Introduction
Lung cancer is the most common cancer worldwide and
is the leading cause of cancer-associated mortality despite
improvements in diagnosis and therapy (1). More than 1 million mortalities are
attributable to lung cancer each year and <10% of patients with
the disease survive >5 years after diagnosis (2). Lung cancer can be divided into two
main groups: Small-cell lung cancer (SCLC) and non-SCLC (NSCLC)
(3). NSCLC accounts for 75% of all
lung cancers and, despite advances in treatment, the prognosis of
patients with NSCLC remains poor, with a 5-year overall survival
rate of only 15% (4). Therefore,
it is vital to investigate the molecular mechanisms involved in
lung carcinogenesis, and to identify diagnostic and prognostic
markers for the early detection and targeted treatment of lung
cancer.
Fibroblast growth factor receptor 1 (FGFR1) is a
member of the FGFR receptor tyrosine kinase family, consisting of
FGFR1, 2, 3 and 4 (5). FGFR
proteins interact with fibroblast growth factor to initiate a
cascade of downstream signaling pathways that ultimately regulate
cell proliferation, survival, migration and differentiation
(6). In particular, increased
expression and sustained activation of FGFR1 has been reported in
several human cancers, including oral and tongue squamous cell
carcinoma (7), esophageal squamous
cell carcinoma (8) and breast
cancer (9). Increased expression
of FGFR1 has been observed in 10–20% of patients with lung cancer,
and is correlated with the level of cigarette smoking and poor
clinical outcomes in patients with resected smoking-associated
squamous cell lung cancer (10).
FGFR1 has been identified as a promising molecular target for the
treatment of NSCLC, thus providing a novel therapeutic target for
these tumors (11). Recently, Pu
et al (12) reported
increased expression levels of FGFR1 are associated with a subgroup
of NSCLCs, and FGFR1 was identified as an independent prognostic
factor associated with poor prognosis, particularly in early stage
disease.
MicroRNAs (miRNAs/miRs) are a group of small,
noncoding, endogenous single-stranded RNAs that regulate the
expression of ~60% of human genes (13,14).
miRNAs reduce gene expression by binding to complementary regions
of target mRNA sequences and either inhibit their translation or
mediate their degradation via the RNA-induced silencing complex
(15). The aberrant expression of
miRNAs leads to the altered expression of their target genes, which
has been demonstrated to contribute to cancer initiation and
progression (16–18). In addition to gene amplification,
several miRNAs have been reported to directly or indirectly control
the expression of FGFRs to mediate cancer development (19–21).
In NSCLC, next generation sequencing and microarray-based analyses
have revealed the role of several miRNAs in lung carcinogenesis
(22,23). However, how these differentially
expressed miRNAs promote cancer progression remains elusive.
In the present study, miR-497 expression was
observed to be decreased in tumor tissues derived from patients
with NSCLC, and was negatively correlated with FGFR1 mRNA levels.
Overexpression of miR-497 led to a reduction in the growth and
migration of A549 cells, and reduction in FGFR1 at the mRNA and
protein levels. FGFR1 was predicted and validated to be a target
gene of miR-497. Through regulation of FGFR1, miR-497 inactivated
the protein kinase B (AKT) and c-Jun N-terminal kinase (JNK)
signaling pathways and repressed matrix metallopeptidase 26 (MMP26)
expression. Transfection of recombinant FGFR1 reversed the miR-497
mimics-induced inhibition of A549 cell growth and migration. The
results of the present study suggest that miR-497 may exert a tumor
suppressive role in NSCLC by binding to FGFR1 mRNA.
Materials and methods
Tissues
A total of 20 matched NSCLC tumor tissues and normal
lung tissues were collected from patients with NSCLC (aged
62.65±8.92 years old, male:female=13:7) admitted to Jingzhou First
People's Hospital (Jingzhou, China) between February 2015 and May
2016. All participants provided written consent prior to the study.
The experiments were conducted under the supervision of the
Jingzhou First People's Hospital Ethics Committee. Following
surgery, the tissue samples were immediately stored at −80°C prior
to RNA extraction.
Cell lines
The 293 cell line, human BEAS-2B normal lung
epithelial cells and the human A549 NSCLC cell line were purchased
from the American Type Culture Collection (Manassas, VA, USA), and
used within 6 months of receipt. 293 cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc, Waltham,
MA, USA) and A549 cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.). All of the
above cell culture media was supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), and cells were
maintained in a humidified incubator at 37°C with 5%
CO2. BEAS-2B normal lung epithelial cells were cultured
in LHC-8 medium (Gibco; Thermo Fisher Scientific, Inc.) and
maintained in a humidified incubator at 37°C with 5%
CO2.
Cell proliferation assay
The growth rate of A549 cells was analyzed using
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's protocol. Briefly,
cells (1×103 cells/well) were seeded in 96-well plates
and transfected with 50 nM miR-negative control (NC,
5′-UAACCACUUUCACAUGGUCCUA-3′) or miR-497 mimics
(5′-CAGCAGCACACUGUGGUUUGU-3′) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). At 0, 24, 48 and 72 h
following transfection, 10 µl CCK-8 solution was added into the
indicated wells and the cells were incubated for a further 1 h. The
medium containing CCK-8 was then transferred to a fresh 96-well
plate and the absorbance of each well was measured using a
microplate reader at 450 nm (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) to determine the cell number.
Cell migration assay
The migration ability of A549 cells was analyzed
using a wound healing assay. Briefly, the cells were cultured in a
6-well plate, transfected with miR-NC or miR-497 mimics and
cultured until 90% confluency was reached. A 10 µl pipette was then
used to scrape the surface monolayer of cells. The cells were
subsequently washed with PBS, followed by the addition of medium
containing 1% FBS. Images were captured using a Nikon TE2000
microscope (Nikon Corporation, Tokyo, Japan) at 0 and 30 h
following generation of the scratch-wound. The relative migration
area was determined using a light microscope (SZX16-3111; Olympus
Corporation, Tokyo, Japan).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
At 24 h after transfection, RNA from tissues and
cells was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). For the detection of miRNA, RNA was first
reverse transcribed using the PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd.) at 37°C for 15 min followed by incubation
at 85°C for 5 sec. qPCR was then performed to detect miR-497 levels
in cells and tissues using the SYBR Premix Ex Taq (Takara
Biotechnology, Co., Ltd.). The thermocycling conditions were:
Initial denaturation at 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec and 60°C for 30 sec. U6 was used as an internal
control for the normalization of miRNA. The primer sequences used
to detect U6 and miR-497 were as listed: U6 forward:
5′-TGCGGGTGCTCGCTTCGCAGC-3′; reverse: 5′-CCAGTGCAGGGTCCGAGGT-3′;
miR-497 forward: 5′-TGGTGTGAATGATAGGTTATTTTATT-3′; reverse:
5′-TCCATCTCTCTAAATCCCTACAAAA-3′. For the detection of mRNA
expression, RNA was first reverse transcribed into first-strand
cDNA using the PrimeScript RT reagent Kit (Takara Biotechnology,
Co., Ltd.) at 37°C for 15 min followed by incubation at 85°C for 5
sec, followed by RT-PCR using SYBR Premix Ex Taq (Takara
Biotechnology, Co., Ltd.). The thermocycling conditions were:
Initial denaturation at 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec and 60°C for 30 sec. Gene expression was quantified
using the 2−ΔΔCq method (24). β-actin served as an internal
control for mRNA expression. The primer sequences used to detect
β-actin and FGFR1 were: β-actin forward:
5′-CTCCATCCTGGCCTCGCTGT-3′; reverse: 5′-GCTGTCACCTTCACCGTTCC-3′;
FGFR1 forward: 5′-GCTAGGTGCCGAGGGTGTT-3′; reverse:
5′-ACTGCAGGCTCCTTCAGAAC-3′.
Western blotting
At 30 h after cell transfection, protein lysates
were prepared using radioimmunoprecipitation assay lysis buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to
manufacturer's instructions. The western blot was performed using
standard procedures. Firstly, protein concentration was quantified
by the BCA method. Proteins (15 µg/lane) were separated by 8–10%
SDS-PAGE, transferred to a polyvinylidene difluoride membrane and
then blocked with 5% non-fat milk at room temperature for 2 h. The
membranes were then incubated with primary antibodies at 4°C
overnight, washed with PBS and then incubated with the appropriate
secondary antibodies at room temperature for 1 h. Antibodies
against FGFR1 [rabbit monoclonal antibody (mAb); cat. no. 9740;
1:1,000], AKT (rabbit mAb; cat. no. 4691; 1:1,000), phosphorylated
(p)-AKT (rabbit mAb; cat. no. 13038; 1:1,000), JNK (rabbit mAb;
cat. no. 9252; 1:1,000), p-JNK (rabbit mAb; cat. no. 4668; 1:1,000)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA), while the β-actin antibody (mouse mAb; cat. no. A1978;
1:2,000) was purchased from Sigma-Aldrich (Merck KGaA). Secondary
antibodies against mouse (cat. no. sc2005; 1:500) and rabbit (cat.
no. sc2004; 1:500) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Finally, the membranes were developed using
enhanced chemiluminescence western blot substrate (Vazyme,
Piscataway, NJ, USA) and images were captured using an ImageQuant
400 (GE Healthcare, Chicago, IL, USA). Relative band intensity was
quantified with ImageJ software (version. 1.8.0; National
Institutes of Health).
Dual relative luciferase activity
assay
The binding of miR-497 to the 3′-untranslated region
(UTR) of FGFR1 was predicted by TargetScan release 7.1 (http://www.targetscan.org/vert_71/) and validated
using a dual luciferase reporter assay. The 3′-UTR of FGFR1 mRNA
was amplified from cDNA obtained from 293 cells and BEAS-2B cells
and annealed into the pGL3-basic vector (Promega Corporation,
Madison, WI, USA) between KpnI and Xhol restriction
enzyme sites. Two site mutations were introduced into the
pGL3-FGFR1 3′-UTR-wild-type (WT) sequence to generate the
pGL3-FGFR1 3′-UTR-Mutant using a Quick Site-directed mutation kit
(Agilent Technologies, Inc., Santa Clara, CA, USA). 293 cells
(1×105) and A549 cells (1×105) were seeded
into 24-well plates, and co-transfected with pGL3-FGFR1 3′-UTR-WT
(0.4 mg) or pGL3-FGFR1 3′UTR-Mutant (0.4 mg) together with miR-NC
(20 nm) or miR-497 mimics (20 nm) by Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), respectively. At 48 h
after transfection, the relative luciferase activity of each group
was measured using a dual luciferase reporter system kit normalized
to Renilla luciferase activity (Promega Corporation).
Overexpression of FGFR1
The full length open reading frame of FGFR1 was
amplified from 293 cell cDNA digested with HindIII and
XhoI (New England Biolabs, Inc.) and ligated into the pcDNA3
plasmid (Invitrogen; Thermo Fisher Scientific, Inc.). The primer
sequences for FGFR1 were: Forward, 5′-AAGCTTATGTGGAGCTGGAAGTG-3′;
reverse, 5′-CTCGAGGGAGGGCGTGTGGGTG-3′. For FGFR1 overexpression, 2
µg pcDNA3-FGFR1 was mixed with Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) and added to the
2×106 cells in cell culture medium. At 8 h after
transfection, the medium was replaced with fresh medium and the
cells were maintained for a further 48 h prior to western blotting
analysis.
Statistical analysis
Each experiment was repeated ≥3 times in the present
study. Statistical analysis was performed using the GraphPad Prism
7.0 software program (GraphPad Software, Inc., La Jolla, CA, USA).
The Pearson correlation test was performed to determine the
association between miR-497 and FGFR1 mRNA expression levels.
Differences between two groups were analyzed using the Student's
t-test. For comparisons among three groups, one-way analysis of
variance was performed followed by Newman-Keuls test. All P-values
obtained in this study were two-tailed. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-497 is negatively correlated with
FGFR1 in NSCLC tumor tissues
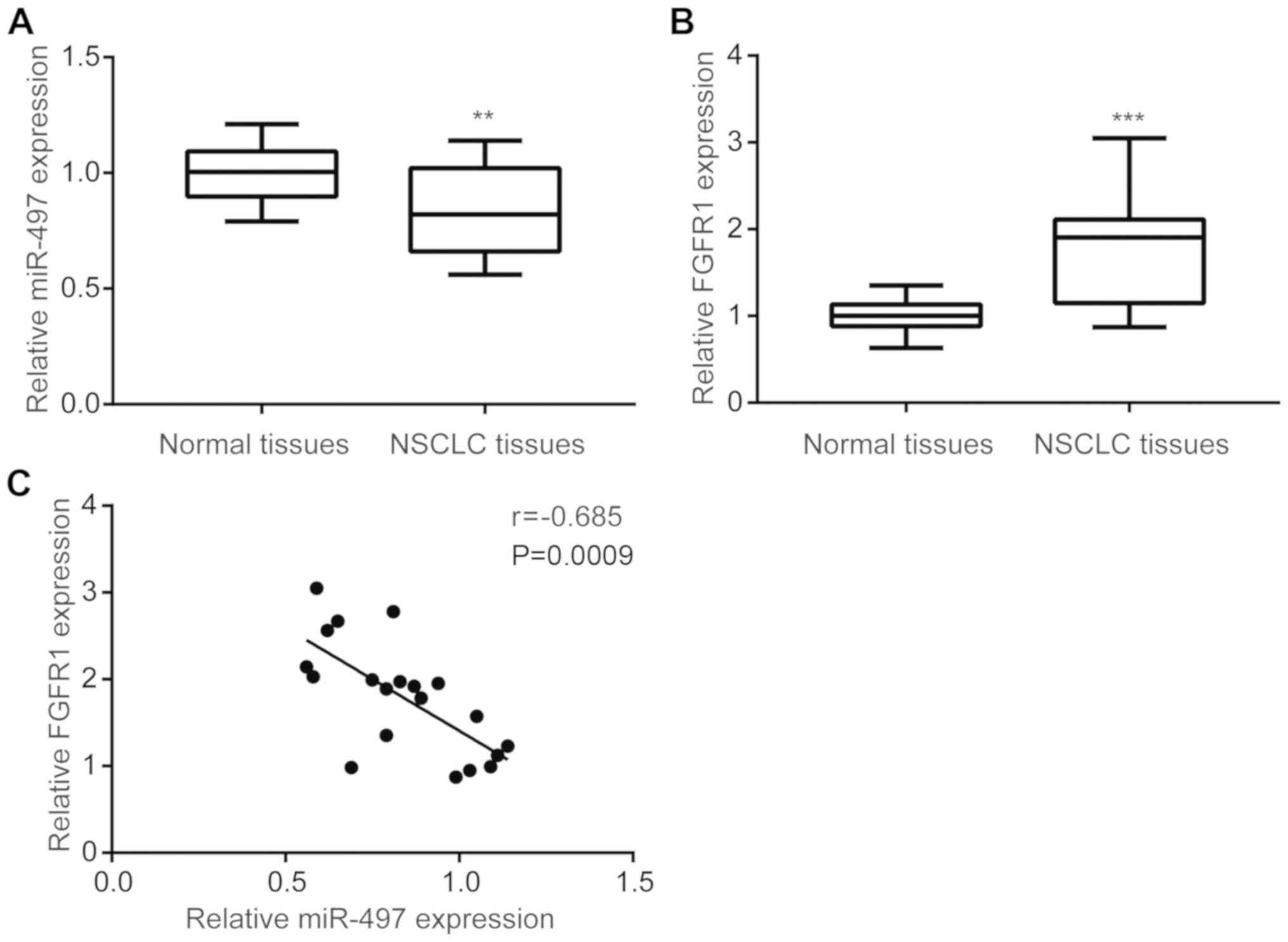
To investigate the expression of miR-497 in NSCLC
tumor tissues, RT-qPCR was performed to compare miR-497 levels in
tumor and matched normal tissues from 20 NSCLC patients. miR-497
expression was significantly downregulated in tumor tissues
compared with matched normal tissues (Fig. 1A). In addition, FGFR1 mRNA levels
were increased in tumor tissues compared with matched normal
tissues (Fig. 1B). A negative
correlation between miR-497 and FGFR1 mRNA levels was observed in
NSCLC tumor tissues (Fig. 1C).
Overexpression of miR-497 inhibits the
proliferation and migration of NSCLC cells
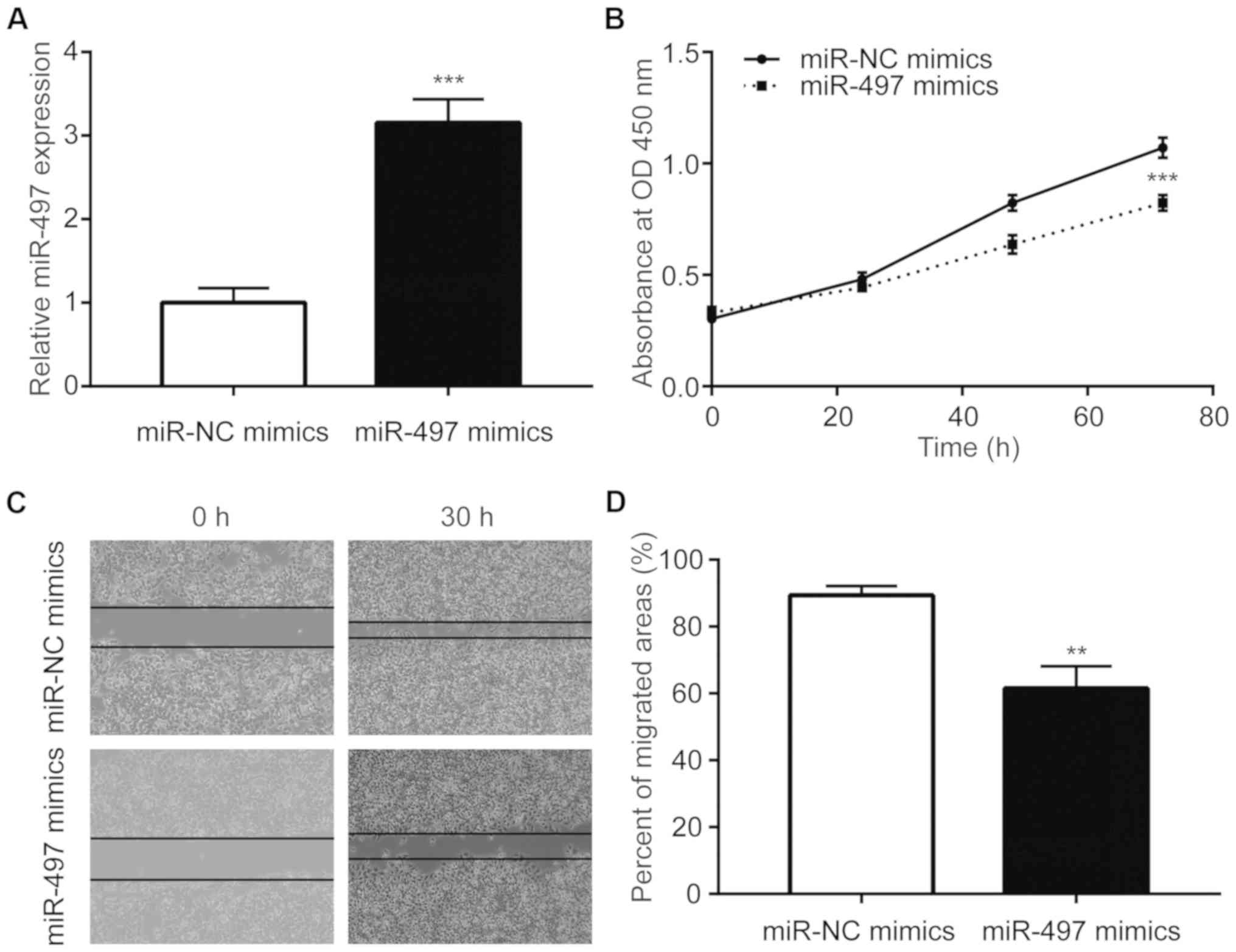
To investigate the function of miR-497 in NSCLC
cells, miR-497 was overexpressed in A549 cells via transfection of
miR-497 mimics (Fig. 2A).
Overexpression of miR-497 inhibited the proliferative ability of
A549 cells (Fig. 2B).
Additionally, miR-497 mimics suppressed the migration of A549 cells
(Fig. 2C and D).
miR-497 represses FGFR1 expression and
inactivates FGFR1-regulated signaling pathways in A549 cells
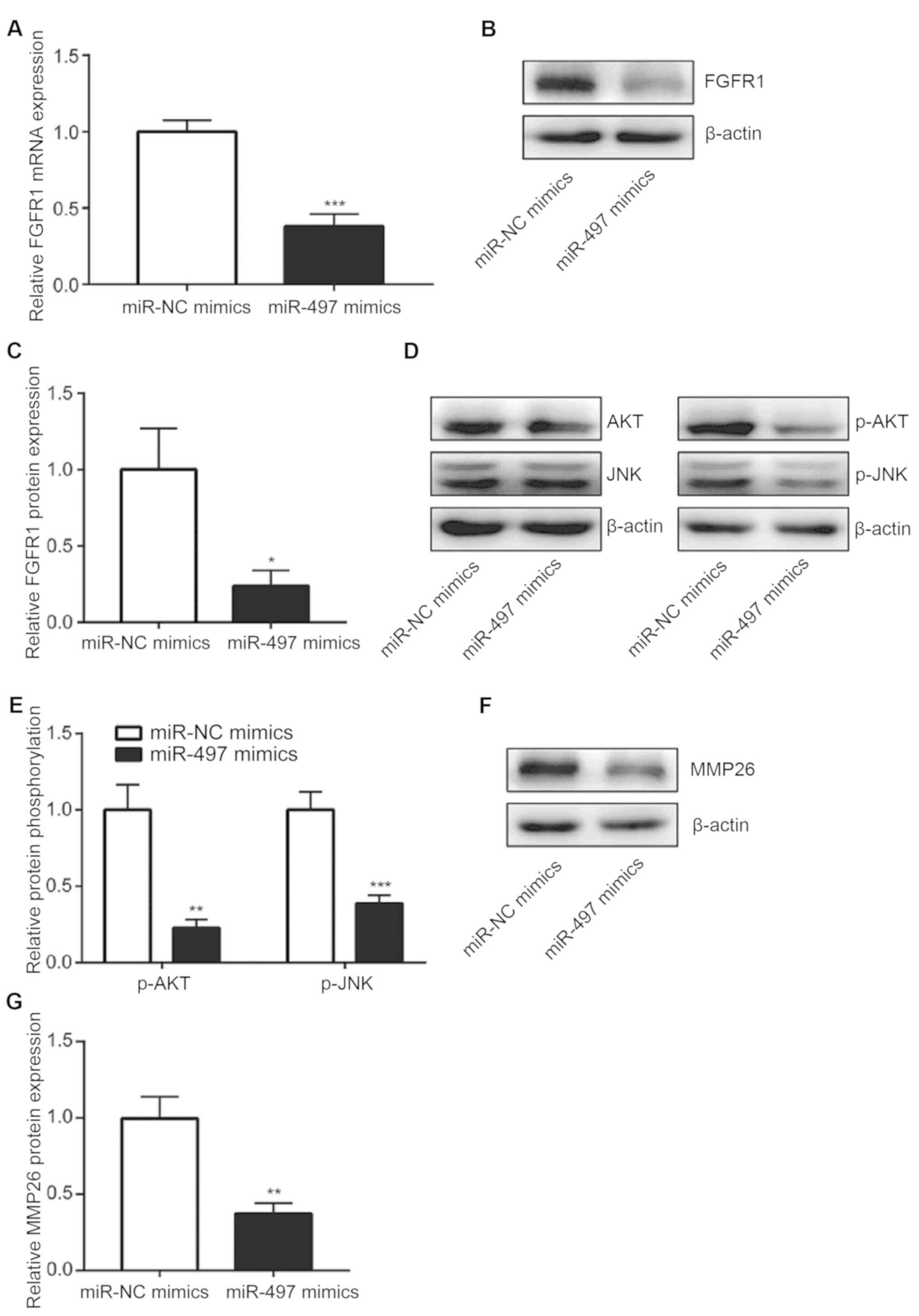
Subsequent experiments sought to investigate the
mechanisms underlying the observed effects of miR-497
overexpression in NSCLC cells. RT-qPCR analysis demonstrated that
miR-497 overexpression led to a significant reduction in FGFR1 mRNA
levels in A549 cells (Fig. 3A). In
addition, western blot analysis revealed that FGFR1 protein levels
were downregulated following miR-497 overexpression (Fig. 3B and C). It has been previously
reported that FGFR1 activates the phosphoinositide 3-kinase
(PI3K)-AKT and JNK signaling pathways promote NSCLC progression
(25,26). In the present study, miR-497 mimics
reduced p-AKT and p-JNK levels, but not those of AKT or JNK, via
regulation of FGFR1, which suggested that PI3K-AKT and JNK
signaling pathway activities were repressed following miR-497
overexpression in A549 cells (Fig. 3D
and E). MMP26 is an oncogene and is tightly controlled by
FGFR1/JNK signaling in NSCLC cells (27). Overexpression of miR-497 in A549
cells decreased MMP26 protein expression in the current study
(Fig. 3F and G). Therefore, these
results indicate that miR-497 may function as a tumor suppressor in
NSCLC via regulation of FGFR1 and the downstream targets of
FGFR1.
miR-497 binds to the 3′-UTR of FGFR1
mRNA
Using TargetScan software, predicted complementary
binding sites between miR-497 and the FGFR1 3′-UTR sequence were
identified, indicating that miR-497 may bind to 3′-UTR of FGFR1
mRNA (Fig. 4A). The subsequent
dual luciferase reporter assay analysis demonstrated that
transfection with miR-497 mimics repressed luciferase activity in
239 and A549 cells transfected with pGL3-FGFR1 3′-UTR-WT vector,
but not the pGL3-FGFR1-Mutant vector containing mutations in two
potential binding nucleotides (Fig. 4B
and C).
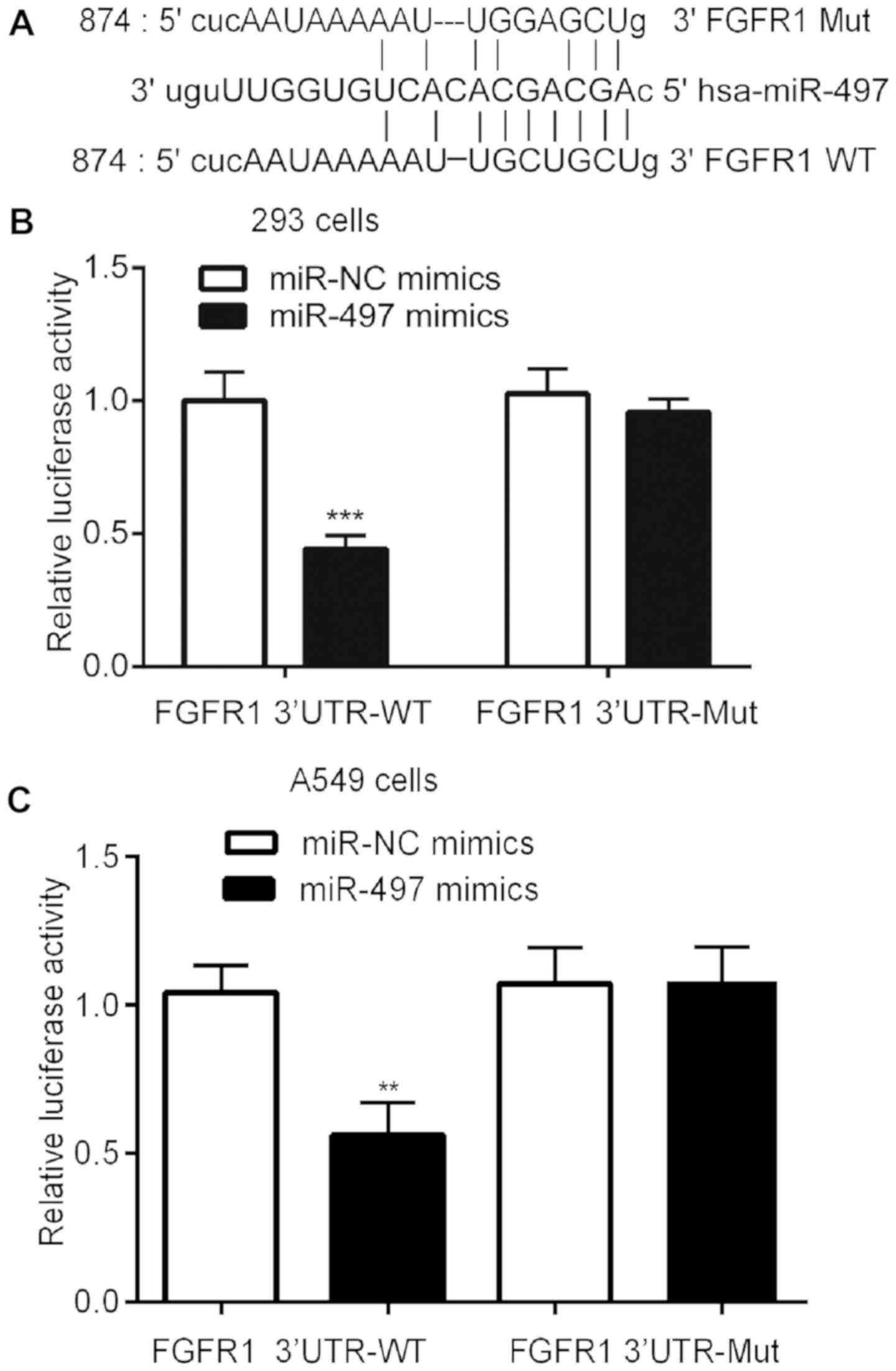 | Figure 4.miR-497 directly binds the 3′-UTR of
FGFR1 mRNA. (A) Alignment of the miR-497 and the FGFR1 3′-UTR
sequences indicates a complementary binding site. In (B) 293 cells
and (C) A549 cells, dual luciferase reporter assay analysis
indicates that miRNA-497 reduces the relative luciferase activity
of wild-type, not mutant, FGFR1 3′-UTR mRNA sequences. The
experiment was repeated ≥3 times. **P<0.01, ***P<0.0001.
FGFR1, fibroblast growth factor receptor 1; Mut, mutant; WT,
wild-type; miR, microRNA; NC, negative control; UTR, untranslated
region. |
FGFR1 is involved in miR-497
mimic-induced arrest of cell growth and migration in A549
cells
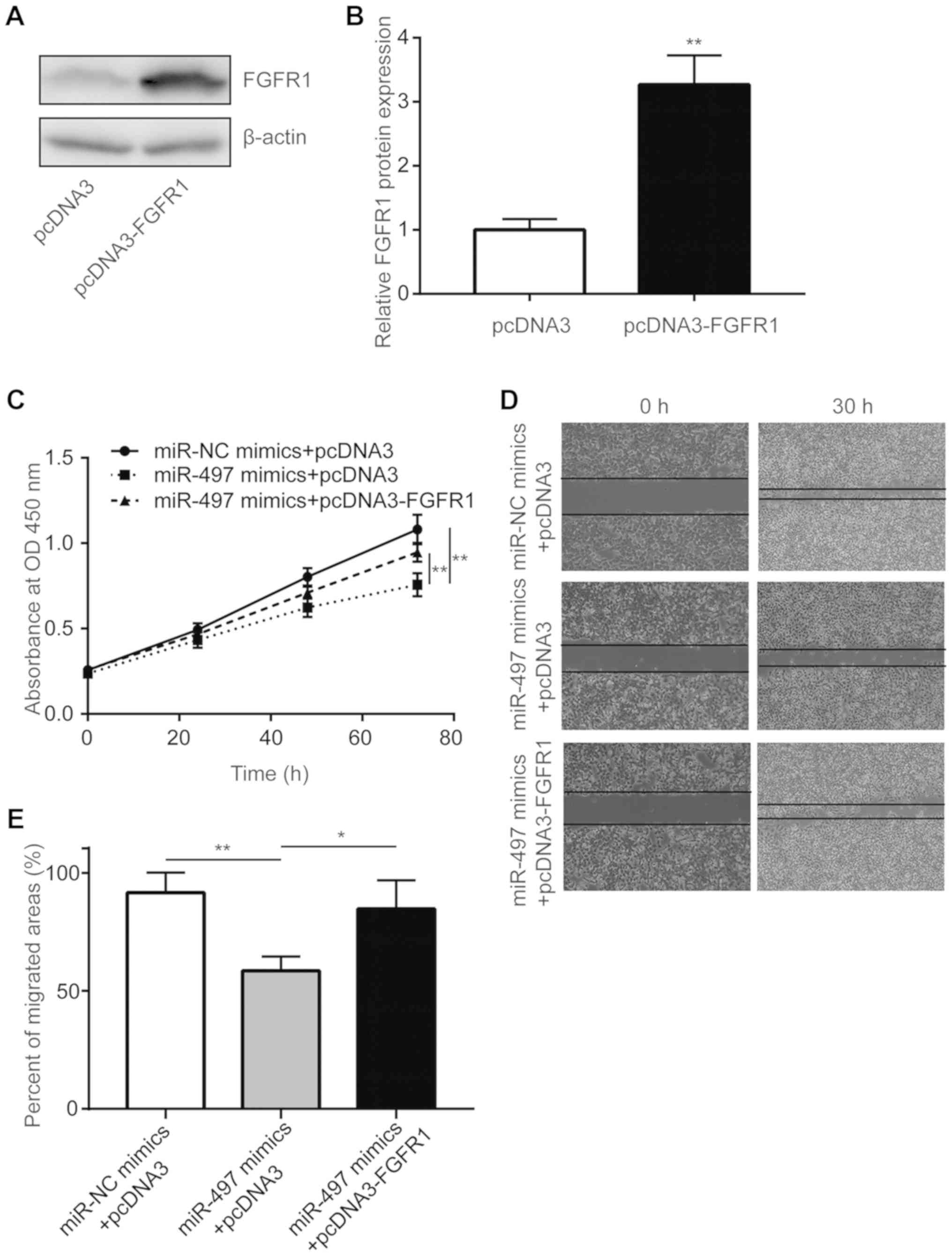
To elucidate the role of FGFR1 in the
miR-497-mediated inhibition of cancer progression, a pcDNA3-FGFR1
plasmid was used to overexpress FGFR1 in A549 cells. As shown in
Fig. 5A and B, transfection of
pcDNA3-FGFR1 elevated FGFR1 protein levels in A549 cells.
Overexpression of FGFR1 significantly reversed cell proliferation
inhibition induced by miR-497 mimics in A549 cells (Fig. 5C). Furthermore, miR-497
mimics-induced inhibition of cell migration was rescued by
transfection with pcDNA3-FGFR1 (Fig.
5D and E). Collectively, these data demonstrate that miR-497
inhibited NSCLC progression via the regulation of FGFR1.
Discussion
Overexpression of FGFR1 is frequently observed in
lung cancer and is considered to be a biomarker of response to
FGFR-tyrosine kinase inhibitor (TKI) therapy in patients with lung
cancer (28,29). FGFR1 gene amplification is known to
be an underlying cause of increased FGFR1 expression in lung
cancer, and gene copy number (GCN) is used to predict patient
response to FGFR1-TKI in the clinic (30,31).
However, a recent study reported that FGFR1 mRNA levels may be an
improved indicator of patient response to FGFR-TKI therapy compared
with GCN, suggesting that gene amplification may sufficiently
explain the increased FGFR1 expression levels in lung cancer
(32). Indeed, a subsequent study
demonstrated that FGFR1 expression was also regulated by miR-198 in
lung cancer (33). In the present
study, a negative correlation between miR-497 and FGFR1 mRNA levels
in NSCLC tumor tissues was observed. In A549 cells, RT-qPCR and
western blot analyses demonstrated that miR-497 repressed FGFR1 at
the mRNA and protein expression levels. In addition, the PI3K-AKT
and JNK signaling pathways, which are two downstream signaling
pathways of FGFR1, were inactivated following miR-497
overexpression. The expression of the MMP26 oncogene was also
negatively regulated by miR-497 in A549 cells. The present study
also predicted and validated FGFR1 as a target gene of miR-497 in
A549 cells.
Previous studies have demonstrated that miR-497
expression is downregulated in NSCLC, and that miR-497 targets
multiple oncogenes to promote NSCLC progression (34,35).
In the present study, overexpression of miR-497 inhibited the
proliferation and migration of NSCLC cells, which is consistent
with previous reports (36,37).
Notably, overexpression of FGFR1 reversed the miR-497 mimic-induced
arrest of cell growth and migration in A549 cells, suggesting that
miR-497 may primarily rely on the regulation of FGFR1 to inhibit
NSCLC progression. In addition, there are a number of studies on
the function of miR-497 in lung cancer by targeting other mRNA
targets. For instance, miR-497 was reported to inhibit the
epithelial-mesenchymal transition of NSCLC by targeting Metadherin
(38), miR-497 was discovered to
inhibit the tumor growth of NSCLC by targeting YAP1 (34) and miR-497 was found to inhibit cell
growth and invasion of NSCLC by targeting VEGF-A (36).
In conclusion, the results of the current study
suggest that miR-497 may function as a tumor suppressor in NSCLC
via regulation of FGFR1. In NSCLC cells, miR-497 binds directly to
the 3′-UTR of FGFR1 mRNA, leading to reduction in FGFR1 protein
levels, inactivation of the PI3K-AKT and JNK signaling pathways and
the downregulation of MMP26 expression. These findings enrich the
current understanding of miR-497 and FGFR1 in mediating NSCLC
progression, and implicate miR-497 as a putative therapeutic target
for patients with NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The present study was designed by HL. The
experiments were carried out by QH, XD, DZ, BG and WX. The data
were analyzed by QH, HL and XD. The manuscript was written by HL,
and all authors approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jingzhou First People's Hospital. Written informed
consent was provided by the patients or their family.
Patient consent for publication
The present study received consent for publication
from each patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marshall AL and Christiani DC: Genetic
susceptibility to lung cancer-light at the end of the tunnel?
Carcinogenesis. 34:487–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wistuba II, Gelovani JG, Jacoby JJ, Davis
SE and Herbst RS: Methodological and practical challenges for
personalized cancer therapies. Nat Rev Clin Oncol. 8:135–141. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tiong KH, Mah LY and Leong CO: Functional
roles of fibroblast growth factor receptors (FGFRs) signaling in
human cancers. Apoptosis. 18:1447–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monaco SE, Rodriguez EF, Mahaffey AL and
Dacic S: FGFR1 amplification in squamous cell carcinoma of the lung
with correlation of primary and metastatic tumor status. Am J Clin
Pathol. 145:55–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young RJ, Lim AM, Angel C, Collins M, Deb
S, Corry J, Wiesenfeld D, Kleid S, Sigston E, Lyons B, et al:
Frequency of fibroblast growth factor receptor 1 gene amplification
in oral tongue squamous cell carcinomas and associations with
clinical features and patient outcome. Oral Oncol. 49:576–581.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishizuka T, Tanabe C, Sakamoto H, Aoyagi
K, Maekawa M, Matsukura N, Tokunaga A, Tajiri T, Yoshida T, Terada
M and Sasaki H: Gene amplification profiling of esophageal squamous
cell carcinomas by DNA array CGH. Biochem Biophys Res Commun.
296:152–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gru AA and Allred DC: FGFR1 amplification
and the progression of non-invasive to invasive breast cancer.
Breast Cancer Res. 14:1162012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HR, Kim DJ, Kang DR, Lee JG, Lim SM,
Lee CY, Rha SY, Bae MK, Lee YJ, Kim SH, et al: Fibroblast growth
factor receptor 1 gene amplification is associated with poor
survival and cigarette smoking dosage in patients with resected
squamous cell lung cancer. J Clin Oncol. 31:731–737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Cai Y, Ji J, Liu Z, Zhao C, Zhao
Y, Wei T, Shen X, Zhang X, Li X and Liang G: Discovery and
identification of new non-ATP competitive FGFR1 inhibitors with
therapeutic potential on non-small-cell lung cancer. Cancer Lett.
344:82–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pu D, Liu J, Li Z, Zhu J and Hou M:
Fibroblast growth factor receptor 1 (FGFR1), partly related to
vascular endothelial growth factor receptor 2 (VEGFR2) and
microvessel density, is an independent prognostic factor for
non-small cell lung cancer. Med Sci Monit. 23:247–257. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawahara Y: Human diseases caused by
germline and somatic abnormalities in microRNA and microRNA-related
genes. Congenit Anom (Kyoto). 54:12–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kasinski AL and Slack FJ: miRNA-34
prevents cancer initiation and progression in a therapeutically
resistant K-ras and p53-induced mouse model of lung adenocarcinoma.
Cancer Res. 72:5576–5587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fortunato O, Boeri M, Moro M, Verri C,
Mensah M, Conte D, Caleca L, Roz L, Pastorino U and Sozzi G:
Mir-660 is downregulated in lung cancer patients and its
replacement inhibits lung tumorigenesis by targeting MDM2-p53
interaction. Cell Death Dis. 5:e15642014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng Y, Dai Y, Hitchcock C, Yang X, Kassis
ES, Liu L, Luo Z, Sun HL, Cui R, Wei H, et al: Insulin growth
factor signaling is regulated by microRNA-486, an underexpressed
microRNA in lung cancer. Proc Natl Acad Sci USA. 110:15043–15048.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Li J, Wang X, Zheng C and Ma W:
Downregulation of microRNA-214 and overexpression of FGFR-1
contribute to hepatocellular carcinoma metastasis. Biochem Biophys
Res Commun. 439:47–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang H, Qu L, Wang Y, Cong J, Wang W and
Yang X: miR-99a promotes proliferation targeting FGFR3 in human
epithelial ovarian cancer cells. Biomed Pharmacother. 68:163–169.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishijima N, Seike M, Soeno C, Chiba M,
Miyanaga A, Noro R, Sugano T, Matsumoto M, Kubota K and Gemma A:
miR-200/ZEB axis regulates sensitivity to nintedanib in non-small
cell lung cancer cells. Int J Oncol. 48:937–944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen QY, Jiao DM, Yan L, Wu YQ, Hu HZ,
Song J, Yan J, Wu LJ, Xu LQ and Shi JG: Comprehensive gene and
microRNA expression profiling reveals miR-206 inhibits MET in lung
cancer metastasis. Mol Biosyst. 11:2290–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang R, Chen XF and Shu YQ: Prediction of
non-small cell lung cancer metastasis-associated microRNAs using
bioinformatics. Am J Cancer Res. 5:32–51. 2014.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu F, Liu H, Xie X, Mei J and Wang M:
Activated cdc42-associated kinase is up-regulated in non-small-cell
lung cancer and necessary for FGFR-mediated AKT activation. Mol
Carcinog. 55:853–863. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung SY, Yi JY, Kim MH, Song KH, Kang SM,
Ahn J, Hwang SG, Nam KY and Song JY: IM-412 inhibits the invasion
of human breast carcinoma cells by blocking FGFR-mediated
signaling. Oncol Rep. 34:2731–2737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao D, Lu Y, Yang C, Zhou X and Xu Z:
Activation of FGF receptor signaling promotes invasion of
non-small-cell lung cancer. Tumour Biol. 36:3637–3642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Theelen WS, Mittempergher L, Willems SM,
Bosma AJ, Peters DD, van der Noort V, Japenga EJ, Peeters T, Koole
K, Šuštić T, et al: FGFR1, 2 and 3 protein overexpression and
molecular aberrations of FGFR3 in early stage non-small cell lung
cancer. J Pathol Clin Res. 2:223–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Göke A, Franzen A, Menon R, Goltz D,
Kirsten R, Boehm D, Vogel W, Scheble V, Ellinger J, Gerigk U, et
al: Rationale for treatment of metastatic squamous cell carcinoma
of the lung using fibroblast growth factor receptor inhibitors.
Chest. 142:1020–1026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tran TN, Selinger CI, Kohonen-Corish MR,
McCaughan BC, Kennedy CW, O'Toole SA and Cooper WA: Fibroblast
growth factor receptor 1 (FGFR1) copy number is an independent
prognostic factor in non-small cell lung cancer. Lung Cancer.
81:462–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dutt A, Ramos AH, Hammerman PS, Mermel C,
Cho J, Sharifnia T, Chande A, Tanaka KE, Stransky N, Greulich H, et
al: Inhibitor-sensitive FGFR1 amplification in human non-small cell
lung cancer. PLoS One. 6:e203512011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wynes MW, Hinz TK, Gao D, Martini M, Marek
LA, Ware KE, Edwards MG, Böhm D, Perner S, Helfrich BA, et al:
FGFR1 mRNA and protein expression, not gene copy number, predict
FGFR TKI sensitivity across all lung cancer histologies. Clin
Cancer Res. 20:3299–3309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Zhao H, Xin Y and Fan L:
MicroRNA-198 inhibits proliferation and induces apoptosis of lung
cancer cells via targeting FGFR1. J Cell Biochem. 115:987–995.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang C, Ma R, Yue J, Li N, Li Z and Qi D:
MiR-497 suppresses YAP1 and inhibits tumor growth in non-small cell
lung cancer. Cell Physiol Biochem. 37:342–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao WY, Wang Y, An ZJ, Shi CG, Zhu GA,
Wang B, Lu MY, Pan CK and Chen P: Downregulation of miR-497
promotes tumor growth and angiogenesis by targeting HDGF in
non-small cell lung cancer. Biochem Biophys Res Commun.
435:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu A, Lu J, Wang W, Shi C, Han B and Yao
M: Role of miR-497 in VEGF-A-mediated cancer cell growth and
invasion in non-small cell lung cancer. Int J Biochem Cell Biol.
70:118–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang
Y, Yang C and Jiang Y: miR-497 and miR-34a retard lung cancer
growth by co-inhibiting cyclin E1 (CCNE1). Oncotarget.
6:13149–13163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin Q, Han Y, Zhu D, Li Z, Shan S, Jin W,
Lu Q and Ren T: miR-145 and miR-497 suppress TGF-β-induced
epithelial-mesenchymal transition of non-small cell lung cancer by
targeting MTDH. Cancer Cell Int. 18:1052018. View Article : Google Scholar : PubMed/NCBI
|