Introduction
Osteoarthritis (OA) is a common degenerative joint
disease associated with progressive loss of articular cartilage,
formation of osteophytes and synovial inflammation, particularly in
elderly patients (1–3). It is widely recognized that
inflammatory cytokines serve pivotal roles in the pathogenesis of
OA. Among these, interleukin (IL)-1β is considered the most
important inflammatory cytokine in OA. Stimulation of IL-1β leads
to decreased expression of collagen II and transcription factor
SOX-9 (SOX9), which are phenotypic markers of chondrocytes
(4,5). IL-1β may also induce the expression
of inducible nitric oxide synthase (INOS) and cyclooxygenase-2
(COX-2) in chondrocytes, which leads to increased levels of nitric
oxide (NO) and prostaglandin E2 (PGE2) (6). The action of PGE2 has been confirmed
in joint pain and bone resorption (7,8). NO
is produced in excess by INOS, and has been demonstrated to
increase the production of inflammatory cytokines and matrix
metalloproteinases (MMPs) in OA (9,10).
Cytokines cause degradation of cartilage matrix by upregulating the
expression of MMPs (11).
The MMP family is considered to serve a major role
in the pathophysiology of OA, as MMPs lead to the breakdown of the
extracellular matrix (ECM) and their expression is increased in the
cartilage of patients with OA (12). Among the members of the MMP family,
MMP-1, MMP-13 and MMP-3 are indispensable for cartilage
degradation. The primary role of MMP-1 and MMP-13 is to degrade
aggrecans and collagens, which are the major components of the
cartilage matrix (13). Although
numerous drugs have been approved for treating this disease, none
appear to delay the progression of OA. Corticosteroids,
nonsteroidal anti-inflammatory drugs (NSAIDs) and hyaluronan are
drugs currently used for the treatment of OA. However, they cannot
prevent subsequent cartilage degradation, but only relieve OA
symptoms. Additionally, multiple patients with OA may eventually
require surgery. Therefore, there is a necessity for more optimal
agents to treat OA (14,15).
Costunolide is a sesquiterpene lactone, which is a
group of bioactive compounds that can be isolated from various
plants (16). The pharmacological
activities of costunolide include anti-inflammatory (17), antitumor (18), antimicrobial (19) and antioxidant (20) effects. Previous studies focused on
the molecular mechanisms of costunolide in effectively decreasing
the activation of inflammatory signaling pathways, including the
NF-κB and Wnt/β-catenin signaling pathways (21,22).
It is well recognized that these pathways are involved in the
progression of OA and may be an effective target for OA therapy
(22,23). The anti-inflammatory activity of
costunolide may exert a potential therapeutic effect on diseases
associated with inflammatory mediators. However, to the best of our
knowledge, no studies have examined the effects of costunolide in
the treatment of OA at present. Therefore, the present study
investigated whether costunolide inhibited the progression of OA
via the Wnt/β-catenin and NF-κB signaling pathways by analyzing the
effects of costunolide in an OA rat model in vivo and in rat
chondrocytes in vitro.
Materials and methods
Reagents
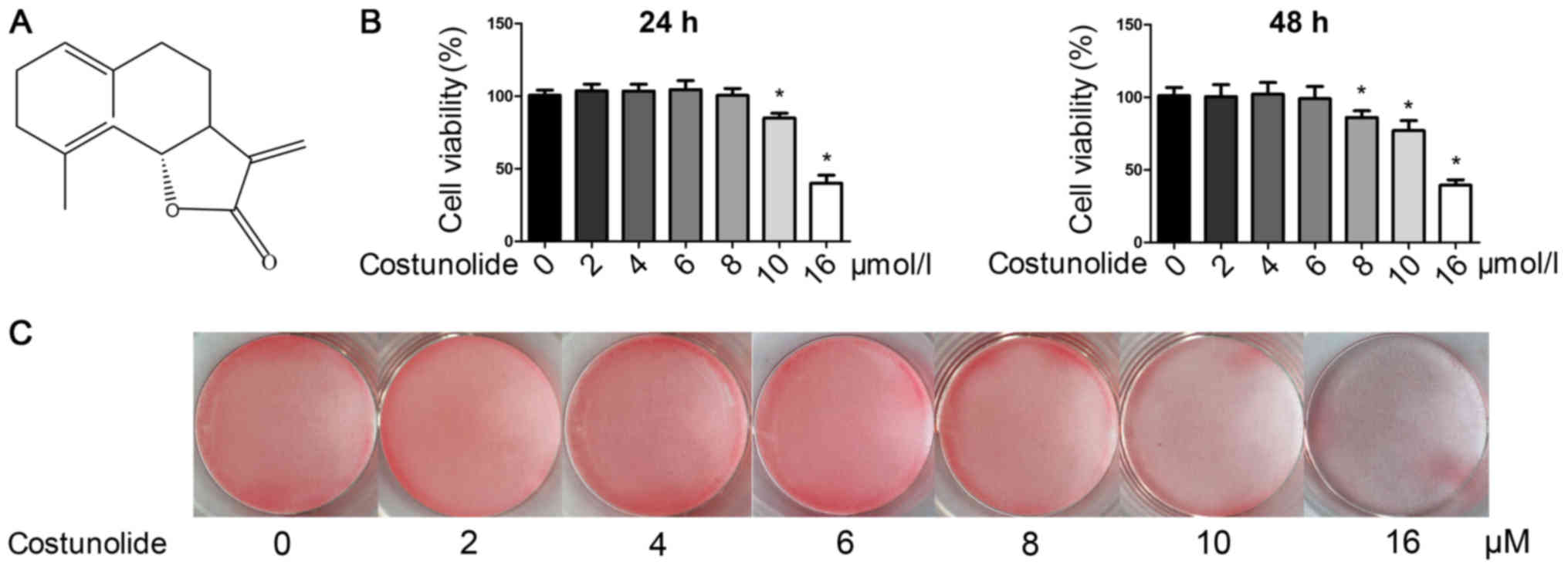
Costunolide (purity >98%; Fig. 1A) was obtained from Nantong Jingwei
Fine Chemical Co., Ltd., and was dissolved in dimethyl sulfoxide
(DMSO). Rat IL-1β was purchased from R&D Systems, Inc. Chloral
hydrate and DMSO were purchased from Sigma-Aldrich (Merck KGaA).
FBS, Dulbecco's modified Eagle's medium (DMEM), streptomycin,
penicillin, 0.25% trypsin and collagenase II were obtained from
Gibco (Thermo Fisher Scientific, Inc.).
Cell culture
The articular cartilage was harvested from the
femoral heads of rats under sterile conditions. The obtained
cartilage was cut into small pieces and digested with 0.25% trypsin
for 15 min to remove unwanted tissues and cells, followed by
digestion with 0.2% collagenase II in an incubator at 37°C for 5 h
to obtain dispersed chondrocytes. Subsequently, the chondrocytes
were suspended in DMEM containing 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin. Then, the chondrocytes were seeded in
tissue culture flasks at 37°C with 95% air and 5% CO2.
These cells were considered to be passage 0 (P0). In order to
increase the number of chondrocytes, cells were split at a ratio of
1:3 when cells were at 80% confluence. To avoid phenotype loss, all
experiments were conducted using chondrocytes of P2-P3.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 kit (Nanjing KeyGen Biotech Co., Ltd.) was
used according to the manufacturer's instructions to assess the
cytotoxicity of various concentrations of costunolide. The cells,
which were in the logarithmic growth phase, were seeded into
96-well plates (4×103 cells/ml). The culture medium was
replaced with medium containing costunolide (0, 2, 4, 6, 8, 10 or
16 µM) for 24 and 48 h. Then, 10 µl CCK-8 solution was added to
each well and incubated at 37°C for 4 h. Following incubation, the
absorbance of each well was measured at a wavelength of 450 nm
using a microplate reader. Each experiment was repeated 3 times
independently.
Safranin O staining
To analyze the effect of costunolide on chondrocyte
phenotype changes, safranin O staining was used. Upon seeding in
12-well plates (5×105 cells/ml), chondrocytes were
treated with different concentrations (0, 2, 4, 6, 8, 10 and 16 µM)
of costunolide for 48 h. The cells were then stained with 0.1%
safranin O solution for 5 min followed by fixation with 4%
paraformaldehyde solution for 10 min (both at room temperature).
Images of the cells were captured using a gross camera (ILCE-7M3K;
Sony Corporation) upon washing with PBS for 3 times.
Cell treatment
Chondrocytes were plated overnight in 6-well plates
at a density of 2×105 cells/well. Next, chondrocytes
were preincubated with different concentrations of costunolide (2,
4 and 6 µM) at 37°C for 1 h followed by stimulation with IL-1β (10
ng/ml) for 24 h to analyze the mRNA expression of MMPs, INOS, IL-6
and COX-2. Similarly, other chondrocytes were seeded in 25
cm2 flasks (5×105 cells/ml) to analyze the
levels of protein expression.
The cells were also pretreated with costunolide (2,
4 and 6 µM) for 1 h and then stimulated with IL-1β for 30 min to
analyze the NF-κB signaling pathway, or for 3 h to analyze the
Wnt/β-catenin signaling pathway. Then, total intracellular proteins
were extracted used RIPA lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) to investigate the activation of the
NF-κB and Wnt/β-catenin signaling pathways.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from chondrocytes using a
TRIzol® Plus RNA Purification kit (Invitrogen; Thermo
Fisher Scientific, Inc.). The absorbance at 260 nm (A260)/A280
ratio was calculated to verify the quality and purity of RNA. Total
RNA was used to synthesize cDNA by RT with PrimeScript™ RT Master
Mix (Takara Biotechnology Co., Ltd.); the reaction was conducted at
37°C for 15 min, 85°C for 5 sec, and then terminated at 4°C. The
mRNA levels of the target gene were analyzed by RT-qPCR using SYBR
Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) as follows: 30 sec at 95°C for the initial denaturation, then
40 cycles of 15 sec at 95°C, 32 sec at 60°C and 1 min at 72°C,
followed by 5 min at 72°C. The level of target mRNA was normalized
to the level of 18S and compared with the control. The primers used
are listed in Table I. All gene
analyses were performed in triplicate, and the data were analyzed
using the 2−ΔΔCq method (24).
 | Table I.Reverse transcription quantitative
polymerase chain reaction primer sequences. |
Table I.
Reverse transcription quantitative
polymerase chain reaction primer sequences.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| Rat MMP3 |
CAGGCATTGGCACAAAGGTG |
GATAACCATCCGAGCGACCTTT |
| Rat MMP9 |
GCAAACCCTGCGTATTTCCAT |
GATAACCATCCGAGCGACCTTT |
| Rat MMP13 |
GCAAACCCTGCGTATTTCCAT |
GATAACCATCCGAGCGACCTTT |
| Rat IL-6 |
AGCGATGATGCACTGTCAGA |
GGAACTCCAGAAGACCAGAGC |
| Rat INOS |
CCTTACGAGGCGAAGAAGGACAG |
CAGTTTGAGAGAGGAGGCTCCG |
| Rat COX-2 |
GAGAGATGTATCCTCCCACAGTCA |
GACCAGGCACCAGACCAAAG |
| Rat 18S |
CCTGAGAAACGGCTACCACA |
ACCAGACTTGCCCTCCAATG |
Western blot analysis
Upon washing twice with PBS, 100 µl RIPA lysis
buffer (Beyotime Institute of Biotechnology) containing protease
and phosphatase inhibitors was added to stimulated cells. The
extracted protein was analyzed by using a BCA quantification kit,
and protein (30 µg//lane) was resolved by 10% SDS-PAGE prior to
being transferred to a polyvinylidene difluoride membrane.
Following blocking with 5% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA) for 2 h at room temperature, the
membranes were incubated overnight at 4°C with antibodies against
MMP-3 [rabbit monoclonal antibody (mAb); cat. no. ab52915 Abcam],
MMP-9 (rabbit mAb; cat. no. ab76003; Abcam), MMP-13 (rabbit mAb;
cat. no. sc-30073; Santa Cruz Biotechnology, Inc.), collagen II
(rabbit mAb; cat. no. ab34712; Abcam), GAPDH (rabbit mAb; cat. no.
ab70699; Abcam), INOS (rabbit mAb; cat. no. ab3523; Abcam), IL-6
(10E5; mouse mAb; cat. no. sc-57315; Santa Cruz Biotechnology,
Inc.), COX-2 (D5H5; rabbit mAb; cat. no. 12282; Cell Signaling
Technology, Inc.), SOX9 (rabbit mAb; cat. no. ab185966Ab; Abcam),
β-actin (mouse mAb; cat. no. ab8226; Abcam), β-catenin (D10A8;
rabbit mAb; cat. no. 8480p; Cell Signaling Technology, Inc.),
active non-phosphorylated (p)-β-catenin (Ser45; D2U8Y; rabbit mAb;
cat. no. 19807S; Cell Signaling Technology, Inc.), transcription
factor p65 (p65; C22B4; rabbit mAb; cat. no. 4764S; Cell Signaling
Technology, Inc.), p-p65 (Ser536; rabbit Ab; cat. no. 3031; Cell
Signaling Technology, Inc.), NF-κB inhibitor α (IκB-α; rabbit mAb;
cat. no. 4812; Cell Signaling Technology, Inc.) and p-IκB-α (Ser32;
14D4; rabbit mAb; cat. no. 2859; Cell Signaling Technology, Inc.).
All primary antibodies were used at a 1:1,000 dilution. Then,
horseradish peroxidase (HRP)-conjugated goat anti-rabbit and
anti-mouse secondary antibodies (1:1,000; cat. nos., A0208 and
A0216; Beyotime Institute of Biotechnology) was incubated with the
membranes at room temperature for 2 h. Protein bands were
visualized using an ECL kit (Immobilon Western Chemiluminescent HRP
Substrate; cat. no. WBKLS0050; Merck KGaA) and analyzed with
Quantity One software v4.6.6 (Bio-Rad Laboratories, Inc.). GAPDH or
β-actin were used as controls in all western blot analyses.
Immunofluorescence staining
Upon fixing with 4% paraformaldehyde for 15 min at
room temperature, chondrocytes were permeabilized with PBS
containing 0.3% Triton X-100 for 15 min and then blocked with 5%
BSA for 1 h at room temperature. Chondrocytes were then incubated
with rabbit monoclonal anti-p65 antibody (1:500) or rabbit
monoclonal anti-β-catenin antibody (1:500) at 4°C overnight, and
then incubated with Alexa Fluor 555-labeled Donkey Anti-Rabbit IgG
(H+L) (cat. no. A0453; Beyotime Institute of Biotechnology) or
Alexa Fluor 488-labeled Goat Anti-Rabbit IgG (H+L) (cat. no. A0423;
Beyotime Institute of Biotechnology) secondary antibodies (1:1,000)
for 2 h in the dark at room temperature. Cells were counterstained
with DAPI (1:1,000) for 5 min and analyzed using a Leica
fluorescence microscope (magnification, ×100; Leica Microsystems,
Inc.).
Animal experiments
Sprague-Dawley rats (6-week-old; Animal Center of
Zhejiang University) weighing 1.8–2.4 kg were used in the present
study. All rats were housed 3 animals/cage at room temperature
(24±2°C) and at a relative humidity of 55±5% with controlled
lighting (12 h light/dark cycle). Food and water were routinely
provided in the facility ad libitum. There were a total of
30 rats included, and 20 of them underwent surgical destabilization
of the medial meniscus (DMM) in the knee joints to construct a rat
model of OA. The remaining 10 rats (sham group) received sham
surgeries. Prior to surgery, all rats were anesthetized by an
intraperitoneal injection of 10% chloral hydrate (300 mg/kg)
without observing any signs of peritonitis, and then the effect of
the anesthetic was evaluated by measuring the breathing, nerve
reflexes and muscle relaxation. A total of 1 week after the
surgeries, the rats in the costunolide group were intra-articularly
injected with 6 µM costunolide once a week for 8 weeks, while the
OA group was injected with the same volume of PBS in both knees
under the same conditions. The health and behavior of rats were
monitored every day from the first postoperative day until
sacrifice. Following the final intra-articular injection of
costunolide, rats were euthanized with 100% CO2. The
flow rate of CO2 was 20% of the chamber volume per
minute. Loss of breathing and fading of eye color were monitored
during the procedure, which usually takes 2–3 min. Following
observation of these events, the flow of CO2 was
maintained for 1 min, and then the animals were removed from the
chamber. A combination of criteria was used to confirm death,
including lack of pulse, breathing and inability to hear heartbeat
by use of stethoscope, in compliance with AVMA guidelines (25). This experiment was conducted
according to the National Institutes of Health guidelines (26), and the protocol was approved by the
Ethics Committee of the Second Affiliated Hospital, School of
Medicine, Zhejiang University (Hangzhou, China; approval no.
2015-107).
Histological examination
Knee joint samples from each group were first fixed
in 4% paraformaldehyde and decalcified until becoming soft
following ~2 months at room temperature. Subsequently, the samples
were dehydrated in a graded alcohol series (95% followed by 100%),
embedded in paraffin and cut into 3-µm sections. Paraffin sections
were stained with safranin O-fast green (1:100) for ~5 min at room
temperature and graded according to the Mankin scoring system
(27) to assess the degree of
histological change in the different groups. A total of five fields
per sample were analyzed for the different groups (magnification,
×40; BX51-P; Olympus Corporation).
Immunohistochemistry
Immunohistochemical analyses were performed to
evaluate MMP-13 and COX-2 expression on cartilage. The tissue
sections were permeabilized with xylene for 10 min twice and then
rehydrated in a graded alcohol series. Then, the sections were
treated with pepsin for 20 min for antigen retrieval after the
peroxidase activity in the samples had been quenched by incubation
with 3% H2O2 for 10 min. The sections were
then incubated with primary antibodies (1:500) against MMP-13
(rabbit mAb; cat. no. sc-30073; Santa Cruz Biotechnology, Inc.) and
COX-2 (rabbit mAb; cat. no. 12282; Cell Signaling Technology, Inc.)
overnight at 4°C following blocking with 5% BSA for 1 h at room
temperature. HRP-conjugated secondary antibodies (1:1,000) were
then added to the sections for 1 h at room temperature, and
3,3′-diaminobenzidine (1:1,000; Sigma-Aldrich; Merck KGaA) was used
as a chromogenic agent at room temperature. A total of five fields
per sample were analyzed for the different groups (magnification,
×400; BX51-P; Olympus Corporation).
Statistical analysis
All data are presented as the mean ± standard
deviation of 3 experiments used GraphPad Prism 5 (GraphPad
Software, Inc.). One-way analysis of variance followed by Tukey's
post hoc test was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of costunolide on chondrocyte
viability and phenotype maintenance
To evaluate the cytotoxicity of costunolide,
chondrocytes were treated with various concentrations of
costunolide (0, 2, 4, 6, 8 and 16 µM) and a CCK-8 assay was
performed 24 or 48 h later. As demonstrated in Fig. 1B, the results of CCK-8 indicate
that concentrations ≤6 µM had no noticeable toxic effects on the
viability of chondrocytes after 24 or 48 h. Chondrocyte phenotype
was detected by safranin O staining, and the images revealed that
costunolide did not affect the loss of safranin O staining at
concentrations ranging from 0–6 µM (Fig. 1C). Therefore, the subsequent
experiments were performed with 2, 4 and 6 µM of costunolide to
avoid cytotoxicity, and 6 µM costunolide was used for the animal
experiments.
Effects of costunolide on gene
expression and protein levels in chondrocytes
Release of inflammatory mediators and matrix
degradation are representative features of OA. Therefore, the
present study evaluated the effect of costunolide on the expression
of inflammatory genes including INOS, IL-6 and COX-2, and
matrix-degrading genes including MMP-3, MMP-9 and MMP-13, at the
mRNA (Fig. 2A-F) and protein
levels (Fig. 3A-C). IL-1β
significantly upregulated the mRNA and protein expression levels of
INOS, IL-6, COX-2, MMP-3, MMP-9 and MMP-13, whereas pretreatment
with costunolide resulted in significant inhibition of IL-1β
induction at the mRNA and protein levels. Western blot analysis
revealed that costunolide reversed the downregulation of SOX9 and
collagen II at the protein level, which was induced by IL-1β
(Fig. 3A-C). Therefore, the effect
of costunolide involved the inhibition of the IL-1β-induced
expression of matrix-degrading genes while maintaining chondrocyte
gene expression in vitro to protect rat chondrocytes.
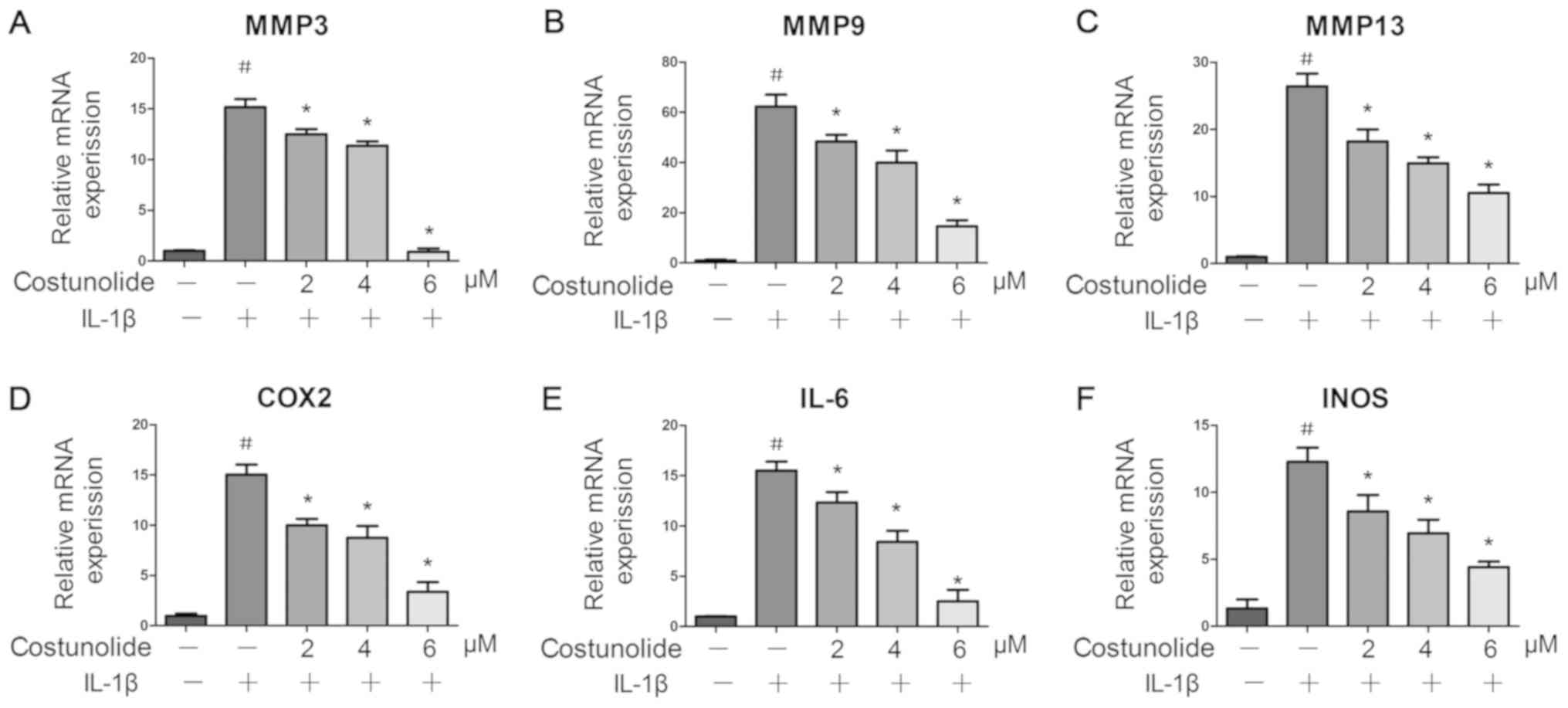 | Figure 2.Effects of costunolide on
IL-1β-induced gene expression. Levels of (A) MMP-3, (B) MMP-9, (C)
MMP-13, (D) COX-2, (E) IL-6 and (F) INOS were measured by reverse
transcription-quantitative polymerase chain reaction. The
chondrocytes were pretreated for 1 h with various concentrations of
costunolide (0, 2, 4 and 6 µM) and then stimulated with or without
IL-1β (10 ng/ml) for 24 h. The data are expressed as the mean ±
standard deviation of three experiments (n=3). *P<0.05 vs.
samples stimulated with IL-1β in the absence of costunolide.
#P<0.05 vs. the control group. MMP, matrix
metalloproteinase; COX2, cyclooxygenase-2; IL, interleukin; INOS,
inducible nitric oxide synthase. |
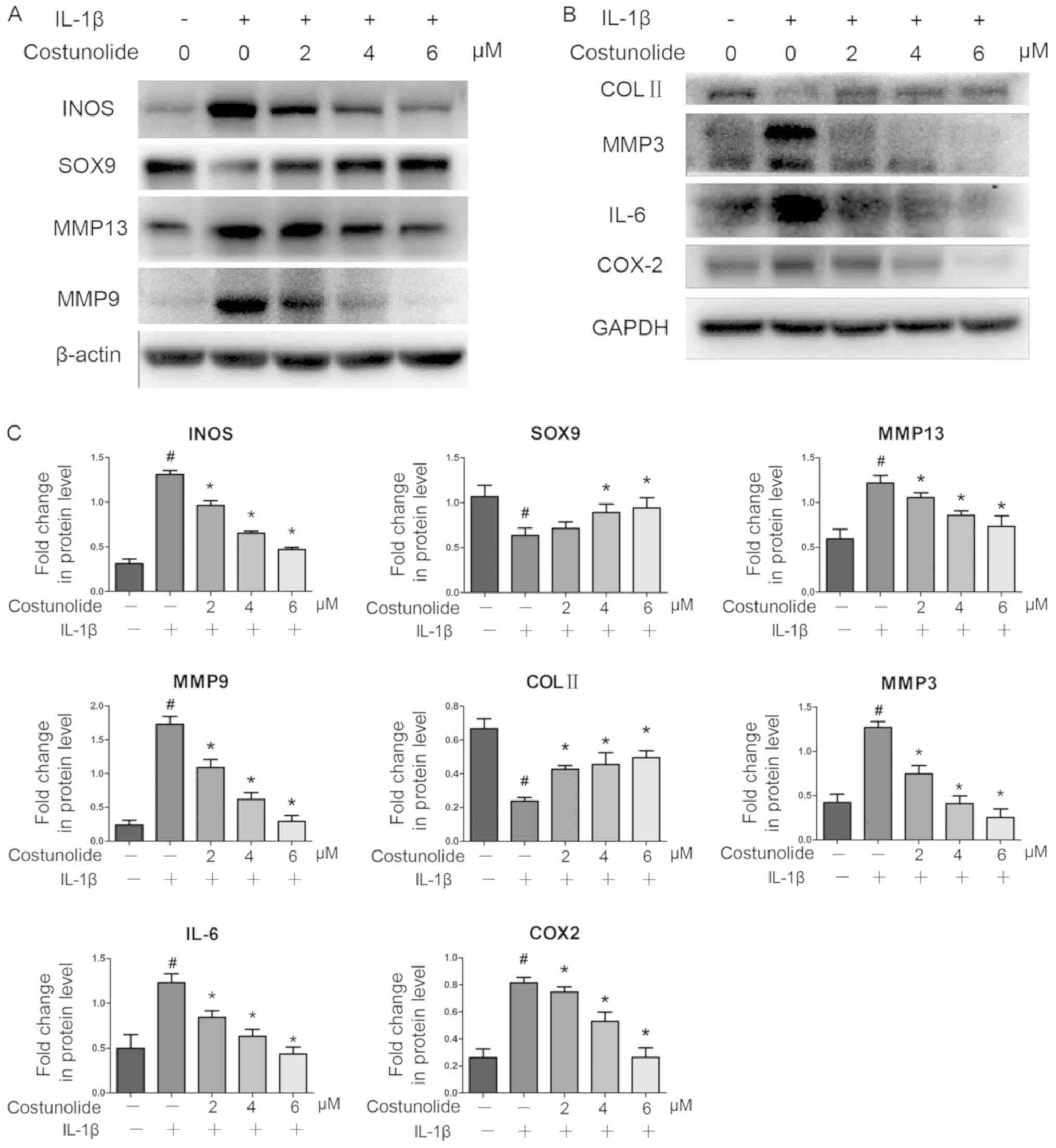 | Figure 3.Effects of costunolide on
inflammation-associated protein expression. IL-1β-pretreated
chondrocytes were incubated with various concentrations of
costunolide for 24 h in the presence (+) or absence (−) of IL-1β.
(A) Representative gel of INOS, SOX9, MMP-13 and MMP-9 expression.
(B) Representative gel of COL II, MMP-3, IL-6 and COX-2 expression.
(C) Densitometric quantification of the protein concentrations. The
data are presented as the mean ± standard deviation of three
experiments (n=3). *P<0.05 vs. samples stimulated with IL-1β in
the absence of costunolide. #P<0.05 vs. the control
group. INOS, inducible nitric oxide synthase; SOX9, transcription
factor SOX-9; MMP, matrix metalloproteinase; IL, interleukin; COL
II, collagen II; COX2, cyclooxygenase-2. |
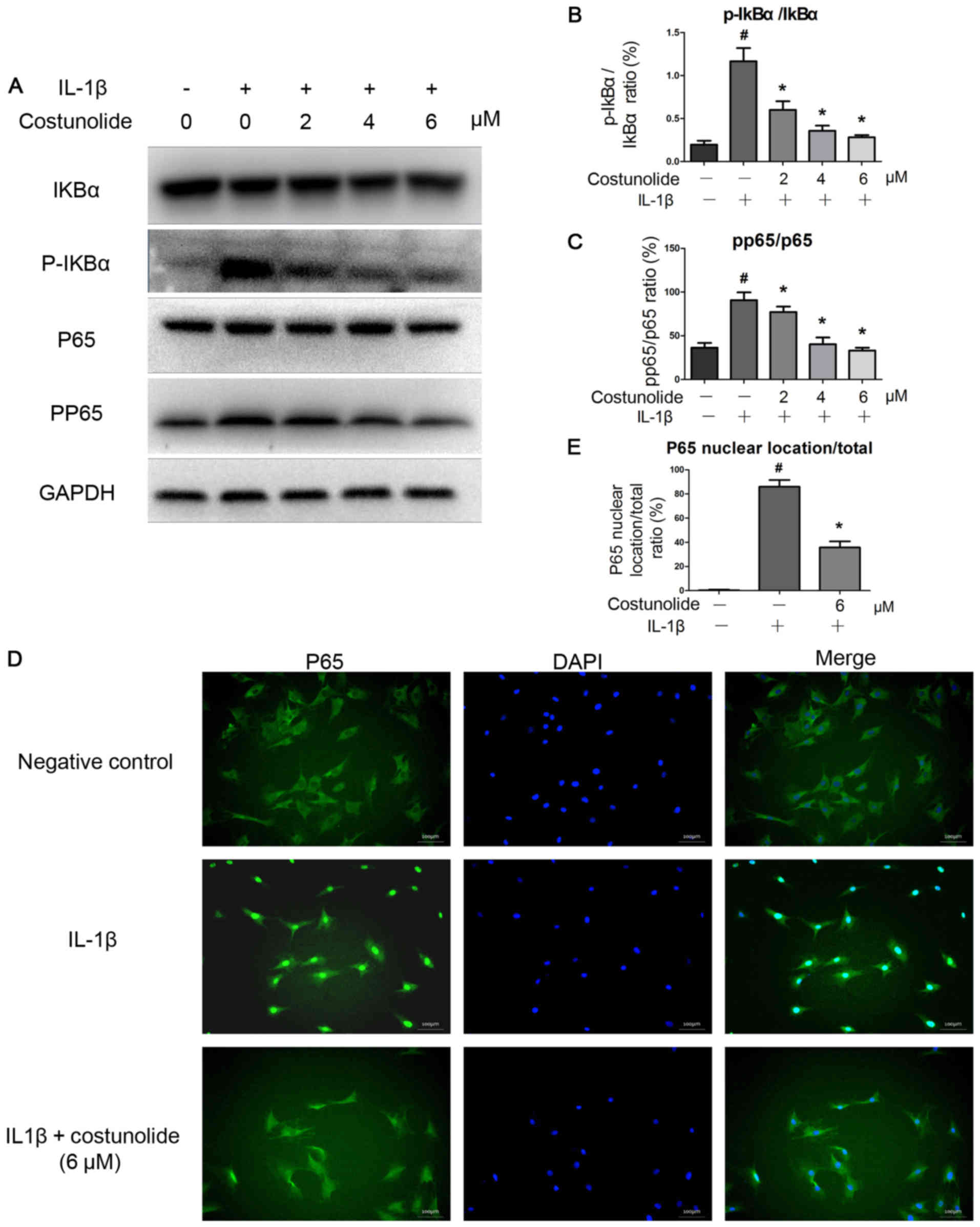
IL-1β activated the NF-κB signaling pathway.
However, costunolide was able to decrease the IL-1β-induced
activation of the NF-κB signaling pathway. Western blot analysis
demonstrates decreased levels of p-p65 compared with the IL-1β
group (Fig. 4A). The levels of
p-IκBα were downregulated by costunolide in a dose-dependent
manner, which was induced by IL-1β (Fig. 4A). Costunolide significantly
inhibited the increase of the p-p65/p65 and p-IκBα/IκBα ratios
induced by IL-1β stimulation (Fig. 4B
and C). Furthermore, immunofluorescence staining revealed that
IL-1β-induced p65 translocation into the nucleus was significantly
inhibited by pretreatment with 6 µM costunolide (Fig. 4D and E). These results demonstrated
that costunolide effectively inhibited IL-1β-induced NF-κB
signaling activation in rat chondrocytes in vitro.
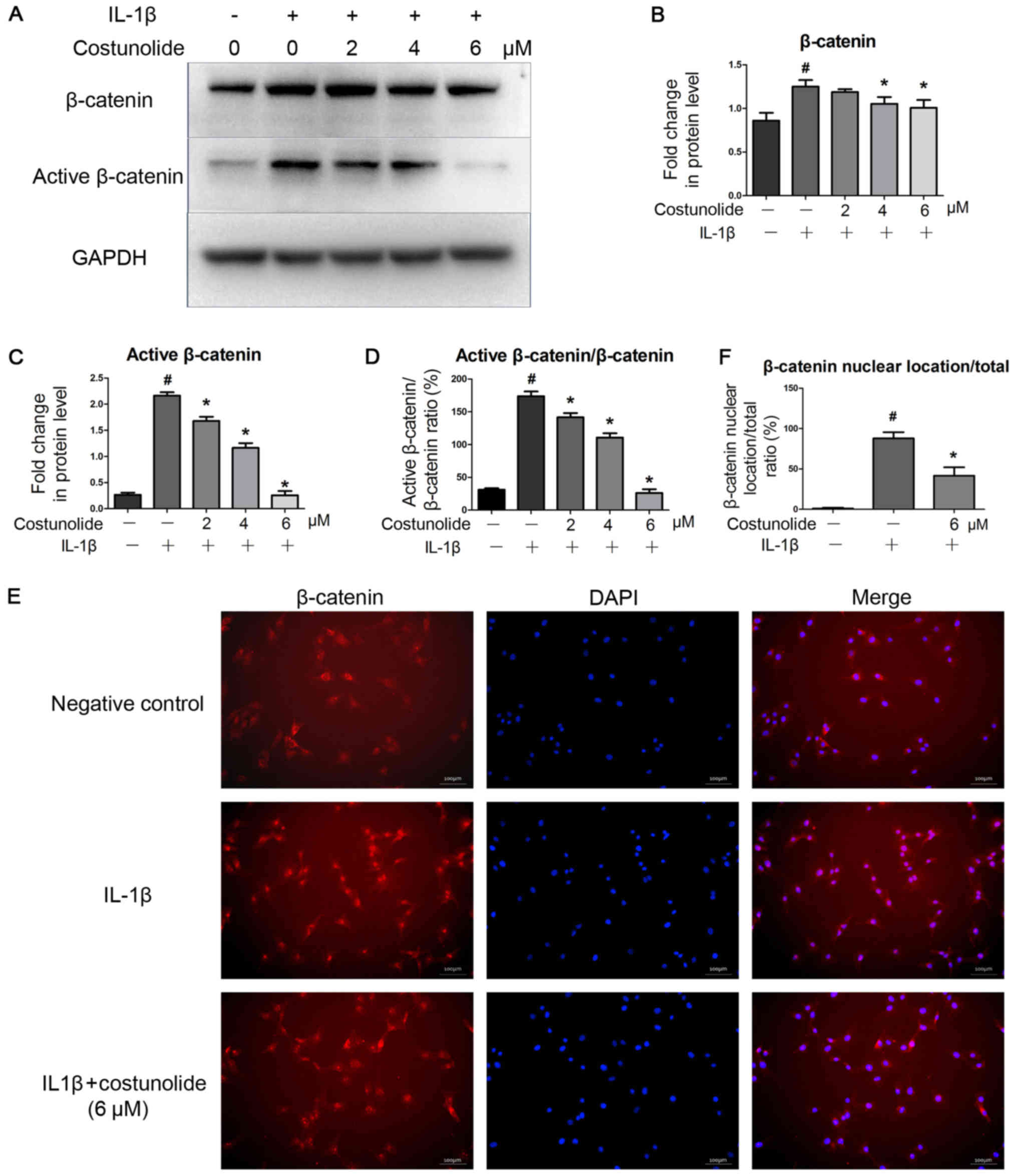
Effect of costunolide on IL-1β-induced
Wnt/β-catenin activation in chondrocytes
To confirm the inhibitory effects of costunolide on
the Wnt/β-catenin signaling pathway, β-catenin protein levels and
its distribution in chondrocytes were determined by western blot
analysis and immunofluorescence staining. IL-1β stimulation
significantly activated the Wnt/β-catenin signaling pathway by
inhibiting the degradation of β-catenin, which was suppressed by
costunolide in a concentration-dependent manner (Fig. 5A and B). Active β-catenin (non
p-β-catenin) levels were also decreased by costunolide treatment
(Fig. 5A and C). In addition, a
decrease in the active β-catenin: β-catenin ratio was also observed
(Fig. 5D). Immunofluorescence
staining revealed that the translocation of β-catenin into the
nucleus decreased, which was induced by IL-1β stimulation (Fig. 5E and F).
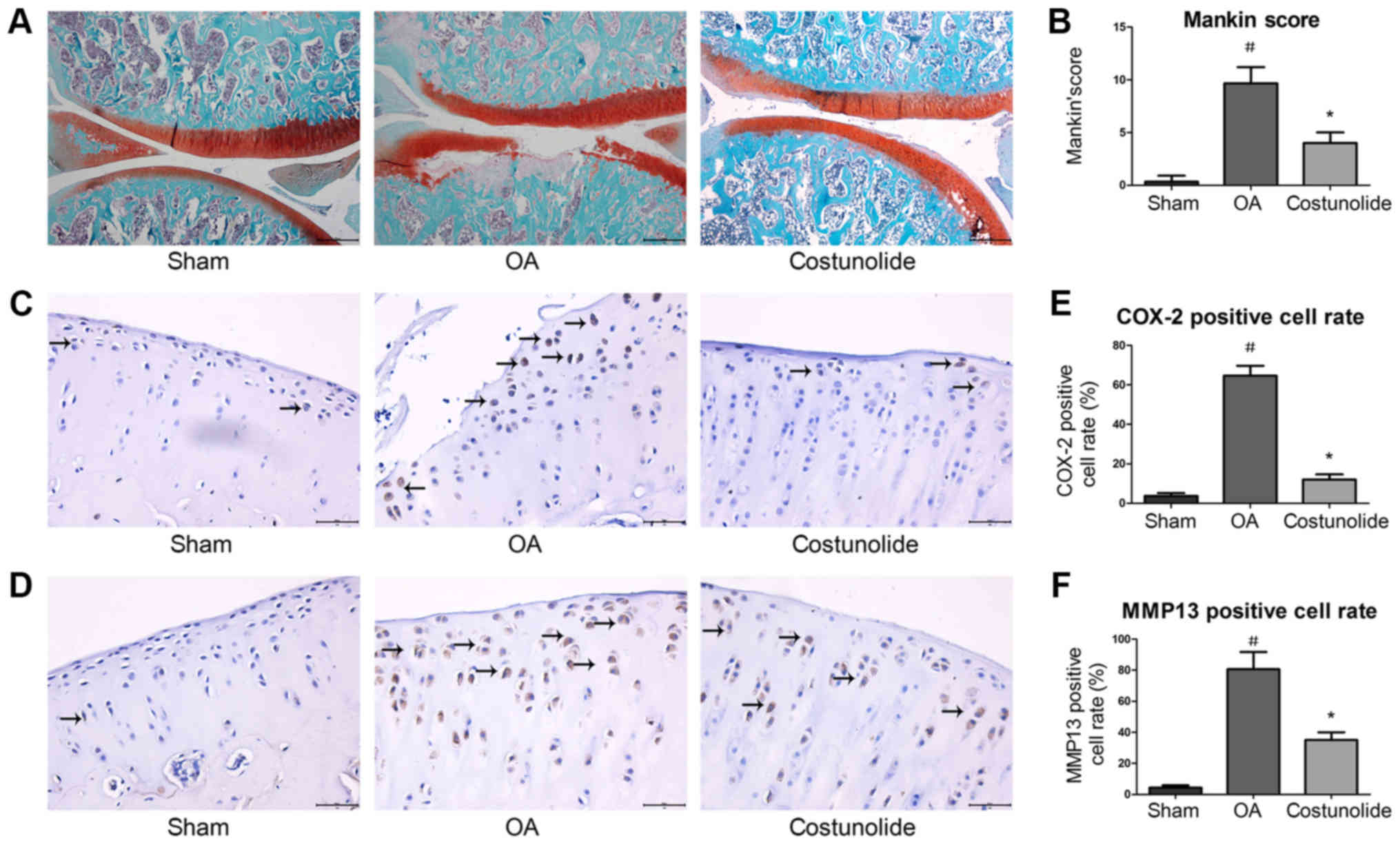
Histopathological and
immunohistochemical changes in articular cartilage
To investigate the protective effects of costunolide
on OA development in vivo, a surgically-induced rat OA model
involving DMM was established. Histopathological changes in
cartilage were assessed by safranin O-fast green staining. Fissure
in the matrix and loss of safranin O staining in chondrocytes were
observed in rats following surgery. Intra-articular injection of
costunolide suppressed cartilage degradation, thereby delaying OA
progression (Fig. 6A). The Mankin
score of the costunolide group was decreased compared with that of
the OA group (Fig. 6B).
Immunohistochemistry revealed that COX-2 and MMP-13 expression were
significantly decreased in the costunolide group compared with the
OA group (Fig. 6C-F). These
experimental results demonstrated that costunolide ameliorated the
progression of OA in vivo.
Discussion
OA is a progressive degenerative joint disease
characterized by synovial inflammation, destruction of subchondral
bone, formation of osteophytes and degradation of articular
cartilage. At present, the treatments for OA include
pharmacological and non-pharmacological therapies. NSAIDs are
commonly used drugs for the treatment of OA to relieve the symptom
of patients, but cannot effectively prevent cartilage degeneration,
and replacement surgery is usually performed during end-stage
disease.
Costunolide, a sesquiterpene lactone, exhibits
anti-oxidant and anti-inflammatory properties. A previous study
demonstrated that costunolide significantly inhibited RANKL-induced
bone marrow-derived macrophage differentiation into osteoclasts in
a dose-dependent manner without affecting cytotoxicity (28). Certain studies have hypothesized
that osteoclasts serve an important role in the pathogenesis of OA,
indicating that agents that can effectively suppress subchondral
bone loss and chondrocyte degradation may aid in the treatment of
OA (29). However, whether
costunolide is able to suppress cartilage degeneration remains
unclear at present. The present study indicated that costunolide
ameliorated cartilage degeneration via suppression of the NF-κB and
Wnt/β-catenin signaling pathways.
The process of matrix degradation in OA is
attributed to the release of MMPs, which are primarily responsible
for degrading the ECM, particularly MMP-1 and MMP-13 (30). It has been suggested that certain
risk factors associated with OA include the activation of catabolic
factors, including the pro-inflammatory cytokine IL-1β. Previous
studies have revealed that the expression of IL-1β is increased in
joints with OA compared with in normal joints (31). IL-1β stimulation may upregulate
MMPs expression and aggravate chondrocyte apoptosis, which causes
OA (32). Downregulation of MMPs
expression and chondrocyte inflammation leads to a therapeutic
effect in OA (33). In the cell
viability assay performed in the present study, it was observed
that costunolide treatment at ≤6 µM had no effect on the viability
of rat chondrocytes, and did not alter gene expression under
nonpathological conditions, which indicates that a low
concentration of costunolide is not harmful to normal cartilage.
The data from the present study also indicated that IL-1β promoted
the levels of IL-6, INOS, COX-2, MMP-3, MMP-9 and MMP-13, and
decreased the levels of collagen II and SOX9 in chondrocytes.
Costunolide reversed IL-1β-induced inflammatory and
matrix-degrading gene expression and maintained cartilage
phenotype. In addition, histological evaluation of safranin O
staining revealed that the OA rat model resulted in cartilage
degradation, while injection of costunolide for 8 weeks markedly
improved the structure of the cartilage, with a lower Makin score
in the costunolide treatment group compared with the OA group.
Specifically, the effect of costunolide on the treatment of OA
manifested through a decrease in OA-specific gene expression,
including COX-2 and MMP-13, as analyzed by immunohistochemistry.
The results from these analyses confirmed the protective role of
costunolide in ameliorating cartilage erosion and decreasing matrix
degeneration in vitro and in vivo.
There are multiple signaling pathways involved in
the progression of OA. As shown in Fig. 7, the present study elucidated the
mechanism by which costunolide exhibits anti-inflammatory and
anti-catabolic effects in the ECM of chondrocytes via the Wnt and
NF-κB signaling pathways, which have been reported to be involved
in the progression of OA (34,35).
In the classic sequence of NF-κB activation, IL-1β activates the
NF-κB signaling pathway by triggering the phosphorylation of
members of the inhibitor of κB family, which are ubiquitinated upon
phosphorylation by IκB kinase, and p65 heterodimers are
subsequently released (36,37).
p-p65 is translocated from the cytosol to the nucleus, which
results in the expression of inflammatory genes including MMPs,
INOS and IL-6 (38). According to
the results of immunofluorescence microscopy and western blot
analysis, costunolide significantly suppressed the phosphorylation
of IκB and p65 in chondrocytes and decreased the nuclear
translocation of p65 upon treatment with IL-1β stimulation.
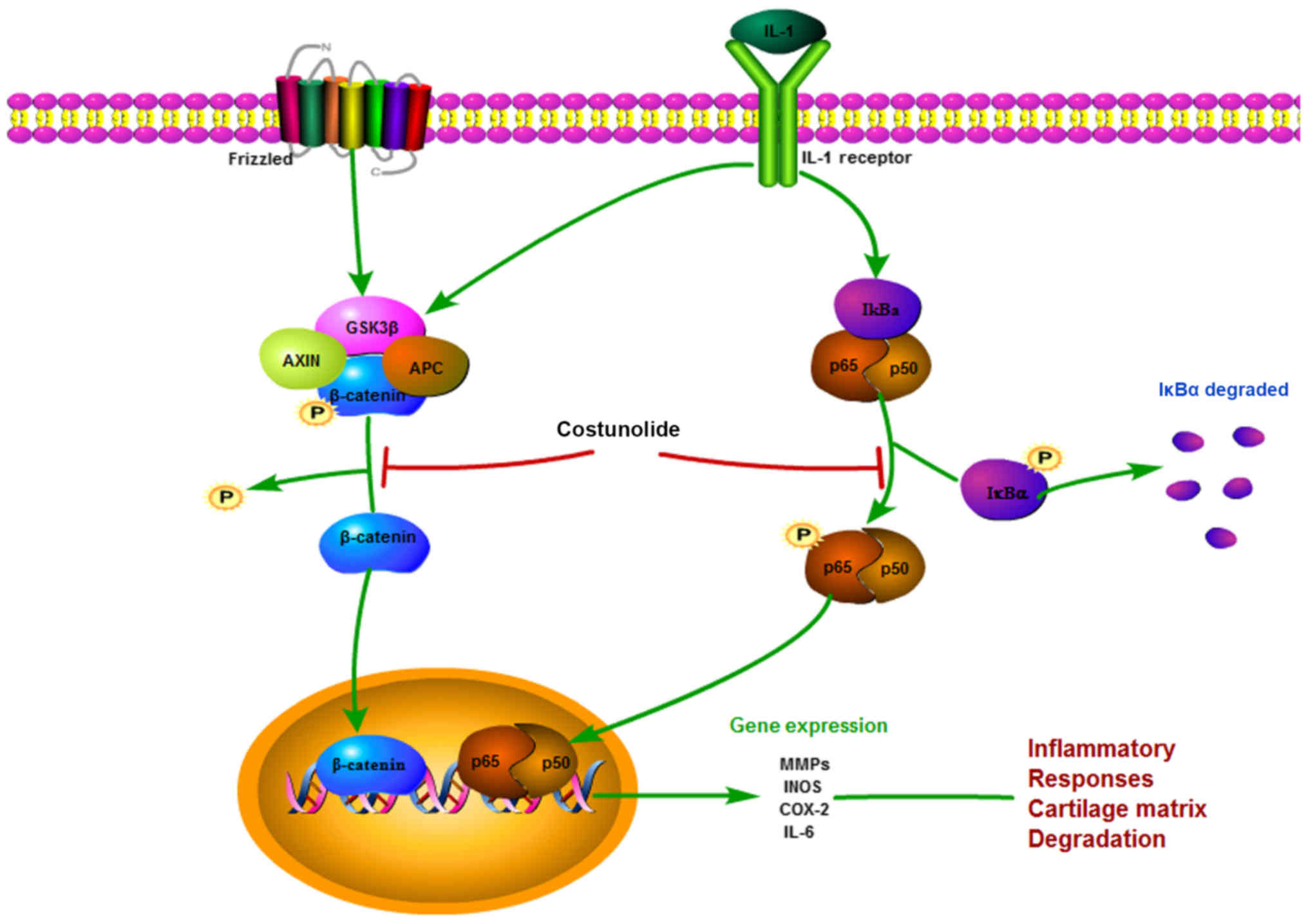 | Figure 7.Schematic illustration of the
potential protective effects of costunolide in osteoarthritis
development. IL, interleukin; GSK3β, glycogen synthase kinase-3β;
AXIN, Axin-1; APC, adenomatous polyposis coli; IκB-α, NF-κB
inhibitor α; p65, transcription factor p65; p50, NF-κB p105
subunit; MMPs, matrix metalloproteinases, INOS, inducible nitric
oxide synthase; COX-2, cyclooxygenase-2. |
The Wnt/β-catenin signaling pathway regulates
crucial aspects of bone metabolism and formation, and the
reconstruction and development of cartilage tissue, which has been
acknowledged as important in the progression of OA (39,40).
β-catenin is the most important component of the Wnt signaling
pathway. During the basal status, β-catenin is steadily
phosphorylated by a destructive complex composed of casein kinase
1, glycogen synthase kinase-3β (GSK-3β), Axin-1 and adenomatous
polyposis coli. GSK-3β phosphorylates β-catenin to cause its
degradation, ultimately inhibiting the activation of the Wnt
signaling pathway. Due to the stimulation of IL-1β, β-catenin is
stabilized and translocated into the nucleus to activate target
genes (41,42). The results from the present study
revealed that costunolide promoted total β-catenin degradation
while inhibiting the production of non-p-β-catenin (active), which
was translocated into the nucleus.
To the best of our knowledge, the present study was
the first to examine the effect of costunolide in preventing
cartilage degeneration. The underlying mechanism of this effect is
associated with the inhibition of the NF-κB and Wnt/β-catenin
signaling pathways induced by IL-1β. Additional studies are
required to elucidate the exact mechanism by which costunolide
regulates the NF-κB and Wnt/β-catenin signaling pathways.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81371996 and
81572173).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors made a significant contribution to the
study and were in agreement with the content of the article. YH and
SM conceived and designed the experiments. YH and CM performed the
experiments, and JR and KX analyzed the data. LX, JB, WC and LJ
interpreted the data, and contributed reagents and materials. YH,
YX and LW interpreted the data and drafted the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Affiliated Hospital, School of Medicine,
Zhejiang University (approval no. 2015-107).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dubin A: Managing osteoarthritis and other
chronic musculoskeletal pain disorders. Med Clin North Am.
100:143–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loeuille D, Chary-Valckenaere I
Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, Goebel JC,
Mainard D, Blum A, Pourel J, et al: Macroscopic and microscopic
features of synovial membrane inflammation in the osteoarthritic
knee: Correlating magnetic resonance imaging findings with disease
severity. Arthritis Rheum. 52:3492–3501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krajewska-Wlodarczyk M,
Owczarczyk-Saczonek A, Placek W, Osowski A and Wojtkiewicz J:
Articular cartilage aging-potential regenerative capacities of cell
manipulation and stem cell therapy. Int J Mol Sci. 19:E6232018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Igarashi M, Sakamoto K and Nagaoka I:
Effect of glucosamine on expression of type II collagen, matrix
metalloproteinase and sirtuin genes in a human chondrocyte cell
line. Int J Mol Med. 39:472–478. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu ZG, Xu N Wang WB, Pan SH, Li KS and Liu
JK: Interleukin-1 inhibits Sox9 and collagen type II expression via
nuclear factor-kappaB in the cultured human intervertebral disc
cells. Chin Med J (Engl). 122:2483–2488. 2009.PubMed/NCBI
|
|
6
|
Chabane N, Zayed N Afif H, Mfuna-Endam L,
Benderdour M, Boileau C, Martel-Pelletier J, Pelletier JP, Duval N
and Fahmi H: Histone deacetylase inhibitors suppress
interleukin-1beta-induced nitric oxide and prostaglandin E2
production in human chondrocytes. Osteoarthritis Cartilage.
16:1267–1274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyaura C, Inada M, Suzawa T, Sugimoto Y,
Ushikubi F, Ichikawa A, Narumiya S and Suda T: Impaired bone
resorption to prostaglandin E2 in prostaglandin E receptor
EP4-knockout mice. J Biol Chem. 275:19819–19823. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dannhardt G and Kiefer W: Cyclooxygenase
inhibitors-current status and future prospects. Eur J Med Chem.
36:109–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salvemini D, Misko TP, Masferrer JL,
Seibert K, Currie MG and Needleman P: Nitric oxide activates
cyclooxygenase enzymes. Proc Natl Acad Sci USA. 90:7240–7244. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasaki K, Hattori T, Fujisawa T, Takahashi
K, Inoue H and Takigawa M: Nitric oxide mediates
interleukin-1-induced gene expression of matrix metalloproteinases
and basic fibroblast growth factor in cultured rabbit articular
chondrocytes. J Biochem. 123:431–439. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi M, Squires GR, Mousa A, Tanzer
M, Zukor DJ, Antoniou J, Feige U and Poole AR: Role of
interleukin-1 and tumor necrosis factor alpha in matrix degradation
of human osteoarthritic cartilage. Arthritis Rheum. 52:128–135.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freemont AJ, Hampson V, Tilman R, Goupille
P, Taiwo Y and Hoyland JA: Gene expression of matrix
metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic
human knee articular cartilage is zone and grade specific. Ann
Rheum Dis. 56:542–549. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okada Y, Shinmei M, Tanaka O, Naka K,
Kimura A, Nakanishi I, Bayliss MT, Iwata K and Nagase H:
Localization of matrix metalloproteinase 3 (stromelysin) in
osteoarthritic cartilage and synovium. Lab Invest. 66:680–690.
1992.PubMed/NCBI
|
|
14
|
Ji B, Guo W, Ma H, Xu B, Mu W, Zhang Z,
Amat A and Cao L: Isoliquiritigenin suppresses IL-1β induced
apoptosis and inflammation in chondrocyte-like ATDC5 cells by
inhibiting NF-κB and exerts chondroprotective effects on a mouse
model of anterior cruciate ligament transection. Int J Mol Med.
40:1709–1718. 2017.PubMed/NCBI
|
|
15
|
Ran J, Ma C, Xu K, Xu L, He Y, Moqbel SAA,
Hu P, Jiang L, Chen W, Bao J, et al: Schisandrin B ameliorated
chondrocytes inflammation and osteoarthritis via suppression of
NF-κB and MAPK signal pathways. Drug Des Devel Ther. 12:1195–1204.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pitchai D, Roy A and Banu S: In vitro and
in silico evaluation of NF-κB targeted costunolide action on
estrogen receptor-negative breast cancer cells--a comparison with
normal breast cells. Phytother Res. 28:1499–1505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HC, Chou CK, Lee SD, Wang JC and Yeh
SF: Active compounds from Saussurea lappa Clarks that suppress
hepatitis B virus surface antigen gene expression in human hepatoma
cells. Antiviral Res. 27:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kreuger MR, Grootjans S, Biavatti MW,
Vandenabeele P and D'Herde K: Sesquiterpene lactones as drugs with
multiple targets in cancer treatment: Focus on parthenolide.
Anticancer Drugs. 23:883–896. 2012.PubMed/NCBI
|
|
19
|
Duraipandiyan V, Al-Harbi Na, Ignacimuthu
S and Muthukumar C: Antimicrobial activity of sesquiterpene
lactones isolated from traditional medicinal plant, Costus
speciosus (Koen ex.Retz.) Sm. BMC Complement Altern Med. 12:132012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eliza J, Daisy P and Ignacimuthu S:
Antioxidant activity of costunolide and eremanthin isolated from
Costus speciosus (Koen ex. Retz) Sm. Chem Biol Interact.
188:467–472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong GZ, Shim AR, Hyeon JS, Lee HJ and Ryu
JH: Inhibition of Wnt/β-catenin pathway by dehydrocostus lactone
and costunolide in colon cancer cells. Phytother Res. 29:680–686.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roshak AK, Callahan JF and Blake SM:
Small-molecule inhibitors of NF-κB for the treatment of
inflammatory joint disease. Curr Opin Pharmacol. 2:316–321. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Held A, Glas A, Dietrich L, Bollmann M,
Brandstädter K, Grossmann TN, Lohmann CH, Pap T and Bertrand J:
Targeting β-catenin dependent Wnt signaling via peptidomimetic
inhibitors in murine chondrocytes and OA cartilage. Osteoarthritis
Cartilage. 26:818–823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
American Veterinary Medical Association.
AVMA Guidelines for the Euthanasia of Animals, . 2013 Edition. J Am
Veterinary Med Association. 2013.
|
|
26
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. National Academies Press. 1996.
|
|
27
|
van der Sluijs JA, Geesink RG, van der
Linden AJ, Bulstra SK, Kuyer R and Drukker J: The reliability of
the Mankin score for osteoarthritis. J Orthop Res. 10:58–61. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheon YH, Song MJ, Kim JY, Kwak SC, Park
JH, Lee CH, Kim JJ, Kim JY, Choi MK, Oh J, et al: Costunolide
inhibits osteoclast differentiation by suppressing c-Fos
transcriptional activity. Phytother Res. 28:586–592. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanwar JR, Samarasinghe RM, Kumar K, Arya
R, Sharma S, Zhou SF, Sasidharan S and Kanwar RK: Cissus
quadrangularis inhibits IL-1β induced inflammatory responses on
chondrocytes and alleviates bone deterioration in osteotomized rats
via p38 MAPK signaling. Drug Des Devel Ther. 9:2927–2940. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woessner JF Jr and Gunja-Smith Z: Role of
metalloproteinases in human osteoarthritis. J Rheumatol Suppl.
27:99–101. 1991.PubMed/NCBI
|
|
31
|
Blumenfeld I and Livne E: The role of
transforming growth factor (TGF)-beta, insulin-like growth factor
(IGF)-1, and interleukin (IL)-1 in osteoarthritis and aging of
joints. Exp Gerontol. 34:821–829. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pascarelli NA, Collodel G, Moretti E,
Cheleschi S and Fioravanti A: Changes in ultrastructure and
cytoskeletal aspects of human normal and osteoarthritic
chondrocytes exposed to interleukin-1β and cyclical hydrostatic
pressure. Int J Mol Sci. 16:26019–26034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sabatini M, Lesur C, Thomas M, Chomel A,
Anract P, de Nanteuil G and Pastoureau P: Effect of inhibition of
matrix metalloproteinases on cartilage loss in vitro and in a
guinea pig model of osteoarthritis. Arthritis Rheum. 52:171–180.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Corr M: Wnt-beta-catenin signaling in the
pathogenesis of osteoarthritis. Nat Clin Pract Rheumatol.
4:550–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu L, Huang X, Li L, Huang H, Xu R and
Luyten W: Insights on biology and pathology of HIF-1α/-2α,
TGFβ/BMP, Wnt/β-catenin, and NF-κB pathways in osteoarthritis. Curr
Pharm Des. 18:3293–3312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schottelius AJ, Mayo MW, Sartor RB and
Baldwin AS Jr: Interleukin-10 signaling blocks inhibitor of kappaB
kinase activity and nuclear factor kappaB DNA binding. J Biol Chem.
274:31868–31874. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vincenti MP, Coon CI and Brinckerhoff CE:
Nuclear factor kappaB/p50 activates an element in the distal matrix
metalloproteinase 1 promoter in interleukin-1beta-stimulated
synovial fibroblasts. Arthritis Rheum. 41:1987–1994. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y, Wang T, Hamilton JL and Chen D:
Wnt/β-catenin signaling in osteoarthritis and in other forms of
arthritis. Curr Rheumatol Rep. 19:532017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/beta-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu W, Shi J, Zhang J, Lv Z, Guo F, Huang
H, Zhu W and Chen A: CXCL12/CXCR4 axis regulates aggrecanase
activation and cartilage degradation in a post-traumatic
osteoarthritis rat model. Int J Mol Sci. 17:E15222016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sassi N, Laadhar L, Allouche M, Achek A,
Kallel-Sellami M, Makni S and Sellami S: WNT signaling and
chondrocytes: From cell fate determination to osteoarthritis
physiopathology. J Recept Signal Transduct Res. 34:73–80. 2014.
View Article : Google Scholar : PubMed/NCBI
|