Introduction
Liver ischemia-reperfusion (I/R) injury is a common
clinical issue that occurs as a consequence of liver
transplantation, hepatic resection and trauma (1,2).
Compared with pure ischemia, liver I/R injury can result in more
serious liver damage and even liver failure after reperfusion. A
large number of studies have shown that the mechanism of liver I/R
injury involves complex and multiple pathways, including oxidative
stress, inflammation and cell apoptosis (3–5).
Recent studies have found that gastrin-releasing peptide (GRP) and
the GRP receptor, bone marrow mesenchymal stem cells,
cobalt-protoporphyrin and glycogen synthase kinase 3β are also
involved in liver I/R injury (6–8). In
addition, various approaches have been reported towards the
prevention or attenuation of liver I/R injury, including ischemic
preconditioning (IPC), antioxidant preconditioning, pharmaceutical
preconditioning and a gene targeting approach (9). However, effective strategies and
drugs for liver I/R injury are still lacking. Therefore,
elucidating the possible molecular mechanism underlying liver I/R
injury is necessary for the sake of developing effective drugs and
approaches.
In recent years, several studies have suggested that
endoplasmic reticulum stress (ERS) plays a critical role in the
progression of liver IR injury (10,11).
ERS, also known as the unfolded protein response, contributes to
the protection of cells against toxic stimuli or cellular
stress-induced accumulation of misfolded proteins (12,13).
Under ERS, the BiP (GRP78) chaperone binds to misfolded proteins to
generate an adaptive mechanism by activating a series of signaling
pathways such as PERK, ATF6 and IRE1α (14,15).
However, excessive ERS can lead to cell damage or death by
activating pro-apoptotic factors, including the C/EBP homologous
protein (CHOP) and caspase-12 (16,17).
Thus, inhibiting ERS may provide novel insight into the treatment
of liver I/R injury (18).
MicroRNAs are a class of small non-coding RNAs that
bind to the 3′UTR (untranslated region) of target mRNAs, thereby
regulating target gene expression (19–21).
Several reports have suggested that microRNAs (miRs) can contribute
to liver I/R injury by the regulation of several key signaling
pathways (22–25). miR-27a is a member of the
miR-23a~27a~24-2 cluster, and plays a crucial role in cell survival
and death (26–28). In addition, it has been
demonstrated that peroxisome proliferator-activated receptor γ
(PPARγ) is a target of miR-27a, and PPARγ is a therapeutic target
for liver I/R injury (29–32). Recently, miR-27a-5p was found to
have protective effects against liver I/R-induced apoptosis by
targeting Bach1 (33). However,
many aspects of the molecular mechanisms underlying the effect of
miR-27a in liver I/R injury remain largely unknown, and whether
this effect is associated with PPARγ and ERS needs to be further
clarified. Therefore, in this study, we aimed to investigate the
effect and relevant molecular mechanism of miR-27a in response to
liver I/R injury in vitro and in vivo. Our study may
provide new insight into the development of novel therapeutic
strategies for clinical interventions to reduce liver I/R
injury.
Materials and methods
Materials and reagents
miR-27a mimics, inhibitors, PPARγ siRNA and matched
negative control (NC) were synthesized by GenePharma, Shanghai,
China: miR-27a mimics (5′-AGGGCUUAGCUGCUUGUGAGCA-3′ and
3′-CUCACAAGCAGCUAAGCCCUUU-5′); miR-27a mimic NC
(5′-UUUGUACUACACAAAAGUACUG-3′ and 3′-AAACAUGAUGUGUUUUCAUGAC-5′);
miR-27a inhibitor (5′-UGCUCACAAGCAGCUAAGCCCU-3′); miR-27a inhibitor
NC (5′-CAGUACUUUUGUGUAGUACAAA-3′); PPARγ siRNA
(5′-AATATGACCTGAAGCTCCAAGAATAAG-3′); siRNA NC
(5′-GAGGCGGACTAATATCTAACAACAAAT-3′). Malondialdehyde (MDA) and
superoxide dismutase (SOD) commercial kits were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu,
China). Annexin V-FITC/propidium iodide (PI) apoptosis kit was
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). PPARγ (cat. no. sc-271392; dilution 1:200), GRP78 (cat. no.
sc-13539; dilution 1:200), CHOP (cat. no. sc-7351; dilution 1:200)
and GAPDH (cat. no. sc-66163; dilution 1:200) antibodies were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Secondary antibodies used in the western blot analysis included
Alexa Fluor Plus 800 anti-rabbit IgG (H+L) (cat. no. A32735;
dilution 1:1,000) and Alexa Fluor Plus 800 anti-mouse IgG (H+L)
(cat. no. A32730; dilution 1:1,000). A dual luciferase reporter
assay kit was purchased from Promega (Madison, WI, USA).
Cell culture and transfection
AML12 cells, which were obtained from the Cell Bank
of the Chinese Academy of Sciences, were cultured in Dulbecco's
modified Eagle's medium (DMEM, Gibco; Thermo Fisher Scientific,
Inc.) that was supplemented with 10% fetal bovine serum (FBS,
Hyclone, South Logan, UT, USA) plus 1% penicillin and streptomycin
(Thermo Fisher Scientific, Inc.). The cells were cultured in a
humidified atmosphere containing 5% CO2. at 37°C. AML12
cells were seeded into 6-well plates and transfected with miR-27a
mimics, miR-27a mimic NC, miR-27a inhibitor, or miR-27a inhibitor
NC using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. For gene
silencing, 50 nm PPARγ siRNA or siRNA NC was transfected into AML12
cells using Lipofectamine 2000 according to the manufacturer's
instructions. Subsequent experiments including H/R, RNA/protein
extraction and apoptosis analysis were performed 24 h after miRNA
transfection.
In vitro hypoxia/reoxygenation (H/R)
model
Briefly, AML12 cells were firstly perfused in normal
Hank's solution with a gas mixture of 95% O2−5%
CO2. at 37°C, at pH 7.4. To simulate ischemia, the
Hank's solution was switched to pH 7.4 at 37°C without glucose or
calcium and then the cells were aerated with a gas mixture of 95%
N2−5% CO2. for 4 h. To simulate reperfusion,
the cells were again treated with normal Hank's solution with a gas
mixture of 95% O2−5% CO2. at 37°C at pH 7.4.
Cells under normoxia throughout the experiments were included as a
control.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was determined by the CCK-8 assay
kit (Dojindo, Kumamoto, Japan). Briefly, 10 µl CCK-8 reagent was
added to transfected AML2 cells at 0, 2, 6, 12 and 24 h,
respectively and incubated for 2 h. The absorbance was detected at
450 nm. Each group was analyzed in triplicate.
Flow cytometry
The Annexin V-FITC/PI apoptosis detection kit was
used to determine the cell apoptosis, according to the
manufacturer's instructions. After transfection, cells were
harvested and resuspended in 200 µl binding buffer. Then, the cells
were incubated with 10 µl Annexin V-FITC and 5 µl PI in the dark
for 15 min. The stained cells were analyzed by flow cytometry (FACS
Calibur, BD Biosciences, San Jose, CA, USA).
Luciferase reporter assay
The predicted 3′UTR sequences of PPARγ that
interacts with miR-27a and its mutated sequences within the
predicted target sites were synthesized and inserted into the
pRL-TK control vector. AML12 cells were transfected with 120 ng of
miR-27a mimics, miR-27a inhibitors or the negative control (NC),
followed by co-transfection with 30 ng of the wild-type or mutant
3′UTR of PPARγ using 0.45 µl of Fugene (Promega). The luciferase
assay was carried out on cell extracts 24 h post-transfection, and
the signals were measured using the Dual-Luciferase Assay System.
The pRL-TK expressing Renilla luciferase was co-transfected
as an internal control (29). The
relative luciferase activity (RLA) was calculated as the ratio of
firefly luciferase activity (Ff) to Renilla luciferase
activity (Rn).
Animals and experimental design
Male Sprague-Dawley (SD) rats (weight, 150–200 g;
age, 8 weeks) were purchased from Shanghai SLAC Laboratory Animal
Co., Ltd. Rats were housed under appropriate conditions (25±2°C and
12-h light/dark cycle) with free access to water and food before
the experiment. All of the animal procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals and approved by the Fuzhou General Hospital for
Accreditation of Laboratory Animal Care.
The rats were randomly divided into four groups (n=8
per group) as follows: i) Sham group; ii) I/R group; iii) I/R +
miR-NC group and iv) I/R + miR-27a antagomir group. The I/R group
was anesthetized by an intraperitoneal injection of 3 ml/kg chloral
hydrate. The liver hilum was subsequently exposed, and the portal
structures of the left and median lobes were occluded. After 60 min
of ischemia, the clamp was removed, and the liver was reperfused
for 6 h. The sham group underwent abdominal surgery without liver
ischemia/reperfusion. In the miR-27a antagomir group, the rats were
given the miR-27a antagomir (20 µl of 500 pmol miR-27a
antagomir/day) by intraperitoneal injection for 7 days before
ischemia. Rats in the I/R + miR-NC group were given an equal amount
of miR-NC.
After the surgery, the animals were immediately
sacrificed. The serum was prepared and stored at −80°C until the
biochemical assays, and the left liver tissues were used for
biochemical analyses, real-time PCR and western blot analysis.
ALT/AST assessment
Serum alanine transaminase (ALT) and aspartate
transaminase (AST) levels were determined using a commercial
reagent kit (Jiancheng Bioengineering Institute, Nanjing, China)
according to the manufacturer's protocol.
H&E staining and TUNEL assay
Liver tissues were fixed in 10% formalin for 24 h,
dehydrated and embedded in paraffin. The liver sections were
subsequently cut from each paraffin-embedded tissue and were
stained with hematoxylin and eosin (H&E) to evaluate the degree
of liver damage. The sections were imaged under a microscope
(Olympus, Tokyo, Japan). TUNEL staining was performed using a
commercial reagent kit (Beyotime Biotechnology) according to the
protocols in the manual. The nuclei were stained with DAPI to
assess nuclear morphology. All of the slices were imaged with a
microscope (Olympus, Tokyo, Japan; magnification, ×100).
Real-time PCR (qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and was reverse
transcribed into cDNA using the PrimeScriptRT reagent kit (Takara,
Tokyo, Japan). The expression of miRNA was quantified using the
SYBR Green PCR Master Mix (Takara). The qPCR protocol was 95°C for
5 min followed by 40 cycles of 95°C for 30 sec and 60°C for 1 min
conducted in the Step One Real-Time PCR System (Applied Biosystems,
Warrington, UK). The following primers were synthesized by Sangon
Biotech (Shanghai, China) and were used in the PCR: miR-27a-F,
5′-TTCACAGTGGCTAAG-3′ and miR-27a-R, 5′-CCAGTGCAGGGTCCGAGGT-3′;
U6-F, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and U6-R,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The relative expression of miR-27a
was determined in relation to U6 by the 2−ΔΔCq method
(34).
Western blot analysis
Total proteins were extracted using lysis buffer
(Beyotime), and the protein concentration was quantified with the
BCA protein kit (Beyotime). Equal amounts of protein (10 µg total
protein/well) were mixed with the SDS loading buffer and boiled for
5 min at 100°C. The proteins were resolved on a 10% SDS-PAGE gel
for 30 min at 80 V and then at 120 V for 1 h. The proteins were
transferred onto an NC membrane for 60 min at 250 ma. The NC
membranes were subsequently blocked with 5% skim milk at room
temperature for 2 h and incubated with primary antibodies at 4°C
overnight. The membranes were washed with TBST thrice for 5 min and
incubated with the corresponding secondary antibodies for 1 h.
Protein expression was imaged using the Odyssey Infrared Imaging
System (Lincoln, NE, USA). Protein levels were measured using the
Quantity One software (version 4.62; Bio-ra Bio-Rad Laboratories,
Inc.).
Statistical analysis
Experiments were performed in triplicate, and the
data are expressed as the mean ± standard deviation (SD). The
differences between two groups was analyzed using the Student's
t-test. The differences among multiple groups were determined by
ANOVA followed by Student-Newman-Keuls post hoc test in GraphPad
Prism 5 (GraphPad Software, Inc.) P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-27a upon liver I/R
injury
To investigate the potential effect of miR-27a on
liver I/R injury, the I/R-induced liver injury model was
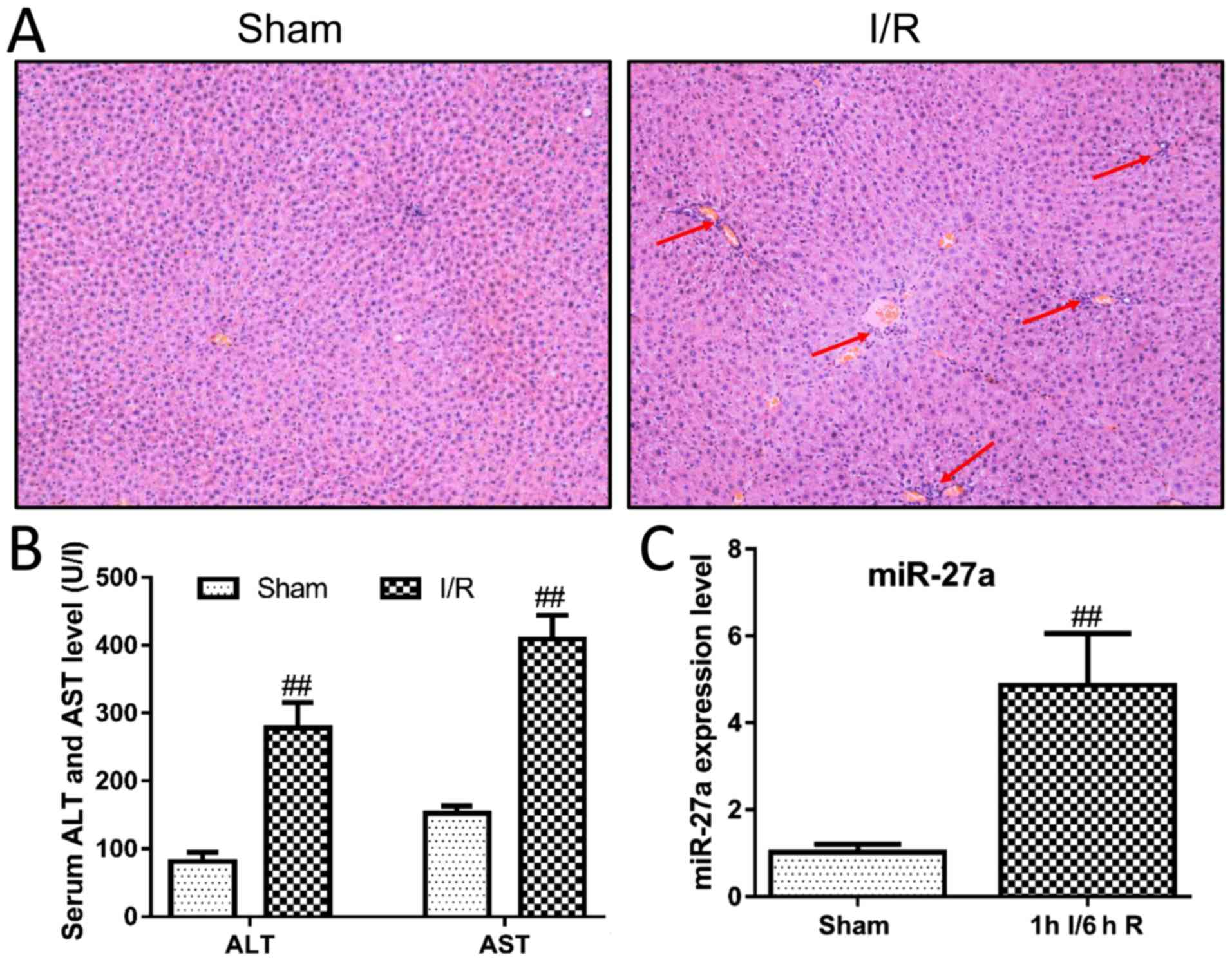
constructed in rats. As shown in Fig.
1A, H&E staining of livers revealed large areas of
hepatocyte necrosis and inflammatory infiltration in the I/R group.
Moreover, the serum levels of ALT and AST were significantly
increased after liver I/R surgery compared with the sham group
(Fig. 1B). These results suggest
that we successfully constructed a rat model of I/R in this study.
We then examined the expression levels of miR-27a in the I/R model.
As shown in Fig. 1C, miR-27a in
the I/R group was significantly upregulated at least 5-fold when
compared with the sham group. These findings indicate that miR-27a
may play an important role in I/R-induced liver injury.
Effects of miR-27a on H/R-induced
hepatocyte injury
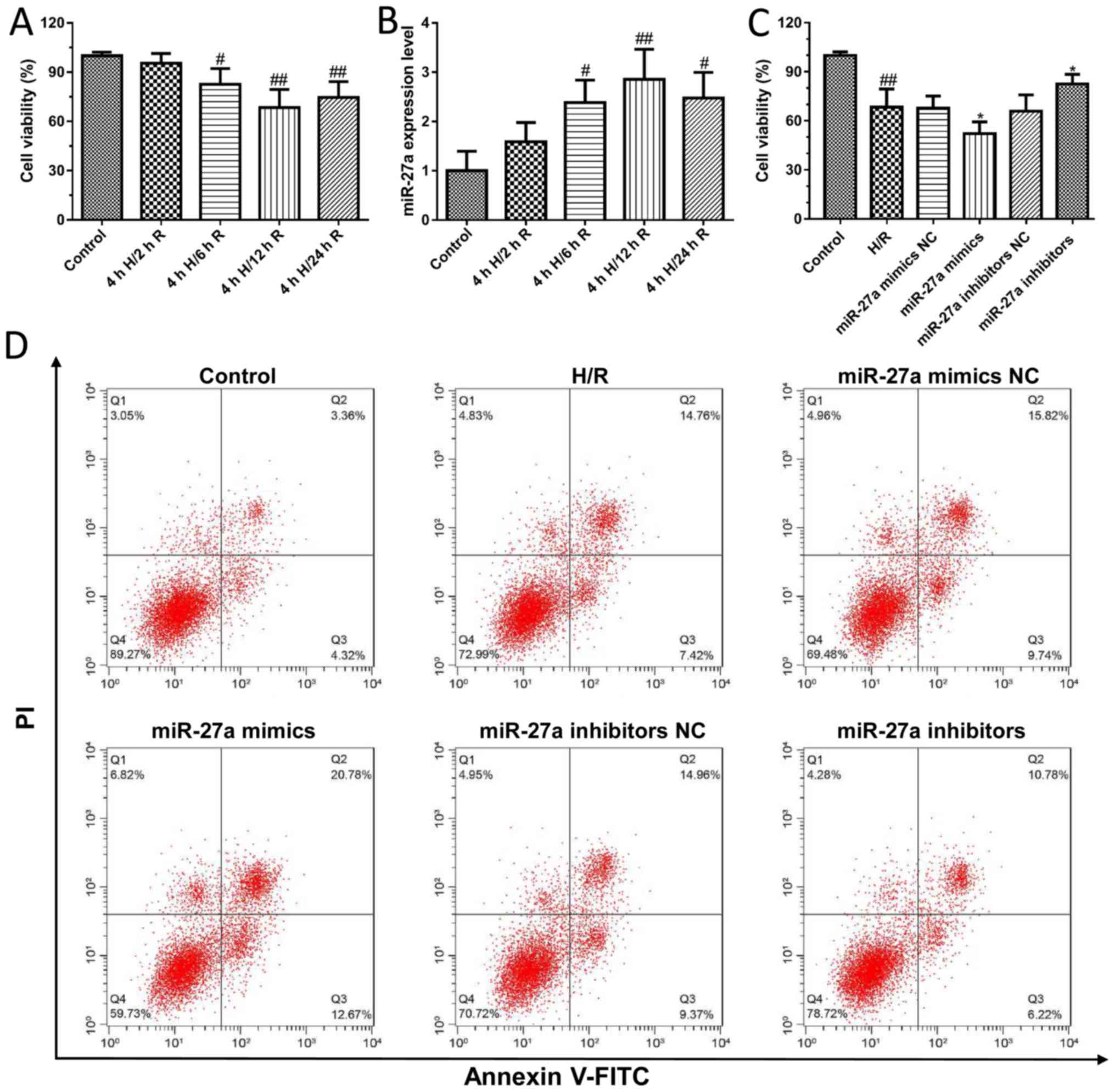
To further confirm the effect of miR-27a on liver
I/R injury, an H/R-induced cell model in AML12 cells was
established. As shown in Fig. 2A,
the viability of AML12 cells was significantly decreased after H/R
treatment compared to the control group. In addition, we found that
the expression of miR-27a was significantly increased in a
time-dependent manner after H/R treatment compared with control
cells (Fig. 2B). Furthermore,
AML12 cells were transfected with miR-27a mimics and inhibitors,
and the cell viability was detected using the CCK-8 kit. We first
detected the effects of miR-27a mimics and inhibitors on the
expression of miR-27a by qPCR. The results found that miR-27a
mimics significantly increased the expression of miR-27a, and the
miR-27a inhibitor decreased the expression of miR-27a, which showed
that transfection of the miR-27a mimics and inhibitors was
successfully carried out (Fig.
S1). The results showed that miR-27a inhibitors significantly
increased cell viability compared with what was observed in the
control group. In contrast, the cell viability was significantly
decreased following treatment with miR-27a mimics (Fig. 2C). In addition, we detected their
effect on cell apoptosis by flow cytometry. Compared with the
control group, the apoptotic cells were markedly increased in the
H/R group, which was effectively attenuated with the miR-27a
inhibitor or significantly aggravated by miR-27a mimics (Fig. 2D). These results suggest that
suppression of miR-27a may exert a protective effect against liver
I/R injury.
Effects of miR-27a on H/R-induced
oxidative stress and ERS
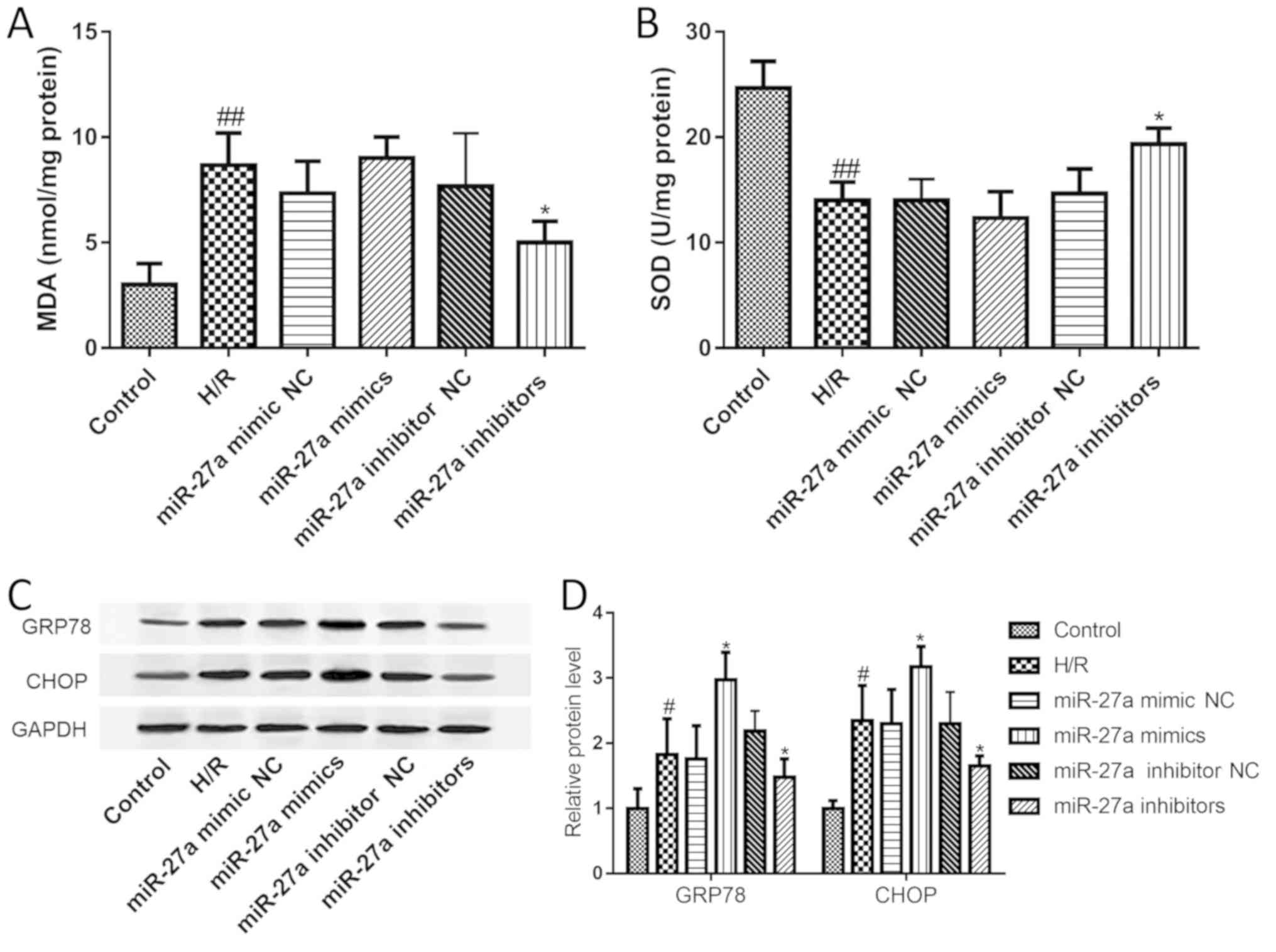
A large number of studies have suggested that
oxidative stress and ERS play an important role in the development
of liver I/R injury. First, the MDA and SOD levels were examined to
investigate the effect of miR-27a on oxidative stress. The results
showed that compared with the control group, MDA levels were
significantly elevated, while SOD was significantly decreased.
However, these changes were restored with the miR-27a inhibitor
(Fig. 3A and B). Furthermore, the
expression of GRP78 and CHOP was assessed to study the effect of
miR-27a on ERS. As shown in Fig. 3C
and D, the expression levels of GRP78 and CHOP were both
significantly increased after H/R treatment. As expected, the
expression levels of GRP78 and CHOP were also significantly
decreased with miR-27a inhibitor treatment or further increased by
treatment with miR-27a mimics. Our data suggest that miR-27a
regulates oxidative stress and ERS signaling in H/R-induced
hepatocyte injury.
PPARγ is a potential target gene of
miR-27a
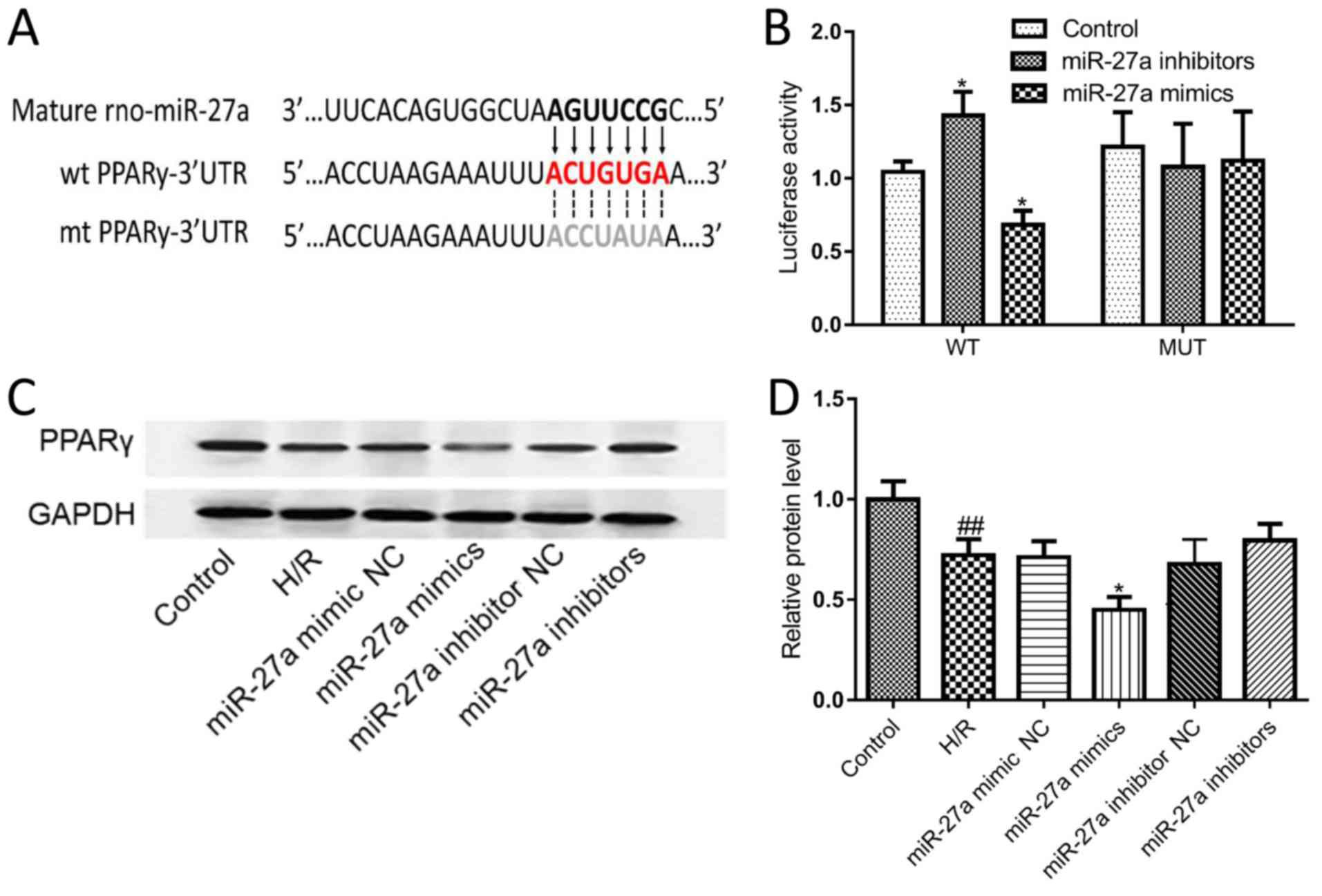
Bioinformatic analysis revealed that the 3′UTR of
PPARγ mRNA contains one binding site for miR-27a; thus, PPARγ is
one of the candidate target genes of miR-27a (Fig. 4A). To validate this prediction, the
PPARγ luciferase reporter gene assay was used to analyze the
relationship between miR-27a and PPARγ. The results showed that
miR-27a mimics significantly suppressed the luciferase activity of
the 3′UTR of wild-type (WT) PPARγ, which had no effect on the
mutated (MUT) 3′UTR of the PPAR-γ transfected group. By contrast,
the miR-27a inhibitor markedly increased the luciferase activity in
WT-transfected cells (Fig. 4B).
Furthermore, the expression of the PPARγ protein was detected by
western blot analysis. As shown in Fig. 4C and D, the expression of PPARγ was
significantly downregulated after transfection with the miR-27a
mimic, indicating a negative association between miR-27a and PPARγ
expression. Taken together, these results indicate that PPARγ is a
target gene of miR-27a.
miR-27a regulates ERS by PPARγ
To verify that miR-27a regulates ERS via PPARγ,
PPARγ siRNA was used to determine the effect of miR-27a on the ERS
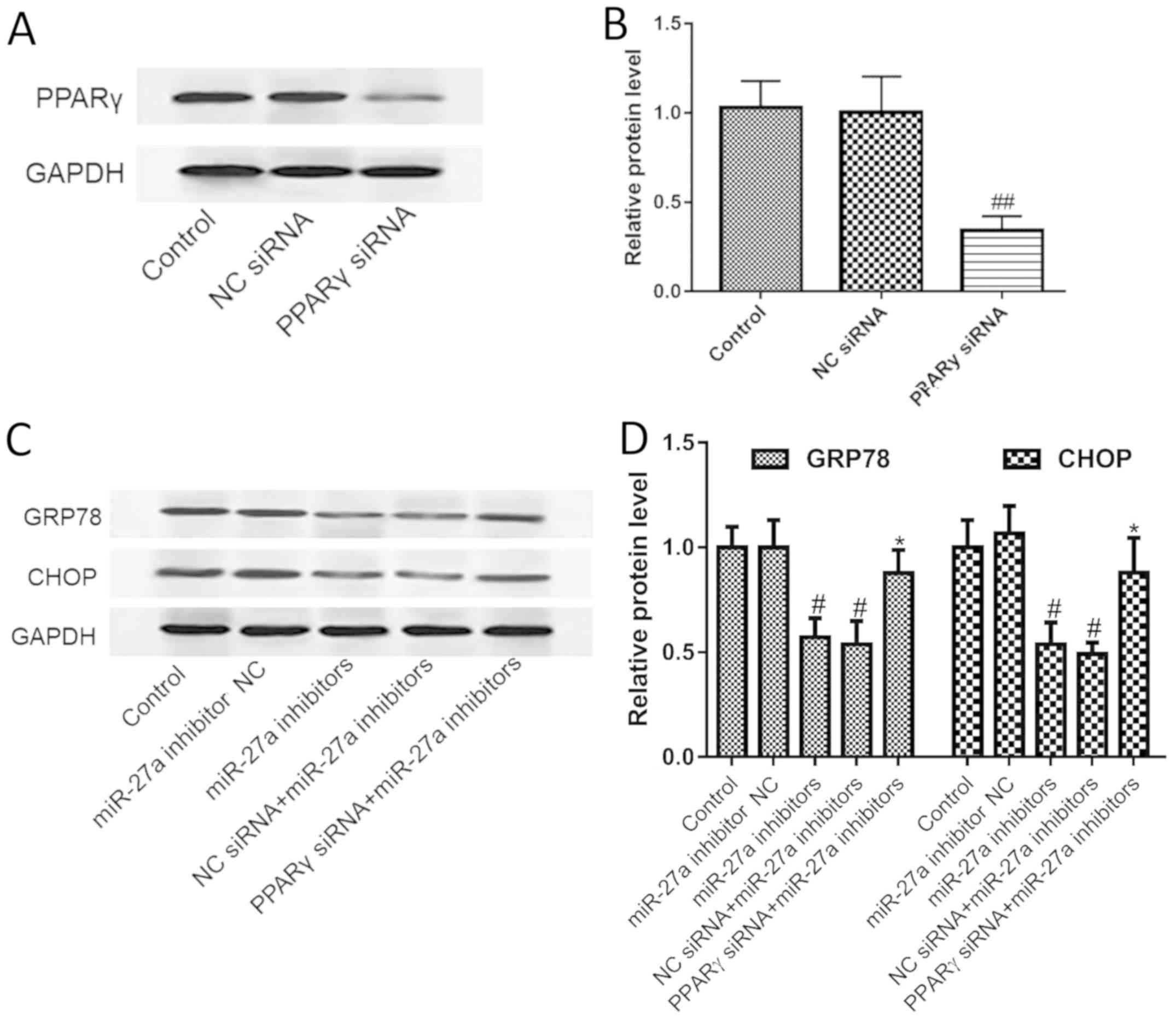
pathway. We first tested the effect of PPARγ siRNA on PPARγ
expression, and the results showed that PPARγ siRNA could
significantly silence the expression of PPARγ (Fig. 5A and B). Furthermore, we found that
silencing of PPARγ significantly blocked the inhibitory effect of
the miR-27a inhibitor on the ERS pathway, as indicated by the
increased expression of CHOP and GRP78 (Fig. 5C and D). The results suggest that
miR-27a regulates the ER stress pathway by targeting PPARγ.
Suppression of miR-27a protects
against liver I/R injury in rats
To study the therapeutic effect of miR-27a on liver
I/R injury in vivo, the I/R rats were treated with the
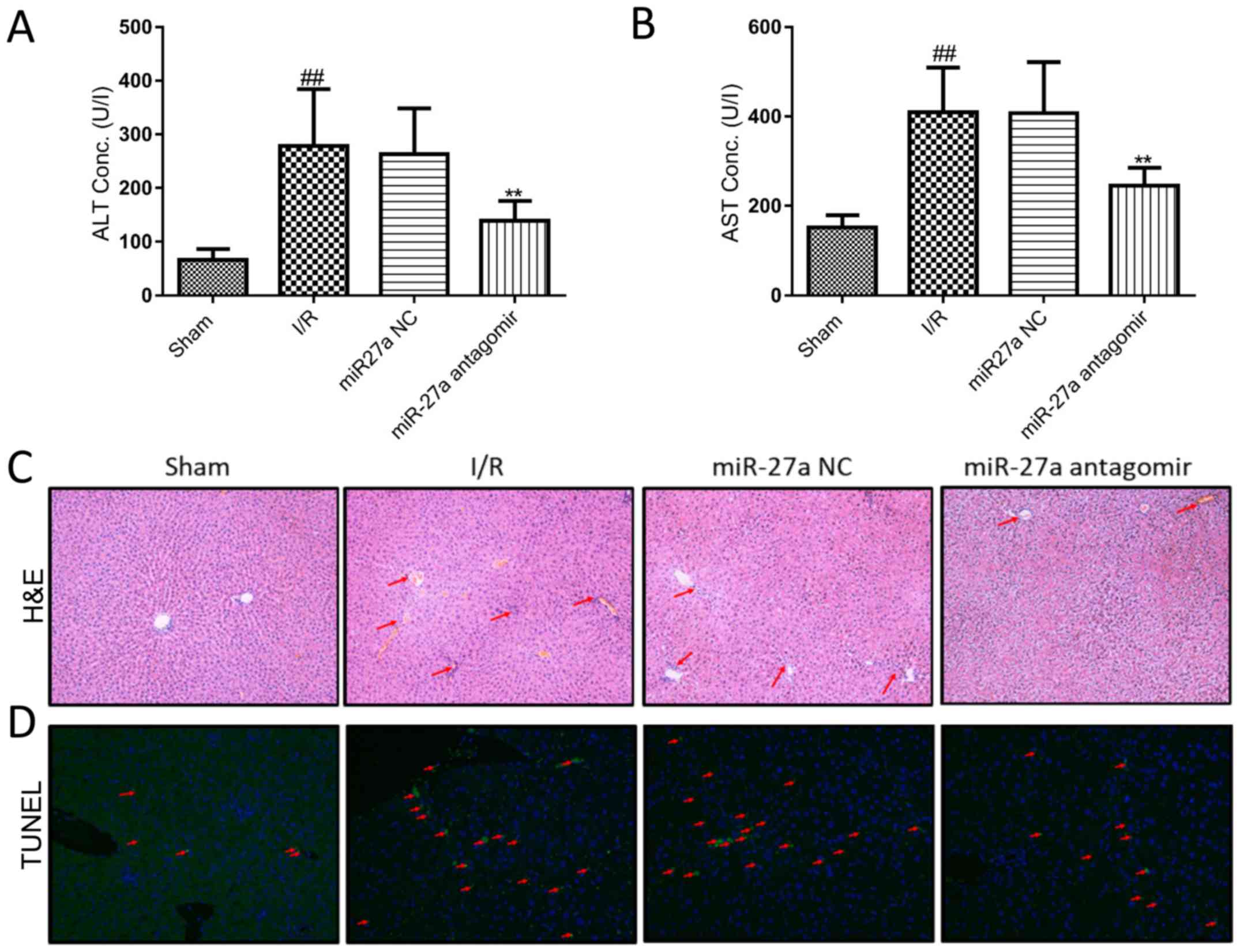
miR-27a antagomir and miR-NC by intraperitoneal injections. As
shown in Fig. 6A and B, serum ALT
and AST levels in the miR-27a antagomir group were significantly
decreased, compared with the miR-NC group. Moreover, the liver
histology of H&E staining showed an obvious reduction in
I/R-induced hepatocellular necrosis and an improvement in cell
integrity in the miR-27a antagomir group (Fig. 6C). In addition, the TUNEL results
demonstrated that miR-27a antagomir markedly decreased I/R-induced
apoptosis (Fig. 6D). These results
suggest that the miR-27a antagomir effectively alleviated liver I/R
injury in rats.
Suppression of miR-27a inhibits
I/R-induced oxidative stress and ERS in rats
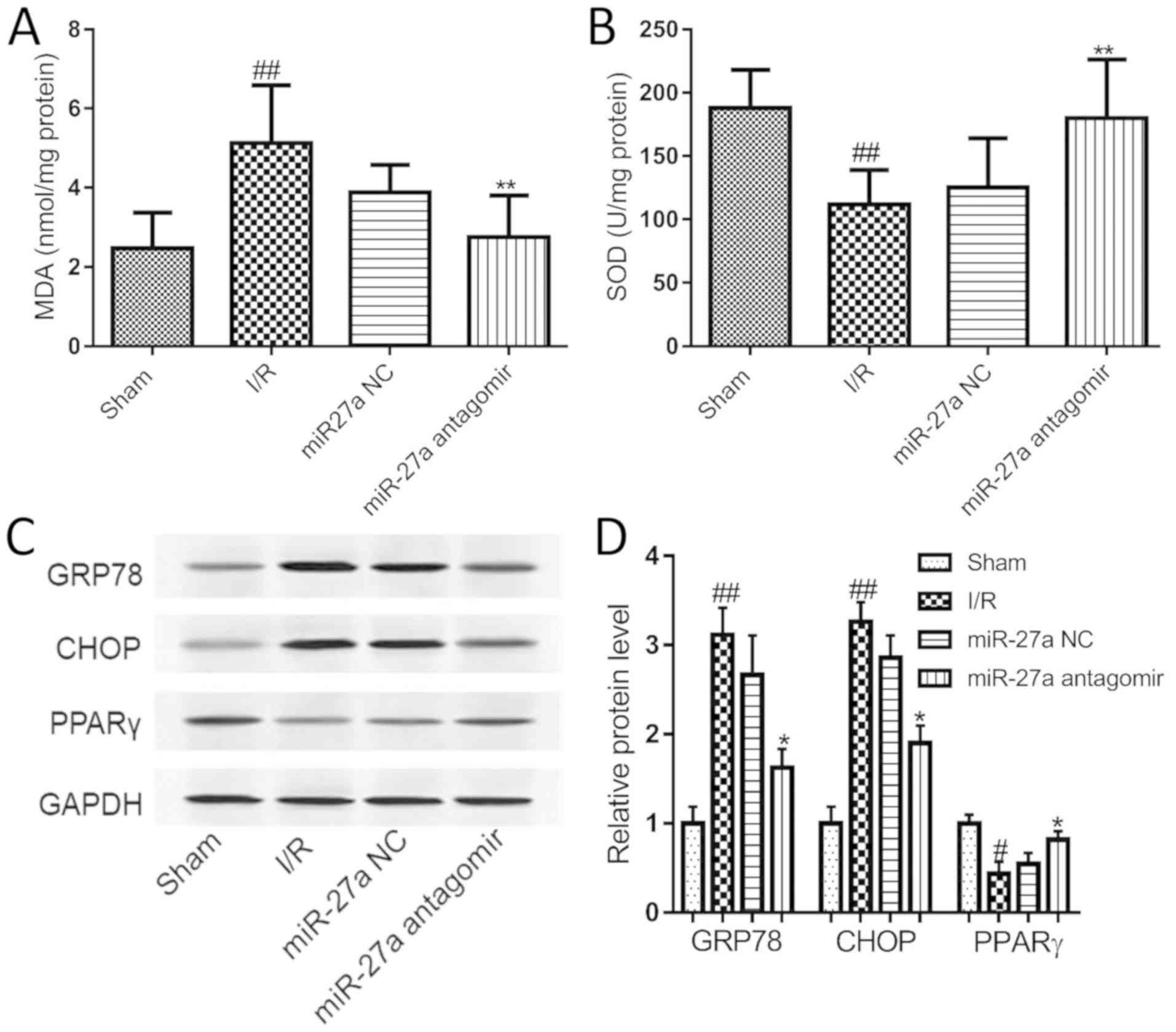
We further explored the molecular mechanism
underlying the effect of miR-27a in I/R rats. Compared with the
sham group, I/R significantly elevated the level of MDA, but
miR-27a antagomir treatment significantly inhibited I/R-induced
elevation of MDA in the liver (Fig.
7A). Meanwhile, the activity of SOD was decreased in
I/R-exposed livers, and this decrease was significantly increased
by miR-27a antagomir treatment (Fig.
7B). Furthermore, the expression of CHOP, GRP78 and PPARγ was
examined in rat liver. As shown in Fig. 7C and D, the PPARγ level was
decreased in the I/R rats, and the expression of CHOP and GRP78 was
significantly increased compared with the sham group. After miR-27a
antagomir treatment, the PPARγ level in the rat liver was
significantly upregulated, and CHOP and GRP78 was significantly
reduced compared to the miR-NC group. This finding indicated that
the inhibition of miR-27a protected the rat liver against I/R
injury by regulating oxidative stress and the ERS signaling
pathway.
Discussion
Liver ischemia-reperfusion (I/R) injury is one of
the major issues during liver surgery and greatly affects surgical
outcomes. Recent studies suggest that miRNAs play an important role
in liver I/R injury, and miRNAs have become potential molecular
targets for therapeutic intervention (35–38).
In the present study, it was demonstrated that inhibition of
miR-27a protects the liver against I/R injury by targeting PPARγ
and inhibiting the endoplasmic reticulum stress (ERS) pathway in
vitro and in vivo.
In the present study, a model of liver I/R injury
was successfully constructed in rats according to the findings from
a previous study (7), as evidenced
by the elevated serum activities of ALT/AST and extensive
hepatocyte necrosis. Importantly, it was found that miR-27a was
markedly increased at least 5-fold in the liver I/R group when
compared with the sham group. To investigate the potential role of
miR-27a in liver I/R injury, a hypoxia/reoxygenation (H/R)-induced
cell model in hepatic AML12 cells was used to simulate liver I/R
injury in vitro. The results showed that the expression of
miR-27a was time-dependent and was increased in hepatic AML12 cells
during H/R, a finding that was consistent with the in vivo
results. Furthermore, it was also found that miR-27a inhibitors
significantly improved proliferation and decreased apoptosis in the
H/R-exposed AML12 cells when compared with the NC group. These
results indicate that the suppression of miR-27a exerted a
protective role against H/R-induced hepatocyte injury in AML12
cells.
Oxidative stress is an important factor that leads
to liver I/R injury (39,40). In this study, miR-27a inhibitors
reduced MDA content and enhanced SOD activity. This trend was
reversed by miR-27a mimics. Since SOD is an antioxidant enzyme that
acts against superoxide, increased SOD activity resulted in
decreased oxidative stress. Previous research has also shown that
miR-27a-induced cell apoptosis was associated with the ERS
signaling pathway in 293T cells. The ERS pathway is an important
signaling pathway in the regulation of cell survival and apoptosis
(5). Studies have shown that
inhibition of the ERS pathway can significantly prevent I/R-induced
cell apoptosis (41–43). To validate the possible role of
miR-27a in ERS in liver I/R injury, the expression of GRP78 and
CHOP (the main signaling pathways of ERS) was detected. In line
with the findings in previous studies (15), the protein levels of GRP78 and CHOP
were increased during liver I/R. Our study showed that the
expression levels of GRP78 and CHOP were significantly decreased by
the miR-27a inhibitor or were further increased by treatment with
miR-27a mimics. These results suggest that the suppression of
miR-27a exerted a protective role against H/R-induced hepatocyte
injury, which may inhibit oxidative stress and the ERS pathway.
Next, the target gene of miR-27a was investigated.
The results of our bioinformatic analysis revealed that PPARγ is
one of the target genes of miR-27a. Furthermore, a dual-luciferase
reporter assay also confirmed that miR-27a specifically targeted
the 3′UTR of the PPARγ gene, findings that are consistent with
those in previous studies (43,44).
In addition, in vitro experiments showed that the protein
expression of PPARγ was markedly decreased after treatment with the
miR-27a mimics and was increased after treatment with the miR-27a
inhibitor in vitro, further confirming PPARγ as a downstream
target of miR-27a. Several reports have suggested that PPARγ plays
an important role in the regulation of ERS and cell apoptosis
(45,46). To confirm that miR-27a regulates
the ERS pathway through PPARγ, PPARγ was knocked down using PPARγ
siRNA. The results showed that knockdown of PPARγ significantly
abrogated the inhibitory effect of the miR-27a inhibitor on the ERS
pathway. Taken together, these findings indicate that miR-27a
increased ERS by negatively regulating PPARγ, and PPARγ is a target
gene of miR-27a.
To study the therapeutic effect of miR-27a on liver
I/R injury in vivo, we treated I/R rats with
antagomir-miR-27a by intraperitoneal injection. It was found that
the miR-27a antagomir alleviated liver I/R injury as evidenced by
lower serum ALT/AST levels and improved liver morphology and
histology. In addition, I/R increased the number of TUNEL-positive
cells compared with what was observed in normal rats, while miR-27a
antagomir pretreatment decreased this trend. Furthermore, oxidative
stress and the ERS pathway were evaluated to explore the molecular
mechanism of miR-27a. The results demonstrated that miR-27a
antagomir treatment significantly decreased MDA content and
increased SOD activity. Moreover, our study showed that the miR-27a
antagomir reduced CHOP and GRP78 and increased PPARγ expression.
These results suggest that the suppression of miR-27a effectively
alleviated liver I/R injury by regulating oxidative stress and ERS
in vivo.
In conclusion, the persent study demonstrated that
miR-27a mediates liver I/R injury by oxidative stress and ERS, and
the suppression of miR-27a protects against liver I/R injury.
Therefore, miR-27a inhibitors have therapeutic potential for liver
I/R injury and warrant further research interest. Macrophage
infiltration plays a critical role in the pathogenesis of liver I/R
injury and inflammation response (47), thus the effect of miR-27a on the
inflammatory response and macrophage infiltration will be
investigated in future research.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by Key Projects of the
Logistics Department of the Army (grant no. CNJ15J002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and YJ conceived and designed the study. XC, YC,
FY, QC and FP performed the experiments. XZ and LL analyzed the
data. XC and LL wrote the paper. XC, XZ and YJ reviewed and edited
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All of the animal procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals and approved by the Fuzhou General Hospital for
Accreditation of Laboratory Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhai Y, Petrowsky H, Hong JC, Busuttil RW
and Kupiec-Weglinski JW: Ischaemia-reperfusion injury in liver
transplantation-from bench to bedside. Nat Rev Gastroenterol
Hepatol. 10:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Irie T, Ito K, Ozasa H, Noda Y, Ikeda S,
Tanaka S, Arii S and Horikawa S: Splenic artery ligation: A
protection against hepatic ischemia/reperfusion injury in partially
hepatectomized rats. Hepatol Res. 42:819–827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhai Y, Busuttil RW and Kupiec-Weglinski
JW: Liver ischemia and reperfusion injury: New insights into
mechanisms of innate-adaptive immune-mediated tissue inflammation.
Am J Transplant. 11:1563–1569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lentsch AB, Kato A, Yoshidome H, McMasters
KM and Edwards MJ: Inflammatory mechanisms and therapeutic
strategies for warm hepatic ischemia/reperfusion injury.
Hepatology. 32:169–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brenner C, Galluzzi L, Kepp O and Kroemer
G: Decoding cell death signals in liver inflammation. J Hepatol.
59:583–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo L, Wu X, Zhang Y, Wang F, Li J and Zhu
J: Protective effects of gastrin-releasing peptide receptor
antagonist RC-3095 in an animal model of hepatic
ischemia/reperfusion injury. Hepatol Res. 49:247–255. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong D, Hua X, Qin T, Zhang J and He K:
Inhibition of glycogen synthase kinase 3β protects liver against
ischemia/reperfusion injury by activating 5′ adenosine
monophosphate-activated protein kinase-mediated autophagy. Hepatol
Res. 49:462–472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Wu B, Teng D, Sun X, Li J, Li J,
Zhang G and Cai J: Cobalt-protoporphyrin enhances heme oxygenase 1
expression and attenuates liver ischemia/reperfusion injury by
inhibiting apoptosis. Mol Med Rep. 17:4567–4572. 2018.PubMed/NCBI
|
|
9
|
Suyavaran A and Thirunavukkarasu C:
Preconditioning methods in the management of hepatic ischemia
reperfusion- induced injury: Update on molecular and future
perspectives. Hepatol Res. 47:31–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Ren F, Cheng Q, Bai L, Shen X, Gao
F, Busuttil RW, Kupiec-Weglinski JW and Zhai Y: Endoplasmic
reticulum stress modulates liver inflammatory immune response in
the pathogenesis of liver ischemia and reperfusion injury.
Transplantation. 94:211–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malhi H and Kaufman RJ: Endoplasmic
reticulum stress in liver disease. J Hepatol. 54:795–809. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malhotra JD and Kaufman RJ: The
endoplasmic reticulum and the unfolded protein response. Semin Cell
Dev Biol. 18:716–731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J and Kaufman RJ: From acute ER stress
to physiological roles of the unfolded protein response. Cell Death
Differ. 13:374–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Folch-Puy E, Panisello A, Oliva J, Lopez
A, Castro Benítez C, Adam R and Roselló-Catafau J: Relevance of
endoplasmic reticulum stress cell signaling in liver cold ischemia
reperfusion injury. Int J Mol Sci. 17(pii): E8072016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hammadi M, Oulidi A, Gackiere F,
Katsogiannou M, Slomianny C, Roudbaraki M, Dewailly E, Delcourt P,
Lepage G, Lotteau S, et al: Modulation of ER stress and apoptosis
by endoplasmic reticulum calcium leak via translocon during
unfolded protein response: Involvement of GRP78. FASEB J.
27:1600–1609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui FF, Pan YY, Xie HH, Wang XH, Shi HX,
Xiao J, Zhang HY, Chang HT and Jiang LP: Pressure combined with
ischemia/reperfusion injury induces deep tissue injury via
endoplasmic reticulum stress in a rat pressure ulcer model. Int J
Mol Sci. 17:2842016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peralta C and Brenner C: Endoplasmic
reticulum stress inhibition enhances liver tolerance to
ischemia/reperfusion. Curr Med Chem. 18:2016–2024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: Disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
20
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Ren J and Sun Q: The expression of
microRNA-23a regulates acute myocardial infarction in patients and
in vitro through targeting PTEN. Mol Med Rep. 17:6866–6872.
2018.PubMed/NCBI
|
|
22
|
Zheng X, Zhou H, Qiu Z, Gao S, Wang Z and
Xiao L: Gene microarray analysis of expression profiles in liver
ischemia and reperfusion. Mol Med Rep. 16:3299–3307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng W, Men H, Li J, Xing Y, Wu B, Wang
Z, Li J, Teng D, Shi Y, Li J, et al: Global MicroRNA expression
profiling of mouse livers following ischemia-reperfusion injury at
different stages. PLoS One. 11:e01486772016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu CF, Yu CH and Li YM: Regulation of
hepatic microRNA expression in response to ischemic preconditioning
following ischemia/reperfusion injury in mice. OMICS. 13:513–520.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raza A, Dikdan G, Desai KK, Shareef A,
Fernandes H, Aris V, de la Torre AN, Wilson D, Fisher A,
Soteropoulos P and Koneru B: Global gene expression profiles of
ischemic preconditioning in deceased donor liver transplantation.
Liver Transpl. 16:588–599. 2010.PubMed/NCBI
|
|
26
|
Chen Q, Xu J, Li L, Li H, Mao S, Zhang F,
Zen K, Zhang CY and Zhang Q: MicroRNA-23a/b and microRNA-27a/b
suppress Apaf-1 protein and alleviate hypoxia-induced neuronal
apoptosis. Cell Death Dis. 5:e11322014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv X, Yan J, Jiang J, Zhou X, Lu Y and
Jiang H: MicroRNA-27a-3p suppression of peroxisome
Proliferator-Activated receptor-γ contributes to cognitive
impairments resulting from sevoflurane treatment. J Neurochem.
143:306–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chhabra R, Dubey R and Saini N:
Cooperative and individualistic functions of the microRNAs in the
miR-23a~27a~24-2 cluster and its implication in human diseases. Mol
Cancer. 9:2322010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Z, Wan J, Hou X, Geng J, Li X and Bai
X: MicroRNA-27a promotes podocyte injury via PPARγ-mediated
β-catenin activation in diabetic nephropathy. Cell Death Dis.
8:e26582017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao Y, Li B and Liu J: miRNA27a regulates
arthritis via PPARγ in vivo and in vitro. Mol Med Rep.
17:5454–5462. 2018.PubMed/NCBI
|
|
31
|
Akahori T, Sho M, Hamada K, Suzaki Y,
Kuzumoto Y, Nomi T, Nakamura S, Enomoto K, Kanehiro H and Nakajima
Y: Importance of peroxisome proliferator-activated receptor-gamma
in hepatic ischemia/reperfusion injury in mice. J Hepatol.
47:784–792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuboki S, Shin T, Huber N, Eismann T,
Galloway E, Schuster R, Blanchard J, Zingarelli B and Lentsch AB:
Peroxisome proliferator-activated receptor-gamma protects against
hepatic ischemia/reperfusion injury in mice. Hepatology.
47:215–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xing Y, Li J, Li SP, Xi J, Ma N, Liu L,
Wang JS and Cai JZ: MiR-27a-5p regulates apoptosis of liver
ischemia-reperfusion injury in mice by targeting Bach1. J Cell
Biochem. 119:10376–10383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Ma D, Wang Z and Yang J:
MicroRNA-155 Deficiency in kupffer cells ameliorates liver
Ischemia-Reperfusion injury in mice. Transplantation.
101:1600–1608. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Li G, Yu C, Shen Z, Xu C, Feng Z,
Zhang X and Li Y: A role of microRNA-370 in hepatic
ischaemia-reperfusion injury by targeting transforming growth
factor-β receptor II. Liver Int. 35:1124–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang W, Liu G and Tang W: MicroRNA-182-5p
Ameliorates Liver Ischemia-Reperfusion injury by suppressing
toll-like receptor 4. Transplant Proc. 48:2809–2814. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Farid WR, Verhoeven CJ, de Jonge J,
Metselaar HJ, Kazemier G and van der Laan LJ: The ins and outs of
microRNAs as biomarkers in liver disease and transplantation.
Transpl Int. 27:1222–1232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Imarisio C, Alchera E, Bangalore Revanna
C, Valente G, Follenzi A, Trisolini E, Boldorini R and Carini R:
Oxidative and ER stress-dependent ASK1 activation in steatotic
hepatocytes and Kupffer cells sensitizes mice fatty liver to
ischemia/reperfusion injury. Free Radic Biol Med. 112:141–148.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo Y, Hu B, Huang H, Tsung A, Gaikwad NW,
Xu M, Jiang M, Ren S, Fan J, Billiar TR, et al: Estrogen
Sulfotransferase is an oxidative stress-responsive gene that
gender-specifically affects liver ischemia/reperfusion injury. J
Biol Chem. 290:14754–14764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu J, Hua X, Li D, Zhang J and Xia Q:
Rapamycin attenuates mouse liver ischemia and reperfusion injury by
inhibiting endoplasmic reticulum stress. Transplant Proc.
47:1646–1652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu R, Chen ZF, Yan J, Li QF, Huang Y, Xu
H, Zhang XP and Jiang H: Endoplasmic reticulum stress of
neutrophils is required for Ischemia/Reperfusion-Induced acute lung
injury. J Immunol. 195:4802–4809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xia JG, Xu FF, Qu Y, Song DG, Shen H and
Liu XH: Atorvastatin post-conditioning attenuates myocardial
ischemia reperfusion injury via inhibiting endoplasmic reticulum
stress-related apoptosis. Shock. 42:365–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu L, Wang Q, Guo F, Ma X, Ji H, Liu F,
Zhao Y and Qin G: MicroRNA-27a induces mesangial cell injury by
targeting of PPARγ, and its in vivo knockdown prevents progression
of diabetic nephropathy. Sci Rep. 6:260722016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu B, Ferry CH, Markell LK, Blazanin N,
Glick AB, Gonzalez FJ and Peters JM: The nuclear receptor
peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) promotes
oncogene-induced cellular senescence through repression of
endoplasmic reticulum stress. J Biol Chem. 289:20102–20119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang WX, Wang LK, Wang YQ, Zong ZJ, Gao
ZX, Liu XS, Shen YJ, Shen YX and Li YH: Peroxisome
proliferator-activated receptor-α activation protects against
endoplasmic reticulum stress-induced HepG2 cell apoptosis. Mol Cell
Biochem. 385:179–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee CM, Peng HH, Yang P, Liou JT, Liao CC
and Day YJ: C-C Chemokine Ligand-5 is critical for facilitating
macrophage infiltration in the early phase of liver
ischemia/reperfusion injury. Sci Rep. 7:36982017. View Article : Google Scholar : PubMed/NCBI
|