Introduction
Cerebrovascular disease is becoming a prominent
public health concern. Due to its high rates of disability,
morbidity and mortality, cerebrovascular disease not only has a
strong impact on a patient's quality of life, but is also
accompanied by heavy economic burdens for the patients' families
and society (1). Currently,
treatment of cerebrovascular disease consists in the surgical
removal of blocked blood vessels in order to improve the blood
supply in and around the lesion site (2). Once the blood supply improves, the
infarcted area is prone to develop severe cerebral
ischemia/reperfusion (CIR) injury. There are several pathological
mechanisms involved in the process of CIR damage, including
glutamate-induced excitotoxicity, loss of ionic homeostasis, energy
failure, inflammatory response, increased oxidative stress and
apoptosis (3). The accumulation of
reactive oxygen species (ROS) in cells can directly lead to cell
necrosis and indirectly induce cell senescence, apoptosis and
necrosis by activating oxidative signaling pathways (4). Local excessive inflammatory reactions
after cerebral ischemia-reperfusion also cause tissue damage. In
recent years, calcium ion antagonist, radical scavengers and other
neuroprotective drugs have been developed and used for the
treatment of CIR injury (5).
However, the possible side effects of these treatments, including
drug resistance, cerebral hemorrhage and gastrointestinal
irritation, may exceed the clinical benefits of long-term
therapy.
Ras homolog family member A (RhoA) has GTPase
activity and acts as a signal transducer or as a molecular switch
in cell signaling pathways. It may also act on the cytoskeleton and
regulates the response of actin to extracellular signals. Rho
associated coiled-coil containing protein kinase (ROCK) can
catalyze the conversion of inactive RhoA to active RhoA (6). In neurons, activation of RhoA leads
to axonal retraction; conversely, inactivation of RhoA or ROCK
promotes the growth of neurons and the movement of neuron growth
cones. Therefore, RhoA/ROCK pathway is closely associated with the
growth of neuron and their axons (7). Y-27632 is a type of synthetic
pyridine complex that can be introduced in cells via vectors.
Y-27632 is a cell-permeable, highly potent and selective inhibitor
of Rho-associated, coiled-coil containing protein kinase (ROCK). It
binds intracellularly to the catalytic site of the upstream
effector of the Rho protein ROCK (both ROCK1 and 2), thereby
inhibiting its kinase activity. Y-27632 inhibits both ROCK1 (Ki=220
nM) and ROCK2 (Ki=300 nM) by competing with ATP for binding to the
catalytic site. Y-27632 has been shown to inhibit ROCK kinase
activity in epithelial cells, endothelial cells, smooth muscle
cells and neurons (8). It has also
been found that Y-27632 promotes neurite outgrowth in PC12 cells
(9). Y-27632 has also been used in
treatments following spinal cord injury, for it is known to promote
neurite outgrowth and axonal regeneration in neurons (10). However, the effect of Y-27632 on
CIR injury needs to be studied. IR injury is also known to
stimulate the activation and proliferation of astrocytes and
microglia to protect brain tissues (11,12).
Glial fibrillary acidic protein (GFAP), found in astrocytes and
ependymal cells, is a type of cellular microfilament with multiple
functions in cell activity, and its expression may be affected
following CIR.
Therefore, the present study, aimed to investigate
the effects of the ROCK inhibitor Y-27632 on CIR injury and to
explore its underlying mechanism of action. The cognitive ability,
cerebral infarct, inflammation, oxidative stress and apoptosis in
rats with middle cerebral artery occlusion (MCAO) were used to
evaluate the effect of Y-27632 on CIR. The levels GFAP and
allograft inflammatory factor 1 (AIF1) in neurons were assessed by
western blotting to evaluate the activation of astrocytes and
microglia in the context of CIR.
Materials and methods
Animals
A total of 310 male Sprague-Dawley rats, weighing
230–250 g, were purchased from Tianjin Medical University animal
center. Animals were housed at 25°C with a 12-h day/night cycle in
the Animal Center of Baodi District People's Hospital with food and
water available ad libitum. The procedures of the present
study followed international guidelines of animal care (NIH
publication No. 92-3415, revised 1999) and were approved by the
Animal Care Committee in Baodi District People's Hospital.
Experimental protocol
The experimental protocol is summarized in Fig. 1. For biochemical evaluation,
hematoxylin and eosin (H&E) staining, western blotting and
behavioral assessment, 80 rats were randomly grouped into Control,
Y-27632, MCAO + Vehicle and MCAO + Y-27632 groups, with 20 animals
in each group. Rats in the Control group were operated on like the
experimental MCAO ones, but the nylon filament was not introduced
to the carotid artery. Rats in the Y-27632 group received Y-27632
injection and the same operation as Control. Rats in MCAO + Vehicle
group received MCAO surgery and were injected with saline 120 min
later. Rats in MCAO + Y-27632 group received MCAO surgery and were
injected with Y-27632 120 min later. After MCAO, animals were
allowed to rest for 24 h. Then, half the animals in each group were
sacrificed and used in for biochemical, H&E and western blot
analyses, while the remaining half was used for neurological
deficit scoring. After 48 h, these animals were tested in the water
maze. For survival rate studies, 64 rats were randomly grouped into
the Control group (n=12), Y-27632 group (n=12), MCAO + Vehicle
group (n=20) and MCAO + Y-27632 group (n=20). Survival checks were
performed at 24, 48 h and 7 days after MCAO surgery. The animal
health and behavior were monitored twice a day during the
experiments. Survival checks were performed at 1, 2 and 7 days
following MCAO surgery; the inability of rats to right themselves
in 30 sec after being placed on their side was considered a humane
endpoint and were euthanized by overdose of sodium pentobarbital
(150 mg/kg). All Rats were euthanatized with intraperitoneal
injection of an overdose of sodium pentobarbital (150 mg/kg) at the
end of the study. Death was verified by checking the heartbeat.
Rats would be euthanatized if serious infection occurred and
remained uncontrollable. However, no rat was euthanized prior to
the end of the study due to ill health.
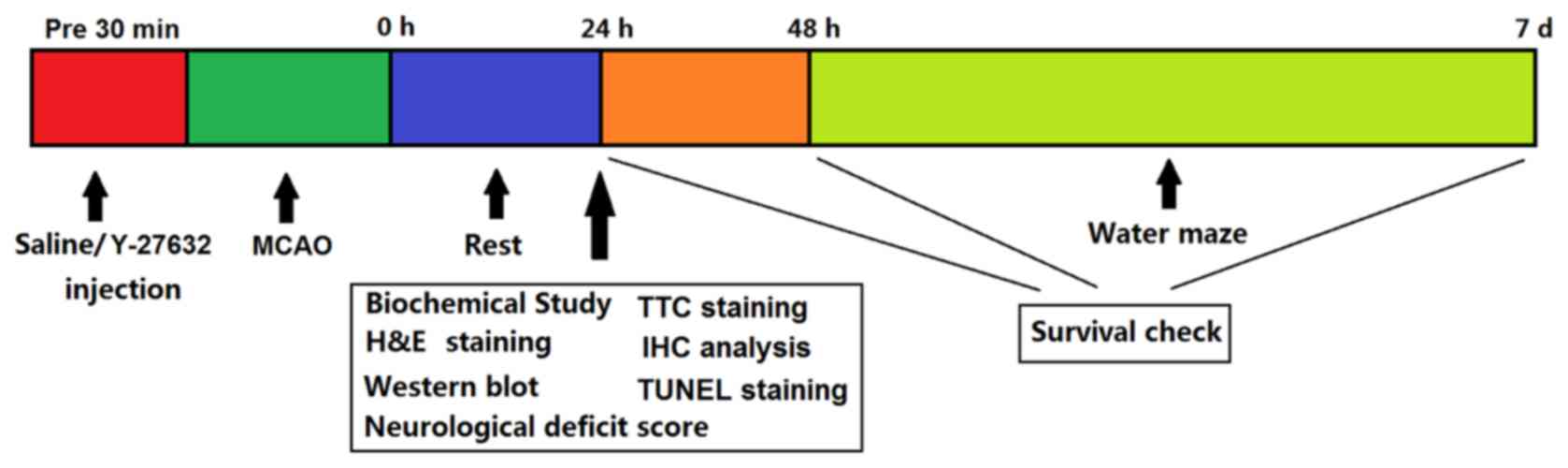 | Figure 1.Experimental protocol. A total of 120
min after MCAO, rats were injected with saline or Y-27632. Rats
were allowed to rest for 24 h after MCAO, then half of the animals
were sacrificed and used for biochemical, hematoxylin and eosin,
2,3,5-triphenyltetrazolium chloride, immunohistochemical, terminal
deoxynucleotidyl transferase dUTP nick end labeling and western
blot analyses. The remaining rats were used for neurological
deficit scoring. After 48 h, these animals were tested in a water
maze study. Survival checks were performed at 24, 48 h and 7 days
after MCAO surgery. MCAO, middle cerebral artery occlusion. |
MCAO procedure
The MCAO procedure was similar to that of Yang et
al (13). Briefly, rats were
anesthetized with ketamine (100 mg/kg, intra-muscularly) and
xylazine (7.5 mg/kg, intra-muscularly) and mounted on a wooden
plate. A 3 cm long incision was made in the center of neck to
expose the common carotid and external carotid arteries. After
these two arteries were ligated, a nylon filament (diameter, 0.25
mm) was inserted into the internal carotid artery and advanced for
20 mm. MCAO lasted for 60 min to generate ischemia, and the nylon
filament was pulled out to allow reperfusion of the injured site.
The body temperature of animals was kept at 37±1°C with a heating
blanket during the IR process.
Intracerebroventricular injection
After 30 min since reperfusion, rats were
anesthetized with ketamine (100 mg/kg, intra-muscularly) and
xylazine (7.5 mg/kg, intra-muscularly). After the head of each rat
was mounted in a stereotaxic instrument, the skull was revealed by
a midline incision. A craniotomy was drilled in the right lateral
cerebral ventricle at the following stereotaxic coordinates
relative to the bregma: 1.00 mm anteroposterior, 1.5 mm
mediolateral and 2.00 mm dorsoventral. In the MCAO + Vehicle group,
10 µl artificial cerebrospinal fluid was injected into the right
lateral cerebral ventricle with a microinjector. In the Y-27632 and
MCAO + Y-27632 groups, 10 µl Y-27632 (10−6 mol/l) was
injected in the same manner. Afterwards, the incision was closed
with a wound clip. Ampicillin (20 mg/kg, twice per day) and
meloxicam (0.2 mg/kg, once per day) were injected intra-muscularly
to prevent wound infection and pain.
2,3,5-Triphenyltetrazolium chloride
(TTC) staining
Following experiments, the brains of animals were
collected for histological analysis. After 20 min freezing at
−20°C, 5 to 6–1.7 mm thick coronal sections were obtained and
placed in 2% TTC phosphate buffer solution (23.9 mM TTC in 0.05 M
phosphate buffer solution, pH 7.5). These sections were stained at
37°C for 30 min in the dark, and then images were capture with a
digital camera. The normal tissue was stained red, while the
infarcted tissue exhibited white coloration. The picture analyzed
using a pathological image analysis computer system, and the
infarct size of each layer was measured to calculate the infarct
volume.
Neurological deficit scoring
Neurological deficit scoring was performed as
previously described (14). At 48
h after MCAO, the potential deficit points of animals were
evaluated as follows: no nerve injury symptom, 0 points;
contralateral papillary flexion, 1 point; reduced contralateral
forepaw clenched grip, 2 points; spontaneous movement in all
directions and circle to the opposite side when pulling their
tails, 3 points; loss of consciousness, 4 points.
Water maze study
The water maze study was performed at 48 h after
MCAO following the methodology described by Ahmadi et al
(15). Briefly, a black circular
tank (120 cm diameter, 80 cm high) was divided into four equal
quadrants and used as the water maze. The depth and the temperature
of water were kept at 30 cm and 25±1°C, respectively. A circular
platform was placed 1.5 cm under the water in a quadrant. A video
camera was used to track the travel path and duration of rats.
Beginning at 48 h after MCAO, the animals were trained for five
consecutive days with the platform located in the same position.
The escape latency, traveled length and mean speed of animals were
calculated from the recorded video. There was no significant
difference between groups in the mean speed, indicating that
physical deficit was not caused by the MCAO procedure. On the sixth
day, the platform was removed. The rats were allowed to swim in the
water maze for 60 s, and the number of times they crossed the
platform location (crosses) and the duration they spent in the
target quadrant were recorded by the video camera.
Measurement of oxidative and
pro-inflammatory factors in the brain tissue
At 24 h after MCAO, animals were sacrificed and
brains were harvested and sliced into 1.0 mm thick slices. A total
100 mg brain tissue of the infarcted area was collected and
homogenized in PBS (10 mM, pH=7.4). Next, they were centrifuged at
12,000 × g at 4°C for 30 min before the supernatant was collected.
The levels of oxidative markers [malondialdehyde (MDA),
(8-hydroxy-2-deoxyguanosine (8-OHdG), 3-nitrotyrosine (3-NT)] and
pro-inflammatory factors [tumor necrosis factor (TNF)-α,
interleukin (IL)-1β, IL-6] in the supernatant were measured using
corresponding assay kits (Sigma-Aldrich; Merck KGaA), following the
manufacturer's protocol. Their concentration was calculated with
absorbance values which were detected by a microplate reader (Azure
Biosystems, Inc.). The protein levels were measured with a protein
assay kit (Nanjing Jiancheng Bioengineering Institute) using the
bicinchoninic acid method. All the concentrations were normalized
to the protein level.
Hematoxylin and eosin (H&E)
staining
A total of 24 h following MCAO, rats were
anesthetized with ketamine (100 mg/kg, intra-muscularly) and
xylazine (7.5 mg/kg, intra-muscularly) and perfused with cold
saline containing heparin (40 mg/l) and 4% paraformaldehyde. After
the rats were fixed on a wooden plate, the needle used for
perfusion was inserted into the aortic root of the rats from the
left apex. After the needle was fixed, the perfusion with cold
saline containing heparin started. When no blood was seen in the
perfusion solution, which indicated that the blood in the animal
was completely replaced by cold saline containing heparin, the
perfusion solution was changed to 4% paraformaldehyde. 1 h later,
the brains were removed, fixed in 4% paraformaldehyde and embedded
in paraffin at room temperature. Coronal sections (6 µm thick) were
taken every millimeter from −1.3 to −6.3 mm relative to the bregma.
Hippocampal sections of the ipsilateral side of the MCAO from eight
rats in each group were collected and stained with H&E at room
temperature for 5 min to analyze the neuronal injury. The numbers
of nucleoli and cytoplasmic Nissl bodies and pyknosis-positive
neurons were compared within groups. A VS120 Virtual Slide
Microscope (Olympus Corporation) and cellSens Imaging Software
(version 2.2; Olympus Corporation) were used to capture and analyze
the images.
Immunohistochemical (IHC)
analysis
IHC analysis of neuron specific enolase (NSE), a
marker of cerebral damage, was performed to evaluate CIR.
Hippocampal sections of the ipsilateral side of the MCAO from eight
rats in each group were collected. Dewaxing and hydration were
first carried out with xylene and alcohol. Endogenous peroxidase
activity was then blocked by the addition of 0.3% hydrogen peroxide
at room temperature for 30 min, and antigen retrieval was performed
using 0.01 M citrate buffer. Next, non-specific antibodies were
blocked by incubation with 5% goat serum (Beyotime Institute of
Biotechnology) at 37°C for 45 min. They were then incubated with
primary antibody against NSE (cat. no. SAB4500768; 1:1,000;
Sigma-Aldrich; Merck KGaA) at 4°C overnight and with the goat
anti-rabbit IgG secondary antibody conjugated to biotin (cat. no.
SAB4600006, 1:5,000, Sigma-Aldrich; Merck KGaA) at 37°C for 1 h.
Afterwards, the sections were washed with PBS three times and
incubated with streptavidin conjugated to horseradish peroxidase
(cat. no. 18-152, 10 µg/ml, Sigma-Aldrich; Merck KGaA) at room
temperature for 1 h. The color was developed using DAB coloring
solution, then counterstained with hematoxylin at room temperature
for 10 min. Finally, the slices were dehydrated and sealed with
xylene and alcohol. Representative images were taken from the
hippocampal region in a total of four different sections per animal
using a VS120 Virtual Slide Microscope (Olympus Corporation) at a
magnification of ×200. The number of immunoreactive signals of NSE
was counted in four randomly selected fields per section.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
Hippocampal sections of the ipsilateral side of the
MCAO from eight rats in each group were collected. Dewaxing and
hydration were first carried out with xylene and alcohol.
Afterwards, the paraffin sections were digested with 20 µg/ml
proteinase K (Sigma-Aldrich; Merck KGaA) at room temperature for 10
min. Next, they were incubated with TUNEL assay solution (Promega
Corporation) at 37°C for 90 min, according to the manufacturer's
protocol. Finally, representative images were taken from the
hippocampal region in a total of four different sections per animal
using a VS120 Virtual Slide Microscope (Olympus Corporation) at a
magnification of ×200. Five randomly selected fields in each image
were examined. The brown colored apoptotic cells were identified as
‘TUNEL positive cells’ and the percentage of the TUNEL positive
cells were calculated.
Western blotting
A total of 100 mg infarcted cerebral cortex tissue
was cut into small pieces, and 1 ml RIPA lysis buffer (Beyotime
Institute of Biotechnology) and 1 ml PMSF (final concentration, 1
mM; Beyotime Institute of Biotechnology) were added to the tissue,
which was then homogenized until fully lysed. After centrifugation
at 30,000 × g for 3 min at 4°C, the supernatant was collected. The
protein concentration in supernatants was determined using a
standard BCA protein assay kit (Beyotime Institute of
Biotechnology), then 40 µg protein in the supernatant was
transferred to polyvinylidene difluoride (PVDF) membranes using
Mini-Protean Tetra Electrophoresis System (Bio-Rad Laboratories,
Inc.). Afterwards, PVDF membranes were blocked in 5% skimmed milk
at room temperature for 2 h. They were incubated with primary
antibodies against Caspase-3 (cat. no. sc-271759; 1:1,000; Santa
Cruz Biotechnology, Inc.), Caspase-8 (cat. no. sc-5263; 1:1,000;
Santa Cruz Biotechnology, Inc.), Caspase-9 (cat. no. sc-133109;
1:1,000; Santa Cruz Biotechnology, Inc.), Bax (sc-20067; 1:1,000;
Santa Cruz Biotechnology, Inc.), Bcl-2 (cat. no. sc-56015; 1:1,000;
Santa Cruz Biotechnology, Inc.), GFAP (cat. no. sc-33673; 1:1,000,
Santa Cruz Biotechnology, Inc.) and AIF1 (cat. no. sc-32725;
1:1,000; Santa Cruz Biotechnology, Inc.) at 4°C overnight. The next
day, they were incubated with a horseradish peroxidase-conjugated
secondary antibody (cat. no. G-21040; 1:10,000; Thermo Fisher
Scientific, Inc.) at 37°C for 2 h. The enhanced chemiluminescent
substrate was applied to the blot membrane and incubated at room
temperature for 5 min. The blot membrane was then placed in a
chemiluminescence imager (Bio-Rad Laboratories, Inc.) for image
acquisition, and densitometry analysis was performed using ImageJ
1.43 software (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using two-way
analysis of variance method followed by Turkey post hoc test with
SPSS software 17.0 (SPSS, Inc.). Data were represented as the mean
± standard error of the mean. The number of experimental repeats
was 12. P<0.05 was considered to indicate a statistically
significant difference.
Results
Treatment with the ROCK inhibitor
increases survival following MCAO
Survival checks were performed at 24 and 48 h and 7
days after MCAO surgery. As shown in Table I, the survival rates at 24 and 48 h
and 7 days in Control and Y-27632 groups were 100%, suggesting that
Y-27632 alone did not affect overall survival. In the MCAO +
Vehicle group, the survival rates at 24 and 48 h and 7 days were
60, 50 and 35%, respectively, with only seven animals surviving
until the seventh day after the MCAO surgery. In the MCAO + Y-27632
group, however, the survival rates at 24, 48 h and 7 days were
significantly higher at 90, 80 and 50%, respectively, with 10 rats
surviving until the seventh day after the MCAO surgery.
 | Table I.Effect of Y-27632 on rat survival
rate. |
Table I.
Effect of Y-27632 on rat survival
rate.
|
| Survival |
|---|
|
|
|
|---|
|
| 24 h | 48 h | 7 days |
|---|
|
|
|
|
|
|---|
|
| n | % | n | % | N | % |
|---|
| Control | 12/12 | 100 | 12/12 | 100 | 12/12 | 100 |
| Y-27632 | 12/12 | 100 | 12/12 | 100 | 12/12 | 100 |
| MCAO+Vehicle | 12/20a | 60 | 10/20a | 50 | 7/20a | 35 |
| MCAO+Y-27632 | 18/20b | 90 | 16/20b | 80 | 10/20b | 50 |
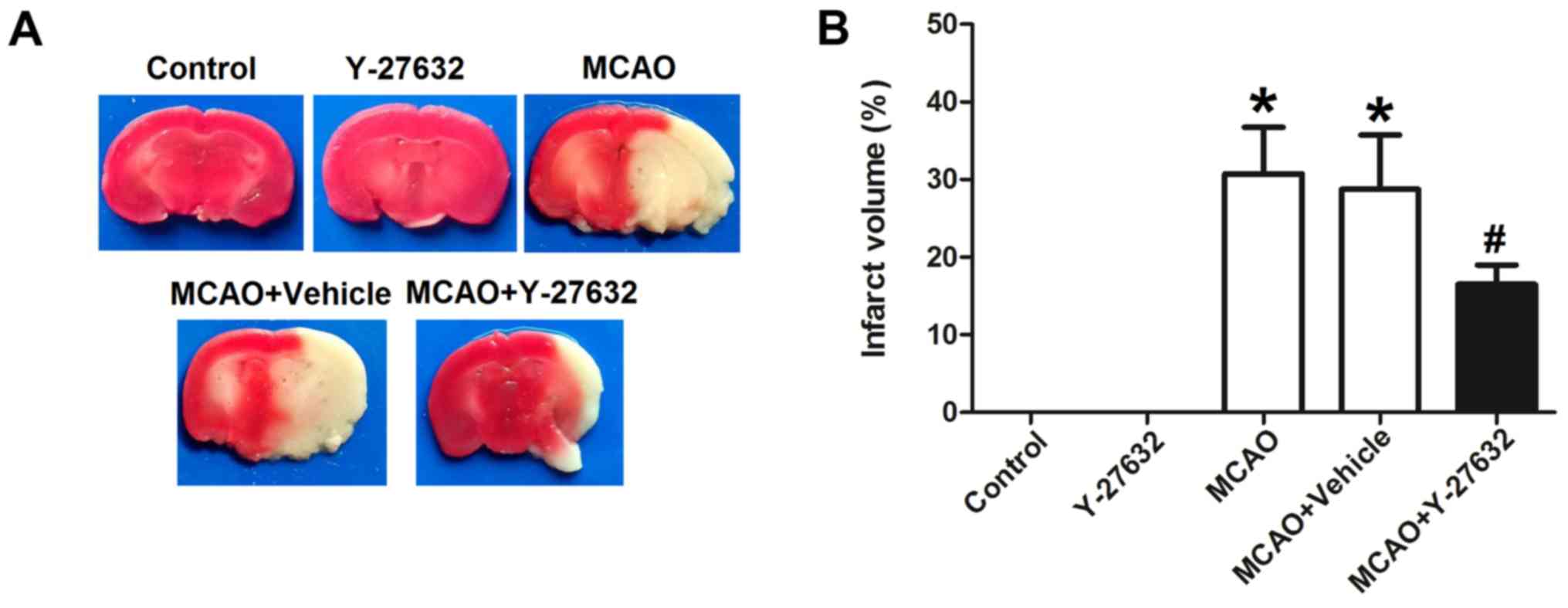
Y-27632 reduces the MCAO-induced
infarct volume in the brain
To assess the effect of ROCK inhibitor Y-27632 on
the infarct caused by MCAO, the infarcted brain area was stained
with TTC and compared between groups. As shown in Fig. 2, the infarct volume in the MCAO
group was 31.5±3.6%, MCAO + Vehicle group was 28.3±5.1%, while the
infarct volume in the MCAO + Y-27632 group was 18.2±3.3%. There was
no significant difference between MCAO group and MCAO + Vehicle
group. The infarct size difference between MCAO + Vehicle and MCAO
+ Y-27632 group was statistically significant (P<0.05).
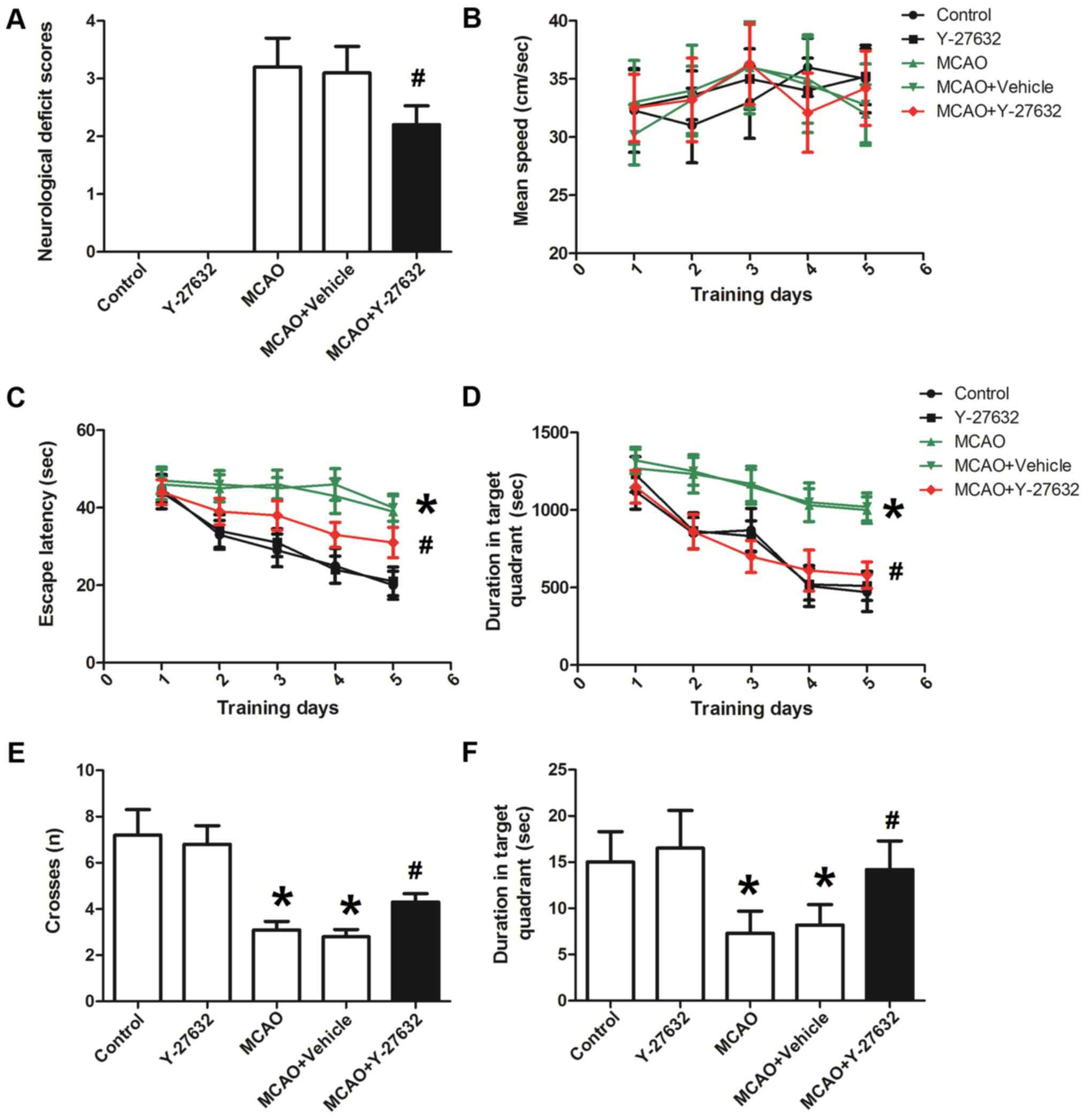
Y-27632 prevents neurological
impairments caused by CIR
As shown in Fig.
3A, MCAO induced significant neurological deficits. The deficit
score in the MCAO + Vehicle group was 3.1±0.23 (P<0.05 compared
to Control). There was no significant difference between MCAO group
and MCAO + Vehicle group. However, the application of the ROCK
inhibitor Y-27632 significantly decreased the neurological deficit
score to 2.2±0.31 (P<0.05 compared to MCAO + Vehicle). The
parameters evaluated with the water maze test are presented in
Fig. 3B-F. The mean swimming speed
(Fig. 3B) was not different
between these four groups, suggesting that the motor ability of
rats were not altered by MCAO or Y-27632. Following treatment with
Y-27632, the escape latency and travel length during training days
(Fig. 3C and D) were significantly
decreased compared to MCAO + Vehicle (P<0.05). Rats in MCAO +
Vehicle group on the test day (sixth day; Fig. 3E) crossed the hidden platform
significantly less, while Y-27632 treated animals significantly
increased the number of crosses (P<0.05 compared to MCAO +
Vehicle). There was no significant difference between MCAO group
and MCAO + Vehicle group. The time rats spent in target quadrant on
the test day (sixth day; Fig. 3F)
was significantly longer in the MCAO + Y-27632 group compared with
the MCAO + Vehicle group (P<0.05). Y-27632 alone did not affect
all these behavior test results (P>0.05 compared to
Control).
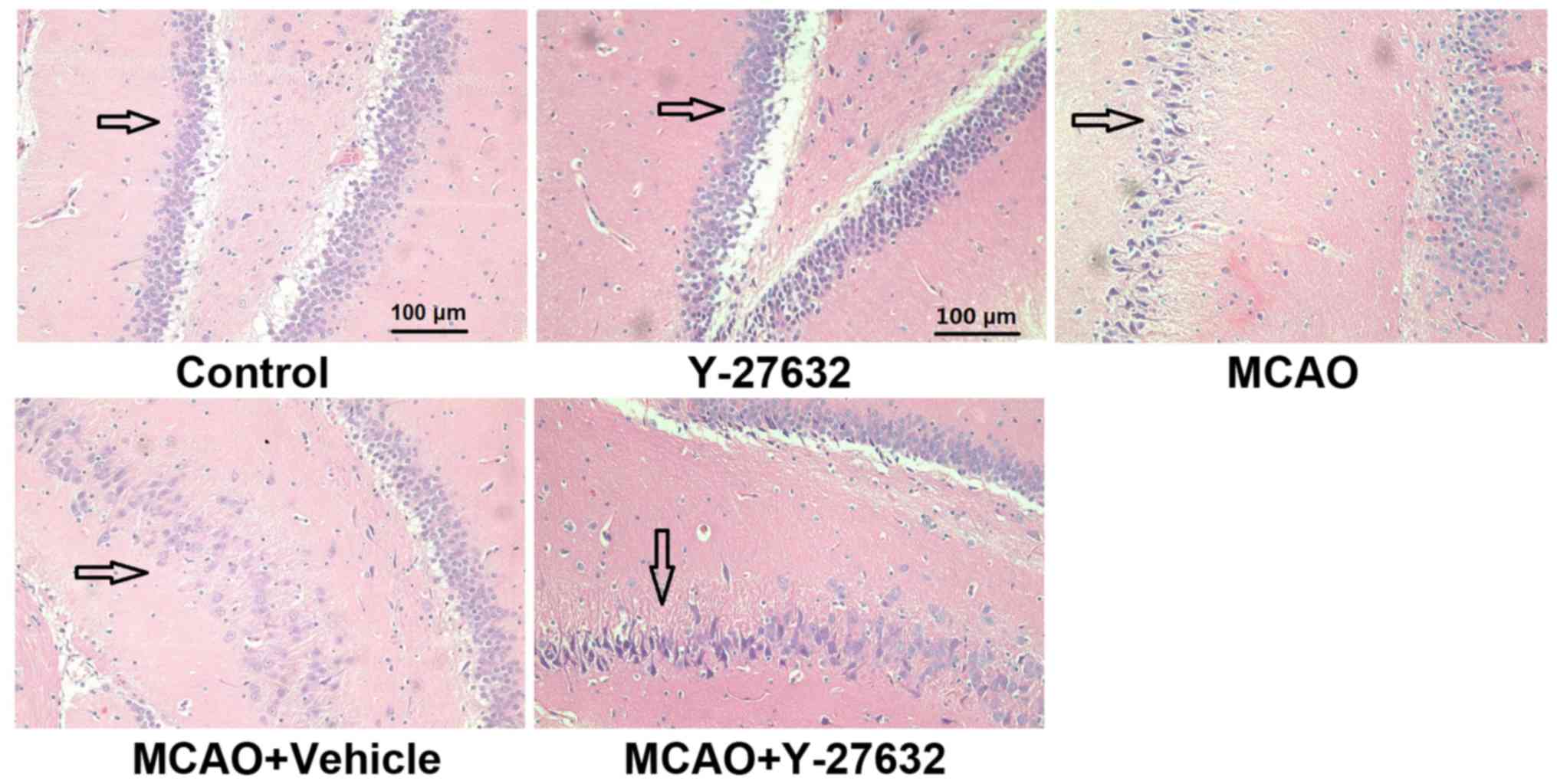
ROCK inhibitor attenuates the
histological changes caused by CIR
The histological changes to the hippocampus across
the four groups are shown in Fig.
4. In the MCAO and MCAO + Vehicle groups, the brain tissue
became less compact, the extracellular space was widened. In the
MCAO + Y-27632 group, these observations were significantly less
common.
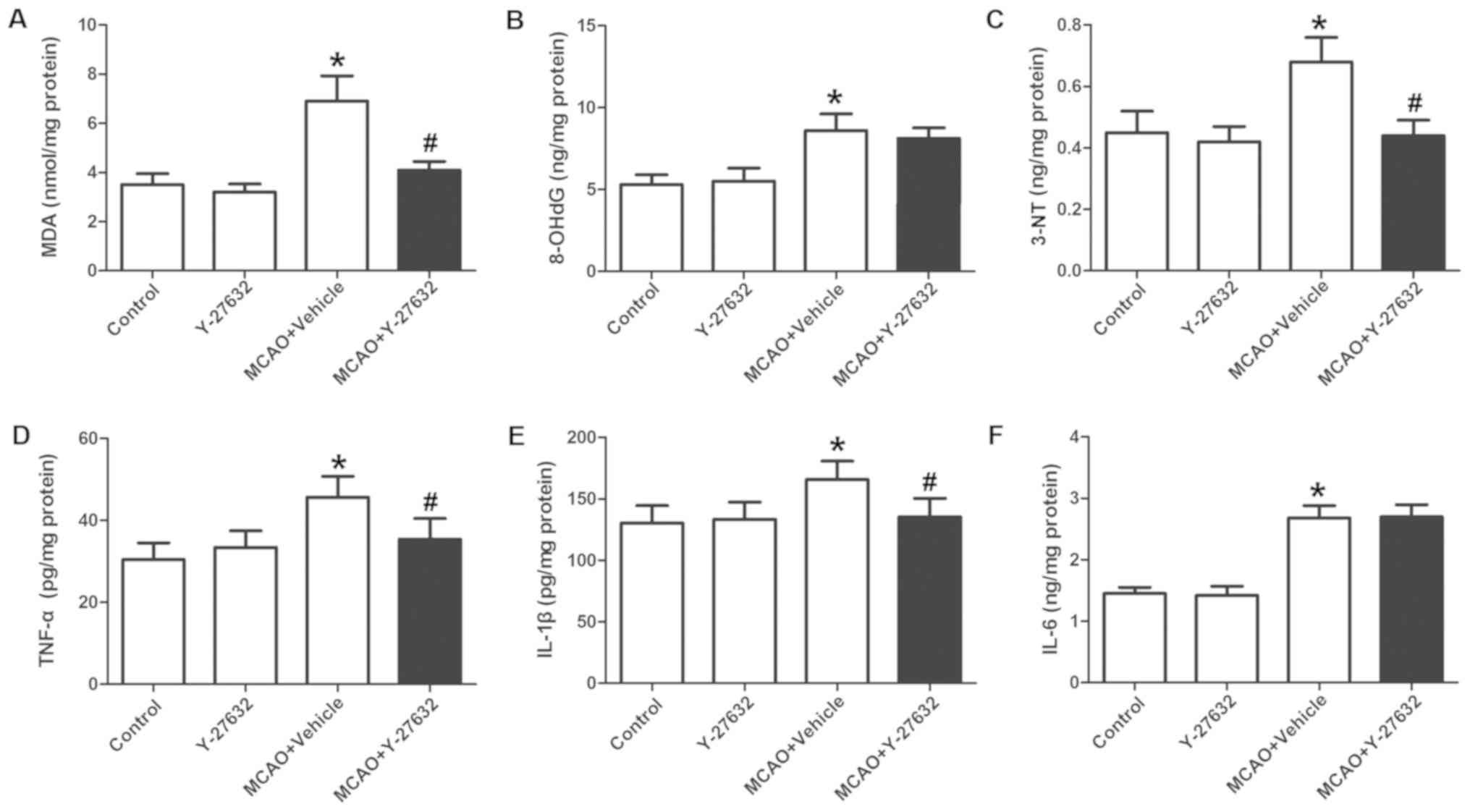
Treatment with the ROCK inhibitor
attenuates the oxidative injury and inflammation
The changes in oxidative injury indicators across
groups are displayed in Fig. 5A-C.
The levels of MDA and 3-NT (Fig. 5A
and C) in the MCAO + Vehicle group were greatly increased
compared to Control, and were significantly inhibited by
co-treatment with Y-27632. The levels of 8-OHdG (Fig. 5B) was greatly increased compared to
Control, but not changed by Y-27632. The changes in inflammatory
marker expression across groups are shown in Fig. 5D-F. The levels of TNF-α and IL-1β
(Fig. 5D and E) in the MCAO +
Vehicle group were significantly increased compared to Control, and
were inhibited by co-treatment with Y-27632 (P<0.05 compared to
MCAO + Vehicle). The IL-6 levels (Fig.
5F) were also increased compared to Control, but not
significantly changed by Y-27632. Y-27632 alone did not affect
oxidative injury or inflammation (P>0.05 compared to
Control).
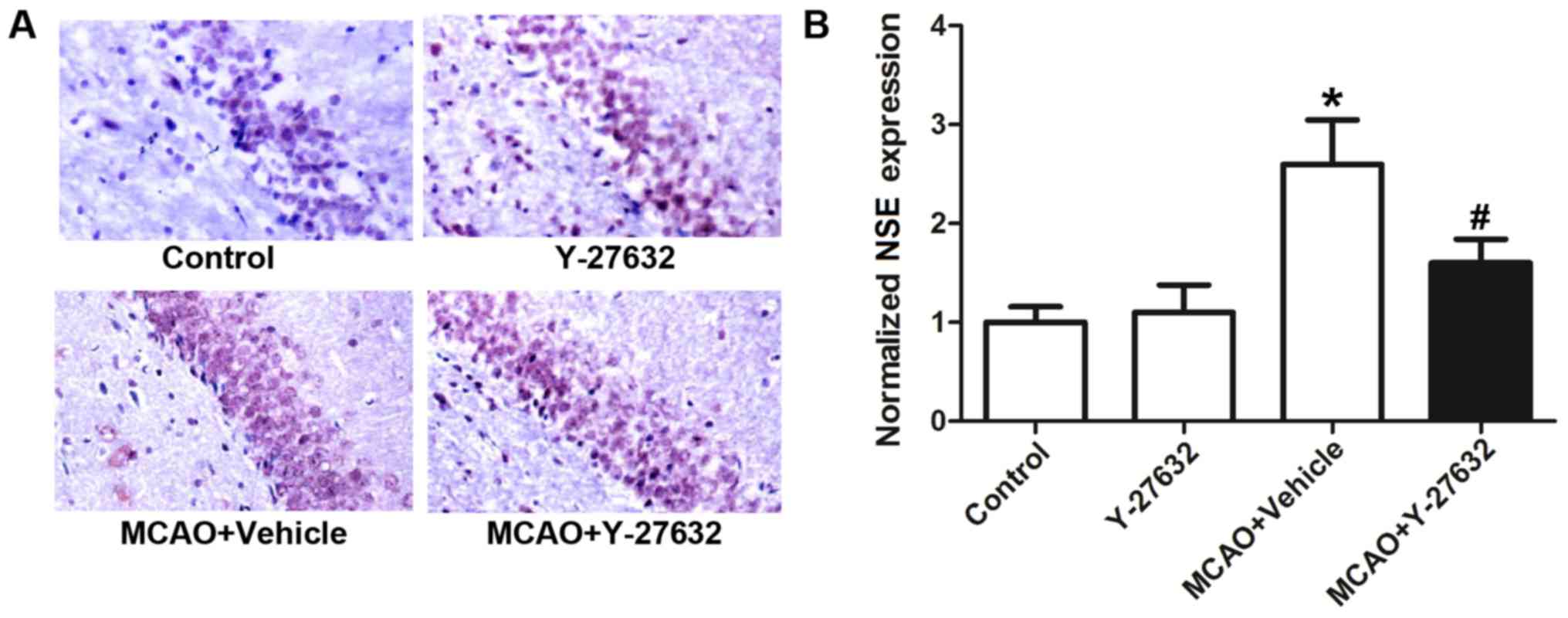
Y-27632 attenuates the expression of
NSE in hippocampus tissue
The expression of NSE, a marker of CIR-induced
damage, in the hippocampus of Control, Y-27632, MCAO + Vehicle and
MCAO + Y-27632 groups is shown in Fig.
6. Representative images of IHC staining for NSE are shown in
Fig. 6A, while Fig. 6B shows the quantification results
of NSE expression. The results indicated that the expression of NSE
was significantly increased following MCAO, but inhibited by the
treatment with Y-27632 (P<0.05 compared to MCAO + Vehicle).
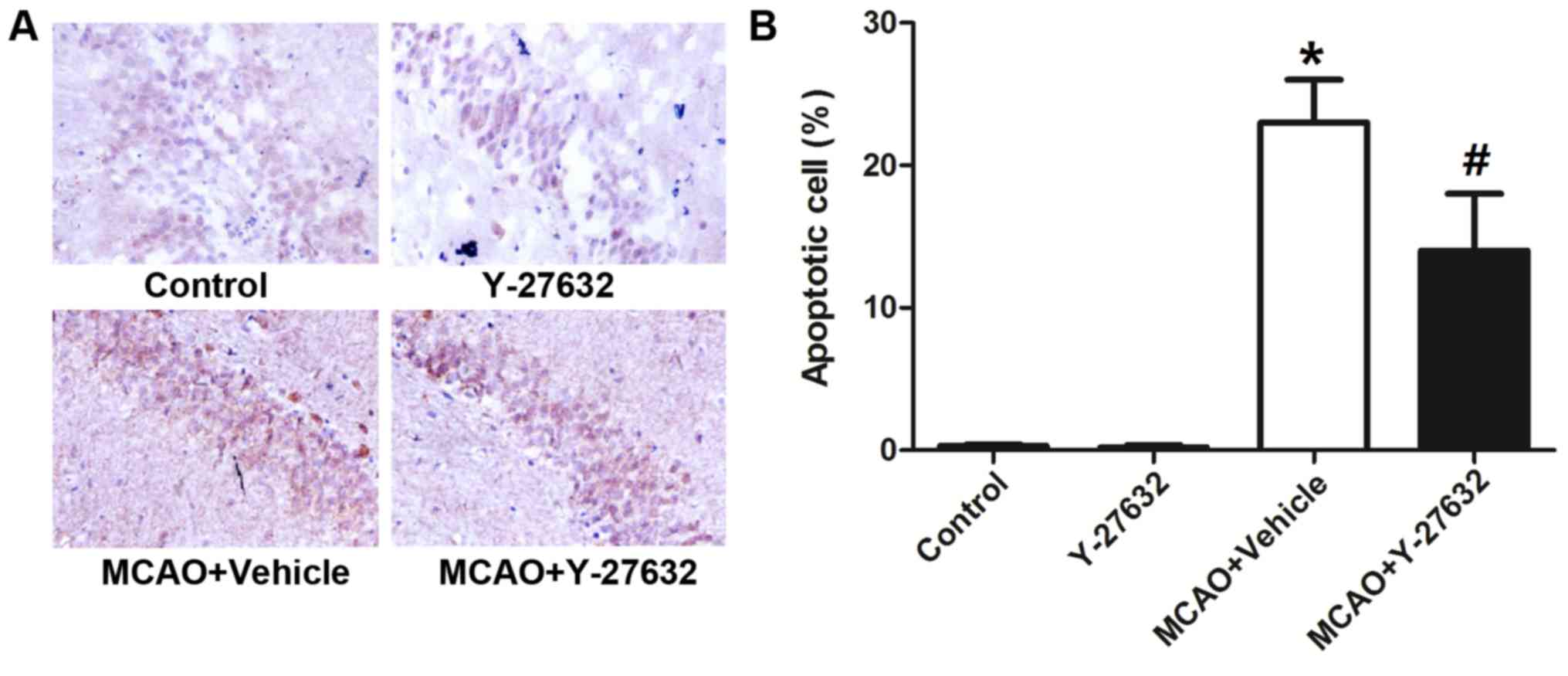
Y-27632 attenuates neuron apoptosis
caused by CIR
To analyze the effect of ROCK inhibition on neuron
apoptosis caused by CIR, the apoptosis rate in the hippocampus
tissue and the protein levels of Caspase-3, Caspase-8 and Caspase-9
and the Bax/Bcl-2 ratio in the infarcted brain tissues were
evaluated (Fig. 7). Representative
images of the TUNEL assay are shown in Fig. 7A, while Fig. 7B indicates the quantification of
apoptotic numbers in the hippocampus. The rate of apoptosis was
greatly increased by MCAO, but reduced with Y-27632 treatment
(P<0.05 compared to MCAO + Vehicle). Y-27632 alone did not
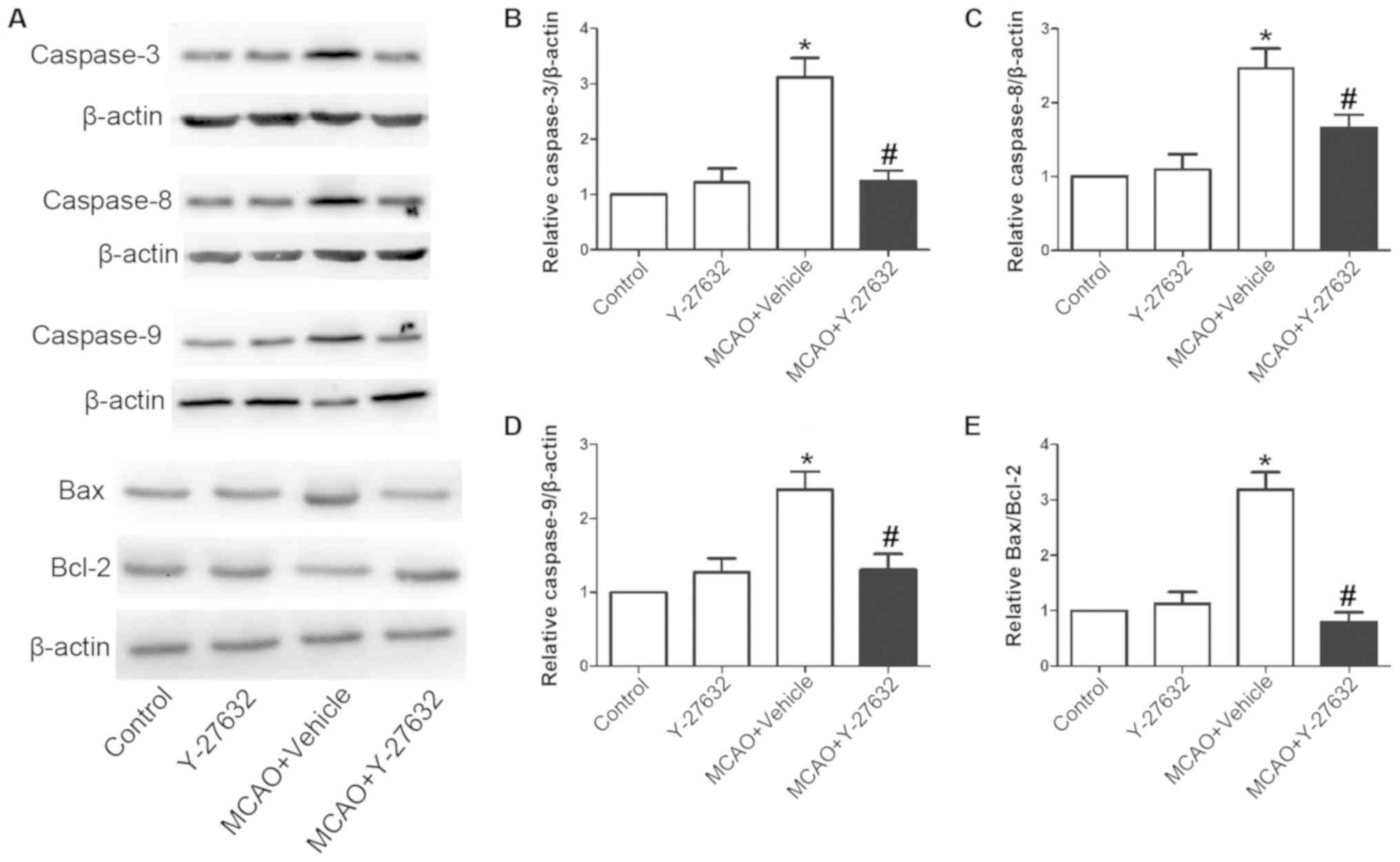
change the rate of apoptosis. As illustrated in Fig. 8, the protein levels of Caspase-3,
Caspase-8 and Caspase-9 were also increased following MCAO, but
reduced with Y-27632 treatment. Furthermore, Y-27632 significantly
decreased the Bax/Bcl-2 ratio in infarcted brain tissues. Y-27632
alone did not affect neuron apoptosis (P>0.05 compared to
Control).
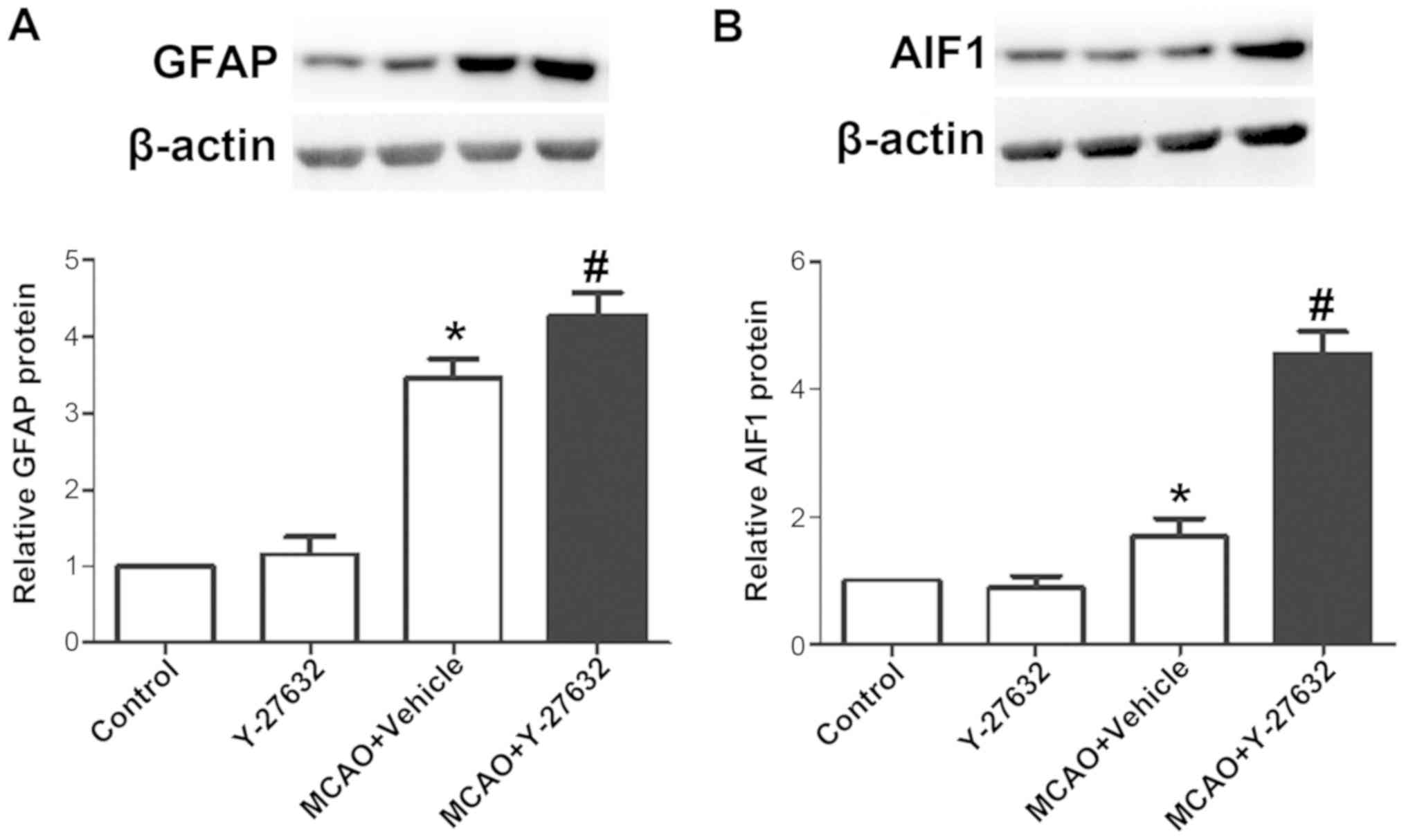
Y-27632 increases the levels of GFAP
and AIF1 following MCAO
To explore the effect of ROCK inhibition on
astrocytes and microglia function, the levels of GFAP and AIF1 were
evaluated in infarcted brain tissues by western blotting.
Representative images of western blotting for GFAP and the
quantification of its expression are shown in Fig. 9A, while Fig. 9B presents representative images of
AIF1 western blot analysisAIF1 and respective quantification of its
expression. The results demonstrated that the protein levels of
GFAP and AIF1 were both increased by MCAO, and the treatment of
Y-27632 further increased these protein levels to a higher level
following MCAO. Y-27632 alone did not affect GFAP and AIF1 protein
levels (P>0.05 compared to Control).
Discussion
The present study investigated the effects of the
ROCK inhibitor Y-27632 on CIR injury and explored its underlying
mechanism of action. It demonstrated that ROCK inhibition by
Y-27632 significantly improved the cognitive ability that was
impaired following CIR damage. Y-27632 also reduced the expression
of oxidative injury and inflammation markers in the brain and
attenuated the histological changes in the hippocampus caused by
the injury. The level of neuronal apoptosis in CIR-injured rats was
also significantly decreased by Y-27632 treatment. Lastly, the
protein levels of GFAP and AIF1 were further increased following
Y-27632 exposure and Y-27632 significantly increased the survival
rates at 24, 48 h and 7 days after CIR.
ROCK is an important kinase involved in cell
mitosis, adhesion, cytoskeleton regulation, muscle cell
contraction, tumor cell infiltration/migration and other cell
functions (16). ROCK participates
in the signaling pathway that regulates the presence of its
respective receptors in the cytoplasm and is also involved in the
regulation of the cytoskeletal structure (17). In recent years, ROCK has been
increasingly studied in context of IR injury (18–20).
The pretreatment of heart tissue with fusadil may prevent the
endothelial dysfunction and significantly reduce the degree of
myocardial infarction (21). In
the early stages of reperfusion, upregulation of Rho expression in
the ischemic myocardium activates ROCK (22). To evaluate the potential beneficial
effect of ROCK inhibition on CIR, rats with MCAO were treated with
the ROCK inhibitor Y-27632 and their survival rates and
neurological performance were examined. The survival rate of
animals treated with MCAO and Vehicle at 24, 48 h and 7 days was
60, 50 and 35%, respectively. In contrast, MCAO animals treated
with Y-27632 group exhibited significantly higher survival rates
(90, 80 and 50% at 24 and 48 h and 7 days respectively).
Furthermore, Y-27632 significantly decreased the neurological
deficit score and enhanced the performance of rats in the water
maze tests compared to MCAO + Vehicle animals. These results
indicated that ROCK inhibition may effectively prevent death and
the neurological impairment caused by CIR.
Different factors, including ischemia, hypoxia,
hyperglycemia and infection, may activate tyrosine kinases and G
protein-coupled receptors, which in turn activate RhoA. Activation
of the RhoA/ROCK signaling pathway may have detrimental effects
during IR. Firstly, activation of the RhoA/ROCK signaling pathway
in the vascular endothelium is known to inhibit P13K/AKT
activation, decrease nitric oxide synthase 3 phosphorylation and
nitric oxide production, which may reduce regional cerebral blood
flow following CIR (23).
Secondly, activation of RhoA/ROCK signaling pathway may increase
blood flow viscosity. The exact mechanism remains unclear, but is
hypothesized to be associated with erythrocyte deformability
(24). Thirdly, activation of the
RhoA/ROCK signaling pathway promotes the expression of serpin
family E member 2 in vascular endothelial cells and worsens
intracapillary circulation (25).
Phosphorylation of myosin light-chain in vascular endothelial cells
can lead to a decrease in tight junctions between cells and
increased endothelial cell permeability (26). Lastly, activation of the RhoA/ROCK
signaling pathway may increase the infiltration of inflammatory
(neutrophils) into ischemic tissue, aggravating the inflammatory
response during IR, and reducing the integrity of the blood-brain
barrier (27). The mechanism
underlying fusadil's protection against cerebral infarction
includes increased local cerebral blood flow by vasodilation and
reduced inflammatory response (28). To evaluate the effect of ROCK
inhibition on inflammatory response induced following CIR injury,
rats with MCAO were treated with Y-27632 and the levels of
pro-inflammatory factors TNF-α, IL-1β and IL-6 in the infarcted
brain tissue were examined (29–30).
The levels of TNF-α and IL-1β in MCAO + Vehicle group were
significantly increased compared to Control, and treatment with
Y-27632 significantly inhibited their increase, suggesting the
involvement of ROCK signaling pathway in the inflammatory response
induced by CIR. MCAO also induced significant histological changes
in the hippocampus, and these were significantly attenuated
following treatment with the ROCK inhibitor. Overall, these results
indicated that Y-27632 may have directly attenuated the
inflammatory response and reduced hippocampal injury induced by
CIR.
Free oxygen radicals are an important product in IR
injuries. These may increase the activity of arginase in
endothelial cells through Protein kinase C-activated RhoA/ROCK
signaling pathway, reduce the production of nitric oxide, increase
superoxide production and disturb normal endothelial function
(31). Moreover, the RhoA/ROCK
signaling pathway has been shown to reduce the production of free
oxygen radicals following cerebral infarction (32). As the present study showed, the
levels of MDA and 3-NT in MCAO + Vehicle group were greatly
increased compared to Control, and treatment of Y-27632 prevented
this increase. This result indicated that the protective effect of
Y-27632 against CIR injury may also be associated with reduced
oxidative stress.
When the central nervous system is injured,
astrocytes will transform from a resting state to an activated
state (and are hence termed ‘reactive astrocytes’). Reactive
gliosis results in the hypertrophy of glial cells and in the
proliferation of microglia (33).
The response of astrocytes to injury is thought to promote the
protection of the nervous system and the isolation of the injury
area from the surrounding healthy tissue. After the injury,
activated astrocytes and microglia may increase the secretion of
neurotrophic factors (including nerve growth factor,
neurotrophin-3, brain-derived neurotrophic factor), which can
maintain the survival of neurons and accelerate the growth of
neurites (34). The release of
these factors also allows activated glial cells to provide neurons
with ‘scaffolds’ that help restore disrupted neural connections. In
the infarcted area, the expression level of a variety of growth
factors secreted by astrocytes increases significantly, which
provides direct nutritional support for neurons and
oligodendrocytes and limits secondary damage (35). Hypoxia, inflammatory response and
trauma can promote activated astrocytes to secrete vascular
endothelial growth factor (VEGF). VEGF may also protect neurons and
promote angiogenesis and formation of new blood vessels, which is
beneficial for the repair of damaged areas (36). By eliminating glial cells or by
selectively blocking a signal pathway, a study has demonstrated
that the key to barrier remodeling is to activate proliferation and
migration of astrocytes (37).
Therefore, to explore the potential role of astrocytes and
microglia in the Y-27632-mediate protection against CIR injury, the
protein levels of GFAP (a marker of astrocyte) and AIF1 (a marker
of microglia) in the infarcted area were evaluated. The results
demonstrated that the protein levels of GFAP and AIF1 were greatly
increased by CIR, as expected considering the damage caused to the
neuronal tissue. However, the treatment of Y-27632 further
increased these protein levels. These results confirmed that
astrocytes and microglia would are more active in response to CIR.
It also revealed that Y-27632 may stimulate their response and
exert protective effects via activation of both astrocytes and
microglia. Previous studies have found that the inhibition of the
ROCK pathway attenuated methylmercury-induced astrocyte death
(38). It was also revealed that
ROCK inhibitors Y-27632 and fasudil promote microglial migration in
the spinal cord via the ERK signaling pathway (39). Consistent with these studies, the
present study confirmed that activation of astrocytes and microglia
may be a possible mechanism by which ROCK inhibition may protect
brain tissue against CIR injury.
However, the present study had some limitations.
Firstly, multiple mechanisms may be associated with Y-27632-induced
protection against CIR. The present study focused on the activation
of astrocytes and microglia, but this may not be the only
mechanism. Secondly, this is a primary animal study on the
protective effect of Y-27632 in CIR injury. Many factors should be
investigated when considering its clinical application against CIR,
including, but not limited to, its effects on human beings,
toxicity and dosage. Thirdly, further mechanism should be
investigated in future studies, such as how Y-27632 regulates the
activation of astrocytes and microglia or the interaction between
ROCK and astrocytes and microglia. Finally, the study would be more
perfect if the formation of the glial scar was shown by
immunohistochemical examination, but because of the limitation of
time and the technology, we are unable to perform it.
In conclusion, the present study confirmed that
Y-27632, a ROCK inhibitor, effectively increased the survival rate
and behavioral performance of rats with CIR damage. It also
decreased the oxidative stress, cerebral inflammation, neuron
apoptosis and hippocampal damage in animals with MCAO. The
activated proliferation of astrocytes and microglia may be
responsible for this beneficial effect.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL conceived and designed the study and revised the
manuscript; BL performed the experiments and performed the
statistical analysis. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Baodi District People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iadecola C and Anrather J: Stroke research
at a crossroad: Asking the brain for directions. Nat Neurosci.
14:1363–1368. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Breuer L, Knott M, Struffert T, Kloska S,
Kurka N, Schwab S, Dörfler A, Köhrmann M and Engelhorn T: Limited
versus whole-brain perfusion for the indication of thrombolysis in
the extended time window of acute cerebral ischemia. J Stroke
Cerebrovasc Dis. 24:2491–2496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong XY, Liu L and Yang QW: Refocusing
neuroprotection in cerebral reperfusion era: New challenges and
strategies. Front Neurol. 9:2492018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panieri E, Gogvadze V, Norberg E,
Venkatesh R, Orrenius S and Zhivotovsky B: Reactive oxygen species
generated in different compartments induce cell death, survival, or
senescence. Free Radic Biol Med. 57:176–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue J, Zhang X, Zhang C, Kang N, Liu X, Yu
J, Zhang N, Wang H, Zhang L, Chen R, et al: Protective effect of
Naoxintong against cerebral ischemia reperfusion injury in mice. J
Ethnopharmacol. 182:181–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Julian L and Olson MF: Rho-associated
coiled-coil containing kinases (ROCK): Structure, regulation, and
functions. Small GTPases. 5:e298462014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmandke A, Schmandke A and Strittmatter
SM: ROCK and Rho: Biochemistry and neuronal functions of
Rho-associated protein kinases. Neuroscientist. 13:454–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong H and Yang CY: Morphological and
hydrodynamic correlations with increasing outflow facility by
rho-kinase inhibitor Y-27632. J Ocul Pharmacol Ther. 30:143–153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oda T, Kume T, Izumi Y, Ishihara K,
Sugmimoto H and Akaike A: Na+/Ca2+ exchanger
inhibitors inhibit neurite outgrowth in PC12 cells. J Pharmacol
Sci. 116:128–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Li H, Yao Y, Ren Y, Lin J, Hu J,
Zheng M, Song X, Zhao T, Chen YY, et al: β-elemene enhances GAP-43
expression and neurite outgrowth by inhibiting RhoA kinase
activation in rats with spinal cord injury. Neuroscience.
383:12–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pang Y, Chai CR, Gao K, Jia XH, Kong JG,
Chen XQ, Vatcher G, Chen JG and Yu AC: Ischemia preconditioning
protects astrocytes from ischemic injury through 14-3-3γ. J
Neurosci Res. 93:1507–1518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao JJ, Hu JX, Lu DX, Ji CX, Qi Y, Liu
XY, Sun FY, Huang F, Xu P and Chen XH: Soluble cpg15 from
astrocytes ameliorates neurite outgrowth recovery of hippocampal
neurons after mouse cerebral ischemia. J Neurosci. 37:1628–1647.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang C, Zhang X, Fan H and Liu Y: Curcumin
upregulates transcription factor Nrf2, HO-1 expression and protects
rat brains against focal ischemia. Brain Res. 28:133–141. 2009.
View Article : Google Scholar
|
|
14
|
Jia D, Deng Y, Gao J, Liu X, Chu J and Shu
Y: Neuroprotective effect of Panax notoginseng plysaccharides
against focal cerebral ischemia reperfusion injury in rats. Int J
Biol Macromol. 63:177–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmadi M, Rajaei Z, Hadjzadeh MA, Nemati H
and Hosseini M: Crocin improves spatial learning and memory
deficits in the Morris water maze via attenuating cortical
oxidative damage in diabetic rats. Neurosci Lett. 642:1–6. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mueller BK, Mack H and Teusch N: Rho
kinase, a promising drug target for neurological disorders. Nat Rev
Drug Discov. 4:387–398. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka T, Nishimura D, Wu RC, Amano M, Iso
T, Kedes L, Nishida H, Kaibuchi K and Hamamori Y: Nuclear Rho
kinase, ROCK2, targets p300 acetyltransferase. J Biol Chem.
281:15320–15329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen JY, Gao SS, Chen FL, Chen S, Wang M
and Chen ZW: Role of CSE-produced H2S on cerebrovascular relaxation
via RhoA-ROCK inhibition and cerebral ischemia-reperfusion injury
in Mice. ACS Chem Neurosci. 10:1565–1574. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun XQ, Chen S, Wang LF and Chen ZW: Total
flavones of Rhododendron simsii Planch flower protect isolated rat
heart from ischaemia-reperfusion injury and its mechanism of
UTR-RhoA-ROCK pathway inhibition. J Pharm Pharmacol. 70:1713–1722.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen F, Liu Z, Peng W, Gao Z, Ouyang H,
Yan T, Ding S, Cai Z, Zhao B, Mao L and Cao Z: Activation of EphA4
induced by EphrinA1 exacerbates disruption of the blood-brain
barrier following cerebral ischemia-reperfusion via the Rho/ROCK
signaling pathway. Exp Ther Med. 16:2651–2658. 2018.PubMed/NCBI
|
|
21
|
Yada T, Shimokawa H, Hiramatsu O, Kajita
T, Shigeto F, Tanaka E, Shinozaki Y, Mori H, Kiyooka T, Katsura M,
et al: Beneficial effect of hydroxyfasudil, a specific Rho-kinase
inhibitor, on ischemia/reperfusion injury in canine coronary
microcirculation in vivo. J Am Coll Cardiol. 45:599–607. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamid SA, Bower HS and Baxter GF: Rho
kinase activation plays a major role as a mediator of irreversible
injury in reperfused myocardium. Am J Physiol Heart Circ Physiol.
292:H2598–H2606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin HK, Salomone S, Potts EM, Lee SW,
Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA and
Ayata C: Rho-kinase inhibition acutely augments blood flow in focal
cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow
Metab. 27:998–1009. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tiftik RN, Baskurt OK, Kul S and
Buyukafsar K: The functional significance of the rho/rho-kinase
pathway in human erythrocytes. Turk J Haematol. 31:168–174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwasaki H, Okamoto R, Kato S, Konishi K,
Mizutani H, Yamada N, Isaka N, Nakano T and Ito M: High glucose
induces plasminogen activator inhibitor-1 expression through
Rho/Rho-kinase-mediated NF-kappaB activation in bovine aortic
endothelial cells. Atherosclerosis. 196:22–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen L, Black ED, Witkowski ED, Lencer WI,
Guerriero V, Schneeberger EE and Turner JR: Myosin light chain
phosphorylation regulates barrier function by remodeling tight
junction structure. J Cell Sci. 119:2095–2106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Satoh S, Utsunomiya T, Tsurui K, Kobayashi
T, Ikegaki I, Sasaki Y and Asano T: Pharmacological profile of
hydroxy fasudil as a selective rho kinase inhibitor on ischemic
brain damage. Life Sci. 69:1441–1453. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rikitake Y, Kim HH, Huang Z, Seto M, Yano
K, Asano T, Moskowitz MA and Liao JK: Inhibition of Rho kinase
(ROCK) leads to increased cerebral blood flow and stroke
protection. Stroke. 36:2251–2257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu Z, Cheng D, Yin J, Wu R, Zhang G, Zhao
Q, Wang N, Wang F and Liang M: Antithrombin III protects against
contrast-induced nephropathy. EBioMedicine. 17:101–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin J, Wang F, Kong Y, Wu R, Zhang G, Wang
N, Wang L, Lu Z and Liang M: Antithrombin III prevents progression
of chronic kidney disease following experimental
ischaemic-reperfusion injury. J Cell Mol Med. 21:3506–3514. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chandra S, Romero MJ, Shatanawi A,
Alkilany AM, Caldwell RB and Caldwell RW: Oxidative species
increase arginase activity in endothelial cells through the
RhoA/Rho kinase pathway. Br J Pharmacol. 165:506–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kahles T, Luedike P, Endres M, Galla HJ,
Steinmetz H, Busse R, Neumann-Haefelin T and Brandes RP: NADPH
oxidase plays a central role in blood-brain barrier damage in
experimental stroke. Stroke. 38:3000–3006. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao Z, Zhu Q, Zhang Y, Zhao Y, Cai L,
Shields CB and Cai J: Reciprocal modulation between microglia and
astrocyte in reactive gliosis following the CNS injury. Mol
Neurobiol. 48:690–701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cragnolini AB, Montenegro G, Friedman WJ
and Masco DH: Brain-region specific responses of astrocytes to an
in vitro injury and neurotrophins. Mol Cell Neurosci. 88:240–248.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Williams A, Piaton G and Lubetzki C:
Astrocytes-friends or foes in multiple sclerosis? Glia.
55:1300–1312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo D, Murdoch CE, Xu H, Shi H, Duan DD,
Ahmed A and Gu Y: Vascular endothelial growth factor signaling
requires glycine to promote angiogenesis. Sci Rep. 7:147492017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okada S, Nakamura M, Katoh H, Miyao T,
Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y
and Okano H: Conditional ablation of Stat3 or Socs3 discloses a
dual role for reactive astrocytes after spinal cord injury. Nat
Med. 12:829–834. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dos Santos AA, Lopez-Granero C, Farina M,
Rocha JBT, Bowman AB and Aschner M: Oxidative stress, caspase-3
activation and cleavage of ROCK-1 play an essential role in
MeHg-induced cell death in primary astroglial cells. Food Chem
Toxicol. 113:328–336. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu PC, Tang RH, Yu ZY, Xie MJ, Wang W and
Luo X: The Rho-associated kinase inhibitors Y27632 and fasudil
promote microglial migration in the spinal cord via the ERK
signaling pathway. Neural Regen Res. 13:677–683. 2018. View Article : Google Scholar : PubMed/NCBI
|