23:69–92. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwan P, Hori K, Ding J and Tredget EE:
Scar and contracture: Biological principles. Hand Clin. 25:511–528.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Babalola O, Mamalis A, Lev-Tov H and
Jagdeo J: The role of microRNAs in skin fibrosis. Arch Dermatol
Res. 305:763–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Y, Ji C, Guo S, Su X, Zhao X, Zhang
S, Liu G, Qiu X, Zhang Q, Guo H and Chen H: The miR-486-5p plays a
causative role in prostate cancer through negative regulation of
multiple tumor suppressor pathways. Oncotarget. 8:72835–72846.
2017.PubMed/NCBI
|
|
34
|
Fu SJ, Chen J, Ji F, Ju WQ, Zhao Q, Chen
MG, Guo ZY, Wu LW, Ma Y, Wang DP, et al: MiR-486-5p negatively
regulates oncogenic NEK2 in hepatocellular carcinoma. Oncotarget.
8:52948–52959. 2017.PubMed/NCBI
|
|
35
|
Youness RA, El-Tayebi HM, Assal RA, Hosny
K, Esmat G and Abdelaziz AI: MicroRNA-486-5p enhances
hepatocellular carcinoma tumor suppression through repression of
IGF-1R and its downstream mTOR, STAT3 and c-Myc. Oncol Lett.
12:2567–2573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Viñas JL, Burger D, Zimpelmann J, Haneef
R, Knoll W, Campbell P, Gutsol A, Carter A, Allan DS and Burns KD:
Transfer of microRNA-486-5p from human endothelial colony forming
cell-derived exosomes reduces ischemic kidney injury. Kidney Int.
90:1238–1250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eppert K, Scherer SW, Ozcelik H, Pirone R,
Hoodless P, Kim H, Tsui LC, Bapat B, Gallinger S, Andrulis IL, et
al: MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related
protein that is functionally mutated in colorectal carcinoma. Cell.
86:543–552. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Riggins GJ, Thiagalingam S, Rozenblum E,
Weinstein CL, Kern SE, Hamilton SR, Willson JK, Markowitz SD,
Kinzler KW and Vogelstein B: Mad-related genes in the human. Nat
Genet. 13:347–349. 1996. View Article : Google Scholar : PubMed/NCBI
|