Introduction
Osteosarcoma is the most common type of primary
malignant tumor of the bone, particularly in children and
adolescents (1), and exhibits a
strong tendency for pulmonary metastasis (2,3).
Pulmonary metastasis is the main cause of failure in the treatment
of patients with osteosarcoma (4,5).
Patients with pulmonary metastasis exhibit an increased rate of
mortality compared with those without metastasis (5). The 5-year overall survival (OS) rate
was 60–70% in patients with localized disease, but only 23% in
patients with metastatic osteosarcoma (4,6,7).
Chou et al (8) reported
that the addition of liposomal muramyl tripeptide
phosphatidylethanolamine (MTP-PE) to conventional chemotherapy
improved the event-free survival and OS of patients with metastatic
osteosarcoma. Notably, the European Medical Agency has approved the
use of MTP-PE for patients with osteosarcoma. The investigation of
pulmonary metastasis may provide novel insight for the
identification of appropriate treatment strategies. Thus, it would
be of clear clinical significance to identify biomarkers of
pulmonary metastasis or prognostic factors in patients with
osteosarcoma.
Growth and differentiation factor 15 (GDF15) is a
novel divergent member of the transforming growth factor-β (TGF-β)
superfamily, and has been reported to be involved in numerous
physiological processes and cellular events, including metabolism,
tissue differentiation and maintenance, apoptosis, angiogenesis,
cell cycle arrest and tumor dissemination (9). Previous studies have identified GDF15
to be involved in various pathologies, including cancer,
inflammation, cardiovascular diseases and dyserythropoietic
diseases (10–12). Overexpression of GDF15 has been
reported in numerous types of cancer and was associated with poor
clinical outcomes (11,13,14).
Of note, there were significant increases in serum GDF15 levels
reported following progression to carcinoma and metastasis in
prostate, colon and endometrial cancer, suggesting that GDF15 may
serve as an independent predictor of metastasis (11,13,15);
however, the role of GDF15 in osteosarcoma metastasis and its
clinical relevance remain unclear.
In the present study, it was observed that the
expression of GDF15 was increased in metastatic osteosarcoma
tissues. The upregulated expression of GDF15 was associated with
poorer OS and reduced pulmonary metastasis-free survival (PMFS)
compared with low GDF15 expression, and GDF15 was a significant
prognostic factor in multivariate analysis. Furthermore, the
downregulation of GDF15 effectively suppressed the migration and
invasion of osteosarcoma cells. Additionally, it was revealed that
the TGF-β signaling pathway was involved in the GDF15-mediated
metastasis of osteosarcoma cells. Increased serum GDF15 levels
exhibited clear prognostic value for poor clinical outcome in
osteosarcoma, and may serve as a promising biomarker for pulmonary
metastasis, enabling the identification of high-risk patients and
the selection of appropriate treatment strategies.
Materials and methods
Cell culture
The osteosarcoma cell lines U-2 OS, SaOS-2, 143B,
MG-63 and HOS, and the human hFOB1.19 osteoblast and mouse NIH3T3
fibroblast cell lines, were purchased from the American Type
Culture Collection, and cultured in Dulbecco's modified Eagle's
medium (DMEM, Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences)
in a 37°C incubator containing 5% CO2. MG-63 and U-2 OS
cells were selected for subsequent experiments (transfection,
migration and invasion assays, and luciferase assays) due to their
increased GDF15 expression. For simulating extracellular secretion
of GDF15, cells were treated with 50 ng/ml recombinant human GDF15
(rhGDF15; R&D Systems, Inc.) at 37°C for 24 h.
Patient information and tissue
samples
The present study was approved by the Ethics
Committee of the Affiliated Foshan Chancheng District Center
Hospital of Guangdong Medical University according to the 1975
Declaration of Helsinki (ethics approval no. IRB-ATT-001-24). All
clinical samples were collected from the Affiliated Foshan
Chancheng District Center Hospital of Guangdong Medical University.
Patient written informed consent forms were obtained prior to the
use of clinical specimens for research purposes. In the present
study, 106 serum samples and 10 fresh tissues were collected from
106 patients (67 males and 39 females, aged 12–58 years), diagnosed
pathologically with osteosarcoma, between January 2005 and December
2009. All patients received standard neoadjuvant chemotherapy,
followed by resection of the tumor and postoperative chemotherapy.
Clinical follow-up information, including Enneking staging (a
system for staging bone cancer) (16), overall survival and pulmonary
metastasis-free survival time, was available for all patients
enrolled in the study. All serum samples (67 non-metastatic and 39
pulmonary metastatic osteosarcoma samples) were collected from the
peripheral blood of patients with osteosarcoma prior to systemic
treatment. The serum was obtained from blood samples by
centrifugation at 1,000 × g for 10 min at 4°C, and then stored at
−80°C and thawed prior to analyses. Fresh osteosarcoma samples for
reverse transcription-quantitative PCR (RT-qPCR) and ELISA
analyses, including 4 non-metastatic and 4 pulmonary metastatic
osteosarcoma tissues, and 2 adjacent non-tumor soft tissues, were
obtained from 10 of the 106 aforementioned patients with
osteosarcoma at the time of surgical resection, and immediately
frozen at −80°C until further use. In the present study, 106 serum
samples and 10 fresh tissues were collected between January 2005
and December 2009. All patients received standard neoadjuvant
chemotherapy, followed by resection of the tumor and postoperative
chemotherapy.
Total RNA extraction and RT-qPCR
Total RNA samples from osteosarcoma cell lines and
fresh surgical osteosarcoma tissues were extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. The isolated RNA was
pretreated with RNase-free DNase. Then, the mRNA levels of GDF15
were evaluated by RT-qPCR. Total RNA (2 µg) from samples was
reverse transcribed to cDNA using M-MLV Reverse Transcriptase
(Promega Corporation) according to the manufacturer's protocols.
Briefly, total RNA (2 µg) was incubated with random primers, heated
to 70°C for 5 min and then placed on ice. A mix containing 1X M-MLV
reaction buffer, dNTPs (all 0.5 mM), Recombinant RNasin®
Ribonuclease Inhibitor (25 U) and M-MLV reverse transcriptase (200
U; Promega Corporation) was added to each sample at 37°C for 1 h.
All samples, including cDNAs, gene-specific primers and SYBR-Green
(Roche Applied Sciences) were heated to 95°C for 5 min and then
amplified for 35 cycles consisting of 95°C for 30 sec, a 30 sec
annealing step at a primer-specific annealing temperature, and 72°C
for 45 sec. All reactions were then incubated at 72°C for 7 min and
cooled to 4°C. qPCR analysis was performed on an ABI Prism 7500
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The results were normalized to the expression of
the housekeeping gene GAPDH. Relative expression levels were
determined using the following formula: 2−[(Cq of gene)-(Cq of
GAPDH)], where Cq represents the threshold cycle for each
transcript (17). The
oligonucleotide primers are listed in Table I.
 | Table I.Reverse transcription-quantitative PCR
primers. |
Table I.
Reverse transcription-quantitative PCR
primers.
| Name | Sequence (5′-3′) |
|---|
| GDF15-UP |
CTCCAGATTCCGAGAGTTGC |
| GDF15-DOWN |
AGAGATACGCAGGTGCAGGT |
| SNAI1-UP |
TTTACCTTCCAGCAGCCCTA |
| SNAI1-DOWN |
CCCACTGTCCTCATCTGACA |
| SNAI2-UP |
TCGGACCCACACATTACCTT |
| SNAI2-DOWN |
TTGGAGCAGTTTTTGCACTG |
| IL11-UP |
AGCCACCACCGTCCTTCCAAA |
| IL11-DOWN |
CCTCCGTCCCCACCCCAACAT |
| MMP13-UP |
TTAAGGAGCATGGCGACTTCT |
| MMP13-DOWN |
CCCAGGAGGAAAAGCATGAG |
| TWIST1-UP |
GCTCTTCTCCTCTGCCCCGG |
| TWIST1-DOWN |
CATCTAGGTCTCCGGCCCTG |
| ZEB1-UP |
ACCTCTTCACAGGTTGCTCCT |
| ZEB1-DOWN |
AGTGCAGGAGCTGAGAGTCA |
| COL1A1-UP |
CCTTTCTGCTCCTTTCTCCA |
| COL1A1-DOWN |
AGCAACACAGTTACACAAGG |
| VEGFA-UP |
ATGATTCTGCCCTCCTCCTT |
| VEGFA-DOWN |
CCTTGCTGCTCTACCTCCAC |
| GAPDH-UP |
GCACCGTCAAGGCTGAGAAC |
| GAPDH-DOWN |
TGGTGAAGACGCCAGTGGA |
ELISA
The concentrations of GDF15 in serum and cell
culture medium samples were determined using ELISA kits (1:250;
cat. no. DGD150; R&D Systems, Inc.). ELISAs were conducted
according to the manufacturer's instructions. Briefly, the
supernatant was transferred to a well coated with GDF15 monoclonal
antibody (cat. no. MAB957; R&D Systems, Inc.) and immunosorbed
using biotinylated polyclonal anti-human GDF15 antibody (1:1,000;
cat. no. BAF940, R&D Systems, Inc.) at room temperature for 1
h. The color development was catalyzed by horseradish peroxidase,
and the absorption was detected at 450 nm. The protein
concentration was determined by comparing the relative absorbance
of each sample with the standards. Patients were assigned to high
and low GDF15 expression groups based on the results of the ELISA.
Patients with serum GDF15 concentrations <1 ng/ml were assigned
to the low expression group; patients with serum GDF15
concentrations ≥1 ng/ml were assigned to the high expression
group.
Plasmids, virus constructs and
retroviral infection of target cells
Short-hairpin RNA (shRNA) against GDF15 (sense
sequence: 5′-CTATGATGACTTGTTAGCCAA-3′) in a Plko.1-puro vector was
commercially purchased (Sigma-Aldrich; Merck KGaA). Retroviruses
containing pLKO.1-puro-GDF15-Ri or pLKO.1-puro-vector were
generated by transfecting 293FT cells (Invitrogen; Thermo Fisher
Scientific, Inc.). Virus particles were then examined by
spectrophotometry at 260 nm and the infectivity of adenovirus
stocks was evaluated using an Adeno-X Rapid Titre kit (Clontech
Laboratories, Inc.), which determines infectious replication in
293FT cells. The virus particle/infectious focus-forming units
(IFU) ratio was then calculated. The virus stocks used in the paper
were GDF15-Ri (IFU ratio 43). MG-63 or U-2 OS cells
(2×105) were seeded and infected with 500 µl/day
retroviral particles for 3 days. The stable cell lines were
identified following treatment with 0.5 µg/ml puromycin for 10
days. The (CAGAC) 12/pGL3 TGF-β/SMAD-responsive luciferase reporter
plasmid and control plasmids (Clontech Laboratories, Inc.) were
used to quantitatively evaluate the transcriptional activity of
TGF-β signaling components. All transfection experiments were
performed using the Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols, and cells were collected 24 h later for
subsequent experiments.
Western blot analysis
Nuclear fractions were prepared using the Nuclear
Extraction kit (Active Motif, Inc.) according to the manufacturer's
protocols. Western blotting was conducted according to a standard
method, as previously described (18). Briefly, cells were lysed, and
protein sample (20 µg/lane) was separated via 10% SDS-PAGE and
transferred to PVDF membranes. Following blocking with 5% (w/v)
skim milk for 1 h at room temperature, membranes were incubated
overnight at 4°C with primary antibodies. Antibodies against
phosphorylated (p)-mothers against decapentaplegic homolog (SMAD)-2
(1:1,000; cat. no. 3108), p-SMAD3 (1:1,000; cat. no. 9520), SMAD2
(1:1,000; cat. no. 5339), and SMAD3 (1:1,000; cat. no. 9513) were
purchased from Cell Signaling Technology, Inc. The GDF15 monoclonal
antibody (1:1,000; cat. no. MAB957) was purchased from R&D
Systems, Inc. Anti-α-tubulin (1:2,000; cat. no. T6199,
Sigma-Aldrich; Merck KGaA) and anti-P84 (1:1,000; cat. no.
ab102684; Abcam) antibodies were used as loading controls. Then the
membrane was incubated with a secondary antibody for 1 h at room
temperature. The secondary antibodies, goat anti-rabbit
immunoglobulin G (1:2,000; cat. no. 31460, Pierce; Thermo Fisher
Scientific, Inc.), and goat anti-mouse immunoglobulin G (1:2,000;
cat. no. 31430, Pierce; Thermo Fisher Scientific, Inc.), were used
in this study. Protein bands were visualized using an enhanced
chemiluminescence kit (EMD Millipore).
Wound-healing and cell invasion assays.
Wound-healing and cell invasion assays were conducted as described
previously (19) to determine the
migratory and invasive abilities of osteosarcoma cells. For the
wound healing assay, in brief, 2×105 cells were seeded
in 6-well plates, and the cell layer at ~90% confluence was wounded
using a sterile tip. The extent of wound closure was evaluated
using a light microscope (magnification, ×100) following incubation
at 37°C for 24 h. For the invasion assay, in brief,
2×103 cells suspended in serum-free DMEM were seeded
into the upper Transwell chambers coated with Matrigel, whereas
DMEM with 20% FBS was seeded into the lower chambers. Then, cells
were incubated at 37°C for 24 h. The cells were fixed at 4%
paraformaldehyde for 20 min and stained with 1% crystal violet for
1 min (both at room temperature), and the number of cells that
reached the lower membrane was counted using a light microscope
(magnification, ×200). All experiments were performed in
triplicate.
Luciferase assay
Luciferase assays were conducted as described
previously (20). Briefly, cells
(1×104) were seeded in triplicate in 48-well plates, and
cultured at 37°C for 24 h. Then, 100 ng of (CAGAC) 12/pGL3 reporter
luciferase plasmid or control luciferase plasmid, and 3 ng pRL-TK
Renilla plasmid (Promega Corporation) were transfected into
shRNA- or rhGDP15-treated cells using Lipofectamine®
3000 (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. The luciferase and Renilla signals
were measured 24 h following transfection using a Dual Luciferase
Reporter Assay kit (Promega Corporation) according to the
manufacturer's protocols; luciferase activity was normalized to
Renilla activity.
Public microarray analyses
The microarray gene expression profiles in GSE9508
(21) were downloaded from the
Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/). The datasets were
analyzed for the expression of GDF15. Information on the clinical
characteristics, including metastasis status, were obtained from
the respective clinical information files.
Heatmap
Gene expression was graphed into heatmaps using MeV
version 4.9 software (http://mev.tm4.org). The pseudocolours represent the
intensity scale of fold change of GDF15-Ri vs. vector, or GDF15-Ri
+ rhGDF15 vs. GDF15-Ri in MG-63 and U-2 OS, generated by log2
transformation.
Statistical analysis
All statistical analyses were conducted using SPSS
version 17.0 software (SPSS, Inc.). Data are presented as the mean
± standard deviation of three independent experiments. The
associations between GDF15 protein expression and the
clinicopathological characteristics of osteosarcoma patients were
analyzed using χ2 tests. Survival curves were generated
using the Kaplan-Meier method and survival was analyzed using the
log-rank test. The significance of various variables for survival
was analyzed to predict prognostic value using the Cox proportional
hazards regression model in univariate and multivariate analyses.
For the comparison of two groups, P-values were calculated with a
Student's t-test. One-way analysis of variance followed by a
Newman-Keuls multiple comparison test was used to perform
comparisons between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
GDF15 is overexpressed in metastatic
osteosarcoma tissues
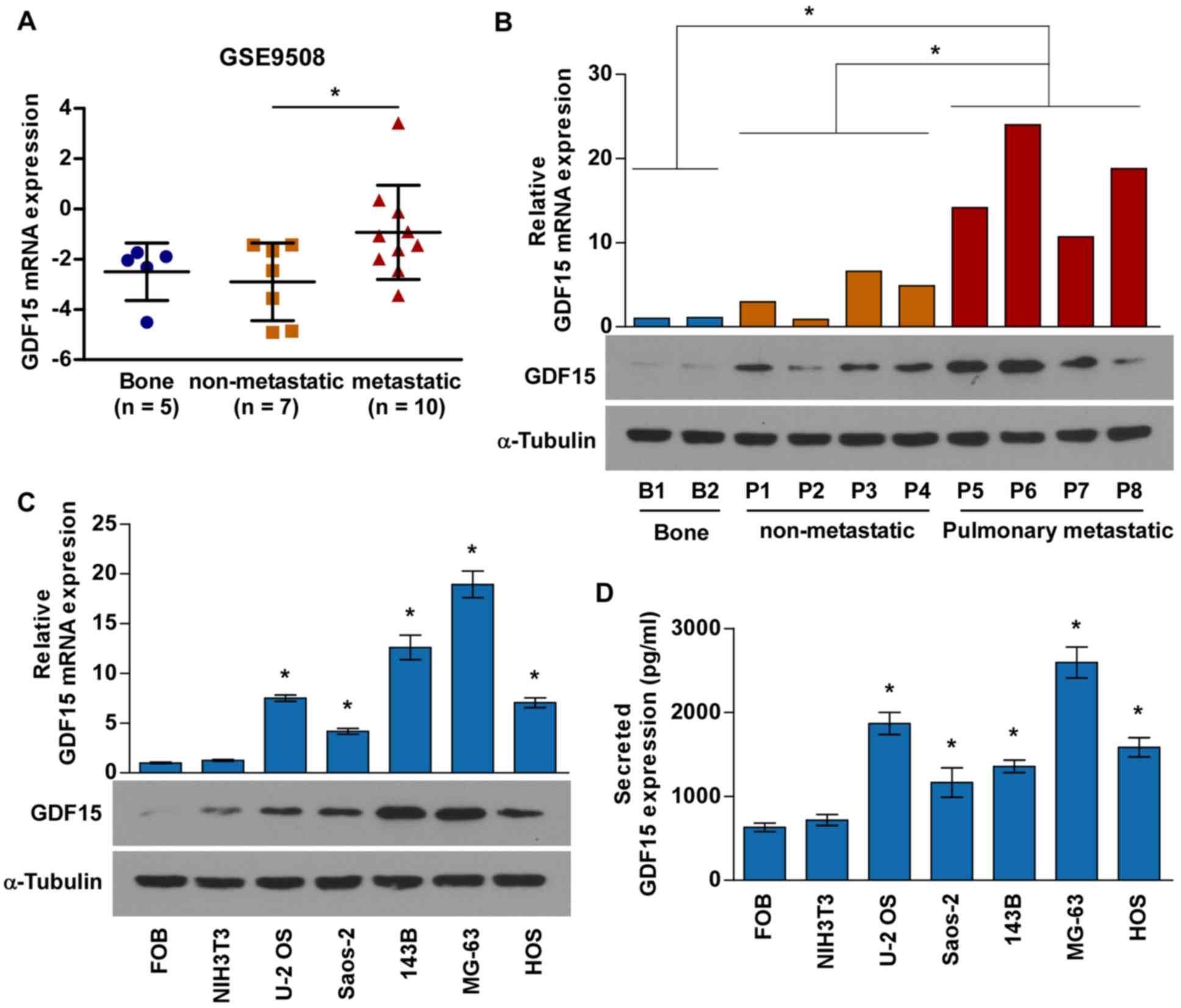
GDF15 expression was first investigated in human
osteosarcoma tissues by analyzing the GEO dataset GSE9508,
containing gene expression profiles for metastatic and
non-metastatic osteosarcoma biopsies. Notably, the mRNA levels of
GDF15 were significantly increased in metastatic osteosarcoma
tissues compared with in non-metastatic osteosarcoma tissues
(Fig. 1A). Similar results were
obtained when analyzing the mRNA and protein expression of GDF15 in
4 non-metastatic osteosarcoma tissues, 4 pulmonary metastatic
osteosarcoma tissues and 2 adjacent non-tumor soft tissues
(Fig. 1B). RT-qPCR, western blot
and ELISA analyses were conducted to determine GDF15 mRNA and
protein expression in five osteosarcoma cell lines (MG-63, HOS, U-2
OS, Saos-2 and 143B), human hFOB1.19 osteoblasts and mouse NIH3T3
fibroblasts. As presented in Fig. 1C
and D, GDF15 was expressed at high levels in osteosarcoma cell
lines compared with FOB and NIH3T3 cell lines.
High serum levels of GDF15 are
associated with poor prognosis in patients with osteosarcoma
To further investigate the clinical relevance of
serum GDF15 levels in patients with osteosarcoma, a total of 106
serum samples were examined for GDF15 expression by ELISA (Table II). χ2 tests revealed
that high serum levels of GDF15 were significantly associated with
vital status (P=0.010) and pulmonary metastasis (P<0.001) in
patients with osteosarcoma (Table
III); however, there were no significant associations between
GDF15 protein expression and other clinicopathologic
characteristics.
 | Table II.Clinicopathological characteristics
in 106 patients with osteosarcoma. |
Table II.
Clinicopathological characteristics
in 106 patients with osteosarcoma.
|
Characteristics | Number of
cases | % |
|---|
| Sex |
|
|
|
Female | 39 | 36.8 |
|
Male | 67 | 63.2 |
| Age, years |
|
|
|
≤20 | 77 | 72.6 |
|
21-40 | 25 | 23.6 |
|
>40 | 4 | 3.8 |
| Location |
|
|
| Distal
femur | 60 | 56.6 |
|
Proximal tibia | 24 | 22.6 |
|
Proximal humerus | 11 | 10.4 |
|
Proximal femur | 5 | 4.7 |
|
Others | 6 | 5.7 |
| Enneking |
|
|
|
IIB | 79 | 74.5 |
|
III | 27 | 25.5 |
| Relapse |
|
|
|
Yes | 8 | 7.5 |
| No | 98 | 92.5 |
| Pulmonary
metastasis |
|
|
|
Yes | 39 | 36.8 |
| No | 67 | 63.2 |
| Vital status (at
follow-up) |
|
|
|
Alive | 61 | 57.5 |
|
Deceased | 45 | 42.5 |
 | Table III.Association between GDF15 expression
and clinicopathological characteristics of 106 patients with
osteosarcoma. |
Table III.
Association between GDF15 expression
and clinicopathological characteristics of 106 patients with
osteosarcoma.
|
| GDF15
expression |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low | High | P-value |
|---|
| Sex |
|
| 0.218 |
|
Female | 29 | 10 |
|
|
Male | 42 | 25 |
|
| Age, years |
|
| 0.893 |
|
≤20 | 52 | 25 |
|
|
21-40 | 16 | 9 |
|
|
>40 | 3 | 1 |
|
| Location |
|
| 0.464 |
| Distal
femur | 42 | 18 |
|
|
Proximal tibia | 14 | 10 |
|
|
Proximal humerus | 8 | 3 |
|
|
Proximal femur | 2 | 3 |
|
|
Others | 5 | 1 |
|
| Enneking |
|
| 0.323 |
|
IIB | 55 | 24 |
|
|
III | 16 | 11 |
|
| Relapse |
|
| 0.231 |
|
Yes | 4 | 4 |
|
| No | 69 | 29 |
|
| Pulmonary
metastasis |
|
| <0.001 |
|
Yes | 18 | 21 |
|
| No | 53 | 14 |
|
| Vital status (at
follow-up) |
|
| 0.010 |
|
Alive | 47 | 14 |
|
|
Deceased | 24 | 21 |
|
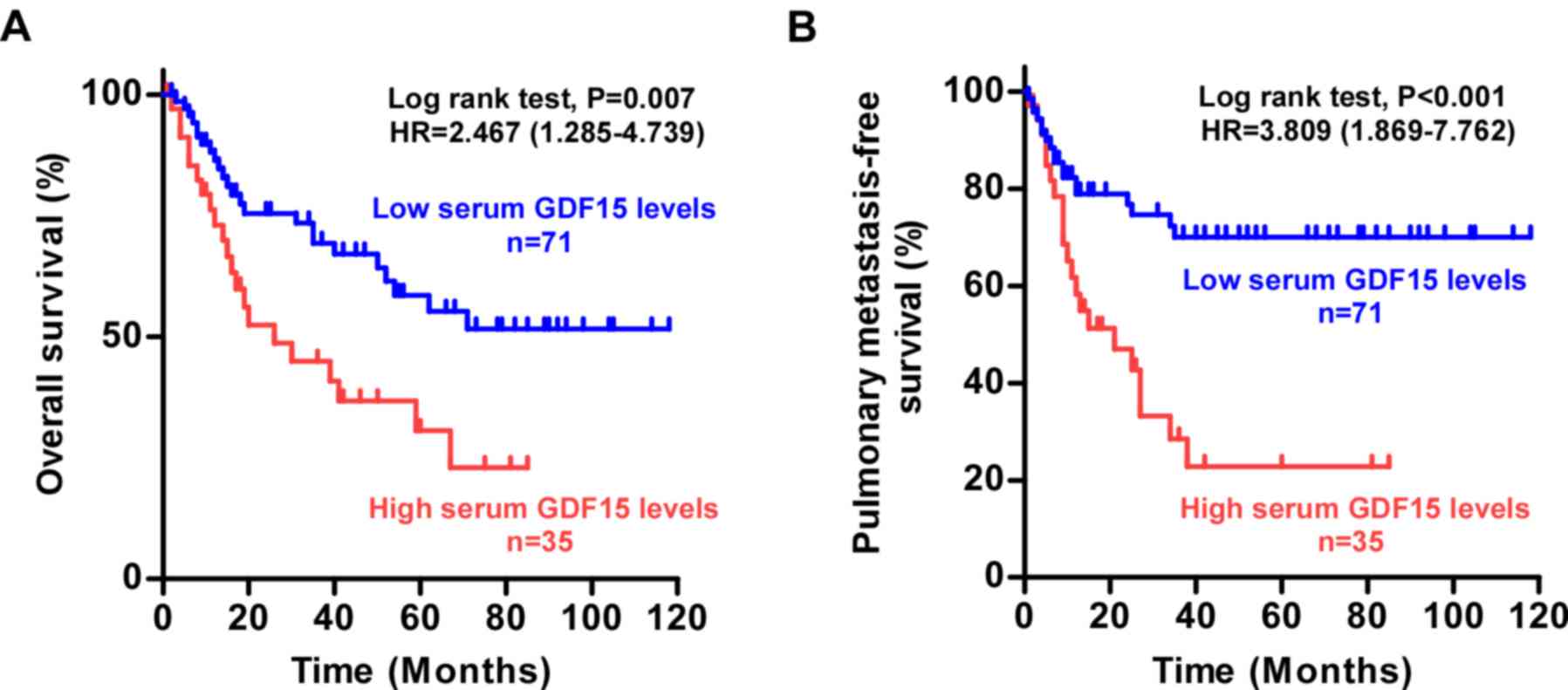
Kaplan-Meier survival analysis and log-rank tests
indicated that patients with osteosarcoma possessing high serum
levels of GDF15 exhibited significantly poorer OS and reduced PMFS
compared with those with low serum GDF15 levels (Fig. 2A and B). In addition, multivariate
survival analyses revealed that serum GDF15 expression (P=0.004)
was an independent prognostic factor for poor OS (Table IV), and that serum GDF15
expression (P<0.001) and Enneking stage (P=0.009) were
independent prognostic factors for short PMFS (Table V). Thus, these results suggested
that serum GDF15 may be a valuable prognostic marker in
osteosarcoma.
 | Table IV.Univariate and multivariate analysis
of factors associated with overall survival in 106 patients with
osteosarcoma. |
Table IV.
Univariate and multivariate analysis
of factors associated with overall survival in 106 patients with
osteosarcoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.145 | 0.664–1.975 | 0.627 | 0.907 | 0.515–1.600 | 0.737 |
| Sex | 0.850 | 0.459–1.573 | 0.605 | 0.693 | 0.366–1.315 | 0.262 |
| Location | 0.789 | 0.598–1.040 | 0.093 | 0.761 | 0.570–1.016 | 0.064 |
| Enneking stage | 0.926 | 0.446–1.926 | 0.838 | 1.001 | 0.474–2.111 | 0.999 |
| GDF15 | 2.206 | 1.224–3.977 | 0.008 | 2.474 | 1.345–4.550 | 0.004 |
 | Table V.Univariate and multivariate analysis
of factors associated with pulmonary metastasis survival in 106
osteosarcoma patients. |
Table V.
Univariate and multivariate analysis
of factors associated with pulmonary metastasis survival in 106
osteosarcoma patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.102 | 0.623–1.948 | 0.739 | 0.770 | 0.424–1.398 | 0.391 |
| Sex | 0.890 | 0.462–1.713 | 0.727 | 0.727 | 0.371–1.426 | 0.354 |
| Location | 0.886 | 0.677–1.160 | 0.378 | 0.837 | 0.632–1.108 | 0.213 |
| Enneking stage | 2.339 | 1.225–4.467 | 0.010 | 2.436 | 1.250–4.747 | 0.009 |
| GDF15 | 3.077 | 1.630–5.808 | 0.001 | 3.234 | 1.684–6.211 | <0.001 |
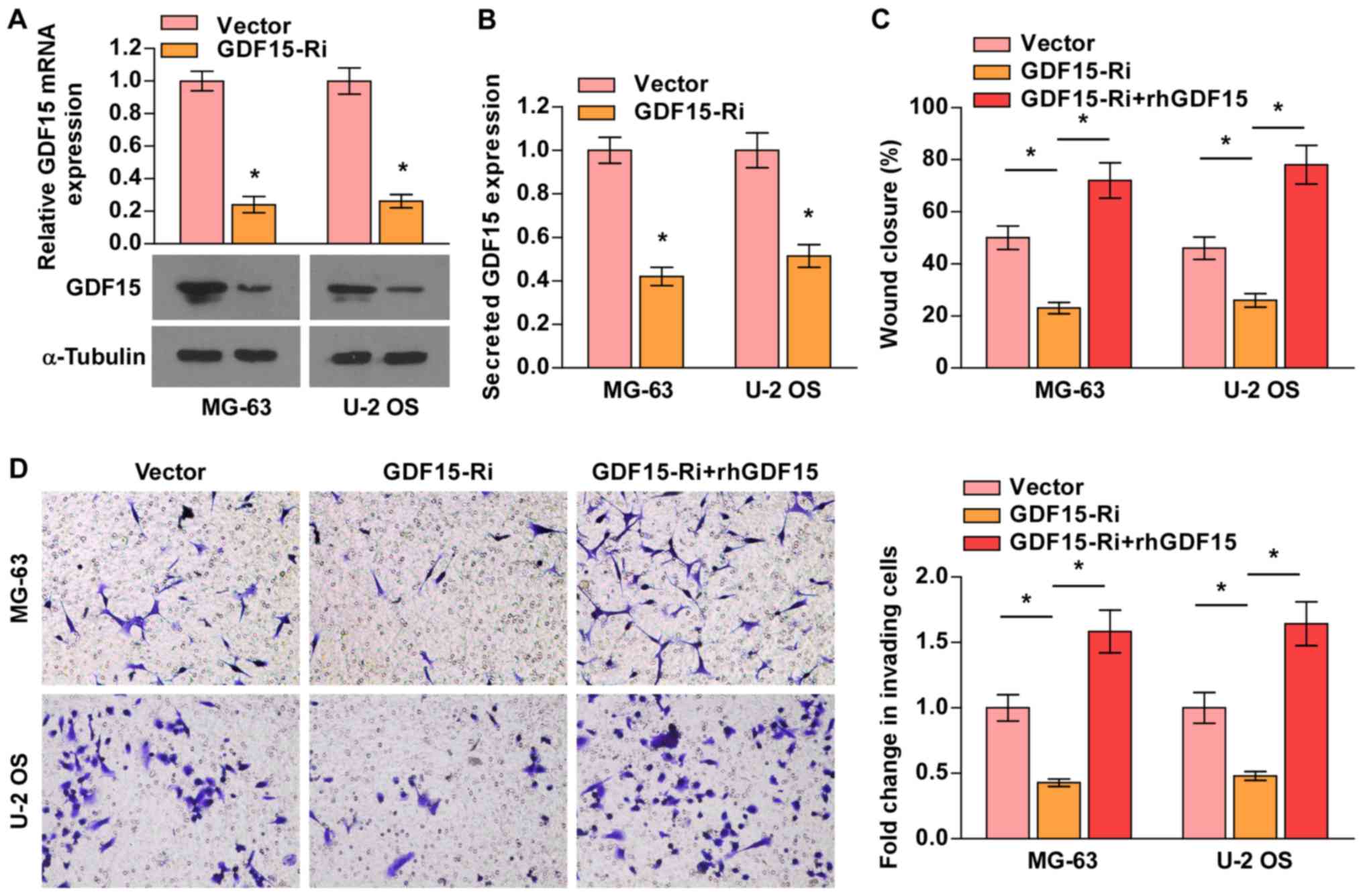
GDF15 knockdown attenuates
osteosarcoma cell migration and invasion
To explore the potential oncogenic functions of
GDF15 in osteosarcoma cells, migration and invasion assays were
performed. MG-63 and U-2 OS cells (selected for their high GDF15
expression) were transduced with lentiviruses carrying GDF15-shRNA
or vector control, with downregulated expression demonstrated via
RT-qPCR, western blot and ELISA analyses (Fig. 3A and B). As presented in Fig. 3C and D, GDF15-shRNA significantly
suppressed the migration and invasion of MG-63 and U-2 OS
osteosarcoma cells, whereas treatment with rhGDF15 rescued the
migratory and invasive abilities of GDF15-silenced cells.
Collectively, these results suggested that GDF15 knockdown
inhibited the metastatic ability of osteosarcoma cells.
GDF15 knockdown suppresses the TGF-β
signaling pathway
GDF15 is a member of the TGF-β superfamily (22), and TGF-β signaling is an important
pathway for maintaining metastatic phenotypes in osteosarcoma
(23). Therefore, whether GDF15
promotes the migratory and invasive abilities of osteosarcoma cells
via the activation of the TGF-β signaling pathway was subsequently
investigated. As presented in Fig.
4A-C, silencing GDF15 notably inhibited p-SMAD2/3 nuclear
translocation, TGF-β signaling activity and downstream target gene
expression, whereas rhGDF15 reversed these effects in
GDF15-silenced cells. These findings indicated that GDF15 knockdown
suppressed the TGF-β signaling pathway, and that the TGF-β
signaling may be involved in the GDF15-induced metastasis of
osteosarcoma cells (Fig. 4D).
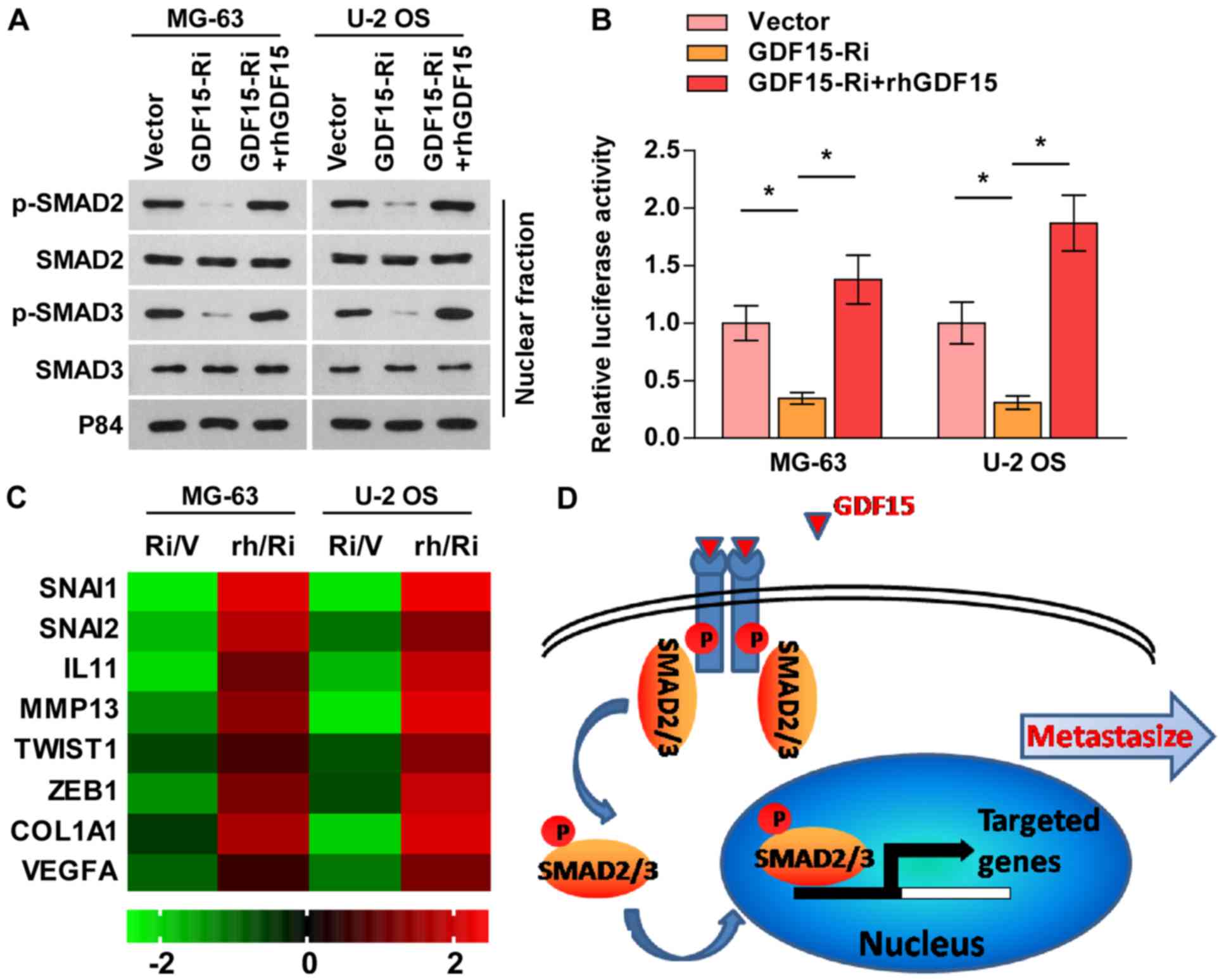 | Figure 4.GDF15 knockdown suppresses the TGF-β
signaling pathway. (A) Nuclear p-SMAD2/3 expression as determined
by western blot analysis. P84 was used as a loading control. (B)
Transcriptional activity of a TGF-β/SMAD-responsive luciferase
reporter, as determined by a luciferase assay. (C) Fold-change in
the mRNA expression of TGF-β signaling-associated genes, as
determined via reverse transcription-quantitative PCR analysis. (D)
Schematic model. GDF15 activates the TGF-β signaling pathway,
leading to the metastasis of osteosarcoma. Data are presented as
the mean ± standard deviation of three independent experiments.
*P<0.05, as indicated. GDF15, growth and differentiation factor
15; GDF15-Ri or Ri, GDF15 shRNA treatment; Vector/V, vector
control; rhGDF15/rh, recombinant human GDF15; TGF-β, transforming
growth factor-β; p-, phosphorylated; SNAI, snail family
transcriptional repressor; IL11, interleukin 11; MMP13, matrix
metallopeptidase 13; TWIST1, twist family bHLH transcription factor
1; ZEB1, zinc finger E-box binding homeobox 1; COL1A1, collagen
type I α1 chain; VEGFA, vascular endothelial growth factor A. |
Discussion
At the time of diagnosis, ~18% of patients with
osteosarcoma present with metastatic disease, with the most common
(81.2%) site being the lung (2,24).
The problem is even more serious, as ~80% of patients with
osteosarcoma are predicted to possess micrometastases, which are
subclinical or undetectable using current diagnostic instruments
(25). Furthermore, despite
combined therapeutic modality, 40% of patients with localized
osteosarcoma will experience treatment failure within 5 years
following diagnosis and develop a local or distant relapse
(4). It has been reported that
~81.4% of relapses are pulmonary metastases, which usually occur
within the first 2–3 years (4). At
present, metastatic osteosarcoma is incurable and responsible for
the majority of patient mortalities. Thus, there is an urgent
requirement for the improved understanding of the molecular
mechanisms underlying the regulation of osteosarcoma pulmonary
metastasis, and for a promising diagnostic and prognostic biomarker
for metastatic osteosarcoma.
An increasing body of evidence has indicated that
GDF15 is overexpressed in prostate, thyroid, pancreatic, and
colonic cancers, and may possess potential clinical use in the
diagnosis and/or monitoring of the progression of these diseases
(11,15,26).
Brown et al (11) reported
that GDF15 is an independent predictor of metastasis and overall
survival in colorectal carcinoma. Similar results were obtained in
prostate cancer and uveal melanoma (15,27);
however, its clinical relevance in osteosarcoma is yet to be
determined. In the present study, it was reported that the
expression of GDF15 was upregulated in pulmonary metastatic
osteosarcoma tissues compared with non-metastatic osteosarcoma
tissues and adjacent non-tumor soft tissues. Furthermore, it was
demonstrated that the serum levels of GDF15 were increased in
patients with metastatic osteosarcoma compared with those with
non-metastatic osteosarcoma. High serum levels of GDF15 predicted
poor OS and short PMFS. Thus, these results suggested that GDF15
may be useful for evaluating the prognosis of patients, and aid in
the early diagnosis of pulmonary metastasis, which may support
follow-up therapy in the future.
The effects of GDF15 on tumors appear to be
contradictory, as GDF15 has been described as an antitumorigenic
gene in previous studies. Li et al (28) first reported that GDF15
overexpression reduced MDA-MB-468 breast cancer cell viability by
inducing G1 cell cycle arrest and apoptosis. GDF15-transfected
HCT-116 cells exhibited increased basal apoptosis, reduced soft
agar cloning efficiency and decreased tumorigenicity in athymic
nude mice (29). Similarly,
ectopic expression of GDF15 completely abolished the tumorigenicity
of glioblastoma cells in nude mice (30). Conversely, emerging evidence has
indicated that GDF15 also facilitated the migratory and invasive
abilities of tumor cells. Lee et al (31) reported that GDF15 promoted the
malignant progression of gastric cancer by inducing tumor cell
invasion via the upregulation of urokinase-type plasminogen
activator (uPA) and uPA receptor expression. In prostate cancer,
recombinant GDF15 treatment reduced tumor cell adhesion and
consequently accelerated tumor cell dissemination by downregulating
Rho family GTPase 3 and catenin δ1 (32). These apparently contradictory
effects of GDF15 may be due to tumor heterogeneity and the tumor
environment, but this remains unclear. In the present study, it was
observed that silencing GDF15 significantly suppressed the
migratory and invasive abilities of osteosarcoma cell lines,
whereas rhGDF15 rescued these effects in GDF15-silenced cells.
Furthermore, GDF15 knockdown suppressed the TGF-β signaling
pathway. These findings suggested that GDF15 contributed to the
progression of osteosarcoma and pulmonary metastasis, and that the
TGF-β signaling pathway may be involved in the GDF15-induced
metastasis in osteosarcoma cells; however, the detailed molecular
mechanisms require further investigation.
In conclusion, it was revealed that GDF15 was
upregulated in pulmonary metastatic osteosarcoma tissues and
patients' serum, and significantly associated with progression and
prognosis in osteosarcoma. GDF15 is an important regulator of the
TGF-β signaling pathway and may promote osteosarcoma metastasis.
Therefore, serum GDF15 may be a potential biomarker for pulmonary
metastasis in osteosarcoma, enabling the identification of patients
at high risk and informing the selection of appropriate therapeutic
strategies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and analyzed during the present
study are included in this published article.
Authors' contributions
GC and XL conceived and designed the current study.
MW and XL performed the experiments. GC was responsible for data
collection and analysis, and wrote the manuscript. All authors read
and approved the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients, and ethical approval was granted by the Ethics Committee
of The Affiliated Foshan Chancheng District Center Hospital of
Guangdong Medical University (Guangdong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marko TA, Diessner BJ and Spector LG:
Prevalence of metastasis at diagnosis of osteosarcoma: An
international comparison. Pediatr Blood Cancer. 63:1006–1011. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duchman KR, Gao Y and Miller BJ:
Prognostic factors for survival in patients with high-grade
osteosarcoma using the Surveillance, Epidemiology, and End Results
(SEER) Program database. Cancer Epidemiol. 39:593–599. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kempf-Bielack B, Bielack SS, Jürgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch H, et al: Osteosarcoma relapse after combined modality
therapy: An analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu PK, Chen WM, Chen CF, Lee OK, Haung CK
and Chen TH: Primary osteogenic sarcoma with pulmonary metastasis:
Clinical results and prognostic factors in 91 patients. Jpn J Clin
Oncol. 39:514–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goorin AM, Schwartzentruber DJ, Devidas M,
Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE and Link MP:
Presurgical chemotherapy compared with immediate surgery and
adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric
oncology group study POG-8651. J Clin Oncol. 21:1574–1580. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyers PA, Heller G, Healey J, Huvos A,
Lane J, Marcove R, Applewhite A, Vlamis V and Rosen G: Chemotherapy
for nonmetastatic osteogenic sarcoma: The Memorial Sloan-Kettering
experience. J Clin Oncol. 10:5–15. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chou AJ, Kleinerman ES, Krailo MD, Chen Z,
Betcher DL, Healey JH, Conrad EU III, Nieder ML, Weiner MA, Wells
RJ, et al: Addition of muramyl tripeptide to chemotherapy for
patients with newly diagnosed metastatic osteosarcoma: A report
from the Children's Oncology Group. Cancer. 115:5339–5348. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauskin AR, Brown DA, Kuffner T, Johnen H,
Luo XW, Hunter M and Breit SN: Role of macrophage inhibitory
cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res.
66:4983–4986. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown DA, Breit SN, Buring J, Fairlie WD,
Bauskin AR, Liu T and Ridker PM: Concentration in plasma of
macrophage inhibitory cytokine-1 and risk of cardiovascular events
in women: A nested case-control study. Lancet. 359:2159–2163. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown DA, Ward RL, Buckhaults P, Liu T,
Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B and
Breit SN: MIC-1 serum level and genotype: Associations with
progress and prognosis of colorectal carcinoma. Clin Cancer Res.
9:2642–2650. 2003.PubMed/NCBI
|
|
12
|
Tamary H, Shalev H, Perez-Avraham G,
Zoldan M, Levi I, Swinkels DW, Tanno T and Miller JL: Elevated
growth differentiation factor 15 expression in patients with
congenital dyserythropoietic anemia type I. Blood. 112:5241–5244.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Staff AC, Trovik J, Eriksson AG, Wik E,
Wollert KC, Kempf T and Salvesen HB: Elevated plasma growth
differentiation factor-15 correlates with lymph node metastases and
poor survival in endometrial cancer. Clin Cancer Res. 17:4825–4833.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blanco-Calvo M, Tarrío N, Reboredo M,
Haz-Conde M, García J, Quindós M, Figueroa A, Antón-Aparicio L,
Calvo L and Valladares-Ayerbes M: Circulating levels of GDF15, MMP7
and miR-200c as a poor prognostic signature in gastric cancer.
Future Oncol. 10:1187–1202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Winand FJ, Boegemann M, Gallitz I, Hertle
L, Semjonow A, Eveslage M, Van Aken HK, Herrmann E and Steinbicker
AU: GDF15 and Hepcidin as prognostic factors in patients with
prostate cancer. J Mol Biomark Diagn. 5:1992014.
|
|
16
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 106–120. 1980.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Ling MT, Guan XY, Tsao SW, Cheung
HW, Lee DT and Wong YC: Identification of a novel function of
TWIST, a bHLH protein, in the development of acquired taxol
resistance in human cancer cells. Oncogene. 23:474–482. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong KL, Kwong DL, Fu L, Chan TH, Chen L,
Liu H, Li Y, Zhu YH, Bi J, Qin YR, et al: Characterization of a
candidate tumor suppressor gene uroplakin 1A in esophageal squamous
cell carcinoma. Cancer Res. 70:8832–8841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Ye L, Guo W, Wang M, Huang S and
Peng X: PHF21B overexpression promotes cancer stem cell-like traits
in prostate cancer cells by activating the Wnt/β-catenin signaling
pathway. J Exp Clin Cancer Res. 36:852017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Endo-Munoz L, Cumming A, Rickwood D,
Wilson D, Cueva C, Ng C, Strutton G, Cassady AI, Evdokiou A,
Sommerville S, et al: Loss of osteoclasts contributes to
development of osteosarcoma pulmonary metastases. Cancer Res.
70:7063–7072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Böttner M, Suter-Crazzolara C, Schober A
and Unsicker K: Expression of a novel member of the TGF-beta
superfamily, growth/differentiation factor-15/macrophage-inhibiting
cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res.
297:103–110. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sung JY, Park SY, Kim JH, Kang HG, Yoon
JH, Na YS, Kim YN and Park BK: Interferon consensus
sequence-binding protein (ICSBP) promotes epithelial-to-mesenchymal
transition (EMT)-like phenomena, cell-motility, and invasion via
TGF-β signaling in U2OS cells. Cell Death Dis. 5:e12242014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kager L, Zoubek A, Pötschger U, Kastner U,
Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M,
Winkelmann W, et al: Primary metastatic osteosarcoma: Presentation
and outcome of patients treated on neoadjuvant Cooperative
Osteosarcoma Study Group protocols. J Clin Oncol. 21:2011–2018.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
26
|
Koopmann J, Buckhaults P, Brown DA,
Zahurak ML, Sato N, Fukushima N, Sokoll LJ, Chan DW, Yeo CJ, Hruban
RH, et al: Serum macrophage inhibitory cytokine 1 as a marker of
pancreatic and other periampullary cancers. Clin Cancer Res.
10:2386–2392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suesskind D, Schatz A, Schnichels S,
Coupland SE, Lake SL, Wissinger B, Bartz-Schmidt KU and Henke-Fahle
S: GDF-15: A novel serum marker for metastases in uveal melanoma
patients. Graefes Arch Clin Exp Ophthalmol. 250:887–895. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li PX, Wong J, Ayed A, Ngo D, Brade AM,
Arrowsmith C, Austin RC and Klamut HJ: Placental transforming
growth factor-beta is a downstream mediator of the growth arrest
and apoptotic response of tumor cells to DNA damage and p53
overexpression. J Biol Chem. 275:20127–20135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baek SJ, Kim KS, Nixon JB, Wilson LC and
Eling TE: Cyclooxygenase inhibitors regulate the expression of a
TGF-beta superfamily member that has proapoptotic and
antitumorigenic activities. Mol Pharmacol. 59:901–908. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Albertoni M, Shaw PH, Nozaki M, Godard S,
Tenan M, Hamou MF, Fairlie DW, Breit SN, Paralkar VM, de Tribolet
N, et al: Anoxia induces macrophage inhibitory cytokine-1 (MIC-1)
in glioblastoma cells independently of p53 and HIF-1. Oncogene.
21:4212–4219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee DH, Yang Y, Lee SJ, Kim KY, Koo TH,
Shin SM, Song KS, Lee YH, Kim YJ, Lee JJ, et al: Macrophage
inhibitory cytokine-1 induces the invasiveness of gastric cancer
cells by up-regulating the urokinase-type plasminogen activator
system. Cancer Res. 63:4648–4655. 2003.PubMed/NCBI
|
|
32
|
Liu T, Bauskin AR, Zaunders J, Brown DA,
Pankhurst S, Russell PJ and Breit SN: Macrophage inhibitory
cytokine 1 reduces cell adhesion and induces apoptosis in prostate
cancer cells. Cancer Res. 63:5034–5040. 2003.PubMed/NCBI
|