Introduction
Dementia is a brain disorder in which learning and
memory are compromised by various complex factors (1). Alzheimer's disease (AD), the most
common form of dementia, is a typical age-related degenerative
brain disease characterized by loss of neurons in the hippocampus
and cortex (2–4). According to a previous study,
approximately 34 million people suffer from AD, among them more
than 5 million were diagnosed with AD patients (5). In the current aging society,
neurodegenerative diseases such as AD have become critical medical
issue worldwide (6). The
pathogenesis of AD involves amyloid plaque accumulation, tau
protein aggregation, and cholinergic dysfunction that induces
neurotoxicity, accompanied by the impairment of cognition,
behavior, and emotion (3,5,7).
Amyloid beta (Aβ) is generated from the cleavage of
amyloid precursor protein (APP) by the combination of enzymes,
β-secretase, and γ-secretase. Aβ 1–42 protein is a fragment of the
full-length Aβ that can cause inflammation and synaptic toxicity by
initiating different biochemical cascades (8,9). In
addition to Aβ accumulation, hyperphosphorylation of the tau
protein is a pathological feature observed in AD that triggers the
neurodegenerative process (10).
Furthermore, memory dysfunction is also triggered by cholinergic
system damage that includes cholinergic neurons, neurotransmitters,
and their receptors (5,11). Therefore, it is important to
maintain the acetylcholine (Ach) level by inhibiting the
acetylcholinesterase (AchE) activity. Various AchE inhibitors such
as tacrine, donepezil, rivastigmine, and galantamine have been used
to treat cognitive impairment by inhibiting AchE activity at the
cholinergic synapses and thus, increasing the Ach content. However,
the clinical efficacy remains unexplored; moreover, various side
effects have been reported (12).
Citrus fruits have been traditionally used as
medicine to increase immunity, alleviate indigestion, and reduce
inflammation in Asia (13). The
peels of Citrus aurantium contain a variety of active
components, including naringin, hesperidin, narirutin, and
polymethoxylated flavone (PMF) such as nobiletin and tangeretin.
Previous studies, reported that nobiletin has various biological
properties such as anti-cancer activity, anti-oxidant capacity,
anti-inflammatory effects, and anti-obesity activity (13–15)
as well as anti-dementia activity (16). Nobiletin is known to increase the
levels of neprilysin protein, an enzyme that demonstrated Aβ
degradation activity in SK-N-SH cells and activation of the protein
kinase A (PKA)/extracellular signal-regulated kinase (ERK)/cAMP
response element (CRE)-binding protein (CREB) intracellular
signaling pathway in PC12D cells (17,18).
However, this has not yet been tested on memory loss in an Aβ
1–42-induced normal mouse model.
In the present study, we administered 50 or 100
mg/kg Citrus aurantium extract (CAE) and 30 mg/kg nobiletin
based on various references (3,13,19).
The nobiletin concentration was determined by calculating the
amount of nobiletin contained in the CAE in this experiment. We
hypothesized whether CAE and nobiletin treatments have similar
anti-dementia effects and underlying anti-apoptotic activity
against β-amyloid-induced memory impairments in mice.
Materials and methods
Sample preparation
The CAE was supplied by KPLC Group (Paris, France)
and prepared as described previously. The main component of CAE,
nobiletin, was analyzed by high-performance liquid chromatography
according to followed method: The solvent was a mixture of water
(A) and Methanol (B) and delivered at 0.7 ml/min in a gradient flow
as follows: 50–25% (20 min), 25–10% (24 min), and 10–50% (35 min)
A. The nobiletin compound (purity; >98) used in the experiment
was purchased from Wako Pure Chemical Industries, Ltd. (Osaka,
Japan).
Animals
Eight week-old 40 male C57BL/6J mice (weight, 20–24
g) were purchased from Orient-bio Co. (Seongnam, South Korea). The
mice were individually housed in stainless steel cages under
controlled conditions with a 12-h light-dark cycle at 23±3°C and 55
± 15% humidity and given free access to water and food. After a
7-day acclimation period, they were used in the experiment. All
experiments were approved by an Ethics Committee (no. 2018-07-008)
of ChemOn Inc. (Yongin, Korea) and performed in accordance with the
national guidelines for the care and use of laboratory animals and
the mice were maintained according to their guidelines. We
monitored changes in body weight once a week, and observed changes
in feed and water intake. In additions, appearance changes such as
hair coat, activity, and posture were monitored once a day after
β-amyloid injection over the course of the experiments. To improve
animal well-being, we provided a sanitary environment to prevent
disease and proper breeding and management and used appropriate
painkillers and anesthetics to reduce pain. The humane endpoints
were set by observing body weight change, hair coat, movement and
posture of mice and applied in consultation with experimental
committee. After the experiment, the mice were anaesthetized by the
100% carbon dioxide inhalation for 2–3 min and a fill rate of about
10–30% of the chamber volume per minute with carbon dioxide. When
both sings as lack of respiration and faded eye color were
observed, the mice were removed from the CO2
chamber.
Aβ 1–42 injection and drug
administration
Aβ 1–42 was dissolved in sterile 0.1 M
phosphate-buffered saline (PBS) and incubated for 7 days at 37°C to
disrupt the aggregates. The mice were anesthetized with 1 ml/kg
body weight Zoletil (40 mg/kg) and Rompun (5 mg/kg) (4:1, v/v)
without any adverse events and the aggregates of Aβ 1–42 protein or
vehicle (sterile 0.9% saline) was injected (5 µl/2.5 min, i.c.v.)
using stereotaxic apparatus coordinates [anteroposterior (AP), −1.0
mm; mediolateral (ML), +1.0 mm; dorsoventral (DV), −2.5 mm]. After
the injection of Aβ 1–42 peptide, the mice were divided into five
groups: 1) control (vehicle), 2) Aβ 1–42 alone, 3) Aβ 1–42 + CAE
(50 mg/kg), 4) Aβ 1–42 + CAE (100 mg/kg), and 5) Aβ 1–42 +
nobiletin (30 mg/kg). The respective drugs were given orally (p.o.)
for 4 weeks (Day 28). Based on the results of previous studies, the
concentrations of CAE and nobiletin were set (3,13,19).
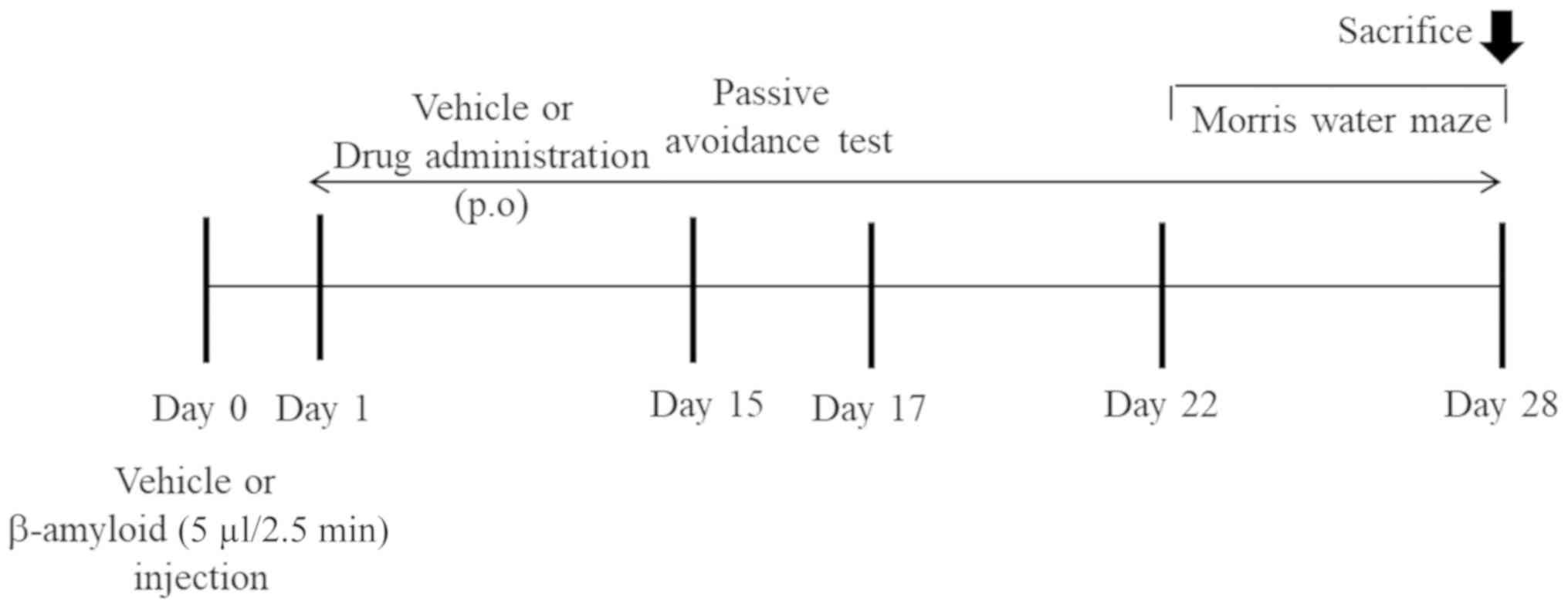
The passive avoidance test was conducted in all groups for 3 days
(Days 15–17) and Morris water maze (MWM) task for 7 days (Days
22–28). The mice were performed by cardiac puncture for
exsanguination after carbon dioxide anesthesia at Day 28 and were
confirmed the death by observing stop breathing and cardiac
dysfunction through CO2 anesthesia and exsanguination.
Their cortex and hippocampus were stored at −80°C for further
analysis. The entire experimental schedule is shown in Fig. 1.
Step-through passive avoidance
test
To determine learning and memory ability, the
passive avoidance test was conducted in an acrylic shuttle box with
two compartments and a guillotine door in the middle. The box
consists of illuminated and non-illuminated compartments with an
electric grid floor that allows for electronic shock stimuli. The
test was performed on 3 consecutive days at 24-h intervals. On the
first day (Day 15), the animals were allowed to explore the
non-illuminated compartment for 2 min and then transferred back to
the illuminated compartment. They were then allowed to access the
illuminated and non-illuminated compartment freely for 60 sec. If
the mice moved to the non-illuminated compartment, they were
immediately removed and trained to adapt to the illuminated
compartment. After the end of adaptation the time, on the second
day, the mice moved between the two compartments for 120 sec, but
when they entered the non-illuminated compartment, the guillotine
door automatically closed and a scrambled shock was given for 2
sec. On the last day of the test, Day 17, the mice were placed in
the illuminated compartment and the time taken for them to move to
the non-illuminated compartment after the door opened was
measured.
MWM task
On Day 22, the mice were tested using the MWM task.
A circular water pool was divided into quadrants; the platform was
randomly located within one quadrant. The mice were trained to find
the platform for 60 sec. When the mice reached the platform, they
were allowed to remain on the platform for 30 sec; if they could
not find the platform within 60 sec, they were guided and placed on
it for 30 sec to learn the extra maze cues. All animals performed
two trials per day and the position of the platform in the circular
pool was randomly changed. The task consisted of 2 days of
training; 4 days of acquisition, during which the time to find the
platform was recorded; and 1 day of probe trial. For the probe
trial, the platform was removed and the animals were allowed to
search it for 1 min. The number of crossings by the mice in the
quadrant with the removed platform was measured on Day 28.
AchE activity
At the end of the experiment, the mice were
sacrificed, and the cortex and hippocampus were dissected from the
brain. Both tissues were rapidly homogenized and sonicated in 0.1 M
phosphate buffer (pH 7.5), followed by centrifugation at 14,000 rpm
for 5 min. The supernatant was collected and stored at −80°C until
further analysis. AchE activity was determined using commercial
assay kits (Abnova; cat. no. KA-1607, Taiwan) according to the
manufacturer's instructions. The activity was calculated as the
optical density (OD) at 412 nm and represented as OD values per
milligram of protein.
Western blotting
The dissected cortex and hippocampus tissues were
lysed in radioimmunoprecipitation assay buffer containing 50 mM
Tris-HCl pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid,
1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl
sulfate, 1 mM phenylmethylsulfonyl fluoride and 1% protease
inhibitor cocktail (Roche, Germany), followed by centrifugation at
12,000 rpm for 15 min at 4°C. The supernatant was collected, and
the protein concentration was calculated by bicinchoninic acid
protein assay kit (Thermo, USA). Equal amount of proteins (20
µg/lane) was separated on a 12% SDS-polyacrylamide gel and
transferred to polyvinylidene difluoride membranes (Bio-Rad, USA).
The membranes were blocked with commercial blocking buffer (Thermo,
USA) for 1 h at room temperature and washed thrice with
Tris-buffered saline containing 0.1% Tween 20 (TBS-T). After
washing, the membrane was incubated at 4°C overnight with the
following appropriate antibodies: B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax; 1:1,000; cat. no. 2772; Cell
Signaling Technology, Inc., USA), B-cell lymphoma 2 (Bcl-2;
1:1,000; cat. no. 3498; Cell Signaling Technology, Inc., USA),
Cleaved caspase-3 (1:1,000; cat. no. 9664; Cell Signaling
Technology, Inc., USA), and β-actin (1:2,000; cat. no. 8457; Cell
Signaling Technology, Inc., USA) The next day, the membranes were
washed thrice with TBS-T and incubated with horseradish
peroxidase-conjugated secondary antibodies for 1 h at room
temperature. The membranes were again washed thrice and enhanced
using chemi-luminescence reagents. The protein bands on the
membrane were detected by a chemi-luminometer (ATTO, Japan).
Densitometry was performed using the Image-Pro Plus soft-ware
(version 6.0; Media Cybernetics, Inc., USA).
Statistical analysis
Data are expressed as the mean ± standard error and
were analyzed with SPSS Statistics 22.0. Different treatment groups
were compared using one-way analysis of variance followed by
multiple comparisons using Dunnett's post hoc test using Origin 7.0
software (Microcal, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Composition of CAE
The composition in CAE were investigated the
chromatographic profiles of a standard on HPLC analysis. We
confirmed the CAE contained 27% nobiletin, and 22% tangeretin
respectively. The optimized CAE was used in all subsequent
experiments.
Observation made regarding the human
endpoints
The mice did not show any change in body weight
during the experiment, but hair coat showed rough condition.
Response to weak stimuli decreased and activity ability also
decreased. Also, the mice was sitting on the floor with a curved
posture was observed and through these symptoms, the humane
endpoint was set. The animals were sacrificed by meeting the
defined endpoint.
Effect of CAE and nobiletin on
step-through passive avoidance task in Aβ 1–42-induced memory
impairment
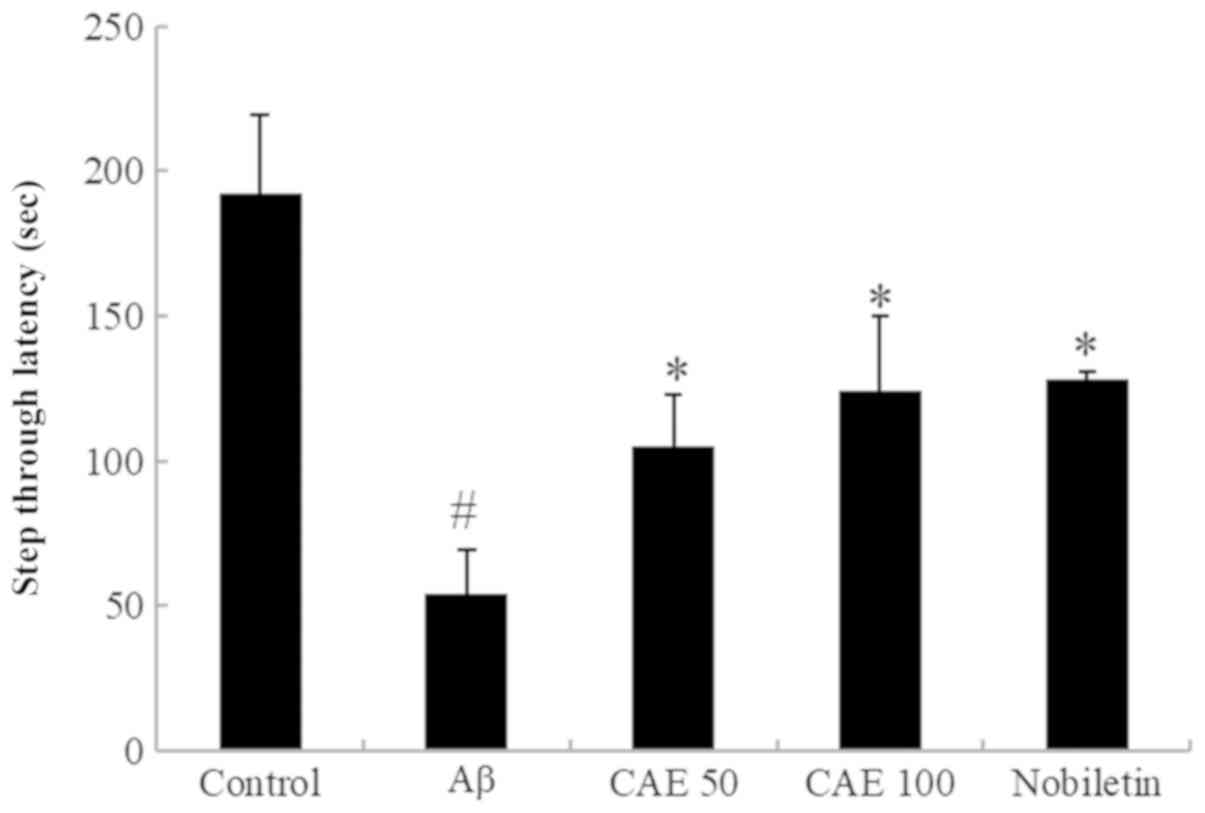
The effect of CAE and nobiletin on the Aβ
1–42-induced memory impairment was measured using a passive
avoidance task. The step-through latency of the Aβ 1–42-only
treated group was significantly shortened compared to the control
group (Fig. 2). However, the
reduced step latency with Aβ 1–42 was restored by the
administration of CAE and nobiletin. CAE 50 and 100 mg/kg treatment
increased the step-through latency by 49.7% (105.06±17.75) and
57.3% (123.92±26.20), and nobiletin (30 mg/kg) recovered the
induced memory impairment up to 58.8% (128.32±2.43) compared with
Aβ 1–42-only treated mice.
Effect of CAE and nobiletin on the MWM
task in Aβ 1–42-induced memory loss in mice
The efficacy of CAE and nobiletin in protection from
the spatial memory impairment via Aβ 1–42 injection was further
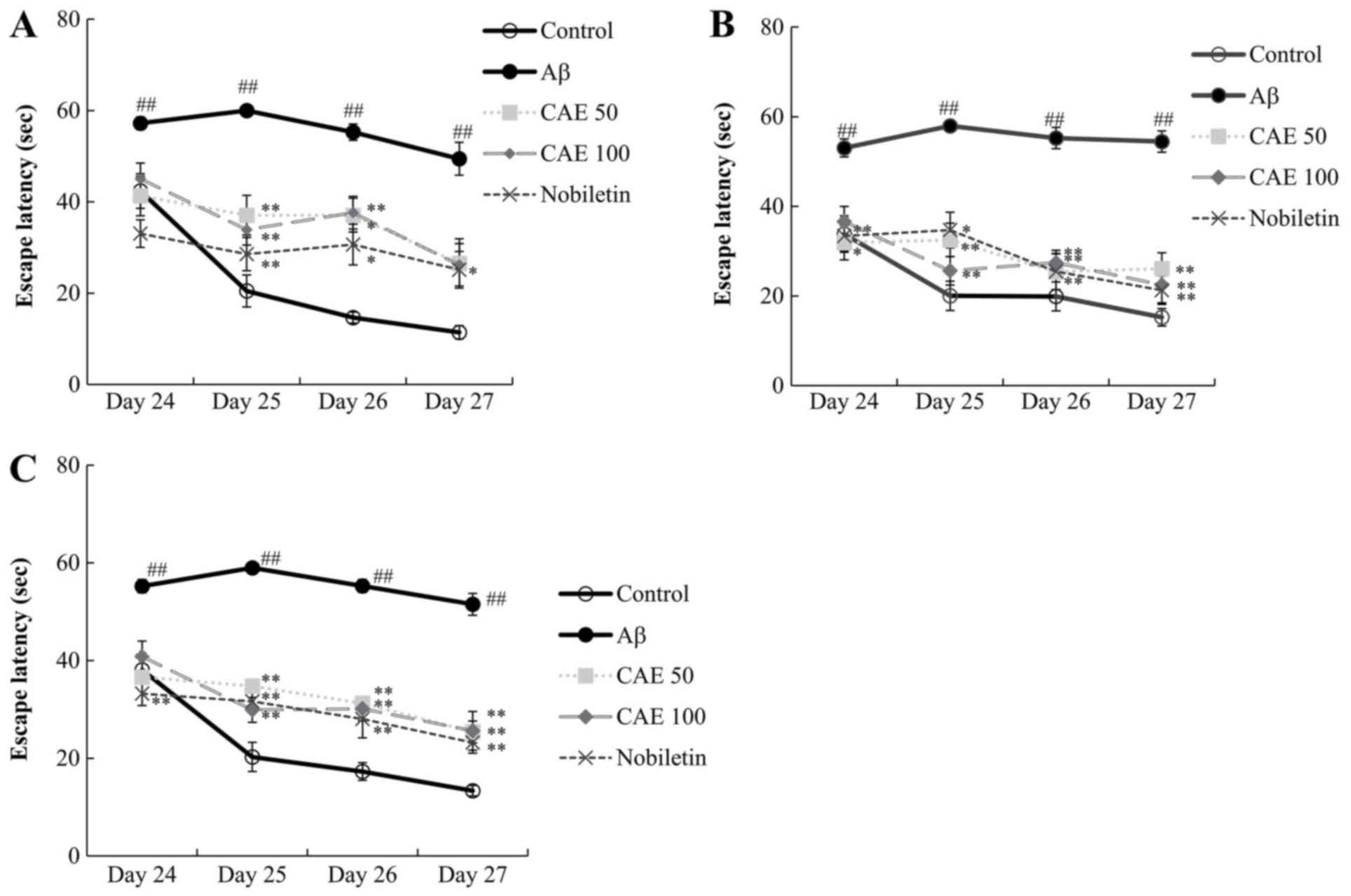
confirmed. The escape latency assessment was performed twice a day.
During the test period, escape latency decreased slightly in the
second trial compare to the first trial in all experimental groups
(Fig. 3A and B). No difference was
observed in the escape latency for 4 days in the amnesic mice,
which were treated with Aβ 1–42. By contrast, the control group
showed significantly decreased escape latency in two trials over 4
days (Fig. 3A). It is
well-established that Aβ 1–42 induces memory loss and increases the
escape latency. The CAE 50 and 100 mg/kg groups demonstrated a
statistically significant (P<0.01) reduction in the escape
latency from Day 25 to Day 27 compared to than the Aβ 1–42-only
treated group (Fig. 3C).
Similarly, the escape latency in mice administered nobiletin
significantly decreased for 4 days compared to the Aβ 1–42-only
injected group.
Effect of CAE and nobiletin on swim
distance in the MWM task
The effect of CAE and nobiletin treatment on the
swim distance to locate the platform in the MWM task is shown in
Table I. The control group mice
were able to swiftly locate the platform and reached the platform
during the training session. However, the Aβ 1–42-treated group had
difficulty learning to locate the platform. The swim distance was
significantly increased compared to that of the control group. The
swim distance of the CAE 50 and 100 mg/kg-treated groups was
significantly reduced compared with those in the Aβ 1–42 group
during the training session. The mice in the nobiletin-treated
group were also able to find the platform easily with a short swim
distance, especially on Day 24.
 | Table I.Effect of nobiletin and CAE on the
distance swum by Aβ 1–42 treated mice to find the platform in the
water maze task. |
Table I.
Effect of nobiletin and CAE on the
distance swum by Aβ 1–42 treated mice to find the platform in the
water maze task.
|
| Distance (mm) |
|---|
|
|
|
|---|
| Treatment | Day 24 | Day 25 | Day 26 | Day 27 |
|---|
| Control | 1,033.69±167.08 | 539.00±179.58 | 454.69±114.75 | 339.88±67.56 |
| Aβ | 1,297.44±152.80 |
1,570.13±83.02c |
1,449.63±109.44c |
1,232.38±116.74c |
| CAE 50 | 910.69±199.40 |
935.13±158.16b |
869.88±230.11a |
637.06±136.15b |
| CAE 100 |
1,056.81±172.04 |
663.75±105.84b |
791.06±87.19b |
610.38±142.27b |
| Nobiletin | 923.19±131.32 |
847.94±121.70b |
752.50±210.10a |
512.81±187.10b |
Effect of CAE and nobiletin on probe
trial in the MWM task
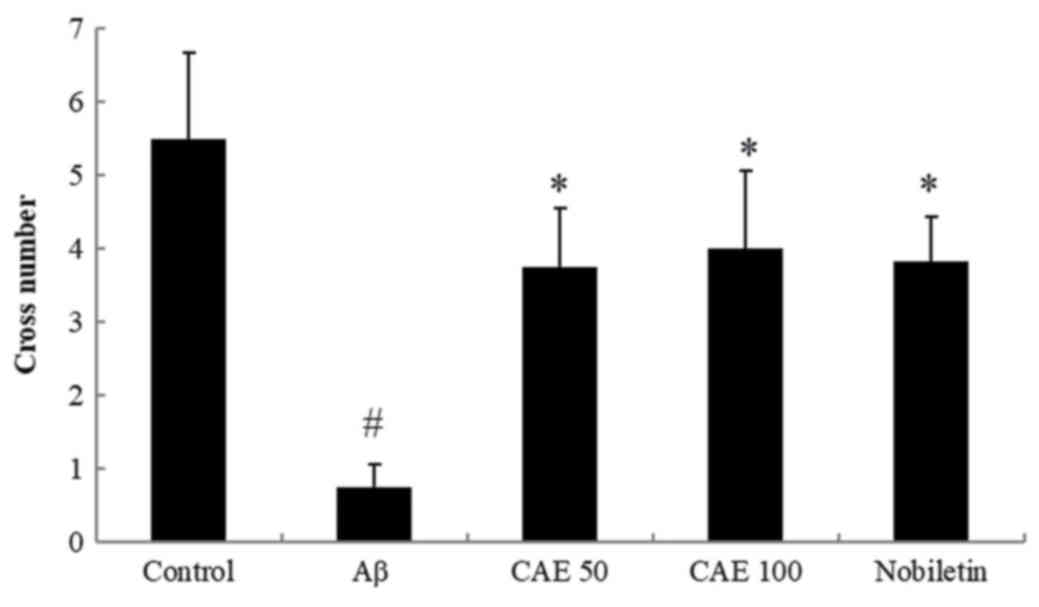
To evaluate the spatial memory of the mice, the
number of crossings to the platform was measured in the probe trial
on Day 28. As shown in Fig. 4, a
significantly increased number of crossings were observed in the
control group than the mice treated with Aβ 1–42, which implies
that the Aβ 1–42 group with memory impairment showed less learning
than the control group. However, the administration of CAE 50 and
100 mg/kg enhanced the number of crossings by 5-fold and 5.33-fold
and nobiletin treatment increased up to 5.17-fold compared with the
Aβ 1–42-treated group. These results show that these drugs enhance
spatial cognition, learning, and memory functions against Aβ
1–42-induced memory impairment.
Effect of CAE and nobiletin on AchE
inhibitory activity in the cortex and hippocampus
To investigate the neuroprotective effect of CAE and
nobiletin on brain tissue, AchE activity was measured in the cortex
and hippocampus (Table II). The
AchE activity in the Aβ 1–42-treatment group was significantly
increased compared to that in control group. CAE 50 and 100 mg/kg
administration reduced the AchE activity in a dose-dependent manner
by 56.6 and 58.8% in the cortex and 8.8 and 35.6% in the
hippocampus, respectively compared to the Aβ 1–42-treated group.
Similarly, the AchE activity in the nobiletin treatment group also
decreased significantly by 56.5% in the cortex and 72.2% in the
hippocampus.
 | Table II.Effect of nobiletin and CAE on AchE
activity in the cortex and hippocampus of mice. |
Table II.
Effect of nobiletin and CAE on AchE
activity in the cortex and hippocampus of mice.
|
| U/mg protein |
|---|
|
|
|
|---|
| Treatment | Cortex | Hippocampus |
|---|
| Control | 38.2902±3.8548 | 24.7195±4.0319 |
| Aβ |
90.2940±10.2591d |
45.6556±2.3807c |
| CAE 50 |
40.7787±3.9846a | 41.5168±2.5505 |
| CAE 100 |
38.0118±1.5175a |
29.8937±2.7889a |
| Nobiletin |
39.2531±2.1394b |
12.7880±0.4159b |
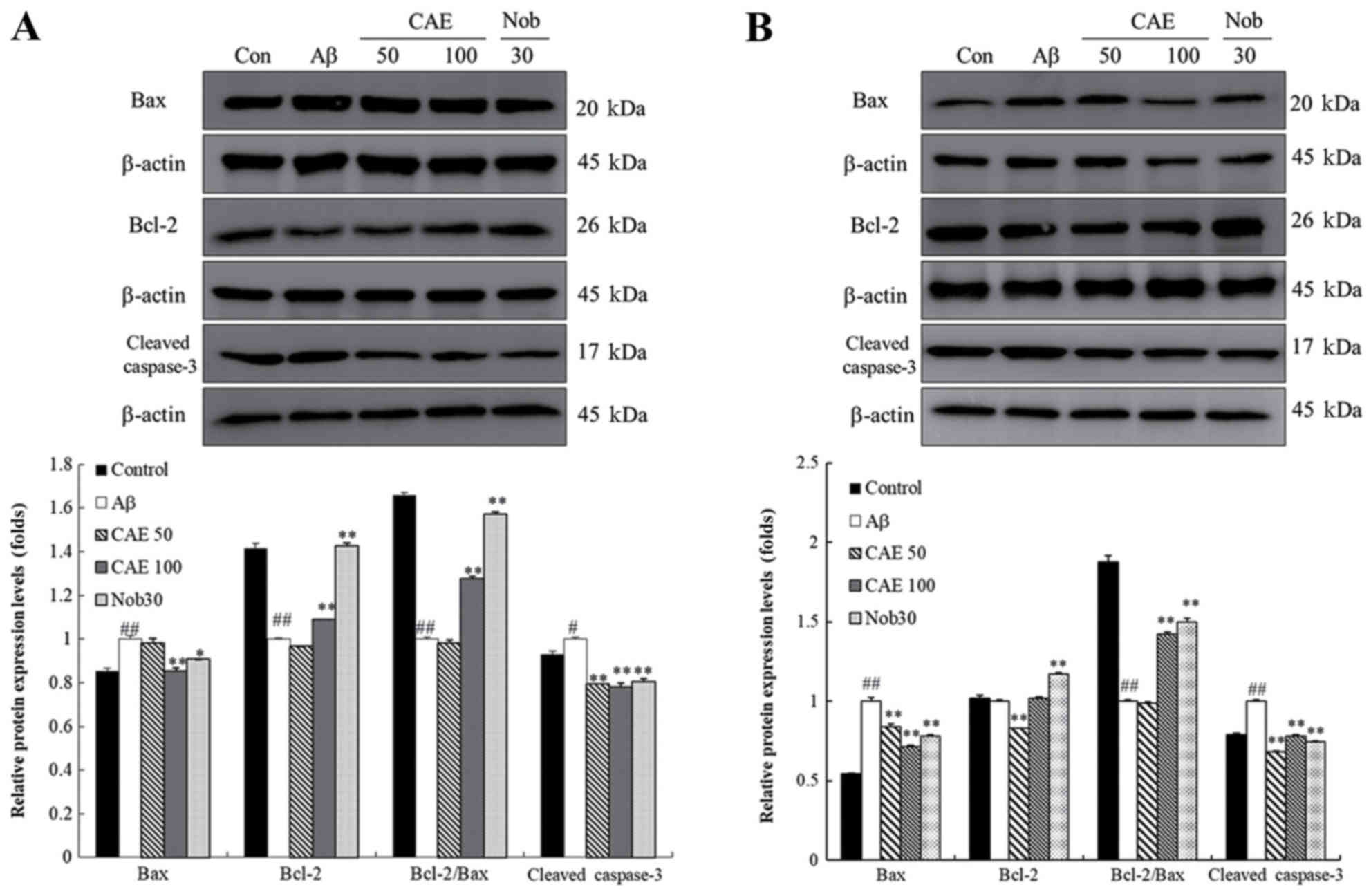
Effect of CAE and nobiletin on
expression levels of Bax, Bcl-2 and cleaved caspase-3 proteins in
the cortex and hippocampus
The effect of CAE and nobiletin on the Bcl-2 family
and caspase pathway was investigated in the cortex and hippocampus.
As shown in Fig. 5, Bax and
cleaved caspase-3 protein levels in the Aβ 1–42-treated group were
decreased in the cortex and hippocampus compared to those in the
control group. In contrast, Bcl-2 protein expression was higher in
the control group than in the Aβ 1–42-only treated group in the
cortex and hippocampus. In the hippocampus, the Bax protein levels
in the CAE 100 mg/kg and nobiletin administration group was
significantly reduced by 15 and 10%, while Bcl-2 expression was
up-regulated in the CAE 100 mg/kg and nobiletin-treated group up to
1.4- and 2-fold compared to the Aβ 1–42-treated mice. In addition,
the ratio of Bcl-2/Bax protein expression was significantly higher
than in the Aβ 1–42-only treated group. The CAE 50 and CAE 100
mg/kg and nobiletin-treated groups showed down-regulation of the
cleaved caspase-3 protein expression by 21, 22, and 20%
respectively (Fig. 5A). A similar
protein expression pattern was observed in the cortex. The Bax and
cleaved caspase-3 protein level decreased with CAE 50 mg/kg, CAE
100 mg/kg, and nobiletin treatment, while the Bcl-2 and Bcl-2/Bax
protein expression was upregulated compared to the levels in the
mice treated with Aβ 1–42 only (Fig.
5B).
Discussion
The present study is the first report to evaluate
the neuroprotective effects in Aβ 1–42-induced memory impairment
animal model and not the transgenic or senescence accelerated mouse
model. Our results showed that the Aβ 1–42-injection resulted in
severe performance deficits in the passive avoidance and Morris
water task as well as neurodegeneration in the mice brain that was
evident from increased AchE activity in the hippocampus and cortex.
In this study, we treated the amnesic mice with CAE and nobiletin
and confirmed the anti-amnesic effect by regulating of apoptotic
signaling.
Aβ plays a major role in the development of AD,
particularly the neurotoxic Aβ 1–42 (10). The direct injection of Aβ 1–42 in
the rodent brain has been used to cause apparent memory deficits,
and Aβ-exposed rats have shown hippocampus-dependent spatial
learning dysfunction in long and short-term tasks (4). Also, the brains of AD patients
demonstrated a high Aβ level compared with normal aged brain
samples (20). The deposition of
Aβ in the cortex and hippocampus, which are responsible for
learning and memory performance, resulted in neuronal apoptosis
(21,22).
To examine the protective effect of CAE and
nobiletin, we performed the passive avoidance and MWM tasks to
investigate learning and memory function. The passive avoidance
task is a method that is used to measure the escape time from the
space that induces pain and fear by electronic shock in rodents
(23). It is commonly used to
confirm the memory function, and we found in this study that CAE
and nobiletin administration significantly increased the
step-through latency to similar levels, a phenomenon that was
reduced by the Aβ 1–42 injection. The MWM is an assessment method
to evaluate hippocampal-dependent learning abilities and cognitive
deficits in rodents. The animals were trained to learn spatial
working information at the learning stage and assisted to build
future memory (24). These results
are consistent with those of previous studies that show Aβ-induced
memory deficits in an MWM task than those in the saline group
(25). As a result of two trials
for 4 days on the MWM task, CAE treatment reduced escape latency in
the second trial compared to the first trial, and escape time
decreased over training days. The nobiletin administration group
showed similar escape latency for 4 days in the first trial, and
the escape latency decreased rapidly on Day 26 in the second trial.
Although the pattern of escape latency of the CAE and nobiletin
group was slightly different, the mean escape latency was decreased
to a similar pattern. This means that CAE and nobiletin
administration showed significant decreases in escape latency,
improvement in cognitive performance, and amelioration of the
memory deficits.
Furthermore, to investigate the neuroprotective
effect of CAE and nobiletin, we examined the changes in the Ach
system in the hippocampus and cortex. Ach is an essential enzyme
that maintains the normal function of the nervous system and is
hydrolyzed by AchE. In addition, Aβ deposition is increased in the
presence of AchE, and AchE activity in AD is related to Aβ
deposition (23). Therefore, it is
important to reduce the level of AchE, which is used as a marker
for the cholinergic nervous system. Here we found that AchE
activity in the cortex was similar to that of CAE and nobiletin.
However, nobiletin administration showed significantly lower AchE
activity than CAE administration in the hippocampus. CAE and
nobiletin administration benefits on the cholinergic
neurotransmission by decreasing AchE activities in the cortex and
hippocampus.
AchE can also be used as a marker of apoptosis. AchE
expression or activity is increased when the cells undergo
apoptosis, and enhanced AchE expression levels are detected in the
brain of focal cerebral ischemic rats (26,27).
AchE is usually present in the cytoplasm and moves to the nucleus
before nuclear morphological changes occur. It then accelerates
chromatin condensation and fragmentation by modulating nuclear
components Therefore, AchE can be detected on the fragmented nuclei
of apoptotic cells, and increased AchE activity implies the
occurrence of cell death (28).
Apoptosis is triggered via two major pathways: The
mitochondrial (intrinsic) pathway and the death receptor-mediated
(extrinsic) pathway. In this study, we focused on the mitochondrial
pathway, which is regulated by Bcl-2 family and caspases (29). Bcl-2 is a known anti-apoptotic
protein, while Bax is a pro-apoptotic protein that promotes
apoptosis. These two proteins are the major factors responsible for
cell death regulation. The Bcl-2 and Bax ratio determines whether a
cell undergoes or escapes apoptosis (23,30).
When the Bcl-2/Bax ratio is lower, the caspase pathway triggers
apoptosis and results in the release of apoptosis-promoting factors
such as cleaved caspase-3. Also, in the previous study, the
decreased Bax/Bcl-2 ratio was seen in the AchE deficiency-mice
model and inhibited the activation of cleaved caspase-3 when AchE
was deficient and inhibited (31).
In our study, the Aβ 1–42 injection group had increased Bax and
cleaved caspase-3 protein expressions compared with the control
group, while Bcl-2 protein levels were increased in the control
group and reduced in the Aβ 1–42-treated group. CAE treatment
significantly decreased the Bax and cleaved caspase-3 protein
expression levels and simultaneously increased Bcl-2 protein
expression in the hippocampus and cortex. Likewise, the treatments
also increased the expression ratio of Bcl-2 to Bax in the cortex
and hippocampus. The nobiletin treatment group displayed reduced
expression levels of Bax and cleaved caspase-3 protein in the
hippocampus to a level similar to that of the CAE 100 mg/kg group.
On the other hand, Bcl-2 protein expression in the nobiletin
administration group was increased to a level similar to that of
the control group. Bax and cleaved caspase-3 protein expressions in
the cortex were significantly inhibited by nobiletin treatment,
while the Bcl-2 protein level was significantly enhanced compared
the control group. Similar to this result, the Bcl-2/Bax ratio also
increased significantly in the hippocampus and cortex.
In conclusion, our results indicate that the
administration of CAE and nobiletin had a similar neuroprotective
effect against Aβ-induced cognitive impairment through reduction of
AchE activity and anti-apoptotic activity and regulating the Bcl-2
family and caspase pathway in the cortex and hippocampus. Our
results provide evidence of the dietary intake of CAE or nobiletin
as a valuable functional food since it has the ability to reduce
cognitive impairment and memory dysfunction. However, to confirm
the neuroprotective effects of CAE and nobiletin, we have to
confirm the morphological change of brain tissues in further study.
In addition, the effects of CAE and nobiletin on Aβ accumulation in
cortex and hippocampus should be studied.
Acknowledgements
Not applicable.
Funding
The present study was supported by Korea Institute
of Planning and Evaluation for Technology in Food, Agriculture,
Forestry and Fisheries (IPET) through High Value-added Food
Technology Development Program, funded by Ministry of Agriculture,
Food and Rural Affairs (MAFRA; grant no. 116014033SB010).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request with the permission of Nutrapharm Tech who granted the use
of this data in the present study.
Authors' contributions
HJL carried out the experiments and wrote the
original manuscript. SKL analyzed the experimental data. DRL and BL
performed the data processing and quality control assessment. BKC
and SHY designed the study, and proofread and finalized the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were approved by an Ethics Committee
(no. 2018-07-008) of ChemOn Inc. (Yongin, Korea) and performed in
accordance with the national guidelines for the care and use of
laboratory animals and the mice were maintained according to their
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bae D, Kim J, Na JR, Kim Y, Lee JY and Kim
S: Anti-amnesic effect of Eriobotrya japonica leaf extract on
scopolamine-induced memory impairment in rats. J Korean Soc Food
Sci Nutr. 43:799–806. 2014. View Article : Google Scholar
|
|
2
|
Jung EY, Lee MS, Ahn CJ, Cho SH, Bae H and
Shim I: The neuroprotective effect of gugijihwang-tang on
trimethyltin-induced memory dysfunction in the rat. Evid Based
Complement Alternat Med. 2013:5420812013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim MJ, Lee J, Seong AR, Lee YH, Kim YJ,
Baek HY, Kim YJ, Jun WJ and Yoon HG: Neuroprotective effects of
Eriobotrya japonica against β-amyloid-induced oxidative stress and
memory impairment. Food Chem Toxicol. 49:780–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang SJ, Luo D, Li L, Tan RR, Xu QQ, Qin
J, Zhu L, Luo NC, Xu TT, Zhang R, et al: Ethyl acetate extract
components of bushen-yizhi formula provides neuroprotection against
scopolamine-induced cognitive impairment. Sci Rep. 7:98242017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnes DE and Yaffe K: The projected
effect of risk factor reduction on Alzheimer's disease prevalence.
Lancet Neurol. 10:819–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HL, Lim SA, Lee HW, Yoo HR and Kim HG:
Yuk-Mi- Jihwang-Tang, a traditional Korean multiple herbal
formulae, improves hippocampal memory on scopolamine injection-
induced amnesia model of C57BL/6 mice. Evid Based Complement
Alternat Med. 2018:28210402018.PubMed/NCBI
|
|
7
|
Yeon SW, You YS, Kwon HS, Yang EH, Ryu JS,
Kang BH and Kang JH: Fermented milk of Lactobacillus
helveticus IDCC3801 reduces beta-amyloid and attenuates memory
deficit. J Funct Foods. 2:143–152. 2010. View Article : Google Scholar
|
|
8
|
Chu YF, Chang WH, Black RM, Liu JR, Sompol
P, Chen Y, Wei H, Zhao Q and Cheng IH: Crude caffeine reduces
memory impairment and amyloid β1-42 levels in an Alzheimer's mouse
model. Food Chem. 135:2095–2102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ali T, Yoon GH, Shah SA, Lee HY and Kim
MO: Osmotin attenuates amyloid beta-induced memory impairment, tau
phosphorylation and neurodegeneration in the mouse hippocampus. Sci
Rep. 5:117082015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng WH, Bastianetto S, Mennicken F, Ma W
and Kar S: Amyloid beta peptide induces tau phosphorylation and
loss of cholinergic neurons in rat primary septal cultures.
Neuroscience. 115:201–211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee GY, Lee C, Park GH and Jang JH:
Amelioration of scopolamine-induced learning and memory impairment
by α-pinene in C57BL/6 mice. Evid Based Complement Alternat Med.
2017:49268152017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SK, Jin DE, Park CH, Seung TW, Guo
TJ, Song JW, Kim JH, Kim DO and Heo HJ: Ameliorating effects of
ethyl acetate fraction from onion (Allium cepa L.) flesh and peel
in mice following trimethyltin-induced learning and memory
impairment. Food Res Int. 75:53–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi BK, Kim TW, Lee DR, Jung WH, Lim JH,
Jung JY, Yang SH and Suh JW: A polymethoxy flavonoids-rich Citrus
aurantium extract ameliorates ethanol-induced liver injury through
modulation of AMPK and Nrf2-related signals in a binge drinking
mouse model. Phytother Res. 29:1577–1584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim TW, Lee DR, Choi BK, Kang HK, Jung JY,
Lim SW, Yang SH and Suh JW: Hepatoprotective effects of
polymethoxyflavones against acute and chronic carbon tetrachloride
intoxication. Food Chem Toxicol. 91:91–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawahata I, Suzuki T, Rico EG, Kusano S,
Tamura H, Mimaki Y and Yamakuni T: Fermented Citrus reticulata
(ponkan) fruit squeezed draff that contains a large amount of
4′-demethylnobiletin prevents MK801-induced memory impairment. J
Nat Med. 71:617–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakajima A, Ohizumi Y and Yamada K:
Anti-dementia activity of nobiletin, a citrus flavonoid: A review
of animal studies. Clin Psychopharmacol Neurosci. 12:75–82. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujiwara H, Kimura J, Sakamoto M, Yokosuka
A, Mimaki Y, Murata K, Yamaguchi K and Ohizumi Y: Nobiletin, a
flavone from Citrus depressa, induces gene expression and increases
the protein level and activity of neprilysin in SK-N-SH cells. Can
J Physiol Pharmacol. 92:351–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura J, Shimizu K, Kajima K, Yokosuka A,
Mimaki Y, Oku N and Ohizumi Y: Nobiletin reduces intracellular and
extracellular β-Amyloid in iPS cell-derived Alzheimer's disease
model neurons. Biol Pharm Bull. 41:451–457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han HY, Lee SK, Choi BK, Lee DR, Lee HJ
and Kim TW: Preventive effect of Citrus aurantium peel extract on
high-fat diet-induced non-alcoholic fatty liver in mice. Biol Pharm
Bull. 42:255–260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Funato H, Yoshimura M, Kusui K, Tamaoka A,
Ishikawa K, Ohkoshi N, Namekata K, Okeda R and Ihara Y:
Quantitation of amyloid beta-protein (A beta) in the cortex during
aging and in Alzheimer's disease. Am J Pathol. 152:1633–1640.
1998.PubMed/NCBI
|
|
21
|
Wang Q, Wang C, Shu Z, Chan K, Huang S, Li
Y, Xiao Y, Wu L, Kuang H and Sun X: Valeriana amurensis improves
Amyloid-beta 1–42 induced cognitive deficit by enhancing cerebral
cholinergic function and protecting the brain neurons from
apoptosis in mice. J Ethnopharmacol. 153:318–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, Chen L, Pan X, Chen J, Wang L,
Wang W, Cheng R, Wu F, Feng X, Yu Y, et al: The effect of
resveratrol on beta amyloid-induced memory impairment involves
inhibition of phosphodiesterase-4 related signaling. Oncotarget.
7:173802016.PubMed/NCBI
|
|
23
|
Talesa VN: Acetylcholinesterase in
Alzheimer's disease. Mech Ageing Dev. 122:1961–1969. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clementi M, Pezzotti M, Orsini F,
Sampaolese B, Mezzogori D, Grassi C, Giardina B and Misiti F:
Alzheimer's amyloid beta-peptide (1–42) induces cell death in human
neuroblastoma via bax/bcl-2 ratio increase: An intriguing role for
methionine 35. Biochem Biophys Res Commun. 342:206–213. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Fang Y, Xu Y, Lian Y, Xie N, Wu
T, Zhang H, Sun L, Zhang R and Wang Z: Curcumin improves amyloid
β-peptide (1–42) induced spatial memory deficits through BDNF-ERK
signaling pathway. PLoS One. 10:e01315252015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu T, Fu Q, Liu X, Zhang H and Dong M:
Increased acetylcholinesterase and capase-3 expression in the brain
and peripheral immune system of focal cerebral ischemic rats. J
Neuroimmunol. 211:84–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang XJ and Greenberg DS:
Acetylcholinesterase involvement in apoptosis. Front Mol Neurosci.
5:402012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Yang L, Zhao Q, Caen JP, He HY,
Jin QH, Guo LH, Alemany M, Zhang LY and Shi YF: Induction of
acetylcholinesterase expression during apoptosis in various cell
types. Cell Death Differ. 9:790–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang M, Li Y, Ni C and Song G: Honokiol
attenuates oligomeric amyloid β1-42-induced Alzheimer's disease in
mice through attenuating mitochondrial apoptosis and inhibiting the
nuclear factor kappa-B signaling pathway. Cell Physiol Biochem.
43:69–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paradis E, Douillard H, Koutroumanis M,
Goodyer C and LeBlanc A: Amyloid beta peptide of Alzheimer's
disease downregulates Bcl-2 and upregulates Bax expression in human
neurons. J Neurosci. 16:7533–7539. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye W, Gong X, Xie J, Wu J and Zhang X,
Ouyang Q, Zhao X, Shi Y and Zhang X: AChE deficiency or inhibition
decreases apoptosis and p53 expression and protects renal function
after ischemia/reperfusion. Apoptosis. 15:474–487. 2010. View Article : Google Scholar : PubMed/NCBI
|