Introduction
Tissue engineering is a multidisciplinary
biotechnological science, the aim of which is to produce in
vitro (and then possibly in vivo) tissues resembling at
best the original tissues, to be used for the therapeutic
replacement/regeneration of damaged tissues, and as models for
functional and toxicological in vitro studies.
In the attempt to construct suitable tissue models,
a critical step is the setting of 3D-scaffolds that simulate the
extracellular matrix (ECM) into which cells are normally embedded.
Importantly, cell behavior is controlled by signals originating
from the ECM, but the cells themselves are also the producers of
the ECM. Therefore a suitable scaffold is expected to be
metabolized by cells and is gradually substituted with ECM
molecules that can then be synthesized and secreted.
In this context, the generation of 3D cultures of
brain cells is of particular interest. Cells of the nervous system
(including neurons, astrocytes, oligodendrocytes, pericytes,
microglial cells and endothelial cells) form a highly complex
system in vivo in which cells continuously exchange
information in many ways, including through extracellular vesicles
(EVs). EVs are comprised of different types of membrane structures,
the primary types include: i) Ectosomes (or microvesicles) that bud
directly from the plasma membrane; and ii) exosomes, which
originate from the endosomal compartment via the exocytosis of
multivesicular bodies (MVBs) (1).
Given the difficulties encountered in discriminating the different
classes of vesicles (2), they are
often collectively called EVs. EVs contain proteins, nucleic acids,
lipids and metabolites, and many of which are clearly enriched in
EVs when compared with the producing cells, thereby suggesting the
existence of specific sorting mechanisms (3). By releasing their contents to the ECM
or directly into the surrounding cells, EVs have the potential to
affect the structure and function of the brain, both in
physiological and pathological conditions (4–7). EVs
can also be involved in the distribution of pathological proteins,
such as the prion protein (8),
amyloid β peptide (9) and the
hyperphosphorylated tau protein (10).
In addition to their role in cell-to-cell
communication (11), EVs are also
present in biological fluids such as blood, saliva and urine, and
it has been suggested that they and their contents could be used as
biomarkers of specific pathologies (12).
We previously reported that both neurons (13) and astrocytes (14) are able to release EVs in 2D culture
systems. However, in 2D-cultures the topology of the cell membranes
could be different than in vivo. This could also mean a
different distribution of membrane lipids and lipid-metabolizing
enzymes. Given the suggested importance of these latter molecules
in the specific sorting of molecules to EVs and in EV formation
itself, it was reasoned that a 3D model could be more suitable to
study these processes.
Previous studies have revealed that it is possible
to carry out 3D astrocyte culture on substrates as different as
hydrogel (15,16), collagen (Coll) (17–19)
and polymeric scaffolds (20,21).
For example, Hyysalo et al (22) demonstrated that astrocytes are able
to grow on aligned poly(ε-caprolactone) nanofibers, spreading over
them.
However, in all of the reported cases, the effect of
the morphology of the adopted devices on cellular growth is still
unclear. On the other hand, it is well known that the morphology of
the scaffold markedly influences cell functions as well as tissue
regeneration, which are both dependent on the size of the pores, as
demonstrated in other cell types such as endothelial cells and
chondrocytes, cultured on poly-L-lactic acid (PLLA) (23,24).
For these reasons, it is mandatory to rely on procedures that allow
for the precise and reproducible control of the 3D support
morphology, and, above all, of the pore size and porosity.
Among the possible techniques available to produce
porous scaffolds, thermally-induced phase separation (TIPS) is
probably one of the most versatile. This technique is based on the
separation of the homogeneous polymer solution induced by a
variation in temperature. Through TIPS, a porous structure with a
high degree of interconnection can be obtained (25,26).
Furthermore, using a targeted temperature instead of time protocols
it is possible to cover a wide range of pore dimensions (from 10 to
200 µm) (27,28).
Therefore, 3D-monocultures of brain capillary
endothelial cells (BCECs), as previously reported (29), and astrocytes, which were analyzed
in the present study, were tested on PLLA scaffolds with the aim to
set a 3D-model to be enriched over time by co-culturing more than
one brain cell type, in order to study in detail intercellular
communications, especially those based on EVs. Specifically in the
present in vitro study, PLLA scaffolds produced via TIPS
were utilized as substrates for primary rat astrocyte 3D growth.
Different scaffold morphologies and coatings were tested in order
to evaluate their influence on astrocyte growth, morphology and EV
production.
Materials and methods
Scaffold preparation and
characterization
All of the porous devices employed for the 3D growth
of neural cells are characterized by a very small average pore size
(<30–40 microns) (30–32). In order to achieve scaffolds with a
low average pore size, the present study slightly modified the
protocol described in a previous study (28). Specifically, experiments were
performed at a demixing temperature of 0°C in order to ensure that
a spinodal decomposition mechanism occurred (33). Two ternary solutions composed of
PLLA Resomer® L 209 S (Evonik Industries), 1,4 dioxane
(Sigma-Aldrich; Merck KGaA) and distilled water were prepared, with
the same dioxane to water weight ratio of 87/13 wt/wt and different
polymer concentrations (4 and 6% wt/wt, respectively). Briefly, the
solution was kept at 60°C and subsequently poured into a
cylindrical high density polyethylene mold. Then the mold was
sealed and placed in a thermal bath at 0°C for 10 min. Quenching
was conducted via immersion in an ethyl alcohol bath at a
temperature of −20°C for 15 min in order to freeze the
structure.
The obtained samples were removed from the mold and
washed in deionized water for 24 h to eliminate the residual
dioxane. Then, the samples were dried in a vacuum at 30°C for 24 h.
Finally, smaller samples (diameter 5 mm, thickness 1 mm) were
obtained by cutting the dried samples with a surgical blade.
Scaffold pore size and topography were analyzed via scanning
electron microscopy (SEM; Phenom Pro X, Phenom-World; Thermo Fisher
Scientific, Inc.) at 5 kV along the cross-section. Astrocytes were
seeded on both types of scaffold and, at well-defined culture
times, cell proliferation tests were conducted.
Cells and cell culture
The present study did not use animals as the
astrocytes used had already been isolated from the brain cortices
of 2-day old Wistar newborn rats (Harlan, Udine, Italy), and frozen
in a solution containing 93% heat-inactivated fetal calf serum
(FCS) and 7% dimethyl-sulfoxide (both from Sigma-Aldrich; Merck
KGaA), as previously described (34).
Astrocytes were thawed and cultured in DMEM/Ham's
F-12 (2/1), supplemented with 10% heat-inactivated FCS
(Sigma-Aldrich; Merck KGaA), and 100,000 units penicillin, 100 mg
streptomycin, and 250 µg amphotericin B (Sigma-Aldrich; Merck KGaA)
per liter. The cells were then maintained in humidified 5%
CO2/95% air, at 37°C. To assess astrocyte purity, cells
were cultured on coverslips, fixed with 96% ethanol on ice for 10
min and permeabilized for 5 min with 0.1% Triton X-100 in PBS.
Cells were then incubated with polyclonal rabbit anti-glial
fibrillary acidic protein (GFAP) antibodies (Sigma-Aldrich; Merck
KGaA; cat. no. G9269; used at 1:200 dilution).
Subconfluent cultures were plated on scaffolds
(100.000/scaffold; 5,000 cells/µl of medium), previously sterilized
with 70% ethanol in a vacuum for 24 h and pretreated for 1 h with
Coll type I (Coll I; final concentration 1 µg/µl; Sigma-Aldrich;
Merck KGaA) in 0.01 M CH3COOH, or Coll type IV (Coll IV;
Sigma-Aldrich; Merck KGaA; 31 µg/µl) in 0.05 N HCl, or with
fibronectin (0.05 µg/ml) in Ham's F-12.
After incubation at 37°C and 5% CO2 for
90 min to promote cell adhesion (35–37),
each scaffold was transferred to a well of a 24-well plate and
fresh medium was added.
Immunofluorescence
Cells were fixed in 96% ethanol, and immunostained
with rabbit anti-GFAP antibodies (Sigma-Aldrich; Merck KGaA; cat.
no. G9269; used at 1:200 dilution). The secondary antibody was
rhodamine-isothiocyanate-conjugated anti-rabbit-IgG (Sigma-Aldrich;
Merck KGaA; cat. no. T6778; used at a 1:100 dilution). Nuclei were
stained with 4′,6-diamidino-2-phenylindole dihydrochloride (H1200;
Vector Laboratories, Inc.). Cells were finally observed under a
fluorescence microscope (Olympus BX-50).
Cell proliferation assay
Cell proliferation on scaffolds was evaluated via
Cell Counting Kit-8 (CCK8; Sigma-Aldrich; Merck KGaA); a sensitive
colorimetric kit containing WST-8, a salt reduced by mitochondrial
dehydrogenases to orange formazan, used to evaluate the absorbance
at 450 nm. At each time-point, scaffolds were transferred into
other wells and each sample was incubated at 37°C and 5%
CO2 for 3 h with 500 µl of the 1:10 diluted reagent in
fresh medium. After incubation, the medium was collected and
analyzed with a spectrophotometer at an absorbance of 450 nm. The
assays were carried out in triplicate for each time-point.
Non-seeded scaffolds were used as the negative controls in each
measurement.
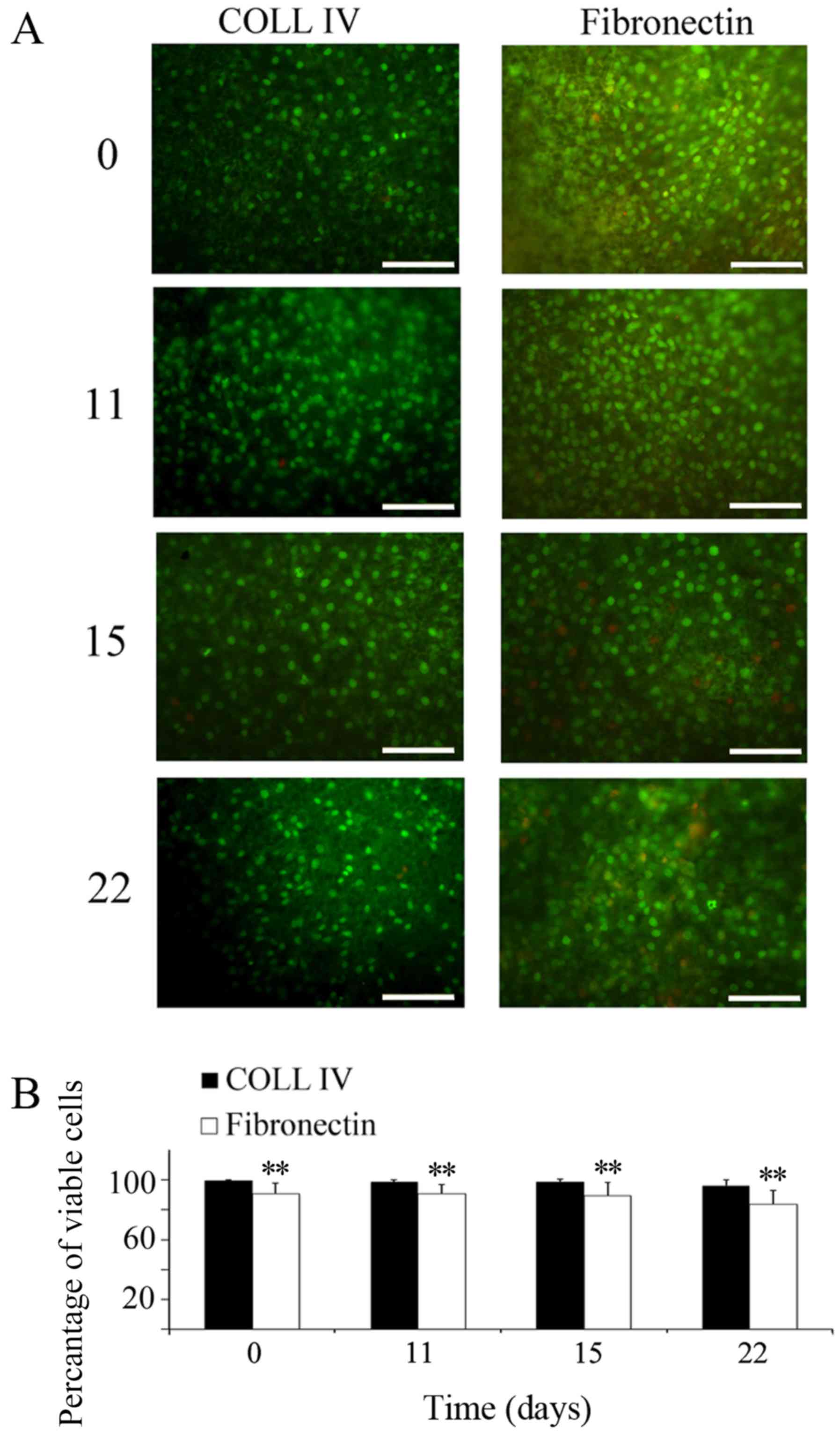
Viability assay
The general condition of cells was evaluated by
acridine orange/ethidium bromide (AO/EtBr) staining, using
fluorescence microscopy (Olympus BX-50), in order to identify
apoptotic and/or necrotic cells. Cells were washed with PBS and
stained with AO/EtBr solution (concentration of each compound: 100
µg/ml in PBS). Counting of viable cells was performed after
dividing each picture into quarters. Cells in each quarter were
counted by two different operators. Finally, the values were used
to calculate a mean value.
Transmission electron microscopy
(TEM)
For ultrastructure evaluation with a TE microscope,
cells plated on scaffolds were placed for 1 h at 4°C in 4%
glutaraldehyde buffered with 0.05 M sodium cacodylate (pH 7.3),
followed by post-fixation in 1% osmium tetroxide. Subsequently,
after rinsing, all specimens were progressively dehydrated in
ethanol solutions, cleared in propylene oxide and embedded in Epon
resin. Thin sections were stained and examined in a Jeoll 1400 TE
microscope operated at 80 kV.
SEM
Seeded scaffolds were fixed in 4% glutaraldehyde for
30 min at 4°C, rinsed in PBS and finally dehydrated through a
graded series of ethanol (15, 30, 50, 70, 90 and 100%, for 3 min
each). After complete dehydration, the scaffolds were mounted on an
aluminum stub, coated in gold and observed using SEM (Phenom Pro X,
Phenom-World; Thermo Fisher Scientific, Inc.) at an accelerating
voltage of 10 kV.
Statistical analysis
All of the experiments were repeated three times for
each time-point. Data were expressed as the mean ± standard
deviation. Statistical analyses were conducted using GraphPad Prism
software version 8.0 (GraphPad Software, Inc.). Multiple
comparisons were assessed by one way analysis of variance with post
hoc Bonferroni multiple analysis and for comparisons between two
groups data were analyzed by Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
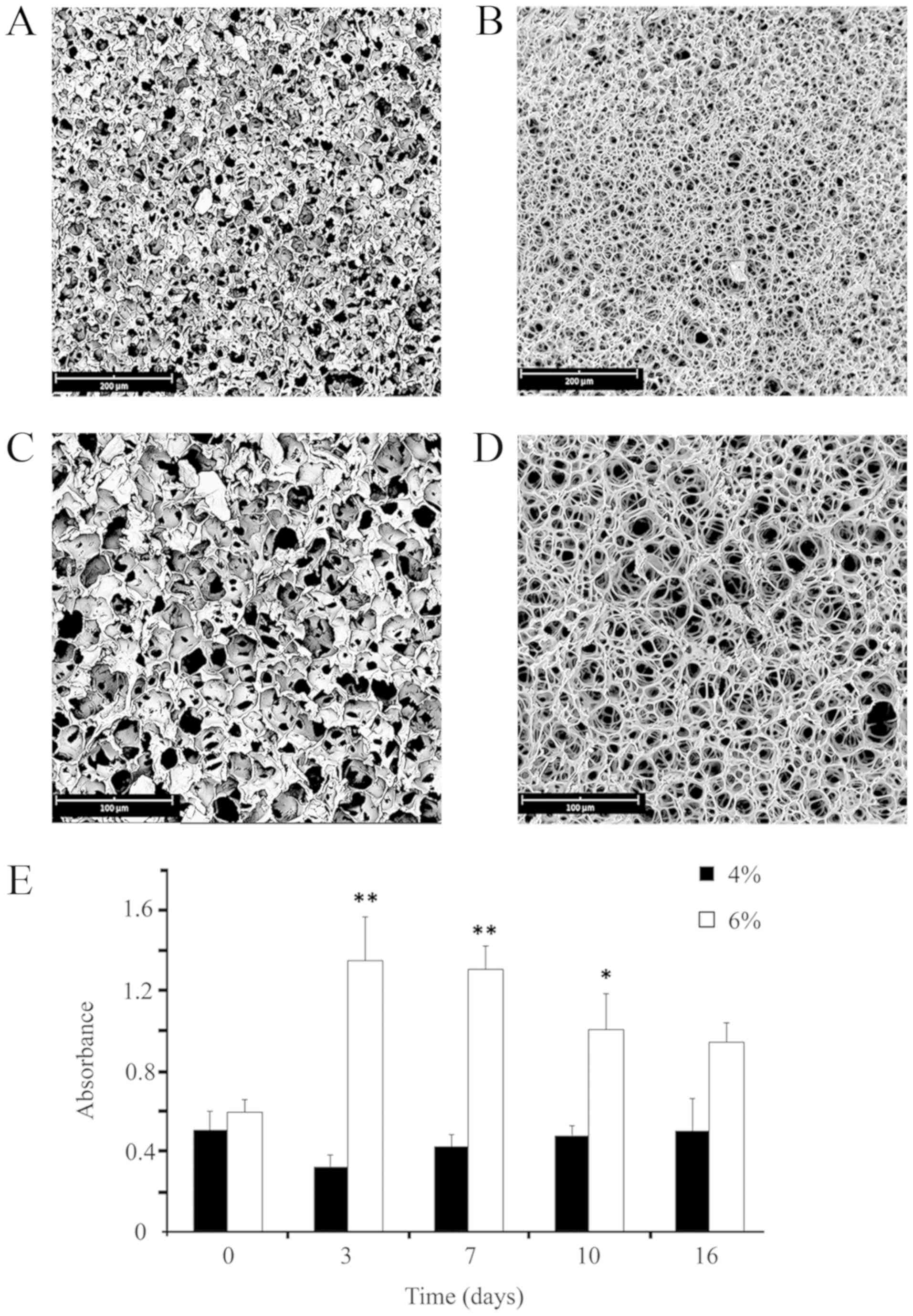
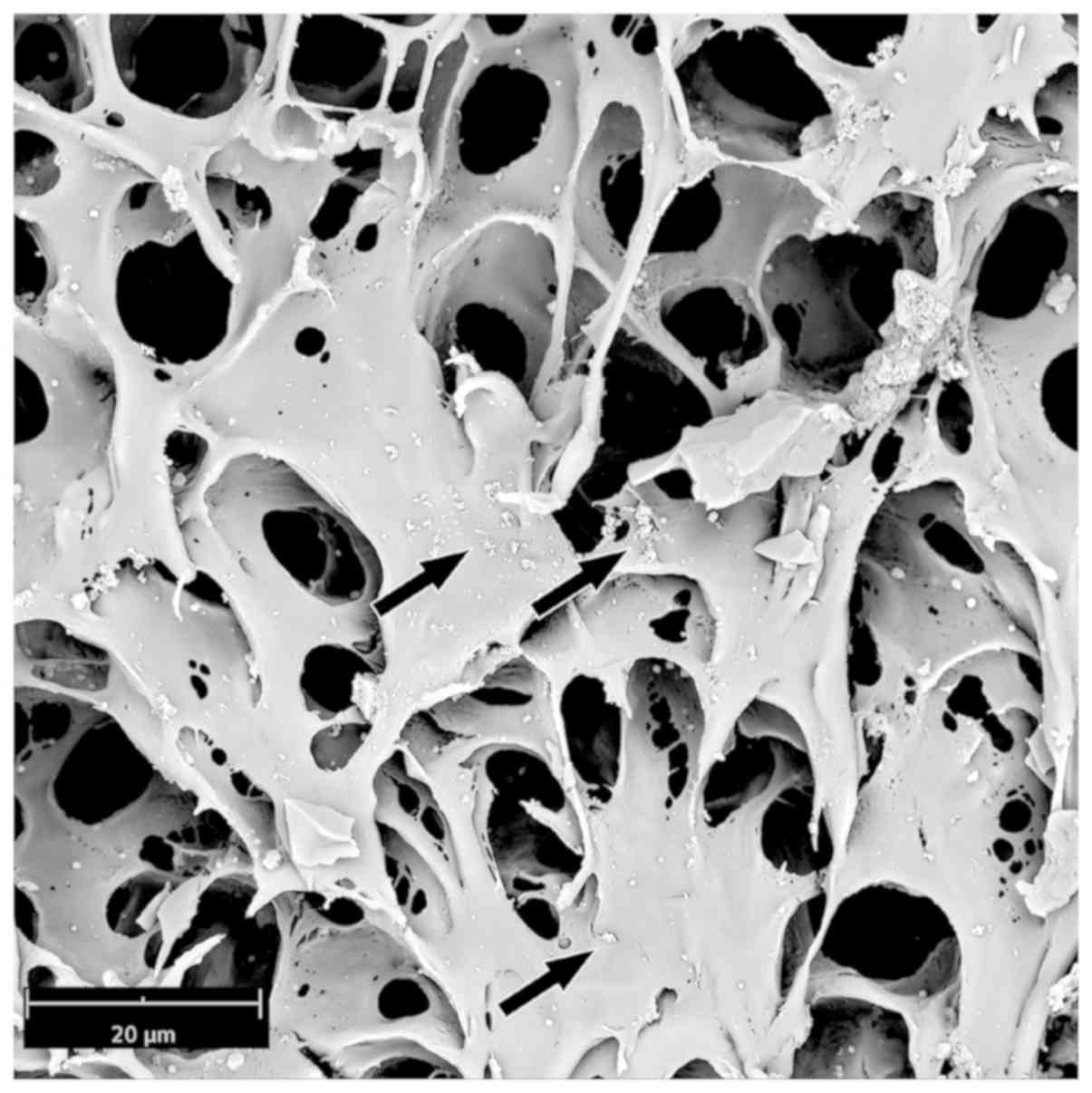
Fig. 1 presents the
morphologies of the resulting scaffolds. Although in both cases
porous structures were obtained, evident differences in terms of
morphology were detected between the two types of samples. As
expected, both samples presented a very low average pore size, but
a higher level of pore interconnection was observed in the 6%
samples when compared with the 4% group. At a higher magnification
(Fig. 1C and D), it is clearly
visible that the pore walls of the 6% scaffolds are considerably
thinner and, consequently, had a more open structure. On the other
hand, when observing the pore morphology of the 4% samples, it
would seem that nucleation and growth mechanism have occurred.
Fig. 1E presents
the absorbance values of astrocytes grown on the two different
types of scaffolds at the different time-points analyzed. When
considering the whole period (except for T0), the number of cells
on the 6% scaffold was higher when compared with the 4% scaffold.
Based on this experimental evidence, the present study utilized the
6% scaffolds for the subsequent studies and analyses.
A preliminary analysis was also conducted to
evaluate which substrate was the most suitable for the assessment
of the adhesion and growth of astrocytes on the scaffolds. The low
concentration of astrocytes and their non-homogeneous distribution
on the scaffolds pretreated with Coll I (data not shown), together
with the fact that the ECM of the nervous tissue is rich in Coll IV
and Fibronectin (FN), led to the selection of the latter substrates
for use in the subsequent experiments.
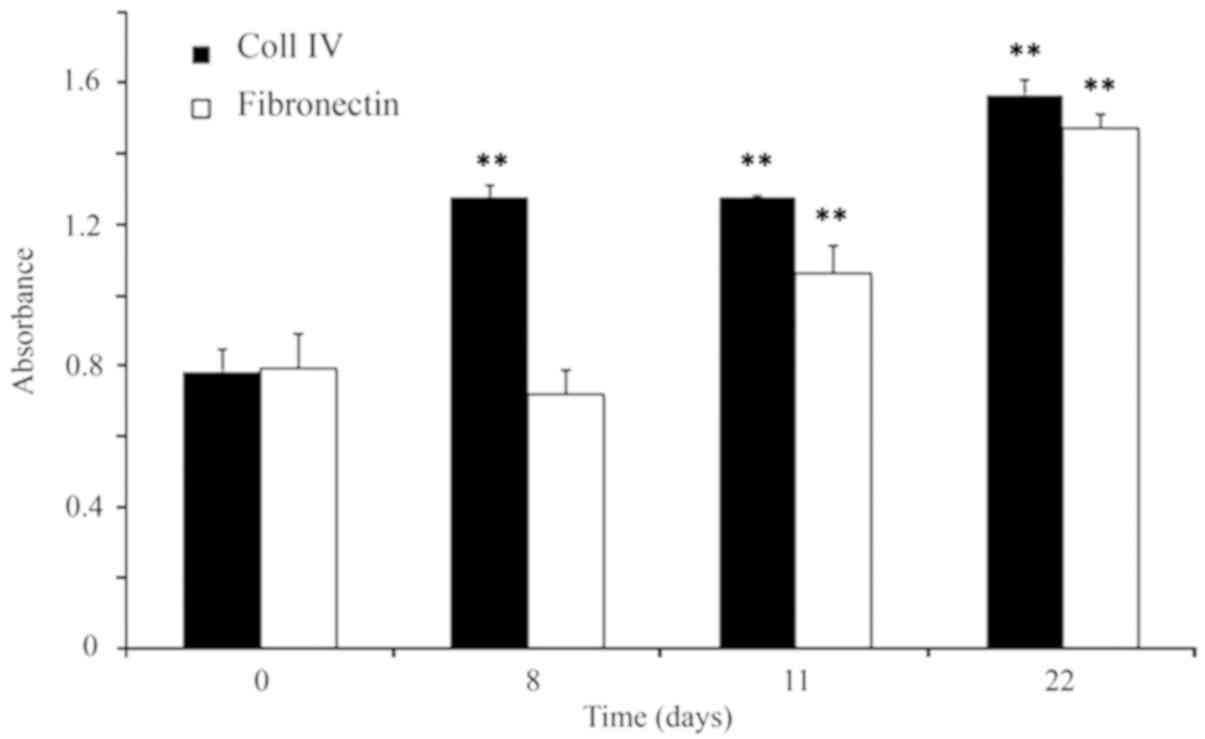
To verify whether the scaffolds coated with Coll IV
or FN provided adequate support to the cells, the present study
evaluated the cell proliferation by CCK8 assay (Fig. 2) and cell viability by AO/EtBr
staining (Fig. 3). The analysis of
the growth curve evidenced that astrocytes adapted better on Coll
IV than on FN. Images taken at various time-points revealed that
the cells were viable over a time interval of 22 days in culture
(Fig. 3). Furthermore, a
preference for Coll IV, compared to FN, was also visible; cell
viability on Coll IV was higher at all of the time-points (for
example: Coll IV 96±4%; FN 83±9%, at 22 days of culture), and the
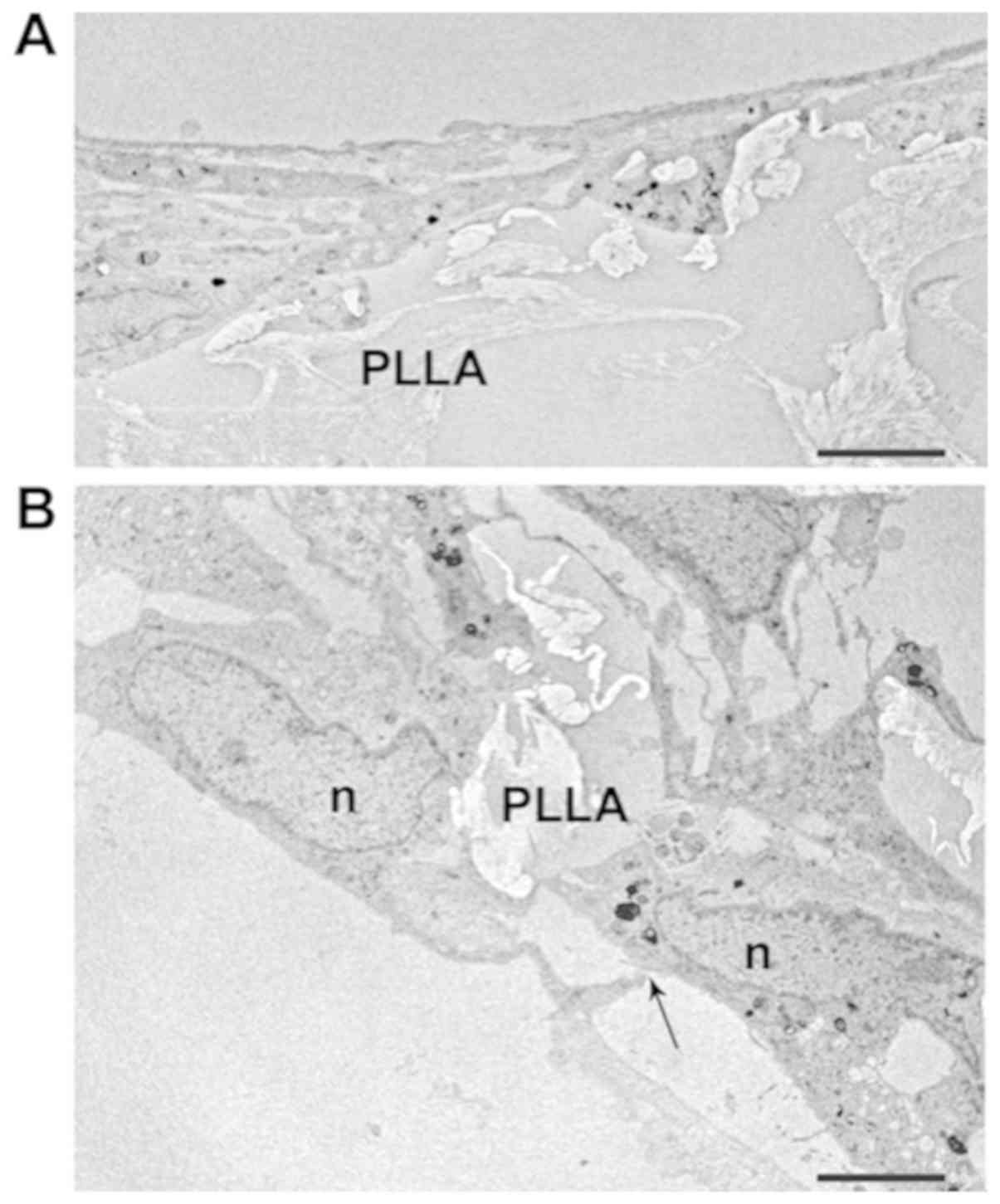
differences were significant (P<0.01). Astrocyte morphology on
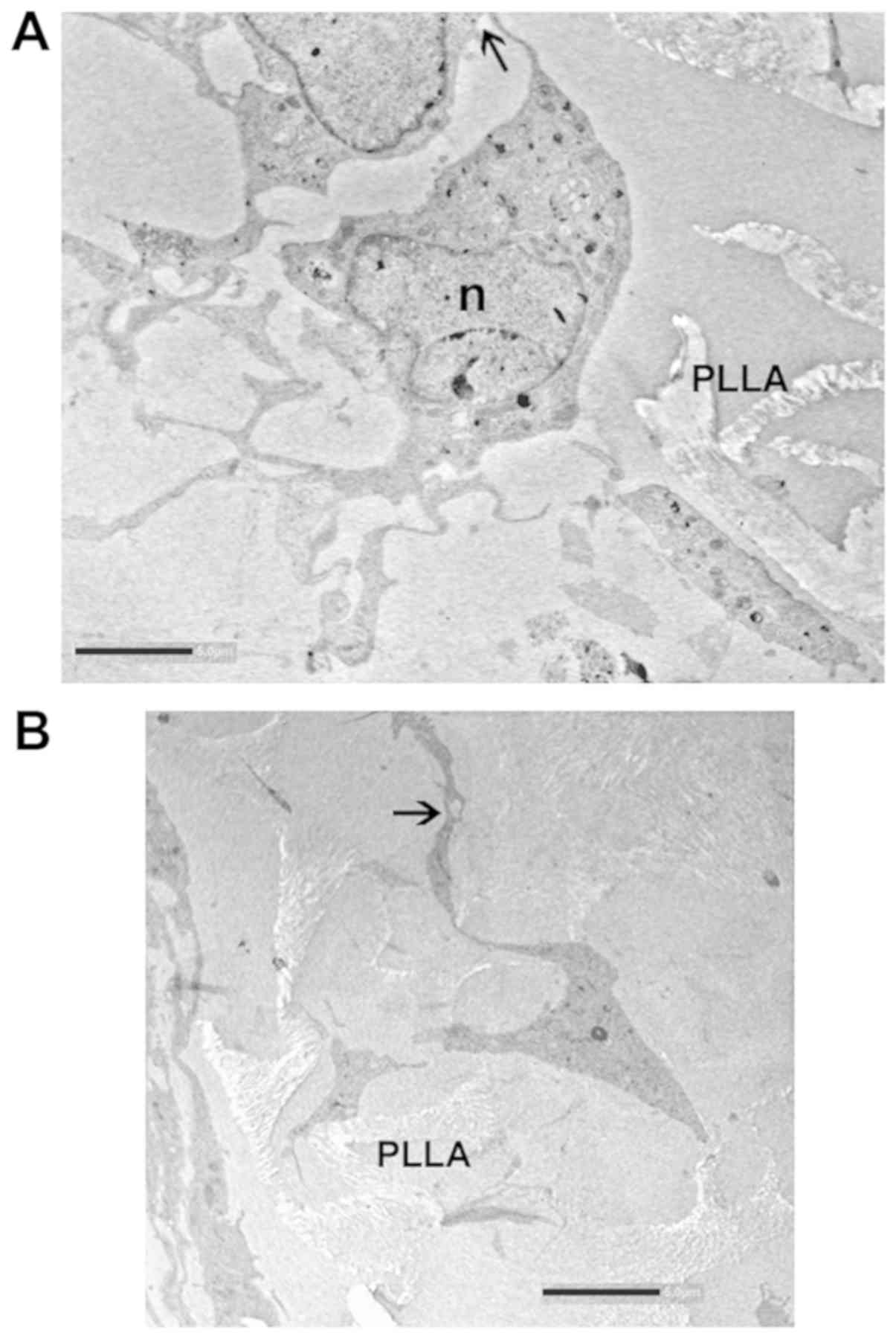
the PLLA scaffolds was also visualized by TEM. The images obtained
confirmed that the cells colonized inside the porous matrices
(Fig. 4A), although those that
remained on the surface were also abundant (Fig. 4B).
Electron microscopy images provided further evidence
of cell attachment onto the scaffold. Cells appeared well
differentiated with their typical elongated shape and long
processes (Figs. 4B and 5A). The cell body contained an
irregularly round or oval nucleus, and the cytoplasm around the
nucleus was abundant. The nucleoplasm of astrocytes was finely
granular and of moderate density. It was evenly distributed through
the nucleus, except for at the edge of the nuclear profile where it
aggregated into clumps just under the nuclear membrane (Fig. 4B).
In the TEM micrographs, migration into the scaffold
was easily detectable. Astrocytes can be seen throughout the
homogenously distributed pores between polymer fibers. The
interaction between the cells and scaffold as well as the star-like
shape of the cells are visible in Fig.
5. At the same time, astrocytes formed intercellular junctions,
including contacts between cell processes from distal astrocytes
(arrows in Figs. 4 and 5).
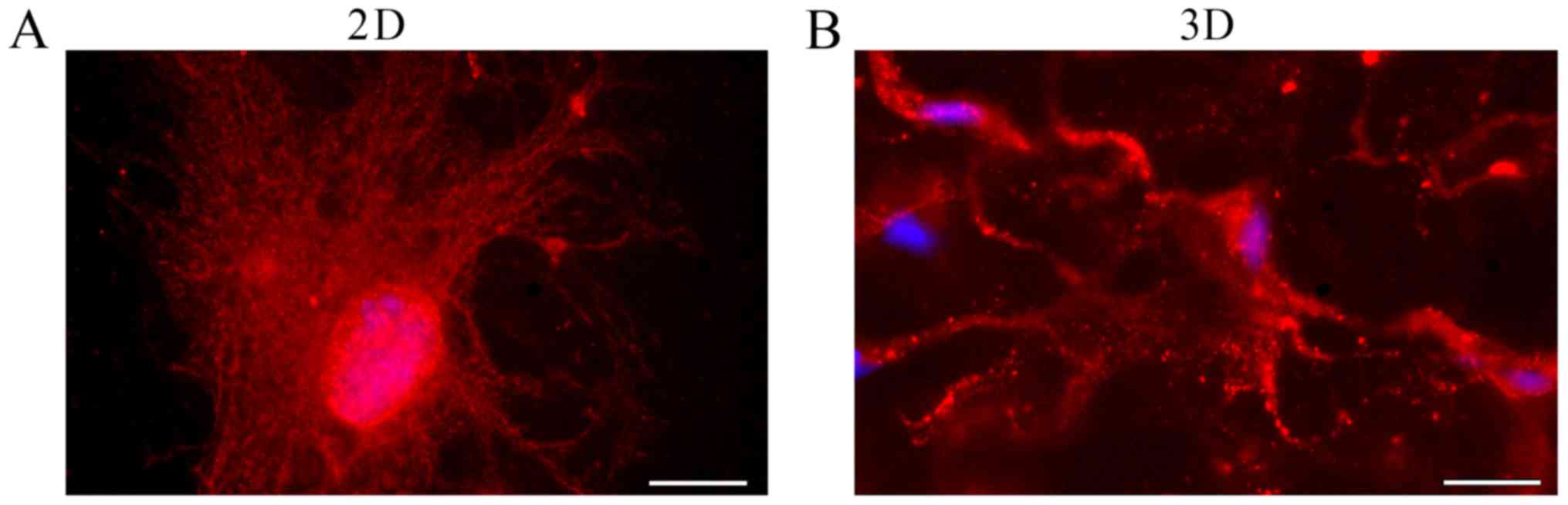
Astrocyte morphology was also analyzed by staining
the cells with antibodies against GFAP, an astrocyte-specific
member of the intermediate filament family of proteins (Fig. 6). After 20 days of culture, the
astrocytes began to assume their classic ‘starry’ morphology, with
longer and thinner extensions, as it normally occurs in vivo
(Fig. 6B). This phenotype is
especially evident if the cells are kept in culture for a
sufficiently long period of time (20–25 days). Interestingly, when
grown in 2D, astrocytes maintain a more flattened shape, less
similar to the in vivo shape (Fig. 6A).
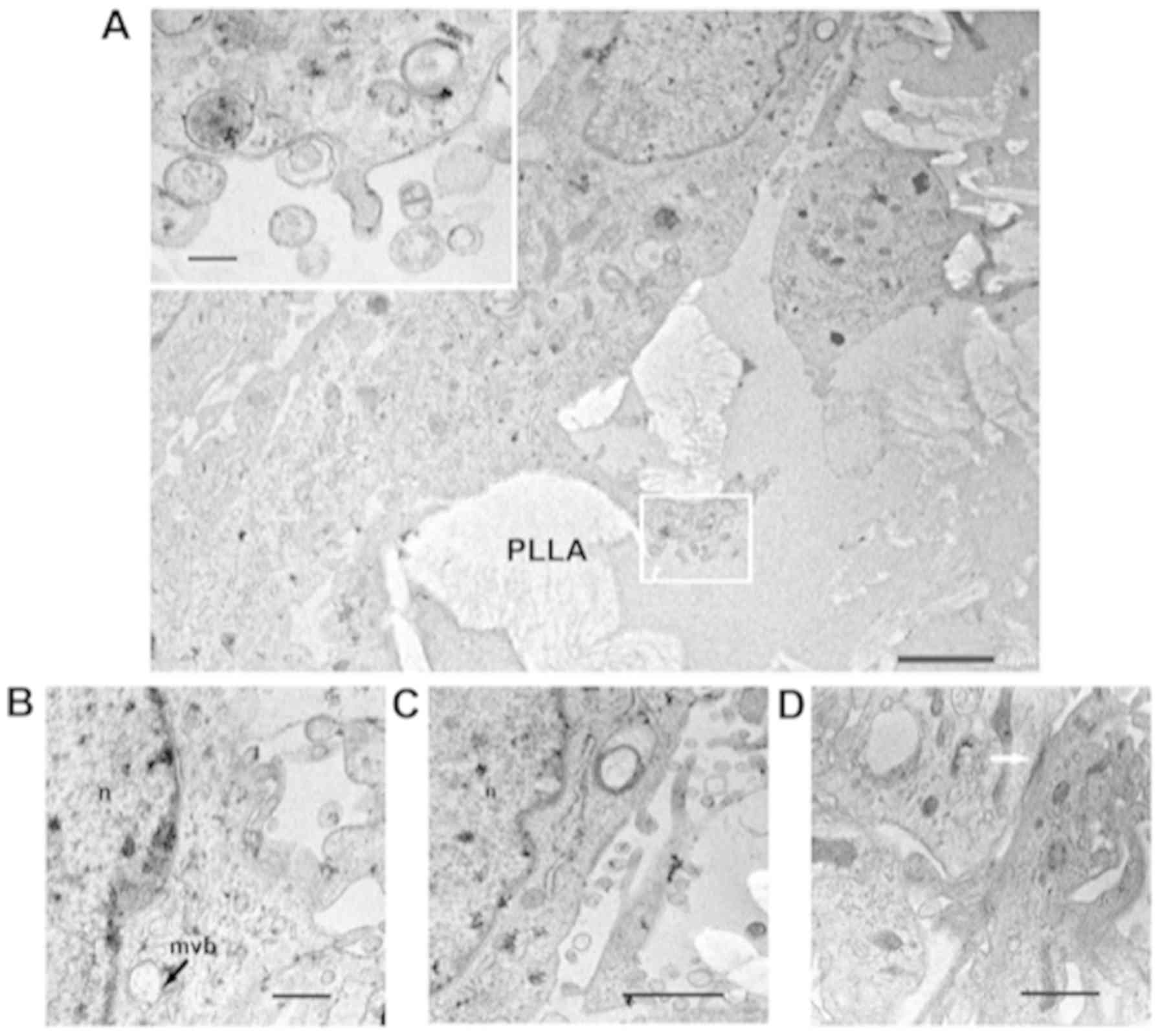
A final important point was the observation that
astrocytes grown on the 3D PLLA scaffold were able to produce EVs,
as shown by both TEM and SEM analyses (Figs. 7 and 8). In Fig.
7A-D, some invaginations of the plasma membrane and small
vesicles adjacent to the cell surface, or immediately outside the
cell, were observed. In addition, intracellular membrane-bound
bodies known as MVBs, with vesicles inside were clearly
identifiable (Fig. 7B, arrow). The
production of vesicles was also evident in the SEM analyses
(Fig. 8).
Discussion
In the last few decades it has become more and more
accepted that glial cells have more functions than expected.
Astrocytes, in particular, contribute to the majority of neuronal
activities, from recapturing neurotransmitters to the transfer of
critical metabolites, such as lactate (38). Interestingly, astrocytes do not
behave like single cells, but, thanks to a net of long
intercellular contacts based on gap junction channels (39,40)
formed by connexins (41), they
seem to constitute networks embracing neurons; these networks may
control many aspects of neuronal metabolism, such as ion and water
transport, and energy metabolite exchange, probably in a synaptic
activity-dependent manner. Notably, neuron-glial cell
communications are possibly also mediated in both directions by EVs
(5). Most importantly, vesicle
production by glial cells seems to be regulated by
neurotransmission (42). To date,
many reports have suggested that astrocytes cultured under 2D
conditions, although they are able to attach to the substrate, to
grow and to express the astrocyte-specific GFAP, do not assume all
of the morphological and functional properties of astrocytes in
vivo. For this reason, the present study decided to set a 3D
system more similar to the tridimensional environment of the brain.
In particular, a PLLA scaffold was used as many cell types have
already been shown to be able to grow on this substrate and to
metabolize it (43). The PLLA
matrix was first prepared starting from two different
concentrations of polymer in the solution. At different initial
concentrations, the polymers obtained showed different
morphologies. Scaffolds with pores of an average size of <20 µm,
formed from a 6% starting solution, had the best effect on
astrocyte survival and adaptation.
Another set of experiments aimed to identify the
biological macromolecules able to improve cell adhesion on the
scaffolds. To this end, immediately before cell seeding, the
scaffolds were treated with two different substrates (Coll IV and
FN). The results demonstrated that the best degree of adhesion
could be obtained with Coll IV-coated scaffolds, though FN also had
a good effect on astrocyte survival. These results agree with a
previous study, which demonstrated that astrocytes showed only
moderate affinity for surfaces covered with FN (44). In addition, in another study Coll
was demonstrated to support both initial adhesion and the growth of
astrocytes because of its favorable gelation properties, the
presentation of bioactive adhesive sites, and the ability to be
remodeled by astrocytes (18).
Accordingly, the present study revealed that
astrocytes adhere to Coll IV-coated scaffolds, and are able to grow
on them and to colonize the matrix, acquiring a typical star-like
morphology. In addition, they formed cell contacts both at the
level of the cell bodies, and among their long and thin
processes.
In addition, they also secrete EVs, compatible in
size with exosomes. Their ability to produce exosomes was
demonstrated by both TEM and SEM analyses, which revealed EVs and
intracellular MVBs. This observation is of the most importance as
it confirms that, as proposed years ago (14), astrocytes can produce EVs even in
cell culture. It is now well recognized that all of the brain cell
types release exosomes in vivo (5,45,46),
and that these particles can also be transported across the
blood-brain barrier (47). At
present, we do not know whether the amount of EVs produced by
astrocytes in the scaffold was comparable with the amount of EVs
released in 2D cultures nor whether it was comparable with the
amount of EVs released in vivo by comparable numbers of
cells; similarly, we currently do not know the composition of EVs
released from astrocytes in our culture system. However, once
established, a 3D scaffold such as that described in the present
study, appears to work well for astrocytes; future experiments are
already in progress, aiming to compare the rate of production and
the components of astrocyte-derived EVs, in vivo and in
culture. These experiments will be run in parallel with the attempt
to co-culture astrocytes with other brain cell types. In our
opinion, it is unlikely that the properties of EVs released from
astrocytes cultured alone are completely comparable with those of
EVs produced in vivo; many researchers have already proposed
that EV properties are as a result of continuous cross-talk between
all of the different brain cell types (48).
For the development of these complex systems, the
availability of a stable 3D model for brain cell culture was an
essential starting point. The use of polymeric structures as
substrates able to drive cell behavior and to reproduce tissue
environment has widely diffused in the last few years (49). The present study proposes that PLLA
scaffolds constitute a good model for the 3D growth of astrocytes.
The main advantage of using PLLA is that it relies on its
properties, including biocompatibility, processability, and
degradation rates. In addition, this polymer is characterized by
long biodegradation times (~1 year) (28,50),
that could allow for the performance of long-term in vitro
tests. As a further contribution to the field, the present study
presents a simple and feasible modelling method which allows to
tune the properties of the scaffold in terms of pore dimensions.
However, the model requires further improvements and upgrading in
further studies.
In conclusion, the results of the present study
together with those obtained in the previous analysis on BCECs
(29), suggest that the chosen
conditions could be a good starting point for the preparation of 3D
brain cell co-culture systems, more suitable for studying brain
cell-cell interaction routes, including the physiology of EV
production and delivery.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Italian
Ministry of Education, Universities and Research (grant no.
PJ_RIC_FFABR_2017_160958).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VBl, FZ and IV produced the scaffold and carried out
the cell seeding experiments. MADB produced the TEM images and
contributed to the analysis of the results. FCP performed all of
the SEM experiments. GS performed the fluorescence experiments.
CMDL, GG and VBr contributed to the analysis of the results. FCP,
GS, CMDL and IDL conceived and designed the study, and contributed
to the analysis of the results. The manuscript was written by IDL,
GS and FCP with close consultation with all of the other authors.
All authors have approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cocucci E and Meldolesi J: Ectosomes and
exosomes: Shedding the confusion between extracellular vesicles.
Trends Cell Biol. 25:364–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mateescu B, Kowal EJ, van Balkom BW,
Bartel S, Bhattacharyya SN, Buzás EI, Buck AH, de Candia P, Chow
FW, Das S, et al: Obstacles and opportunities in the functional
analysis of extracellular vesicle RNA-an ISEV position paper. J
Extracell Vesicles. 6:12860952017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Liegro CM, Schiera G and Di Liegro I:
Extracellular vesicle-associated RNA as a carrier of epigenetic
information. Genes (Basel). 8:E2402017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiera G, Di Liegro CM, Saladino P, Pitti
R, Savettieri G, Proia P and Di Liegro I: Oligodendroglioma cells
synthesize the differentiation-specific linker histone H1.0; and
release it into the extracellular environment through shed
vesicles. Int J Oncol. 43:1771–1776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiera G, Di Liegro CM and Di Liegro I:
Extracellular membrane vesicles as vehicles for brain cell-to-cell
interactions in physiological as well as pathological conditions.
Biomed Res Int. 2015:1529262015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schiera G, Di Liegro CM, Puleo V, Colletta
O, Fricano A, Cancemi P, Di Cara G and Di Liegro I: Extracellular
vesicles shed by melanoma cells contain a modified form of H1.0
linker histone and H1.0 mRNA-binding proteins. Int J Oncol.
49:1807–1814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maas SLN, Breakefield XO and Weaver AM:
Extracellular vesicles: Unique intercellular delivery vehicles.
Trends Cell Biol. 27:172–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vella LJ, Sharples RA, Nisbet RM, Cappai R
and Hill AF: The role of exosomes in the processing of proteins
associated with neurodegenerative diseases. Eur Biophys J.
37:323–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajendran L, Honsho M, Zahn TR, Keller P,
Geiger KD, Verkade P and Simons K: Alzheimer's disease beta-amyloid
peptides are released in association with exosomes. Proc Natl Acad
Sci USA. 103:11172–11177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saman S, Kim WH, Raya M, Visnick Y, Miro
S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC and Hall GF:
Exosome-associated tau is secreted in tauopathy models and is
selectively phosphorylated in cerebrospinal fluid in early
Alzheimer disease. J Biol Chem. 287:3842–3849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camussi G, Deregibus MC, Bruno S,
Cantaluppi V and Biancone L: Exosomes/microvesicles as a mechanism
of cell-to-cell communication. Kidney Int. 78:838–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rennert RC, Hochberg FH and Carter BS:
ExRNA in biofluids as biomarkers for brain tumors. Cell Mol
Neurobiol. 36:353–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schiera G, Proia P, Alberti C, Mineo M,
Savettieri G and Di Liegro I: Neurons produce FGF2 and VEGF and
secrete them at least in part by shedding extracellular vesicles. J
Cell Mol Med. 11:1384–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Proia P, Schiera G, Mineo M, Ingrassia AM,
Santoro G, Savettieri G and Di Liegro I: Astrocytes shed
extracellular vesicles that contain fibroblast growth factor-2 and
vascular endothelial growth factor. Int J Mol Med. 21:63–67.
2008.PubMed/NCBI
|
|
15
|
Knight VB and Serrano EE: Hydrogel
scaffolds promote neural gene expression and structural
reorganization in human astrocyte cultures. PeerJ. 5:e28292017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi W, Huang CJ, Xu XD, Jin GH, Huang RQ,
Huang JF, Chen YN, Ju SQ, Wang Y, Shi YW, et al: Transplantation of
RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal
stem cells forced with CXCR4 and activated astrocytes for repair of
traumatic brain injury. Acta Biomater. 45:247–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katiyar KS, Winter CC, Struzyna LA, Harris
JP and Cullen DK: Mechanical elongation of astrocyte processes to
create living scaffolds for nervous system regeneration. J Tissue
Eng Regen Med. 11:2737–2751. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winter CC, Katiyar KS, Hernandez NS, Song
YJ, Struzyna LA, Harris JP and Cullen DK: Transplantable living
scaffolds comprised of micro-tissue engineered aligned astrocyte
networks to facilitate central nervous system regeneration. Acta
Biomater. 38:44–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Führmann T, Hillen LM, Montzka K, Wöltje M
and Brook GA: Cell-Cell interactions of human neural
progenitor-derived astrocytes within a microstructured 3D-scaffold.
Biomaterials. 31:7705–7715. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ugbode CI, Hirst WD and Rattray M:
Astrocytes grown in alvetex® three dimensional scaffolds
retain a non-reactive phenotype. Neurochem Res. 41:1857–1867. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lau CL, Kovacevic M, Tingleff TS, Forsythe
JS, Cate HS, Merlo D, Cederfur C, Maclean FL, Parish CL, Horne MK,
et al: 3D Electrospun scaffolds promote a cytotrophic phenotype of
cultured primary astrocytes. J Neurochem. 130:215–226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hyysalo A, Ristola M, Joki T, Honkanen M,
Vippola M and Narkilahti S: Aligned poly(ε-caprolactone) nanofibers
guide the orientation and migration of human pluripotent stem
cell-derived neurons, astrocytes, and oligodendrocyte precursor
cells in vitro. Macromol Biosci. 17:2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conoscenti G, Schneider T, Stoelzel K,
Carfì Pavia F, Brucato V, Goegele C, La Carrubba V and
Schulze-Tanzil G: PLLA scaffolds produced by thermally induced
phase separation (TIPS) allow human chondrocyte growth and
extracellular matrix formation dependent on pore size. Mater Sci
Eng C Mater Biol Appl. 80:449–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narayan D and Venkatraman SS: Effect of
pore size and interpore distance on endothelial cell growth on
polymers. J Biomed Mater Res A. 87:710–718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carfì Pavia F, Palumbo FS, La Carrubba V,
Bongiovì F, Brucato V, Pitarresi G and Giammona G: Modulation of
physical and biological properties of a composite PLLA and
polyaspartamide derivative obtained via thermally induced phase
separation (TIPS) technique. Mater Sci Eng C Mater Biol Appl.
67:561–569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carfì Pavia F, La Carrubba V and Brucato
V: Polymeric scaffolds based on blends of poly-l-lactic acid (PLLA)
with poly-d-l-lactic acid (PLA) prepared via thermally induced
phase separation (TIPS): Demixing conditions and morphology. Polym
Bull. 70:563–578. 2013. View Article : Google Scholar
|
|
27
|
Mannella GA, Conoscenti G, Carfì Pavia F,
La Carrubba V and Brucato V: Preparation of polymeric foams with a
pore size gradient via Thermally Induced Phase Separation (TIPS).
Mater Lett. 160:31–33. 2015. View Article : Google Scholar
|
|
28
|
Carfì Pavia F, La Carrubba V, Piccarolo S
and Brucato V: Polymeric scaffolds prepared via thermally induced
phase separation: Tuning of structure and morphology. J Biomed
Mater Res A. 86:459–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Bella MA, Zummo F, Carfì Pavia F,
Brucato VM, Di Liegro I and Schiera G: Migration of brain capillary
endothelial cells inside poly (lactic acid) 3D scaffolds.
Microscopy and imaging science: Practical approaches to applied
research and education. Méndez-Vilas A: Formatex Research Center;
Barcelona: pp. 260–264. 2017
|
|
30
|
Murphy AR, Laslett A, O'Brien CM and
Cameron NR: Scaffolds for 3D in vitro culture of neural lineage
cells. Acta Biomater. 54:1–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Connor SM, Stenger DA, Shaffer KM, Maric
D, Barker JL and Ma W: Primary neural precursor cell expansion,
differentiation and cytosolic Ca(2+) response in three-dimensional
collagen gel. J Neurosci Methods. 102:187–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seidlits SK, Khaing ZZ, Petersen RR,
Nickels JD, Vanscoy JE, Shear JB and Schmidt CE: The effects of
hyaluronic acid hydrogels with tunable mechanical properties on
neural progenitor cell differentiation. Biomaterials. 31:3930–3940.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mannella GA, Carfì Pavia F, Conoscenti G,
La Carrubba V and Brucato V: Evidence of mechanisms occurring in
thermally induced phase separation of polymeric systems. J Polym
Sci Part B Polym Phys. 52:979–983. 2014. View Article : Google Scholar
|
|
34
|
Schiera G, Bono E, Raffa MP, Gallo A,
Pitarresi GL, Di Liegro I and Savettieri G: Synergistic effects of
neurons and astrocytes on the differentiation of brain capillary
endothelial cells in culture. J Cell Mol Med. 7:165–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carfì Pavia F, Conoscenti G, Greco S, La
Carrubba V, Ghersi G and Brucato V: Preparation, characterization
and in vitro test of composites poly-lactic acid/hydroxyapatite
scaffolds for bone tissue engineering. Int J Biol Macromol.
119:945–953. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liverani C, Mercatali L, Cristofolini L,
Giordano E, Minardi S, Porta GD, De Vita A, Miserocchi G, Spadazzi
C, Tasciotti E, et al: Investigating the mechanobiology of cancer
cell-ECM interaction through collagen-based 3D scaffolds. Cell Mol
Bioeng. 10:223–234. 2017. View Article : Google Scholar
|
|
37
|
Villalona GA, Udelsman B, Duncan DR,
McGillicuddy E, Sawh-Martinez RF, Hibino N, Painter C, Mirensky T,
Erickson B, Shinoka T and Breuer CK: Cell-seeding techniques in
vascular tissue engineering. Tissue Eng Part B Rev. 16:341–350.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Proia P, di Liegro CM, Schiera G, Fricano
A and Di Liegro I: Lactate as a metabolite and a regulator in the
central nervous system. Int J Mol Sci. 17(pii): E14502016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giaume C, Koulakoff A, Roux L, Holcman D
and Rouach N: Astroglial networks: A step further in neuroglial and
gliovascular interactions. Nat Rev Neurosci. 11:87–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pannasch U and Rouach N: Emerging role for
astroglial networks in information processing: From synapse to
behavior. Trends Neurosci. 36:405–417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bosone C, Andreu A and Echevarria D: GAP
junctional communication in brain secondary organizers. Dev Growth
Differ. 58:446–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frohlich D, Kuo WP, Fruhbeis C, Sun JJ,
Zehendner CM, Luhmann HJ, Pinto S, Toedling J, Trotter J and
Krämer-Albers EM: Multifaceted effects of oligodendroglial exosomes
on neurons: impact on neuronal firing rate, signal transduction and
gene regulation. Philos Trans R Soc Lond B Biol Sci.
369:201305102014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carfì-Pavia F, Turturici G, Geraci F,
Brucato V, La Carrubba V, Luparello C and Sconzo G: Porous poly
(L-lactic acid) scaffolds are optimal substrates for internal
colonization by A6 mesoangioblasts and immunocytochemical analyses.
J Biosci. 34:873–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma SH, Lepak LA, Hussain RJ, Shain W and
Shuler ML: An endothelial and astrocyte co-culture model of the
blood-brain barrier utilizing an ultra-thin, nanofabricated silicon
nitride membrane. Lab Chip. 5:74–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frühbeis C, Fröhlich D, Kuo WP and
Krämer-Albers EM: Extracellular vesicles as mediators of
neuron-glia communication. Front Cell Neurosci. 7:1822013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zagrean AM, Hermann DM, Opris I, Zagrean L
and Popa-Wagner A: Multicellular crosstalk between exosomes and the
neurovascular unit after cerebral ischemia. Therapeutic
implications. Front Neurosci. 12:8112018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Balusu S, Van Wonterghem E, De Rycke R,
Raemdonck K, Stremersch S, Gevaert K, Brkic M, Demeestere D,
Vanhooren V, Hendrix A, et al: Identification of a novel mechanism
of blood-brain communication during peripheral inflammation via
choroid plexus-derived extracellular vesicles. EMBO Mol Med.
8:1162–1183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Basso M and Bonetto V: Extracellular
vesicles and a novel form of communication in the brain. Front
Neurosci. 10:1272016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
O'Brien FJ: Biomaterials and scaffolds for
tissue engineering. Mater Today. 14:88–95. 2011. View Article : Google Scholar
|
|
50
|
Tajbakhsh S and Hajiali F: A comprehensive
study on the fabrication and properties of biocomposites of
poly(lactic acid)/ceramics for bone tissue engineering. Mater Sci
Eng C Mater Biol Appl. 70:897–912. 2017. View Article : Google Scholar : PubMed/NCBI
|