Introduction
Esophageal carcinoma is a malignant tumor
originating from esophageal epithelium, which is characterized by
strong invasiveness and a high mortality rate (1). The incidence of esophageal cancer
ranks eighth among all types of cancer worldwide (2). A total of 80–85% of esophageal cancer
cases occur in developing countries, including China (3). Esophageal squamous cell carcinoma
(ESCC) is the major histopathological subtype of esophageal cancer
(4,5), and accounts for the majority of
esophageal cancer cases in China (6,7).
Despite great advances in medical and surgical treatments, the
prognosis of patients remains poor, with an overall 5-year survival
rate of 15–25% (8,9).
Currently, the clinical diagnosis of ESCC is mainly
based on serum protein tumor markers, endoscopy and
histopathological methods (10).
The application of serological tumor markers in clinical diagnosis,
including SCC antigen and carcinoembryonic antigen (CEA), is
limited due to low specificity and accuracy (11). As well as reduced sensitivity for
small-diameter tumor lesions, endoscopy causes patients a certain
degree of pain (10,12,13).
In addition, although histopathology remains the gold standard for
tumor diagnosis, it cannot be used to dynamically monitor treatment
response, since patients who have undergone surgery are unable to
provide diseased tissue during this process (14). Therefore, an effective adjuvant
diagnostic method is required to detect early esophageal cancer,
monitor treatment response and predict prognosis.
Circulating tumor cells (CTCs) are tumor cells that
have moved from the primary tumor site into the circulatory system,
which usually have a strong invasive and metastatic capacity
(15–17). The enumeration of CTCs and their
phenotypes may provide useful information for the diagnosis,
treatment and prognosis of malignant tumors (15,18–20).
The CellSearch® system was previously the only
instrument approved by the US Food and Drug Administration for
evaluating CTCs in patients with breast cancer (21), and is based on epithelial cell
adhesion molecule (EpCAM) and cytokeratin (CK) expression in the
tumor cell itself (22). However,
EpCAM is highly heterogeneous and dynamically expressed in various
epithelial tumor cells, and epithelial-mesenchymal transition may
reduce EpCAM and CK expression, resulting in failure of CTC
detection (23). Furthermore, the
system cannot detect tumor cells that are hyperdiploid and tumor
marker-negative.
To promote further research and clinical
applications of CTC detection, other platforms, including
immunostaining-fluorescence in situ hybridization (iFISH)
have been introduced. Compared with CellSearch®, iFISH
can effectively detect chromosome ploidy, and tumor markers in the
cytoplasm or on the cell surface (24,25).
Furthermore, numerous studies have indicated that iFISH has a high
CTC detection rate (15,19,24,25).
Therefore, iFISH may be considered a more promising method for
detecting CTCs in patients with ESCC.
In the present study, subtraction enrichment (SE)
and iFISH were used to detect CTCs from the peripheral blood (PB)
of patients with ESCC. SE was used to acquire the CTCs, whereas
iFISH was performed to identify them. The association between CTC
status and the clinicopathological features or prognosis of
patients with ESCC was subsequently evaluated.
Materials and methods
Patients and sample collection
The present study was performed at Anyang Cancer
Hospital. A total of 63 patients with confirmed esophageal cancer
(stage 0 and I, 11; stage II, 27; stage III, 25) and 50 healthy
donors (age, 40–72; male:female, 35:15) were enrolled in the
present study between November 2015 and July 2017. A blood (7.5 ml)
CTC test was performed on patients with ESCC at the time of first
diagnosis, after neoadjuvant chemoradiotherapy (NCRT), 24 h and 13
days post-surgery, and every 3 months during the follow-up
period.
This study was approved by the Ethics Committee of
Anyang Cancer Hospital. Written informed consent was obtained from
all subjects. Clinical data were collected with regards to sex,
age, primary tumor site, tumor size, lymph node metastasis (LNM),
histological type, CEA and SCC antigen levels, and progressive
disease. Tumor-node-metastasis (TNM) staging was performed
according to the American Joint Committee on Cancer 2010 staging
system (26). Patients were
subjected to endoscopic ultrasound along-with chest and abdominal
enhanced computerized tomography (CT) scans to carry out
pre-operative assessments of the clinical stage of ESCC and the
status of distant metastasis. To evaluate tumor responses, an
endoscopic biopsy and chest CT were carried out one month following
the completion of therapy. A follow-up CT and an esophagography
were carried out every 3 months for the first 2 years, and every 6
months thereafter. Serial PB samples (7.5 ml) were collected from
each subject. The samples were tested within 48 h of
collection.
SE analysis
CTC enrichment was performed using the Human
Circulating Rare Cell Subtraction Enrichment kit (Cytelligen,
Inc.), according to the manufacturer's protocol. PB samples (7.5
ml) were centrifuged at 800 × g for 8 min at room temperature, and
the supernatant above the red blood cell layer was discarded. hCTC
Separation Matrix (3 ml) was mixed with the remaining components,
and the mixture was centrifuged at 450 × g for 8 min at room
temperature. After centrifugation, the white buffy coat was
collected and incubated with 150 µl anti-CD45 monoclonal
antibody-conjugated immunomagnetic particles at room temperature
for 20 min with gentle shaking, which then underwent magnetic
separation to remove leukocytes. The solution without beads was
transferred to a clean centrifuge tube and centrifuged at 450 × g
for 8 min at room temperature, and then washed twice. The resulting
cell pellet was mixed with cell fixative and applied to coated CTC
slides. After drying at 32°C for 4 h, the slides were identified
using iFISH.
Immunofluorescence staining of
CTCs
CTC identification was performed according to the
instructions of the Cytelligen CTC Enrichment kit (cat. no.
SEH-003; Cytelligen, Inc.). The slides were immersed in 2X
saline-sodium citrate buffer for 10 min and dehydrated in ethanol
for 2 min. Centromere Probe 8 (CEP8) Spectrum Orange (Abbott
Laboratories) was added to the slides, denatured at 76°C for 5 min
and hybridized for 90 min at 37°C. Subsequently, the slides were
incubated with Antibody Preparation Solution-1, Alexa
Fluor® 594-conjugated anti-CD45 immunoglobulin G (IgG)
and Alexa Fluor® 488-conjugated anti-CK18 IgG (1:200).
After incubation at room temperature for 2 h in the dark, the
slides were washed and mounted with DAPI (Vector Laboratories,
Inc.) containing mounting medium. Finally, the CTCs were detected
under a fluorescence microscope with a ×100 oil immersion objective
(Olympus Corporation).
Statistical analysis
Data were analyzed using SPSS 23.0 (IBM Corp.).
Differences in CTC numbers between the healthy controls and
patients were compared using nonparametric Mann-Whitney U test.
One-way ANOVA and Bonferroni multiple comparisons test were
performed to analyze differences in CTC counts among tumor stages.
Graphical plots were generated using GraphPad Prism 6.0 (GraphPad
Software, Inc.) and OriginPro8.1 (OriginLab Corporation). Receiver
operating characteristic (ROC) curves were plotted to analyze the
sensitivity and specificity of CTCs between patients with ESCC and
healthy controls. Spearman's correlation analysis was applied to
analyze the correlation between CTC and serological tumor markers.
Kaplan-Meier survival curves and log-rank test were used to compare
the differences in progression-free survival (PFS) rate between two
groups. χ2 was used to determine the relationship
between CTCs and clinicopathological characteristics of esophageal
SCC. Univariate and multivariate Cox proportional hazards
regression analyses were carried out to identify independent risk
factors for clinical outcomes. A two-sided P<0.05 was considered
to indicate a statistically significant difference.
Results
Identification of CTCs from patients
with ESCC
The cells obtained through SE were identified based
on chromosome ploidy, existence of a nucleus, a hematopoietic white
blood cell (WBC) marker and an epithelial marker, using CEP8, DAPI,
CD45 and CK, respectively. CTCs were characterized as hyperdiploid
cells without detectable CD45 expression. In particular, CTCs in
the present study were defined as follows:
DAPI+/CD45−/CK+/CEP8 >2 spots,
DAPI+/CD45−/CK+/CEP8=2 spots, or
DAPI+/CD45−/CK−/CEP8 >2 spots.
WBCs were defined as
CK−/CD45+/DAPI+/CEP8=2 spots, and
indeterminate cells were defined as
CK−/CD45−/DAPI+/CEP8=2 spots
(Fig. 1A-E). Only FISH signals
with homogeneous light and size were counted as a spot as
previously described (27,28).
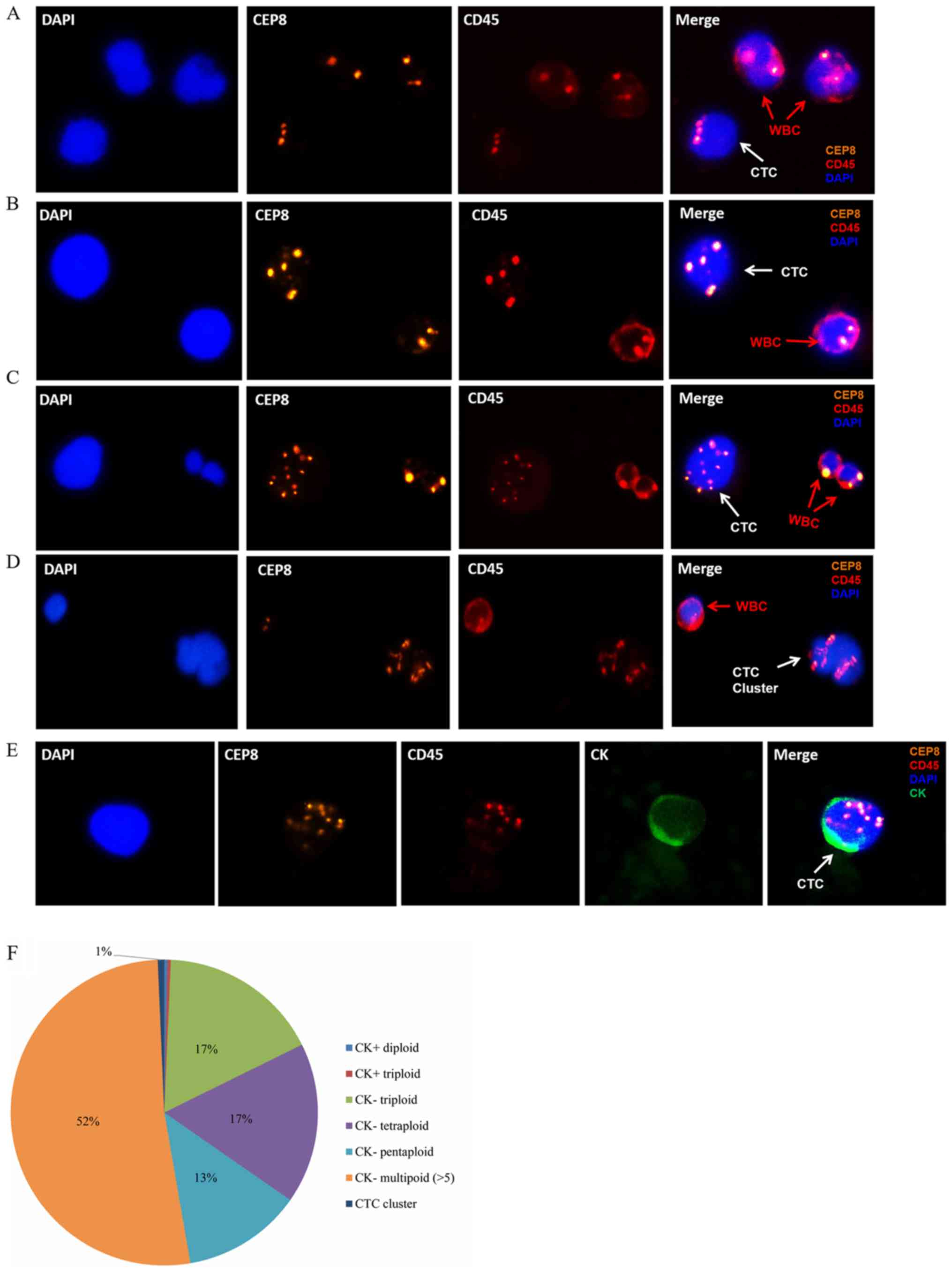 | Figure 1.Identification of CTCs in ESCC by
subtraction enrichment and immunostaining-fluorescence in
situ hybridization assay. (A)
CK−/CD45−/DAPI+/CEP8=3 spots
(white arrow), (B)
CK−/CD45−/DAPI+/CEP8=4 spots
(white arrow), (C)
CK−/CD45−/DAPI+/CEP8 >5 spots
(white arrow), (D) CTC cluster (white arrow), defined as >2 CTCs
adhered together. (E)
CK+/CD45−/DAPI+/CEP8 >2 spots
(white arrow). CK, green staining; CEP8, orange staining; DAPI,
blue staining; CD45, red staining. Magnification, ×400. (F)
Distribution of CK expression and ploidy in the 292 CTCs from 63
patients with ESCC at first diagnosis. CEP8, Centromere Probe 8;
CTC, circulating tumor cells; CK, cytokeratin; ESCC, esophageal
squamous cell carcinoma; WBC, white blood cell. |
According to the current criteria, a total of 292
CTCs were detected from the 63 patients with ESCC. CK+
CTCs have been reported to account for a relatively small
proportion of CTCs in breast and colorectal cancer (28,29).
As expected, only two CTCs were found to be CK+,
occurring in two patients (Fig.
1E). The majority of CTCs were
CK−/CD45−/DAPI+/CEP8 >2, which
accounted for 99.3% (290/292) of all CTCs in the first diagnosis
group. Among them, 50 CTCs (n=22 patients) were triploid (Fig. 1A), 50 CTCs (n=25 patients) were
tetraploid (Fig. 1B), 37 CTCs
(n=14 patients) were pentaploid and 153 CTCs (n=37 patients) were
multiploid (>5 copies of chromosome 8; Fig. 1C and F). In addition, two CTC
clusters were identified in two patients at first diagnosis
(Fig. 1D). CTC clusters are
recognized as oligoclonal precursors of breast cancer metastasis
(30); however, to the best of our
knowledge, they have not yet been studied in ESCC. Although no
direct association was observed in the present study, due to
limited sample size, further studies with a larger sample size are
required, in order to provide further insight into the effects of
CTCs on ESCC prognosis.
CTCs in patients and controls
The distribution of CTCs in patients with ESCC and
healthy controls was subsequently examined. There was a significant
difference between the CTC status of patients and that of controls
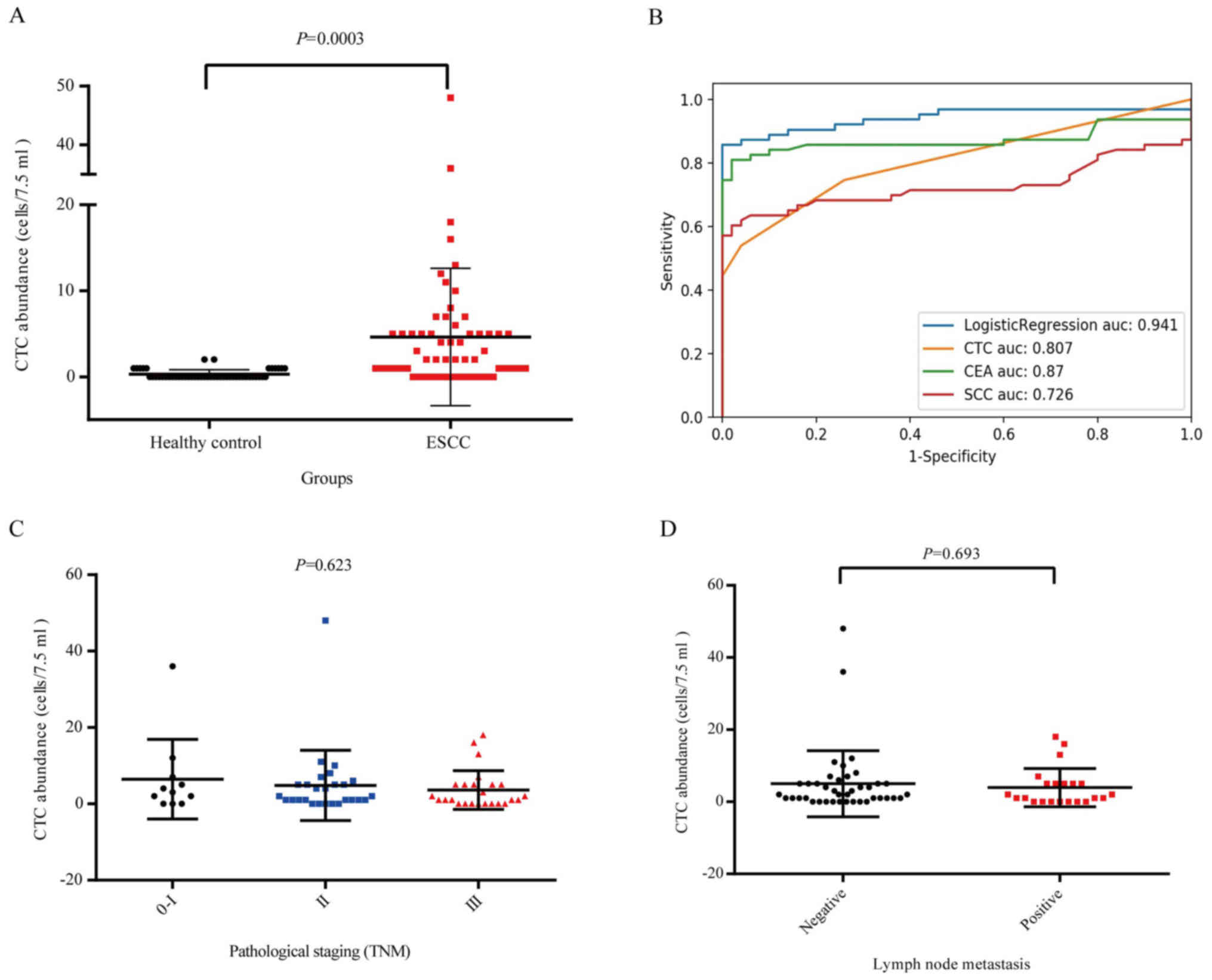
(P=0.0003; Fig. 2A). To evaluate
the validity of CTC detection in discriminating between patients
and healthy controls, ROC curves were used. The cut-off value of 1
CTC/7.5 ml yielded 74.6% sensitivity and 74.0% specificity. The
area under the ROC curve (AUC) was 0.807 [95% confidence interval
(CI), 0.727–0.887], which is similar to that of CEA and SCC antigen
(Fig. 2B). Taking into
consideration the actual cut-off value of CEA (5 µg/l) and SCC (2.5
µg/l) in terms of clinical application, the sensitivity of CTCs
with a cut-off value of 1 CTC/7.5 ml was much higher (SCC, 22.2%;
CEA, 4.8%). In addition, using a logistic regression model, it was
predicted that the optimal AUC could be further improved to 0.941
by combining all three biomarkers; suggesting that CTC may serve as
a better biomarker when combined with traditional serum biomarkers.
The association between CTC counts and several ESCC
clinicopathological variables was also compared. The median CTC
count per 7.5 ml PB in stage 0-I, stage II and stage III disease
was six (range, 0–12), five (range, 0–10), and nine (range, 0–18),
respectively; however, the difference was not significant
(P>0.05; Fig. 2C). The median
CTC count per 7.5 ml PB in patients with positive and negative LNM
was nine (range, 0–18) and six (range, 0–12), respectively, and the
difference was not significant (P=0.693; Fig. 2D). Similarly, CTC status was not
significantly associated with sex, age, pathological stage, tumor
location, tumor depth or lymph node involvement (P>0.05;
Table I). In addition, the
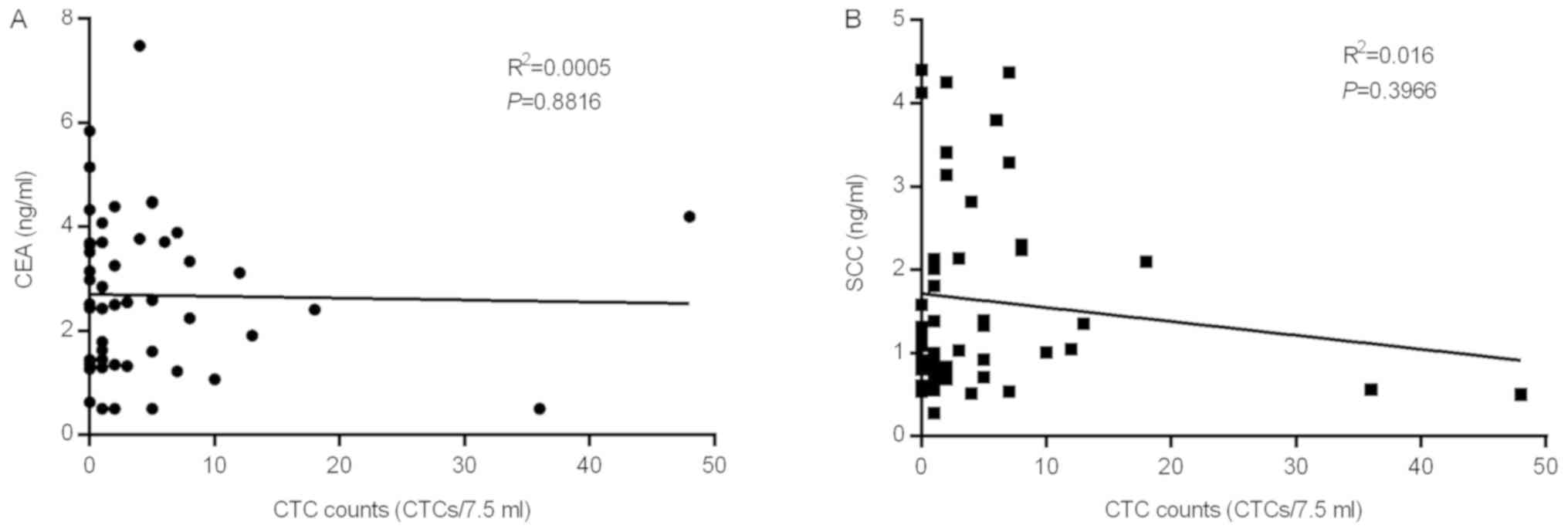
correlation between CTC counts and the commonly used protein tumor
markers, CEA and SSC, was investigated in patients, and it was
suggested to be a relatively independent factor (Fig. 3).
 | Table I.Relationship between CTCs and
clinicopathological characteristics of esophageal SCC. |
Table I.
Relationship between CTCs and
clinicopathological characteristics of esophageal SCC.
|
Characteristics | N | CTC >0 (%) | CTC=0 (%) |
P-valuea |
|---|
| Sex |
|
Male | 46 | 32 | 14 | 0.131 |
|
Female | 17 | 15 | 2 |
|
| Age (years) |
|
≥65 | 26 | 21 | 5 | 0.346 |
|
<65 | 37 | 26 | 11 |
|
| Smoking
history |
|
Yes | 34 | 25 | 9 | 0.832 |
| No | 29 | 22 | 7 |
|
| Pathologic
stage |
|
0-I | 11 | 8 | 3 | 0.53 |
| II | 27 | 22 | 5 |
|
|
III | 25 | 17 | 8 |
|
| Tumor location |
|
Upper | 9 | 8 | 1 | 0.257 |
|
Middle | 45 | 34 | 11 |
|
|
Lower | 9 | 5 | 4 |
|
| Tumor depth |
|
Tis+T1 | 10 | 8 | 2 | 0.669 |
|
T2+T3+T4 | 53 | 39 | 14 |
|
| Lymph nodes |
|
Negative | 41 | 32 | 9 | 0.391 |
|
Positive | 22 | 15 | 7 |
|
| Serum CEA
(ng/ml) |
|
≥5.09 | 3 | 1 | 2 | 0.114 |
|
<5.09 | 56 | 42 | 14 |
|
|
Missing | 4 | 4 | 0 |
|
| Serum SCC antigen
(ng/ml) |
|
≥2.5 | 13 | 11 | 2 | 0.468 |
|
<2.5 | 44 | 33 | 11 |
|
|
Missing | 6 | 3 | 3 |
|
CTCs and other circulating biomarkers
for tumor monitoring
The presence of CTCs may provide important
information for evaluating the clinical response to treatment,
including surgery, chemotherapy and NCRT. To evaluate the
differences in CTC counts, the CTCs of patients with ESCC were
detected at numerous time points pre- or post-surgery (Fig. S1). The CTC counts of patients
receiving NCRT (n=12) were decreased compared with at first
diagnosis (n=63), suggesting that NCRT may exert effects on tumor
regression. CTCs were detected in 51 out of 63 patients 24 h
post-surgery and the counts were even higher compared with at first
diagnosis. Further analysis of CTCs from 43 patients at the two
time points is presented in Fig.
S2. CTCs were detected in 43 patients prior to surgery and 24 h
post-surgery. In accordance with the aforementioned results, CTC
counts increased following surgery in >50% of patients (22/43),
whereas only 17 patients exhibited reduced CTC counts, and four had
consistent CTC counts. No significant association with disease
progression was observed. This finding may be explained by
dislodged cells from surgery, which may affect the accuracy of the
prognostic prediction. In addition, CTC counts gradually stabilized
on day 13, and remained relatively stable between 3 and 12 months
post-surgery.
To identify patients likely to suffer from
recurrence following surgical intervention, serial plasma samples
from six of the 63 patients with relapsed ESCC were collected and
CTC counts were periodically detected across ≥2 time points during
18 months of follow-up. The alterations in CTCs and other
biomarkers, including SCC antigen and CEA, were compared alongside
the results of clinical CT scans, which is the gold standard for
measurement of disease status, as defined by the Response
Evaluation Criteria in Solid Tumors 1.0 (RECIST 1.0) (31). CTCs were detected and exhibited
serial alterations, alongside fluctuations associated with
treatment response observed by CT. During follow-up, recurrence was
detected by CT in several patients with increased CTC counts as
early as 13 days post-surgery (Fig.
4A-C, E and F), suggesting that CTC counts on day 13
post-surgery may serve as a better indicator for prognosis. In the
case of Patient 12, although the level of CTC on day 13
post-surgery was missing, the CTC counts appeared higher at day
1–180 and then gradually reduced. In addition, a marked increase in
CTCs was observed at day 360 compared with day 270 post-surgery in
Patient 12 (Fig. 4D), who had
progressive disease according to a CT scan performed 90 days later.
Reduced CEA/SCC antigen levels and a smaller dynamic range neither
predicted nor reflected the presence of progressive disease in
these patients. These data suggested that alterations in CTC counts
may be a highly specific approach for the early detection of
residual or recurrent disease following surgical resection.
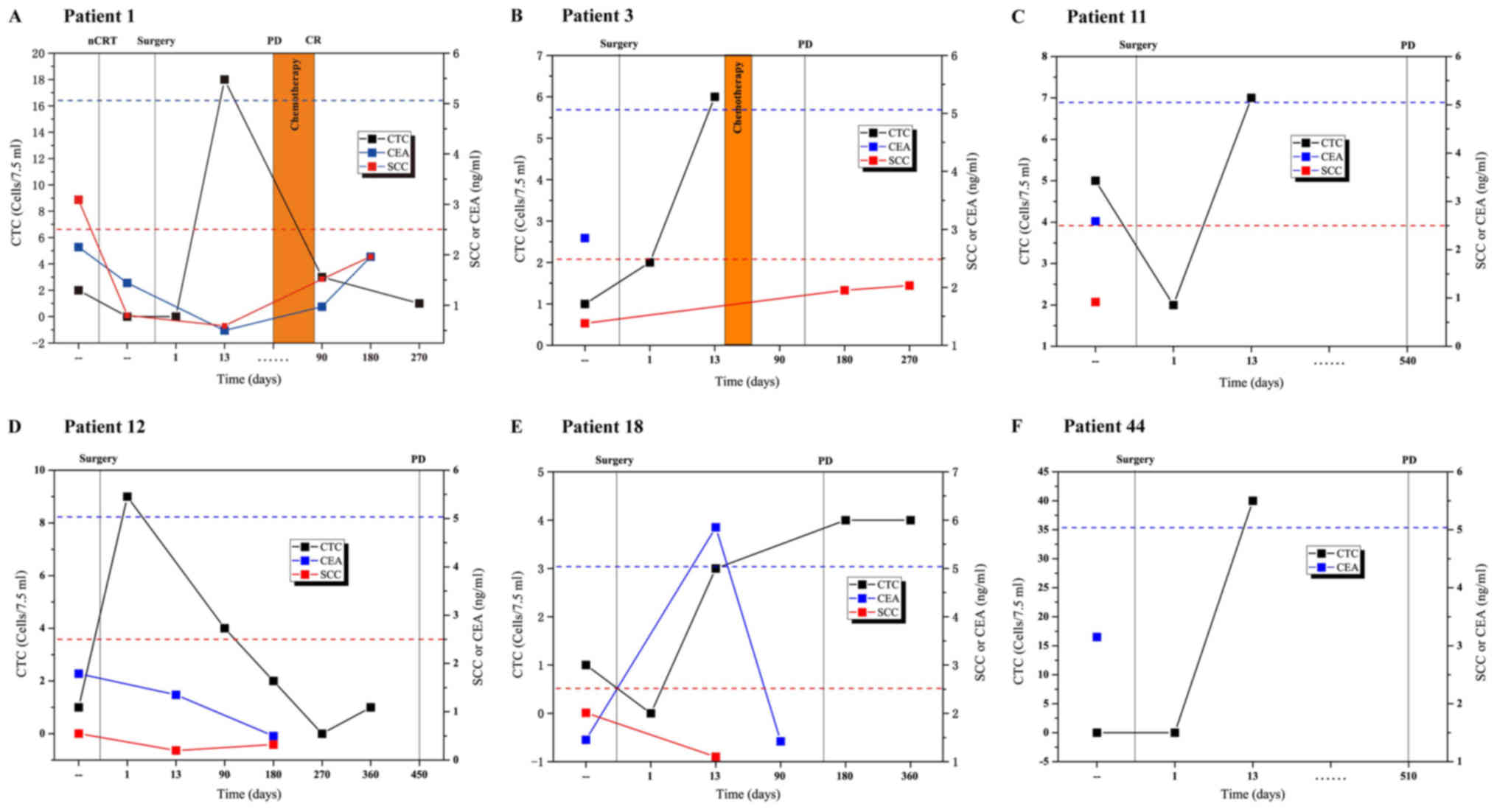 | Figure 4.Dynamic monitoring of CTC counts and
serological tumor markers in six patients with esophageal cancer
pre- and post-operative care. The six patients were selected since
they periodically underwent CTC detection and were identified as
having relapsed ESCC during the follow-up period. Patient (A) 1,
(B) 3, (C) 11, (D) 12, (E) 18 and (F) 44 were selected. The dotted
blue line indicates CEA cut-off level (5.09 ng/ml); the dotted red
line indicates SCC antigen cut-off level (2.5 ng/ml). CEA,
carcinoembryonic antigen; CR, complete response; CTCs, circulating
tumor cells; NCRT, neoadjuvant chemoradiotherapy; PD, progressive
disease; SCC, squamous cell carcinoma. |
Survival analysis
The median follow-up time of the 63 patients with
ESCC was 21 months (range, 0–32 months). A total of 15 patients
with ESCC had died or exhibited disease progression at the end
point of PFS analysis (follow up time: median, 10 months; range
0–18 months). The median follow-up time of the remaining ESCC
patients without progression was 24 months (range, 12–32 months).
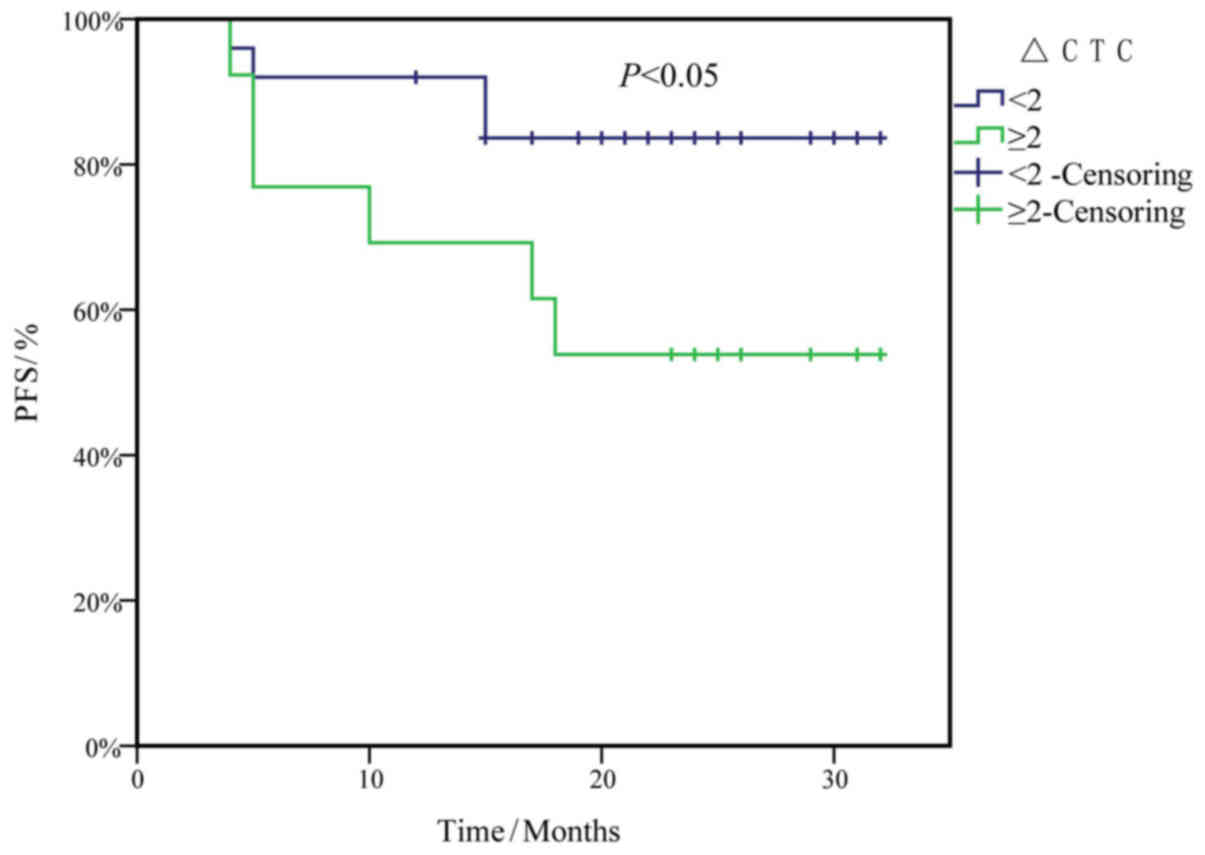
PFS survival curves were plotted to evaluate the risk of CTC counts
at first diagnosis, and the alterations in CTC counts 13 days
post-surgery (CTC13) compared with at first diagnosis
(CTCFD) (ΔCTC=CTC13-CTCFD), using
Kaplan-Meier analysis and the log-rank test. The results indicated
that ΔCTC ≥2/7.5 ml was an independent risk factor for reduced PFS
in patients with ESCC (P<0.05; Fig.
5).
CTC enumeration and other clinical factors,
including sex, age, TNM staging, LNM and CEA levels were subjected
to univariate Cox proportional hazards regression analysis to
evaluate the potential association with PFS. Only factors with
P<0.1 were included in the multivariate Cox regression analysis
(Table II). As a result, ΔCTC ≥2,
TNM staging, LNM, smoking and drinking qualified for further
analysis. Consistent with the Kaplan-Meier analysis, ΔCTC ≥2/7.5 ml
remained a strong predictor of poor prognosis [hazard ratio, (HR),
3.922; 95% CI: 0.907–16.951; P=0.047] in the multivariate Cox
proportional hazards regression analysis.
 | Table II.Univariate and multivariate Cox
proportional hazards regression analyses for prediction of
progression-free survival. |
Table II.
Univariate and multivariate Cox
proportional hazards regression analyses for prediction of
progression-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Risk factor | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| ΔCTC ≥2 | 0.073 | 3.184 |
0.898–11.294 | 0.047 | 3.922 | 0.907–16.951 |
| Advanced stage | 0.072 | 2.583 | 0.919–7.265 | 0.101 | 12.001 | 0.613–234.850 |
| LNM | 0.093 | 2.387 | 0.865–6.588 | 0.206 | 0.128 | 0.005–3.101 |
| Smoking | 0.102 | 2.559 | 0.815–8.039 | 0.082 | 7.447 | 0.775–71.595 |
| Drinking | 0.042 | 2.924 | 1.040–8.217 | 0.951 | 1.048 | 0.240–4.574 |
Discussion
CTCs are tumor cells that enter the bloodstream from
primary or metastatic lesions, which form at the early stage of
cancer occurrence and metastasis (32). CTCs in the peripheral circulation
exist independently or as cell clumps (30). Compared with tissue biopsy, CTC
detection, the gold standard for cancer detection, is relatively
noninvasive, easy to perform and can be performed repeatedly
(33). CTC detection has been
evaluated as a novel prognostic method and as a tumor marker for
patients with various types of solid tumors, including esophageal
cancer (25).
CTC detection involves the enrichment and
discrimination of CTCs (34).
Conventional enrichment, depending on surface antigens (35) and tumor cell size (36), can easily result in the loss of a
number of CTCs. In the present study, SE was used to obtain CTCs.
In this method, interfering components can be removed effectively,
including WBCs, red blood cells and plasma proteins, through
centrifugation and immunomagnetic separation (28). All types of CTCs and circulating
tumor microemboli can be obtained, with the extraction having a
less detrimental effect (28,37).
Therefore, SE is considered an ideal method for CTC enrichment and
provides a good basis for further CTC analysis; Qiao et al
(20) also applied this method to
evaluate the prognostic value of CTCs in the PB of patients with
ESCC. The SE-enriched CTCs were then stained with anti-CK8/18/19
and anti-CD45 antibodies. Compared with previous literature
(20), the present study used a
different staining technique for the enriched CTCs.
In the present study, iFISH was used to identify
CTCs. Cells that were
CK+/DAPI+/CD45−/CEP8=2 spots,
CK+/DAPI+/CD45−/CEP8 >2 spots,
and CK−/CD45−/DAPI+/CEP8 >2
spots were considered CTCs, since they were hyperdiploid and/or
tumor marker-positive. Cells that were
CK−/DAPI+/CD45+/CEP8=2 spots were
considered to be WBCs (38,39),
whereas cells that were
CK−/CD45−/DAPI+/CEP8=2 spots were
recognized as indeterminate cells, which could be either
CK− tumor cells or WBCs with unstained CD45. In the
present study, CK+ CTCs accounted for a very small
proportion of identified CTCs; this has also been reported by
previous studies (28,29,40).
Due to its higher detection rate, iFISH may be more clinically
useful than the CK staining method using anti-CK8/18/19 and
anti-CD45 antibodies used in other studies (19,20).
CTCs are derived from primary or metastatic lesions,
and metastasis begins with epithelial-mesenchymal transition (EMT).
Chen et al (41) and Han
et al (42) demonstrated
the importance of the EMT process in tumor metastasis and CTC
production; however, CTC clusters, also known as microemboli, were
not detected in any of the cohorts enrolled in these studies
(41,42). Clustered cancer cells in the blood
have been reported to serve as an indicator of poor prognosis and
early recurrence in lung cancer (43,44),
and high malignancy in breast cancer (30,45);
however, to the best of our knowledge, they have not been studied
in ESCC. In the present study, two CTC clusters in two patients
were identified separately prior to surgery, and another three
clustered CTCs were detected in three patients following resection
(data not shown). Among these patients, CT detected recurrence in
only one patient from whom clustered CTCs were detected prior to
pre-operative chemoradiotherapy. No CTC clusters were detected in
the enrolled patients 3 months post-surgery. A total of two
clustered CTCs were detected in patients 24 h following resection,
which suggested that surgery may dislodge cells, resulting in their
movement from the resection site to the circulation, thus inducing
the formation of clustered CTCs. Inflammatory conditions generated
by resection may also contribute to the increased clusters
(46); this may also explain why
no clusters were detected in any of the patients 3 months
post-surgery when inflammation was reduced. These clusters could be
more apoptosis-resistant and metastatic once they co-express
epithelial and mesenchymal markers (30). A more in-depth approach would be to
characterize these EMT markers during the entire study, since it
may help to explain why the three patients with clusters following
surgery had no recurrence, whereas one patient with clustered CTCs
prior to treatment exhibited recurrence. The significance of the
present findings is limited by the lack of EMT marker assessment
and the small number of patients with detected clusters; therefore,
no significant association between CTC clusters and prognosis or
PFS was identified. Nevertheless, further research is required in
order to confirm that pre-operative, instead of post-operative CTC
clusters, are associated with ESCC recurrence, as such insight may
facilitate the full understanding of clustered CTCs and ESCC
prognosis.
Another clinical problem the present study aimed to
solve was the monitoring time points. To understand at which stage
CTCs are associated with treatment response, the detection of CTCs
or other serum protein markers was performed at multiple time
points, during the pre-operative and post-operative period.
Compared with SCC antigen, CEA or CT imaging, CTC fluctuation in
the patients reflected earlier recurrence of esophageal cancer. CTC
counts increased in >50% of patients 24 h post-surgery, which
further verified the hypothesis that the dislodged primary tumor
cells from surgery may enter the circulation and interfere with CTC
enumeration. In this case, the results of CTC detection cannot be
applied for evaluating therapeutic effect or prognosis. Compared to
the other time points, CTC counts 13 days post-resection were a
more favorable indicator, since the dislodged tumor cells have
probably undergone apoptosis during this period; therefore, only
the resistant cells may survive and induce metastasis, which is
responsible for potential disease progression.
Taking this into consideration, Kaplan-Meier and Cox
analyses were performed to analyze the predictive effects of CTC
counts on PFS. Instead of considering a certain CTC number prior to
surgery the cut-off value to predict prognosis, multivariate
analysis revealed that ΔCTC counts ≥2/7.5 ml PB may be a strong
prognostic indicator of PFS (HR, 3.922; 95% CI, 0.907–16.951;
P<0.05) in patients with ESCC. Patients with a change in CTC
status from negative (0 CTC at first diagnosis) to negative (0 CTC
on day 13 post-surgery) had the best prognosis [0% progressive
disease (PD)], whereas patients with positive CTCs at pre- and
post-operative times points had the worst prognosis (36% PD, data
not shown). Even patients with a variation in CTC counts from
positive to negative underwent metastasis or recurrence, which was
contradictory to the results of previous studies (19,20).
This finding is of great clinical significance, as the cut-off
value for predicting prognosis was established with variation of
CTC counts (ΔCTC=CTC13-CTCFD), instead of CTC
number at a certain time point.
In conclusion, the findings of the present study are
limited due to the small number of patients and the short follow-up
period. A prospective study with a larger sample size and a longer
follow-up period is necessary, in order to evaluate the clinical
significance of the SE-iFISH system in CTC detection for patients
with ESCC. In addition, the clusters of CTCs need to be isolated
and characterized for different cell types present, and the results
subsequently compared with patient prognosis. In addition, the
application of next-generation sequencing and single-cell
sequencing CTCs to monitor genomic variations in disease
progression, and decipher tumor evolution during treatment, as well
as the EMT mechanism, has been reported in several types of cancer
(47–49). These aforementioned findings
provide potential direction for future studies. With further
technical improvements in detection and sequencing methods, CTC
analyses may exhibit greater potential as biomarkers for evaluating
cancer prognosis and treatment response.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Project of
National Natural Science Foundation of China (grant no. U1504814),
Major Projects of Science and Technology Department in Henan
Province (grant nos. 16110311200 and 161100311300), the Project of
Anyang Science Foundation of Henan province (grant no. 2016) and
the Major Projects of Special Development Funds in Zhangjiang
National Independent Innovation Demonstration Zone, Shanghai (grant
no. ZJ2017-ZD-012).
Availability of data and materials
The datasets used and/or analyzed during the current
study available from the corresponding author on reasonable
request.
Authors' contributions
FZ and RF developed the study concepts and design,
and were responsible for quality control of data and algorithms.
PH, JX, JZ, JX, LDW, FS and AZ participated in data acquisition.
YZ, LW and JZ conducted the statistical analysis. YZ, PM and JL
were responsible for interpreting data and preparation of the
manuscript, including drafting and revising the manuscript. All
authors edited and reviewed the final manuscript. All authors read
and approved the final manuscript and agree to be accountable for
all aspects of the work.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Anyang Cancer Hospital, and written informed consent was obtained
from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bohanes P, Yang D, Chhibar RS, Labonte MJ,
Winder T, Ning Y, Gerger A, Benhaim L, Paez D, Wakatsuki T, et al:
Influence of sex on the survival of patients with esophageal
cancer. J Clin Oncol. 30:2265–2272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abnet CC, Arnold M and Wei WQ:
Epidemiology of esophageal squamous cell carcinoma.
Gastroenterology. 154:360–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsushima K, Isomoto H, Yamaguchi N,
Inoue N, Machida H, Nakayama T, Hayashi T, Kunizaki M, Hidaka S,
Nagayasu T, et al: MiRNA-205 modulates cellular invasion and
migration via regulating zinc finger E-box binding homeobox 2
expression in esophageal squamous cell carcinoma cells. J Transl
Med. 9:302011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagaraja V and Eslick GD: Forthcoming
prognostic markers for esophageal cancer: A systematic review and
meta-analysis. J Gastrointest Oncol. 5:67–76. 2014.PubMed/NCBI
|
|
6
|
Pera M, Manterola C, Vidal O and Grande L:
Epidemiology of esophageal adenocarcinoma. J Surg Oncol.
92:151–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan YJ, Song X, Li JL, Li XM, Liu B, Wang
R, Fan ZM and Wang LD: Esophageal and gastric cardia cancers on
4238 Chinese patients residing in municipal and rural regions: a
histopathological comparison during 24-year period. World J Surg.
32:1980–1988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Domper Arnal MJ, Ferrandez Arenas A and
Lanas Arbeloa A: Esophageal cancer: Risk factors, screening and
endoscopic treatment in Western and Eastern countries. World J
Gastroenterol. 21:7933–7943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Zhu Z, Liu Y, Jin X, Xu Z, Yu Q
and Li K: Diagnostic value of multiple tumor markers for patients
with esophageal carcinoma. PLoS One. 10:e01169512015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He Z, Liu Z, Liu M, Guo C, Xu R, Li F, Liu
A, Yang H, Shen L, Wu Q, et al: Efficacy of endoscopic screening
for esophageal cancer in China (ESECC): Design and preliminary
results of a population-based randomised controlled trial. Gut.
68:198–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Xu R, Liu M, Cai H, Cao C, Liu F, Li
F, Guo C, Pan Y, He Z and Ke Y: Lugol chromoendoscopy detects
esophageal dysplasia with low levels of sensitivity in a high-risk
region of china. Clin Gastroenterol Hepatol. 16:1585–1592. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smyth EC, Lagergren J, Fitzgerald RC,
Lordick F, Shah MA, Lagergren P and Cunningham D: Oesophageal
cancer. Nat Rev Dis Primers. 3:170482017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Wang F, Ning N, Chen Q, Yang Z,
Guo Y, Xu D, Zhang D, Zhan T and Cui W: Patterns of circulating
tumor cells identified by CEP8, CK and CD45 in pancreatic cancer.
Int J Cancer. 136:1228–1233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marx V: Tracking metastasis and tricking
cancer. Nature. 494:133–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MY, Oskarsson T, Acharyya S, Nguyen
DX, Zhang XH, Norton L and Massagué J: Tumor self-seeding by
circulating cancer cells. Cell. 139:1315–1326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pierga JY, Bidard FC, Mathiot C, Brain E,
Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A
and Marty M: Circulating tumor cell detection predicts early
metastatic relapse after neoadjuvant chemotherapy in large operable
and locally advanced breast cancer in a phase II randomized trial.
Clin Cancer Res. 14:7004–7010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsushita D, Uenosono Y, Arigami T,
Yanagita S, Nishizono Y, Hagihara T, Hirata M, Haraguchi N, Arima
H, Kijima Y, et al: Clinical significance of circulating tumor
cells in peripheral blood of patients with esophageal squamous cell
carcinoma. Ann Surg Oncol. 22:3674–3680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiao Y, Li J, Shi C, Wang W, Qu X, Xiong
M, Sun Y, Li D, Zhao X and Zhang D: Prognostic value of circulating
tumor cells in the peripheral blood of patients with esophageal
squamous cell carcinoma. Onco Targets Ther. 10:1363–1373. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lianidou ES and Markou A: Circulating
tumor cells as emerging tumor biomarkers in breast cancer. Clin
Chem Lab Med. 49:1579–1590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Swennenhuis JF, van Dalum G, Zeune LL and
Terstappen LW: Improving the CellSearch(R) system.
Expert Rev Mol Diagn. 16:1291–1305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andree KC, van Dalum G and Terstappen LW:
Challenges in circulating tumor cell detection by the CellSearch
system. Mol Oncol. 10:395–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sheng Y, Wang T, Li H, Zhang Z, Chen J, He
C, Li Y, Lv Y, Zhang J, Xu C, et al: Comparison of analytic
performances of Cellsearch and iFISH approach in detecting
circulating tumor cells. Oncotarget. 8:8801–8806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reeh M, Effenberger KE, Koenig AM,
Riethdorf S, Eichstädt D, Vettorazzi E, Uzunoglu FG, Vashist YK,
Izbicki JR, Pantel K and Bockhorn M: Circulating tumor cells as a
biomarker for preoperative prognostic staging in patients with
esophageal cancer. Ann Surg. 261:1124–1130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stephen Edge DRB and Carolyn C: AJCC
Cancer Staging Manual. 7th. Edge S, Byrd DR, Compton CC, Fritz AG,
Greene F and Trotti A: Springer; New York, NY: 2010
|
|
27
|
Liu X, Zhang Z, Zhang B, Zheng Y, Zheng C,
Liu B, Zheng S, Dong K and Dong R: Circulating tumor cells
detection in neuroblastoma patients by EpCAM-independent enrichment
and immunostaining-fluorescence in situ hybridization.
EBioMedicine. 35:244–250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu W, Zhang Z, Gao XH, Shen Z, Jing Y, Lu
H, Li H, Yang X, Cui X, Li Y, et al: Clinical significance of
detecting circulating tumor cells in colorectal cancer using
subtraction enrichment and immunostaining-fluorescence in situ
hybridization (SE-iFISH). Oncotarget. 8:21639–21649.
2017.PubMed/NCBI
|
|
29
|
Xu L, Jia S, Li H, Yu Y, Liu G, Wu Y, Liu
X, Liu C, Zhou Y, Zhang Z and Sheng Y: Characterization of
circulating tumor cells in newly diagnosed breast cancer. Oncol
Lett. 15:2522–2528. 2018.PubMed/NCBI
|
|
30
|
Aceto N, Bardia A, Miyamoto DT, Donaldson
MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al:
Circulating tumor cell clusters are oligoclonal precursors of
breast cancer metastasis. Cell. 158:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Masuda T, Hayashi N, Iguchi T, Ito S,
Eguchi H and Mimori K: Clinical and biological significance of
circulating tumor cells in cancer. Mol Oncol. 10:408–417. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yap TA, Lorente D, Omlin A, Olmos D and de
Bono JS: Circulating tumor cells: A multifunctional biomarker. Clin
Cancer Res. 20:2553–2568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression and survival in metastatic breast cancer. N Engl J Med.
351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vona G, Sabile A, Louha M, Sitruk V,
Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et
al: Isolation by size of epithelial tumor cells: A new method for
the immunomorphological and molecular characterization of
circulatingtumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xue F, Shi S, Zhang Z, Xu C, Zheng J, Qin
T, Qian Z, Zhao X, Tong Y, Xia L and Xia Q: Application of a novel
liquid biopsy in patients with hepatocellular carcinoma undergoing
liver transplantation. Oncol Lett. 15:5481–5488. 2018.PubMed/NCBI
|
|
38
|
Roach T, Slater S, Koval M, White L, Cahir
McFarland ED, Okumura M, Thomas M and Brown E: CD45 regulates Src
family member kinase activity associated with macrophage
integrin-mediated adhesion. Curr Biol. 7:408–417. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Court CM, Ankeny JS, Hou S, Tseng HR and
Tomlinson JS: Improving pancreatic cancer diagnosis using
circulating tumor cells: Prospects for staging and single-cell
analysis. Expert Rev Mol Diagn. 15:1491–1504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ge F, Zhang H, Wang DD, Li L and Lin PP:
Enhanced detection and comprehensive in situ phenotypic
characterization of circulating and disseminated heteroploid
epithelial and glioma tumor cells. Oncotarget. 6:27049–27064. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen W, Li Y, Yuan D, Peng Y and Qin J:
Practical value of identifying circulating tumor cells to evaluate
esophageal squamous cell carcinoma staging and treatment efficacy.
Thorac Cancer. 9:956–966. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Han D, Chen K, Che J, Hang J and Li H:
Detection of epithelial-mesenchymal transition status of
circulating tumor cells in patients with esophageal squamous
carcinoma. Biomed Res Int. 2018:76101542018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Wang K, Xu J, Huang J and Zhang T:
Prognostic significance of circulating tumor cells in
non-small-cell lung cancer patients: A meta-analysis. PLoS One.
8:e780702013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Funaki S, Sawabata N, Abulaiti A, Nakagiri
T, Shintani Y, Inoue M, Minami M and Okumura M: Significance of
tumour vessel invasion in determining the morphology of isolated
tumour cells in the pulmonary vein in non-small-cell lung cancer.
Eur J Cardiothorac Surg. 43:1126–1130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jolly MK, Boareto M, Huang B, Jia D, Lu M,
Ben-Jacob E, Onuchic JN and Levine H: Implications of the Hybrid
epithelial/mesenchymal phenotype in metastasis. Front Oncol.
5:1552015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao Y, Ni X, Guo H, Su Z, Ba Y, Tong Z,
Guo Z, Yao X, Chen X, Yin J, et al: Single-cell sequencing
deciphers a convergent evolution of copy number alterations from
primary to circulating tumor cells. Genome Res. 27:1312–1322. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
D'Avola D, Villacorta-Martin C,
Martins-Filho SN, Craig A, Labgaa I, von Felden J, Kimaada A,
Bonaccorso A, Tabrizian P, Hartmann BM, et al: High-density single
cell mRNA sequencing to characterize circulating tumor cells in
hepatocellular carcinoma. Sci Rep. 8:115702018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu Z, Qiu S, Shao K and Hou Y: Progress
and challenges of sequencing and analyzing circulating tumor cells.
Cell Biol Toxicol. 34:405–415. 2017. View Article : Google Scholar : PubMed/NCBI
|