Introduction
Chronic obstructive pulmonary disease (COPD) is an
important and growing cause of morbidity and mortality, and is
predicted to be the third leading cause of mortality globally by
2020 (1). Patients with COPD can
suffer from episodes of symptom exacerbations during the course of
the disease that negatively affect their prognosis; however, COPD
and its exacerbations are both heterogeneous conditions that are
linked to complex and heterogeneous immune responses (2,3).
Autoimmunity has been suggested to be an influential
factor in the progression of patients who have suffered from COPD
for >10 years (4,5), as COPD shares numerous
pathophysiological and clinical characteristics with autoimmune
diseases (4). Increasing evidence
indicates that autoimmune responses serve a role in the development
and progression of COPD (6–10).
Autoantibodies in stable COPD have been comprehensively reviewed
recently (11); however, the
heterogeneity of autoimmunity should also be considered. Kim et
al (12) reported abnormal
blood T-lymphocyte subsets in a subgroup of patients with COPD. Our
recent study demonstrated that sputum autoantibody levels were
associated with exacerbation risk in a subgroup of COPD patients
(13), suggesting that
autoimmunity is highly heterogeneous in COPD.
Network-based analysis is a novel integrative
research approach that is suitable for the study of complex and
heterogeneous conditions, such as COPD and its exacerbations
(14–17). Divo et al (18) used network analysis to investigate
the association between multiple comorbidities in patients with
stable COPD. The authors included 79 comorbidities and various
demographic, clinical and functional parameters in the network
analysis, and observed that the comorbidities were significantly
interlinked and formed a complex network in which six sub-networks
(also termed modules) were identified. Grosdidier et al
(19) used an integrative
network-based approach to investigate the biological associations
between COPD, its comorbidities and the chemical products contained
in tobacco smoke. They revealed that comorbidities shared genes,
proteins and biological pathways with COPD. Faner et al
(20) explored the association
between comorbidities and patients with exacerbated COPD from a
molecular viewpoint (also termed a molecular diseasome) using
network analysis. Noell et al (21) explored the pathobiological
mechanisms of exacerbations and biomarkers by comparing multi-level
(clinical, physiological, biological, imaging and microbiological)
correlation networks determined during exacerbation and
convalescence in patients with COPD; however, no known study has
investigated the interrelationships between airway and circulating
autoantibody responses, and clinical parameters in immunological
subgroups of patients with COPD. It was hypothesised that network
analysis, an analytical approach that involves the comparison of
clinical, functional, biological and immunological correlation
networks, may provide a novel insight into the complex association
between autoantibody profiles and COPD clinical parameters. To
properly adjust for the redundancy of autoantibody profiles and the
heterogeneity of autoantibody responses, principal component
analysis (PCA) and hierarchical clustering were performed prior to
network analysis. Thus, in the present proof-of-concept study,
network analysis based on unsupervised classification was used to:
i) Compare the network structures of different COPD subgroups
identified by sputum and serum autoantibody profiles; and ii)
identify a series of exacerbation risk-associated factors.
Materials and methods
Patients
This was a prospective cross-sectional study. A
total of 102 patients with COPD with stable disease were enrolled
at the First Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China) between March 2017 and October 2017. A group of
18 non-smoking healthy controls was also enrolled for comparison.
Inclusion criteria for patients with COPD were: i) Aged >40
years; and ii) confirmed diagnosis of COPD according to the Global
Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines
(22) [post-bronchodilator forced
expiratory volume in 1 sec (FEV1)/forced vital capacity (FVC) ratio
<0.7]. Exclusion criteria were: i) Diagnosis of known
respiratory disorders other than COPD; ii) history of significant
inflammatory disease other than COPD; iii) COPD exacerbation within
4 weeks of enrolment; iv) history of lung surgery and tuberculosis;
v) diagnosis of cancer; vi) having undergone a blood transfusion
within 4 weeks of enrolment; vii) diagnosis of autoimmune diseases;
and viii) enrolment in a blinded drug trial. The
clinicopathological data of the patients and healthy controls are
presented in Table I.
 | Table I.Subject demographics and clinical
characteristics. |
Table I.
Subject demographics and clinical
characteristics.
| Characteristic | Non-smoking healthy
controls, n=18 | COPD patients,
n=102 |
|---|
| Age, years | 58.33±7.67 | 66.46±8.10 |
| Sex (M/F) | 10/8 | 98/4 |
| BMI,
kg/m2 | 25.26±3.65 | 21.86±4.11 |
| Smoking, n
(never/ex/current) | 18/0/0 | 10/73/19 |
| Pre-BD FEV1,
litres | 2.51±0.79 | 1.27±0.57 |
| Pre-BD
FEV1pred% | 96.94±16.79 | 49.02±21.40 |
| Pre-BD FVC,
litres | 3.15±0.98 | 2.57±0.73 |
| Pre-BD
FEV1/FVC | 0.80±0.06 | 0.49±0.13 |
| Post-BD FEV1,
litres | ND | 1.40±0.59 |
| Post-BD
FEV1pred% | ND | 53.34±22.41 |
| Post-BD FVC,
litres | ND | 2.74±0.73 |
| Post-BD
FEV1/FVC | ND | 0.51±0.14 |
| CAT score | NA | 11.67±6.44 |
| mMRC | NA | 2 (1–2) |
| Respiratory
medications |
|
|
|
ICS | NA | 66 (64.7%) |
|
LABA | NA | 66 (64.7%) |
|
LAMA | NA | 41 (40.2%) |
Inclusion criteria for non-smoking healthy controls
were: i) Aged >40 years; and ii) without any known respiratory
disorders and significant inflammatory diseases. Subjects with one
or more of the following criteria were excluded: i) Diagnosis of
known respiratory diseases; ii) history of significant inflammatory
disease; iii) diagnosis of cancer; iv) blood transfusion within 4
weeks of enrolment; v) inability to walk; or vi) current
participation in an intervention trial.
Written informed consent was obtained from all
patients. The study was approved by the ethics committee of the
First Affiliated Hospital of Guangzhou Medical University (permit
no. 2017-22) and was registered with www.clinicaltrials.gov (NCT 03240315).
Clinical and functional
parameters
Data collected at enrolment included demographic
characteristics, lung function, COPD assessment test (CAT), and
modified Medical Research Council Dyspnea Scale (mMRC) of subjects
prior to sputum induction. Spirometry was performed according to
the American Thoracic Society guidelines (23).
Blood samples, sputum collection and
processing
Peripheral venous blood samples (4 ml per subject)
were collected into a vacuum tube, and serum was obtained by
centrifuging whole blood at 1,057 × g (3,000 rpm) for 10 min at
room temperature. Sputum induction was performed according to
guidelines suggested by the Task Force of the European Respiratory
Society (24). A two-step
procedure was conducted to process the sputum as previously
described (25). Sputum
supernatant and serum were stored at −80°C.
Autoantibody detection
Based on a literature search, ten autoantigens with
known or putative links to COPD were selected (26–29),
including Smith antigen (Sm), ribosomal phosphoprotein P0 (P0),
Ro/Sjögren syndrome type A antigen (SS-A), La/Sjögren syndrome type
B antigen (SS-B), DNA topoisomerase I (Scl70), histidyl-tRNA
synthetase (Jo1), U1 small nuclear ribonucleoprotein (U1-SnRNP),
thyroid peroxidase (TPO), proteinase-3 (PR3) and myeloperoxidase
(MPO). Autoantigens (DIARECT AG) were coupled with multiplex
magnetic beads (Bio-Rad Laboratories, Inc.) and incubated with
sputum supernatant and serum samples diluted 1:10 and 1:180,
respectively, at 37°C for 1 h. The beads were washed using the
Bio-Plex Pro™ wash station (Bio-Rad Laboratories, Inc.), and then
incubated at 37°C for 1 h with biotin-conjugated anti-human IgG
(1:1,000; cat. no. A24474; Thermo Fisher Scientific, Inc.).
Subsequently, they were washed and then reacted for 15 min at 37°C
with streptavidin-R-phycoerythrin (Bio-Rad Laboratories, Inc.).
After the microspheres were washed and resuspended, the median
fluorescence intensity of each encoded microsphere was measured
using Bio-Plex 200 with an excitation wavelength at 532 nm and
emission wavelength at 575 nm (Bio-Rad Laboratories, Inc.).
Bio-Plex Manager™ 6.0 software (Bio-Rad Laboratories, Inc.) was
used to generate the result files.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 19.0; IBM Corp.). PCA was performed on
autoantibody profiles in sputum and serum, and components with
eigenvalues >1 were extracted. Unsupervised agglomerative
hierarchical clustering was performed on the above components,
using the un-centred correlation as the similarity metric (Cluster
version 3.0) (30). The dendrogram
and resulting heatmap were visualised using TreeView (version 1.60)
(31). Shapiro-Wilk test was
performed to access the normality of distribution of each
continuous variable, and depending on the distribution of the data,
ANOVA or Kruskal-Wallis test were used to compare the clusters.
Then, Fisher's Least Significant Difference (LSD) test or the
Nemenyi test was performed to analyse the differences between
clusters. Correlation networks integrating 45 clinical and
molecular parameters were then established using Gephi software
(version 0.9.1) (32). Networks
integrating clinical and autoantibody parameters in each group were
constructed using Spearman's correlation test. Correlation
coefficients with P>0.05 were excluded. Network clustering was
conducted using the ‘fast unfolding’ algorithm within the Gephi
software.
Results
Patient information
The clinical characteristics of 102 patients with
COPD and 18 non-smoking healthy controls are presented in Table I. The mean ages of the patients and
controls were 66.46±8.10 and 58.33±7.67 years, respectively.
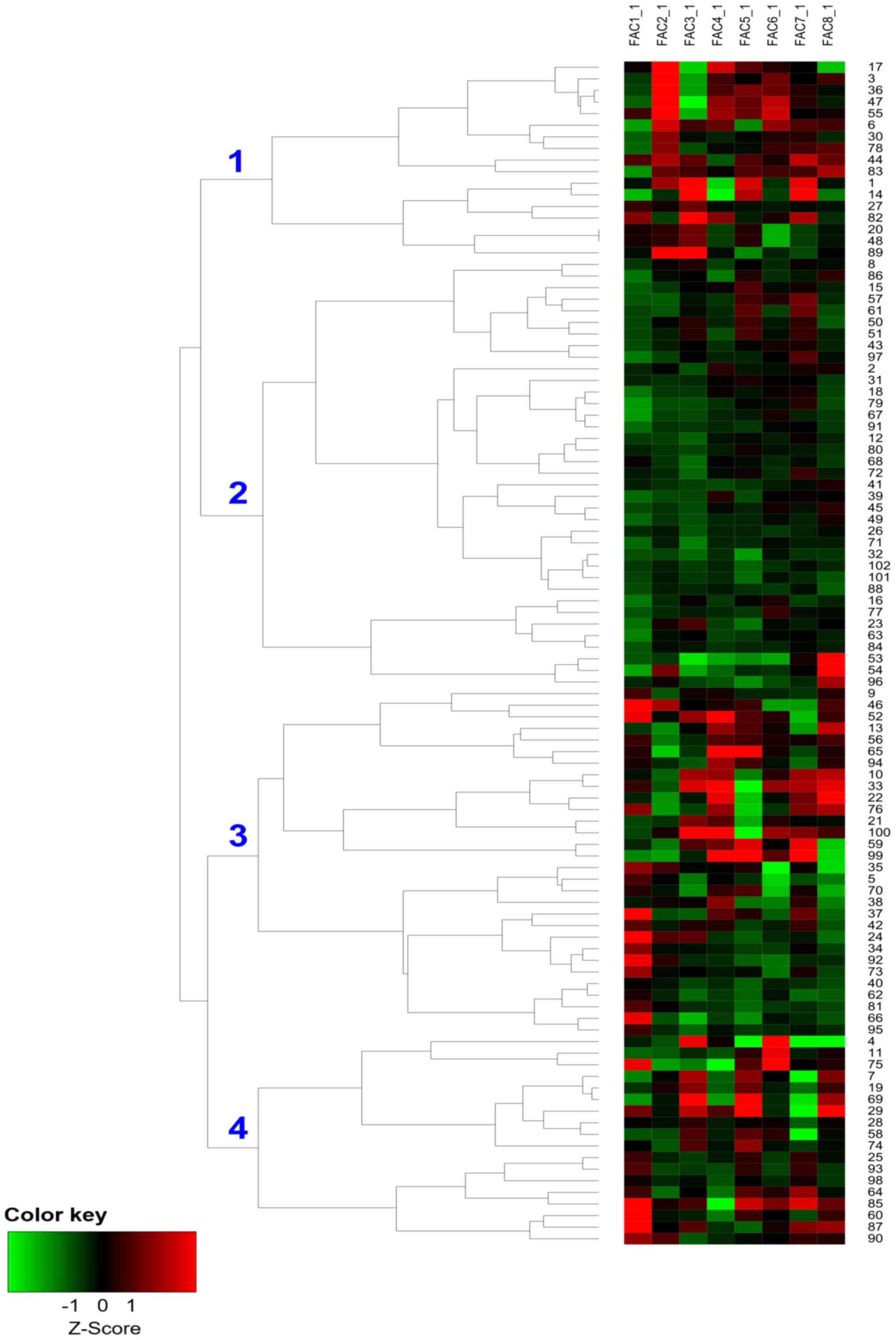
Hierarchical clustering based on
PCA
Sputum and serum autoantibody profile data were
processed with PCA: The eight largest principal components
extracted were able to account for 69.54% of the variability
contained in the original data (Fig.
S1; Table SI), suggesting
that these eight components alone contributed to the majority of
the information among the groups. Components and coefficient sets
used in the analysis are presented in Table SII. Using hierarchical cluster
analysis, four clusters of patients with COPD were identified based
on the above components (Fig.
1).
Clinical characteristics of the four
clusters
To determine whether the patients within these
clusters represented clinically distinct subgroups of COPD, the
clinical parameters of the four clusters were analysed (Table II). The average CAT score and mMRC
of individuals in Cluster 2 were significantly increased compared
with those in Cluster 4. Conversely, there were no significant
differences in various other clinical characteristics [age, number
of exacerbations in the previous year (AE), FEV1, FEV1 as a
percentage of the predicted value (FEV1pred%), maximal
mid-expiratory flow (MMEF), and body mass index (BMI)] among
clusters. Autoantibody levels of the four subtypes were also
analysed (Figs. S2 and S3; Table
SIII).
 | Table II.Comparison of clinical parameters
among clusters. |
Table II.
Comparison of clinical parameters
among clusters.
| Clinical
parameter | Cluster 1,
n=17 | Cluster 2,
n=37 | Cluster 3,
n=30 | Cluster 4,
n=18 | P-value |
|---|
| Age, years | 66.65±8.21 | 66.95±8.58 | 67.03±8.40 | 64.33±6.67 | 0.681 |
| BMI,
kg/m2 | 21.76±5.62 | 22.47±3.63 | 20.79±4.07 | 22.49±3.37 | 0.355 |
| Number of
exacerbations in the previous year | 0 (0–1) | 1 (0–1.5) | 1 (0–2) | 0 (0–1) | 0.109 |
| Blood neutrophil
count, ×109/l |
4.4±1.11 |
4.76±2.45 |
4.04±1.71 | 4.46±1.69 | 0.527 |
| FEV1pred% | 45.16±18.72 |
43.65±17.25 |
53.26±24.11 | 56.63±24.46 | 0.095 |
| MMEF | 0.43±0.25 |
0.46±0.30 |
0.62±0.49 | 0.68±0.46 | 0.105 |
| CAT | 9.88±4.85 | 13.84±6.90 | 11.80±5.46 | 8.67±7.01 | 0.022a |
| mMRC | 1 (1–2) | 2 (1–2) | 1 (1–2.25) | 1 (0–2) | 0.029a |
Differential network analysis
Fig. 2 presents the
Spearman correlation networks integrating clinical and autoantibody
parameters in healthy controls (Fig.
2A) and the aforementioned four clusters (Fig. 2B-E). Table III presents the comparisons of
the topological properties of the five groups. Notable observations
included: i) The five networks exhibited different topological
properties (the cluster 2 network displayed high density, whereas
the cluster 1 network displayed low density); ii) there were seven
modules in cluster 1, five modules in clusters 2 and 3, and six
modules in cluster 4, but all modules appeared markedly
heterogeneous in their clinical and biological content, as the
majority contained nodes of distinct functional and immunological
categories (Fig. 2); and iii) the
retrospective exacerbation-associated factors (AE-nodes in Fig. 2) were significantly different among
the four clusters. In cluster 1 (Fig.
2B), the AE was only positively associated with the CAT score.
In cluster 2 (Fig. 2C), the AE was
negatively associated with age, lung function (FEV1pred% and MMEF),
sputum autoantibodies (P0, Scl70, Sm, U1-SnRNP, PR3 and Ro/SSA) and
serum globulin (Glb), and positively associated with blood cell
counts (peripheral white blood cell, neutrophil and monocyte) and
blood haemoglobin. In cluster 3 (Fig.
2D), the AE was positively associated with the CAT score and
sputum autoantibodies (U1-SnRNP, PR3, MPO and Ro/SSA), and in
cluster 4 (Fig. 2E), the AE was
negatively associated with serum uric acid and blood neutrophil
count (Table III). Additionally,
sputum anti-PR3, sputum anti-Ro/SSA, and sputum anti-U1-SnRNP were
significantly negatively correlated with AE in cluster 2 (Fig. 2C), but were positively correlated
with AE in cluster 3 (Fig. 2D;
Table III). The network of
non-smoking controls had a lower density, lower average path
length, lower average degree and longer diameter than those in COPD
groups, reflecting normal immunological condition (Fig. 2A; Table III).
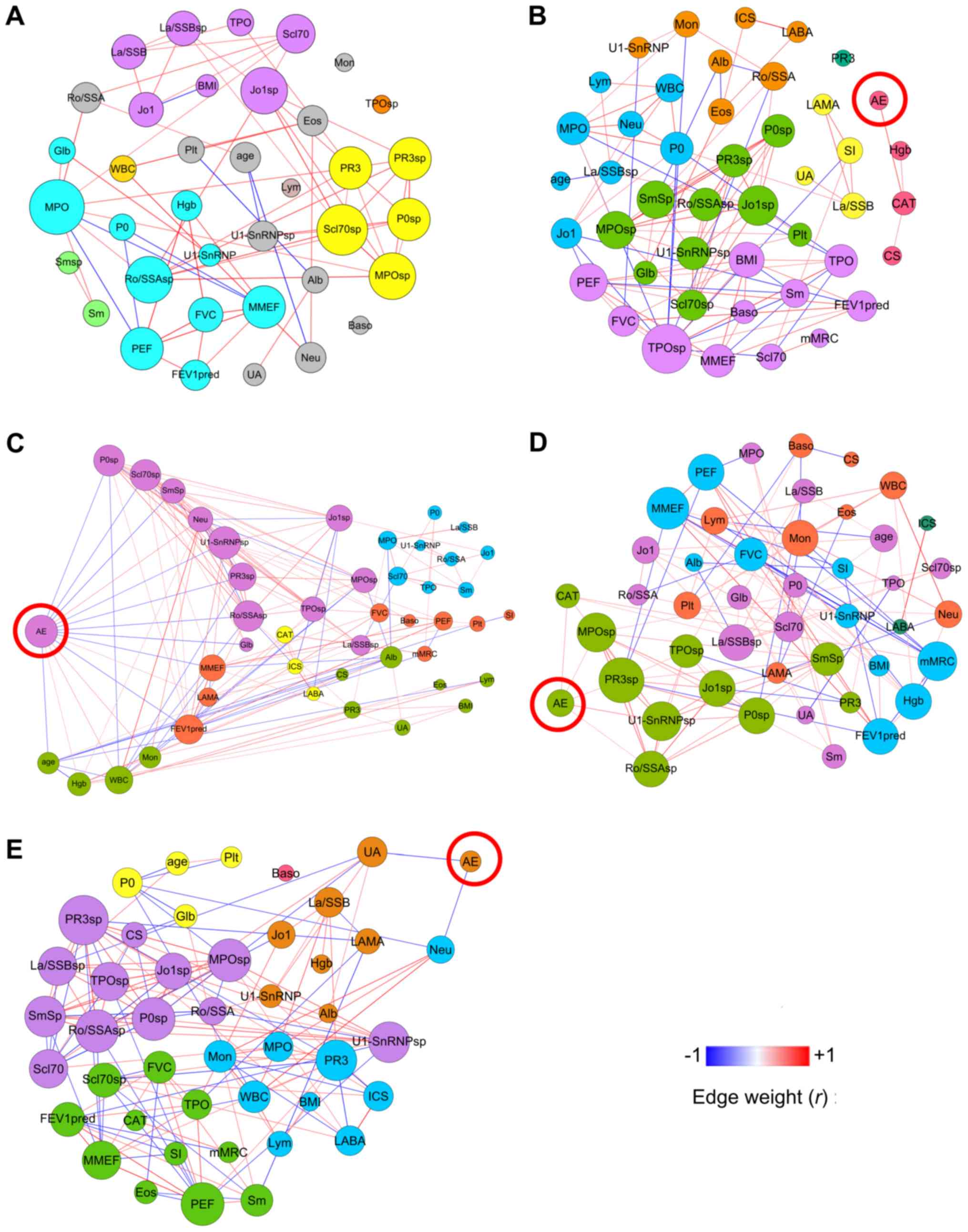 | Figure 2.Network analysis of non-smoking
healthy controls and the four clusters. (A) Non-smoking healthy
controls and clusters (B) 1, (C) 2, (D) 3 and (E) 4. The size of
each node is proportional to its weighted degree value. The colour
of each node represents the corresponding module. Correlation
coefficients with P>0.05 were filtered out. The colour of each
edge indicates the correlation coefficient (edge weight) between
two nodes. AE, number of exacerbations in the previous year; BMI,
body mass index; Alb, serum albumin; CAT, chronic obstructive
pulmonary disease assessment test score; FEV1pred, forced
expiratory volume in 1 sec as percentage of predicted; CS, current
smoker; Hgb, haemoglobin; Glb, serum globulin; MMEF, maximal
mid-expiratory flow; mMRC, modified Medical Research Council
Dyspnea Scale; Mon, peripheral blood monocyte count; Neu,
peripheral blood neutrophil count; sp, sputum; UA, serum uric acid;
WBC, peripheral white blood cell count; Sm, Smith antigen; P0,
ribosomal phosphoprotein P0; Ro/SSA, Ro/Sjögren syndrome type A
antigen; La/SSB, La/Sjögren syndrome type B antigen; Scl70, DNA
Topoisomerase I; Jo1, histidyl-tRNA synthetase; U1-SnRNP, U1 small
nuclear ribonucleoprotein; TPO, thyroid peroxidase; PR3,
proteinase-3; MPO, myeloperoxidase. |
 | Table III.Topological properties of the four
correlation networks. |
Table III.
Topological properties of the four
correlation networks.
| A, Network
properties |
|---|
|
|---|
| Factor | Non-smoking healthy
controls | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 |
|---|
| Number of
nodes | 37 | 45 | 45 | 45 | 45 |
| Average degree | 3.459 | 4.711 | 6.711 | 5.156 | 6.311 |
| Number of
edges | 64 | 106 | 151 | 116 | 142 |
| Network
diameter | 9 | 7 | 6 | 8 | 6 |
| Graph density | 0.096 | 0.107 | 0.153 | 0.117 | 0.143 |
| Average path
length | 3.64 | 3.043 | 2.555 | 2.908 | 2.552 |
| Average Clustering
coefficient | 0.45 | 0.533 | 0.46 | 0.441 | 0.45 |
| Modularity | 0.535 | 0.56 | 0.367 | 0.582 | 0.447 |
| Module number | 10 | 7 | 5 | 5 | 6 |
| Hubs (nodes with
degree within top 10%) | Serum anti-MPO,
sputum anti-Scl70, sputum anti-MPO, sputum anti-Jo1, sputum
anti-Ro/SSA | Sputum anti-TPO,
sputum anti-Jo1, sputum anti-Sm, sputum anti-MPO, PEF | Sputum
anti-U1-SnRNP, AE, sputum anti-P0, sputum anti-Ro/SSA, sputum
anti-Scl70 | Sputum anti-PR3,
MMEF, mMRC, sputum anti-MPO, sputum anti-U1-SnRNP | Sputum anti-PR3,
sputum anti-P0, sputum anti-Ro/SSA, sputum anti-MPO, PEF |
|
| B, AE-node
properties |
|
| Factor | Non-smoking
healthy controls | Cluster
1 | Cluster
2 | Cluster
3 | Cluster
4 |
|
| Degree | NA | 1 | 15 | 5 | 2 |
| Betweenness
centrality | NA | 0 | 70.59 | 3.59 | 7.47 |
| Eccentricity | NA | 2.0 | 5 | 5 | 5.0 |
| Closeness
centrality | NA | 0.6 | 0.49 | 0.35 | 0.32 |
| Clustering
coefficient | NA | 0 | 0.41 | 0.7 | 0 |
| Correlated
nodes | NA | Positive: CAT
Negative: none | Positive: Neu, Mon,
WBC, Hgb Negative: FEV1pred%, MMEF, sputum anti-P0, sputum
anti-Scl70, sputum anti-Sm, sputum anti-U1-SnRNP, sputum anti-PR3,
sputum anti-Ro/SSA, Glb, age | Positive: CAT,
sputum anti-PR3, sputum anti-MPO, sputum anti-Ro/SSA, sputum
anti-U1-SnRNP Negative: none | Positive: none
Negative: UA, Neu |
Exacerbation-related module
analysis
In each cluster, network clustering yielded modules,
and the module containing retrospective exacerbation (AE-node) was
extracted for further analysis (Fig.
3). Table IV presents the
topological properties of the four AE modules of the corresponding
clusters. These modules exhibited significant heterogeneity in
terms of their biological and clinical contents, in addition to
their topological properties. Module 2 demonstrated very high
network density, whereas module 4 demonstrated low density. Module
2 contained 13 nodes with a high average degree, whereas module 1
contained only three nodes with a low average degree. In module 1,
AE was only positively associated with the CAT score. In module 2,
AE was negatively associated with sputum autoantibodies (P0, Scl70,
Sm, U1-SnRNP, PR3 and Ro/SSA) and Glb, and positively associated
with neutrophil counts. In module 3, AE was positively associated
with the CAT score and sputum autoantibodies (U1-SnRNP, PR3, MPO
and Ro/SSA). In module 4, AE was negatively associated with serum
uric acid (Fig. 3, Table IV). Sputum anti-PR3, sputum
anti-Ro/SSA and sputum anti-U1-SnRNP were significantly negatively
correlated with AE in module 2, but were positively correlated with
AE in module 3.
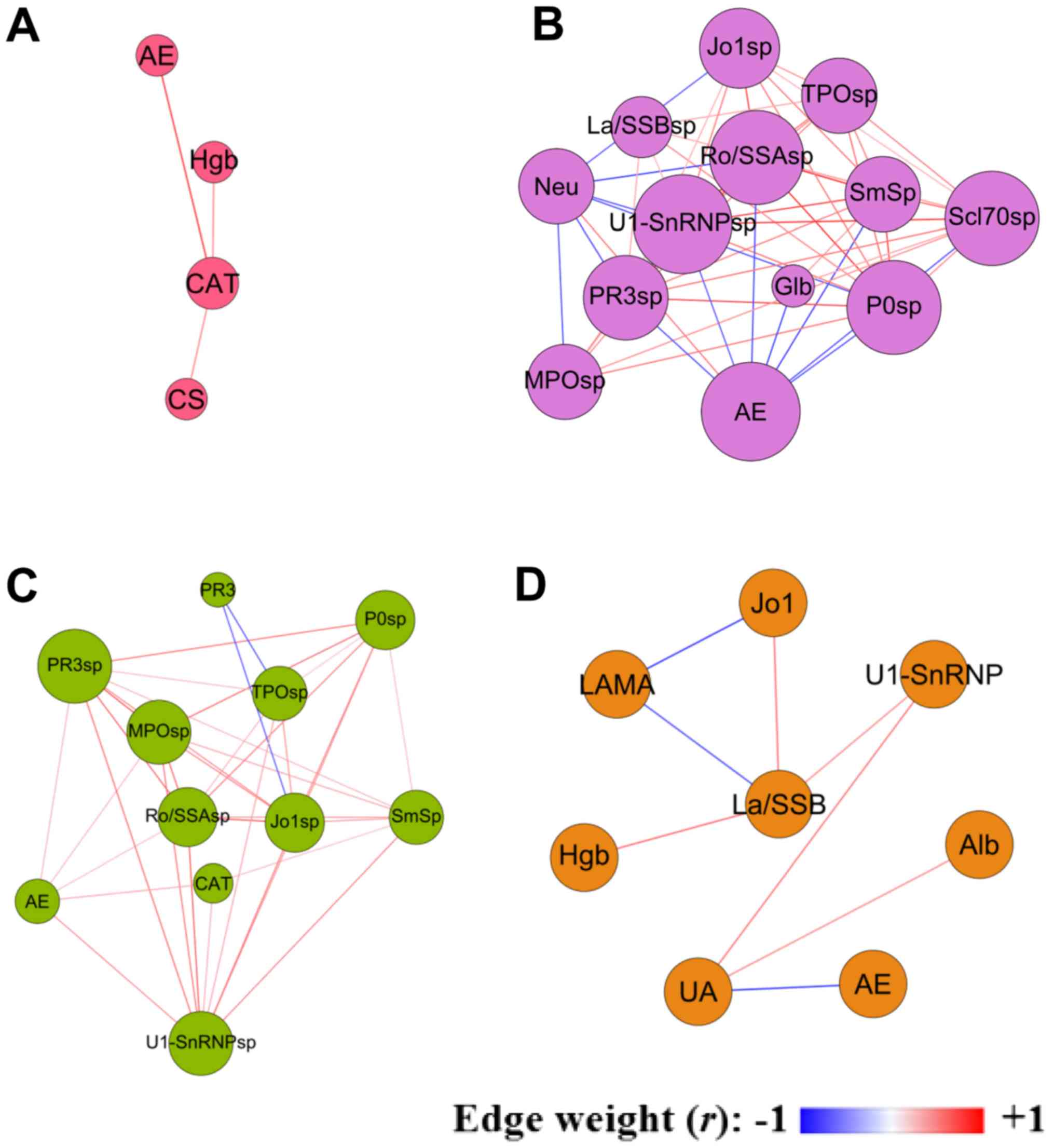 | Figure 3.Exacerbation-associated modules. (A)
Cluster 1; (B) cluster 2; (C) cluster 3; and (D) cluster 4. The
size of each node is proportional to its weighted degree value.
Correlation coefficients with P>0.05 were filtered out. The
colour of each edge indicates the correlation coefficient (edge
weight) between two nodes. AE, number of exacerbations in the
previous year; Alb, serum albumin; CAT, chronic obstructive
pulmonary disease assessment test score; CS, current smoker; Hgb,
haemoglobin; Glb, serum globulin; Neu, peripheral blood neutrophil
count; sp, sputum; UA, serum uric acid; Sm, Smith antigen; P0,
ribosomal phosphoprotein P0; Ro/SSA, Ro/Sjögren syndrome type A
antigen; La/SSB, La/Sjögren syndrome type B antigen; Scl70, DNA
Topoisomerase I; LAMA, long-acting muscarinic antagonist; Jo1,
histidyl-tRNA synthetase; U1-SnRNP, U1 small nuclear
ribonucleoprotein; TPO, thyroid peroxidase; PR3, proteinase-3; MPO,
myeloperoxidase. |
 | Table IV.Topological properties of the
exacerbation-related module of the four clusters. |
Table IV.
Topological properties of the
exacerbation-related module of the four clusters.
| Factor | Module 1 (Cluster
1) | Module 2 (Cluster
2) | Module 3 (Cluster
3) | Module 4 (Cluster
4) |
|---|
| Number of
nodes | 4 | 13 | 11 | 8 |
| Average degree | 1.5 | 8.308 | 6.364 | 2 |
| Number of
edges | 3 | 54 | 35 | 8 |
| Network
diameter | 2 | 2 | 3 | 4 |
| Graph density | 0.5 | 0.692 | 0.636 | 0.286 |
| AE-node degree | 1 | 8 | 5 | 1 |
| AE-correlated
nodes | Positive: CAT | Positive: Neu | Positive: CAT | Positive: none |
|
| Negative: none | Negative: sputum
anti-P0 anti-Scl70 sputum anti-Sm sputum anti-U1-SnRNP sputum
anti-PR3 sputum anti-Ro/SSA Glb | sputum anti-PR3
sputum anti-MPO sputum anti-Ro/SSA sputum anti-U1-SnRNP Negative:
none | Negative: UA
sputum |
Discussion
Autoimmune components in COPD have received
increasing attention, as COPD shares various pathophysiological and
clinical characteristics with autoimmune diseases (4,8,10,11);
however, there remains a lack of medical literature regarding the
relationship between airway/circulating autoantibody responses and
clinical parameters in COPD, particularly in different heterogenous
subgroups. In the present proof-of-concept study, three methods
were employed to investigate the interrelationships among
autoantibody profiles and clinical variables in various COPD
subgroups. First, a highly sensitive detection method was used to
simultaneously investigate autoantibody profiles in sputum and
serum. Second, unsupervised clustering was performed on the
PCA-transformed autoantibody profile data, independent of clinical
parameters, to identify immunological subgroups of COPD. Third, a
network-based analysis was applied to investigate the association
between immunological and clinical parameters, and COPD
exacerbation risks, in each cluster, followed by comparison of the
networks and module properties of these clusters. The following
main findings were reported: i) Four stable COPD subgroups with
distinguished immunological features were identified, although
there were no significant differences among subgroups for the
majority of clinical characteristics; ii) the networks of the four
subgroups exhibited distinct topological properties; iii) the
exacerbation risk-associated factors were significantly different
among the four clusters; and iv) sputum anti-PR3, sputum
anti-Ro/SSA and sputum anti-U1-SnRNP were significantly negatively
associated with exacerbation risk in cluster 2, but positively
associated in cluster 3, suggesting the heterogeneity and dual
nature of the airway autoantibody responses in COPD.
A number of previous studies have investigated
autoantibodies in COPD from a clinical point of view. For example,
Cheng et al (33) detected
circulating IgG, IgA and IgM against human bronchial epithelial
cells (anti-HBEC) in stable patients with COPD using indirect
immunofluorescence, and observed an increased positive rate of
anti-HBEC expression in patients with COPD compared with in healthy
controls. Sigari et al (34) reported increased serum levels of
anti-cyclic citrullinated peptide antibody levels in
wood-smoke-induced COPD compared with in tobacco-induced COPD and
controls. Xiong et al (35)
reported that the plasma autoantibody levels of IgG, IgA and IgM
against cytokeratin-18 and −19 were elevated in patients with COPD
compared with healthy controls. Luo et al (36) investigated the presence of
anti-CD80 autoantibodies in the serum of patients with stable COPD
and controls, and observed that serum levels of anti-CD80 were
increased in patients with COPD compared with those in controls and
were positively correlated with serum levels of interleukin (IL)-6
and IL-8. Shindi et al (37) detected serum IgM and IgG
autoantibodies in patients with COPD and controls using an antigen
microarray, and reported significant differences in the
autoantigenic specificities of IgM autoantibodies compared with IgG
autoantibodies in COPD serum. Conversely, none of these studies
reported airway autoantibody responses in COPD, and no studies have
investigated the autoantibody responses in different immunological
subgroups of COPD. Therefore, the present study was conducted to
investigate the airway/circulating autoantibody responses in
heterogenous subgroups of COPD.
A number of previous studies have explored the
associations among clinical, functional and biological parameters,
and exacerbation risk, in COPD. For example, it was reported that
the CAT score can assist in the prediction of COPD exacerbations
(38); in the present study, it
was observed that the CAT score was significantly associated with
retrospective exacerbations only in two subgroups (cluster 1 and
cluster 3), suggesting clinical heterogeneity in patients with
COPD. Additionally, it was demonstrated that deteriorating airflow
limitation is associated with an increasing prevalence of
exacerbations (39); however, FEV1
lacks sufficient precision (wide variation) to be used clinically
as a predictor of exacerbation in patients with COPD (40). The present study reported that
airflow limitations were associated with retrospective
exacerbations only in one COPD subgroup, which suggested the
heterogeneity of exacerbation risks and was consistent with
previous reports. Peripheral neutrophil count represents low-grade
systemic inflammation in a number of chronic conditions (41); Hong et al (42) reported that the blood neutrophil
count was significantly correlated with main clinical outcomes in
patients with COPD. Of note, it was observed in the present study
that blood neutrophil count was positively associated with
retrospective exacerbation only in one subgroup of COPD patients
(cluster 2) and was negatively associated with retrospective
exacerbation in another subgroup (cluster 4). These results
indicated that the existence of systemic inflammation is also
heterogeneous; however, verification of this requires further
research. Finally, a previous study reported that serum uric acid
was associated with an increased risk of COPD exacerbation
(43); however, the present study
reported that serum uric acid was negatively associated with
retrospective exacerbation in a subgroup of patients, which may be
connected to population heterogeneity and/or recall bias during the
collection of retrospective information.
Differential module analysis also provided further
insight into COPD by demonstrating relationships between
autoantibody modules and clinical variables in the various
subgroups. Module 1 demonstrated a simple structure where
retrospective exacerbations were only associated with CAT score,
suggesting that it may be easier to prevent exacerbation in this
subgroup (cluster 1). Modules 2 and 3 exhibited complex structures,
high network density and high degrees of their respective AE-nodes,
pointing towards the complexity and difficulty of preventing
exacerbation in these subgroups. Of note, sputum autoantibodies
(U1-SnRNP, PR3, and Ro/SSA) were negatively associated with
exacerbation risk in module 2, but were positively related to
exacerbation risk in module 3, implying the dual character and
heterogeneity of airway autoantibody responses. Thus, these
autoantibodies may mediate tissue injury, but may also serve a
protective role by removing senescent cells and maintaining immune
homeostasis.
The present study had two main strengths. First,
autoantibody profiles were detected in sputum and serum
simultaneously, whereas the majority of previous clinical studies
have detected autoantibodies only in serum or plasma (34,44–47).
In this study and a previous preliminary study (13), it was observed that sputum
autoantibodies were more clinically relevant than serum
autoantibodies, suggesting that studies solely focused on
circulating autoantibodies may provide limited information. Second,
due to the heterogeneity and complexity of autoantibody responses
in cases of COPD, an integrative method was applied based on
unsupervised classification. This method differs from previously
published COPD autoantibody studies in that it provides the
capacity to visualise a wide range of autoantibodies in
heterogeneous subgroups, rather than focusing on a single or small
number of autoantibodies and viewing all patients as homogenous.
Without dividing patients into heterogeneous subgroups, those prior
studies may have generated inconsistent findings (46,47).
A number of limitations of the current study should
be discussed. First, this was a cross-sectional study, so causal
relationships could not be drawn, meaning that
exacerbation-associated factors identified in this study should be
validated using longitudinal cohort data; however, previous studies
reported that the type of inflammatory responses observed during
exacerbation may depend on patient phenotype in stable disease
(48–52), suggesting that patient parameters
in stable disease and exacerbation are closely associated (3,53).
Second, as this preliminary study was performed to investigate the
heterogeneities of airway/circulating autoantibody responses in
patients with COPD, the autoantibody profile data provide limited
clinical information to accurately discriminate the COPD subgroups.
Third, this study was preliminary and was limited to the analysis
of autoantibodies against ten autoantigens. The inclusion of an
increased number of diverse autoantibodies may be more clinically
informative. Furthermore, autoreactive B cells, which are the
source of autoantibodies, should be studied in the future. Finally,
the patients recruited into the present study were predominantly
male, which may have been related to their risk factors. According
to the China Global Adults Tobacco Survey of 2010, 52.9% of males
and 2.4% of females were current smokers (54). In China, cigarette smoking is the
main risk factor for COPD, although in rural Southern China it has
been replaced by exposure to biomass fuel (55). The patients in the cohort were
admitted to a university teaching hospital in Guangzhou (the
largest city in Southern China). Thus, cigarette smoking would have
been the main risk factor for COPD but would have resulted in a
sexual bias, as more males than females are smokers.
In conclusion, using unsupervised clustering and
network analysis, it was demonstrated that: i) Pulmonary
autoantibody responses were heterogeneous and associated with
exacerbation risk in certain subgroups, and therefore their dual
character should be taken into consideration in future research;
and ii) airway and circulating autoantibody profiles can identify
COPD subgroups with various factors associated with exacerbation
risk and distinct network topologies. The present study also
provides support for future strategies involving personalised
predictive biomarker identification and precision management.
Further clinical research should focus on local (airway) autoimmune
responses.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Key R&D
Program of China (grant nos. 2017YFC1310600, 2017YFC1310601 and
2018YFC1311900); Guangzhou Healthcare Collaborative Innovation
Major Project (grant no. 201604020012); National Natural Science
Foundation of China and Canadian Institutes of Health Research
(grant no. 81361128004); Natural Science Foundation of Guangdong
Province (grant no. 2015A030310497); Medical Scientific Research
Foundation of Guangdong Province (grant no. C2017050); Guangzhou
Respiratory Disease Research and Clinical Center Transformation
(grant no. 2014Y2-00540); and 111 Project (grant no. D18010).
Availability of data and materials
All the data generated and analysed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZL, FL, FW, TP and RC designed the study and drafted
the manuscript. ZL, FW, YY, WJ, LZ, JZ, KD, JXX and RC recruited
patients and collected clinical data. FL, JX and TP conducted
Luminex detection. ZL, FW and YY analyzed microarray data. ZL, XC,
WJ, WG, JXX and JZ conducted quality control on the clinical data.
ZL and MJ performed data mining. WG and YY processed biological
samples.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The First Affiliated Hospital of Guangzhou Medical
University (no. 2017-22). Informed consent was obtained from all
patients. The trial registration number for the study was
NCT03240315.
Patient consent for publication
Written informed consent was obtained from the
patients for publication of the data included in the present
manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vogelmeier CF, Criner GJ, Martinez FJ,
Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M,
Fabbri LM, et al: Global strategy for the diagnosis, management and
prevention of chronic obstructive lung disease 2017 report. GOLD
executive summary. Am J Respir Crit Care Med. 195:557–582. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agusti A, Celli B and Faner R: What does
endotyping mean for treatment in chronic obstructive pulmonary
disease? Lancet. 390:980–987. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopez-Campos JL and Agusti A:
Heterogeneity of chronic obstructive pulmonary disease
exacerbations: A two-axes classification proposal. Lancet Respir
Med. 3:729–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agusti A, MacNee W, Donaldson K and Cosio
M: Hypothesis: Does COPD have an autoimmune component? Thorax.
58:832–834. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brusselle GG, Joos GF and Bracke KR: New
insights into the immunology of chronic obstructive pulmonary
disease. Lancet. 378:1015–1026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polverino F, Seys LJ, Bracke KR and Owen
CA: B cells in chronic obstructive pulmonary disease: Moving to
center stage. Am J Physiol Lung Cell Mol Physiol. 311:L687–L695.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kheradmand F, Shan M, Xu C and Corry DB:
Autoimmunity in chronic obstructive pulmonary disease: Clinical and
experimental evidence. Expert Rev Clin Immunol. 8:285–292. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cosio MG, Saetta M and Agusti A:
Immunologic aspects of chronic obstructive pulmonary disease. N
Engl J Med. 360:2445–2454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cosio MG: Autoimmunity, T-cells and STAT-4
in the pathogenesis of chronic obstructive pulmonary disease. Eur
Respir J. 24:3–5. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caramori G, Ruggeri P, Di Stefano A, Mumby
S, Girbino G, Adcock IM and Kirkham P: Autoimmunity and COPD:
Clinical Implications. Chest. 153:1424–1431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wen L, Krauss-Etschmann S, Petersen F and
Yu X: Autoantibodies in chronic obstructive pulmonary disease.
Front Immunol. 9:662018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim WD, Kim WS, Koh Y, Lee SD, Lim CM, Kim
DS and Cho YJ: Abnormal peripheral blood T-lymphocyte subsets in a
subgroup of patients with COPD. Chest. 122:437–444. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang Z, Long F, Deng K, Jian W, Zhou L,
Zheng J, Huang A, Cui D, Jin A, Gao Y, et al: Sputum but not
circulating autoantibodies associated with exacerbations risk in
patients with chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 195:A52482017.
|
|
14
|
Diez D, Agusti A and Wheelock CE: Network
analysis in the investigation of chronic respiratory diseases. From
basics to application. Am J Respir Crit Care Med. 190:981–988.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barabasi AL, Gulbahce N and Loscalzo J:
Network medicine: A network-based approach to human disease. Nat
Rev Genet. 12:56–68. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noell G, Faner R and Agusti A: From
systems biology to P4 medicine: Applications in respiratory
medicine. Eur Respir Rev. 27(pii): 1701102018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watts G: Alvar Agusti: Bringing systems
biology to COPD. Lancet. 390:9272017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Divo MJ, Casanova C, Marin JM, Pinto-Plata
VM, de-Torres JP, Zulueta JJ, Cabrera C, Zagaceta J,
Sanchez-Salcedo P, Berto J, et al: COPD comorbidities network. Eur
Respir J. 46:640–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grosdidier S, Ferrer A, Faner R, Piñero J,
Roca J, Cosío B, Agustí A, Gea J, Sanz F and Furlong LI: Network
medicine analysis of COPD multimorbidities. Respir Res. 15:1112014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faner R, Gutierrez-Sacristan A,
Castro-Acosta A, Grosdidier S, Gan W, Sánchez-Mayor M, Lopez-Campos
JL, Pozo-Rodriguez F, Sanz F, Mannino D, et al: Molecular and
clinical diseasome of comorbidities in exacerbated COPD patients.
Eur Respir J. 46:1001–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noell G, Cosio BG, Faner R, Monsó E,
Peces-Barba G, de Diego A, Esteban C, Gea J, Rodriguez-Roisin R,
Garcia-Nuñez M, et al: Multi-level differential network analysis of
COPD exacerbations. Eur Respir J. 50(pii): 17000752017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Global Strategy for the Diagnosis,
Management and Prevention of COPD - 2015, . Global Initiative for
Chronic Obstructive Lung Disease (GOLD), Fontana, WI. 2015,
http://goldcopd.org
|
|
23
|
Miller MR, Hankinson J, Brusasco V, Burgos
F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP,
Gustafsson P, et al: Standardisation of spirometry. Eur Respir J.
26:319–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paggiaro PL, Chanez P, Holz O, Ind PW,
Djukanović R, Maestrelli P and Sterk PJ: Sputum induction. Eur
Respir J Suppl. 37:3S–8S. 2002.PubMed/NCBI
|
|
25
|
Bafadhel M, McCormick M, Saha S, McKenna
S, Shelley M, Hargadon B, Mistry V, Reid C, Parker D, Dodson P, et
al: Profiling of sputum inflammatory mediators in asthma and
chronic obstructive pulmonary disease. Respiration. 83:36–44. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mackay IR, Leskovsek NV and Rose NR: Cell
damage and autoimmunity: A critical appraisal. J Autoimmun.
30:5–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Packard TA, Li QZ, Cosgrove GP, Bowler RP
and Cambier JC: COPD is associated with production of
autoantibodies to a broad spectrum of self-antigens, correlative
with disease phenotype. Immunol Res. 55:48–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoenderdos K and Condliffe A: The
neutrophil in chronic obstructive pulmonary disease. Am J Respir
Cell Mol Biol. 48:531–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sangaletti S, Tripodo C, Chiodoni C,
Guarnotta C, Cappetti B, Casalini P, Piconese S, Parenza M,
Guiducci C, Vitali C and Colombo MP: Neutrophil extracellular traps
mediate transfer of cytoplasmic neutrophil antigens to myeloid
dendritic cells toward ANCA induction and associated autoimmunity.
Blood. 120:3007–3018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Hoon MJ, Imoto S, Nolan J and Miyano S:
Open source clustering software. Bioinformatics. 20:1453–1454.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Page RD: TreeView: An application to
display phylogenetic trees on personal computers. Comput Appl
Biosci. 12:357–358. 1996.PubMed/NCBI
|
|
32
|
Bastian M, Heymann S and Jacomy M: Gephi:
An open source software for exploring and manipulating networks.
International AAAI Conference on Weblogs and Social Media; San
Jose, CA: pp. p22009
|
|
33
|
Cheng G, Zhang N, Wang Y, Rui J, Yin X and
Cui T: Antibodies of IgG, IgA and IgM against human bronchial
epithelial cell in patients with chronic obstructive pulmonary
disease. Clin Lab. 62:1101–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sigari N, Moghimi N, Shahraki FS,
Mohammadi S and Roshani D: Anti-cyclic citrullinated peptide (CCP)
antibody in patients with wood-smoke-induced chronic obstructive
pulmonary disease (COPD) without rheumatoid arthritis. Rheumatol
Int. 35:85–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiong Y, Gao S, Luo G, Cheng G, Huang W,
Jiang R, Wang Y and Cui T: Increased circulating autoantibodies
levels of IgG, IgA, IgM against cytokeratin 18 and cytokeratin 19
in chronic obstructive pulmonary disease. Arch Med Res. 48:79–87.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo XM, Liu XY, Tang JH, Yang W, Ni ZH,
Chen QG and Wang X: Autoantibodies against CD80 in patients with
COPD. Clin Transl Immunology. 5:e1032016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shindi R, Almehairi A, Negm OH, Kalsheker
N, Gale NS, Shale DJ, Harrison TW, Bolton CE, John M, Todd I, et
al: Autoantibodies of IgM and IgG classes show differences in
recognition of multiple autoantigens in chronic obstructive
pulmonary disease. Clin Immunol. 183:344–353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SD, Huang MS, Kang J, Lin CH, Park MJ,
Oh YM, Kwon N, Jones PW and Sajkov D; Investigators of the
Predictive Ability of CAT in Acute Exacerbations of COPD (PACE)
Study, : The COPD assessment test (CAT) assists prediction of COPD
exacerbations in high-risk patients. Respir Med. 108:600–608. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mullerova H, Maselli DJ, Locantore N,
Vestbo J, Hurst JR, Wedzicha JA, Bakke P, Agusti A and Anzueto A:
Hospitalized exacerbations of COPD: Risk factors and outcomes in
the ECLIPSE cohort. Chest. 147:999–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soriano JB, Lamprecht B, Ramirez AS,
Martinez-Camblor P, Kaiser B, Alfageme I, Almagro P, Casanova C,
Esteban C, Soler-Cataluña JJ, et al: Mortality prediction in
chronic obstructive pulmonary disease comparing the GOLD 2007 and
2011 staging systems: A pooled analysis of individual patient data.
Lancet Respir Med. 3:443–450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liang Z, Liu L, Zhao H, Xia Y, Zhang W, Ye
Y, Jiang M and Cai S: A systemic inflammatory endotype of asthma
with more severe disease identified by unbiased clustering of the
serum cytokine profile. Medicine (Baltimore). 95:e37742016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hong Y, Park J, Jung YJ, Jeong JS, Kim JH
and Kim WJ: Clinical significance of blood neutrophil differential
count in patients with COPD. Eur Respir J. 50:PA39902017.
|
|
43
|
Bartziokas K, Papaioannou AI, Loukides S,
Papadopoulos A, Haniotou A, Papiris S and Kostikas K: Serum uric
acid as a predictor of mortality and future exacerbations of COPD.
Eur Respir J. 43:43–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wood AM, de Pablo P, Buckley CD, Ahmad A
and Stockley RA: Smoke exposure as a determinant of autoantibody
titre in α1-antitrypsin deficiency and COPD. Eur Respir J.
37:32–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brandsma CA, Kerstjens HA, Geerlings M,
Kerkhof M, Hylkema MN, Postma DS and Timens W: The search for
autoantibodies against elastin, collagen and decorin in COPD. Eur
Respir J. 37:1289–1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Greene CM, Low TB, O'Neill SJ and
McElvaney NG: Anti-proline-glycine-proline or antielastin
autoantibodies are not evident in chronic inflammatory lung
disease. Am J Respir Crit Care Med. 181:31–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee SH, Goswami S, Grudo A, Song LZ, Bandi
V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et
al: Antielastin autoimmunity in tobacco smoking-induced emphysema.
Nat Med. 13:567–569. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hurst JR: Exacerbation phenotyping in
chronic obstructive pulmonary disease. Am J Respir Crit Care Med.
184:625–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bhowmik A, Seemungal TA, Sapsford RJ and
Wedzicha JA: Relation of sputum inflammatory markers to symptoms
and lung function changes in COPD exacerbations. Thorax.
55:114–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saetta M, Di Stefano A, Maestrelli P,
Turato G, Ruggieri MP, Roggeri A, Calcagni P, Mapp CE, Ciaccia A
and Fabbri LM: Airway eosinophilia in chronic bronchitis during
exacerbations. Am J Respir Crit Care Med. 150:1646–1652. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bafadhel M, McKenna S, Terry S, Mistry V,
Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, et
al: Acute exacerbations of chronic obstructive pulmonary disease:
Identification of biologic clusters and their biomarkers. Am J
Respir Crit Care Med. 184:662–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gao P, Zhang J, He X, Hao Y, Wang K and
Gibson PG: Sputum inflammatory cell-based classification of
patients with acute exacerbation of chronic obstructive pulmonary
disease. PLoS One. 8:e576782013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Miravitlles M, D'Urzo A, Singh D and
Koblizek V: Pharmacological strategies to reduce exacerbation risk
in COPD: A narrative review. Respir Res. 17:1122016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
GH Y: Global adult tobacco survey (GATS)
China 2010 country report. China Sanxia Press; Beijing, China:
2011
|
|
55
|
Liu S, Zhou Y, Wang X, Wang D, Lu J, Zheng
J, Zhong N and Ran P: Biomass fuels are the probable risk factor
for chronic obstructive pulmonary disease in rural South China.
Thorax. 62:889–897. 2007. View Article : Google Scholar : PubMed/NCBI
|