Introduction
Ankylosing spondylitis (AS) is a common chronic
inflammatory autoimmune disease, which is characterized by
inflammation of the spine, and frequently involves inflammation of
tendons, peripheral joints, the attachment points of tendon
ligaments and other cartilaginous tissue, leading to spinal
rigidity and fibrosis (1). The
incidence of AS varies across regions, ages and genders. It was
previously reported that the incidence of AS was 0.25% in Europe
(2) and 0.23% in China (3). The disease frequently occurs in young
adults (4). AS progresses slowly,
with the development of long-term disability in 20–30% of cases
(5). At present, there is no cure
available for AS, with treatment limited to the relief of symptoms
(6,7). Therefore, further investigation into
the pathogenesis is required.
Toll-like receptors (TLRs) are a class of receptors
involved in non-specific immunity, acting as a bridge to link the
non-specific and specific immune responses (8). TLRs are single transmembrane
domain-containing non-catalytic proteins that identify and bind
conserved molecules from microorganisms (9). When microorganisms infiltrate
physical barriers such as skin and mucous membranes, TLRs bind them
and stimulate immune cell responses (10,11).
TLRs are involved in various immune system diseases, including AS,
rheumatoid arthritis, osteoarthritis and autoimmune myositis
(12–14).
As a regulator of signal transduction, the nuclear
factor-κB (NF-κB) protein complex is involved in various biological
processes, including inflammation, immune response and apoptosis,
and has received increasing attention in recent years (15,16).
The NF-κB signaling pathway in mammals primarily involves the
p65/p50 heteromer, which binds its inhibitory protein [inhibitor of
NF-κB B (IκB) subunit IκBα], masking the nuclear translocation
signal of NF-κB (17). NF-κB
exists in an inactive form in the cytoplasm; however, following
phosphorylation of IκB by IκB kinase, IκBα separates from NF-κB and
activated NF-κB is translocated into the nucleus (17). It has been reported that NF-κB
signaling serves important roles in the progression of AS,
rheumatoid arthritis and various cancers (18–20).
In the present study, the expression levels of
inflammatory cytokines and TLRs were determined in healthy subjects
and patients with AS. The inflammatory cytokines, TLRs and NF-κB
signaling were analyzed in peripheral blood mononuclear cells
(PBMCs) from patients with AS prior to and following treatment.
Materials and methods
Patients
A total of 30 patients (male patients, 21; female
patients, 9) with AS were recruited at Mingzhou Hospital of
Zhejiang University (Ningbo, China) between May 2015 and April
2017, aged between 26 and 69 years old with an average age of
37.25±10.17 years. Patients were diagnosed with AS according to the
New York diagnostic standard of AS revised in 1984 (21). Patients were excluded from the
study based on the following criteria: Serious lesion of the heart,
brain, liver, kidney or other important organs; diagnosis of blood
or endocrine system diseases; the primary lesion was not located in
the spine; diagnosis of psoriasis, inflammatory bowel disease or
uveitis; pregnancy; lactation or diagnosis of acute ophthalmia
requiring corticosteroid therapy. A total of 30 healthy subjects
(male patients, 20; female patients, 10) aged between 27 and 66
years old (average age, 36.94±10.65 years) were included as
controls; these subjects were recruited during physical
examinations between May 2015 and April 2017. Healthy controls were
excluded according to the following criteria: The aforementioned
exclusion criteria; and diseases or symptoms of the spinal joints,
chronic diseases or autoimmune diseases. All patients provided
written informed consent prior to the collection of blood samples,
and all experiments were approved by the Ethics Committee of
Mingzhou Hospital of Zhejiang University. Patients received three
doses of infliximab (5 mg/kg), with subsequent doses received 2 and
6 weeks following the first dose.
Source and culture of cells
PBMCs were obtained from patients with AS by
Ficoll-Hypaque (Ficoll™ Paque Plus; cat. no. 17-1440-03; GE
Healthcare) density gradient centrifugation as previously described
(22). PBMCs were maintained in
RPMI-1640 medium (Shanghai BioSun SciTech Co., Ltd.) containing 10%
fetal bovine serum (HyClone; GE Healthcare Life Sciences) and 1%
penicillin-streptomycin (Beijing Leagene Biotech Co., Ltd.) in an
incubator (Shanghai SANTN Instrument Co., Ltd.) at 37°C with 95%
humidified air and 5% CO2. Following 24 h of culture,
the morphology of the cells was observed under an inverted
microscope (×100 and ×200; XDS-100; Shanghai Caikon Optical
Instrument Co., Ltd.).
Reagents and experimental
grouping
Tumor necrosis factor-α (TNF-α) was purchased from
MedChemExpress. Pomalidomide (anti-TNF-α; Selleck Chemicals) is a
derivative of thalidomide and inhibits TNF-α release induced by
lipopolysaccharide (23). It has
been reported that as an analogue of thalidomide, pomalidomide
exhibits antiapoptotic, antiangiogenic and immunomodulatory
activities (24,25).
PBMCs were collected from three groups: Healthy
individuals (control); patients with AS prior to treatment
(AS/Before treatment) and patients following treatment with
infliximab (After treatment). PBMCs were then assigned to five
groups: PBMCs not treated with TNF-α or anti-TNF-α (control); PBMCs
cultured with 1, 5 and 10 ng/ml TNF-α; and PBMCs cultured with 2 µM
pomalidomide (2 µM anti-TNF-α).
ELISA
Serum samples were isolated from blood samples via
centrifugation at 2,000 × g for 10 min at room temperature. The
levels of interleukin (IL)-6, IL-10, TNF-α and C-reactive protein
(CRP) in serum were detected using ELISA kits (Shanghai
Enzyme-linked Biotechnology Co., Ltd.) according to the
manufacturer's protocols. In brief, the samples were added to wells
coated with polyclonal antibodies against IL-6 (cat. no.
ml058097-1), IL-10 (cat. no. ml064299-1), TNF-α (cat. no.
ml077385-1) and CRP (ml002999-1), and proteins were detected using
biotinylated monoclonal anti-human antibodies at room temperature
for 2 h. After washing with PBS, color development was catalyzed by
horseradish peroxidase conjugated to streptavidin and terminated
using 2 M sulfuric acid. The absorbance was detected at 450 nm, and
the protein content was determined by normalizing the relative
absorbance of the samples to the standards.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from tissues and cells and
extracted using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). RNA (1 µg) was reverse transcribed to obtain
cDNA using a RevertAid™ cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.), with the reaction conditions set at 85°C for 5
min. qPCR was performed using an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and iTaq™ Universal
SYBR®-Green (Bio-Rad Laboratories, Inc.). qPCR was
conducted as follows: Initial denaturation at 92°C for 5 min; 30
cycles of denaturation (at 92°C for 30 sec) and annealing (at 62°C
for 30 sec); and extension at 72°C for 30 sec. The primers were
acquired from Invitrogen (Thermo Fisher Scientific, Inc.) and are
presented in Table I. The internal
reference was β-actin, and gene expression was quantified using the
2−ΔΔCq method (26).
This experiment was repeated at least three times.
 | Table I.Sequences of primers. |
Table I.
Sequences of primers.
| Primer name | Sequence
(5′-3′) |
|---|
| TLR1-Forward |
GGAGGCAATGCTGCTGTT |
| TLR1-Reverse |
GCCCAATATGCCTTTGTTATCCTG |
| TLR2-Forward |
TGTTGCAAGCAGGATCCAAAG |
| TLR2-Reverse |
CACAAAGTATGTGGCATTGTCCAG |
| TLR3-Forward |
GGACTTTGAGGCGGGTGTT |
| TLR3-Reverse |
TGTTGAACTGCATGATGTACCTTGA |
| TLR4-Forward |
AGGATGATGCCAGCATGATGTC |
| TLR4-Reverse |
TCAGGTCCAGGTTCTTGGTTGAG |
| TLR5-Forward |
AAGATGTCGGAGCCTCAGATG |
| TLR5-Reverse |
GGGTCCCTGGTTGTTTAAAGACTTC |
| TLR6-Forward |
CAGAGTGAGTGGTGCCATTACGA |
| TLR6-Reverse |
AGCCTTCAGCTTGTGGTACTTGTTG |
| TLR7-Forward |
TCTTCAACCAGACCTCTACATTCCA |
| TLR7-Reverse |
GGAACATCCAGAGTGACATCACAG |
| TLR8-Forward |
GCGCTGCTGCAAGTTACGGA |
| TLR8-Reverse |
TCGACGATTGCTGCACTCTG |
| TLR9-Forward |
GGGACCTCGAGTGTGAAGCA |
| TLR9-Reverse |
CTGGAGCTCACAGGGTAGGAA |
| IL-6-Forward |
AACCTGAACCTTCCAAAGATGG |
| IL-6-Reverse |
TCTGGCTTGTTCCTCACTACT |
| IL-10-Forward |
CCTGCCTAACATGCTTCGAG |
| IL-10-Reverse |
GAGTTCACATGCGCCTTGAT |
| TNF-α-Forward |
ATGAGCACTGAAAGCATGATCC |
| TNF-α-Reverse |
GAGGGCTGATTAGAGAGAGGTC |
|
β-actin-Forward |
GTGGACATCCGCAAAGAC |
|
β-actin-Reverse |
AAAGGGTGTAACGCAACTAA |
Western blotting
Protein was isolated from cells and extracted using
radioimmunoprecipitation assay lysis buffer (Beijing Leagene
Biotech Co., Ltd.), and the total protein was quantified using a
bicinchoninic acid detection assay kit (Shanghai Yeasen
Biotechnology, Co., Ltd.). Proteins (20 µg/lane) were
separated via 10% SDS-PAGE and transferred to nitrocellulose
membranes. The membranes were blocked in 5% dried skimmed milk in
TBS buffer at 37°C for 1 h and incubated overnight at 4°C with
antibodies against TLR3 (1:700; ab62566, Abcam), TLR4 (1:600;
ab13556, Abcam), TLR5 (1:800; ab62460, Abcam), p65 (1:1,000;
ab32536, Abcam), phosphorylated (p)-p65 (1:600; ab86299, Abcam) and
β-actin (1:1,000; MAB8969, R&D Systems, Inc.). Then, the
membranes were washed in TBS-0.1% Tween-20 three times, and
incubated at room temperature for 1.5 h with the following
secondary antibodies: Mouse anti-rabbit IgG, (1:6,000; cat. no.
3678; Cell Signaling Technology, Inc.); horseradish peroxidase
(HRP)-conjugated rabbit anti-mouse IgG (1:7,000; cat. no. 58802;
Cell Signaling Technology, Inc.) and HRP-conjugated rabbit
anti-goat IgG (1:6,000; sc-2768; Santa Cruz Biotechnology, Inc.).
Bands were visualized using enhanced chemiluminescence detection
reagent (Amersham; GE Healthcare) and imaged using an iBright™
CL1000 imaging system (iBright analysis software, version 1.2.0;
Thermo Fisher Scientific, Inc.).
Statistical analysis
Data were analyzed using IBM SPSS Statistics version
20 (IBM Corp.). Data were presented as the mean ± standard
deviation. All experiments were performed in triplicate.
Differences between groups were determined using one-way analyses
of variance followed by a Tukey's post hoc test. P<0.05 was
considered to indicate a significant difference.
Results
Upregulation of IL-6, TNF-α,
C-reactive protein (CRP), TLR4 and TLR5, and downregulation of
IL-10 and TLR3 in patients with AS
To investigate the levels of inflammatory cytokines
and TLRs in patients with AS, ELISA and RT-qPCR analysis were
performed. It was revealed that the serum levels of IL-6, TNF-α and
CRP were significantly increased in patients with AS compared with
in healthy individuals, whereas those of IL-10 were decreased
(P<0.05; Fig. 1A-D).
Additionally, it was demonstrated that the expression of TLR4 and
TLR5 mRNA was significantly upregulated in patients with AS
compared with the control, whereas that of TLR3 was downregulated
(P<0.05; Fig. 1E). The mRNA
expression levels of the other TLRs were not significantly
different between the two groups.
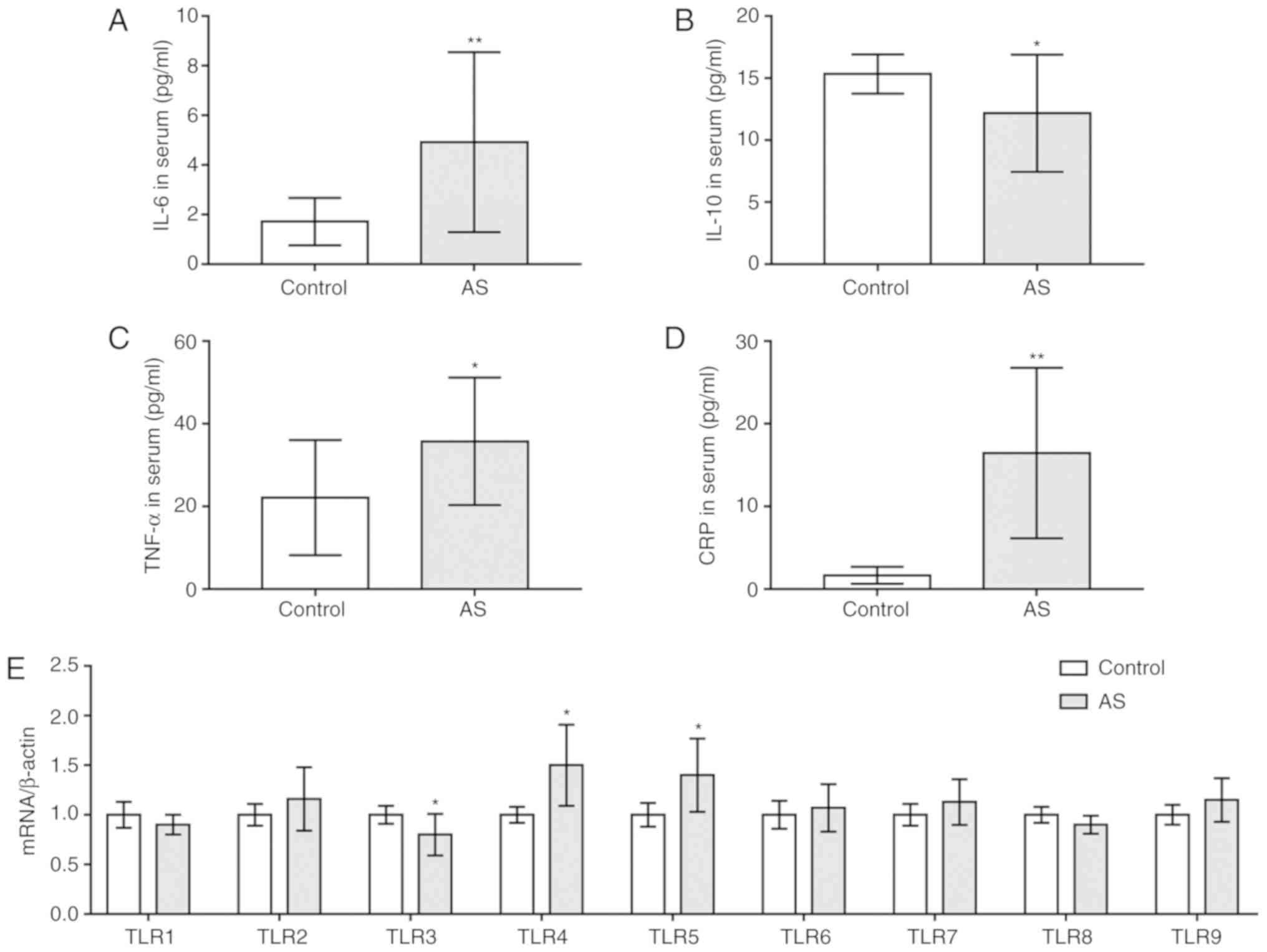 | Figure 1.Upregulation of IL-6, TNF-α, CRP,
TLR4 and TLR5, and downregulation of IL-10 and TLR3 in patients
with AS. Serum contents of (A) IL-6, (B) IL-10, (C) TNF-α and (D)
CRP in patients with AS and healthy subjects as determined by
ELISA. (E) mRNA levels of TLRs as determined via reverse
transcription-quantitative PCR analysis. *P<0.05, **P<0.01
vs. control. AS, ankylosing spondylitis; CRP, C-reactive protein;
IL, interleukin; TLR, Toll-like receptor; TNF-α, tumor necrosis
factor-α. |
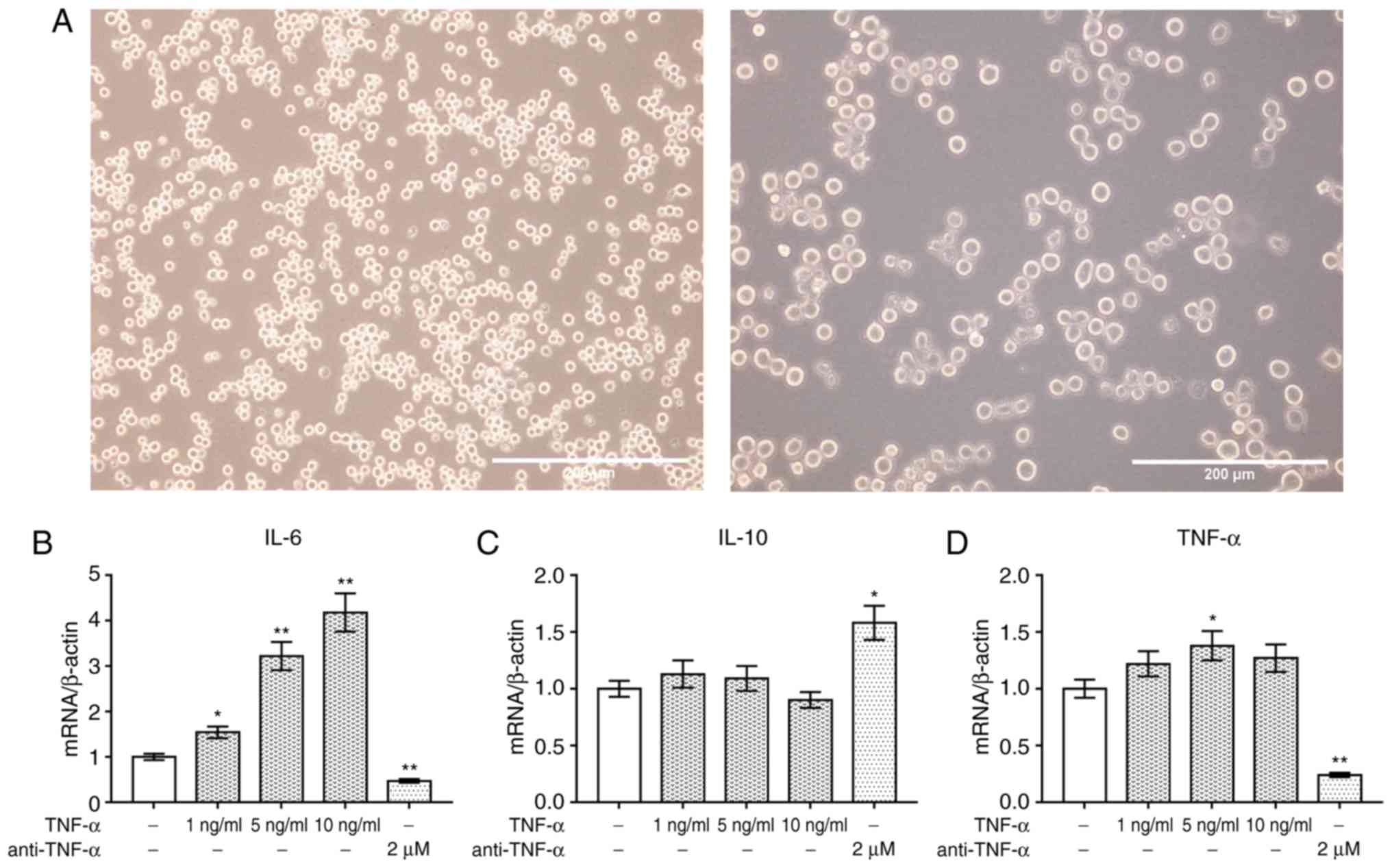
TNF-α regulates the expression of
inflammatory cytokines in PBMCs
PBMCs from patients with AS were identified under an
inverted microscope. The cells were round and clustered together
(Fig. 2A). The levels of
inflammatory cytokines were determined via RT-qPCR analysis to
investigate the effects of TNF-α and anti-TNF-α on PBMCs. It was
revealed that TNF-α significantly promoted the expression of IL-6
mRNA level in a dose-dependent manner compared with the control;
however, IL-10 expression was markedly unaltered following TNF-α
treatment (P<0.05; Fig. 2B and
C). Conversely, anti-TNF-α significantly downregulated the
expression of IL-6 compared with the control, and upregulated that
of IL-10. Furthermore, TNF-α (5 ng/ml) significantly increased the
expression of TNF-α mRNA in PBMCs; however, expression was
significantly downregulated following treatment with anti-TNF-α
(P<0.05; Fig. 2D).
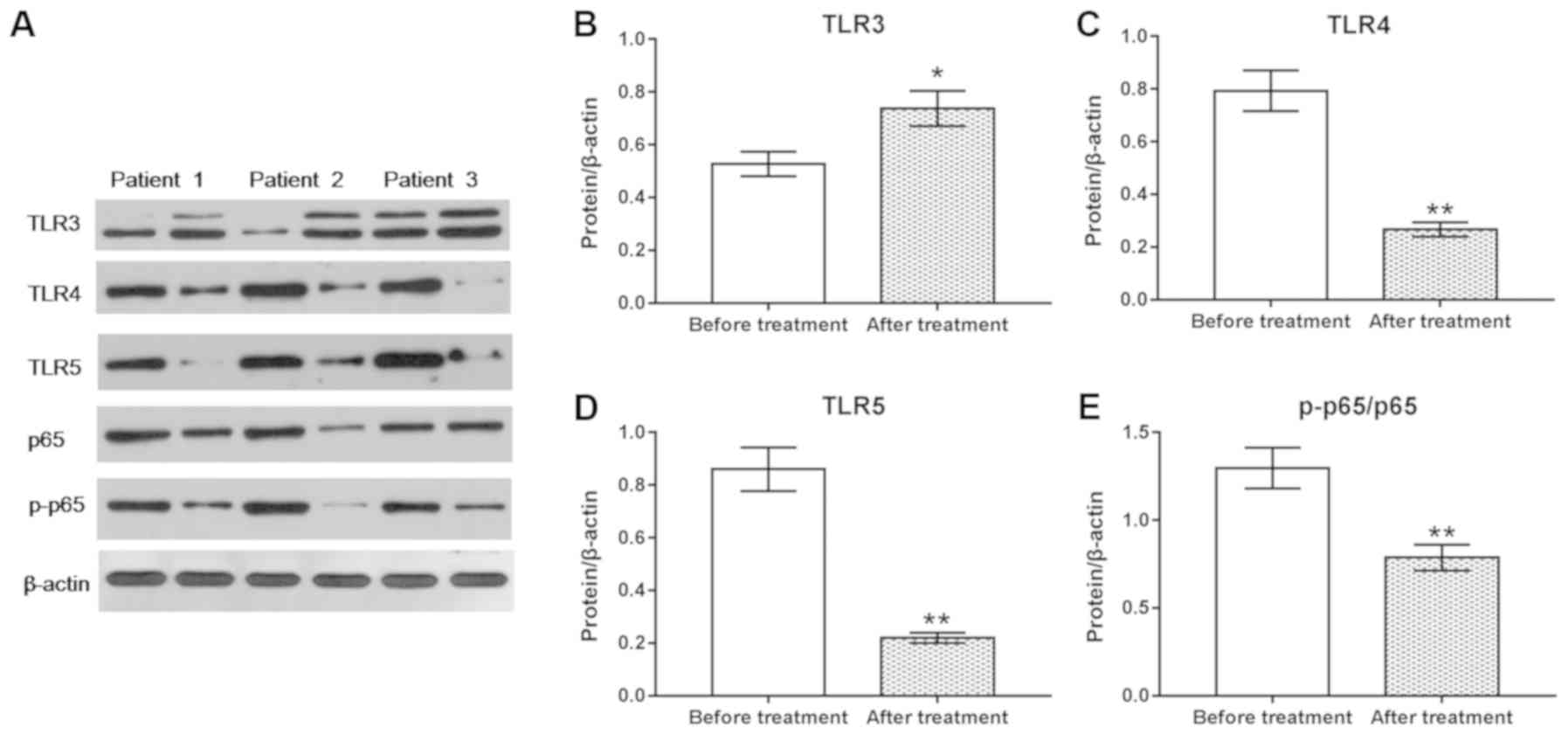
TNF-α regulates the expression of TLRs
and NF-κB signaling in PBMCs
To investigate the effects of TNF-α and anti-TNF-α
on PBMCs, the expression of TLRs and the activation of NF-κB
signaling were determined via Western blotting. It was revealed
that treatment with TNF-α significantly upregulated the expression
of TLR4 and TLR5 protein, and the phosphorylation of p65 compared
with the control, whereas it downregulated the expression of TLR3
and p65 (P<0.05, Fig. 3A-E).
Conversely, anti-TNF-α treatment significantly downregulated the
expression of TLR4 and TLR5, and the phosphorylation of p65; the
expression levels of TLR3 and p65 protein were markedly unaltered
following anti-TNF-α treatment.
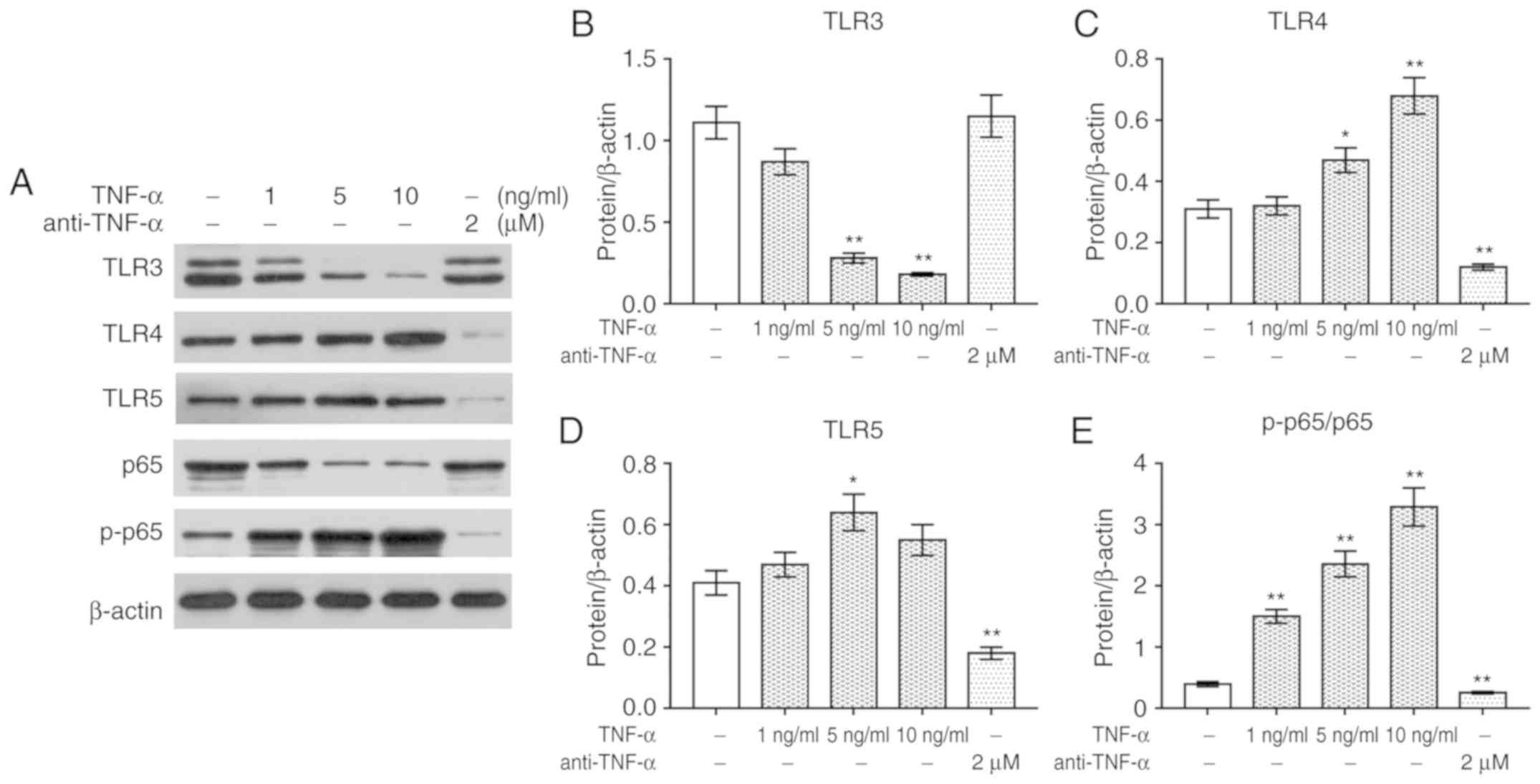 | Figure 3.TNF-α mediates TLR expression and
NF-κB p65 signaling in PBMCs. (A) Western blotting was performed to
detect the protein levels of TLR3, TLR4, TLR5, p65 and p-p65.
Protein expression of (B) TLR3, (C) TLR4, (D) TLR5 and (E)
p-p65/p65 presented as bar diagrams. β-actin was used as an
internal control. *P<0.05, **P<0.01 vs. control. NF-κB,
nuclear factor-κB; p-, phosphorylated; PBMC, peripheral blood
mononuclear cell; TLR, Toll-like receptor; TNF-α, tumor necrosis
factor-α. |
Upregulated expression of IL-10 and
TLR3, and downregulated expression of IL-6, TNF-α, CRP, TLR4 and
TLR5 following the treatment of patients with AS
An ELISA was conducted to investigate alterations in
the serum expression levels of inflammatory cytokines and TLRs
following the treatment of patients with AS. It was revealed that
the levels of IL-6, TNF-α and CRP were significantly reduced
following treatment with infliximab compared with prior to
treatment, whereas those of IL-10 were increased (P<0.05;
Fig. 4A-D). Additionally, RT-qPCR
analysis demonstrated that treatment with infliximab significantly
upregulated the expression of TLR3 mRNA compared with levels prior
to treatment, whereas the expression levels of TLR4 and TLR5 mRNA
were downregulated (P<0.05; Fig.
4E). The mRNA expression levels of the other TLRs were not
significantly altered following treatment.
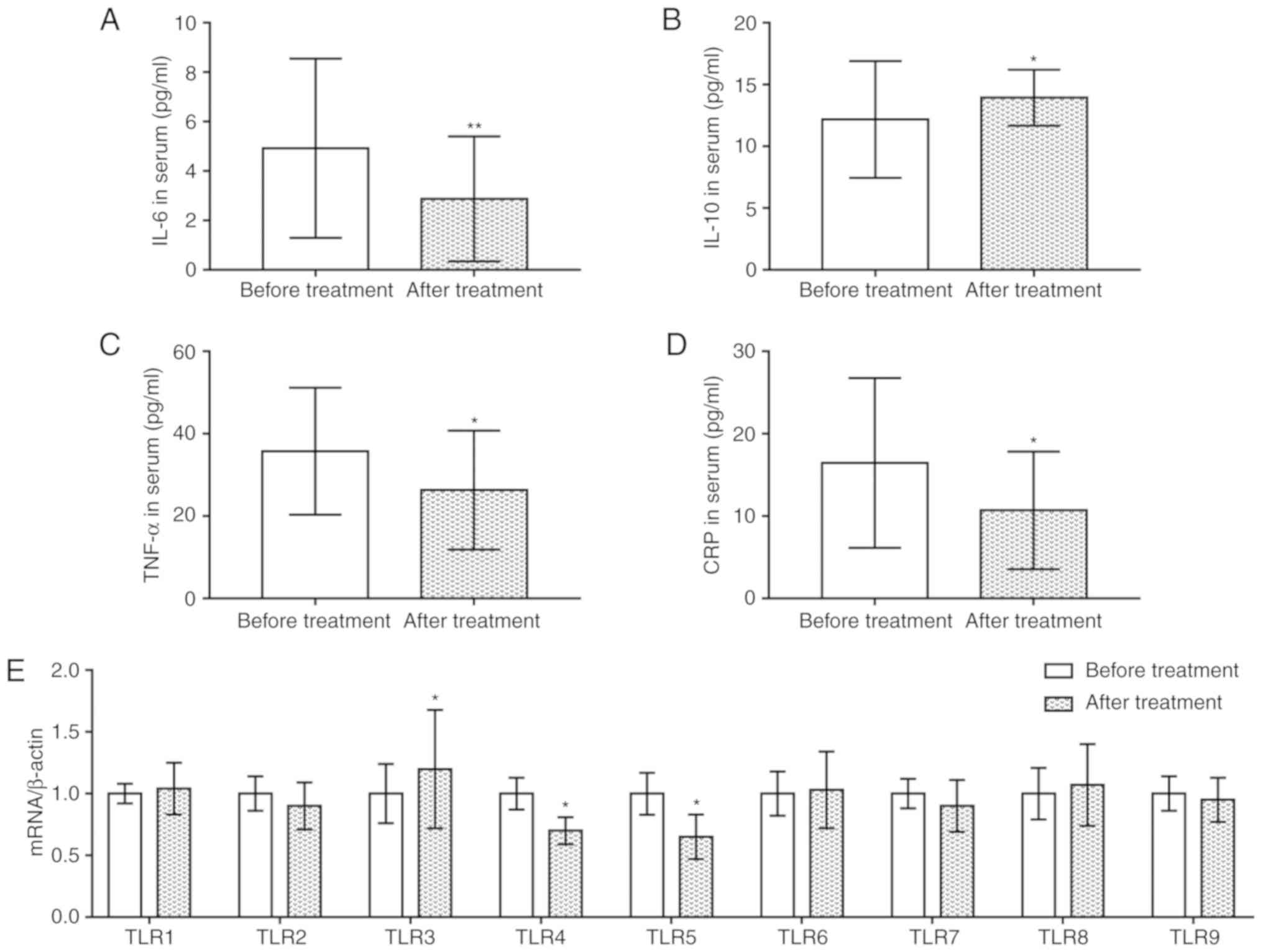 | Figure 4.Upregulation of IL-10 and TLR3, and
downregulation of IL-6, TNF-α, CRP, TLR4 and TLR5 following the
treatment of patients with AS using infliximab. Serum levels of (A)
IL-6, (B) IL-10, (C) TNF-α and (D) CRP in patients with AS before
and after treatment, as determined via ELISA. (E) mRNA levels of
TLRs were detected via reverse transcription-quantitative PCR.
*P<0.05, **P<0.01 vs. before treatment. AS, ankylosing
spondylitis; CRP, C-reactive protein; IL, interleukin; TLR,
Toll-like receptor; TNF-α, tumor necrosis factor-α. |
Upregulated expression of TLR3, and
downregulated expression of TLR4, TLR5, p65 and p-p65 following
treatment of patients with AS
To further investigate the protein expression of
TLRs and NF-κB p65 signaling prior to and following treatment of
patients with AS, western blotting was performed. It was revealed
that TLR3 protein expression was significantly upregulated
(P<0.05; Fig. 5A and B),
whereas the protein levels of TLR4 and TLR5, and phosphorylation of
p65 were significantly downregulated following treatment
(P<0.01; Fig. 5A and C-E),
compared with prior to treatment.
Discussion
AS is a rheumatic disease characterized by
inflammation (1), of which
cytokines are important mediators. It has been hypothesized that
alterations in cell networks are important features of the
pathology of AS (27). Cytokines
can be divided into pro- and anti-inflammatory cytokines, depending
on their function during inflammatory responses. Proinflammatory
cytokines include TNF-α, IL-1, IL-6, IL-8, interferon (IFN)-γ,
macrophage migration inhibitory factors, IL-17A and IL-23 (28,29),
whereas anti-inflammatory cytokines include IL-2, IL-10, IL-13,
transforming growth factor-β (TGF-β) and IL-35 (30–32).
CRP is the first detected inflammatory acute phase reaction
protein, and its levels are closely associated with the degree of
inflammation in the disease. CRP is notably upregulated during
inflammation (33,34). In the present study, increased
levels of IL-6, TNF-α and CRP, and decreased levels of IL-10 were
observed in patients with AS, compared with in healthy subjects.
TNF-α is an important proinflammatory factor mainly produced by
mononuclear macrophages, and is involved in a series of immune
responses (35). A previous study
suggested that TNF-α was important for the inflammatory response to
AS (36). In the present study, it
was revealed that TNF-α inhibitor attenuated the inflammatory
response of PBMCs in patients with AS by suppressing IL-6
expression and promoting IL-10 expression. Data concerning the
effects of TNF-α inhibition on the expression of CRP will enable
the development of more firm conclusions. Furthermore, analysis of
the levels of additional inflammatory factors, including IL-1,
IL-13 and TGF-β, will be considered to further investigate the
inflammatory response to AS.
TLRs serve an important role in the interaction
between the immune system and pathogens, which are similar to the
effects of nucleotide-binding oligomerization domain-containing
protein 2 on diabetic nephropathy disease (37). Following activation, TLRs induce
specific gene expression via cell signal transduction, promoting
the secretion of cytokines and chemokines (38–40).
TLR4, a ligand of LPS, hyaluronic acid and heat shock protein, can
promote the production of TNF-α, IFN, IL-12 and more
proinflammatory factors, inducing inflammatory damage (41). Kragstrup et al (42) reported that TLR2 and TLR4-induced
IL-19 dampened immune reactions, and was inversely associated with
spondyloarthritides (SpA) disease activity. Assassi et al
(43) reported that TLR4 and TLR5
levels were upregulated in the plasma of patients with AS.
Similarly, elevated expression levels of TLR4 and TLR5 were
observed in patients with AS in the present study, compared with in
healthy subjects. De Rycke et al (44) reported that TLR2 and TLR4
expression was enhanced in patients with SpA. Conversely, in the
present study, TLR2 expression was not significantly different in
patients with AS compared with in controls. Furthermore, in
comparison with healthy controls, no significant changes in the
expression of TLR1 or TLR6-9 were observed. Additionally, TLR3
expression was downregulated in patients. Therefore, TLR3, TLR4 and
TLR5 were selected for subsequent investigation. It was then
revealed that pomalidomide significantly promoted TLR3 expression,
and inhibited the expression of TLR4 and TLR5 in PBMCs obtained
from patients with AS. Therefore, it was hypothesized that TLR4 and
TLR5 may promote the progression of AS, whereas TLR3 may suppress
the progression of AS. Inhibitors of TLR4, including VGX-1027 and
Eritoran, have been widely associated with several immune diseases
(45–47). It was proposed that the inhibitors
may also serve an important role in AS treatment; thus, an in-depth
study involving the use of TLR4 inhibitors, such as the
anti-retroviral protease inhibitor Saquinavir, in PBMCs and
patients with AS that are resistant to standard treatment, is
planned for the future.
As an anti-TNF-α drug, infliximab is widely used in
clinical treatment of various inflammatory diseases, including AS
(48–50). In the present study, the levels of
inflammatory factors and TLRs were evaluated in patients with AS
prior to and following infliximab treatment. It was observed that
following infliximab treatment, the inflammatory response in
patients was reduced, as determined by increased levels of IL-6,
TNF-α and CRP, and enhanced levels of IL-10. Furthermore, TLR3
expression was upregulated, whereas the expression of TLR4 and TLR5
was downregulated following infliximab treatment. The findings were
consistent with observations in PBMCs. It should be noted that
infliximab exhibits certain side effects, including dyspnea,
flushing, headache, rash, abdominal pain, diarrhea, back pain,
chest pain and nausea (51–53).
NF-κB is a key transcriptional regulator in the
inflammatory response, and serves an important role in the
development of AS (18,54). TLRs are the potential catalyst for
activation of the NF-κB pathway, which has been reported to be
involved in the occurrence of inflammation (55–57).
Previous studies have demonstrated that β-D-mannuronic acid
inhibited the activity of AS by blocking the TLR2/4/NF-κB pathway
(55,56). Zhao et al (58) reported that astragaloside protected
myocardial cells against cell apoptosis by suppressing the
TLR4/NF-κB pathway. Therefore, the expression of NF-κB pathway in
patients with AS, and PBMCs from these patients. It was revealed
that TNF-α inhibitor decreased the p-p65/p65 ratio in PBMCs from
patients. Additionally, infliximab reduced the phosphorylation of
p65/p65 in patients with AS. The findings suggested that the NF-κB
pathway was involved in the progression of AS; more specifically,
the NF-κB pathway was suppressed when the progression of AS was
blocked by infliximab.
In conclusion, the findings of the present study
revealed that TNF-α inhibitor suppressed inflammatory responses in
AS, increased TLR3 expression, and suppressed the expression of
TLR4 and TLR5, and NF-κB signaling. These observations indicated
that TLRs and the NF-κB pathway contributed to the regulation of
the inflammatory response during AS. These findings provided novel
insight for the potential inhibition of the development of AS. It
was hypothesized that TLR4 and TLR5 may promote the progression of
AS, and that TLR3 may suppress the progression of AS by suppressing
NF-κB signaling; however, this hypothesis requires further
validation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ made substantial contributions to conception and
design. RX, LW and JJ conducted data acquisition, and data analysis
and interpretation. JZ drafted the article and critically revised
it for important intellectual content. All authors gave final
approval of the version to be published, and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All patients provided written informed consent, and
all experiments were approved by the Ethics Committee of Mingzhou
Hospital of Zhejiang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen D, He J, Lu C, Zhou J, Fang K, Liu X
and Xu L: Increased expression of T cell immunoglobulin and mucin
domain 4 is positively associated with the disease severity of
patients with ankylosing spondylitis. Inflammation. 38:935–940.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akkoc N and Khan MA: Overestimation of the
prevalence of ankylosing spondylitis in the Berlin study: Comment
on the article by Braun et al. Arthritis Rheum.
52:4048–4050. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ng SC, Liao Z, Yu DT, Chan ES, Zhao L and
Gu J: Epidemiology of spondyloarthritis in the People's Republic of
China: Review of the literature and commentary. Semin Arthritis
Rheum. 37:39–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taurog JD, Chhabra A and Colbert RA:
Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med.
374:2563–2574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sari I, Öztürk MA and Akkoc N: Treatment
of ankylosing spondylitis. Turk J Med Sci. 45:416–430. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martins NA, Furtado GE, Campos MJ, Leitão
JC, Filaire E and Ferreira JP: Exercise and ankylosing spondylitis
with New York modified criteria: A systematic review of controlled
trials with meta-analysis. Acta Reumatol Port. 39:298–308.
2014.PubMed/NCBI
|
|
7
|
Mau W, Zeidler H, Mau R, Majewski A,
Freyschmidt J, Stangel W and Deicher H: Clinical features and
prognosis of patients with possible ankylosing spondylitis. Results
of a 10-year followup. J Rheumatol. 15:1109–1114. 1988.PubMed/NCBI
|
|
8
|
Husseinzadeh N and Davenport SM: Role of
toll-like receptors in cervical, endometrial and ovarian cancers: A
review. Gynecol Oncol. 135:359–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim KH and Staudt LM: Toll-like receptor
signaling. Cold Spring Harb Perspect Biol. 5:a0112472013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huggins T, Haught JC, Xie S, Tansky CS,
Klukowska M, Miner MC and White DJ: Quantitation of endotoxin and
lipoteichoic acid virulence using toll receptor reporter gene. Am J
Dent. 29:321–327. 2016.PubMed/NCBI
|
|
11
|
Shimizu M: Multifunctions of dietary
polyphenols in the regulation of intestinal inflammation. J Food
Drug Anal. 25:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abhyankar MM, Orr MT, Lin S, Suraju MO,
Simpson A, Blust M, Pham T, Guderian JA, Tomai MA, Elvecrog J, et
al: Adjuvant composition and delivery route shape immune response
quality and protective efficacy of a recombinant vaccine for
entamoeba histolytica. NPJ Vaccines. 3:222018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takakubo Y, Barreto G, Konttinen YT, Oki H
and Takagi M: Role of innate immune sensors, TLRs, and NALP3 in
rheumatoid arthritis and osteoarthritis. J Long Term Eff Med
Implants. 24:243–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zauner D, Quehenberger F, Hermann J,
Dejaco C, Stradner MH, Stojakovic T, Angerer H, Rinner B and
Graninger WB: Whole body hyperthermia treatment increases
interleukin 10 and toll-like receptor 4 expression in patients with
ankylosing spondylitis: A pilot study. Int J Hyperthermia.
30:393–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gómez R, Castro A, Martínez J,
Rodríguez-García V, Burgués O, Tarín JJ and Cano A: Receptor
activator of nuclear factor Kappa B (RANK) and clinicopathological
variables in endometrial cancer: A study at protein and gene level.
Int J Mol Sci. 19(pii): E18482018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang JH, Lee E, Lee B, Cho WK, Ma JY and
Park KI: Ethanolic extracts of Artemisia apiacea hance
improved atopic dermatitis-like skin lesions in vivo and suppressed
TNF-alpha/IFN-gamma(−)induced proinflammatory chemokine production
in vitro. Nutrients. 10(pii): E8062018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang L, Liu J, Wan L, Zhu F, Tan B and
Zhang P: Xinfeng capsule improves hypercoagulative state by
inhibiting miR-155/NF-κB signaling pathway in patients with active
ankylosing spondylitis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
32:1094–1098. 2016.(In Chinese). PubMed/NCBI
|
|
19
|
Noort AR, Tak PP and Tas SW: Non-canonical
NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde?
Arthritis Res Ther. 17:152015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shostak K and Chariot A: EGFR and NF-κB:
Partners in cancer. Trends Mol Med. 21:385–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Misra RS, Bhattacharya S, Huyck HL, Wang
JC, Slaunwhite CG, Slaunwhite SL, Wightman TR, Secor-Socha S, Misra
SK, Bushnell TP, et al: Flow-based sorting of neonatal lymphocyte
populations for transcriptomics analysis. J Immunol Methods.
437:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muller GW, Chen R, Huang SY, Corral LG,
Wong LM, Patterson RT, Chen Y, Kaplan G and Stirling DI: Amino-
substituted thalidomide analogs: Potent inhibitors of TNF-alpha
production. Bioorg Med Chem Lett. 9:1625–1630. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoy SM: Pomalidomide: A review in relapsed
and refractory multiple myeloma. Drugs. 77:1897–1908. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang YT, Cheng CC, Chiu TH and Lai PC:
Therapeutic potential of thalidomide for gemcitabine-resistant
bladder cancer. Int J Oncol. 47:1711–1124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rezaiemanesh A, Abdolmaleki M,
Abdolmohammadi K, Aghaei H, Pakdel FD, Fatahi Y, Soleimanifar N,
Zavvar M and Nicknam MH: Immune cells involved in the pathogenesis
of ankylosing spondylitis. Biomed Pharmacother. 100:198–204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicoletti F, Zaccone P, Di Marco R,
Lunetta M, Magro G, Grasso S, Meroni P and Garotta G: Prevention of
spontaneous autoimmune diabetes in diabetes-prone BB rats by
prophylactic treatment with antirat interferon-gamma antibody.
Endocrinology. 138:281–288. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zaky DS and El-Nahrery EM: Role of
interleukin-23 as a biomarker in rheumatoid arthritis patients and
its correlation with disease activity. Int Immunopharmacol.
31:105–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Wakkad A, Hassan Nel M, Sibaii H and
El-Zayat SR: Proinflammatory, anti-inflammatory cytokines and
adiponkines in students with central obesity. Cytokine. 61:682–687.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Urushima H, Nishimura J, Mizushima T,
Hayashi N, Maeda K and Ito T: Perilla frutescens extract
ameliorates DSS-induced colitis by suppressing proinflammatory
cytokines and inducing anti-inflammatory cytokines. Am J Physiol
Gastrointest Liver Physiol. 308:G32–G41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou C, Wu Q, Ouyang C and Huang T: Effects
of an intravitreal injection of interleukin-35-expressing plasmid
on pro-inflammatory and anti-inflammatory cytokines. Int J Mol Med.
38:713–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji X, Zhou P, Zhong L, Xu A, Tsang ACO and
Chan PKL: Smart surgical catheter for C-reactive protein sensing
based on an imperceptible organic transistor. Adv Sci (Weinh).
5:17010532018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Y, Potempa LA, El Kebir D and Filep JG:
C-reactive protein and inflammation: Conformational changes affect
function. Biol Chem. 396:1181–1197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frasca D and Blomberg BB: Inflammaging
decreases adaptive and innate immune responses in mice and humans.
Biogerontology. 17:7–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rusman T, Ten Wolde S, Euser SM, van der
Ploeg T, van Hall O and van der Horst-Bruinsma IE: Gender
differences in retention rate of tumor necrosis factor alpha
inhibitor treatment in ankylosing spondylitis: A retrospective
cohort study in daily practice. Int J Rheum Dis. 21:836–842. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shang J, Zhang Y, Jiang Y, Li Z, Duan Y,
Wang L, Xiao J and Zhao Z: NOD2 promotes endothelial-to-mesenchymal
transition of glomerular endothelial cells via MEK/ERK signaling
pathway in diabetic nephropathy. Biochem Biophys Res Commun.
484:435–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lalancette-Hebert M, Faustino J,
Thammisetty SS, Chip S, Vexler ZS and Kriz J: Live imaging of the
innate immune response in neonates reveals differential TLR2
dependent activation patterns in sterile inflammation and
infection. Brain Behav Immun. 65:312–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Satoh T and Akira S: Toll-like receptor
signaling and its inducible proteins. Microbiol Spectr. Dec
4–2016.(Epub ahead of print). doi:
10.1128/microbiolspec.MCHD-0040-2016. PubMed/NCBI
|
|
40
|
Sugitharini V, Pavani K, Prema A and Berla
Thangam E: TLR-mediated inflammatory response to neonatal pathogens
and co-infection in neonatal immune cells. Cytokine. 69:211–217.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: Critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kragstrup TW, Andersen T, Holm C,
Schiøttz-Christensen B, Jurik AG, Hvid M and Deleuran B: Toll-like
receptor 2 and 4 induced interleukin-19 dampens immune reactions
and associates inversely with spondyloarthritis disease activity.
Clin Exp Immunol. 180:233–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Assassi S, Reveille JD, Arnett FC, Weisman
MH, Ward MM, Agarwal SK, Gourh P, Bhula J, Sharif R, Sampat K, et
al: Whole-blood gene expression profiling in ankylosing spondylitis
shows upregulation of Toll-like receptor 4 and 5. J Rheumatol.
38:87–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Rycke L, Vandooren B, Kruithof E, De
Keyser F, Veys EM and Baeten D: Tumor necrosis factor alpha
blockade treatment down-modulates the increased systemic and local
expression of toll-like receptor 2 and Toll-like receptor 4 in
spondylarthropathy. Arthritis Rheum. 52:2146–2158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fagone P, Muthumani K, Mangano K, Magro G,
Meroni PL, Kim JJ, Sardesai NY, Weiner DB and Nicoletti F: VGX-1027
modulates genes involved in lipopolysaccharide-induced Toll-like
receptor 4 activation and in a murine model of systemic lupus
erythematosus. Immunology. 142:594–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stojanovic I, Cuzzocrea S, Mangano K,
Mazzon E, Miljkovic D, Wang M, Donia M, Al Abed Y, Kim J, Nicoletti
F, et al: In vitro, ex vivo and in vivo immunopharmacological
activities of the isoxazoline compound VGX-1027: Modulation of
cytokine synthesis and prevention of both organ-specific and
systemic autoimmune diseases in murine models. Clin Immunol.
123:11–323. 2007. View Article : Google Scholar
|
|
47
|
Younan P, Ramanathan P, Graber J, Gusovsky
F and Bukreyev A: The Toll-like receptor 4 antagonist eritoran
protects mice from lethal filovirus challenge. MBio. 8(pii):
e00226–17. 2017.PubMed/NCBI
|
|
48
|
Codreanu C, Šírová K, Jarosova K and
Batalov A: Assessment of effectiveness and safety of biosimilar
infliximab (CT-P13) in a real-life setting for treatment of
patients with active rheumatoid arthritis or ankylosing
spondylitis. Curr Med Res Opin. 34:1763–1769. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Osswald D, Rameau AC, Speeg-Schatz C,
Terzic J and Sauer A: Clinical and epidemiological profile of
pediatric uveitis, course of inflammatory uveitis treated with
anti-TNF alpha. J Fr Ophtalmol. 41:447–452. 2018.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Siljehult F, Ärlestig L, Eriksson C and
Rantapää-Dahlqvist S: Concentrations of infliximab and anti-drug
antibodies in relation to clinical response in patients with
rheumatoid arthritis. Scand J Rheumatol. 47:345–350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wasserman MJ, Weber DA, Guthrie JA, Bykerk
VP, Lee P and Keystone EC: Infusion-related reactions to infliximab
in patients with rheumatoid arthritis in a clinical practice
setting: Relationship to dose, antihistamine pretreatment, and
infusion number. J Rheumatol. 31:1912–1917. 2004.PubMed/NCBI
|
|
52
|
Kim BY and Kim HS: Phlegmonous gastritis
in an ankylosing spondylitis patient treated with infliximab.
Korean J Intern Med. 32:945–946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Maksymowych WP: Ankylosing spondylitis.
Not just another pain in the back. Can Fam Physician. 50:257–262.
2004.PubMed/NCBI
|
|
54
|
Dong M, Yu D, Duraipandiyan V and Abdullah
Al-Dhabi N: The protective effect of chrysanthemum indicum extract
against ankylosing spondylitis in mouse models. Biomed Res Int.
2017:82062812017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aletaha S, Haddad L, Roozbehkia M, Bigdeli
R, Asgary V, Mahmoudi M and Mirshafiey A: M2000 (β-D-Mannuronic
Acid) as a novel antagonist for blocking the TLR2 and TLR4
downstream signalling pathway. Scand J Immunol. 85:122–129. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Roozbehkia M, Mahmoudi M, Aletaha S,
Rezaei N, Fattahi MJ, Jafarnezhad-Ansariha F, Barati A and
Mirshafiey A: The potent suppressive effect of β-d-mannuronic acid
(M2000) on molecular expression of the TLR/NF-kB signaling pathway
in ankylosing spondylitis patients. Int Immunopharmacol.
52:191–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu GJ, Lin YW, Chuang CY, Tsai HC and Chen
RM: Liver nitrosation and inflammation in septic rats were
suppressed by propofol via downregulating TLR4/NF-κB-mediated iNOS
and IL-6 gene expressions. Life Sci. 195:25–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao Y, Liu Z and Zhang H: Astragaloside
protects myocardial cells from apoptosis through suppression of the
TLR4/NF-κB signaling pathway. Exp Ther Med. 15:1505–1509.
2018.PubMed/NCBI
|