Introduction
Annually, in the USA, approximately six million
children require general anesthesia (1,2).
Furthermore, in China, the number of children requiring anesthetics
is increasing (3). The human brain
develops rapidly between the embryonic stage and 2 years after
birth. During this period the number of neurons increases, and
large quantities of axons, dendrites and synapses are formed
(4–6). Previous clinical studies have
demonstrated that anesthesia and surgery may lead to cognitive
impairment (7,8). Therefore, decreasing the brain
neuronal damage caused by narcotic drugs is becoming an
increasingly popular research topic.
A number of previous studies identified that
inhalable narcotic drugs, including midazolam, propofol, isoflurane
and sevoflurane, are used in newborns (9–12).
Propofol is a general anesthetic used in surgical operations.
Previous studies demonstrated that propofol may induce
neurotoxicity in the brains of newborn animals (13–16).
In addition, previous studies identified that propofol-induced
apoptosis in numerous types of cells associated with neurons
(17–19). Therefore, it is crucial to identify
novel effective strategies to decrease the neurotoxicity of
propofol in the developing brain.
Phosphoprotein enriched in astrocytes 15 (PEA15) is
a phosphoprotein that was initially identified in astrocytes
(20). PEA15 is known to mediate
the signal transduction pathway; PEA15 serves as a signal
connection point, thus possessing the potential to directly
regulate cell behavior (21).
Previous studies demonstrated that PEA15 serves roles in numerous
processes, including tumor progression, diabetes, metabolic
disorders and nervous system diseases (22–26).
However, at present, to the best of the authors' knowledge, the
effect of PEA15 on propofol-induced hippocampal nerve injury has
not been investigated.
In the present study, propofol was used to damage
rat hippocampal neurons. Furthermore, the effect and potential
molecular mechanism of PEA15 on propofol-induced hippocampal
neuronal cell damage were examined by Cell Counting Kit-8 (CCK-8),
flow cytometry, western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis.
Materials and methods
Animals
Pregnant Sprague-Dawley (SD) rats (3 months old;
embryonic age E18; weight: 200–230 g) were purchased from Shanghai
SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The rats were
housed in cages at 22±1°C. The animals had free access to food and
water, with a constant humidity (50±10%) and with a 12 h light/dark
cycle. The animal experimental research was approved by the Ethics
Committee of The First People's Hospital of Changzhou, The Third
Affiliated Hospital of Soochow University (Changzhou, China).
Isolation of hippocampal neurons
The extraction and cultivation methods of the
neurons were performed as previously described (27,28).
In total, 5 newborn SD rats (3 male and 2 female; weight, 5.0–6.0
g) were sacrificed by cervical dislocation, within 24 h following
birth. A solution of 75% ethanol was used to disinfect the fetal
rats in sterile trays. Subsequently, the rats were guillotined. The
brain was removed using bent tweezers and placed in a precooled
Hank's balanced salt solution and the hippocampus was separated
from the brain. Subsequently, the hippocampus was digested using 2%
trypsin for 15 min at 37°C in a cell incubator with 5%
CO2. The digestion was stopped by adding neurobasal
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Cellbio, Shanghai, China). The
hippocampal neurons were obtained and maintained in an incubator at
37°C with 5% CO2. The morphology of the cells was
observed daily using a TMM-220 inverted light microscope
(magnification, ×200; Shanghai Tianxing Instrument Co., Ltd.,
Shanghai, China).
Cell treatment and transfection
Propofol was purchased from Fresenius Kabi Austria
GmbH (Graz, Austria). As previously described (29,30),
the hippocampal neurons were exposed to 50 µmol/l propofol for 0,
6, 12 and 24 h. Subsequently, cells were transfected with 50 nM
PEA15 cloned in pcDNA3.1 or empty pcDNA3.1 vectors (Invitrogen;
Thermo Fisher Scientific, Inc.) using Lipofectamine®
2000 (Thermo Fisher Scientific, Inc.) and cultured for 48 h at
37°C. A total of six experimental groups were considered in the
present study: Control group (0.1% PBS), empty-vector group
(transfected with pcDNA3.1 vector), PEA15 group (transfected with
PEA15), propofol group (treated with 50 µmol/l propofol),
empty-vector + propofol group (transfected with pcDNA3.1 empty
vector and treated with 50 µmol/l propofol), and PEA15+propofol
group (transfected with PEA15 and treated with 50 µmol/l
propofol).
Cell viability assay
A CCK-8 (Beyotime Institute of Biotechnology,
Haimen, China) assay was conducted to detect the cell viability. A
total of 2×103 cells/ml in logarithmic phase were
inoculated in 96-well plates and maintained in an incubator at 37°C
with 5% CO2 for 24 h. Subsequently, cells were treated
with propofol at 12.5, 25 and 50 µmol/l for 6 h at 37°C and divided
into the aforementioned experimental groups, and transferred into
the incubator for 6, 12 and 24 h. CCK-8 reagent was added into each
well. Cells were cultured in the incubator at 37°C with 5%
CO2 for 2.5 h. An HBS-1096A microplate reader (Nanjing
Detie Experimental Equipment Co., Ltd., Nanjing, China) was used to
measure the absorbance at 450 nm.
Flow cytometry assay
Cell apoptosis was determined using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit [Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.,
Hangzhou, China]. The cells were digested with 0.25% trypsin
(Beyotime Institute of Biotechnology). The supernatant was removed
and cells were resuspended in an incubation buffer at a density of
2×105 cells/ml. Annexin V-FITC and PI were added to the
cell suspension in the dark at room temperature for 20 min. The
CytoFLEX flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) was
used to detect cell apoptosis. Cell proliferation was assessed
using the CellTrace™ carboxyfluorescein succinimidyl ester (CFSE)
cell proliferation kit (Invitrogen; Thermo Fisher Scientific,
Inc.). The cells were digested with 0.25% trypsin (Beyotime
Institute of Biotechnology). The supernatant was removed and cells
were resuspended in an incubation buffer at a density of
2×105 cells/ml. The CFSE solution was added to the cell
suspension and incubated for 15 min at room temperature. A CytoFLEX
flow cytometer (CytExpert software v1.2; Beckman Coulter, Inc.) was
used to measure the cell proliferation.
Western blotting
Subsequent to being treated, cells were lysed with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). The protein content was analyzed using the Bradford
method. A 10% SDS-PAGE was used to separate (20 µg/lane) proteins,
that were transferred onto a polyvinylidene difluoride (PVDF)
membrane. The PVDF membrane was sealed and blocked using 5% non-fat
milk at 37°C for 1.5 h. Subsequently, the PVDF membrane was
incubated with primary antibodies at 4°C for 24 h. The following
antibodies were used in the present study: Anti-PEA15 [1:800; Cell
Signaling Technology (CST), Inc., Danvers, MA, USA; cat. no. 2780],
anti-pro-caspase-3 (1:1,000; Abcam, Cambridge, UK; cat. no.
ab32150), anti-active caspase-3 (1:600; Abcam; cat. no. ab13847),
anti-B-cell lymphoma-2 (Bcl-2; 1:700; Abcam; cat. no. ab59348),
anti-apoptosis regulator BAX (Bax; 1:1,000; Abcam; cat. no.
ab32503), anti-phosphorylated (p)-extracellular signal-regulated
kinases 1/2 (ERK1/2; 1:1,000; CST, Inc.; cat. no. 4370),
anti-ERK1/2 (1:600; CST, Inc.; cat. no. 9102), anti-ribosomal S6
kinase 2 (RSK2; 1:500; Abcam; cat. no. ab32133),
anti-p-cAMP-response element binding protein 1 (CREB1; 1:800;
Abcam; cat. no. ab32096), anti-CREB1 (1:700; Abcam; cat. no.
ab32515) and anti-β-actin (1:5,000; Abcam; cat. no. ab179467). The
PVDF membrane was subsequently incubated with Horseradish
peroxidase-conjugated secondary antibodies (goat anti-rabbit; cat.
no. ab205718; 1:2,000) at room temperature for 1 h. The proteins
were detected using enhanced chemiluminescent reagents (Thermo
Fisher Scientific, Inc.). The density of bands was determined using
the Quantity One software version 2.4 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
RT-qPCR
Total RNA from the neurons was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was reverse transcribed to cDNA using an RT kit
(HaiGene, Harbin, China) at 37°C for 15 min followed by 85°C for 5
sec. The ABI 7500 Thermocycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to amplify the cDNA with AceQ qPCR
SYBR-Green Master Mix (Vazyme, Piscataway, NJ, USA). qPCR
conditions were set as follows: 10 min pretreatment at 95°C, 96°C
for 15 sec, 63°C for 45 sec (35 cycles) and a final extension at
75°C for 10 min. β-actin was used as the reference gene. The
primers used were purchased from Shanghai ShineGene Molecular
Biotech, Inc. (Shanghai, China) and were the following: PEA15
(forward, 5′-CCTGACCAACAACATCACCC-3′ and reverse,
5′-GATCTTCAGCACACGGGTTC-3′), Bcl-2 (forward,
5′-GCCTTCTTTGAGTTCGGTGG-3′ and reverse), Bax (forward,
5′-GAGACACCTGAGCTGACCTT-3′ and reverse, 5′-CGTCTGCAAACATGTCAGCT-3′)
and β-actin (forward, 5′-CAACATGGATGAGCGGAAGG-3′ and reverse,
5′-GCAGTGTAGCAGCATCGAAA-3′). The 2−ΔΔCq method was used
to calculate gene expression levels (31).
Statistical analysis
SPSS (version 20; IBM Corp., Armonk, NY, USA)
software was used for statistical analysis. The data are presented
as the mean ± standard deviation. The experiments were
independently repeated at least three times. The experimental data
were analyzed by one-way analysis of variance with Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Propofol inhibits the expression level
of PEA15 in hippocampal neurons
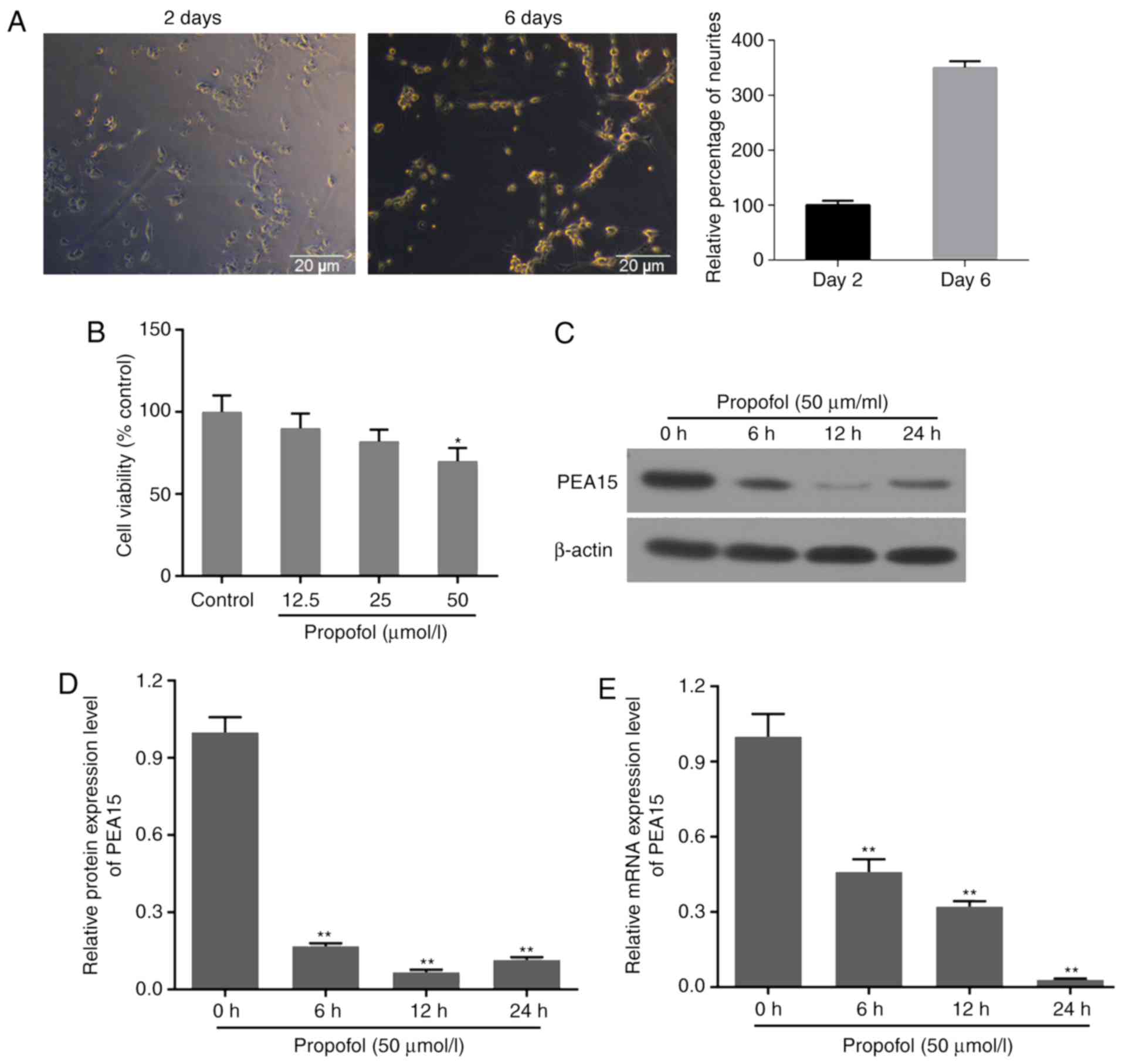
Following hippocampal neuron isolation, the cells
were cultured for 2 days. The neurons presented 2–3 neurites, and
were flat and multilateral. Following 6 days of incubation, the
number of neurites increased, and the cells interconnected with
each other through the neurites. Notably, the majority of the cells
aggregated and exhibited a visible halo surrounding the cells
(Fig. 1A).
Cell viability decreased following treatment with
propofol at 12.5, 25 and 50 µmol/l for 6 h. The decrease in cell
viability was significant in the 50 µmol/l group (Fig. 1B). Therefore, 50 µmol/l propofol
was selected as the working concentration. Western blotting and
RT-qPCR assays were performed to examine the effects of propofol on
the expression level of PEA15 in hippocampal neurons. The western
blotting suggested that the protein expression level of PEA15 was
decreased following treatment with propofol for 6, 12 and 24 h.
Notably, the protein expression level of PEA15 was lower at 12 h
compared with 24 h; however, the difference was not significant
(Fig. 1C and D). Furthermore,
propofol significantly decreased the mRNA expression level of PEA15
in a time-dependent manner (Fig.
1E).
Overexpression of PEA15 reverts the
decreased viability of hippocampal neurons induced by propofol
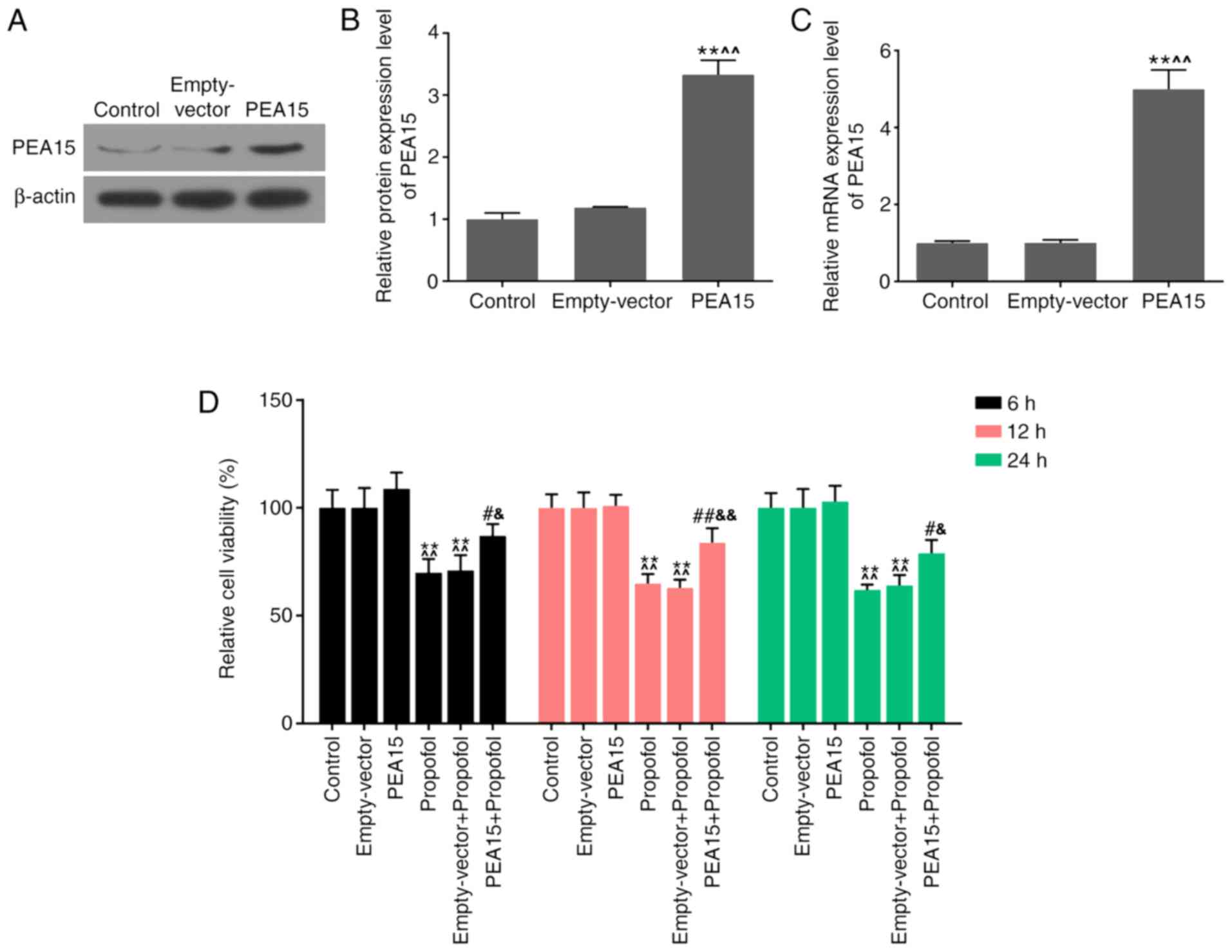
The transfection efficiency of PEA15 in hippocampal
neurons was examined by western blotting and RT-qPCR analysis. The
western blotting results demonstrated that the protein expression
level of PEA15 was significantly increased following transfection
with PEA15 (Fig. 2A and B).
Additionally, the mRNA expression level of PEA15 was consistent
with the protein expression level (Fig. 2C).
CCK-8 was used to test the effects of PEA15 and
propofol on the viability of hippocampal neurons. The results
suggested that propofol significantly decreased cell viability,
compared with the control and empty-vector groups. The cell
viability was unaffected following transfection of PEA15 alone.
However, the cell viability in the PEA15 + propofol group was
significantly increased compared with the propofol and empty-vector
+ propofol groups (Fig. 2D).
Overexpression of PEA15 represses the
propofol-induced apoptosis of hippocampal neurons
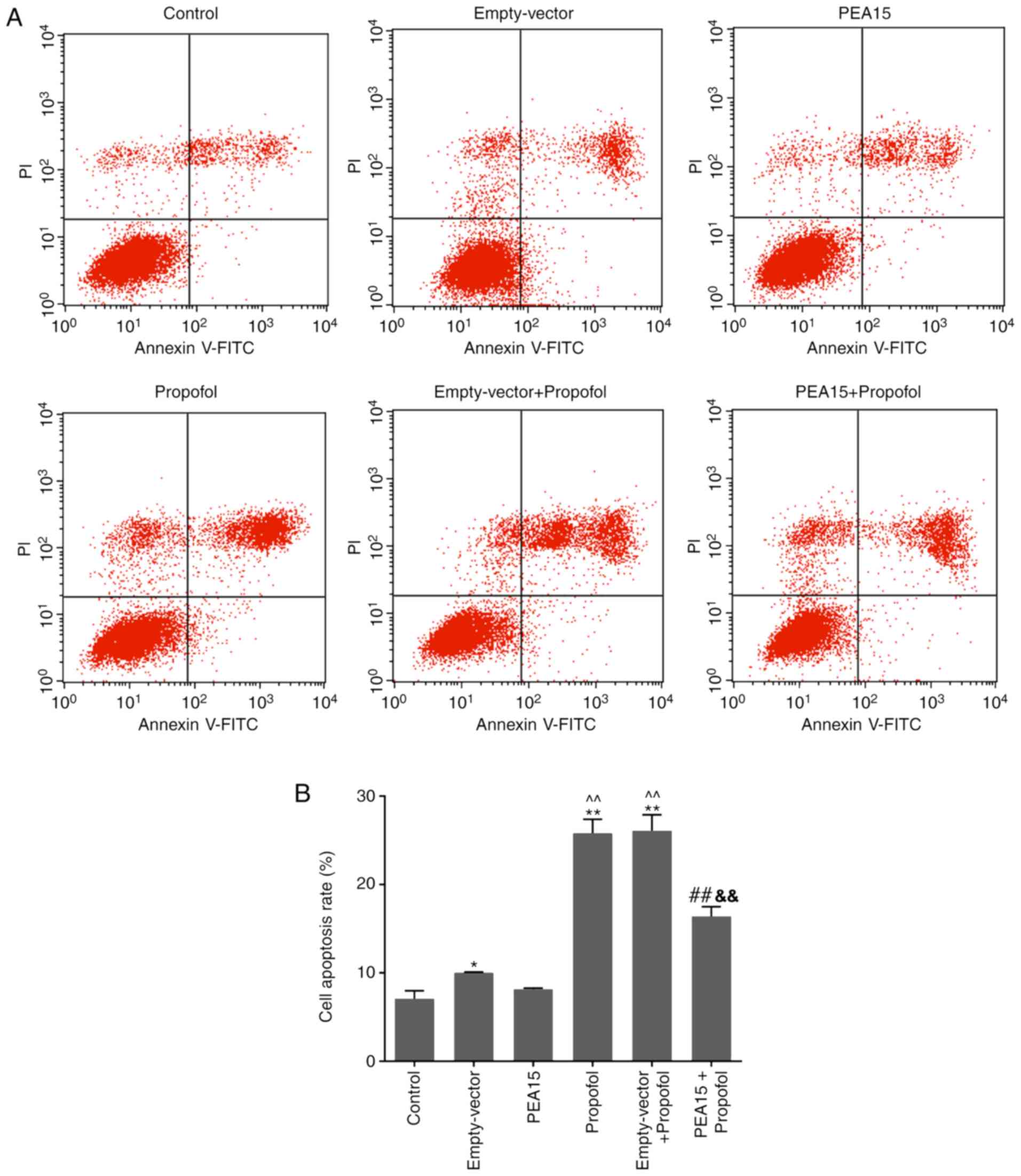
The flow cytometry results suggested that propofol
significantly promoted cell apoptosis. However, PEA15
overexpression was able to decrease the apoptosis of cells treated
with propofol compared with the propofol and empty-vector +
propofol groups (Fig. 3).
Overexpression of PEA15 reverts the
proliferation of hippocampal neurons inhibited by propofol
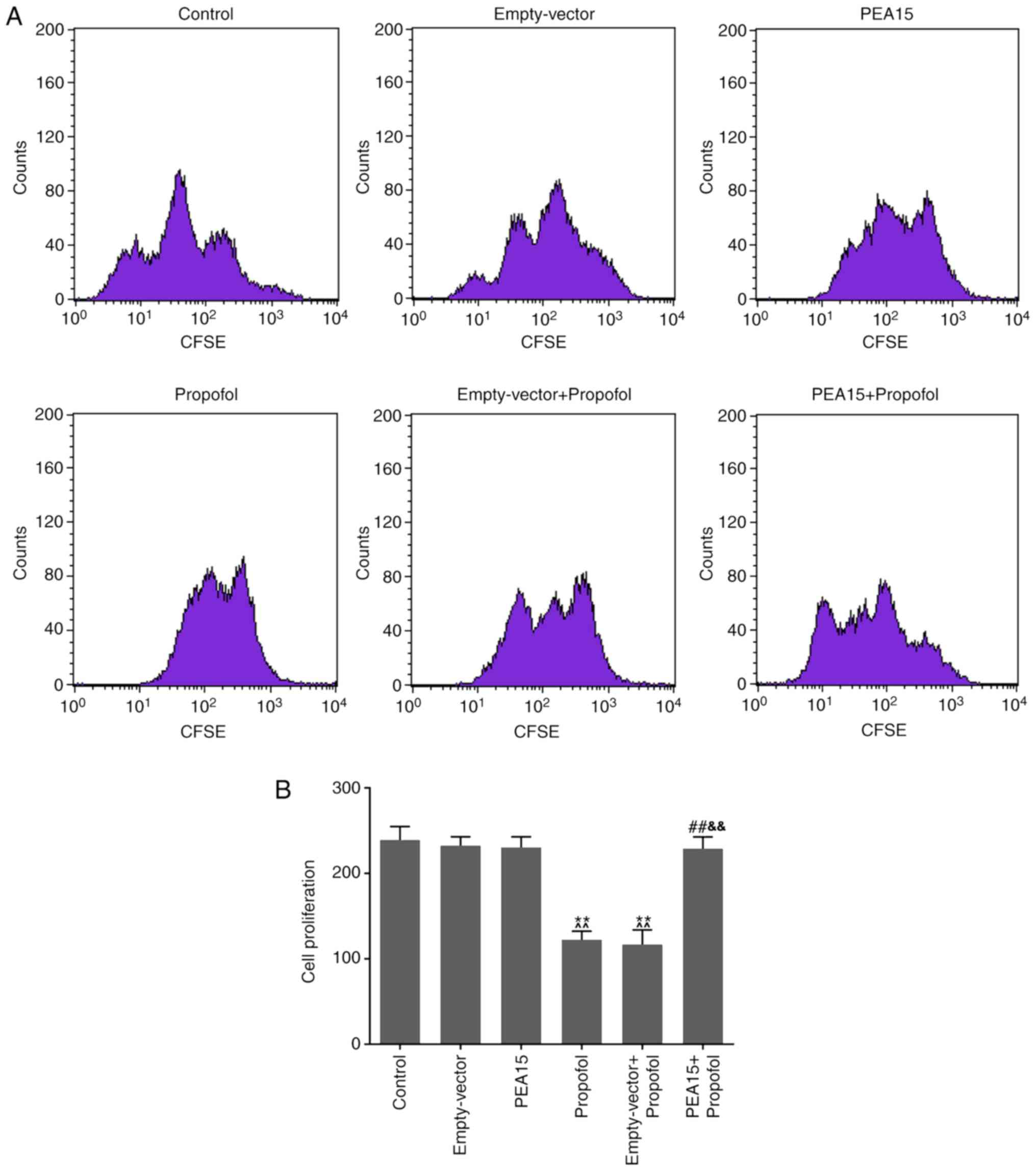
The cell proliferation assay suggested that propofol
significantly decreased the proliferation of hippocampal neurons.
In addition, transfection of PEA15 was sufficient to increase the
cell proliferation of cells treated with propofol compared with the
propofol and empty-vector + propofol groups (Fig. 4).
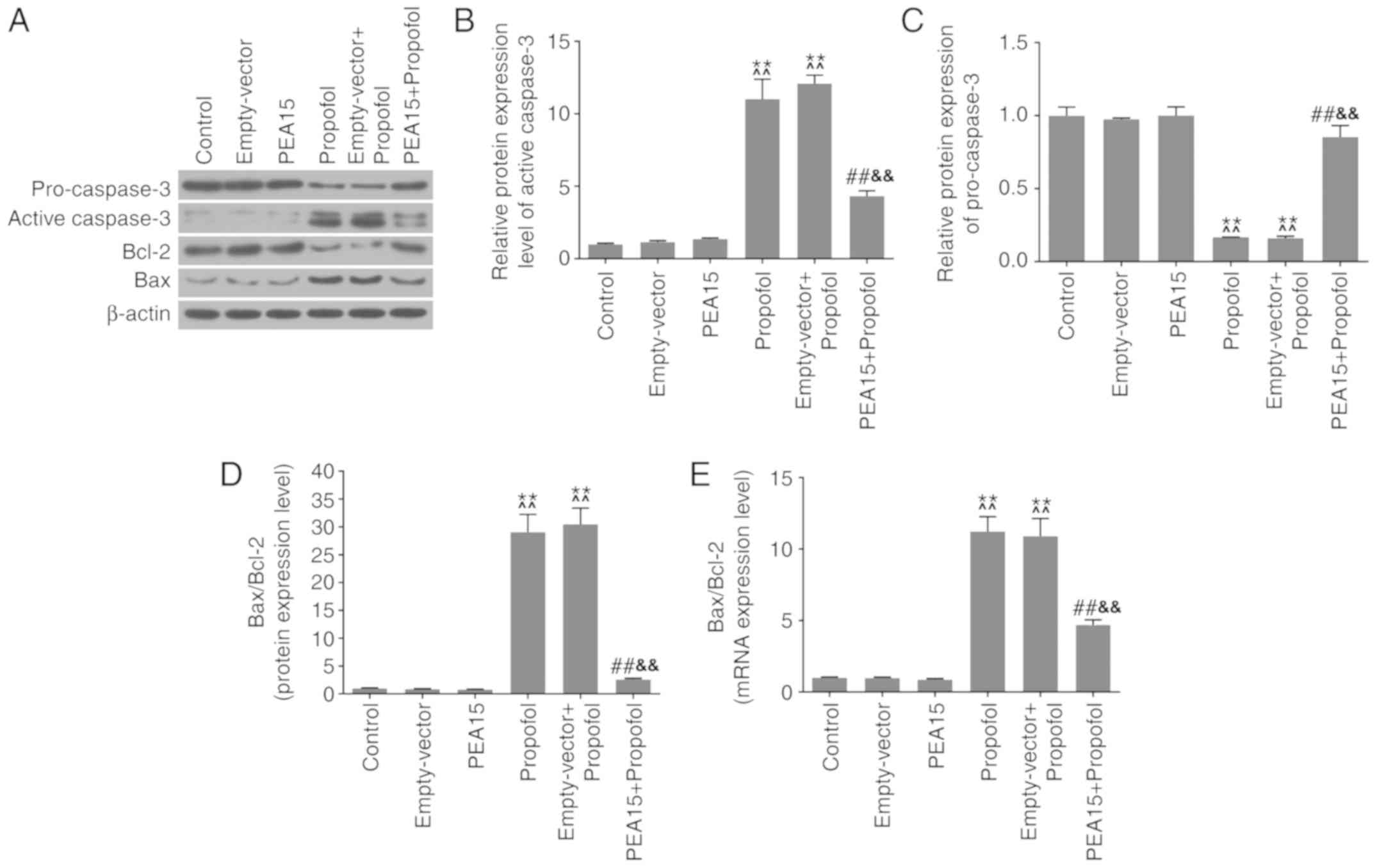
PEA15 and propofol regulate
apoptosis-associated factors in hippocampal neurons
Western blotting and RT-qPCR analysis were performed
to investigate the effects of PEA15 and propofol on
apoptosis-associated factors. The western blotting results
suggested that treatment with propofol promoted the protein
expression levels of active caspase-3 and Bax (Fig. 5A and B), and led to downregulation
of pro-caspase-3 and Bcl-2 protein expression levels (Fig. 5A and C). By analyzing the protein
and the mRNA expression levels of Bax and Bcl-2, it was identified
that the Bax/Bcl-2 ratio was significantly increased following
treatment with propofol (Fig. 5D and
E). Notably, PEA15 overexpression led to opposite effects.
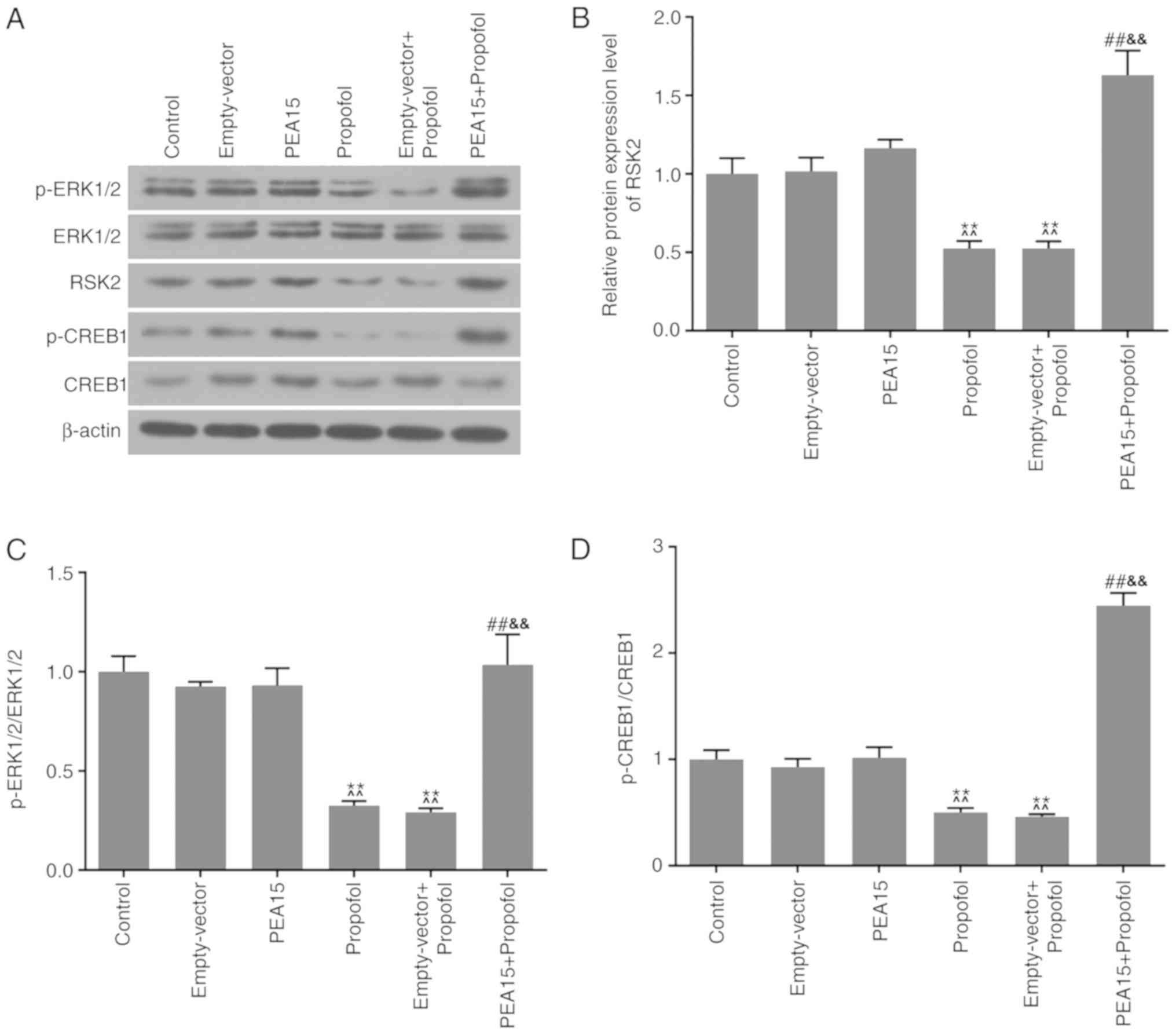
PEA15 and propofol regulate
ERK-CREB-RSK2 signaling in hippocampal neurons
Western blotting was conducted to determine the
protein expression levels of ERK1/2, p-ERK1/2, p-CREB1, CREB1 and
RSK2, in order to examine the molecular mechanisms of PEA15 and
propofol on hippocampal neurons. The present results suggested that
propofol decreased the protein expression levels of p-ERK1/2,
p-CREB1 and RSK2 (Fig. 6A and B).
Additionally, it was identified that the ratios between the
phosphorylated proteins p-ERK1/2 and p-CREB1, and the respective
non-phosphorylated proteins ERK1/2 and CREB1, were significantly
decreased (Fig. 6C and D) when
cells were treated with propofol. Furthermore, PEA15 promoted the
protein expression levels of p-ERK1/2, RSK2 and p-CREB1 in the
hippocampal neurons that were treated with propofol. Notably, the
protein expression levels of ERK1/2 and CREB1 among groups were not
significantly affected.
Discussion
The majority of cultured hippocampal neurons derive
from the hippocampus of neonatal rats, as suckling mice gain an
advantage in finding a location to feed (32,33).
A previous study identified that hippocampal neurons cultured at
day 6 are similar to neurons of a developing human brain (34). During this period, neurons are
sensitive to external stimuli, including neurotoxicity caused by
anesthetics (34). Therefore, in
the present study, primary hippocampal neurons were extracted from
neonatal rats and used as a model of developing neurons. Following
6 days of culturing, the cell morphology remained stable and the
neurons were treated with propofol.
Accumulating evidence suggested that propofol was
neurotoxic in vitro (35–37).
Monni et al (36) observed
that propofol promoted cell death in rat hypoglossal motoneurons.
Yan et al (37)
demonstrated that propofol induced apoptosis of hippocampal neurons
in newborn mice. Furthermore, numerous previous studies
demonstrated that propofol is a potent inducer of neuronal damage
(35,38,39).
Similar to previous studies, the present results suggested that
propofol significantly decreased the viability and proliferation of
neuronal cells, promoting cell apoptosis. Therefore, in the present
study, treatment with propofol was used as a model for damaging
neuronal cells.
A recent study demonstrated that the expression of
PEA15 was downregulated in diabetic SD rats, promoting middle
cerebral artery occlusion (25). A
previous study demonstrated that overexpression of PEA15 decreased
glucose deprivation-induced cell death in neurons (40). Therefore, it was hypothesized that
the overexpression of PEA15 may decrease the neurotoxicity of
propofol, and that PEA15 and propofol may be associated in
hippocampal neurons. As hypothesized, the experimental results
suggested that propofol significantly decreased PEA15 expression.
Furthermore, the CCK-8 and flow cytometry results suggested that
PEA15 overexpression significantly increased the viability and
proliferation of neurons, and decreased the apoptosis of neuronal
cells, reverting the effects of propofol. The present results
suggested that overexpression of PEA15 conferred a neuroprotective
effect on propofol-induced neuronal damage by promoting
proliferation and decreasing apoptosis.
In order to investigate the mechanisms underlying
PEA15 and propofol in neuronal cells, the protein expression levels
of apoptosis-associated factors, including pro-caspase-3, active
caspase-3, Bcl-2 and Bax, were examined by western blotting and
RT-qPCR analysis. Liang et al (35) demonstrated that propofol induced
apoptosis of the developing neurons by upregulating the protein
expression levels of cleaved-caspase-3 and Bax, and by
downregulating the Bcl-2 protein expression level. Similarly, the
present results demonstrated that propofol significantly increased
the protein expression levels of active caspase-3 and Bax, and
decreased pro-caspase-3 and Bcl-2 expression. Furthermore, Ahn
et al (22) observed that
PEA-15 increased Bcl-2 and pro-caspase-3 protein expression levels,
and decreased Bax and cleaved-caspase-3 protein expression levels
in neuroblastoma cells treated with 1-methyl-4-phenylpyridinium.
The present study demonstrated that the overexpression of PEA15 in
neurons treated with propofol, increased the protein expression
levels of pro-caspase-3 and Bcl-2, and Bax and cleaved-caspase-3
expression levels were downregulated. The present results suggested
that overexpression of PEA15 decreased the apoptotic effects
induced by propofol in nerve cells by upregulating pro-caspase-3
and Bcl-2, and by downregulating active caspase-3 and Bax.
ERK-CREB signaling regulates cell growth and
apoptosis of rat hippocampal neurons (41–43).
Furthermore, RSK2 was observed to be involved in the ERK pathway in
the hippocampus (44). Kozinn
et al (45) demonstrated
that propofol inhibited the ERK-CREB pathway in hippocampal
neurons. In the present study, it was hypothesized that the
mechanisms underlying the PEA15 and propofol effects on the
proliferation and apoptosis of hippocampal neurons may be
associated with the ERK-CREB pathway. The present results suggested
that propofol significantly repressed the phosphorylation levels of
ERK1/2 and CREB1, and decreased the protein expression level of
RSK2. However, overexpression of PEA15 was able to revert the
protein expression levels of p-ERK1/2, p-CREB1 and RSK2, which were
suppressed by propofol. The present results suggested that PEA15
overexpression promoted the ERK-CREB-RSK2 signaling pathway.
Collectively, the present results suggested that the cell damage
caused by propofol was attenuated by PEA15 overexpression. However,
testing the effects of PEA15 knockdown may further aid the
understanding of the role of PEA15 in neuronal injury.
In conclusion, the present results suggested that
PEA15 ameliorated propofol-induced hippocampal nerve damage by
promoting cell proliferation, and PEA15 was able to decrease cell
apoptosis by upregulating the ERK-CREB-RSK2 signaling pathway. The
present study suggested that PEA15 may represent a potential
therapeutic agent to treat human diseases characterized by neuronal
damage.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FX provided a substantial contribution to conception
and design of the present study. QL and ZC were involved in data
acquisition, analysis and interpretation. ZC and FX were involved
in drafting the article or critically revising it for important
intellectual content. All authors gave final approval of the
version to be published. All authors agreed to be accountable for
all aspects of the work, and in ensuring that questions related to
the accuracy or integrity of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The animal experimental research was approved by the
Ethics Committee of The First People's Hospital of Changzhou, The
Third Affiliated Hospital of Soochow University (Changzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
King MR and Feldman JM: Optimal management
of apparatus dead space in the anesthetized infant. Paediatr
Anaesth. 27:1185–1192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kotwani MB and Malde AD: Comparison of
maintenance, emergence and recovery characteristics of sevoflurane
and desflurane in pediatric ambulatory surgery. J Anaesthesiol Clin
Pharmacol. 33:503–508. 2017.PubMed/NCBI
|
|
3
|
Wang CH, Luo J, Li J, Zhang JZ, Huang SY,
Shao W and Ma HS: Efficacy of inhalational sevoflurane anesthesia
induction on inhibiting the stress response to endotracheal
intubation in children with congenital heart disease. Eur Rev Med
Pharmacol Sci. 22:1113–1117. 2018.PubMed/NCBI
|
|
4
|
Chimelli L, Moura Pone S, Avvad-Portari E,
Farias Meira Vasconcelos Z, Araújo Zin A, Prado Cunha D, Raposo
Thompson N, Lopes Moreira ME, Wiley CA and da Silva Pone MV:
Persistence of zika virus after birth: Clinical, virological,
neuroimaging, and neuropathological documentation in a 5-month
infant with congenital zika syndrome. J Neuropathol Exp Neurol.
77:193–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hannagan T, Nieder A, Viswanathan P and
Dehaene S: A random-matrix theory of the number sense. Philos Trans
R Soc Lond B Biol Sci. 373:201702532017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Subedi L, Huang H, Pant A, Westgate PM,
Bada HS, Bauer JA, Giannone PJ and Sithisarn T: Plasma
brain-derived neurotrophic factor levels in newborn infants with
neonatal abstinence syndrome. Front Pediatr. 5:2382017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Backeljauw B, Holland SK, Altaye M and
Loepke AW: Cognition and brain structure following early childhood
surgery with anesthesia. Pediatrics. 136:e1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun L: Early childhood general anaesthesia
exposure and neurocognitive development. Br J Anaesth. 105 (Suppl
1):i61–i68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coleman K, Robertson ND, Dissen GA,
Neuringer MD, Martin LD, Cuzon Carlson VC, Kroenke C, Fair D and
Brambrink AM: Isoflurane anesthesia has long-term consequences on
motor and behavioral development in infant rhesus macaques.
Anesthesiology. 126:74–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Dib M and Soul JS: The use of
phenobarbital and other anti-seizure drugs in newborns. Semin Fetal
Neonatal Med. 22:321–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu L, Pan J, Zhang S, Yu J, He K, Shu S
and Wang R: Propofol in combination with remifentanil for cesarean
section: Placental transfer and effect on mothers and newborns at
different induction to delivery intervals. Taiwan J Obstet Gynecol.
56:521–526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Razlevice I, Rugyte DC, Strumylaite L and
Macas A: Assessment of risk factors for cerebral oxygen
desaturation during neonatal and infant general anesthesia: An
observational, prospective study. BMC Anesthesiol. 16:1072016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cattano D, Young C, Straiko MM and Olney
JW: Subanesthetic doses of propofol induce neuroapoptosis in the
infant mouse brain. Anesth Analg. 106:1712–1714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Creeley C, Dikranian K, Dissen G, Martin
L, Olney J and Brambrink A: Propofol-induced apoptosis of neurones
and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br
J Anaesth. 110 (Suppl 1):i29–i38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pearn ML, Hu Y, Niesman IR, Patel HH,
Drummond JC, Roth DM, Akassoglou K, Patel PM and Head BP: Propofol
neurotoxicity is mediated by p75 neurotrophin receptor activation.
Anesthesiology. 116:352–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu D, Jiang Y, Gao J, Liu B and Chen P:
Repeated exposure to propofol potentiates neuroapoptosis and
long-term behavioral deficits in neonatal rats. Neurosci Lett.
534:41–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kahraman S, Zup SL, McCarthy MM and Fiskum
G: GABAergic mechanism of propofol toxicity in immature neurons. J
Neurosurg Anesthesiol. 20:233–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sall JW, Stratmann G, Leong J, Woodward E
and Bickler PE: Propofol at clinically relevant concentrations
increases neuronal differentiation but is not toxic to hippocampal
neural precursor cells in vitro. Anesthesiology. 117:1080–1090.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spahr-Schopfer I, Vutskits L, Toni N,
Buchs PA, Parisi L and Muller D: Differential neurotoxic effects of
propofol on dissociated cortical cells and organotypic hippocampal
cultures. Anesthesiology. 92:1408–1417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Danziger N, Yokoyama M, Jay T, Cordier J,
Glowinski J and Chneiweiss H: Cellular expression, developmental
regulation, and phylogenic conservation of PEA-15, the astrocytic
major phosphoprotein and protein kinase C substrate. J Neurochem.
64:1016–1025. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greig FH and Nixon GF: Phosphoprotein
enriched in astrocytes (PEA)-15: A potential therapeutic target in
multiple disease states. Pharmacol Ther. 143:265–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahn EH, Kim DW, Shin MJ, Kim HR, Kim SM,
Woo SJ, Eom SA, Jo HS, Kim DS, Cho SW, et al: PEP-1-PEA-15 protects
against toxin-induced neuronal damage in a mouse model of
Parkinson's disease. Biochim Biophys Acta. 1840:1686–1700. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greig FH, Kennedy S, Gibson G, Ramos JW
and Nixon GF: PEA-15 (Phosphoprotein Enriched in Astrocytes 15) is
a protective mediator in the vasculature and is regulated during
neointimal hyperplasia. J Am Heart Assoc. 6:e0069362017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohammed HN, Pickard MR and
Mourtada-Maarabouni M: The protein phosphatase 4-PEA15 axis
regulates the survival of breast cancer cells. Cell Signal.
28:1389–1400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sung JH and Koh PO: Hyperglycemia
aggravates decreases of PEA-15 and its two phosphorylated forms in
cerebral ischemia. J Vet Med Sci. 79:654–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei Y: On the quest of cellular functions
of PEA-15 and the therapeutic opportunities. Pharmaceuticals
(Basel). 8:455–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang JY, Wang K, Vermehren-Schmaedick A,
Adelman JP and Cohen MS: PARP6 is a regulator of hippocampal
dendritic morphogenesis. Sci Rep. 6:185122016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang JS, Xie BX, Bian XL, Xue Y, Wei NN,
Zhou JH, Hao YC, Li G, Zhang LR and Wang KW: Identification and in
vitro pharmacological characterization of a novel and selective α7
nicotinic acetylcholine receptor agonist, Br-IQ17B. Acta Pharmacol
Sin. 36:800–812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsu HT, Tseng YT, Hsu YY, Cheng KI, Chou
SH and Lo YC: Propofol attenuates lipopolysaccharide-induced
reactive oxygen species production through activation of Nrf2/GSH
and suppression of NADPH oxidase in human alveolar epithelial
cells. Inflammation. 38:415–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia JH, Shi XY, Xu ZD, Liu G and Wang YH:
In vitro effects of propofol on apoptosis and Bax expression
induced by TNF-α in mouse spinal cord neurons. Acad J Sec Military
Med Univ. 27:169–172. 2006.
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Facci L and Skaper SD: Culture of rodent
cortical, hippocampal, and striatal neurons. Methods Mol Biol.
1727:39–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rivera-Carvantes MC, Jarero-Basulto JJ,
Feria-Velasco AI, Beas-Zárate C, Navarro-Meza M, González-López MB,
Gudiño-Cabrera G and García-Rodríguez JC: Changes in the expression
level of MAPK pathway components induced by monosodium
glutamate-administration produce neuronal death in the hippocampus
from neonatal rats. Neuroscience. 365:57–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eibl JK, Strasser BC and Ross GM:
Structural, biological, and pharmacological strategies for the
inhibition of nerve growth factor. Neurochem Int. 61:1266–1275.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang C, Du F, Cang J and Xue Z: Pink1
attenuates propofol-induced apoptosis and oxidative stress in
developing neurons. J Anesth. 32:62–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Monni L, Ghezzi F, Corsini S and Nistri A:
Neurotoxicity of propofol on rat hypoglossal motoneurons in vitro.
Neurosci Lett. 655:95–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan Y, Qiao S, Kikuchi C, Zaja I, Logan S,
Jiang C, Arzua T and Bai X: Propofol induces apoptosis of neurons
but not astrocytes, oligodendrocytes, or neural stem cells in the
neonatal mouse hippocampus. Brain Sci. 7:E1302017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Yan Y, Inagaki Y, Logan S, Bosnjak
ZJ and Bai X: Insufficient astrocyte-derived brain-derived
neurotrophic factor contributes to propofol-induced neuron death
through Akt/glycogen synthase kinase 3β/mitochondrial fission
pathway. Anesth Analg. 125:241–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lv J, Wei Y, Chen Y, Zhang X, Gong Z,
Jiang Y, Gong Q, Zhou L, Wang H and Xie Y: Dexmedetomidine
attenuates propofol-induce neuroapoptosis partly via the activation
of the PI3k/Akt/GSK3β pathway in the hippocampus of neonatal rats.
Environ Toxicol Pharmacol. 52:121–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Q, Voloudakis G, Ren Y, Yoon Y,
Zhang E, Kajiwara Y, Shao Z, Xuan Z, Lebedev D, Georgakopoulos A
and Robakis NK: Presenilin1/γ-secretase protects neurons from
glucose deprivation-induced death by regulating miR-212 and PEA15.
FASEB J. 32:243–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shahin S, Banerjee S, Swarup V, Singh SP
and Chaturvedi CM: From the cover: 2.45-GHz microwave radiation
impairs hippocampal learning and spatial memory: Involvement of
local stress mechanism-induced suppression of iGluR/ERK/CREB
signaling. Toxicol Sci. 161:349–374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yi LT, Li J, Liu BB, Luo L, Liu Q and Geng
D: BDNF-ERK-CREB signalling mediates the role of miR-132 in the
regulation of the effects of oleanolic acid in male mice. J
Psychiatry Neurosci. 39:348–359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zuo H, Lin T, Wang D, Peng R, Wang S, Gao
Y, Xu X, Zhao L, Wang S and Su Z: RKIP regulates neural cell
apoptosis induced by exposure to microwave radiation partly through
the MEK/ERK/CREB pathway. Mol Neurobiol. 51:1520–1529. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kaphzan H, Doron G and Rosenblum K:
Co-application of NMDA and dopamine-induced rapid translation of
RSK2 in the mature hippocampus. J Neurochem. 103:388–399.
2007.PubMed/NCBI
|
|
45
|
Kozinn J, Mao L, Arora A, Yang L, Fibuch
EE and Wang JQ: Inhibition of glutamatergic activation of
extracellular signal-regulated protein kinases in hippocampal
neurons by the intravenous anesthetic propofol. Anesthesiology.
105:1182–1191. 2006. View Article : Google Scholar : PubMed/NCBI
|