Introduction
The study of mesenchymal stem cells (MSCs) has
advanced considerably throughout the past few decades, and rapid
progress has been made in utilizing MSCs to study disease
pathogenesis, to discover biomarkers and novel targets, and to
validate cell-based tissue engineering therapies (1–4). In
the past few years, synovial mesenchymal stem cells (SMSCs) have
attracted increasing attention as a promising cell source for
tissue engineering as SMSCs have an intriguing multilineage
developmental plasticity in vitro and in vivo
(5–7). Synovial tissue can be obtained by
minimally invasive surgery such as arthroscopy (8), and similar to adult MSCs from other
sources, SMSCs can be isolated and expanded more efficiently in
vitro (8,9). In addition to the ability to
self-replicate and differentiate into multiple lineages, the
immune-privileged nature of SMSCs implies their potential
utilization in allogeneic cell-based settings (10,11).
Articular synovium is avascular and exists in a low oxygen
microenvironment. The oxygen tension ranges from 1 to 7% in the
knee joints of different age groups (12,13).
As oxygen tension seems to be a nonnegligible regulator of cell
proliferation and differentiation of SMSCs, it is therefore
essential to study the survival mechanism of SMSCs in a hypoxic
environment.
Hypoxia-inducible factor-1α (HIF-1α) is a
heterodimeric transcription factor that can be induced under
hypoxic condition (14,15). The stimulation and activity of
HIF-1α have been demonstrated to be mediated at different levels
throughout the cell cycle (16).
Some regulatory pathways, including Wnt, Notch, PTEN, JAK/STAT, LOX
and FBW7 are involved in regulating the metabolism, angiogenesis,
metastasis and invasion of MSCs via HIF-1α mediation (17). HIF-1α undergoes rapid degradation
under normoxic conditions with a half-life of only approximately 5
min (18). In comparison, in
hypoxic conditions, intermediate metabolites stabilize the
expression of HIF-1α by inhibiting the activity of proliferol
hydroxylase, and promotes its migration into the nucleus to combine
with HIF-1β to form HIF-1 heterodimers (19–21).
Numerous studies have demonstrated that HIF-1α participates in the
regulation of angiogenesis, cell growth and glucose metabolism
(22–24).
HIF-1α has been reported to be abundantly expressed
in synovial tissues of patients with rheumatoid arthritis,
osteoarthritis and temporomandibular joint disorders, which are
stimulated by a series of immune factors such as inflammation and
oxidative stress response (25–27).
However, the expression of HIF-1α in normal human synovial membrane
and the role of HIF-1α in synovial membrane adapting to different
oxygen environments have not been reported. In particular, the
effect of low oxygen tension on HIF-1α expression in SMSCs has not
been characterized. Therefore, in order to determine the initial
effects of HIF-1α in SMSCs, we investigated the expression of
HIF-1α in SMSCs under different oxygen conditions and observed the
effect of HIF-1α on the proliferation and apoptosis of human SMSCs
in vitro.
Materials and methods
Tissue harvest and cell culture
Synovial tissue from six patients with a spectrum of
knee conditions including ligament, meniscal, and cartilage injury
were collected by arthroscopy. Ethical approval for this study was
granted by the Institution Review Board of the Affiliated Hospital
of Nanjing Medical University, and all study participants were
recruited after providing informed written consent. SMSCs were
isolated using an enzyme digestion procedure according to a
previously described method (28).
The culture medium (DMEM/Nutrient Mixture F-12 Ham supplemented
with 20% FBS) was changed every 3 days. According to the
manufacturer's protocol, 1.0×106 SMSCs were cultured for
21 days in adipogenic medium (Adipogenic Base Media, StemXVivo;
R&D Systems) for adipogenesis detected by Oil red-O staining.
The same method was used for osteogenesis with osteogenic medium
(Osteogenic Base Media, StemXVivo; R&D Systems) and Alizarin
red staining was conducted.
Hypoxia exposure
The 2nd passage of 1.0×105 SMSCs was
cultured for 7 days under different ambient oxygen tension,
including normoxia (21% O2), hypoxia (5% O2)
or severe hypoxia (0.5% O2) at 37°C in a humidified
incubator with 5% CO2.
Cell transfection
The 2nd passage of 1.0×105 cells were
transfected with small interfering RNA (siRNA) as previously
described (29). SMSCs were placed
into 6-well plates for 24 h. Cells were transfected with specific
siRNA (Ambion; Thermo Fisher Scientific, Inc.) targeting HIF-1α
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). After transfection for 48 h, SMSCs were collected for
further analysis.
Proliferation assay
A Cell Counting Kit-8 (CCK-8) assay was used to
evaluate cell viability. Briefly, the SMSCs were collected and
seeded into 96-well plates at a dose of 5.0×103/ml.
Then, after cell culture for 1 to 7 days, 10 µl of CCK-8 solution
(Nanjing Jiancheng Biotechnology Institute) was added into each
well. Cells were cultured at room temperature for 4 h in the dark.
The absorbance was measured at 450 nm by a microplate reader
(Bio-Rad, Inc.).
Flow cytometry
To determine the phenotypes of the SMSCs, flow
cytometric analysis was used. The 2nd passage of 1.0×106
SMSCs was collected and the cells were suspended in PBS before
being incubated with the following antibodies (Agilent
Technologies, Inc.) for 90 min at 37°C: FITC-conjugated anti-human
CD147 (cat. no. SZB10284; 1:500 dilution), CD90 (cat. no.
bs-10430R; 1:200 dilution), CD105 (cat. no. bs-0579R; 1:10,000
dilution), CD44 (cat. no. K001677P; 1:500 dilution), CD117 (cat.
no. 130-098-570; 1:500 dilution), CD34 (cat. no. bs-0765R-2; 1:200
dilution), CD14 (cat. no. K101533P; 1:200 dilution) and CD45 (cat.
no. bs-10600R; 1:200 dilution). The cell phenotypes were analyzed
using an FC 500 flow cytometer (BD Biosciences).
RT-qPCR
TRIzol reagent (Thermo Fisher Scientific, Inc.) was
used to extract total RNA of the SMSCs. The target gene and an
endogenous control β-actin were amplified by qPCR using the SYBR
Green PCR kit (Takara Biotechnology Co., Ltd., Dalian, China).
GAPDH was used as an internal reference. The primers for PCR were
as follows: GLUT3 forward, 5′-CGGCTTCCTCATTACCTTC-3′ and reverse,
5′-GGCACGACTTAGACATTGG-3′; HIF-1α forward,
5′-TAAAGGAATTTCAATATTTGATGGG-3′ and reverse,
5′-AAAGGGTAAAGAACAAAACACACAG-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The thermocycling conditions were
25°C for 5 min, 42°C for 60 min and at 95°C for 15 sec. Fold
changes were calculated using the 2−ΔΔCq method
(30) normalized to GAPDH.
Western blot analysis
SMSCs were harvested on ice in PBS and centrifuged
at 1.3×104 g for 10 min. Total protein of the SMSCs was
isolated using lysis buffer (Sigma-Aldrich; Merck-Millipore) and
quantified using a bicinchoninic acid (BCA) assay (Beyotime
Biotechnology, Inc., China). Then, 20 µg protein was
electrophoresed on 10% gel with SDS-PAGE and transferred onto a
PVDF membrane (Millipore, Bedford, MA, USA). The membrane was then
blocked with 5% nonfat milk for 2 h at 4°C and was then incubated
with GLUT3 (cat. no. bs-20225R; 1:500 dilution; BIOSS), HIF-1α
(cat. no. K000487P; 1:500 dilution; Beijing Solarbio Science &
Technology Co., Ltd.), cleaved caspase3 (cat. no. 1083-10; 1:500
dilution; BioVision, Inc.), Bax (cat. no. K002397P; 1:500 dilution;
Beijing Solarbio Science & Technology Co., Ltd.) and Bcl-2
(cat. no. K003505P; 1:500 dilution; Beijing Solarbio Science &
Technology Co., Ltd.) antibodies and GAPDH antibody (cat. no.
G5262-1VL; 1:3,000 dilution; Sigma-Aldrich; Merck KGaA) overnight
at 4°C. Then, the membranes were re-incubated with secondary
antibodies (Cell Signaling Technology, Inc., USA). The signal was
visualized using a photographic developer (Invitrogen Life
Technologies, Inc., USA) and densitometry was performed using
ImageJ (version 1.25; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are reported as the means ± standard errors.
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was used for
statistical analysis. Parameters of cells under normoxic (21%
O2), hypoxic (5% O2) and severe hypoxic (0.5%
O2) conditions were determined by one-way ANOVA.
Parameters of cells between groups following siRNA-induced HIF-1α
knockdown were analyzed using a t-test. P<0.05 was considered
statistically significant.
Results
Characterization of the SMSCs
Multilateral form fibre cells and a few
spindle-shaped cells were observed among the primary cells and the
2nd passage of the SMSCs (Fig. 1A and
B). Oil red O-positive lipid droplets could be obviously
observed in the SMSCs after adipogenic induction for 3 weeks
(Fig. 1C). Similarly, Alizarin red
S staining showed that some mineralized nodules were effectively
formed in the isolated SMSCs after three weeks of osteoinduction
(Fig. 1D). These results
demonstrated that the SMSCs exhibited the potential of
multidirectional differentiation. CCK-8 assays demonstrated that
the SMSCs increased over time in exponential growth, and the 2nd
passage of the SMSCs exhibited the highest proliferation (Fig. 1E). The immunophenotypes were
determined via flow cytometry. We found that the surface markers of
CD44, CD90, CD105, CD147, CD14, CD34, CD45 and CD117 were expressed
on average on 96.2, 94.8, 93.6, 98.1, 6.9, 7.6, 4.4 and 3.5% of the
SMSCs, respectively (Fig. 1F),
which fits the criteria that we previously reported (28).
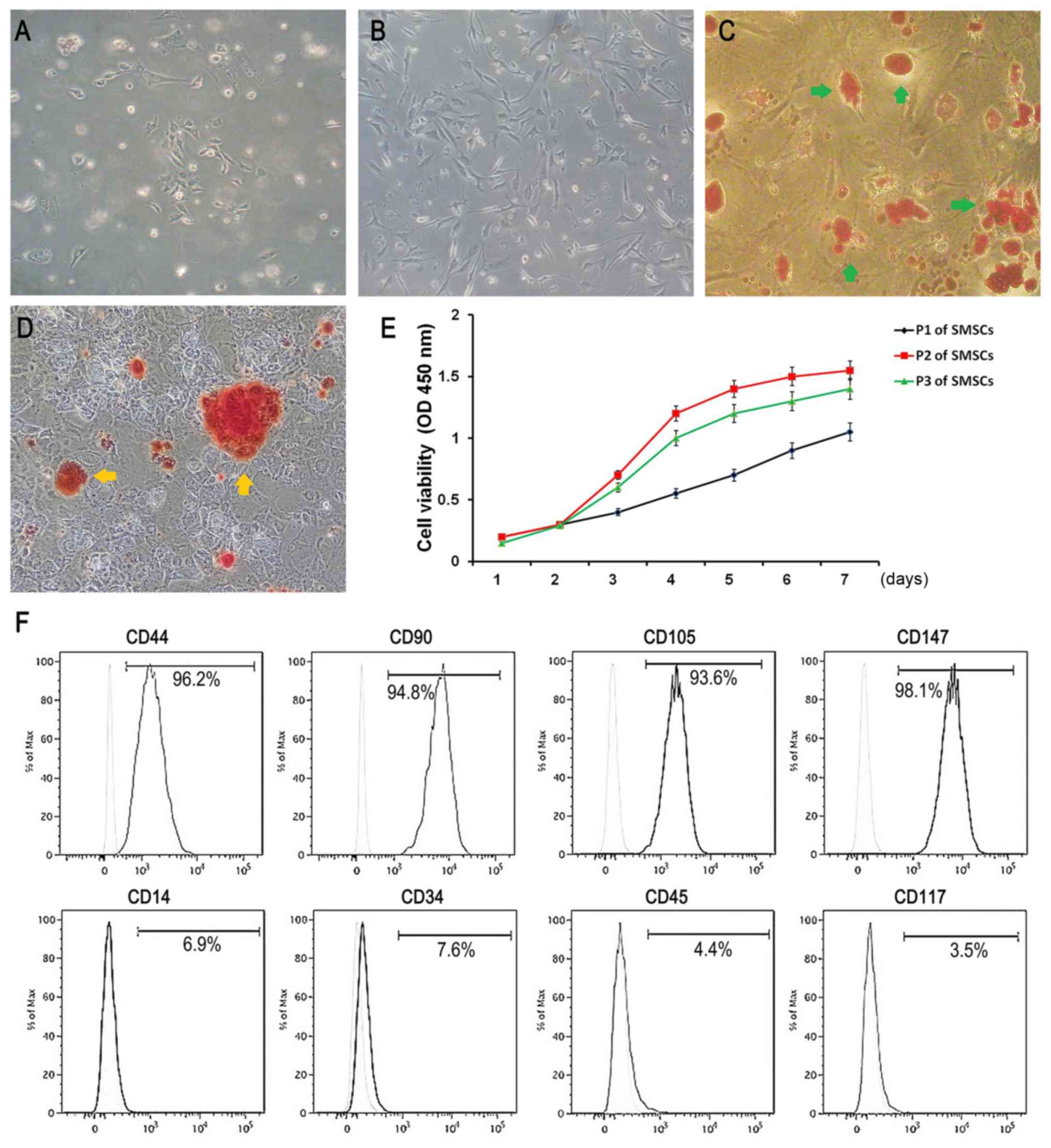 | Figure 1.Characteristics of SMSCs. (A and B)
Observation of primary SMSCs and P2 SMSCs, respectively
(magnification, ×40). (C) Oil red staining indicates lipid droplet
formation (green arrows) after adipogenic differentiation for 14
days (magnification, 40). (D) Alizarin red staining demonstrates
the formation of mineralized nodules (yellow arrows) after
osteogenic induction for 21 days (magnification, ×40). (E) Cell
proliferation of P1 to P3 SMSCs was determined by CCK-8 assay. P2
SMSCs revealed the highest rate of proliferation. (F) Flow
cytometric analysis showed that the surface markers CD44, CD90,
CD105, CD147, CD14, CD34, CD45 and CD117 were expressed on average
on 96.2, 94.8, 93.6, 98.1, 6.9, 7.6, 4.4 and 3.5% of the SMSCs,
respectively. All data are averages ± standard deviations (SD)
(error bars) from 3 to 5 independent experiments. SMSCs, synovial
mesenchymal stem cells. |
Characteristics of the SMSCs under
hypoxic conditions
To observe the effects of different oxygen
concentration microenvironments on the proliferation and apoptosis
of SMSCs, cells were cultured under oxygen environment of normoxia
(21% O2), hypoxia (5% O2) and severe hypoxia
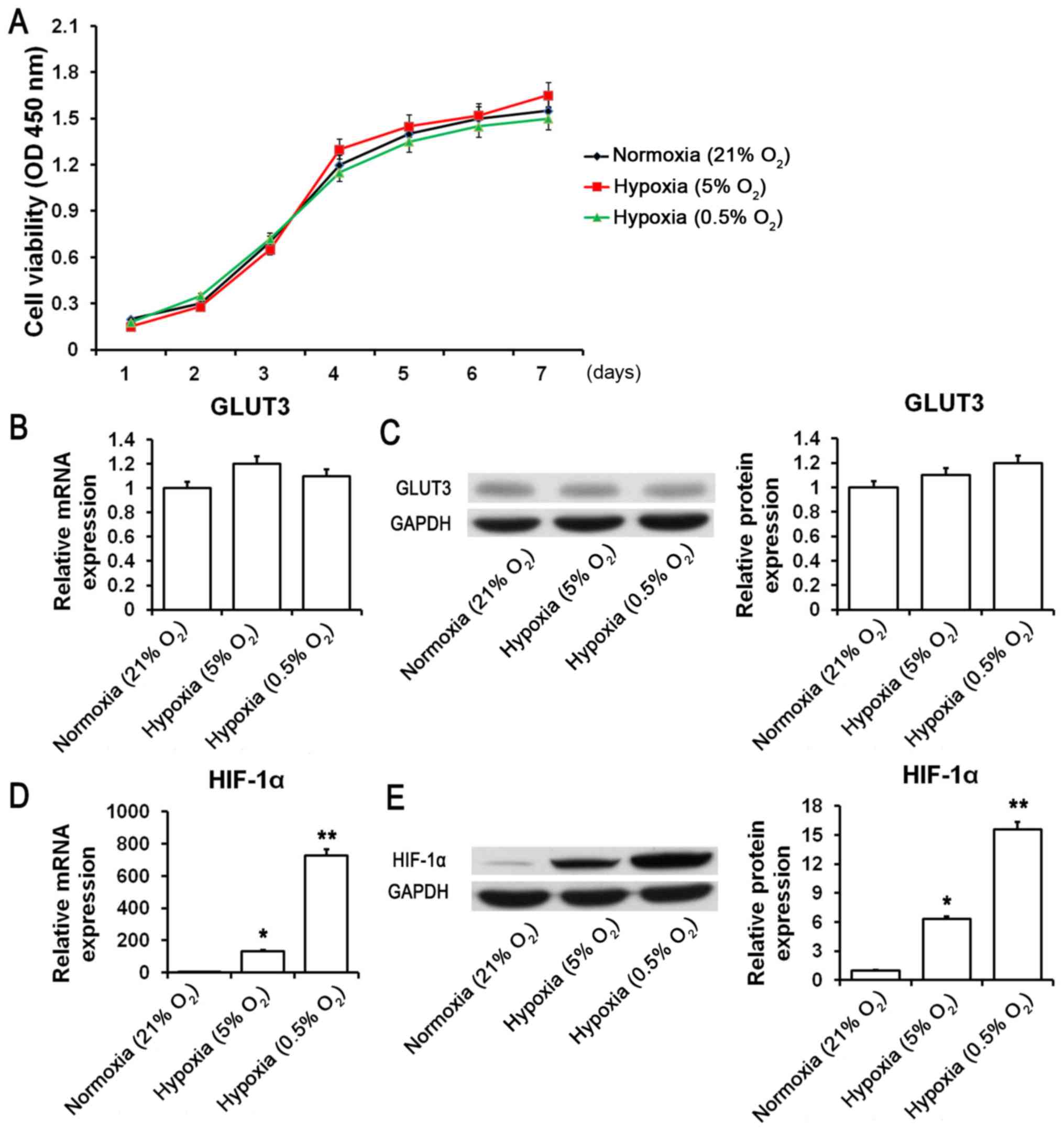
(0.5% O2). Our findings suggested that there were no
significant changes in cell viability or GLUT3 mRNA and protein
expression following incubation under different oxygen conditions
(Fig. 2A-C). Interestingly, the
mRNA and protein expression of HIF-1α was significantly upregulated
under hypoxic (5% O2) and severe hypoxic (0.5%
O2) conditions (Fig. 2D and
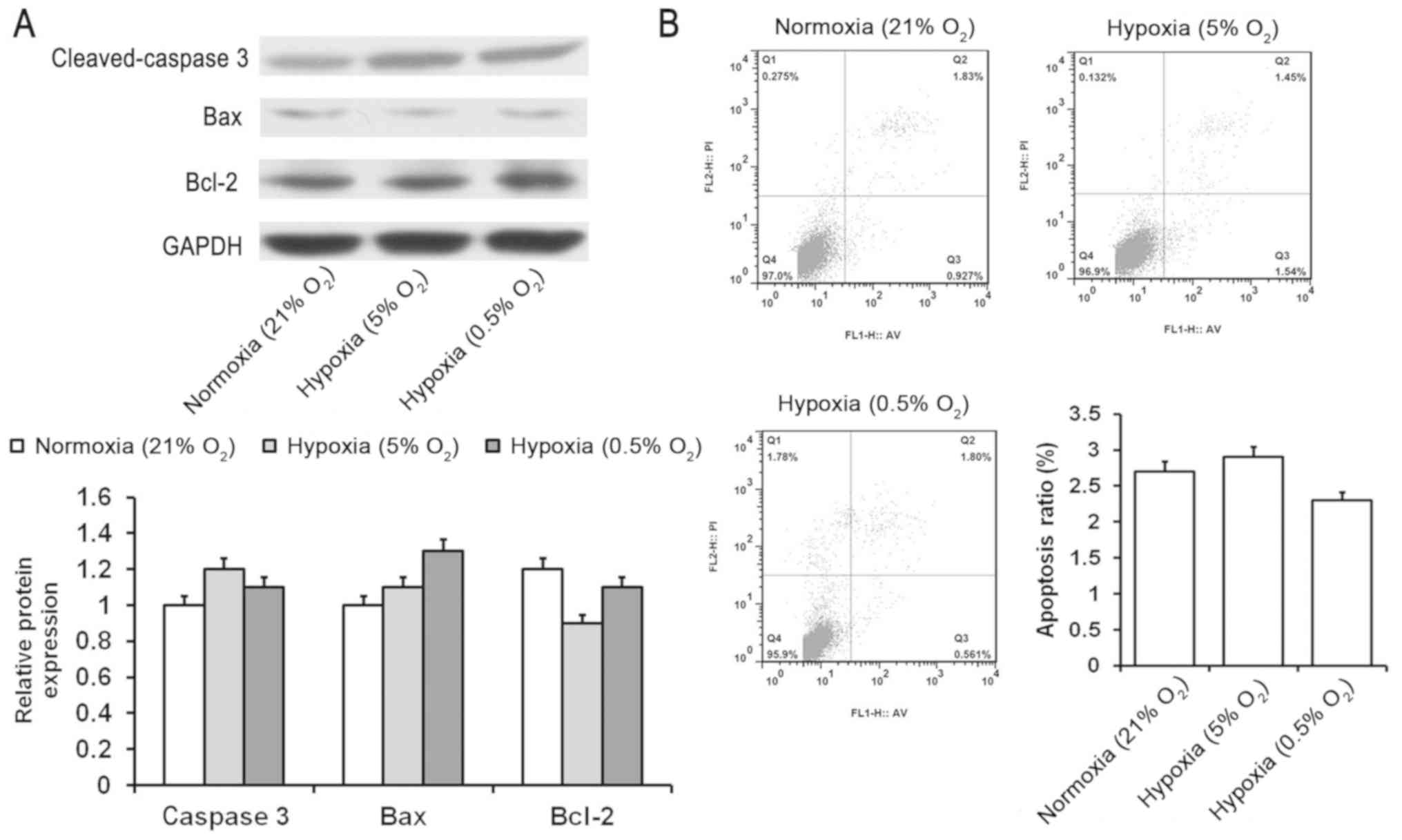
E). Additionally, no obvious changes were found in the protein
expression levels of cleaved caspase 3, Bax and Bcl-2 (Fig. 3A). The level of apoptosis
determined by flow cytometry also revealed no significant changes
(Fig. 3B). These results indicated
that HIF-1α may be engaged in regulating the proliferation and
apoptosis of SMSCs.
Effect of HIF-1α on SMSCs under
hypoxic condition
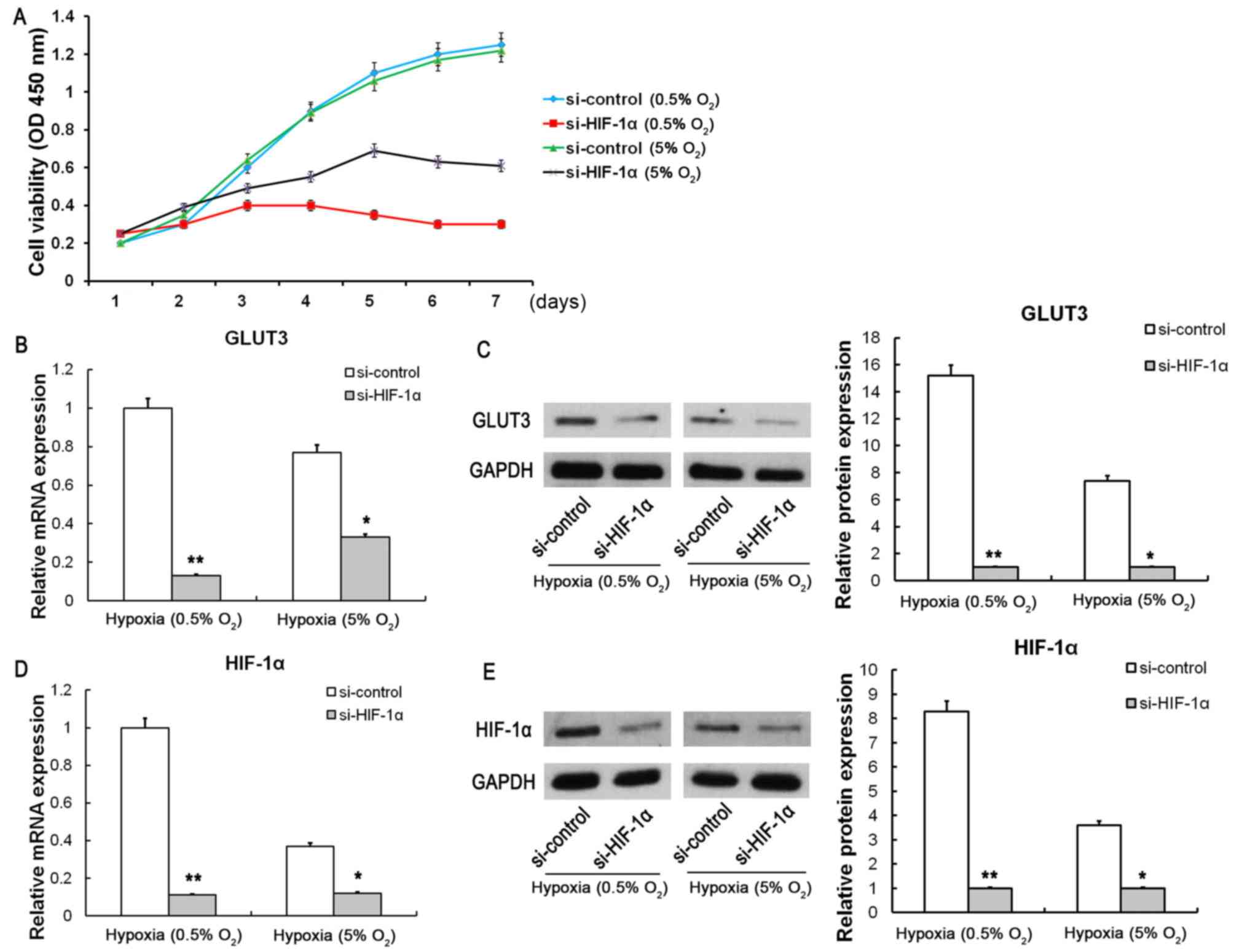
To characterize the specific mechanism of HIF-1α on
SMSCs, siRNA-induced HIF-1α knockdown was conducted under severe
hypoxic (0.5% O2) and hypoxic (5% O2)
conditions. Our findings suggested that the cell viability and
GLUT3 mRNA and protein expression were markedly suppressed
following siRNA-induced HIF-1α knockdown (Fig. 4A-C). The mRNA and protein
expression of HIF-1α were significantly suppressed following
si-HIF-1α knockdown under severe hypoxic (0.5% O2) and
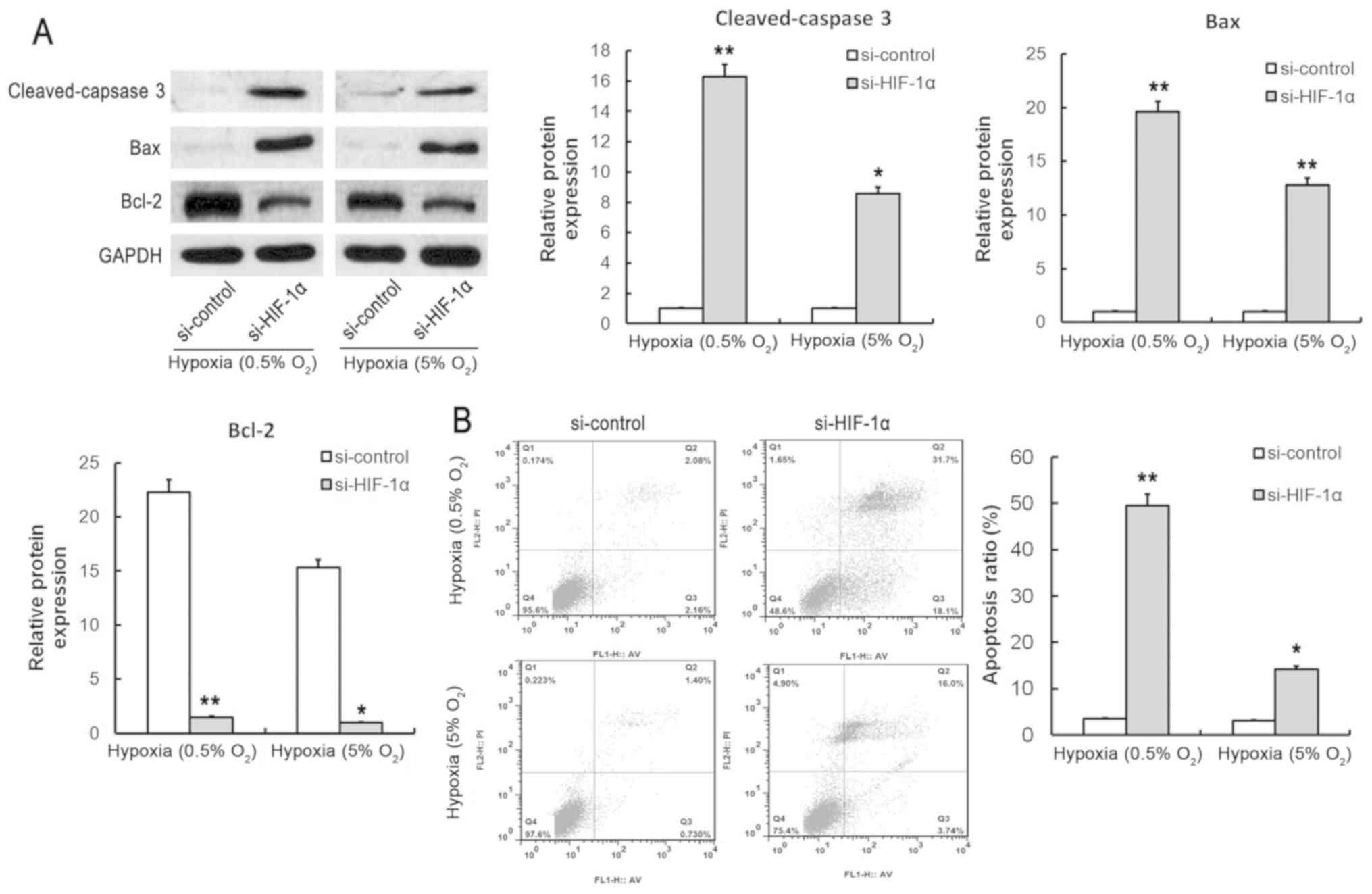
hypoxic (5% O2) conditions (Fig. 4D and E). In addition, the protein
expression of cleaved caspase 3 and Bax was significantly increased
and the Bcl-2 expression was significantly decreased following
HIF-1α knockdown (Fig. 5A). Flow
cytometric analysis revealed that the apoptosis ratio was
significantly increased following siRNA-induced HIF-1α knockdown
(Fig. 5B). These results suggested
that HIF-1α could effectively improve the tolerance to hypoxia of
SMSCs, which might promote cellular proliferation and prevent the
apoptosis of SMSCs under hypoxic conditions.
Discussion
Hypoxia occurs commonly in many types of mammalian
tissues, such as synovium, cartilage and intervertebral discs,
where cells with a poor O2 supply can adapt to these low
oxygen tension conditions by activating survival pathways (31–33).
Synovium is a type of connective tissue covering most of the inner
joint structure, including the inner surface of the joint fibrous
capsule, intra-articular ligaments, tendons, and bone surface
(34). Synovial mesenchymal stem
cells (SMSCs) are the principal cell type of synovial tissue, which
are related to the synthesis of hyaluronate and the secretion of
synovial fluid (35,36). In the present study, to explore the
effects of hypoxia on SMSCs, SMSCs were cultured under normoxic and
hypoxic conditions. We found that there were no significant changes
in the cellular proliferation and apoptosis of SMSCs under normal
and hypoxic conditions. Apoptotic proteins, such as cleaved caspase
3, Bax and Bcl-2 also exhibited no significant change under normal
and hypoxic conditions. However, the levels of HIF-1α mRNA and
protein were significantly increased under hypoxic condition. These
results demonstrated that HIF-1α may be expressed in large
quantities under hypoxic conditions, which could improve the
hypoxic tolerance and inhibit the spontaneous apoptosis of
SMSCs.
To verify the specific role of HIF-1α in SMSCs under
hypoxic conditions and to further explore the relationship between
HIF-1α and the apoptosis of SMSCs, we conducted a selective
inhibition of HIF-1α under 0.5% O2 and 5% O2
hypoxic conditions by siRNA interference. Our findings revealed
that the cellular proliferation was significantly inhibited and the
apoptosis ratio was significantly increased after HIF-1α knockdown.
Western blot assays demonstrated that the protein expression levels
of cleaved caspase 3 and Bax were obviously increased, and Bcl-2
expression was significantly inhibited. These results suggest that
the HIF-1α gene plays a protective role in cellular proliferation
and apoptosis of SMSCs in a hypoxic environment.
The particular mechanisms may be summarized as
follows. In the adaptive response of cells to changes in oxygen,
the activated HIF-1α induced by hypoxia was found to stimulate more
than 100 downstream genes for mediating the process of cell
proliferation and survival (37,38).
These metabolic processes are involved in cell proliferation,
migration, glucose metabolism and angiogenesis (39,40).
Prior studies have noted that HIF-1α mediates adaptive metabolic
responses to hypoxia by decreasing flux via the tricarboxylic acid
cycle and increasing flux via the glycolytic pathway, in order to
meet the energy demands of rapidly growing tissue (23,41).
Therefore, cells under hypoxic conditions tend to burn more glucose
in order to achieve adequate energy requirements for cell survival.
The evidence suggests that HIF-1α mediates this metabolic
transformation by inducing the overexpression of glucose
transporters (GLUTs) and enzymes that are involved in the
glycolysis pathway, thereby increasing glucose entry into cells
(42,43).
In the present study, mRNA and protein expression of
GLUT3 were markedly suppressed following HIF-1α knockdown,
suggesting that HIF-1α may promote the glucose metabolic conversion
of SMSCs under hypoxic condition. As mentioned in a literature
review, the HIF-1α transcriptional induction of a variety of
angiogenic factors, such as vascular endothelial growth factor
(VEGF) and basic fibroblast growth factor (bFGF) promotes the
synthesis of endogenous angiogenesis, which in turn promotes
neovascularization to enrich cells for growth (44). Moreover, HIF-1α was found to
promote cell migration into oxygen-rich regions through inducing
secretion via transcriptional activation growth factors such as
fibroblast growth factor 11 (FGF11), transforming growth factor β3
(TGF-β3), insulin-like growth factor (IGF) and epidermal growth
factor (EGF) (45–47).
In conclusion, we preliminarily explored the effects
of HIF-1α on the proliferation and apoptosis of human SMSCs, and
the results suggest that HIF-1α activation in SMSCs is probably one
of the key mechanisms mediating the ability of SMSCs to adapt to
hypoxic environments.
Acknowledgements
Not applicable.
Funding
This project was supported by Scientific Research
Topics of Jiangsu Provincial Health Commission (grant no.
H2018027).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH and XS conceived and designed the study. DH, XS,
WZ and XG performed the experiments and analyzed the data. WZ and
XG wrote the paper. DH and WZ reviewed and edited the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for this study was granted by the
Institution Review Board of the Affiliated Hospital of Nanjing
Medical University, and all study participants were recruited after
providing informed written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perez JR, Kouroupis D, Li DJ, Best TM,
Kaplan L and Correa D: Tissue engineering and cell-based therapies
for fractures and bone defects. Front Bioeng Biotechnol. 6:1052018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golpanian S, Wolf A, Hatzistergos KE and
Hare JM: Rebuilding the damaged heart: Mesenchymal stem cells,
cell-based therapy, and engineered heart tissue. Physiol Rev.
96:1127–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heathman TR, Nienow AW, McCall MJ, Coopman
K, Kara B and Hewitt CJ: The translation of cell-based therapies:
Clinical landscape and manufacturing challenges. Regen Med.
10:49–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leach JK and Whitehead J:
Materials-directed differentiation of mesenchymal stem cells for
tissue engineering and regeneration. ACS Biomater Sci Eng.
4:1115–1127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toyoda E, Sato M, Takahashi T, Maehara M,
Nakamura Y, Mitani G, Takagaki T, Hamahashi K and Watanabe M:
Multilineage-differentiating stress-enduring (Muse)-like cells
exist in synovial tissue. Regen Ther. 10:17–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hatakeyama A, Uchida S, Utsunomiya H,
Tsukamoto M, Nakashima H, Nakamura E, Pascual-Garrido C, Sekiya I
and Sakai A: Isolation and characterization of synovial mesenchymal
stem cell derived from hip joints: A comparative analysis with a
matched control knee group. Stem Cells Int. 2017:93123292017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng W, Gu X, Sun X, Wu Q and Dan H: FAK
mediates BMP9-induced osteogenic differentiation via Wnt and MAPK
signaling pathway in synovial mesenchymal stem cells. Artifl Cells
Nanomed Biotechnol. 47:2641–2649. 2019. View Article : Google Scholar
|
|
8
|
Baboolal TG, Khalil-Khan A, Theodorides
AA, Wall O, Jones E and McGonagle D: A novel arthroscopic technique
for intraoperative mobilization of synovial mesenchymal stem cells.
Am J Sports Med. 46:3532–3540. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santhagunam A, Dos Santos F, Madeira C,
Salgueiro JB and Cabral JM: Isolation and ex vivo expansion of
synovial mesenchymal stromal cells for cartilage repair.
Cytotherapy. 16:440–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferro T, Santhagunam A, Madeira C,
Salgueiro JB, da Silva CL and Cabral JMS: Successful isolation and
ex vivo expansion of human mesenchymal stem/stromal cells obtained
from different synovial tissue-derived (biopsy) samples. J Cell
Physiol. 234:3973–3984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Segawa Y, Muneta T, Makino H, Nimura A,
Mochizuki T, Ju YJ, Ezura Y, Umezawa A and Sekiya I: Mesenchymal
stem cells derived from synovium, meniscus, anterior cruciate
ligament, and articular chondrocytes share similar gene expression
profiles. J Orthop Res. 27:435–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Juranek I, Stern R and Soltes L:
Hyaluronan peroxidation is required for normal synovial function:
An hypothesis. Med Hypotheses. 82:662–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fearon U, Canavan M, Biniecka M and Veale
DJ: Hypoxia, mitochondrial dysfunction and synovial invasiveness in
rheumatoid arthritis. Nat Rev Rheumatol. 12:385–397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schipani E, Mangiavini L and Merceron C:
HIF-1α and growth plate development: What we really know. Bonekey
Rep. 4:7302015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schönenberger MJ and Kovacs WJ: Hypoxia
signaling pathways: Modulators of oxygen-related organelles. Front
Cell Dev Biol. 3:422015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esteve JM, Launay JM, Kellermann O and
Maroteaux L: Functions of serotonin in hypoxic pulmonary vascular
remodeling. Cell Biochem Biophys. 47:33–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel J, Landers K, Mortimer RH and
Richard K: Regulation of hypoxia inducible factors (HIF) in hypoxia
and normoxia during placental development. Placenta. 31:951–957.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Acker T and Plate KH: Hypoxia and hypoxia
inducible factors (HIF) as important regulators of tumor
physiology. Cancer Treat Res. 117:219–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Warfel NA, Dolloff NG, Dicker DT, Malysz J
and El-Deiry WS: CDK1 stabilizes HIF-1α via direct phosphorylation
of Ser668 to promote tumor growth. Cell Cycle. 12:3689–3701. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alonso D, Serrano E, Bermejo FJ and Corral
RS: HIF-1α-regulated MIF activation and Nox2-dependent ROS
generation promote Leishmania amazonensis killing by macrophages
under hypoxia. Cell Immunol. 335:15–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Zhang C, Zhao Y, Yue X, Wu H, Huang
S, Chen J, Tomsky K, Xie H, Khella CA, et al: Parkin targets HIF-1α
for ubiquitination and degradation to inhibit breast tumor
progression. Nat Commun. 8:18232017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mukund V, Saddala MS, Farran B,
Mannavarapu M, Alam A and Nagaraju GP: Molecular docking studies of
angiogenesis target protein HIF-1α and genistein in breast cancer.
Gene. 701:169–172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palmer CS, Ostrowski M, Balderson B,
Christian N and Crowe SM: Glucose metabolism regulates T cell
activation, differentiation, and functions. Front Immunol. 6:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho SH, Raybuck AL, Blagih J, Kemboi E,
Haase VH, Jones RG and Boothby MR: Hypoxia-inducible factors in
CD4+ T cells promote metabolism, switch cytokine
secretion, and T cell help in humoral immunity. Proc Natl Acad Sci
USA. 116:8975–8984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pfander D and Gelse K: Hypoxia and
osteoarthritis: How chondrocytes survive hypoxic environments. Curr
Opin Rheumatol. 19:457–462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang SJ, Li W, Li YJ, Fang W and Long X:
Dickkopfrelated protein 1 induces angiogenesis by upregulating
vascular endothelial growth factor in the synovial fibroblasts of
patients with temporomandibular joint disorders. Mol Med Rep.
12:4959–4966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fleming AM, Zhu J, Ding Y and Burrows CJ:
Location dependence of the transcriptional response of a potential
G-quadruplex in gene promoters under oxidative stress. Nucleic
Acids Res. 47:5049–5060. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng W, Yang M, Wu C and Su X:
Experimental study on Osteogenesis of synovium-derived mesenchymal
stem cells in vitro and in vivo. Zhongguo Xiu Fu Chong Jian Wai Ke
Za Zhi. 30:102–109. 2016.(In Chinese). PubMed/NCBI
|
|
29
|
Lv B, Hua T, Li F, Han J, Fang J, Xu L,
Sun C, Zhang Z, Feng Z and Jiang X: Hypoxia-inducible factor 1 a
protects mesenchymal stem cells against oxygen-glucose
deprivation-induced injury via autophagy induction and
PI3K/AKT/mTOR signaling pathway. Am J Transl Res. 9:2492–2499.
2017.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernández-Torres J, Martínez-Nava GA,
Gutiérrez-Ruíz MC, Gómez-Quiroz LE and Gutiérrez M: Role of HIF-1α
signaling pathway in osteoarthritis: A systematic review. Rev Bras
Reumatol Engl Ed. 57:162–173. 2017.(In English, Portuguese).
PubMed/NCBI
|
|
32
|
Daniel SK, Sullivan KM, Labadie KP and
Pillarisetty VG: Hypoxia as a barrier to immunotherapy in
pancreatic adenocarcinoma. Clin Transl Med. 8:102019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ichihara S, Li P, Mise N, Suzuki Y, Izuoka
K, Nakajima T, Gonzalez F and Ichihara G: Ablation of aryl
hydrocarbon receptor promotes angiotensin II-induced cardiac
fibrosis through enhanced c-Jun/HIF-1α signaling. Arch Toxicol.
93:1543–1553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Radenska-Lopovok SG: Immunomorphological
characteristics of the synovial membrane in rheumatic diseases.
Arkh Patol. 78:64–68. 2016.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sekiya I, Koga H, Otabe K, Nakagawa Y,
Katano H, Ozeki N, Mizuno M, Horie M, Kohno Y, Katagiri K, et al:
Additional use of synovial mesenchymal stem cell transplantation
following surgical repair of a complex degenerative tear of the
medial meniscus of the knee: A case report. Cell Transplant. Jul
17–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Desando G, Bartolotti I, Cavallo C,
Schiavinato A, Secchieri C, Kon E, Filardo G, Paro M and Grigolo B:
Short-term homing of hyaluronan-primed cells: Therapeutic
implications for osteoarthritis treatment. Tissue Eng Part C
Methods. 24:121–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu W, Xu R, Li X, Zhang H, Wang X and Zhu
J: Downregulating hypoxia-inducible factor-1α expression with
perfluorooctyl-bromide nanoparticles reduces early brain injury
following experimental subarachnoid hemorrhage in rats. Am J Transl
Res. 8:2114–2126. 2016.PubMed/NCBI
|
|
38
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miska J, Lee-Chang C, Rashidi A, Muroski
ME, Chang AL, Lopez-Rosas A, Zhang P, Panek WK, Cordero A, Han Y,
et al: HIF-1α is a metabolic switch between glycolytic-driven
migration and oxidative phosphorylation-driven immunosuppression of
tregs in glioblastoma. Cell Rep. 27:226–237.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bensaad K and Harris AL: Hypoxia and
metabolism in cancer. Adv Exp Med Biol. 772:1–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao L, Mao Y, Zhao Y, Cao Y and Chen X:
Role of multifaceted regulators in cancer glucose metabolism and
their clinical significance. Oncotarget. 7:31572–31585.
2016.PubMed/NCBI
|
|
42
|
Pereira KM, Chaves FN, Viana TS, Carvalho
FS, Costa FW, Alves AP and Sousa FB: Oxygen metabolism in oral
cancer: HIF and GLUTs (Review). Oncol Lett. 6:311–316. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Z, Tian D, Liao X, Zhang Y, Xiao J,
Chen W, Liu Q, Chen Y, Li D, Zhu L and Cai S: Apigenin combined
with gefitinib blocks autophagy flux and induces apoptotic cell
death through inhibition of HIF-1α, c-Myc, p-EGFR, and glucose
metabolism in EGFR L858R+T790M-mutated H1975 cells. Front
Pharmacol. 10:2602019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fu YC and Xin ZM: Inhibited corneal
neovascularization in rabbits following corneal alkali burn by
double-target interference for VEGF and HIF-1α. Biosci Rep.
39(pii): BSR201805522019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Szojka ARA, Lyons BD, Moore CN, Liang Y,
Kunze M, Idrees E, Mulet-Sierra A, Jomha NM and Adesida AB: Hypoxia
and TGF-β3 synergistically mediate inner meniscus-like matrix
formation by fibrochondrocytes. Tissue Eng Part A. 25:446–456.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Knowles HJ: Hypoxia-induced fibroblast
growth factor 11 stimulates osteoclast-mediated resorption of bone.
Calcif Tissue Int. 100:382–391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao FL and Qin CF: EGF promotes HIF-1α
expression in colorectal cancer cells and tumor metastasis by
regulating phosphorylation of STAT3. Eur Rev Med Pharmacol Sci.
23:1055–1062. 2019.PubMed/NCBI
|