Introduction
In the last several years, the importance of
propofol as a short-acting anaesthetic agent has begun to be
recognized in animal models (1,2).
Propofol affects GABAA transmission and decreases
glutamate transmission (3). These
findings have raised questions about how extensively propofol is
used and what other irreversible effects it exerts on the central
nervous system (4–6). Exposure to a subanaesthetic dose of
propofol was demonstrated to alter long non-coding RNA profiles in
the immature mouse hippocampus (7)
and cause disorders in hippocampal circuits resulting in several
diseases, including Alzheimer's disease and Parkinson's disease
(8), while exposure to a high
propofol dose inhibited long-term potentiation in the CA1 area of
the adult hippocampus. A 100 mg/kg dose of propofol induces the
expression of apoptotic proteins, including B-cell lymphoma
2-associated X and caspase-3, in Sprague-Dawley pups (postnatal day
7), followed by adverse effects, such as learning and memory
impairment (9–12). Therefore, it is hypothesized that
neonatal rats have increased sensitivity and are more vulnerable to
a 100 mg/kg dose of propofol than adult rats.
Hypoxic preconditioning (HPC) is the exposure of an
organ to a moderate hypoxic stimulus prior to injury (13). Calcium overload (14) and overproduction of reactive oxygen
species (15) have been identified
by the detection of electrical simulation and neuronal
depolarization during cellular processes in the rat
hippocampus.
HPC has long been recognized to induce
neuroprotection and neuroplasticity in bone marrow stromal cells
(16,17); however, the anti-apoptotic signals
that mediate these processes remain unclear. To address this issue,
immature male Sprague-Dawley rats were exposed to HPC and propofol,
either alone or in the relevant combinations. It was hypothesized
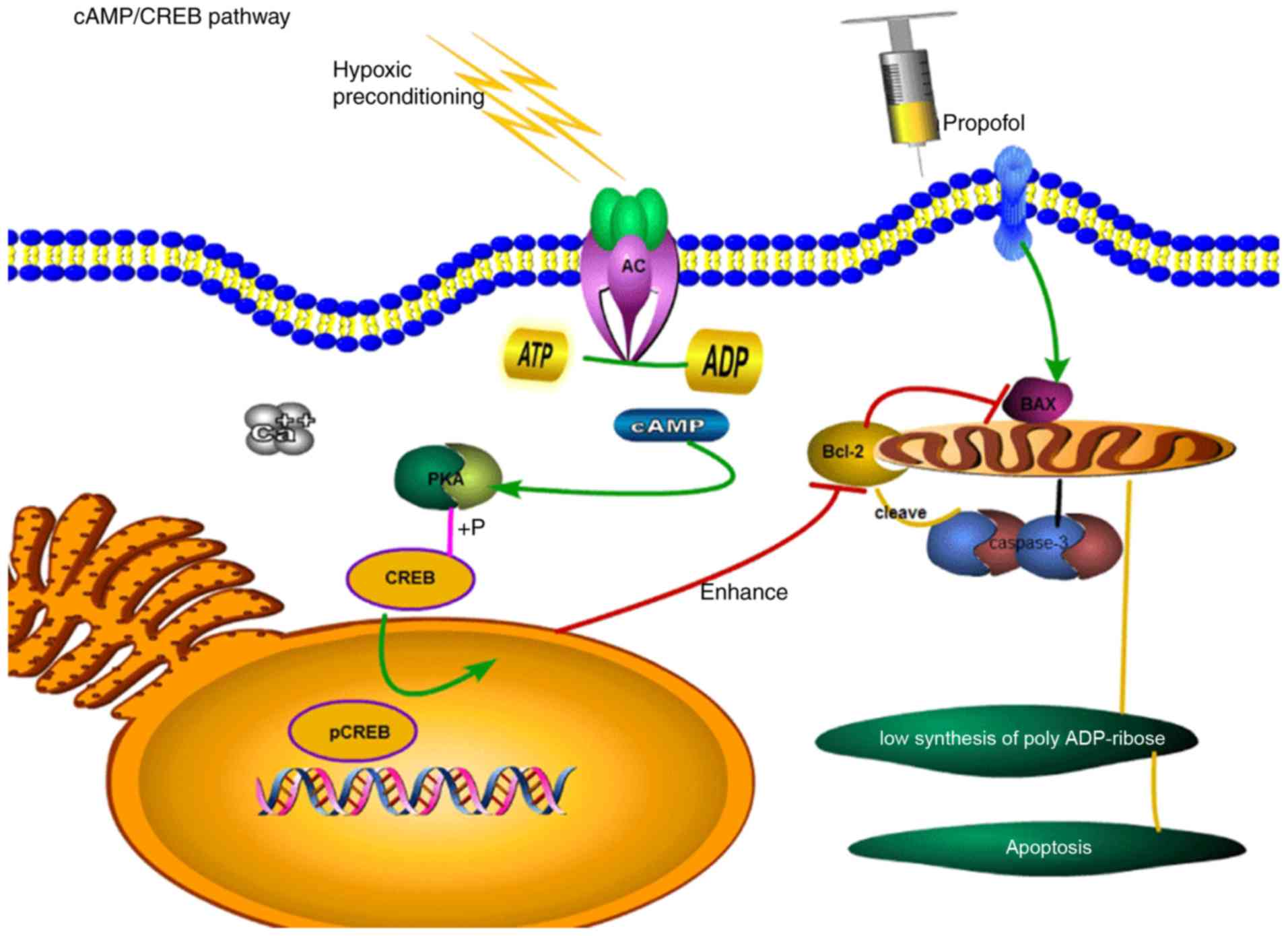
that HPC increases the concentration of cyclic adenosine
monophosphate (cAMP) via direct phosphorylation of effector
proteins and regulation of transcriptional activators or the
corresponding gene transcription. cAMP response element-binding
protein (CREB) is required for neuronal growth within hippocampal
tissues. The role of the cAMP/CREB signalling pathway in intrinsic
apoptosis is illustrated in Fig.
1.
Materials and methods
Rat HPC model
All animal procedures were conducted with the
approval of the Animal Care and Use Committee of Guangxi Medical
University (Nanning, China). Seven-day-old (P7) male Sprague-Dawley
pups (average body weight, 10–15 g, n=70) were identified and
numbered using picric acid, which were revealed to the investigator
only after the completion of experiments and analyses. All pups
were housed in a temperature-controlled room (22±1°C) with a 12-h
light/dark schedule. H89 (Selleck Chemicals) and Sp-cAMP
(Sigma-Aldrich; Merck KGaA) were prepared in 5 µl double-distilled
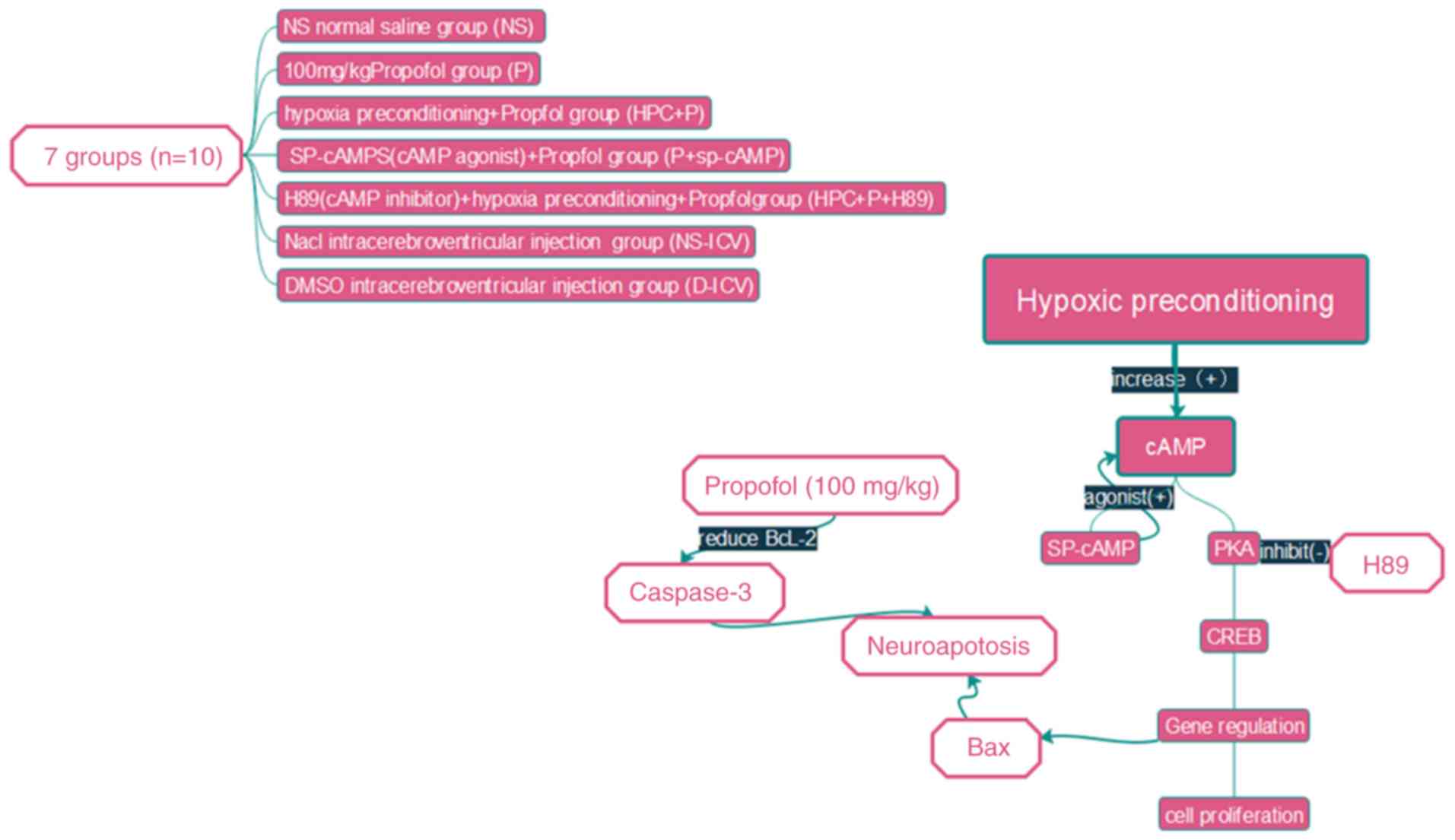
water. The experimental set-up is illustrated in Fig. 2 (n=10) and the following
experimental groupings were used: i) Normal saline group (NS group)
received intraperitoneal injections of an equal volume of normal
saline; ii) propofol group (P group) received intraperitoneal
injections of 100 mg/kg propofol; iii) following the propofol
treatment as in the P group, the propofol + Sp-cAMP group
(P+Sp-cAMP group) received intracerebroventricular injections of 20
nmol/5 µl Sp-cAMP (a cAMP-dependent protein kinase agonist); iv)
HPC+P group rats were placed in a chamber containing 8% oxygen and
92% nitrogen for 10 min, and the pups were subsequently exposed to
room air for a further 10 min, and following five HPC cycles, the
rats received an intraperitoneal injection of 100 mg/kg propofol;
v) HPC+P +H89 group was exposed to 5 µmol/5 µl H89 [a protein
kinase A (PKA) inhibitor] by intracerebroventricular injections,
followed by the same protocol as in the HPC+P group; vi) the
remaining pups in the two blank test groups received
intracerebroventricular injections of dimethyl sulfoxide (D-ICV
group) or normal saline (NS-ICV group). All pups were sacrificed
according to standard protocols (100 mg/kg intraperitoneal sodium
pentobarbital). Brain tissue slices were prepared for
immunohistochemistry and the levels of PKA, CREB, phosopho
(p)-CREB, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein
(Bax) and caspase-3 were evaluated by western blotting.
Morphological and structural changes were evaluated by haematoxylin
and eosin (H&E) staining and transmission electron
microscopy.
Intraventricular injections
As aforementioned, rats were anesthetized with
sodium pentobarbital and centralized coordinates of anterior
fontanel (x=0, y=0, z=0) using stereotaxic apparatus (Ryward Life
Technology Co., Ltd.), the sterile cannula was implanted at AP-2 mm
(front and posterior), MLR-1.5 mm (left and right of the midline),
and H-2 mm (depth from the left ventricle, x=−1.0 mm, y=2 mm, z=0).
After positioning, the skull was drilled, and then Sp-cAMP (5 µl)
or H89 (5 µl) was slowly injected at rate of 0.1 µl/min. The blank
groups following the same protocol with an equal volume of DMSO or
normal saline.
ELISA
The intracellular concentrations of adenylyl cyclase
in the pups was determined by ELISA according to the instructions
of the assay manufacturer (cat. no. S0026; Beyotime Institute of
Biotechnology).
Western blot analysis
All pups were sacrificed to harvest the brain
tissue. The protein was extracted by RIPA Lysis Buffer (Beijing
Solarbio Science & Technology Co., Ltd.) and protein
concentration measured using a bicinchoninic acid protein assay
(Biotype Biotech Co.). The mass of protein loaded per lane was 20
µl. Equal amounts of proteins were loaded onto 12%
SDS-polyacrylamide gels. Electrophoresed proteins were transferred
to polyvinylidene difluoride membranes (0.22-µm pore size; EMD
Millipore). The membranes were blocked using 5% bovine serum
albumin (blocking buffer) for 2 h at room temperature and incubated
with the following primary antibodies overnight at 4°C: β-tubulin
(1:2,000; cat. no. 48885), caspase-3 (1:1,000; cat. no. 48658) and
cleaved caspase-3 (1:1,000; cat. no. 29034; all from Signalway
Antibody) Bcl-2 (1:1,000; cat no. ab196495; Abcam), Bax (cat. no.
27727) PKA (cat. no. 5842S), CREB (cat. no. 9197S) and p-CREB (cat.
no. 9198S) (all 1:1,000; from Cell Signaling Technology, Inc.), and
GAPDH (1:10,000; cat. no. 10494-1-AP; Proteintech, Inc.). The
membranes were washed three times with Tris-buffered saline 1%
Tween-20 (TBST; pH 7.4) and then incubated in horseradish
peroxidase-conjugated secondary antibody (1:10,000; cat. no.
134658; LI-COR Biosciences) for 2 h at room temperature (23–25°C)
and washed three times with TBST. The bands were developed using an
Odyssey infrared imaging system (LI-COR Biosciences) and evaluated
using densitometric analysis (ImageJ 1.52 h, National Institutes of
Health).
H&E and immunohistochemical
staining
Morphological and structural changes were observed
by H&E staining. Tissues were fixed in 4% ice-cold
paraformaldehyde at 4°C for 2 h and paraffin-embedded sections were
obtained. The paraffin sections were dewaxed in xylene for 15 min
and rehydrated using graded ethanol. The sections were immersed in
haematoxylin for 30 sec and then subjected to antigen retrieval
using 0.01 mol/l sodium citrate and incubated with 10% normal goat
serum at room temperature for 30 min to block nonspecific binding,
followed by incubation with the primary antibodies against PKA C
and p-CREB (cat. nos. 5842S and 9198S, 1:1,000; Cell Signaling
Technology, Inc.) at 4°C overnight. The sections were incubated
with streptavidin-horseradish peroxidase at room temperature for 30
min and then stained with 0.05% 3,3-diaminobenzidine substrate,
followed by counterstaining with 1% haematoxylin at 37°C for 30
sec. The sections were observed using a microscope (Olympus BX53;
Olympus Corporation) and four fields of the hippocampus were
randomly selected in every section which represented the areas of
interest and the positive cells were counted using Image-Pro Plus
version 6.0 software (Media Cybernetics Inc.).
Electron microscopy
The ultrastructures of neurocytes were observed by
transmission electron microscopy (HITACHI H-7650; Hitachi, Ltd.).
Briefly, 2.5% glutaraldehyde solution was perfused into the rats,
and the tissues were fixed in 1% OsO4 at 4°C for 1 h,
dehydrated in increasing concentrations of ethanol and embedded in
Epon. Then, the samples were sectioned into semi-thin slices (1 µm)
and stained with 1% uranyl acetate and 5% uranyl acetate at 37°C
for 20 min. The ultrastructures of the entire mitochondria were
measured by manually measuring length using Image Pro Plus (version
6.0.0.260, Media Cybernetics, Inc.).
Statistical analysis
Data are presented as the mean ± standard error, and
were analysed using SPSS version 17.0 (SPSS, Inc.) and GraphPad
Prism 5 software (GraphPad Software Inc.). Multiple comparisons
were performed using one-way analysis of variance (ANOVA), followed
by Dunnett's post hoc test, as appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
HPC induces CREB phosphorylation and
attenuates propofol-induced neurotoxicity in neonatal rats
H&E staining revealed areas of brain cells
affected by propofol, which exhibited less shrunken cell bodies and
pyknotic nuclei (Fig. 3). Compared
with the P group, electron microscopy demonstrated that neurons
were rich in mitochondria and the width of mitochondrion was
decreased in the HPC+P group (F=54.44, P<0.05; Fig. 4). Treatment with H89, a PKA
inhibitor, revealed expansion of the width of mitochondrion similar
to the P group.
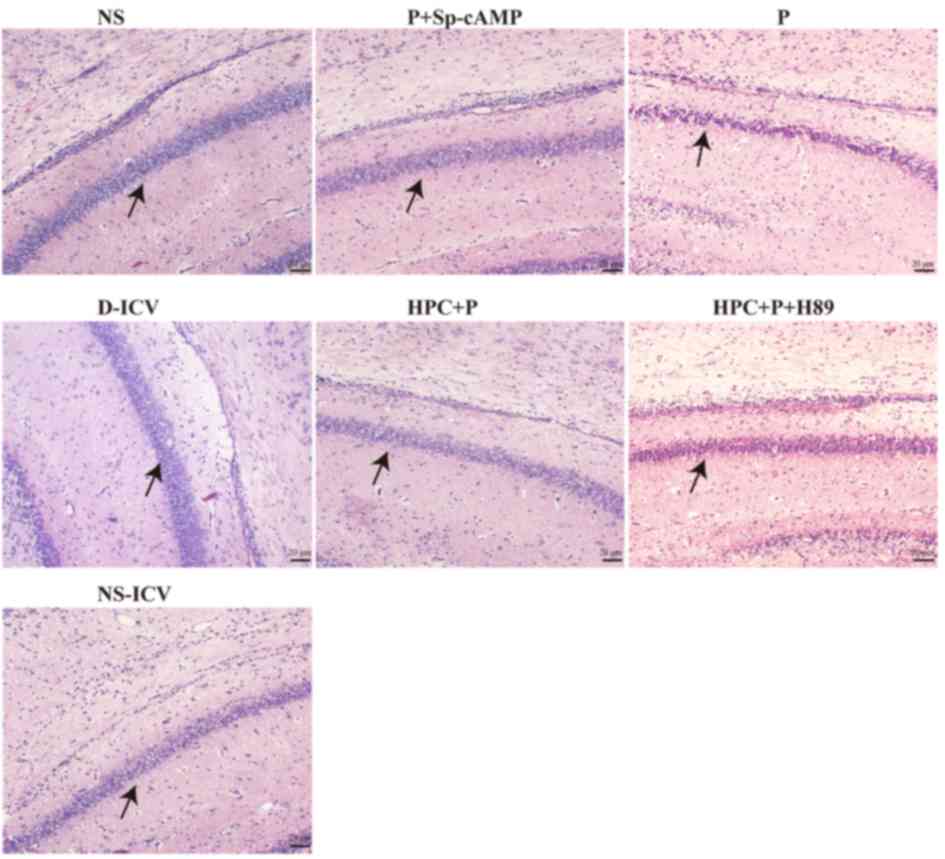 | Figure 3.Haematoxylin and eosin staining of
brain lesions. Examination by light microscopy following a high
dose injection of propofol. Histological analysis revealed normal
cells in the three blank test groups (NS, NS-ICV, and D-ICV) and
disordered cells in the P group, which exhibited different degrees
of degeneration. Furthermore, cellular degeneration in the HPC+P
and P+Sp-cAMP groups was mild. Magnification, ×200. Black arrow
indicates the CA1 area of the Hippocampus. NS, normal saline; ICV,
intracerebroventricular; D, dimethyl sulfoxide; P, propofol; HPC,
hypoxic preconditioning; Sp-cAMP, cyclic adenosine monophosphate
agonist. |
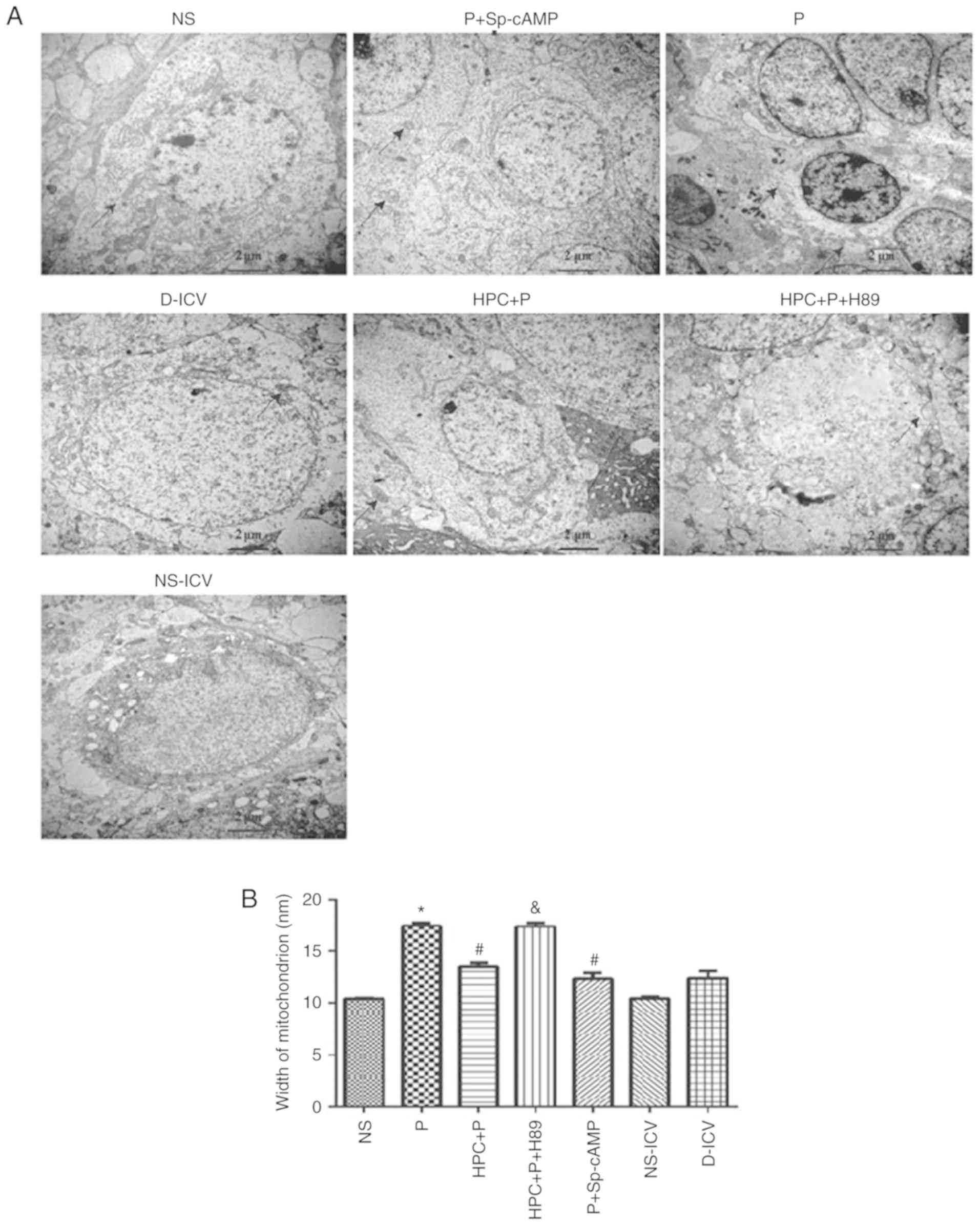 | Figure 4.Examination of the hippocampal CA1
region by transmission electron microscopy (Magnification, ×15,000)
following propofol-induced apoptosis. (A) Dendritic spine lesions,
endoplasmic reticulum degranulated and mitochondrial swelling were
observed. (B) Width of mitochondrion were measured by Image Pro
Plus (version 6.0.0.260), F=54.44; *P<0.05 vs. the NS group;
#P<0.05 vs. the P group; &P<0.05
vs. the HPC+P group. NS, normal saline; P, propofol; HPC, hypoxic
preconditioning; Sp-cAMP, cyclic adenosine monophosphate agonist;
ICV, intracerebroventricular; D, dimethyl sulfoxide. |
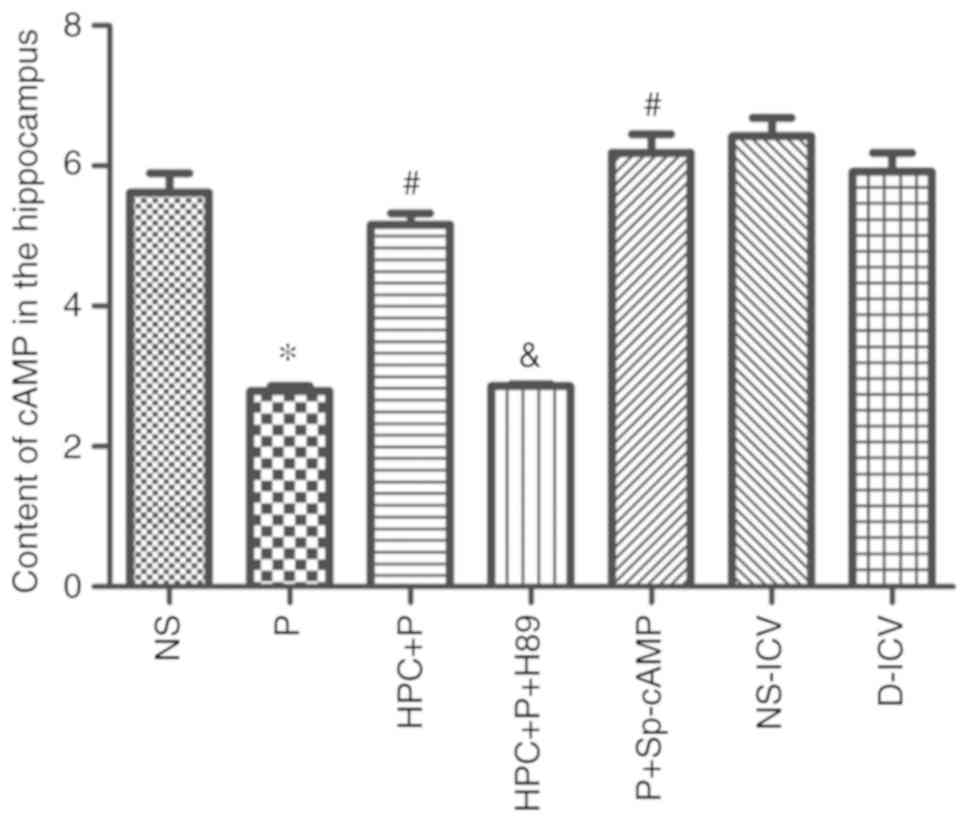
HPC increases the level of cAMP and
PKA, and p-CREB-positive cells
The level of cAMP was significantly upregulated in
the HPC + P group compared with the P group (Fig. 5). Additionally, western blot
analysis revealed a significant difference in PKA levels between
the P group and the NS group. The expression of Bcl-2 was
significantly decreased in the pups treated with propofol; whereas,
the expression of Bax and cleaved caspase-3 was significantly
increased (Fig. 6A-D). Western
blot analysis demonstrated that the level of p-CREB was
significantly increased in the HPC+P group compared with the P
group (1.050±0.083 vs. 1.400±0.111, respectively, F=15.83;
P<0.05; Fig. 6E and F). The
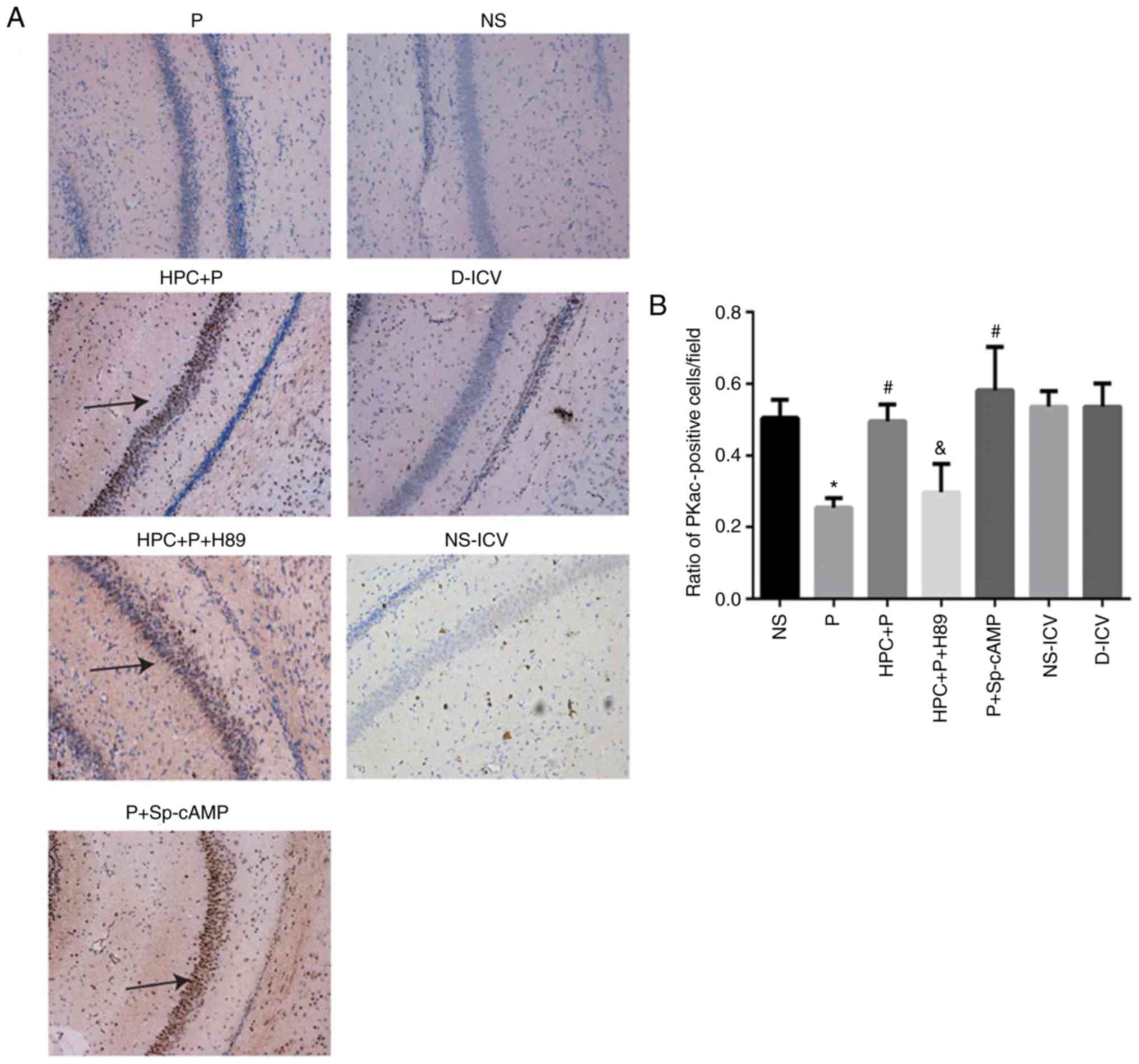
results of immunohistochemical staining indicated that HPC and
treatment with Sp-cAMP (a cAMP-dependent protein kinase agonist)
increased the levels of PKA (F= 17.26; P<0.05; Fig. 7) and p-CREB (F= 14.81; P<0.05;
Fig. 8), whereas H89 abolished
these effects.
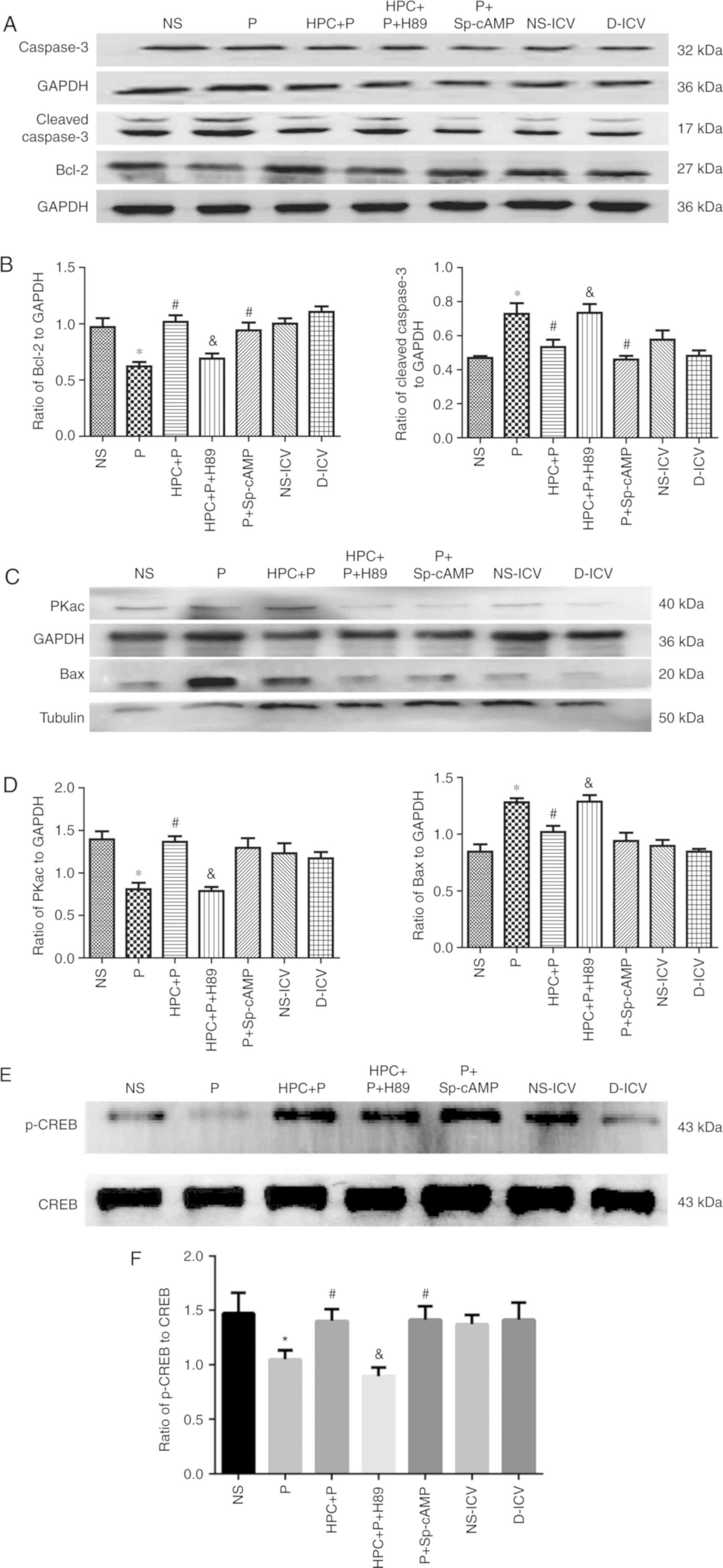 | Figure 6.Activation of cAMP/PKA/CREB by HPC or
cAMP agonists reduces neuroapotosis in the developing brains of
Sprague-Dawley rats (n=10 per group). (A-D) Representative western
blots using 12% SDS-PAGE and densitometry analysis of the ratio of
of Bax to β-tubulin (F=12.81), Bcl-2 (F=9.990), cleaved caspase-3
(F=7.409), PKAc (F=8.366), to GAPDH. Compared with the NS group,
the expression of cleaved caspase-3 was increased to a 2-fold
change in the P group; neonatal exposure to HPC attenuated the
effect of propofol-induced apoptosis with downregulation of cleaved
caspase-3. (E and F) Representative western blots using 12%
SDS-PAGE and densitometry analysis of the ratio of p-CREB to CREB
(F=15.83). The results are expressed as the mean ± standard
deviation. *P<0.05 vs. the NS group; #P<0.05 vs.
the P group; &P<0.05 vs. the HPC+P group. NS,
normal saline; P, propofol; HPC, hypoxic preconditioning; Sp-cAMP,
cyclic adenosine monophosphate agonist; ICV,
intracerebroventricular; D, dimethyl sulfoxide; cAMP, cyclic
adenosine monophosphate; PKAc, protein kinase A catalytic subunit;
CREB, cAMP response element-binding protein; Bcl-2, B-cell
lymphoma-2; Bax, Bcl-2-associated X protein. |
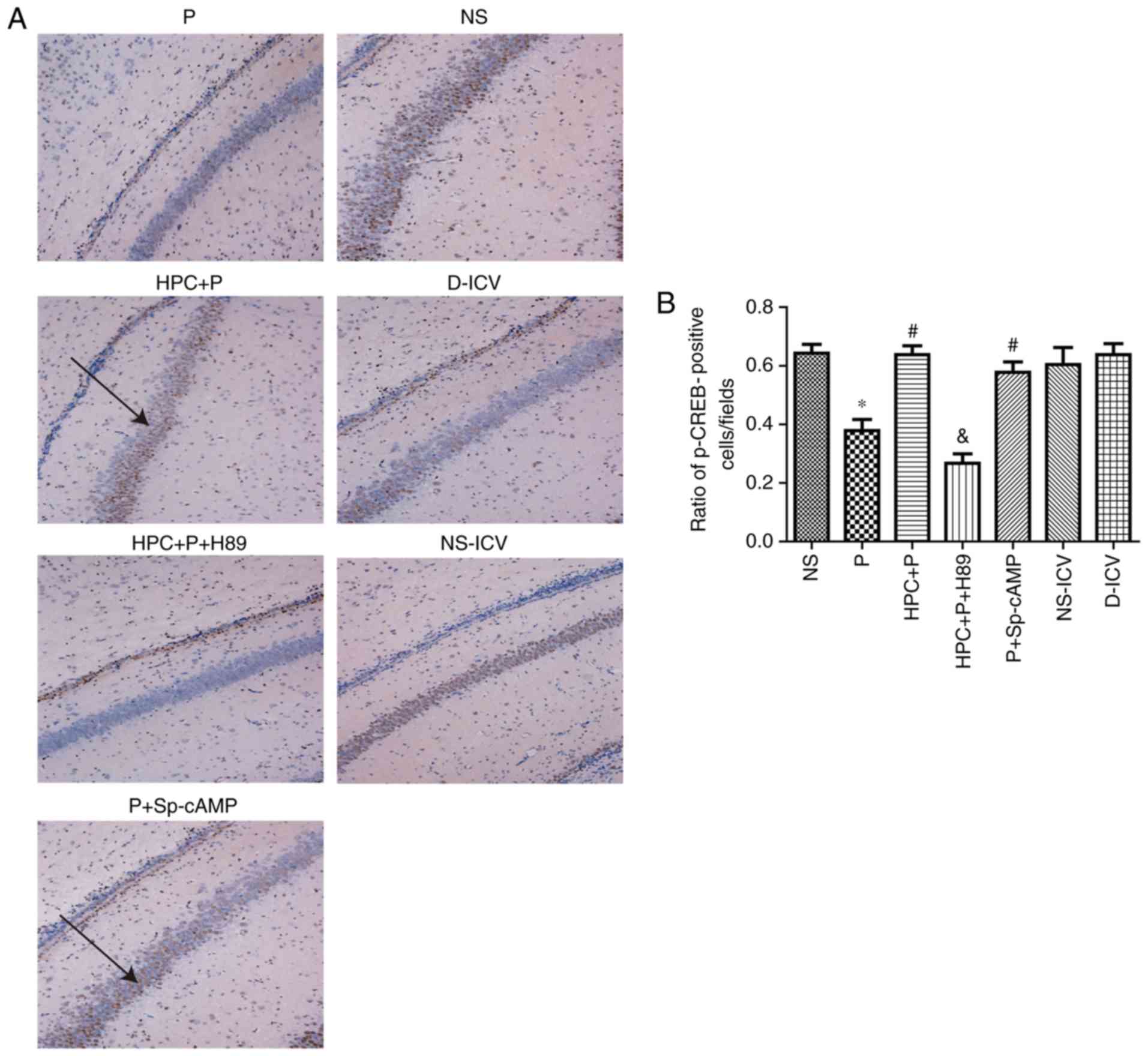 | Figure 8.Effect of propofol on CREB following
HPC. (A) Immunohistochemical staining of p-CREB in the seven groups
(Magnification, ×100). (B) Quantification of p-CREB staining
expressed as the mean ± standard deviation; F=14.81, *P<0.05 vs.
the NS group; #P<0.05 vs. the P group;
&P<0.05 vs. the HPC+P group. Black arrow
indicateS p-CREB positive cells. p, phospho; CREB, cAMP response
element-binding protein; NS, normal saline; P, propofol; HPC,
hypoxic preconditioning; Sp-cAMP, cyclic adenosine monophosphate
agonist; ICV, intracerebroventricular; D, dimethyl sulfoxide. |
Propofol reduces the number of
p-CREB-positive cells in the hippocampal CA1 region
The expression of p-CREB was negatively associated
with the expression of cleaved-caspase-3, and there was no
significant difference in the expression of CREB between the NS and
NS-DIV groups (Fig. 8).
HPC suppresses propofol-induced
neurotoxicity via activation of cAMP-dependent proteins
Compared with the NS group, the activities of
cleaved caspase-3 were upregulated with a 2-fold change in the P
group, and the levels of Bax were examined to investigate
neurotoxicity following treatment with 100 mg/kg propofol. In the
HPC+P group, the Bcl-2 (Fig. 6)
and p-CREB (Fig. 8) levels in
brain tissue were significantly increased compared with the P
group, which was consistent with the Sp-cAMP treatment.
Furthermore, H89 decreased the activation of p-CREB following HPC
compared with the HPC+P group (Fig.
8).
Discussion
The present findings are an extension of previous
studies (18,19) to investigate the cAMP/PKA/CREB
signalling pathway and propofol-induced apoptosis. The results of
the present study revealed that p-CREB promotes cell survival and
long-term synaptic change. Propofol-induced intrinsic apoptosis in
neonatal rats was previously reported to be mediated by Bax
(20) and release of caspase-3,
which are important hallmarks of apoptosis (21). Bax disrupts mitochondrial membrane
potential by affecting the permeability transition pores and
facilitating the release of cytochrome c (22).
PKA expression was reduced in the P group and this
reduction was prevented by HPC, but not when H89 was applied.
Furthermore, cAMP levels were markedly increased in the HPC+P
group. These data confirmed that HPC attenuates propofol-induced
neuroapoptosis by altering the content of cAMP in the hippocampus
of rats via activation of cAMP/PKA/CREB signalling, a reduced
Bax/Bcl-2 ratio and downregulation of caspase-driven apoptosis
downstream. The relative expression of these proteins determines
cell survival. Caspase-3 is one of the key executors of apoptosis
and a widely studied member of the caspase family.
Mitochondrial outer membrane permeabilization is
involved in the neuroprotective effects of HPC against
propofol-induced neurotoxicity in rats. The study by Xu et
al (23) revealed that HPC
promotes the survival and viability of trophoblast cells.
Transmission electron microscopy demonstrated that the
mitochondrial outer membrane and matrix were enhanced by HPC with
less degenerating vacuoles and apoptotic bodies observed. The
findings indicated that pretreament with HPC reduced mitochondrial
apoptosis.
Neuronal cell apoptosis is associated with minor
behavioural changes and cognitive dysfunction in adolescent rats
(24). When an apoptosis signal
appears, cleaved caspase-3 causes degradation of the neuron cell
membrane and prevents the repair of damaged DNA. HPC induces robust
neuroprotection in models of neonatal hypoxia (25). HPC was demonstrated to increase
Bcl-2 expression and reverse the propofol-induced reduction in
neuronal cAMP levels. Additionally, as a potent and selective PKA
inhibitor for evaluation of PKA function in many organs, such as
the brain, muscle and heart, pretreatment with H89 (26), abolished the beneficial effects of
HPC, whereas pretreatment with Sp-cAMP (27,28),
a PKA agonist, increased the protective effects of HPC on
propofol-induced hippocampal apoptosis. Whether Sp-cAMP exhibits a
neuronal protective effect on the hippocampus requires
investigation in future experiments, since our results do not
include H89 or Sp-cAMP alone. Notably, cellular life and death are
mitigated by Bcl-2 (29), which
may be correlated with the cAMP/PKA/CREB pathway balancing the
neuronal proliferation and apoptosis, as the phosphorylation of
CREB promotes synaptic and neural plasticity, and Bcl-2 mediates
mitochondria-induced cellular toxicity (30).
The mechanism underlying the neuroprotective effect
of HPC is associated with the cAMP content. The findings of the
present study revealed that the apoptosis rate was significantly
decreased in the HPC+P and P+Sp-cAMP groups compared with the P
group. Thus, HPC ameliorates propofol-induced neuroapoptosis via an
increase in cAMP levels and phosphorylation of CREB, which prevents
caspase-3 from inducing the apoptosis of hippocampal neurons.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81373498 and
81060277), the Guangxi Key Research and Development Program (grant
no. AB18221031), the Science Study and Technology Development
Program of Guangxi (grant no. 1355005-4-2), and the Science and
Technology Research Project of Guangxi University (grant no.
2013ZD014).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YX was responsible for the conception and design of
the study. YX, RG, JL, YT and FX conducted the experiments. LL
analysed the data, RG drafted the work and revised it critically
for important intellectual content. All authors have read and
approved the final manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All animal procedures were conducted with the
approval of the Animal Care and Use Committee of Guangxi Medical
University (Nanning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miner JR and Burton JH: Clinical practice
advisory: Emergency department procedural sedation with propofol.
Ann Emerg Med. 50:182–187, 187.e1. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vasileiou I, Xanthos T, Koudouna E, Perrea
D, Klonaris C, Katsargyris A and Papadimitriou L: Propofol: A
review of its non-anaesthetic effects. Eur J Pharmacol. 605:1–8.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Cottrell JE and Kass IS: Effects
of desflurane and propofol on electrophysiological parameters
during and recovery after hypoxia in rat hippocampal slice CA1
pyramidal cells. Neuroscience. 160:140–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Creeley C, Dikranian K, Dissen G, Martin
L, Olney J and Brambrink A: Propofol-induced apoptosis of neurones
and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br
J Anaesth. 110 (Suppl 1):i29–i38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ko HM, Kim SY, Joo SH, Cheong JH, Yang SI,
Shin CY and Koo BN: Synergistic activation of
lipopolysaccharide-stimulated glial cells by propofol. Biochem
Biophys Res Commun. 438:420–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun WC and Pei L: rno-miR-665 targets
BCL2L1 (Bcl-xl) and increases vulnerability to propofol in
developing astrocytes. J Neurochem. 138:233–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Logan S, Jiang C, Yan Y, Inagaki Y, Arzua
T and Bai X: Propofol alters long non-coding RNA profiles in the
neonatal mouse hippocampus: Implication of novel mechanisms in
anesthetic-induced developmental neurotoxicity. Cell Physiol
Biochem. 49:2496–2510. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heckman PRA, Blokland A, Bollen EPP and
Prickaerts J: Phosphodiesterase inhibition and modulation of
corticostriatal and hippocampal circuits: Clinical overview and
translational considerations. Neurosci Biobehav Rev. 87:233–254.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Liang Z, Sun W and Pei L:
Repeated propofol anesthesia induced downregulation of hippocampal
miR-132 and learning and memory impairment of rats. Brain Res.
1670:156–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kajimoto M, Atkinson DB, Ledee DR, Kayser
EB, Morgan PG, Sedensky MM, Isern NG, Des Rosiers C and Portman MA:
Propofol compared with isoflurane inhibits mitochondrial metabolism
in immature swine cerebral cortex. J Cereb Blood Flow Metab.
34:514–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui Y, Ling-Shan G, Yi L, Xing-Qi W,
Xue-Mei Z and Xiao-Xing Y: Repeated administration of propofol
upregulated the expression of c-Fos and cleaved-caspase-3 proteins
in the developing mouse brain. Indian J Pharmacol. 43:648–651.
2011.PubMed/NCBI
|
|
12
|
Lv J, Liang Y, Tu Y, Chen J and Xie Y:
Hypoxic preconditioning reduces propofol-induced neuroapoptosis via
regulation of Bcl-2 and Bax and downregulation of activated
caspase-3 in the hippocampus of neonatal rats. Neurol Res.
40:767–773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baillieul S, Chacaroun S, Doutreleau S,
Detante O, Pépin JL and Verges S: Hypoxic conditioning and the
central nervous system: A new therapeutic opportunity for brain and
spinal cord injuries? Exp Biol Med (Maywood). 242:1198–1206. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okuda S, Sufu-Shimizu Y, Kato T, Fukuda M,
Nishimura S, Oda T, Kobayashi S, Yamamoto T, Morimoto S and Yano M:
CaMKII-mediated phosphorylation of RyR2 plays a crucial role in
aberrant Ca2+ release as an arrhythmogenic substrate in
cardiac troponin T-related familial hypertrophic cardiomyopathy.
Biochem Biophys Res Commun. 496:1250–1256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang C, Du F, Cang J and Xue Z: Pink1
attenuates propofol-induced apoptosis and oxidative stress in
developing neurons. J Anesth. 32:62–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei ZZ, Zhu YB, Zhang JY, McCrary MR, Wang
S, Zhang YB, Yu SP and Wei L: Priming of the cells: Hypoxic
preconditioning for stem cell therapy. Chin Med J (Engl).
130:2361–2374. 2017.PubMed/NCBI
|
|
17
|
Tsui YP, Mung AK, Chan YS, Shum DK and
Shea GK: Hypoxic preconditioning of marrow-derived progenitor cells
as a source for the generation of mature schwann cells. J Vis Exp.
Jun 14–2017.doi: 10.3791/55794. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv J, Wei Y, Chen Y, Zhang X, Gong Z,
Jiang Y, Gong Q, Zhou L, Wang H and Xie Y: Dexmedetomidine
attenuates propofol-induce neuroapoptosis partly via the activation
of the PI3k/Akt/GSK3β pathway in the hippocampus of neonatal rats.
Environ Toxicol Pharmacol. 52:121–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong Y, Liang Y, Chen J, Li L, Qin Y,
Guan E, He D, Wei Y, Xie Y and Xiao Q: Propofol inhibits
proliferation and induces neuroapoptosis of hippocampal neurons in
vitro via downregulation of NF-κB p65 and Bcl-2 and upregulation of
caspase-3. Cell Biochem Funct. 32:720–729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galluzzi L and Vanpouille-Box C: BAX and
BAK at the gates of innate immunity. Trends Cell Biol. 28:343–345.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rahmani M, Nkwocha J, Hawkins E, Pei X,
Parker RE, Kmieciak M, Leverson JD, Sampath D, Ferreira-Gonzalez A
and Grant S: Cotargeting BCL-2 and PI3K induces BAX-dependent
mitochondrial apoptosis in AML cells. Cancer Res. 78:3075–3086.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma ZW and Liu DX: Humanin decreases
mitochondrial membrane permeability by inhibiting the membrane
association and oligomerization of Bax and Bid proteins. Acta
Pharmacol Sin. 39:1012–1021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu C, Li X, Guo P and Wang J:
Hypoxia-induced activation of JAK/STAT3 signaling pathway promotes
trophoblast cell viability and angiogenesis in preeclampsia. Med
Sci Monit. 23:4909–4917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karen T, Schlager GW, Bendix I, Sifringer
M, Herrmann R, Pantazis C, Enot D, Keller M, Kerner T and
Felderhoff-Mueser U: Effect of propofol in the immature rat brain
on short- and long-term neurodevelopmental outcome. PLoS One.
8:e644802013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stetler RA, Leak RK, Gan Y, Li P, Zhang F,
Hu X, Jing Z, Chen J, Zigmond MJ and Gao Y: Preconditioning
provides neuroprotection in models of CNS disease: Paradigms and
clinical significance. Prog Neurobiol. 114:58–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bao D, Zhao W, Dai C, Wan H and Cao Y: H89
dihydrochloride hydrate and calphostin C lower the body temperature
through TRPV1. Mol Med Rep. 17:1599–1608. 2018.PubMed/NCBI
|
|
27
|
Yoo SB, Lee S, Lee JY, Kim BT, Lee JH and
Jahng JW: cAMP/PKA agonist restores the fasting-induced
down-regulation of nNOS expression in the paraventricular nucleus.
Korean J Physiol Pharmacol. 16:333–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Laycock JF, Hubbard JI, Schwartz JH,
Stanton BA and Valtin H: The cAMP agonist Sp-cAMPS stimulates
osmotic water transport across rat inner medullary collecting duct
cells. Ann N Y Acad Sci. 689:606–608. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lonze BE and Ginty DD: Function and
regulation of CREB family transcription factors in the nervous
system. Neuron. 35:605–623. 2002. View Article : Google Scholar : PubMed/NCBI
|