Introduction
Diabetes mellitus (DM) is a serious threat to human
health and is complicated by cardiovascular and cerebrovascular
diseases. Disturbances in lipid metabolism are a primary risk
factor for DM and dyslipidemia is a risk factor for type 2 DM
(T2DM) complicated with coronary heart disease (1,2).
Dyslipidemia is characterized by hypertriglyceridemia and increased
lipoprotein levels (3). Long-term
hyperglycemia and hyperlipidemia may cause atherosclerosis and
fatty liver (4). A low-fat and
high-fiber diet can decrease weight gain and the risk of diabetes
(5). Targeted interventions to
correct diabetes-associated dyslipidemia can lower lipid toxicity
and delay the progression of diabetes and its associated
complications.
Hydrogen sulfide (H2S) is a gaseous
signaling molecule that improves the pathophysiology of
hypertension, chronic obstructive pulmonary disease, sepsis,
hemorrhagic shock, Alzheimer's disease, gastric mucosal injury and
liver cirrhosis (6–10). In mouse models of diabetes, the
biosynthesis of H2S decreases with disease progression
(11,12). Moreover, exogenous H2S
decreases fatty liver development in obese rats (13). Clinical studies found that the
levels of H2S in patients with T2DM and obesity are
significantly decreased (14) and
that high levels of H2S may have protective effects
against obesity and diabetes (15). However, the mechanisms underlying
H2S-regulated lipid metabolism in T2DM remain poorly
understood.
AMP-activated protein kinase (AMPK) is an
evolutionarily conserved serine/threonine protein kinase that
promotes short-term energy metabolism, glucose uptake and
glycolysis. AMPK improved insulin resistance by increasing fatty
acid oxidation and decreasing triglyceride (TG) and cholesterol
synthesis (16,17). AMPK was studied as a potential
target for the treatment of T2DM (18); however, the effects of
H2S on AMPK signaling have not been defined. In the
present study, the role of H2S in the regulation of
lipid and TG metabolism in 3T3-L1 adipocytes under high glucose
(HG) conditions was investigated, and the role of AMPK signaling in
mediating the effects of H2S was examined.
Materials and methods
Cell culture
Mouse embryo 3T3-L1 preadipoctyes were obtained from
the American Type Culture Collection and were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Lonsa Science Srl), 0.1 mg/ml streptomycin and 100 U/ml penicillin
at 5% CO2 and 37°C. Preadipocytes were induced to
differentiate into mature adipocytes as described by Lu et
al (19). Confluent
preadipocytes were treated with DMEM containing 10% FBS, 10 µM
insulin, 0.5 mM isobutylmethylxanthine and 0.25 µM dexamethasone
for 2 days, followed by 2 days of treatment with DMEM containing
10% FBS and 10 µM insulin alone. Cells were replenished with fresh
media every other day until day 12 and ≥90% of cells differentiated
into mature adipocytes.
Oil red O staining
Cells were cultured in 6-well plates and Oil Red O
staining was performed as previously described (20). Briefly, cells were fixed in 4%
formalin for 30 min, permeabilized in 60% isopropanol for 20 min,
and stained with Oil Red O for 20 min at room temperature. Cells
were washed 3 times with distilled water, air dried and
counterstained with hematoxylin for 3 min at room temperature.
Slides were imaged on a Nikon 80i microscope (magnification, ×10).
Counts and lipid droplet areas were analyzed using MetaMorph
software (version 6.2; Molecular Devices, LLC). Quantitative
analysis of the lipid droplet in adipocytes was measured by
spectrophotometry. In brief, Oil Red O staining was dissolved with
isopropyl alcohol and the optical density was measured at 510 nm by
spectrophotometry.
Glucose and sodium hydrosulfide (NaHS)
treatment
Mature adipocytes were grown in 60 mm cell culture
dishes and were incubated overnight in M199 medium (Gibco; Thermo
Fisher Scientific, Inc.) containing 2% FBS. Cells were then treated
with normal glucose (NG; 5.5 mM) or HG (25 mM) for the indicated
time periods at 37°C. For NaHS experiments, cells were pretreated
with NaHS (50 µM) for 30 min at 37°C. Mannitol (24.5 mM) was added
to medium containing NG to maintain osmotic pressure (5.5 mM). For
experiments with Compound C (cat. no. 11452, MedChemExpress), the
cells were pre-treated with Compound C (10 µmol/l) at 37°C for 1 h
before stimulation with NG or HG according to the experimental
design.
Endogenous H2S
measurements
Endogenous H2S was measured as previously
described (21) with some
modifications. Briefly, cells were homogenized in 50 mM potassium
phosphate buffer at 4°C, pH 6.8. Tissue homogenates (0.1 ml) were
mixed with 2.5 ml of distilled water, 0.5 ml of 1% zinc acetate,
0.4 ml of 1.2 M hydrochloric acid containing 30 mM iron trichloride
and 0.5 ml of 7.2 M hydrochloric acid containing 20 mM N,
N-dimethyl-p-phenylenediamine sulfate salt for 20 min at room
temperature. Trichloroacetic acid (1 ml of 10% stock) was added to
a total reaction volume of 5 ml. Mixtures were centrifuged at room
temperature at 4,000 × g for 5 min and absorbance values were
measured at 670 nm. The concentrations of H2S (nM) were
calculated against a NaHS calibration curve.
Endogenous TG extraction and
measurements
TG extractions and measurements were performed using
adipogenesis colorimetric/fluorometric assay kits (cat. no.
K610-100; BioVision, Inc.) according to the manufacture's
protocols. Briefly, cells cultured in 96-well plates were washed in
PBS and 100 µl Lipid Extraction (BioVision, Inc.) solution was
added to each well. Plates were heated at 90–100°C for 30 min until
the solution in the wells became cloudy. Plates were cooled at room
temperature and mixed by shaking for 1 min. For the colorimetric
assays, 1 mM TG was used to generate the TG standard curves. Lipase
was added to each well to convert TG to glycerol and fatty acids.
TG reagent (50 µl) was added to each well and plates were incubated
at 37°C for 30 min in the dark. Absorbances were read at 570 nm and
TG concentrations were calculated from standard curves.
ELISA for monocyte chemoattractant
protein-1 (MCP-1) and adiponectin
Cell culture supernatants were collected and MCP-1
and adiponectin were measured by ELISA assay (cat. nos. CSB-E07430m
and CSB-E07272m; CUSABIO Technology LLC) according to the
manufacturer's protocol.
Western blot analysis
Cells were homogenized for proteome extraction in
TNE lysis buffer (10 mM Tris at pH 7.4, 150 mM NaCl, 1 mM EDTA and
1% Nonidet P-40) containing protease and phosphatase inhibitors.
Protein concentrations were determined using BCA assays, 20 mg per
protein sample was used for western blot analysis. Equal amounts of
proteins were subject to 10% SDS-PAGE, transferred to
polyvinylidene fluoride membranes, and were blocked in 5% fat-free
milk for 1 h at room temperature. Membranes were probed with
anti-AMPKα (1:1,000; Cell Signaling Technology, Inc.);
anti-phosphorylated (p)-AMPKα (Thr172; 1:1,000; Cell Signaling
Technology, Inc.), anti-β-actin (1:2,000; Santa Cruz Biotechnology,
Inc.), and anti-MCP1 antibodies (cat. no. ab25124; Abcam) overnight
at 4°C. Following three washing with TBST, membranes were labeled
with HRP-conjugated secondary antibodies (1:5,000, SA00001-1,
HRP-conjugated Affinipure Goat Anti-Mouse IgG (H+L); 1:5,000,
SA00001-2, HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H+L),
ProteinTech Group, Inc.) for 1 h at room temperature. Proteins were
detected using the enhanced chemiluminescence system and
immunoreactive bands were quantified using ImageJ software (version
1.42; National Institutes of Health).
Statistical analysis
Data were analyzed using a Student's t-test or
one-way ANOVA followed by aTukey's post hoc test using the SPSS
software (version 16.0; SPSS, Inc.). All values are presented as
the mean ± SEM. P<0.05 was considered to indicate a
statistically significant difference.
Results
HG increases TG content by
downregulating H2S production
The effects of HG on TG levels in mature adipocytes
were investigated. 3T3-L1 cells were differentiated into mature
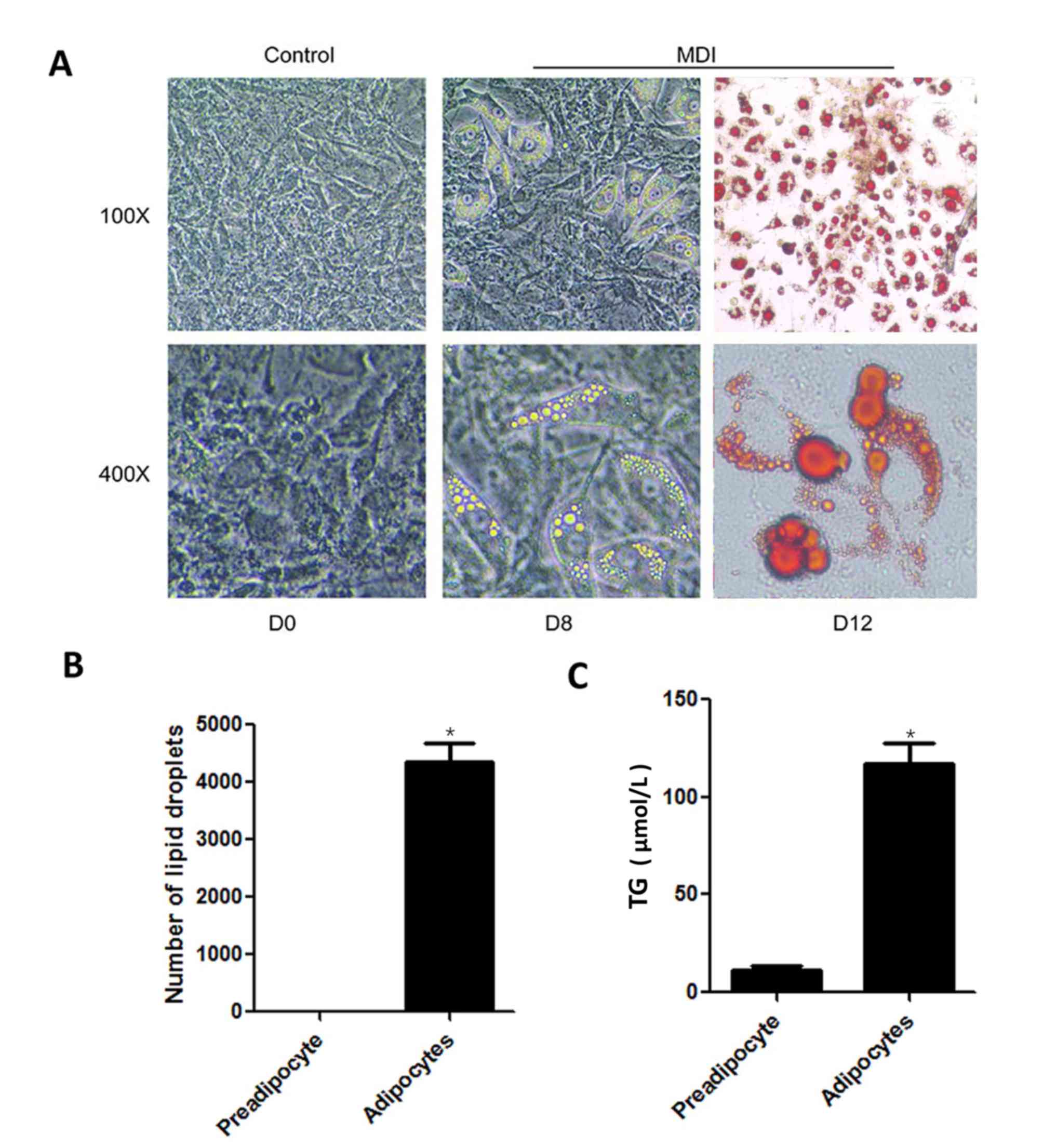
adipocytes, which demonstrated typical characteristic morphology,
round cells containing a high accumulation of Oil Red stained lipid
droplets (Fig. 1A) (19). TG levels were significantly higher
in induced mature adipocytes compared with preadipocytes (Fig. 1B and C).
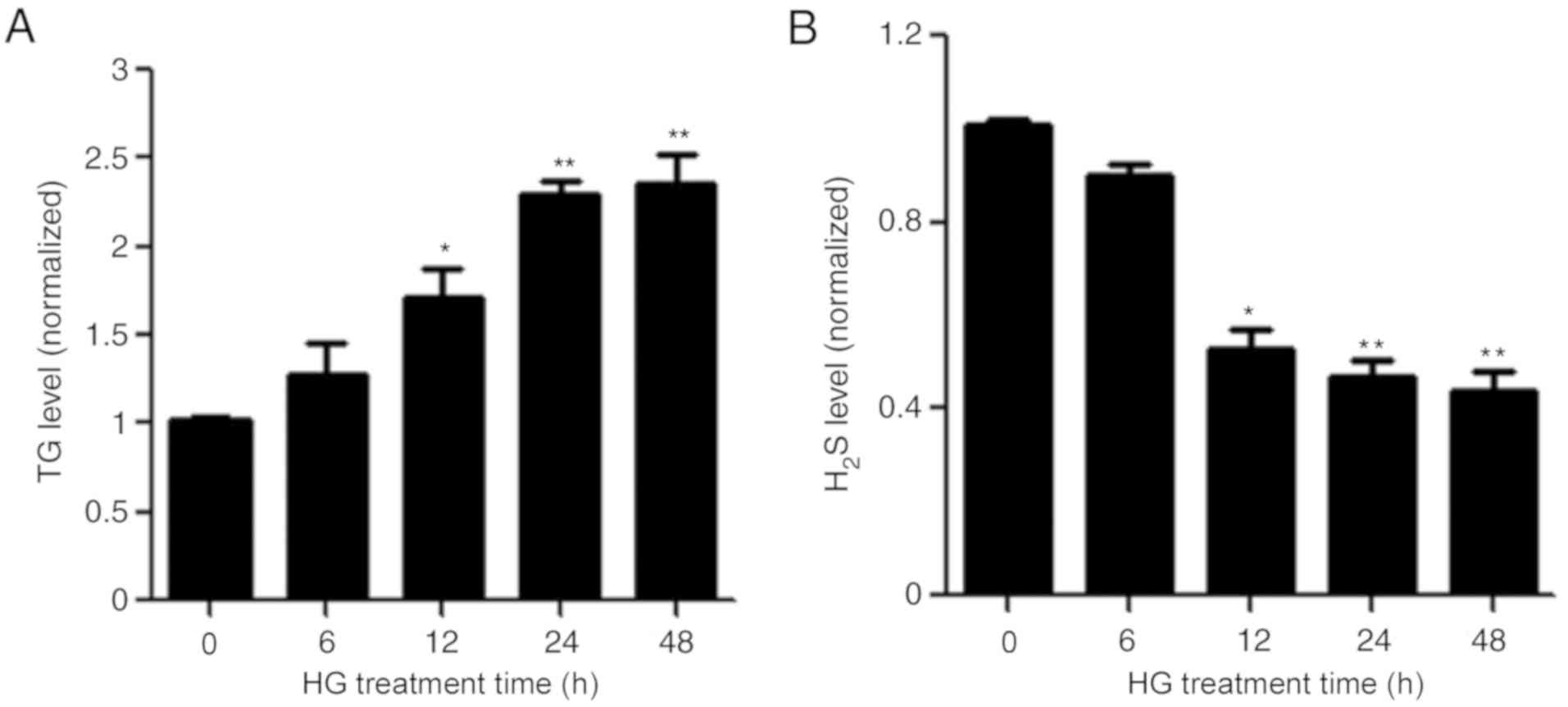
Subsequently, adipocytes were treated with HG for 6,
12, 24 or 48 h. HG significantly increased the content of TG in
cultured adipocytes in a time-dependent manner (Fig. 2A). Interestingly, HG also
significantly decreased the production of H2S in mature
adipocytes (Fig. 2B).
NaHS inhibits HG-induced TG
accumulation and the aberrant secretion of adipokines in mature
adipocytes
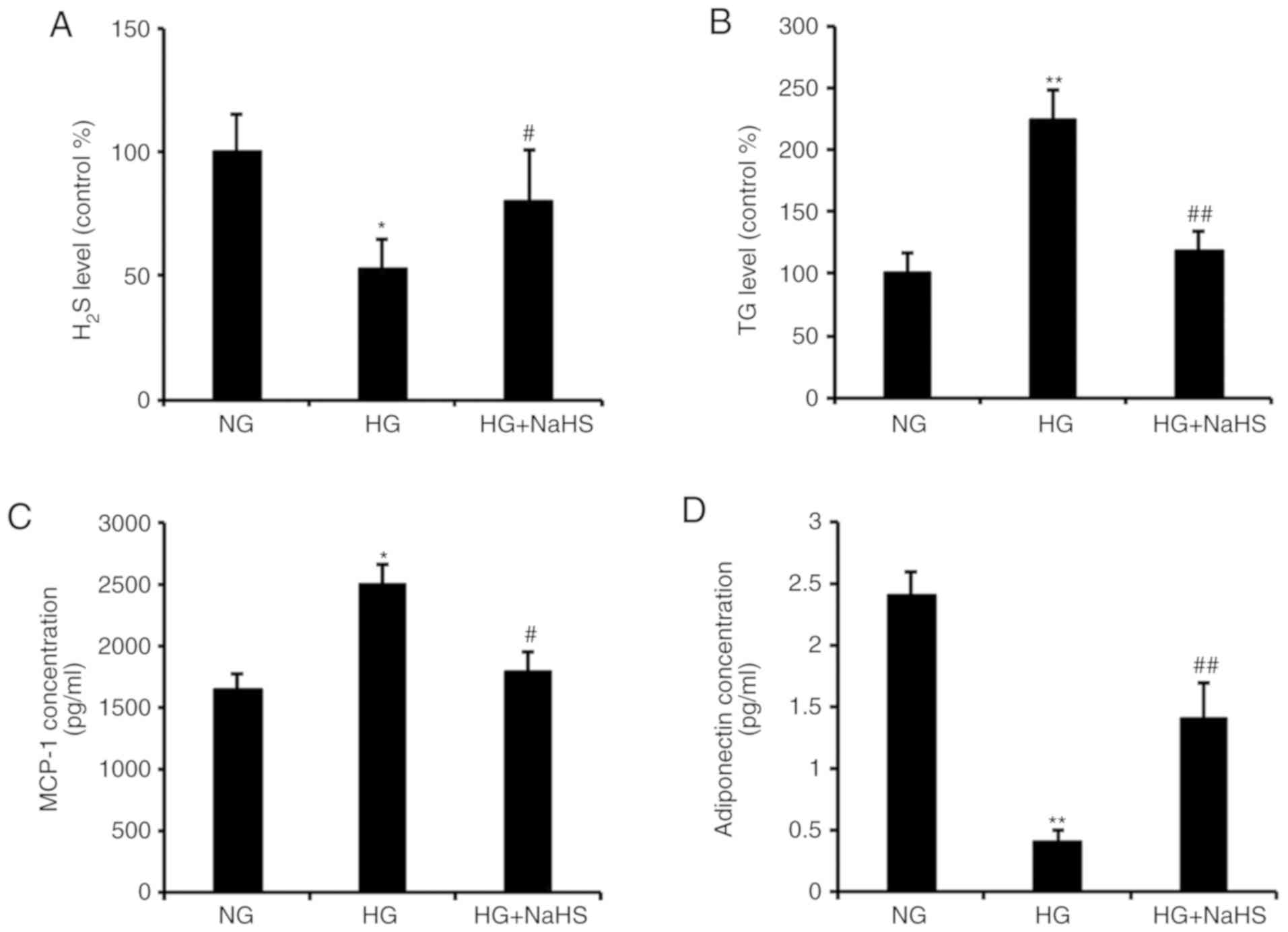
In order to investigate whether the loss in
H2S was responsible for HG-induced TG upregulation in
adipocytes, NaHS was used as an exogenous donor to enhance
H2S production. NaHS pretreatment significantly reversed
the HG-induced loss of H2S in cultured adipocytes
(Fig. 3A). More importantly, NaHS
treatment significantly inhibited the HG-induced increase in TG in
adipocytes (Fig. 3B).
The protective effect of NaHS against HG-induced
adipokine secretion was then investigated. HG significantly
increased the secretion of MCP-1 and decreased the secretion of
adiponectin, which was reversed by the addition of NaHS (Fig. 3C and D).
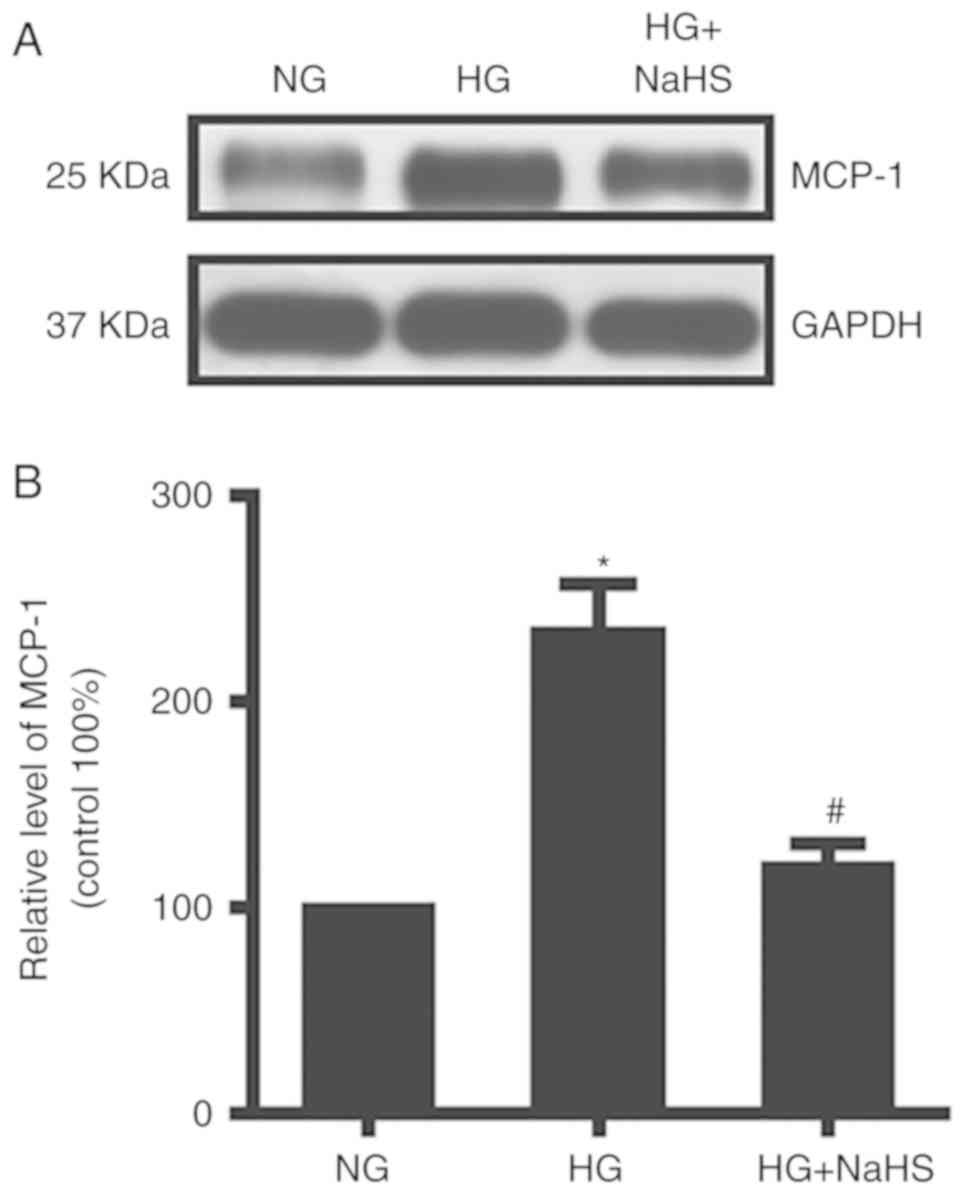
MCP-1 was highly expressed in HG conditions as
assessed by western blot analysis. NaHS treatment decreased MCP-1
levels in mature adipocytes (Fig.
4).
NaHS suppresses the HG-induced
increase in TG through AMPK activation
AMPK activation was previously shown to decrease
lipid synthesis and enhance fatty acid oxidation (18). Therefore, the role of AMPK in the
suppression of HG-induced increase in TG by NaHS was investigated
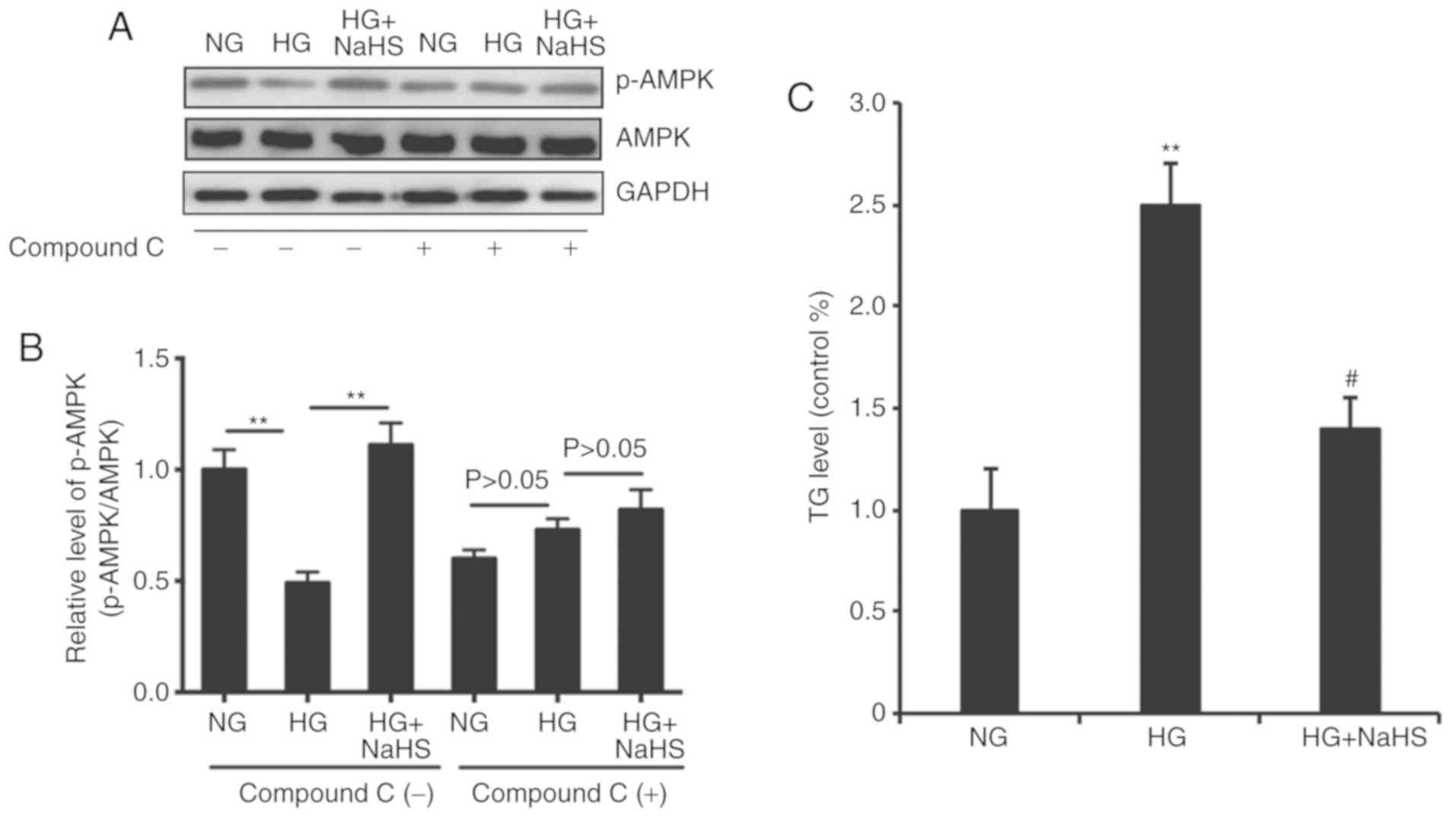
in the present study. Western blot analysis showed that HG
significantly decreased the phosphorylation on Thr172 of AMPKα in
mature adipocytes, which was counteracted by NaHS (Fig. 5). Moreover, the effects of HG and
NaHS on AMPKα phosphorylation were reversed by treatment with
compound C (10 µmol/l), an AMPK inhibitor (Fig. 5A and B). Therefore, the inhibitory
effects of NaHS on the HG-induced increase of TG may be
AMPK-dependent.
Discussion
A comprehensive understanding of the mechanisms
underlying the pathogenesis of diabetic disturbances in lipid
metabolism is required. The present study provided three new
insights into lipid metabolism during diabetes. Firstly, HG
treatment increased TG levels and decreased H2S in
3T3-L1 adipocytes. Secondly, the H2S donor, NaHS,
protected 3T3-L1 adipocytes against HG-induced accumulation of TG.
Finally, NaHS suppressed the HG-induced increase in TG by
activating AMPK.
A previous study showed the association of lipid
metabolic disturbance in DM with the decreased expression of
H2S (15). Previous
studies found that H2S protected against HG-induced
aberrant secretion of adipokines in cultured 3T3-L1 adipocytes
(22). In the present study, the
protective effects of H2S against HG-induced aberrant
lipid metabolism were investigated. The findings of the present
study indicated that HG increased the TG level in cultured
adipocytes and promoted the aberrant secretion of adipokines.
Importantly, the production of H2S also decreased
following HG treatment and exogenous H2S protected
against HG-induced lipid metabolic disturbances in 3T3-L1
adipocytes. Previous studies revealed that H2S can
improve the health of obese individuals with diabetes (15–17)
by promoting, at least in part, the degradation of TG.
A recent study showed that adipose tissue is not
only an energy-storing organ, but also an important endocrine organ
that secretes adipocytokines including adiponectin, leptin,
interleukin (IL)-1, IL-6 and MCP-1 (4). In the present study, HG treatment
increased the levels of MCP-1 and decreased the secretion of
adiponectin in adipocytes. Moreover, NaHS pretreatment blocked the
effects of HG on adipocytokine secretion, which suggested that NaHS
exerted protective effects.
AMPK plays an important role in regulating energy
metabolism. When the ratio of AMP/ATP increases, AMPK is
phosphorylated and activates an array of downstream targets,
increasing the cell catabolism by inhibiting the synthesis of
glycogen and fat, and promoting fatty acid oxidation. In contrast,
when the AMP/ATP ratio decreases, AMPK activity is inhibited and
cell anabolism increases (23).
AMPK is a heterologous trimer consisting of three subunits: α, β
and γ (24). The α subunit plays a
catalytic role, whereas the β and γ subunits play a regulatory
role. AMPKα can be activated by phosphorylation at Thr172.
Activated AMPK subsequently promotes phosphorylation of the
downstream substrate acetyl coenzyme A carboxylase (ACC), which
inhibits ACC activity, thus inhibiting the synthesis of fatty acids
and cholesterol, and increasing fatty acid oxidation (24). In skeletal muscle cells in
vitro, AMPK promotes cellular uptake of sugar, inhibits
glycogen synthesis and promotes glycolysis (25). In hepatocytes, AMPK inhibits
hepatocyte gluconeogenesis and glycolysis (26). Notably, in the liver, AMPK
activation resulted in decreased fat accumulation by upregulating
the expression of lipid oxidation genes (27). Liver-specific AMPKα deletion in
mice led to increased plasma TG content and hepatic lipogenesis
(28). Thus, AMPK is also an
important regulator of lipid metabolism. In the present study, HG
led to decreased phosphorylation of AMPKα and increased the
accumulation of TG in adipocytes, which could be reversed by NaHS
pretreatment, without affecting AMPKα expression. Therefore, AMPKα
may be downstream of H2S signaling, mediating the
function of H2S in lipid metabolism of adipocytes.
Further studies are required to prove whether H2S
promoted lipid metabolism through the activation of AMPKα and to
determine the underlying mechanism of this activation.
Hormone-sensitive lipase and adipose triglyceride lipase were
associated with the mobilization of stored triglycerides from
adipose tissue. The lipolysis-associated genes and proteins include
peroxisome proliferator-activated receptor γ, visfatin and Insig-2.
The protective mechanisms of H2S require further
investigation in the future.
In conclusion, the in vitro experiments of
the present study have shown that AMPK signaling regulates lipid
metabolism and is essential for H2S-induced lipid
metabolic protection against HG injury. Furthermore,
H2S-activated AMPK-dependent signaling protects against
aberrant lipid metabolism. Thus, therapeutic H2S
represents a promising therapeutic strategy for the treatment of
diabetic lipid metabolic disturbances.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shandong Provincial
Medical and Health Science and Technology Development Plan (grant
no. 2017WS626), the National Science Foundation of China (grant
nos. 81670753 and 81070641), the Shandong Provincial Medical and
Health Science and Technology Development Plan (grant no.
2014WS0156), the National Science Foundation for Young Scholars of
China (grant no. 81500554) and the Scientific and Technological
Projects of Shandong Province (grant no. 2014GSF118041).
Availability of data and materials
All data sets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YN made substantial contributions to the design of
the study and wrote paper. ZP and JW conducted research and
analyzed data. MX, SC, XL, AS and NL helped to conduct research and
analyzed data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nadeem A, Mumtaz S, Naveed AK, Aslam M,
Siddiqui A and Lodhi GM: Pattern of dyslipidaemia and impact of
increasing age and duration of type 2 diabetes mellitus on
dyslipidaemia, insulin levels and insulin resistance. J Pak Med
Assoc. 65:928–932. 2015.PubMed/NCBI
|
|
2
|
Tomkin GH and Owens D: Dyslipidaemia of
diabetes and the intestine. World J Diabetes. 6:970–977. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hermans MP and Valensi P: Elevated
triglycerides and low high-density lipoprotein cholesterol level as
marker of very high risk in type 2 diabetes. Curr Opin Endocrinol
Diabetes Obes. 25:118–129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong ND, Zhao Y, Patel R, Patao C, Malik
S, Bertoni AG, Correa A, Folsom AR, Kachroo S, Mukherjee J, et al:
cardiovascular risk factor targets and cardiovascular disease event
risk in diabetes: A pooling project of the atherosclerosis risk in
communities study, multi-ethnic study of atherosclerosis, and
Jackson heart study. Diabetes Care. 39:668–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sylvetsky AC, Edelstein SL, Walford G,
Boyko EJ, Horton ES, Ibebuogu UN, Knowler WC, Montez MG, Temprosa
M, Hoskin M, et al: A high-carbohydrate, high-fiber, low-fat diet
results in weight loss among adults at high risk of type 2
diabetes. J Nutr. 147:2060–2066. 2017.PubMed/NCBI
|
|
6
|
van Goor H, van den Born JC, Hillebrands
JL and Joles JA: Hydrogen sulfide in hypertension. Curr Opin
Nephrol Hypertens. 25:107–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Dai X, Zhu D, Xu X, Gao C and Wu C:
An exogenous hydrogen sulphide donor, NaHS, inhibits the apoptosis
signaling pathway to exert cardio-protective effects in a rat
hemorrhagic shock model. Int J Clin Exp Pathol. 8:6245–6254.
2015.PubMed/NCBI
|
|
8
|
Nagpure BV and Bian JS: Brain, learning,
and memory: Role of H2S in neurodegenerative diseases. Handb Exp
Pharmacol. 230:193–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo SB, Duan ZJ, Wang QM, Zhou Q, Li Q and
Sun XY: Endogenous carbon monoxide downregulates hepatic
cystathionine-γ-lyase in rats with liver cirrhosis. Exp Ther Med.
10:2039–2046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aboubakr EM, Taye A, El-Moselhy MA and
Hassan MK: Protective effect of hydrogen sulfide against cold
restraint stress-induced gastric mucosal injury in rats. Arch Pharm
Res. 36:1507–1515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin S, Pu SX, Hou CL, Ma FF, Li N, Li XH,
Tan B, Tao BB, Wang MJ and Zhu YC: Cardiac H2S generation is
reduced in ageing diabetic mice. Oxid Med Cell Longev.
2015:7583582015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brancaleone V, Roviezzo F, Vellecco V, De
Gruttola L, Bucci M and Cirino G: Biosynthesis of H2S is impaired
in non-obese diabetic (NOD) mice. Br J Pharmacol. 155:673–680.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu D, Zheng N, Qi K, Cheng H, Sun Z, Gao
B, Zhang Y, Pang W, Huangfu C, Ji S, et al: Exogenous hydrogen
sulfide mitigates the fatty liver in obese mice through improving
lipid metabolism and antioxidant potential. Med Gas Res. 5:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki K, Sagara M, Aoki C, Tanaka S and
Aso Y: Clinical implication of plasma hydrogen sulfide levels in
Japanese patients with type 2 diabetes. Intern Med. 56:17–21. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carter RN and Morton NM: Cysteine and
hydrogen sulphide in the regulation of metabolism: Insights from
genetics and pharmacology. J Pathol. 238:321–332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steinberg GR and Schertzer JD: AMPK
promotes macrophage fatty acid oxidative metabolism to mitigate
inflammation: Implications for diabetes and cardiovascular disease.
Immunol Cell Biol. 92:340–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Neill HM, Holloway GP and Steinberg GR:
AMPK regulation of fatty acid metabolism and mitochondrial
biogenesis: Implications for obesity. Mol Cell Endocrinol.
366:135–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coughlan KA, Valentine RJ, Ruderman NB and
Saha AK: AMPK activation: A therapeutic target for type 2 diabetes?
Diabetes Metab Syndr Obes. 7:241–253. 2014.PubMed/NCBI
|
|
19
|
Lu S, Guan Q, Liu Y, Wang H, Xu W, Li X,
Fu Y, Gao L, Zhao J and Wang X: Role of extrathyroidal TSHR
expression in adipocyte differentiation and its association with
obesity. Lipids Health Dis. 11:172012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Howell G III and Mangum L: Exposure to
bioaccumulative organochlorine compounds alters adipogenesis, fatty
acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol
In Vitro. 25:394–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath
RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M and Moore PK:
Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced
inflammation in the mouse. FASEB J. 19:1196–1198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan Z, Wang H, Liu Y, Yu C, Zhang Y, Chen
J, Wang X and Guan Q: Involvement of CSE/H2S in high glucose
induced aberrant secretion of adipokines in 3T3-L1 adipocytes.
Lipids Health Dis. 13:1552014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hardie DG: The AMP-activated protein
kinase pathway-new players upstream and downstream. J Cell Sci.
117:5479–5487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saha AK and Ruderman NB: Malonyl-CoA and
AMP-activated protein kinase: An expanding partnership. Mol Cell
Biochem. 253:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Halse R, Fryer LG, McCormack JG, Carling D
and Yeaman SJ: Regulation of glycogen synthase by glucose and
glycogen: A possible role for AMP-activated protein kinase.
Diabetes. 52:9–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foretz M, Ancellin N, Andreelli F,
Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S and Viollet
B: Short-term overexpression of a constitutively active form of
AMP-activated protein kinase in the liver leads to mild
hypoglycemia and fatty liver. Diabetes. 54:1331–1339. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sabater AG, Ribot J, Priego T, Vazquez I,
Frank S, Palou A and Buchwald-Werner S: Consumption of a mango
fruit powder protects mice from high-fat induced insulin resistance
and hepatic fat accumulation. Cell Physiol Biochem. 42:564–578.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andreelli F, Foretz M, Knauf C, Cani PD,
Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, et
al: Liver adenosine monophosphate-activated kinase-alpha2 catalytic
subunit is a key target for the control of hepatic glucose
production by adiponectin and leptin but not insulin.
Endocrinology. 147:2432–2441. 2006. View Article : Google Scholar : PubMed/NCBI
|