Introduction
Lung cancer, a highly lethal malignant tumor, is a
common cancer and represents a leading cause of cancer-related
deaths worldwide (1).
Approximately 85% of lung cancer cases are classified
histopathologically as non-small cell lung cancer (NSCLC), which
includes lung adenocarcinoma, lung squamous carcinoma, and large
cell lung cancer (2). Notable
improvements in the diagnosis and treatment of NSCLC have been
achieved in the past decades; unfortunately, the prognosis of
patients with NSCLC remains unfavorable, with a 5-year overall
survival rate of only 11% (3).
Tumor recurrence and metastasis are primarily responsible for the
unsatisfying clinical outcomes for NSCLC patients (4,5).
Multiple risk factors and signaling pathways have been implicated
in the pathogenesis of NSCLC; however, the precise molecular
mechanisms are yet to be fully clarified (6). Therefore, elucidation of the
molecular mechanisms underlying the initiation and progression of
NSCLC is urgently necessary and may help to identify novel
diagnostic biomarkers and therapeutic methods to improve the
prognosis of NSCLC patients.
MicroRNAs (miRNAs) are a family of noncoding,
single-stranded, short RNA molecules that have been demonstrated to
serve as an endogenous means of RNA interference (7). miRNAs can directly recognize and
interact with a partially complementary recognition sequence in the
3′ untranslated region (3′-UTRs) of the mRNA of a target gene and
cause degradation of the mRNA and/or translation suppression
(8). It has been well documented
that miRNAs are implicated in a variety of physiological and
pathological processes, including the initiation and progression of
human cancers (9,10). A wide range of miRNAs are
differentially expressed in NSCLCs (11–13).
For example, miR-217 expression is often low in NSCLC. NSCLC
patients with low miR-217 expression exhibit shorter overall
survival than do the patients with high miR-217 expression
(14). These aberrantly expressed
miRNAs may perform oncogenic or tumor-suppressive functions and
contribute to the oncogenesis and progression of NSCLC by
regulating multiple crucial biological processes (15,16).
These findings indicate that an in-depth investigation into the
participation and mechanisms of action of cancer-related miRNAs in
NSCLC may facilitate the search for novel therapeutic targets in
NSCLC.
Certain studies have identified miR-889 as a novel
cancer-associated miRNA that is aberrantly expressed and plays an
important role in esophageal squamous cell carcinoma (17) and hepatocellular carcinoma
(18). Nonetheless, the exact
functions and precise molecular mechanisms via which miR-889
affects NSCLC progression are still unclear. Thus, in the present
study, the quantification of miR-889 expression was attempted and
the potential involvement and mechanism of action of miR-889 in
NSCLC were investigated. The present findings are expected to
provide novel insights into the pathogenesis of NSCLC and/or a
promising target for the treatment of patients with this
disease.
Materials and methods
Patients and tissue specimens
The procedures of our present study were approved by
the Ethics Committee of Weifang People's Hospital (Weifang, China)
and written informed consent was also provided by all patients.
Samples of primary NSCLC tissues and adjacent normal tissues were
collected from 53 patients (30 males, 23 females; age range, 47–71
years) who had received surgical resection at Weifang People's
Hospital between June 2016 and August 2017. None of these patients
had been treated preoperatively with either radiotherapy or
chemotherapy. All tissues were immediately snap-frozen in liquid
nitrogen and then stored at −80°C for further RNA extraction.
Cell culture
A non-tumorigenic bronchial epithelium cell line,
BEAS-2B, and four human NSCLC cell lines (A549, SK-MES-1, H522, and
H460) were purchased from the Shanghai Institute of Biochemistry
and Cell Biology (Shanghai, China). All cells were routinely
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS; both from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 1% penicillin/streptomycin
mixture (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
cultures were maintained at 37°C in a humidified atmosphere
containing 5% CO2.
miRNA mimics, small interfering RNA
(siRNA) and plasmid transfection
miR-889 mimics and corresponding miRNA mimics
negative control (miR-NC), siRNA targeting the expression of TAB1
(si-TAB1) and negative control siRNA (si-NC) were constructed by
GenePharma (Shanghai, China). The si-TAB1 sequence was
5′-GGAUGAGCUCUUCCGUCUUTT-3′ and the si-NC sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. TAB1 expression vector pcDNA3.1-TAB1
(pcTAB1) and empty vector (pcDNA3.1) was obtained from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). Before transfection, cells in
logarithmic phase were plated into 6-well plates with a density of
4×105 cells/well. When the culture confluency reached
60–70%, the cells were transfected with the miR-889 mimics (100
pmol), miR-NC (100 pmol), si-TAB1 (100 pmol), si-NC (100 pmol),
pcTAB1 (4 µg) or pcDNA3 (4 µg) using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). All of the
transfection procedures were based on the product specifications.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and Transwell Matrigel invasion assays were performed 48
h after transfection. The MTT assay and western blotting were
performed at 24 and 72 h post-transfection, respectively.
RNA extraction and RT-qPCR
analysis
Total RNA was extracted from tissue samples or
cultured cell lines using a TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). All-in-One™ miRNA qRT-PCR
Detection kit (GeneCopoeia, Inc., Rockville, MD, USA) was used to
detect miR-889 expression. The thermocycling conditions were as
follows: 95°C for 10 min, and 45 cycles of denaturation at 95°C for
15 sec and annealing/elongation at 60°C for 15 sec. To quantify
TAB1 mRNA expression, total RNA was reverse-transcribed into cDNA
using a PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China). The temperature protocol for TAB1 mRNA reverse
transcription was as follows: 37°C for 15 min and 85°C for 5
second. The quantitative PCR was carried out using an ABI 7500
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and a SYBR Premix Ex Taq™ (Takara Biotechnology
Co., Ltd). The thermocycling conditions for TAB1 mRNA qPCR was as
follows: 5 min at 95°C, followed by 40 cycles of 95°C for 30 sec
and 65°C for 45 sec. U6 small nuclear RNA and GAPDH were used as
the internal controls for miR-889 and TAB1 mRNA, respectively. All
reactions were run in triplicate and relative gene expression was
analyzed using the 2−∆∆Cq method (19). The primers were designed as
follows: miR-889 forward, 5′-ACACTCCAGCTGGGTTAATATCGGACAAC-3′, and
reverse, 5′-TGGTGTCGTGGAGTCG-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′, and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; TAB1 forward,
5′-ATGAGCTCTTCCGTCTTTCG-3′, and reverse, 5′-ATCCCCACCTGCTTGATCT-3′;
and GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
MTT assay
An MTT assay was performed to determine cellular
proliferation. In brief, transfected cells were inoculated in
96-well plates with a density of 3,000 cells/well. Then the cells
were incubated at 37°C supplied with 5% CO2. for 0–3
days. The MTT assay was carried out every day by adding 20 µl of
MTT reagent (5 mg/ml; Beyotime Institute of Biotechnology, Haimen,
China) into each well. Following incubation at 37°C for an
additional 4 h, the culture medium containing MTT solution was
replaced with 150 µl of dimethyl sulfoxide (DMSO). A total of 15
min after incubation, the absorbance value of each well was
detected at a 450 nm wavelength using an enzyme-linked
immunosorbent assay reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Transwell Matrigel invasion assay
Transwell insert chambers (8 µm pore size) covered
with Matrigel (both from BD Biosciences, San Jose, CA, USA) were
utilized to evaluate the invasive ability of NSCLC cells.
Transfected cells were collected and suspended in FBS-free culture
medium. A total of 5×104 cells were added into the upper
chambers. In the lower chambers, 500 µl DMEM containing 20% FBS was
added to serve as a chemoattractant. Subsequent to incubation at
37°C with 5% CO2. for 24 h, the non-invasive cells were
gently wiped away with a cotton swab. The invasive cells that
invaded through the pores were fixed with 100% methanol at room
temperature for 30 min, stained with 0.5% crystal violet at room
temperature for 30 min, and washed with phosphate buffer solution.
Images of five different fields were captured from each insert, and
the number of invasive cells was counted under an inverted light
microscope (×200, magnification; Olympus Corporation, Tokyo,
Japan).
Xenograft assay
A total of eight 6-week-old BALB/c male nude mice
(20 g) were purchased from the Shanghai Laboratory Animal Center
(Chinese Academy of Sciences, Shanghai, China). All animals were
maintained under specific pathogen-free conditions at 25°C, 50%
relative humidity, a 10 h light/14 h dark cycle and had ad
libitum access to food and water. The animals were divided into
two groups (n=4 mice/group), one injected with miR-NC-transfected
cells and the other injected with miR-889 mimic-transfected cells.
The width and length of the xenograft formed was detected with a
vernier caliper. Tumor volume was calculated by the following
formula: Volume (mm3) = width2
(mm2) × length (mm)/2. At the end of the assay, all nude
mice were sacrificed and the excited xenograft was weighted. All
animal experiments were carried out in accordance with the Guide
for Care and Use of Laboratory Animal and all experimental
protocols were approved by the Animal Ethics Committee of the
Weifang People's Hospital.
Target prediction
Three online miRNA target prediction software,
including miRDB (http://www.mirdb.org/), miRanda (http://www.microrna.org), and TargetScan (http://www.targetscan.org/), were used to predict the
potential target genes of miR-889.
Luciferase reporter assay
The 3′-UTR region of TAB1 containing the predicted
wild-type and mutant miR-889 binding sites was amplified by
GenePharma and individually cloned into the pmiR-RB-REPORT™ vector
(Promega Corporation, Madison, WI, USA). For the reporter assays,
cells were plated into 24-well plates, and co-transfected with wild
type or mutant reporter plasmid and miR-889 mimics or miR-NC using
Lipofectamine™ 2000, according to the manufacturer's instructions.
Forty-eight hours later, the luciferase activity of transfected
cells was determined using a Dual-Luciferase Reporter Assay System
(Promega Corporation). Firefly luciferase activity was normalized
against Renilla luciferase activity.
Western blot analysis
Total protein was isolated from tissues or cells
using cell lysis buffer (Cell Signaling Technology, Danvers, MA,
USA). The concentration of total protein was determined using a
bicinchoninic acid protein assay (Pierce; Thermo Fisher Scientific,
Inc.). Equal amounts of protein (30 µg) were separated by 10%
sodium dodecyl sulfate polyacrylamide gels electrophoresis and
transferred onto polyvinylidene fluoride membranes (Beyotime
Institute of Biotechnology), followed by blocking with 5% dried
skimmed milk in Tris-buffered saline and 0.1% Tween-20 (TBS-T) at
room temperature for 1 h. The membranes were subsequently incubated
with the following primary antibodies at 4°C overnight: Rabbit
anti-human TAB1 antibody (cat. no. ab76412; 1:1,000 dilution) and
rabbit anti-human GAPDH antibody (cat. no. ab128915; 1:1,000
dilution; both from Abcam, Cambridge, UK). The membranes were then
probed with horseradish peroxidase-conjugated secondary antibody
(cat. no. ab205718; 1:5,000 dilution; Abcam,) for 2 h at room
temperature. Finally, the protein bands were visualized using an
enhanced chemiluminescence reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). GAPDH was used as the internal control.
Quantity One software version 4.62 (Bio-Rad Laboratories, Inc.) was
used for densitometry analysis.
Statistical analysis
All assays were repeated at least three times. All
data are presented as the mean ± standard deviation, and analyzed
using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). All
functional experiments were repeated three times. Differences
between two groups were examined using an unpaired Student's
t-test, or a paired Student's t-test to compare expression data
from NSCLC and adjacent normal tissues. One-way ANOVA with Tukey's
post-hoc test was used for the comparison between multiple groups.
A χ2 test was performed to evaluate the association
between miR-889 and the clinicopathological characteristics of
NSCLC patients. A correlation between miR-889 and TAB1 mRNA
expression was determined through Spearman's correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-889 is downregulated in NSCLC
tissue samples and cell lines
In total, 53 pairs of NSCLC tissue samples and
adjacent normal tissue samples were collected. Total RNA was
isolated from these tissues, and RT-qPCR was performed to assess
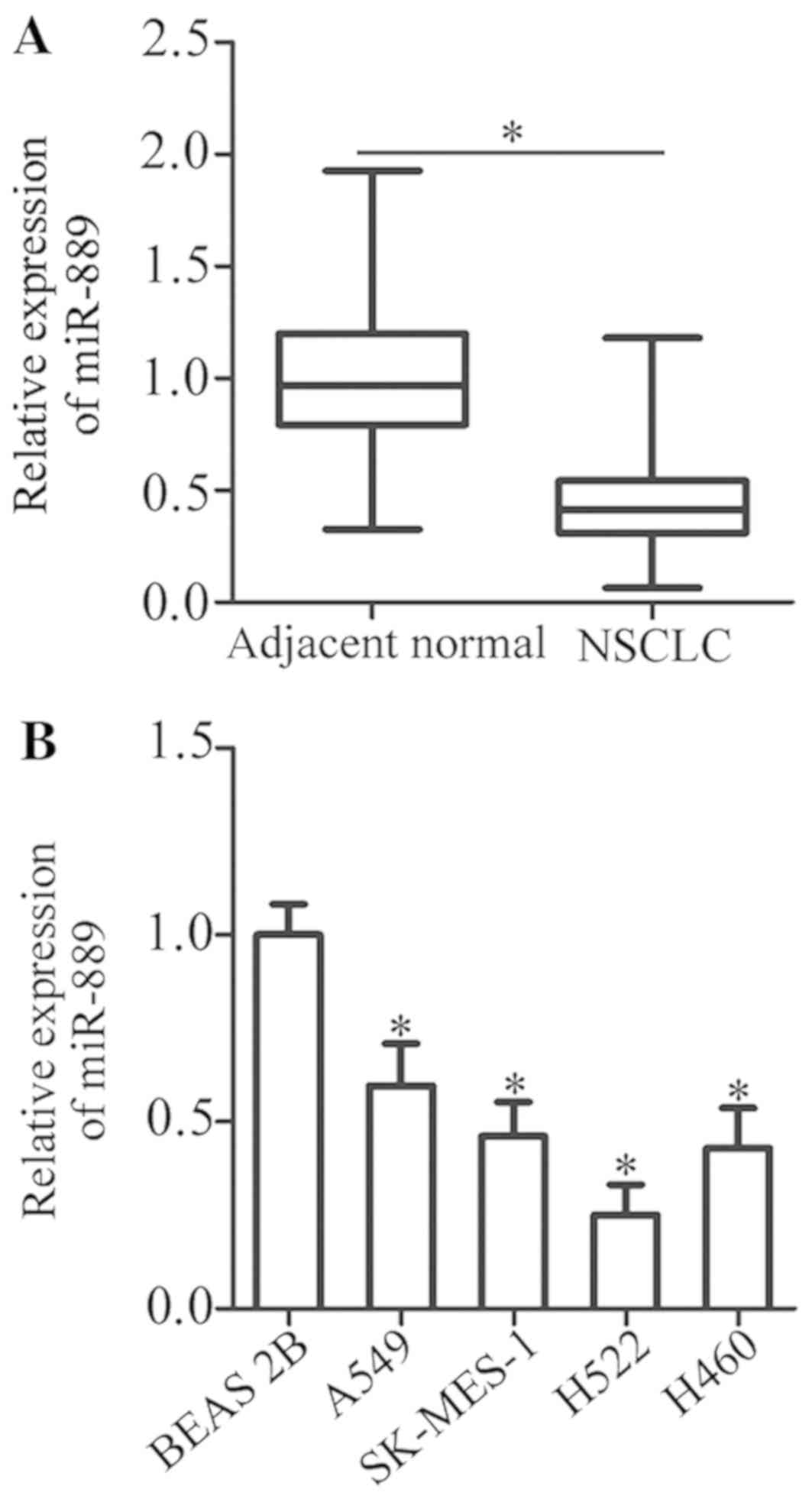
miR-889 expression. The data revealed that the expression of
miR-889 was notably lower in NSCLC tissue samples than in adjacent
normal tissues (P<0.05; Fig.
1A). In addition, miR-889 expression in four human NSCLC cell
lines (A549, SK-MES-1, H522, and H460) was determined by RT-qPCR.
When compared with non-tumorigenic bronchial epithelium BEAS-2B
cells, miR-889 expression was lower in all four NSCLC cell lines to
varying degrees (P<0.05; Fig.
1B). These data indicated that miR-889 was underexpressed in
both NSCLC tissue samples and cell lines.
Downregulated miR-889 is correlated
with the clinical characteristics of patients with NSCLC
To clarify the possible clinical meaning of low
miR-889 expression in NSCLC, all enrolled NSCLC patients were
categorized into two subgroups, ‘low miR-889 expression’ and ‘high
miR-889 expression’, based on the median value of miR-889
expression in NSCLC tissue samples. Statistical analysis revealed
that underexpression of miR-889 was significantly correlated with
TNM stage (P=0.019) and distant metastasis (P=0.020); however, no
significant associations with other pathological characteristics
were observed, including sex (P=0.477), age (P=0.685), tumor size
(P=0.407), histological tumor type (P=0.449), or tumor
differentiation status (P=0.317; Table
I). These results indicated that underexpression of miR-889 may
be implicated in the pathogenesis of NSCLC.
 | Table I.Correlation of miR-889 expression and
clinicopathological characteristics of patients with non-small cell
lung cancer. |
Table I.
Correlation of miR-889 expression and
clinicopathological characteristics of patients with non-small cell
lung cancer.
|
| miR-889 |
|
|---|
| Characteristics | Low expression | High expression | P-value |
|---|
| Sex |
|
| 0.477 |
| Male | 14 | 16 |
|
|
Female | 13 | 10 |
|
| Age (years) |
|
| 0.685 |
|
<65 | 12 | 13 |
|
| ≥65 | 15 | 13 |
|
| Tumor size (cm) |
|
| 0.407 |
|
<5 | 18 | 20 |
|
| ≥5 | 9 | 6 |
|
| Histological tumor
type |
|
| 0.449 |
|
Adenocarcinoma | 16 | 18 |
|
|
Squamous cell carcinoma | 11 | 8 |
|
| Tumor
differentiation |
|
| 0.317 |
|
I–II | 6 | 9 |
|
|
III–IV | 21 | 17 |
|
| TNM stage |
|
| 0.019a |
|
I–II | 7 | 15 |
|
|
III+IV | 20 | 11 |
|
| Distant
metastasis |
|
| 0.020a |
|
Negative | 9 | 17 |
|
|
Positive | 18 | 9 |
|
miR-889 overexpression inhibits the
proliferation and invasiveness of NSCLC cells
To assess whether miR-889 affects NSCLC progression,
cell lines H522 and H460 (possessing relatively lower miR-889
expression among the four assessed NSCLC cell lines) were selected
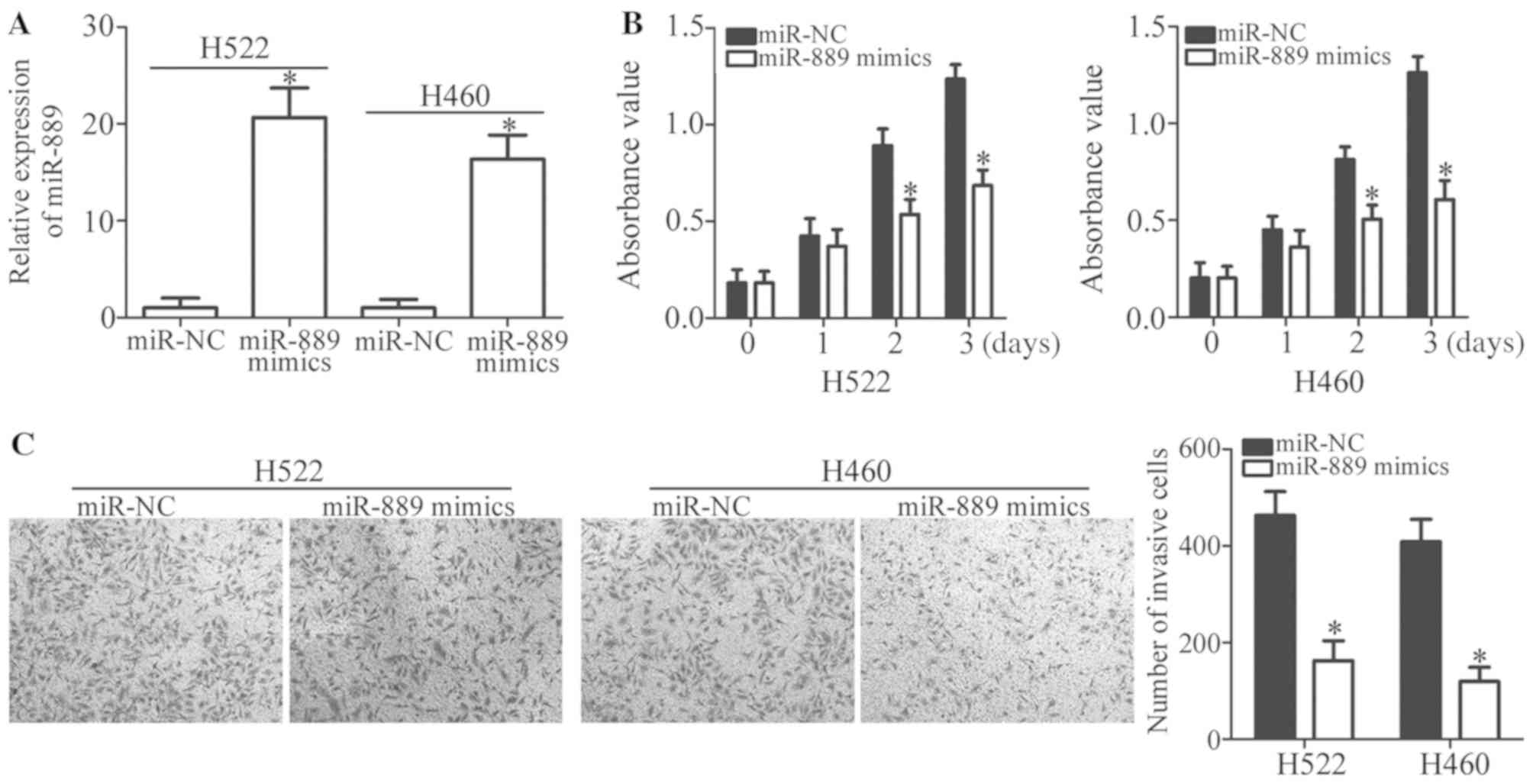
to conduct the following experiments. miR-889 in H522 and H460
cells was overexpressed by transfection with miR-889 mimics
(P<0.05; Fig. 2A). Then, an MTT
assay was carried out to quantify cell proliferation; it was
revealed that miR-889 upregulation significantly inhibited H522 and
H460 cell proliferation when compared with the miR-NC group
(P<0.05; Fig. 2B). Furthermore,
a Transwell Matrigel invasion assay was employed to investigate the
regulation of cell invasion by miR-889 in NSCLC. Ectopic miR-889
expression decreased the capacity for invasion in H522 and H460
cells relative to the cells treated with miR-NC (P<0.05;
Fig. 2C). Collectively, these
findings indicated that miR-889 may perform tumor-suppressive
functions in NSCLC carcinogenesis.
TAB1 is a direct target gene of
miR-889 in NSCLC cells
To illustrate the potential molecular mechanisms by
which miR-889 exerts its influence on NSCLC progression,
bioinformatics tools, including miRDB, miRanda, and TargetScan,
were applied to find a potential target gene (i.e., target mRNA) of
miR-889. This analysis indicated a possible binding site of miR-889
in the 3′-UTR of TAB1 (Fig. 3A).
TAB1 was selected for further analyses since this gene may
participate in the initiation and progression of NSCLC (20). The luciferase reporter assay was
conducted to determine whether miR-889 targets the 3′-UTR of TAB1
mRNA directly. To this end, luciferase reporter vectors were
chemically synthesized and co-transfected into H522 and H460 cells
along with miR-889 mimics or miR-NC. The exogenous miR-889
expression reduced the luciferase activity of the vector that
carried the wild-type 3′-UTR of TAB1 (P<0.05). By contrast,
mutation of the miR-889 binding site in the 3′-UTR of TAB1
abrogated the inhibitory effect of miR-889 overexpression on the
luciferase activity (Fig. 3B).
To confirm that TAB1 contributes to NSCLC
pathogenesis, RT-qPCR analysis was carried out to assess TAB1 mRNA
expression in NSCLC tissue samples. The data indicated that the
mRNA expression of TAB1 was notably higher in NSCLC tissue samples
than in the adjacent normal tissues (P<0.05; Fig. 3C). Furthermore, miR-889 expression
was revealed to be negatively correlated with TAB1 mRNA levels
among NSCLC tissue samples (r=−0.5077, P=0.0001; Fig. 3D). Moreover, TAB1 mRNA and protein
levels in H522 and H460 cells transfected with miR-889 mimics or
miR-NC were examined by RT-qPCR and western blot analysis,
respectively. Transfection of the miR-889 mimics significantly
decreased TAB1 expression in H522 and H460 cells at both the mRNA
(P<0.05; Fig. 3E) and protein
levels (P<0.05; Fig. 3F). Thus,
it was demonstrated that TAB1 mRNA is a direct target of miR-889 in
NSCLC cells.
TAB1 silencing has effects similar to
those of miR-889 mimics on the malignant phenotype of NSCLC cells
in vitro
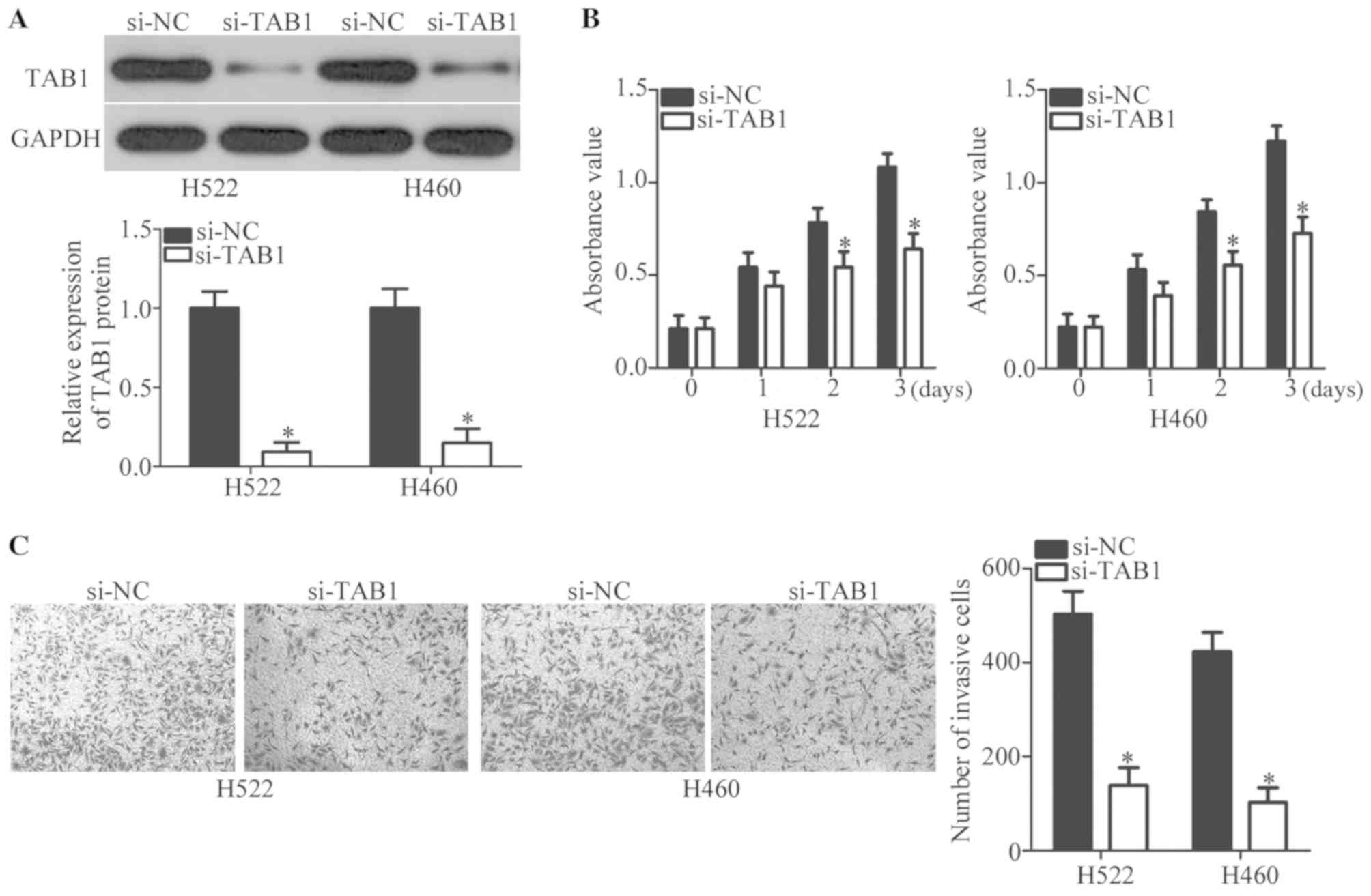
To precisely determine the involvement of TAB1 in
NSCLC, endogenous TAB1 expression was knocked down in H522 and H460
cells by transfection with siRNA against TAB1 (si-TAB1). This
transfection efficiently decreased TAB1 protein expression in H522
and H460 cells, as evidenced by western blot analysis (P<0.05;
Fig. 4A). Then, MTT and Transwell
Matrigel invasion assays were performed to evaluate the changes in
proliferative and invasive abilities of H522 and H460 cells after
transfection with si-TAB1 or si-NC. The knockdown of TAB1 inhibited
the proliferation (P<0.05; Fig.
4B) and invasiveness (P<0.05; Fig. 4C) of H522 and H460 cells. These
observations confirmed that TAB1 inhibition may imitate the
tumor-suppressive roles of miR-889 mimics in NSCLC cells, further
supporting the notion that TAB1 mRNA is a target of miR-889 in
NSCLC cells.
TAB1 reintroduction counteracts the
antitumor actions of miR-889 in NSCLC cells
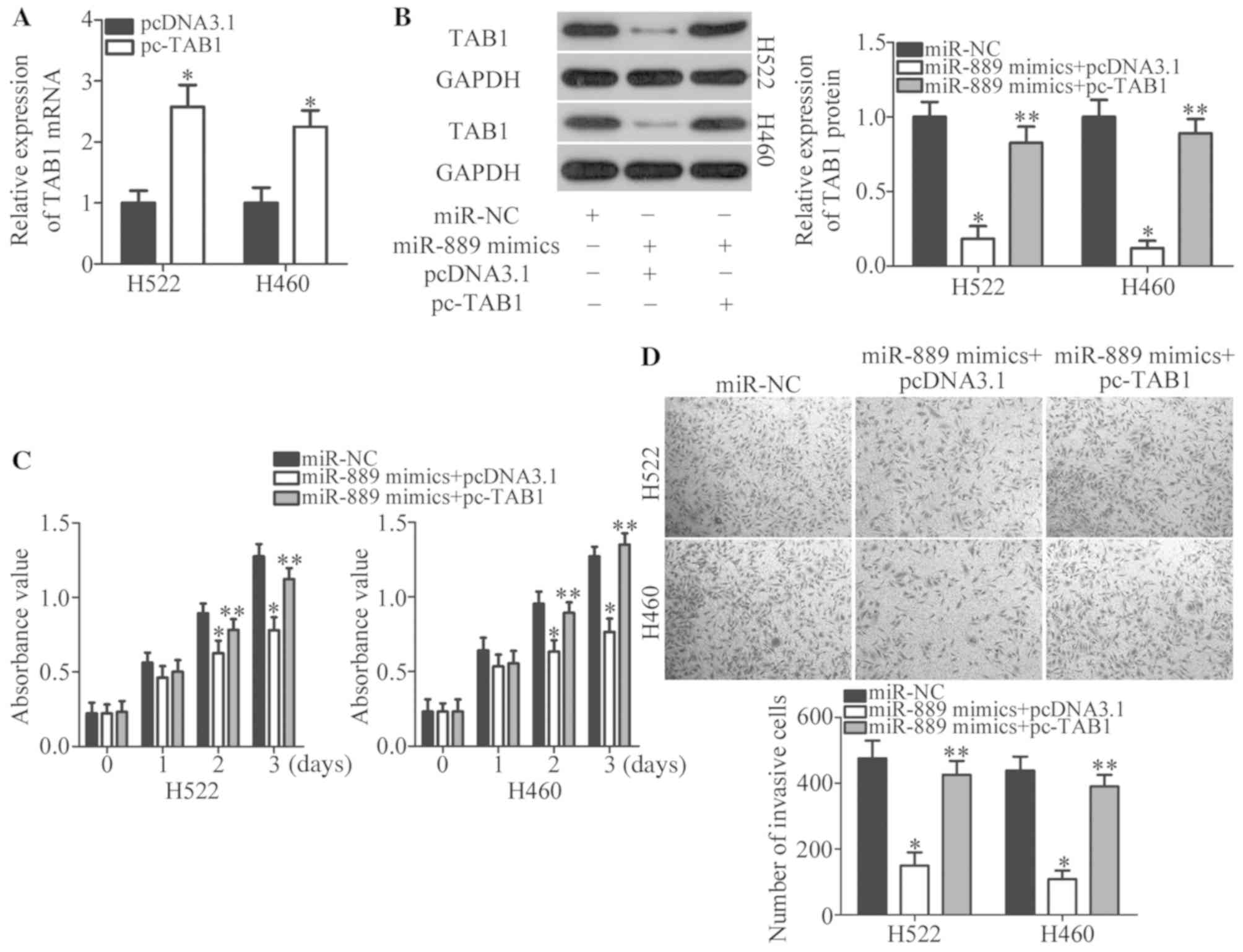
To confirm that TAB1 is involved in the
miR-889-induced tumor-suppressive effects in NSCLC cells, rescue
experiments were conducted next by restoring TAB1 expression in
miR-889 mimic-transfected H522 and H460 cells. Firstly, pcDNA3.1 or
pc-TAB1 was introduced into H522 and H460 cells. RT-qPCR analysis
confirmed that the expression level of TAB1 mRNA was increased in
pc-TAB1-transfected H522 and H460 cells (P<0.05; Fig. 5A). Western blotting indicated that
co-transfection with TAB1 expression vector pcDNA3.1-TAB1 (pc-TAB1)
notably recovered the TAB1 protein amount that was significantly
decreased by miR-889 mimics (P<0.05; Fig. 5B). Functional experiments revealed
that the reintroduction of TAB1 reversed the anti-proliferative
(P<0.05; Fig. 5C) and
anti-invasive (P<0.05; Fig. 5D)
effects of miR-889 upregulation in H522 and H460 cells. Overall,
these results indicated that miR-889 exerts its anticancer actions
on NSCLC at least partly by directly suppressing TAB1
expression.
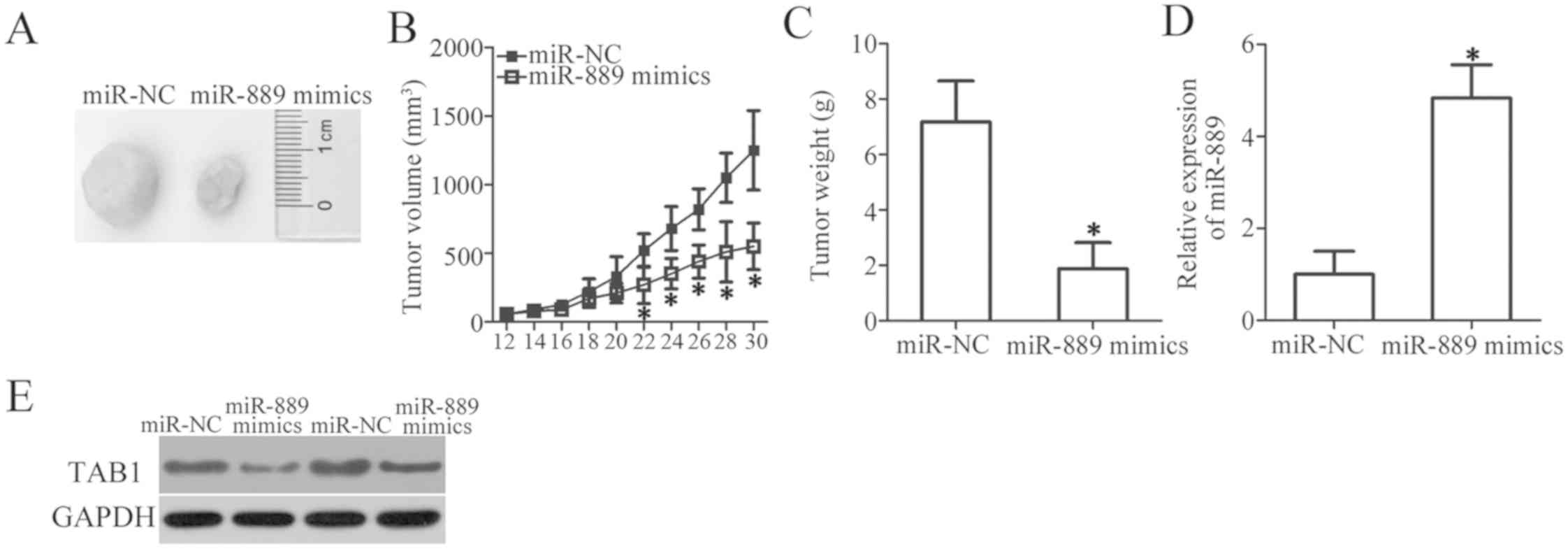
miR-889 inhibits NSCLC tumor growth in
vivo
A xenograft experiment was next conducted to examine
the influence of miR-889 overexpression on in vivo tumor
growth of NSCLC cells. H460 cells transfected with miR-889 mimics
or miR-NC were subcutaneously injected into the flanks of nude
mice. The tumor volume was measured for 1 month. Upregulation of
miR-889 significantly decreased the tumor growth in vivo
(P<0.05; Fig. 6A and B). At the
end of the experiment, all the nude mice were euthanized, and the
excised xenografts were weighed. The results indicated that the
xenograft tumors derived from miR-889-overexpressing H460 cells had
lower weight than did the xenograft tumors in the miR-NC group
(P<0.05; Fig. 6C). Then,
miR-889 expression was quantitated in the excised xenograft tumors
and it was revealed that miR-889 was still overexpressed in the
xenograft tumors of the ‘miR-889 mimics’ group (P<0.05; Fig. 6D). Furthermore, the protein amount
of TAB1 in the xenograft tumors was significantly decreased by
miR-889 overexpression, as revealed by western blot analysis
(Fig. 6E). These observations
indicated that miR-889 upregulation inhibited the tumor growth of
NSCLC cells in vivo by suppressing TAB1 expression.
Discussion
miRNAs are frequently reported to be aberrantly
expressed in NSCLC and closely related with aggressive phenotypes
(21). Accumulating evidence has
validated miRNAs as potential biomarkers for the diagnosis and
prognosis as well as therapeutic targets for patients with NSCLC
(15,22,23).
Hence, identification of cancer-associated miRNAs in NSCLC may be
helpful for improving the curative effects. In the present study,
for the first time, miR-889 expression was detected and its
clinical value in NSCLC was determined. Furthermore, the functional
role and underlying mechanism of miR-889 in NSCLC was investigated.
The results from this study indicated that miR-889 may serve as a
tumor-suppressive miRNA in NSCLC in vitro and in vivo
by directly targeting TAB1.
miR-889 has been revealed to be involved in tumor
development and progression of esophageal squamous cell carcinoma
(17) and hepatocellular carcinoma
(18). miR-889 was revealed to be
expressed at high levels in esophageal squamous cell carcinoma
tissues and cell lines. miR-889 restoration promoted the
proliferation of esophageal squamous cell carcinoma cells in
vitro and in vivo (17). In hepatocellular carcinoma,
expression of miR-889 was negatively regulated by histone
deacetylase inhibitors. Functionally, ectopic miR-889 expression
reduced the susceptibility of hepatocellular carcinoma cells to
natural killer (NK) lysis. However, the expression pattern and
functions of miR-889 in NSCLC are still unknown. In the study,
miR-889 expression was revealed to be decreased in NSCLC tissues
and cell lines. Low miR-889 expression was notably correlated with
the TNM stage and distant metastasis of NSCLC patients.
Overexpression of miR-889 suppressed NSCLC cell proliferation and
invasion in vitro as well as inhibited tumor growth in
vivo. These findings indicated that miR-889 may be an effective
therapeutic target for patients with the aforementioned human
cancer types.
Two genes, DOC-2/DAB2 interactive protein (17) and major histocompatibility complex
class I chain-related gene B (18), have been previously identified as
the direct target genes of miR-889. In the present study, TAB1 was
demonstrated to be a direct and functional downstream target of
miR-889 in NSCLC cells. Increasing evidence has revealed that TAB1
is upregulated in a variety of human cancers, including colorectal
(24), breast (25) and ovarian cancer (26). Upregulated TAB1 expression may be
closely associated with tumorigenesis and tumor development through
regulation of various aggressive behaviors (24,26,27).
In NSCLC, TAB1 was upregulated in tumor tissues, and upregulation
of TAB1 was correlated with clinical stage and lymph node
metastasis (20). Patients with
NSCLC and higher TAB1 expression exhibited a significantly lower
5-year survival rate than those patients with lower TAB1 expression
(20). However, the functional
roles of TAB1 in NSCLC remain largely to be elucidated. Herein, we
revealed that TAB1 silencing inhibited the proliferative and
invasive abilities of NSCLC cells in vitro. miR-889 was
capable of directly targeting TAB1 and inhibiting the malignant
progression of NSCLC cells in vitro and in vivo.
Therefore, TAB1 knockdown via miR-889 reintroduction may be a
suitable therapeutic technique for the management of patients with
NSCLC.
In summary, the significant downregulation of
miR-889 was observed in NSCLC and it was demonstrated that miR-889
expression was associated with TNM stage and distant metastasis. It
was further demonstrated that miR-889 suppressed NSCLC cell
proliferation, invasion in vitro and decreased tumor growth
in vivo, at least partly, by directly targeting TAB1. The
present findings indicate that miR-889 may function as a tumor
suppressor miRNA in NSCLC and holds promise as a potential
diagnosis biomarker and therapeutic target for patients with this
fatal disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors significantly contributed to the
findings and methods. XW designed this research and analyzed the
data. ZD and BL performed all functional experiments. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang People's Hospital (Weifang, China), and was
performed in accordance with the Declaration of Helsinki and the
guidelines of the Ethics Committee of Weifang People's Hospital.
Written informed consent was also provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qin H, Wang F, Liu H, Zeng Z, Wang S, Pan
X and Gao H: New advances in immunotherapy for non-small cell lung
cancer. Am J Transl Res. 10:2234–2245. 2018.PubMed/NCBI
|
|
4
|
Li Z, Song Y, Liu L, Hou N, An X, Zhan D,
Li Y, Zhou L, Li P, Yu L, et al: miR-199a impairs autophagy and
induces cardiac hypertrophy through mTOR activation. Cell Death
Differ. 24:1205–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao M, Wu Z and Chen J: MicroRNA-187-5p
suppresses cancer cell progression in non-small cell lung cancer
(NSCLC) through down-regulation of CYP1B1. Biochem Biophys Res
Commun. 478:649–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I; Eurocare-4 Working
Group, : Recent cancer survival in Europe: A 2000-02 period
analysis of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z and Rana TM: Therapeutic targeting of
microRNAs: Current status and future challenges. Nat Rev Drug
Discov. 13:622–638. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Behm-Ansmant I, Rehwinkel J and Izaurralde
E: MicroRNAs silence gene expression by repressing protein
expression and/or by promoting mRNA decay. Cold Spring Harb Symp
Quant Biol. 71:523–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Florczuk M, Szpechcinski A and
Chorostowska-Wynimko J: miRNAs as biomarkers and therapeutic
targets in non-small cell lung cancer: Current perspectives. Target
Oncol. 12:179–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu BB, Gu ZF, Ma M, Wang JY and Wang HN:
MicroRNA-590-5p suppresses the proliferation and invasion of
non-small cell lung cancer by regulating GAB1. Eur Rev Med
Pharmacol Sci. 22:5954–5963. 2018.PubMed/NCBI
|
|
12
|
Boldrini L, Giordano M, Lucchi M, Melfi F
and Fontanini G: Expression profiling and microRNA regulation of
the LKB1 pathway in young and aged lung adenocarcinoma patients.
Biomed Rep. 9:198–205. 2018.PubMed/NCBI
|
|
13
|
Pan Q, Sun L, Zheng D, Li N, Shi H, Song
J, Shao G and Xu G: MicroRNA-9 enhanced cisplatin sensitivity in
nonsmall cell lung cancer cells by regulating eukaryotic
translation initiation factor 5A2. Biomed Res Int.
2018:17690402018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi YJ, Zha WJ and Zhang W: MicroRNA-217
alleviates development of non-small cell lung cancer by inhibiting
AKT3 via PI3K pathway. Eur Rev Med Pharmacol Sci. 22:5972–5979.
2018.PubMed/NCBI
|
|
15
|
Lu J, Zhan Y, Feng J, Luo J and Fan S:
MicroRNAs associated with therapy of non-small cell lung cancer.
Int J Biol Sci. 14:390–397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fadejeva I, Olschewski H and Hrzenjak A:
MicroRNAs as regulators of cisplatin-resistance in non-small cell
lung carcinomas. Oncotarget. 8:115754–115773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, He J, Wang Y, Zhu X, Pan Q, Xie Q
and Sun F: miR-889 promotes proliferation of esophageal squamous
cell carcinomas through DAB2IP. FEBS Lett. 589:1127–1135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie H, Zhang Q, Zhou H, Zhou J, Zhang J,
Jiang Y, Wang J, Meng X, Zeng L and Jiang X: microRNA-889 is
downregulated by histone deacetylase inhibitors and confers
resistance to natural killer cytotoxicity in hepatocellular
carcinoma cells. Cytotechnology. 70:513–521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu J, Li Q, He JT and Liu GY: Expression
of TAK1/TAB1 expression in non-small cell lung carcinoma and
adjacent normal tissues and their clinical significance. Int J Clin
Exp Pathol. 8:15801–15807. 2015.PubMed/NCBI
|
|
21
|
Zhou Q, Huang SX, Zhang F, Li SJ, Liu C,
Xi YY, Wang L, Wang X, He QQ, Sun CC and Li DJ: MicroRNAs: A novel
potential biomarker for diagnosis and therapy in patients with
non-small cell lung cancer. Cell Prolif. 50:2017. View Article : Google Scholar :
|
|
22
|
Han Y and Li H: miRNAs as biomarkers and
for the early detection of non-small cell lung cancer (NSCLC). J
Thorac Dis. 10:3119–3131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Sun Y, Feng M, Wang L and Liu J:
Clinical significance of blood-based miRNAs as biomarkers of
non-small cell lung cancer. Oncol Lett. 15:8915–8925.
2018.PubMed/NCBI
|
|
24
|
Gong H, Fang L, Li Y, Du J, Zhou B, Wang
X, Zhou H, Gao L, Wang K and Zhang J: miR873 inhibits colorectal
cancer cell proliferation by targeting TRAF5 and TAB1. Oncol Rep.
39:1090–1098. 2018.PubMed/NCBI
|
|
25
|
Neil JR and Schiemann WP: Altered TAB1:I
kappaB kinase interaction promotes transforming growth factor
beta-mediated nuclear factor-kappaB activation during breast cancer
progression. Cancer Res. 68:1462–1470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shuang T, Wang M, Zhou Y, Shi C and Wang
D: NF-kappaB1, c-Rel, and ELK1 inhibit miR-134 expression leading
to TAB1 upregulation in paclitaxel-resistant human ovarian cancer.
Oncotarget. 8:24853–24868. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Isono T, Kim CJ, Ando Y, Sakurai H, Okada
Y and Inoue H: Suppression of cell invasiveness by periostin via
TAB1/TAK1. Int J Oncol. 35:425–432. 2009.PubMed/NCBI
|