Introduction
Renal cell carcinoma (RCC), the most common type of
kidney cancer, originates from the proximal tubule (1), and an increasing incidence of ~21.7
per 100,000 people has been observed over last decade as well as a
5-year survival rate of 79.9% in South Korea (2). The most common initial treatment of
RCC involves removing the affected kidney, and then multiple
therapies are used, including medications when metastasis occurs.
However, RCC is known to be resistant to chemotherapy and
radiotherapy in most cases (3).
There has been substantial interest regarding the
use of microbiota in cancer research. Although in vitro and
animal model studies suggest a protective anticancer effect of
probiotics, the results of human epidemiological studies are still
controversial (4,5). In chronic kidney disease or end-stage
renal disease patients, a correlation has been observed between the
chronic alteration of intestinal microbiota homeostasis, dysbiosis,
and chronic kidney disease (6,7).
Although the relationship among gut microbiota-derived metabolites,
signaling pathways, and kidney diseases remains to be elucidated,
gut microbiota-derived short-chain fatty acids have been revealed
to be involved in kidney diseases through the activation of the
gut-kidney axis. The main beneficial effects of short-chain fatty
acids on kidney function involved decreasing inflammation and
enhancing antioxidant activity (8). Furthermore, dysbiosis could promote
many diseases, including colonic and extracolonic cancers (4). Microorganism fermentation extract has
exhibited a growth inhibitory effect on cancer cells (9).
With the increasing interest in the use of gut
microbiota in the extra-intestinal field, the effects of microbiota
on preventive or therapeutic modality in kidney cancer were
investigated. In our preliminary study, an unknown microbiome-X and
the reinforced clostridial media (RCM) for microbiota culture as a
positive control were used to treat RCC and human kidney proximal
tubular cells (HK-2). RCM unexpectedly exhibited anticancer effects
compared with the microbiome-X, thus the potential growth
inhibitory activity of RCM was studied on primary (Caki-2) and
metastatic (Caki-1) RCC cell lines.
Materials and methods
Cell culture
The HK-2 human kidney proximal tubular cell lines
[American Type Culture Collection (ATCC)] and Caki-1 and Caki-2
clear cell RCC cell lines (Korean Cell Line Bank) were respectively
cultured in RPMI-1640 medium and McCoy's 5A, both supplemented with
10% fetal bovine serum (FBS; Welgene, Inc.) at 37°C with 5%
CO2, as previously described (10) as indicated by ATCC. For the
anchorage-dependent culture, each cell was seeded in a cell culture
dish (90×20 mm; SPL Life Sciences).
Reagents and antibodies
Difco™ reinforced clostridial medium (RCM) was
purchased from BD Biosciences and all ingredients assessed
including yeast extract were obtained from Sigma-Aldrich; Merck
KGaA (Table I).
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)
was purchased from Amresco, Inc. (VWR Inernational LLC).
 | Table I.List of the ingredients of Difco™ RCM
formula and used reagents in the experiments. |
Table I.
List of the ingredients of Difco™ RCM
formula and used reagents in the experiments.
| Difco™ RCM formula
(/liter) | Used ingredient (cat.
no., purchased from Sigma-Aldrich; Merck KGaA) |
|---|
| Agar | 0.5 g | A1296 |
| Beef extract | 10.0 g | – |
| Cysteine HCl | 0.5 g | C1276 |
| Dextrose | 5.0 g | D9434 |
| Peptone | 10.0 g | – |
| Sodium acetate | 3.0 g | S2889 |
| Sodium
chloride | 5.0 g | – |
| Soluble starch | 1.0 g | S9766 |
| Yeast extract | 3.0 g | Y1625 |
The antibodies used were specific for caspase-3
(diluted 1:2,000; product no. 9662) and cleaved caspase-3 (diluted
1:500; product no. 9661; both from Cell Signaling Technology,
Inc.), c-Myc (diluted 1:1,000; cat. no. sc-764) and catalase
(diluted 1:5,000; cat. no. sc-271803; both from Santa Cruz
Biotecnology, Inc.), cyclin B1 (diluted 1:1,000; product no. 4138)
and cyclin D1 (diluted 1:2,000; product no. 2978; both from Cell
Signaling Technology, Inc.), ferritin heavy chain (FTH1; diluted
1:2,000; cat. no. sc-376594) and GAPDH (diluted 1:5,000; cat. no.
sc-25778; both from Santa Cruz Biotecnology, Inc.), glutathione
peroxidase 4 (GPX4; diluted 1:1,000; ID product code ab41787;
Abcam), LC3-I/II (diluted 1:2,000; product no. 12741; Cell
Signaling Technology, Inc.), p21 (diluted 1:1,000; cat. no.
60214-1-Ig; Proteintech Group, Inc.), SLC7A11 (cysteine/glutamate
transporter (xCT); diluted 1:2,000; cat. no. ANT-111; Alomone
Labs), SOD-1 (Cu-ZnSOD; diluted 1:5,000; cat. no. sc-11407),
SOD-2(MnSOD; diluted 1:5,000; cat. no. sc-30080) and transferrin
receptor (CD71, TfRC; diluted 1:2,000; cat. no. sc-65882; all from
Santa Cruz Biotecnology, Inc.).
Cell counting and MTT assay for cell
viability
Cells (5×105/each cell line) were seeded
in appropriate media supplemented with 10% FBS, washed twice with
phosphate-buffered saline (Welgene, Inc.), and then fresh medium
was added. Next, various concentrations of RCM (0.5, 1.0, 5.0, and
10.0%) and yeast extract (1, 5, and 10% dissolved in distilled
water (DW) prior to the experiment) were added to the cells. The
number of viable cells was estimated at various time-points (up to
72 h of culture) using trypan blue staining, as previously
described (11), since an MTT
assay revealed interference when treated with high concentrations
of RCM and yeast extract.
The effect of the ingredients of RCM on cell
viability was evaluated using MTT reduction into its formazan
product as instructed by the manufacturer. Cells
(2×103/each cell line) were seeded in triplicate wells
in 96-well plates and treated with each ingredient and RCM itself.
Next, the cells were incubated for 72 h and then MTT reagent (5
mg/ml in PBS) was added into each well for 2 h, dissolved in DMSO
for 15 min, and the MTT reduction was assessed
spectrophotometrically at 595 and 620 nm as background using a
VERSAmax microplate reader (Molecular Devices Korea LLC). The
absorbance values obtained from the wells of the vehicle
(DW)-treated cells represent 100% cell viability and were used for
comparisons with the treated cells.
Flow cytometry
Cells were treated with or without 5.0% yeast
extract for 72 h. For cell death analysis, suspended cells were
incubated with 5 µl Annexin V-FITC and 5 µl propidium iodide for 15
min at room temperature in the dark using the EzWay Annexin V-FITC
Apoptosis Detection Kit (KOMA Biotech) according to the
manufacturer's protocol. Biding buffer was added to each mixture,
and the samples were analyzed through flow cytometry within 1 h
using the FACSCalibur™ system (BD Biosciences).
For cell cycle analysis, the cells were fixed in 70%
ethanol for 1 h at 4°C, washed with PBS, and treated with 100 µg/ml
RNase A (Sigma-Aldrich; Merck KGaA) for 1 h at 37°C. Next, the
cells were stained with 25 µg/ml propidium iodide (Sigma-Aldrich;
Merck KGaA) for 15 min at 37°C. Flow cytometry was then performed
using the FACSCalibur™ system (BD Biosciences) and analyzed by BD
FACStation software version 6.0 (BD Biosciences), as previously
described (11).
Western blotting
In order to obtain intracellular proteins, cultured
cells were harvested in M-PER mammalian protein extraction reagent
(Thermo Fisher Scientific, Inc.) including 1% protease inhibitor
cocktail set III (EMD Millipore), 0.5% phosphatase inhibitor
cocktail 2 and 0.5% phosphatase inhibitor cocktail 3 (both from
Sigma-Aldrich; Merck KGaA). Protein concentration was assessed
using BCA protein assay (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions.
The electrophoresis of protein in cell lysates on
any TGX Stain-Free FastCast™ Acrylamide Starter Kit (Bio-Rad
Laboratories, Inc.) using tris/glycine buffer systems (product nos.
161-0772 and 161-0771; Bio-Rad Laboratories, Inc.) onto PVDF
membranes was performed as previously described (10).
The membranes were first blocked with 5% skim milk
for 1 h and then incubated with primary antibodies overnight at
4°C. After washing, peroxidase anti-mouse or anti-rabbit IgG
antibodies (cat. no. WB-2000 or WB-1000; Vector Laboratories, Inc.)
were applied for 1 h at room temperature. Next, western lighting
chemiluminescence reagent (product no. NEL101; PerkinElmer, Inc.)
was used to detect proteins. The anti-GAPDH antibody was used as a
loading control on the stripped membranes. The bands were
quantified using AzureSpot analysis software (version 14.2; Azure™
c300; Azure Biosystems, Inc.).
Statistical analysis
All data were compiled from a minimum of three
replicate experiments. Data are expressed as the mean ± standard
deviation. The results were compared between treated and control
cells using Student's t-test (SPSS version 14.0) and compared among
groups or cell lines using ANOVA with a Bonferroni post-hoc test
(both from SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Yeast extract is a candidate for the
reinforced clostridium media (RCM)-related viability on RCC
cells
It was revealed that when compared to the microbiome
‘X’, RCM had antitumor effects on RCC cells. In our preliminary
study, the MTT assay was not a useful method due to interference,
and the results were compared with the cell counting results (data
not shown).
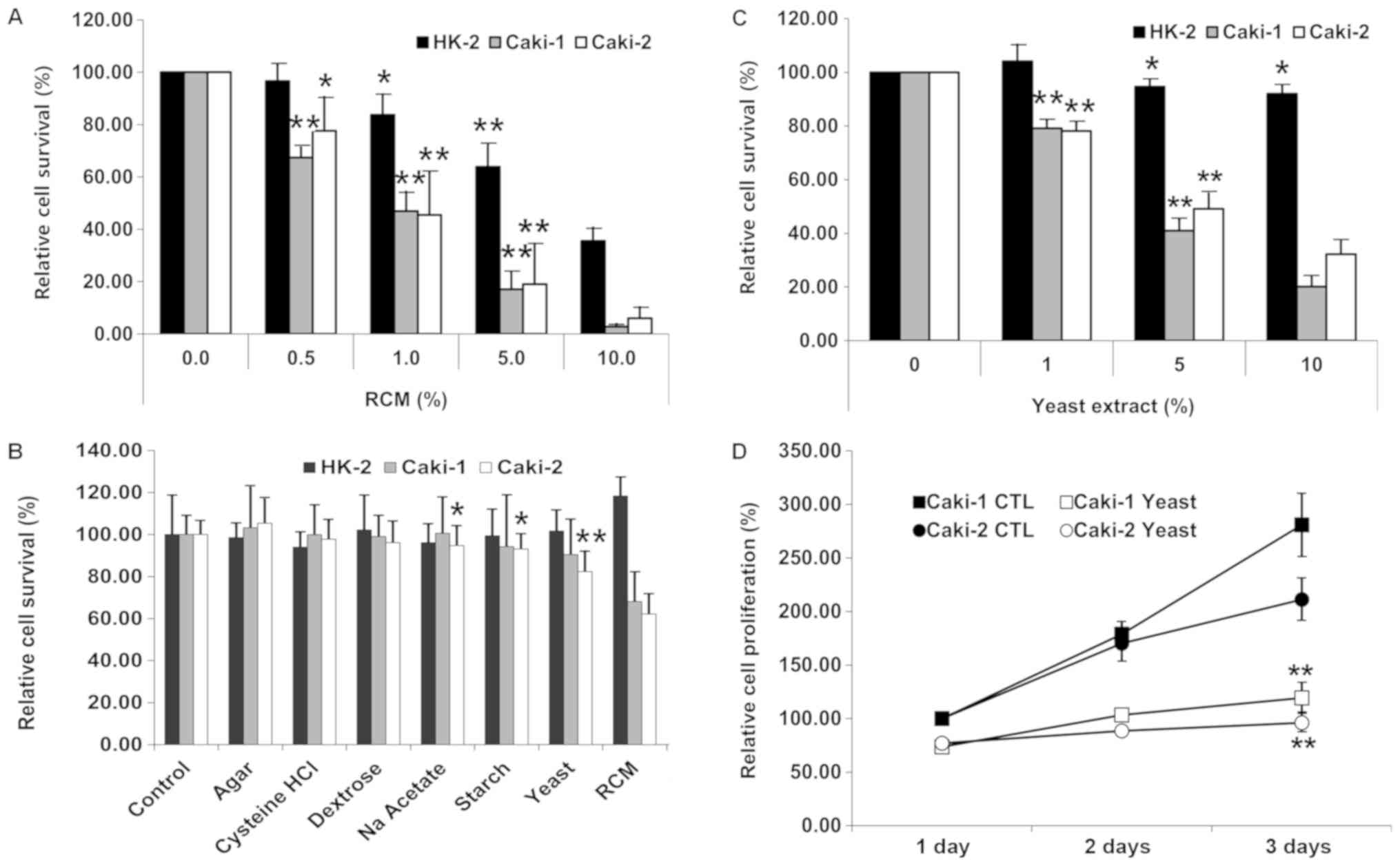
RCM inhibited the growth of all cells examined in a
dose-dependent manner under cell counting. Following treatment with
various concentrations of RCM (0.5, 1.0, 5.0, and 10.0%) for 72 h,
the HK-2 cell viability was reduced to 96.65, 83.73 (P=0.018),
63.83 (P=0.001), and 35.58% of that of the vehicle-treated
condition, respectively. The viability of Caki-1 and Caki-2 were
respectively reduced to 67.43 (P<0.01), 46.92, 17.06, and 2.76%
and 77.63 (P=0.031), 45.48 (P=0.008), 19.03, and 5.94% of those of
the vehicle-treated condition, respectively (Fig. 1A).
In a subsequent experiment, a dose of 1.0% RCM was
used. All ingredients except for peptone and beef extract were
assessed for the antitumor effects of RCM using MTT assay. Compared
to the counting results, HK-2, Caki-1, and Caki-2 cells exhibited
relatively high ratios of viable cells at 1% RCM treatment, with
values of 83.73 vs. 118.67, 46.92 vs. 68.12, and 45.48 vs. 62.17%,
respectively (Fig. 1B). Sodium
acetate (P=0.048), starch (P=0.028), and yeast extract (82.40% vs.
the vehicle, P=0.001) revealed considerable antitumor effects on
Caki-2, but all of the ingredients had only slight effects on
Caki-1, with the lowest viability with yeast extract (91.92% vs.
the vehicle, P=0.202).
Yeast extract exhibits dose-dependent
and time-dependent antitumor effects on RCC cells
Following 72 h of incubation, it was observed that
yeast extract exerted antitumor effects on the RCC cells in a
dose-dependent manner at 1.0, 5.0 and 10.0% concentrations by
volume compared to the normal kidney proximal tubular cells (HK2;
P=0.194) (Fig. 1C).
Yeast extract did not affect HK-2 cells, in which
the viability was counted as 92.04% even with 10% yeast extract
treatment (P=0.018). Yeast extract exhibited significant antitumor
effects in a dose-dependent manner): viabilities of 79.11–41.01%
and 78.16–49.12% at 1.0–5.0% yeast extract treatments on Caki-1 and
Caki-2 cells, respectively. Accordingly, the expected
IC50 was 5.0% of yeast extract on RCC cells. Therefore,
a dose of 5.0% yeast extract was used in all subsequent
experimentation.
With 5.0% yeast extract, time-dependent antitumor
effects were estimated in RCC cells (Fig. 1D). Compared to Caki-1, Caki-2
exhibited relatively slow proliferation. Caki-1 and Caki-2
exhibited decreased cell viability in a time-dependent manner:
after 72 h, the ratios of proliferation were 42.88% (2.81 times vs.
1.19 from the 24-h incubation) and 51.54% (2.11 times vs. 0.96 from
the 24-h incubation) of the control levels in Caki-1 and Caki-2,
respectively.
Yeast extract does not affect the cell
death of RCC cells
In order to investigate the mechanism responsible
for the antitumor effects of yeast extract, cell death and related
protein levels were evaluated.
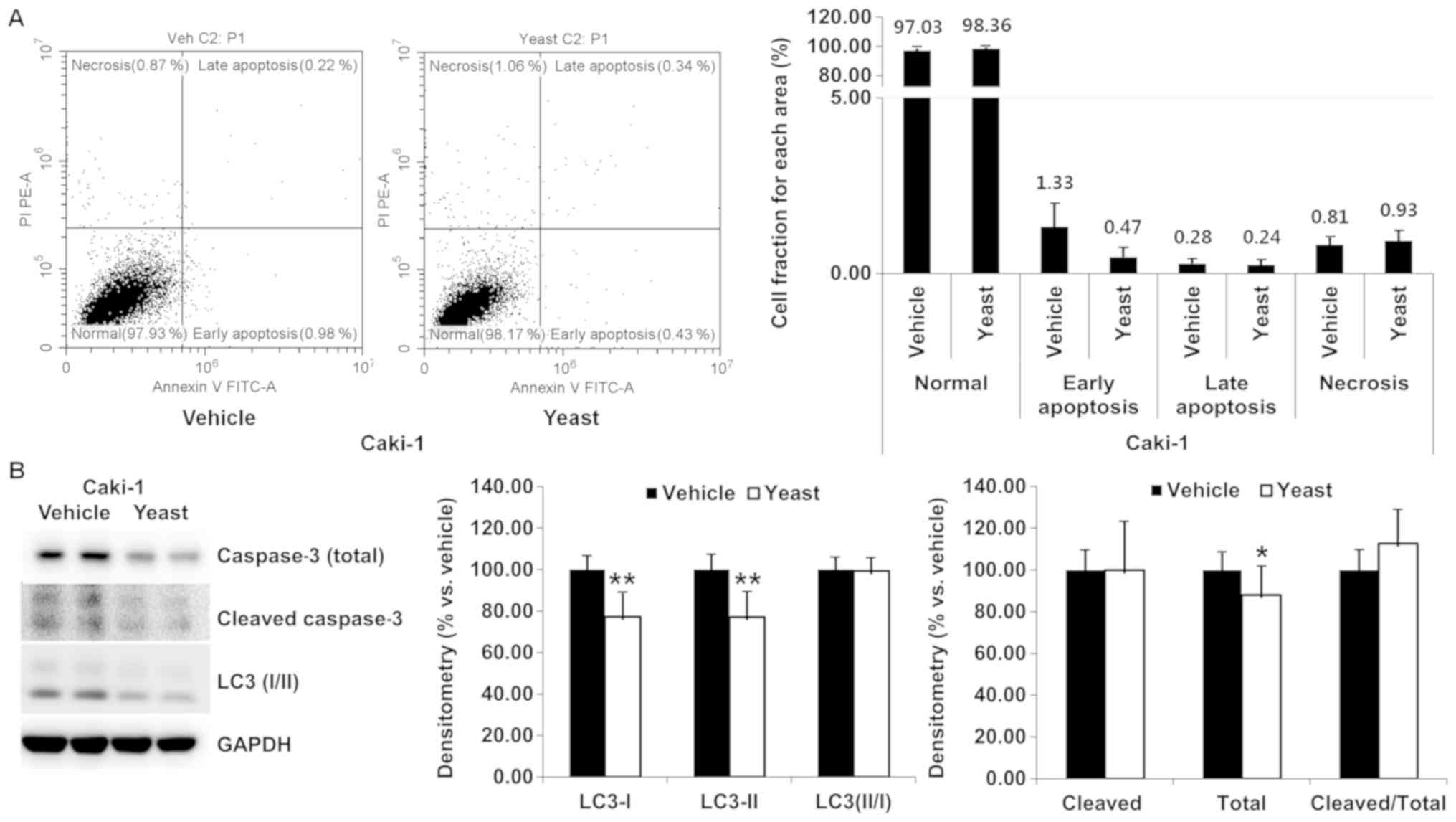
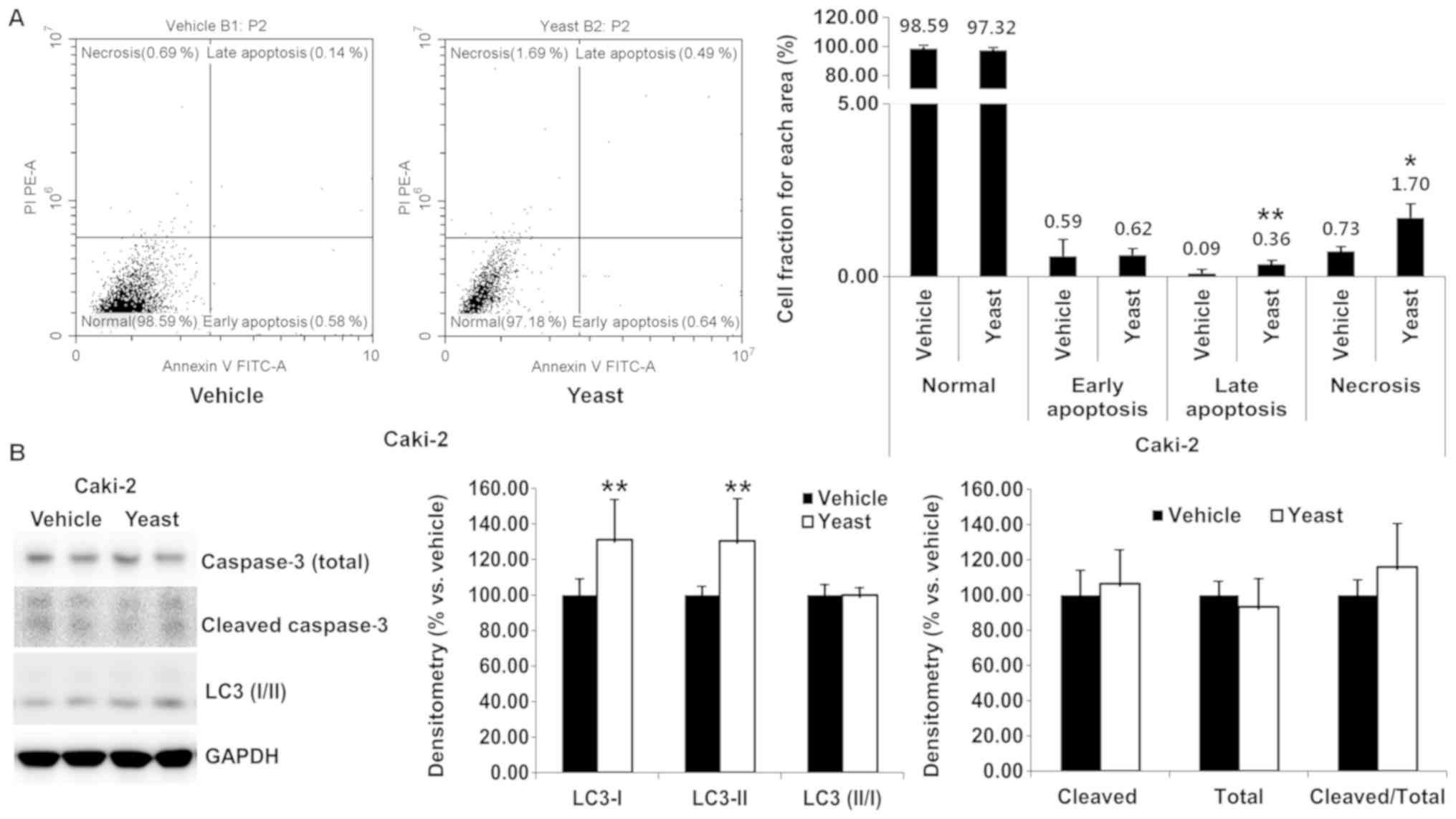
Yeast extract induced necrosis and apoptosis and
were identified through Annexin V/PI staining. Compared to the
control, yeast extract resulted in no marked increase in the rate
of necrosis or apoptosis in RCC cells (Figs. 2A and 3A). Although statistically significant
differences were observed for late apoptosis (0.09–0.36%, P=0.005)
and necrosis (0.73–1.70%, P=0.011), the percentage was extremely
small in the case of Caki-2 (Fig.
3A).
Furthermore, the expression of apoptosis-related
caspase-3 (total vs. cleaved form) and autophagy-related LC3 I/II
was detected (Figs. 2B and
3B). Total caspase-3 was
significantly decreased (P=0.047) in Caki-1, but in the other
proteins was unchanged following yeast extract treatment.
Accordingly, the expression of caspase-3 was not significantly
altered in Caki-1 (P=0.219) or Caki-2 (P=0.283). Both LC3 I and LC3
II were significantly decreased in Caki-1 (P<0.001 and P=0.001,
respectively) and increased in Caki-2 (P=0.005 and P=0.008),
however the expression level of LC3 II/I was sustained under yeast
extract treatment.
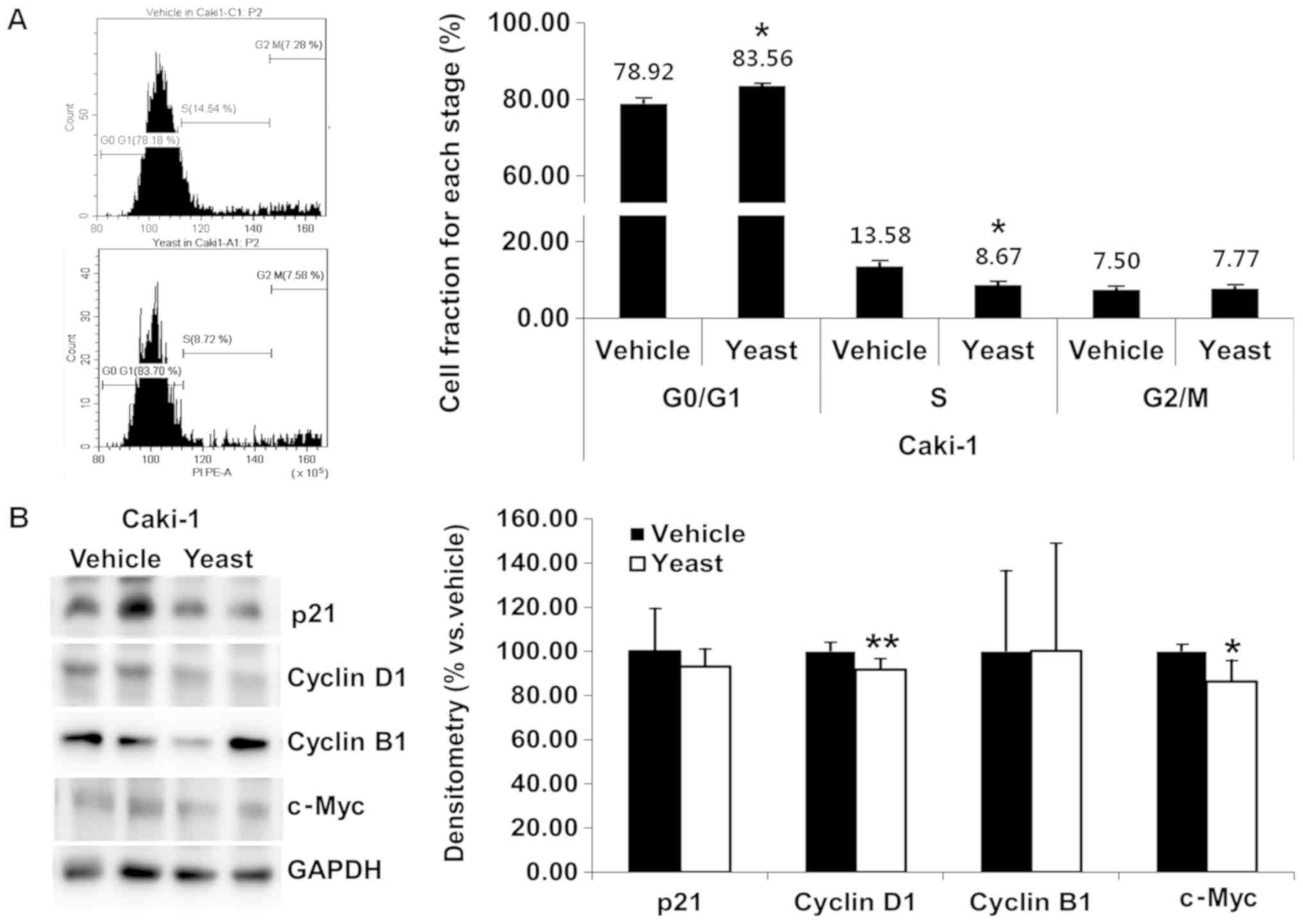
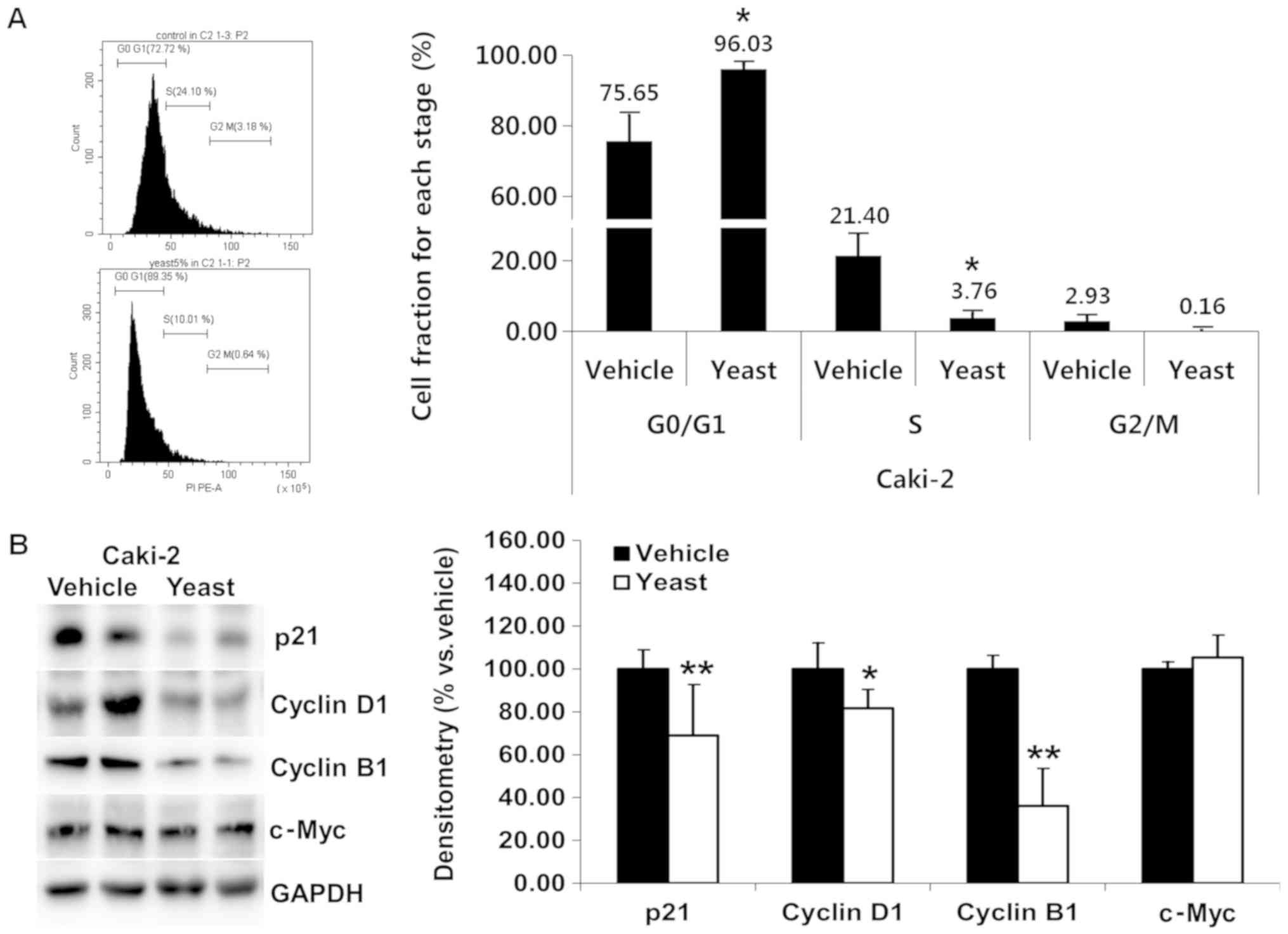
Yeast extract affects the cell cycle
of RCC cells
Yeast extract inhibited the proliferation of RCC
cells. The effects of yeast extract on the cell cycle were assessed
through PI staining. Both Caki-1 (Fig.
4A) and Caki-2 (Fig. 5A) cells
were incubated with 5% yeast extract, revealing a significant
increase in the G0/G1 phase (P=0.019 and
P=0.036, respectively) and a decrease in the S phase (P=0.015 and
P=0.033, respectively).
Compared to the untreated control cells, the
fraction of Caki-1 and Caki-2 cells in the
G0/G1 phase demonstrated a significant upward
trend (4.64% Caki-1, 20.38% increase Caki-2) following treatment
with 5% yeast extract. Specifically, the
G0/G1 fractions were 78.92 and 75.65% in
untreated cells and 83.56 and 96.03% in cells treated with yeast
extract in Caki-1 and Caki-2, respectively.
In order to delineate the mechanisms underlying the
cell cycle arrest induced by yeast extract, p21, cyclin D1, cyclin
B1, and c-Myc were assessed, which all promote cell cycle
progression. Under yeast extract treatment, the expression of
cyclin D1 (P=0.008) and c-Myc (P=0.015) was significantly decreased
in Caki-1 (Fig. 4B), while those
of p21 (P=0.007), cyclin D1 (P=0.017), and cyclin B1 (P<0.001)
were significantly decreased in Caki-2 (Fig. 5B).
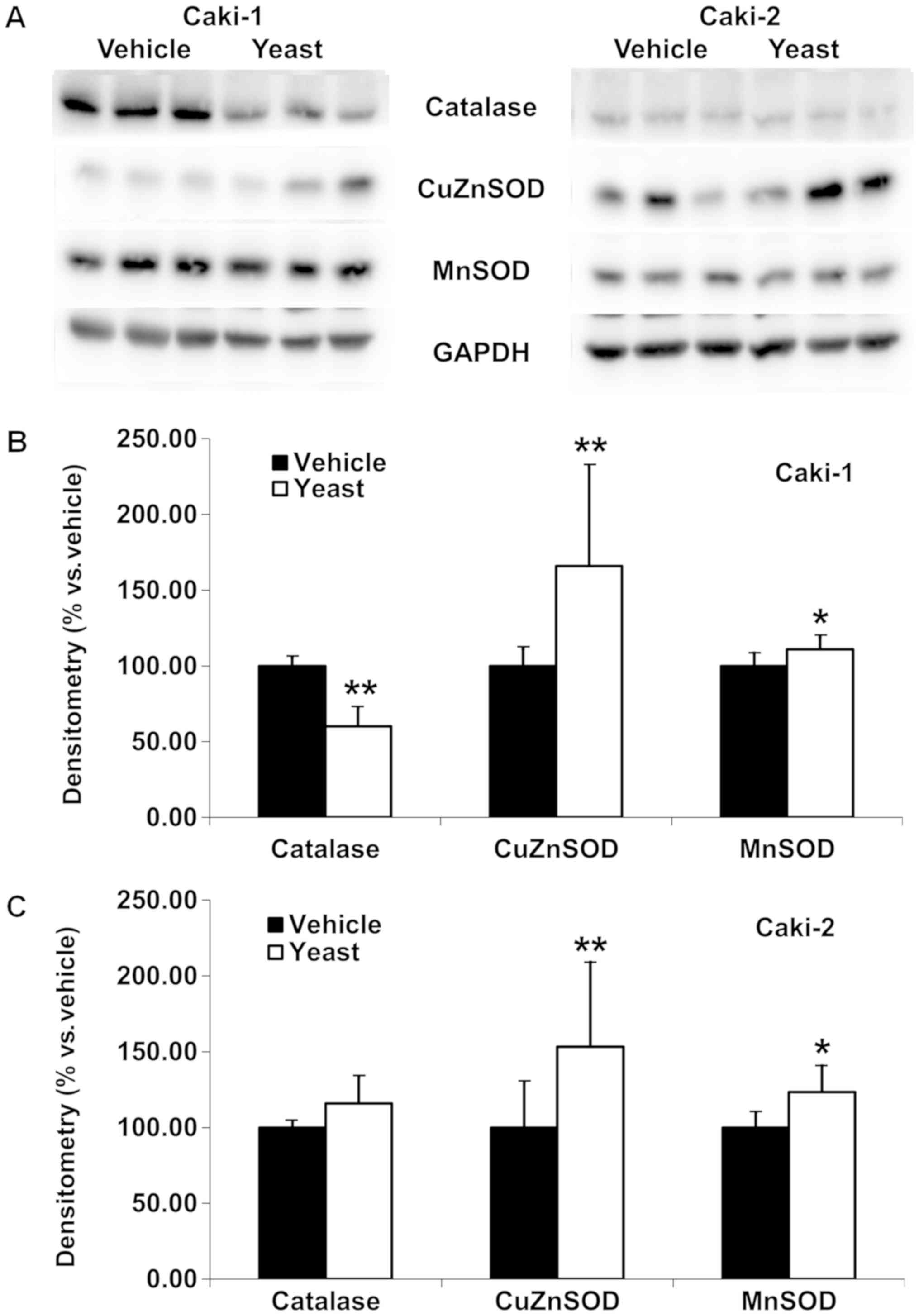
Yeast extract differentially affects
intracellular antioxidant activity in RCC cells
Following yeast extract treatment, potential changes
in the intracellular antioxidant activity were investigated in RCC
cells (Fig. 6). The levels of
catalase, CuZnSOD and MnSOD were revealed to be differentially
altered. While CuZnSOD (P=0.005 in Caki-1, and P=0.010 in Caki-2)
and MnSOD (P=0.048 in Caki-1, and P=0.021 in Caki-2) were
significantly increased in both RCC cells, catalase was
considerably decreased in Caki-1 (P<0.001, Fig. 6B) but was not altered in Caki-2
(Fig. 6C) cells. Specifically,
densitometry revealed 165.84±66.14 and 153.31±55.81% of CuZnSOD,
110.95±8.40 and 123.38±17.31% of MnSOD, and 60.12±12.61 and
115.82±17.61% of catalase in Caki-1 and Caki-2 cells,
respectively.
Yeast extract did affect cell
proliferation via regulating the iron metabolism
Following yeast extract treatment, potential changes
in antioxidant activity, GPX4, and related proteins which were
related to iron metabolism were investigated in RCC cells.
The levels of GPX4, transferrin receptor (TfRC),
ferritin heavy chain (FTH1), and cysteine/glutamate transporter
(xCT) were revealed to be differentially altered (Fig. 7). While a significant increase of
FTH1 (P<0.001 in Caki-1, and P=0.005 in Caki-2) was observed no
change in xCT was observed in both RCC cell lines. In addition, a
significant decrease of TfRC (P=0.001) was revealed in Caki-1
(Fig. 7B) and a significant
decrease of GPX4 was revealed in Caki-2 cells (P=0.007; Fig. 7C). Specifically, densitometry
revealed 208.26±32.78 and 179.43±55.78% of FTH1 in Caki-1 and
Caki-2 cells, respectively, along with 71.45±14.76% of TfRC in
Caki-1 and 76.40±25.26% of GPX4 in Caki-2 cells.
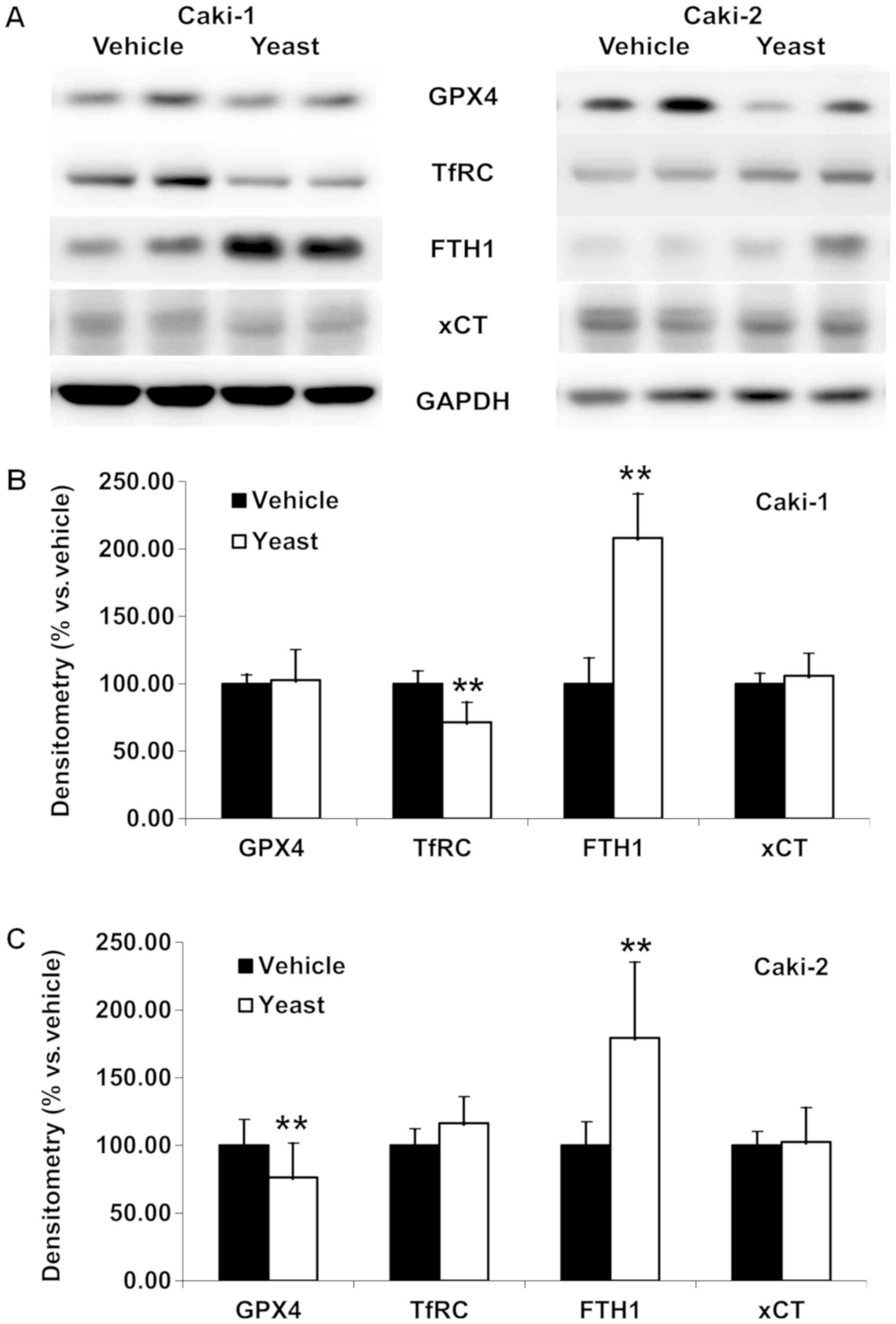 | Figure 7.Yeast extract does affect iron
metabolism and/or iron-dependent cell death. (A) Western blot
analysis revealed GPX4, TfRC, FTH1 and xCT following yeast extract
treatment. (B) Significance was observed with increased FTH1 and
decreased TfRC, while GPX4 and xCT were not altered in metastatic
RCC cells (Caki-1), which is responsible for the growth inhibition
activity via low free iron in the cell. (C) Significance was
observed with increased FTH1 and decreased GPX4, while the TfRC and
xCT were not altered in primary RCC cells (Caki-2), which is
responsible for the iron-dependent cell death, ferroptosis.
Densitometry is presented vs. the vehicle-treated cells following
treatment with yeast extract. Data are expressed as the mean ±
standard deviation. **P<0.01. TfRC, transferrin receptor; FTH1,
ferritin heavy chain; xCT, cysteine/glutamate transporter; RCC,
renal cell carcinoma. |
Discussion
Based on the antitumor effects of RCM, six
ingredients including yeast extract were assessed for their
antitumor effects, and yeast extract was revealed to be the best
candidate. According to the manufacturer (Sigma-Aldrich; Merck
KGaA), yeast extract (product no. Y1625) is a mixture of amino
acids, peptides, water soluble vitamins (including B-complex
vitamins), and carbohydrates, and it is suitable for use as a
nutritional source in microbial culture media. Yeast extract has
previously been revealed to inhibit mitosis of cancer cells while
having no inhibitory effects on non-neoplastic cells (12–14).
We first showed that yeast extract exhibited growth inhibition
activity on RCC cells when compared with a vehicle (DW)-treated
control, which had dose- and time-dependent anti-proliferative
effects, and relatively slight growth inhibition activity in normal
human proximal tubular cells. Since no characteristic features of
necrosis, apoptosis, or autophagy were observed, the cancer cells
may have been arrested at a certain phase of the cell cycle instead
of moving to the sub-G0/G1 phase. Based on
cell cycle analysis, G0/G1 arrest under yeast
extract treatment was induced by the decreased cyclin D1 expression
in RCC cells. Caki-2 exhibited a slower proliferation curve
compared with Caki-1 cells (Fig.
1D), which would be related with the G1/S arrest. In addition,
different cell cycle regulators may also be partially involved in
the anti-proliferative effects of yeast extract including cyclin
B1, the G2/M phase regulator, particularly in Caki-2 cells. These
results were not surprising due to the nature of the yeast extract
of the mixture, but it was not considered to be a major mechanism
for cell cycle arrest since anticancer drugs have exhibited cell
cycle arrest with diminished cyclin D1 and/or cyclin B1 (15). The inhibition of tumor cell growth
can also be attributed to the increase in the steady state levels
of hydrogen peroxide caused by the increased activity of
antioxidant enzymes (16). Yeast
extract-treated cells exhibited higher levels of classic
antioxidant activities with catalase, MnSOD and CuZnSOD, which may
be responsible for the inhibition of cancer growth.
Although the active cell death was unchanged, LC3
was increased in Caki-2 and decreased in Caki-1 cells, indicating
that different cell death pathways may exist in RCC cells,
particularly in Caki-2 cells. Notably, GPX4, which belongs to the
family of glutathione peroxidases, was significantly decreased in
primary clear cell RCC cells (Caki-2). This is worth noting since
the inactivation of GPX4 leads to an accumulation of lipid
peroxides, resulting in ferroptosis, an iron-induced non-apoptotic
and non-necrotic oxidative form of programmed cell death (17–21).
Iron contributes to mutagenicity and malignant transformation, and
then malignant cells require high amounts of iron for
proliferation. For the high requirement of iron, TfRC and FTH1 were
increased in transformed malignant cells (22,23).
Recently, changes in iron profile have been suggested as a
successful marker for chemotherapy of metastatic renal cancer
(24) based on previous
accumulated studies involving serum iron (25), ferritin (26–28)
or TfRC (25,28). As ferroptosis is induced by the
inhibition of cysteine uptake or the inactivation of the lipid
repair enzyme GPX4 (29), xCT was
further examined, but no significant changes were revealed in RCC
cells. Yeast treatment led to decreased transferrin receptors and
increased ferritin in metastatic RCC cells (Caki-1), which may
partially contribute to the growth inhibition activity of yeast
extract via the low free iron level in the cancer cells. However, a
slight increase in transferrin receptors and a decreased GPX4 were
observed in primary RCC cells (Caki-2), suggesting that ferroptosis
may be involved in the antitumor effects of yeast extract in Caki-2
cells. This can be reinforced by the facts that targeted drugs on
RCC, including tyrosine kinase (sorafenib and pazopanib) or
mammalian target of rapamycin (mTOR) inhibitor (everolimus and
temsirolimus), inhibit growth factors that have been revealed to
promote the growth and spread of tumors (30). The tyrosine kinase, sorafenib, is
also known as an inhibitor for cysteine transporter (20), and the mTOR pathway is one of the
regulators of iron homeostasis via iron, ferritin, and TfRC
(24,28,31).
However, recent molecular data (32) insists that Caki-2 is a type of
papillary RCC rather than a clear cell RCC, which may account for
the different responses in this experiment. More RCC cell lines,
including clear cell or papillary cell types, should be included to
determine the possible mechanism of antitumor effects of yeast
extract in future experiments.
In conclusion, yeast extract, a mixture of
compounds, may have a variety of effects on cancer cells requiring
further investigation, however, herein its potential roles in the
growth inhibition activities on RCC cells were clearly revealed.
The anti-proliferative effects of yeast extract were iron-dependent
and resulted in G0/G1 arrest through decreased cyclin D1 and
increased cell death, possibly via ferroptosis in primary RCC
cells. The iron-dependent cell death pathway may be another
mechanism for the antitumor effects of yeast extract and should be
further researched, particularly in primary RCC.
Acknowledgements
Parts of these data were presented at the 8th Asia
Pacific International Congress of Anatomists, October 2018.
Funding
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
2018R1D1A1A02050497).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SPY conceived and designed the present study, and
wrote the manuscript. DM, JK and SPY performed the experiments for
data acquisition and analysis. DM and SPY interpreted the
experimental results. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Inamura K: Renal cell tumors:
Understanding their molecular pathological epidemiology and the
2016 WHO classification. Int J Mol Sci. 18(pii): E21952017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koo KC and Chung BH: Epidemiology and
treatment patterns of urologic cancers in Korea. Korean J Urol
Oncol. 13:51–57. 2015.
|
|
3
|
Rini BI, Rathmell WK and Godley P: Renal
cell carcinoma. Curr Opin Oncol. 20:300–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Compare D and Nardone G: Contribution of
gut microbiota to colonic and extracolonic cancer development. Dig
Dis. 29:554–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pope JL, Tomkovich S, Yang Y and Jobin C:
Microbiota as a mediator of cancer progression and therapy. Transl
Res. 179:139–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramezani A and Raj DS: The gut microbiome,
kidney disease, and targeted interventions. J Am Soc Nephrol.
25:657–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramezani A, Massy ZA, Meijers B, Evenepoel
P, Vanholder R and Raj DS: Role of the gut microbiome in uremia: A
Potential therapeutic target. Am J Kidney Dis. 67:483–498. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li L, Ma L and Fu P: Gut
microbiota-derived short-chain fatty acids and kidney diseases.
Drug Des Devel Ther. 11:3531–3542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chui CH, Cheng GY, Ke B, Lau FY, Wong RS,
Kok SH, Fatima S, Cheung F, Cheng CH, Chan AS and Tang JC: Growth
inhibitory potential of effective microorganism fermentation
extract (EM-X) on cancer cells. Int J Mol Med. 14:925–929.
2004.PubMed/NCBI
|
|
10
|
Yoon SP and Kim J: Exogenous CGRP
upregulates profibrogenic growth factors through PKC/JNK signaling
pathway in kidney proximal tubular cells. Cell Biol Toxicol.
34:251–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JB, Hwang SE and Yoon SP:
Dexamethasone reduces side population fraction through
downregulation of ABCG2 transporter in MCF-7 breast cancer cells.
Mol Med Rep. 16:453–458. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okada T and Yoneyama M: The interaction of
malignant cells with yeast. II. A new yeast extract inhibiting the
growth of malignant cell. Hiroshima J Med Sci. 19:99–117.
1970.PubMed/NCBI
|
|
13
|
Fardon JC, Poydock ME and Tsuchiya Y:
Response of neoplastic and non-neoplastic cells to a yeast extract.
J Surg Oncol. 10:10–21. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poydock ME, Fardon JC, Tsuchiya Y and Cook
ES: Selective effect of fractions of a yeast extract (PCO) on
normal and malignant cells. Exp Cell Biol. 46:231–239.
1978.PubMed/NCBI
|
|
15
|
Heffeter P, Jakupec MA, Körner W, Wild S,
von Keyserlingk NG, Elbling L, Zorbas H, Korynevska A, Knasmüller
S, Sutterlüty H, et al: Anticancer activity of the lanthanum
compound [tris(1,10-phenanthroline)lanthanum(III)]trithiocyanate
(KP772; FFC24). Biochem Pharmacol. 71:426–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KH, Rodriguez AM, Carrico PM and
Melendez JA: Potential mechanisms for the inhibition of tumor cell
growth by manganese superoxide dismutase. Antioxid Redox Signal.
3:361–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kazan HH, Urfali-Mamatoglu C and Gunduz U:
Iron metabolism and drug resistance in cancer. Biometals.
30:629–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu H, Guo P, Xie X, Wang Y and Chen G:
Ferroptosis, a new form of cell death, and its relationships with
tumourous diseases. J Cell Mol Med. 21:648–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin-Sanchez D, Poveda J,
Fontecha-Barriuso M, Ruiz-Andres O, Sanchez-Niño MD, Ruiz-Ortega M,
Ortiz A and Sanz AB: Targeting of regulated necrosis in kidney
disease. Nefrologia. 38:125–135. 2018.(In English, Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fanzani A and Poli M: Iron, oxidative
damage and ferroptosis in rhabdomyosarcoma. Int J Mol Sci. 18(pii):
E17182017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pfeifhofer-Obermair C, Tymoszuk P, Petzer
V, Weiss G and Nairz M: Iron in the tumor
microenvironment-connecting the dots. Front Oncol. 8:5492018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Golčić M and Petković M: Changes in
metabolic profile, iron and ferritin levels during the treatment of
metastatic renal cancer-A new potential biomarker? Med Hypotheses.
94:148–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu CC, Chen KK, Chen MT, Huang JK, Lin AT,
Lee YH and Chang LS: Serum iron as a tumor marker in renal cell
carcinoma. Eur Urol. 19:54–58. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Partin AW, Criley SR, Steiner MS, Hsieh K,
Simons JW, Lumadue J, Carter HB and Marshall FF: Serum ferritin as
a clinical marker for renal cell carcinoma: Influence of tumor
volume. Urology. 45:211–217. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kirkali Z, Esen AA, Kirkali G and Güner G:
Ferritin: A tumor marker expressed by renal cell carcinoma. Eur
Urol. 28:131–134. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Torti SV and Torti FM: Ironing out cancer.
Cancer Res. 71:1511–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Latunde-Dada GO: Ferroptosis: Role of
lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta
Gen Subj. 1861:1893–1900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Santoni M, De Tursi M, Felici A, Lo Re G,
Ricotta R, Ruggeri EM, Sabbatini R, Santini D, Vaccaro V and
Milella M: Management of metastatic renal cell carcinoma patients
with poor-risk features: Current status and future perspectives.
Expert Rev Anticancer Ther. 13:697–709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bayeva M, Khechaduri A, Puig S, Chang HC,
Patial S, Blackshear PJ and Ardehali H: mTOR regulates cellular
iron homeostasis through tristetraprolin. Cell Metab. 16:645–657.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brodaczewska KK, Szczylik C, Fiedorowicz
M, Porta C and Czarnecka AM: Choosing the right cell line for renal
cell cancer research. Mol Cancer. 15:832016. View Article : Google Scholar : PubMed/NCBI
|