Introduction
Diabetes is a metabolic disease, with the number of
people with diabetes estimated to reach 700 million worldwide in
2040 (1). Cardiovascular
complications are the main cause of mortality in diabetic patients,
and ~80% of diabetic patients die from cardiovascular diseases
(2). Diabetic cardiomyopathy (DCM)
has the highest mortality rate associated with diabetic
complications, and the methods for treatment are poor (3). In the early stage of the disease,
patients exhibit symptoms such as ventricular diastolic dysfunction
and myocardial remodeling; as the disease worsens, microvascular
disease and the necrosis of myocardial tissue occurs, eventually
leading to heart failure (4).
Although there are many unknown mechanisms of in
diabetic cardiomyopathy, previous studies have shown that metabolic
disorders have a significant role in the pathogenesis of diabetic
cardiomyopathy. As the body does not normally use glucose to supply
energy, the oxidation of fatty acids is exacerbated, causing
toxicity from sugar and fat. This damages the mitochondria in
myocardial cells (5), inducing
mitochondrial dysfunction and deficits in ATP production, and the
damage can eventually lead to the apoptosis of myocardial cells,
hypertrophy and fibrosis, and, ultimately, cardiac dysfunction
(6). Peroxisome
proliferator-activated receptor γ coactivator 1α (PGC-1α), which is
one of the members of the PGC-1α family, plays a vital role in the
process of biosynthesis in mitochondria (7–9); it
can stimulate nuclear transcription factor nuclear respiratory
factor (NRF) and other downstream factors to promote the
proliferation of mitochondria and the expression of proteins in
mitochondria, regulate cellular energy metabolism and provides ATP
to heart tissue (10). The
increased expression of PGC-1α can improve myocardial metabolic
disorders (11).
Following stimulation by adverse environmental
factors, the apoptosis of cardiac cells is increased, and changes
in cell function and structure lead to cardiac hypertrophy. Under
conditions of high glucose, a series of metabolites generated by
cardiac cells can stimulate the binding of extracellular
apoptosis-associated ligands to specific receptors on the cell
membrane, activating cysteine-containing aspartate-specific
proteases (caspases), triggering a series of apoptosis pathways and
accelerating the process of apoptosis (12). The abnormal structure or function
of mitochondria can lead to abnormal energy metabolism, which not
only damages myocardial function but also causes inflammation and
oxidative stress to further aggravate the damage. Therefore,
restoring mitochondrial function is key to the treatment of
diabetic myocardium (13).
Astragaloside IV (ASIV) is a saponin compound
isolated from Astragalus that has the anti-apoptotic,
anti-oxidative and glucose-controlling effects; therefore it has a
certain therapeutic effect on diabetic cardiomyopathy (14). However, the pharmacological action
of ASIV on diabetic cardiomyopathy is still unclear and requires
further investigation. Previous studies have found that ASIV can
improve energy metabolism dysfunction induced by isoproterenol in
rats by increasing the expression of PGC-1α by isoproterene in rats
(15–20).
The aim of the present study was to investigate the
pharmacological mechanism of ASIV in diabetic cardiomyopathy by
focusing on the aspects of energy metabolism and PGC-1α.
Materials and methods
Reagents
ASIV was purchased from Nanjing Jingzhu
Bio-Technology Co., Ltd. Streptozotocin (STZ) and carboxymethyl
cellulose sodium (CMC-Na) were purchased from Sigma-Aldrich (Merck
KGaA). A TUNEL kit (In Situ Cell Death Detection kit, AP)
was purchased from Roche Molecular Diagnostics. ATP (kt39623), ADP
(kt210319) and AMP (kt28319) ELISA kits were purchased from MSKBIO
Co. Ltd. A BCA Protein Assay kit was purchased from Beyotime
Institute of Biotechnology. TRIzol reagent and a reverse
transcription-PCR (RT-PCR) kit were purchased from Dingguo
Biological Co. Ltd. PGC-1α, NRF1, atrial natriuretic peptide (ANP)
and brain natriuretic peptide (BNP) were purchased from ABclonal.
Cleaved caspase-3, caspase-3 and cytochrome c (Cyt C) were
purchased from Biological Technology Co. Ltd.
Animals and experimental design
Healthy male Sprague-Dawley rats (6–8 weeks old,
180–200 g, n=50) were purchased from the Experimental Animal Center
of Jinzhou Medical University (Jinzhou, China). All experiments and
procedures were approved by the Medical Ethics Committee of Jinzhou
Medical University (approval no. LNMU-2016-121). The rats were
treated in accordance with the Guide for the Care and Use of
Laboratory Animals (8th edition, National Academies Press)
(21). The rats were adapted to
their new environment (at a temperature of 20–23°C, humidity from
30–48%, and a 12-h light/dark cycle) for 1 week before the
experiment. There were 5 groups in the in vivo experiments,
and each group consisted of 10 rats. Healthy male SD rats (n=40)
were injected with STZ through the tail vein at a dose of 35 mg/kg.
The fasting blood glucose level was detected 1 week later. If an
animal presented with a fasting blood glucose level >16.7 mM and
symptoms of polydipsia, polyuria and polyphagia, it was considered
a diabetic model rat. Diabetes was successfully established in 40
rats and 30 of them were randomly selected and randomly divided
into three groups of 10 each. The ASIV-high (H), ASIV-mid (M) and
ASIV-low (L) groups were established by the intraperitoneal
injection of three different doses of ASIV (40, 20 and 10 mg/kg,
respectively) once a day. ASIV was dissolved in 1% CMC. The
remaining 10 rats were used for the diabetic model only group, and
10 SD rats were used as the control group. The same volume of 1%
CMC was administered daily. Blood glucose was measured and recorded
on day 1, and weeks 2, 4, 8, 12 and 16 following administration.
After 16 weeks of ASIV treatment, rats were anesthetized [urethane,
1.5 g/kg, intraperitoneal injection (i.p.)] (22), and hemodynamic parameters were
measured. Rats were euthanized by cervical dislocation under
anesthesia (diazepam, 10 mg/kg and ketamine, 50 mg/kg) and
myocardial tissue was collected. Some of the tissues were fixed
with 4% paraformaldehyde solution at room temperature for 24 h, and
the remaining tissue was stored at −86°C.
Cell culture
H9C2 cells were purchased from Wuhan Boster Biotech
Company, Ltd. and the cells were propagated in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (HyClone; GE
Healthcare Life Sciences) and 1% penicillin-streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.). The H9C2 cells were
divided into six groups: Con (control), mannitol (control +
mannitol 7.5 mM), high glucose (HG; glucose 33.3
mmol·l−1), HG + ASIV-L (20 µmol·l−1), HG +
ASIV-M (40 µmol·l−1) and HG + ASIV-H (80
µmol·l−1).
Detection of cardiac function
indicators
After being anesthetized with 20% urethane (1.5
g/kg, i.p.), all rats were weighed before the beginning of the
experiment. A physiological recorder was used to monitor the left
ventricular systolic pressure (LVSP), the left ventricular end
diastolic pressure (LVEDP) and the maximum left ventricular
ascending/descending rate (± dp/dtmax), after the right
common carotid artery was isolated and intubated into the left
ventricle. The hearts of the rats were harvested and rinsed with
precooled normal saline, and then dissected and weighed. The ratio
of the left ventricle weight to the heart weight (LVW/HW) was
separately calculated. Finally, the heart tissues retained for
slicing were placed in 4% polyformaldehyde and the remaining heart
tissues were stored at −86°C.
Hematoxylin and eosin (H&E) and
TUNEL staining
After soaking in 4% formaldehyde at room temperature
for 24 h, the heart tissues were embedded in paraffin and sectioned
into 5-µm sections. The sections were stained using H&E
(hematoxylin 2–3 min and eosin 1 min, both at room temperature) and
TUNEL kits [50 µl TdT and dUTP (1:9) in a dark moist chamber at
37°C for 1 h], respectively. An inverted microscope (DMI3000B,
Leica Microsystems GmbH) was used for observation (magnification,
×400), and the matching software LAS v4.3 was used to capture and
analyze images. The apoptotic index (%) was calculated as the
number of positive apoptotic cardiomyocytes/the total number of
cardiomyocytes ×100.
Detection of the cell survival by
MTT
H9C2 cells (Cell concentration ~5×104/ml)
were seeded onto 96-well plates (8 wells per group) and cultured at
37°C and 5% CO2 for 24 h. After 48 h, 20 µl MTT (5
mg/ml) was added, and the cells were incubated for 4 h.
Subsequently, 150 µl DMSO was added, and the plates were shaken for
10 min to dissolve the formazan crystals. The absorbance value (OD
value) was measured at a wavelength of 490 nm using an
enzyme-labeling instrument. Cell viability was calculated.
Apoptosis assays
Apoptosis was detected using an Annexin V-FITC
apoptosis detection kit (Annexin V-FITC/PI Apoptosis Detection kit,
Dalian Meilun Biology Technology Co., Ltd.) according to the
manufacturer's instructions. The cells in each group
(5×105-6 cells/ml) were extracted, 100 µl 1X binding
buffer was added, and the cells were resuspended gently. After
adding 5 µl Annexin V-FITC and 5 µl propidium iodide, the cells
were mixed gently and incubated at room temperature without light
for 10 min. Following the addition of 400 µl 1X binding buffer and
suspending the cells gently, Annexin V-FITC staining was detected
using a BD Accuri C6 Plus flow cytometer (BD Biosciences) and
analyzed by FlowJo v10 (FlowJo LLC).
Detection of ATP/ADP and ATP/AMP
According to the ELISA kit instructions, the OD
value was detected and recorded. First, standard curves were
generated, and the related reagents were added to the myocardial
tissues and H9C2 cells. Then, ATP, ADP and AMP were calculated by
measuring the OD values and the standard curves of each group.
Finally, the ATP/ADP and ATP/AMP ratio were obtained.
Preparation of protein extracts and
western blot analysis
Nucleoproteins and mitochondrial proteins were
extracted from the myocardial tissue and H9C2 cells with
corresponding kits (Tissue or Cell Total Protein Extraction kit,
Sangon Biotech Co. Ltd.; Nuclear Protein Extraction kit, Beijing
Solarbio Science & Technology Co., Ltd.; Tissue Mitochondria
Isolation kit, Novland Biopharma Co. Ltd.; www.novland.com.cn) The procedure was performed
according to the manufacturer's instructions. The total protein,
the cytoplasmic protein and the cytoplasmic protein after the
removal of mitochondria were extracted. After the protein
concentration was determined using the BCA method, the samples were
prepared according to the required concentration and dosage (40 µg
of total protein to each lane). SDS-PAGE (10–12% polyacrylamide
gels) was used to separate protein samples. The isolated proteins
were transferred to PVDF membranes (EMD Millipore) and blocked with
1% BSA at room temperature for 2 h. The membranes were incubated
with primary antibodies against ANP (NPPA polyclonal antibody,
ABclonal Biotech Co., Ltd. A14755, 1:1,000), BNP (BNP polyclonal
antibody, ABclonal Biotech Co., Ltd. A2179, 1:1,000), Cyt C
(anti-cytochrome c antibody, Boster Biological Technology,
A03529, 1:1,000), caspase-3 (anti-caspase-3 antibody, Boster
Biological Technology PB9188, 1:1,000), cleaved caspase-3
(anti-Caspase-3 p12 antibody, Boster Biological Technology BM4620,
1:1,000), NRF1 (NRF1 polyclonal antibody, ABclonal Biotech Co.,
Ltd. A5547, 1:1,000), PGC-1α (PGC1 α polyclonal antibody, ABclonal
Biotech Co., Ltd. A12348, 1:1,000) and β-actin (β-actin Antibody,
ProteinTech Group, Inc 66009-1-Ig, 1:20,000) at room temperature
for 1.5 h. Then the PVDF membrane was placed in the corresponding
antibody (HRP-conjugated Affinipure Goat Anti-Rabbit IgG(H+L),
ProteinTech Group, Inc. SA00001-2, 1:20,000; HRP goat anti-mouse
IgG (H+L), ABclonal Biotech Co., Ltd. AS003, 1:10,000) and
incubated at room temperature for 2 h. All antibodies were diluted
with 0.1% BSA. The results were detected with enhanced
chemiluminescence reagents (Wanleibio Co., Ltd.) and analyzed using
Results Quantity One software v6.0.1 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data are expressed as the mean ± SD and were
analyzed using SPSS 19.0 software (IBM Corp.). Statistical analysis
was performed using one-way ANOVA followed by Bonferroni's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ASIV has a regulatory effect on blood
glucose in diabetic rats
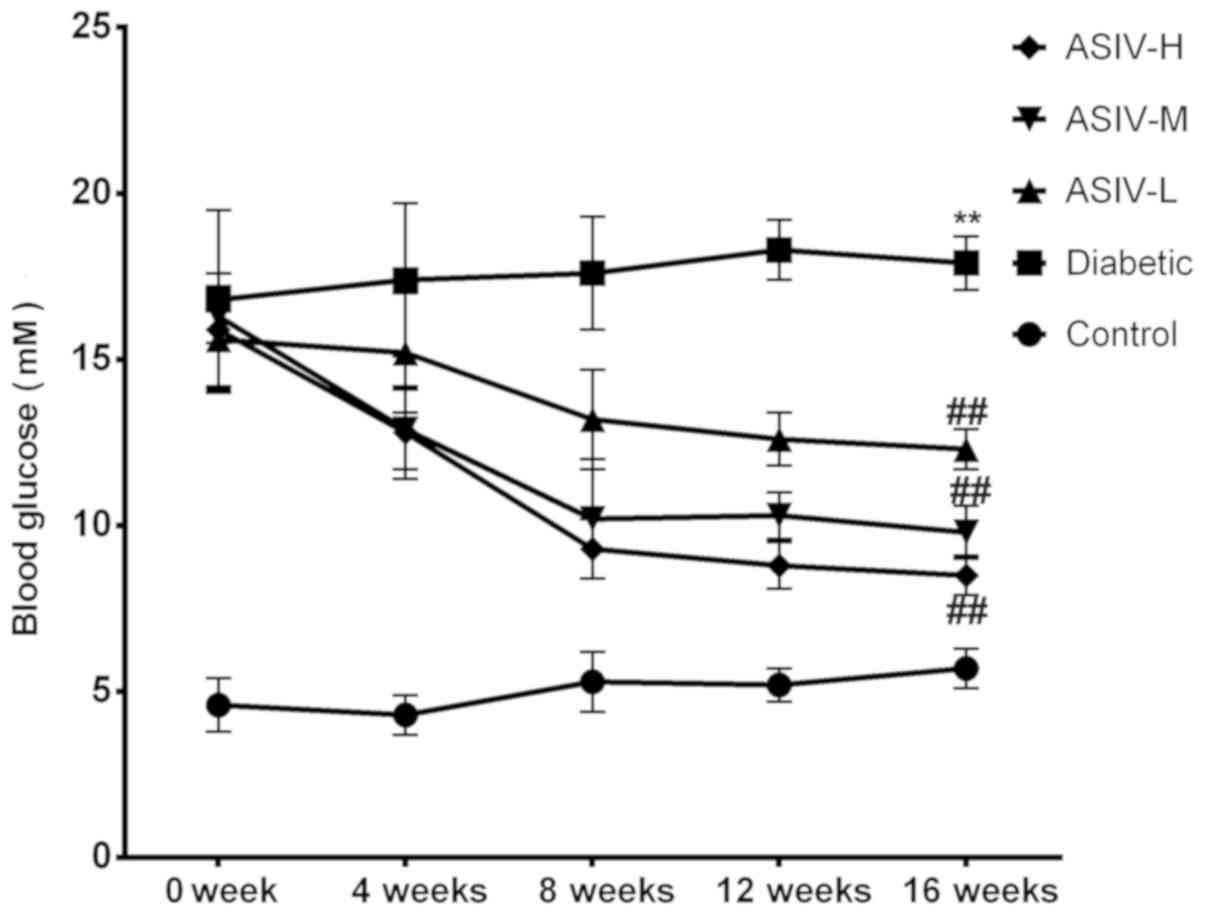
A broken line graph (Fig. 1) of the blood glucose levels was
made to observe the effect of ASIV on blood glucose. No significant
changes in blood glucose level in the control group and the
diabetic group over the 16-week experimental period, indicating
that the solvent did not regulate blood glucose. The blood glucose
of the three groups administered ASIV decreased markedly as the
duration of ASIV administration increased, and the degree of the
decrease was associated with the administered dose, indicating that
ASIV has an effect on hypoglycemia.
ASIV improves the physiological
indexes in STZ-induced diabetic rats and decreases the influence on
tissue structure
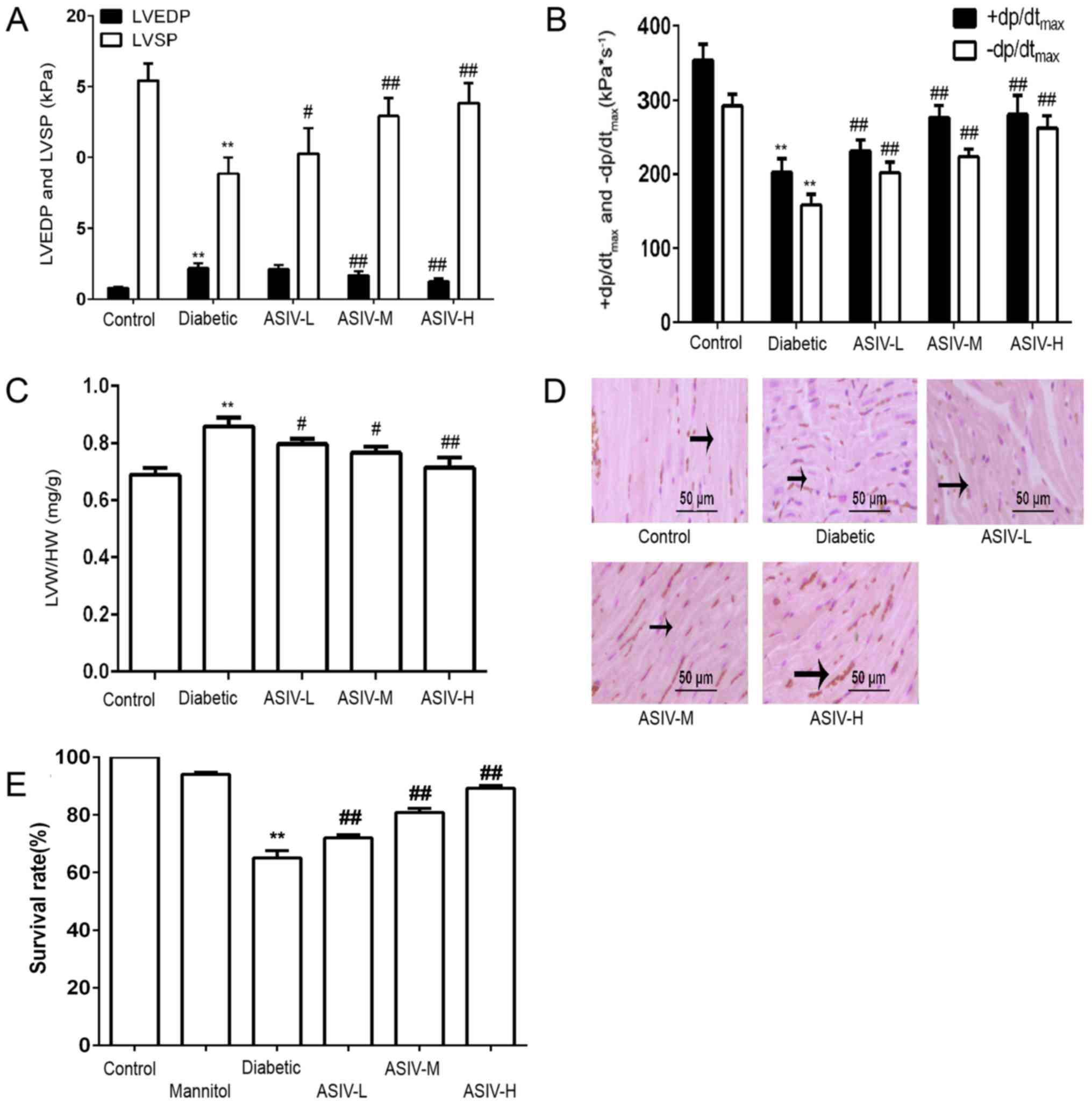
Compared with the control group, the LVSP and
+dp/dtmax in the diabetes model group were significantly
decreased, indicating decreased myocardial systolic ability,
whereas LVEDP was increased and -dp/dtmax decreased,
reflecting decreased myocardial diastolic ability. Combined with
the above signs of diabetic cardiomyopathy, myocardial diastolic
function was abnormal and heart failure occurred. According to the
results, the LVEDP gradually decreased as the dose increased, LVSP,
+dp/dtmax and -dp/dtmax gradually increased,
and myocardial function was significantly improved by ASIV
intervention (Fig. 2A and B). As
shown in Fig. 2C, the LVW/HW was
significantly increased in the diabetic group compared with the
control animals, indicating that diabetes caused the left heart to
increase in weight. Following the administration of ASIV, the
LVW/HW decreased compared with the diabetic model group, and the
degree of the decrease dependent on the ASIV concentration,
demonstrating the effect of ASIV on the degree of myocardial
hypertrophy.
H&E staining showed that the myocardial fibers
in the heart tissues of diabetic rats were disordered and
transversely broken. This damage was improved in the ASIV-treated
groups. The therapeutic effect depended on the dose (Fig. 2D). The results of the cell
viability assay (Fig. 2E) showed
that cells survival in the control and mannitol groups was >95%.
The survival rate in the HG group was significantly decreased
compared to the control group. Furthermore, the survival rate
increased following ASIV intervention. This demonstrated that ASIV
can protect cells and alleviate the damage under high-glucose
conditions.
ASIV decreases the damage of
myocardial tissue damage in diabetic rats and H9C2 cells in
high-glucose conditions
As shown in Fig.
3A-D, the expression of ANP and BNP in the left ventricle of
the diabetic group was significantly higher than that in the
ventricles of the control group, indicating that the diabetic group
exhibited myocardial hypertrophy. The expression of ANP and BNP
decreased in the ASIV group.
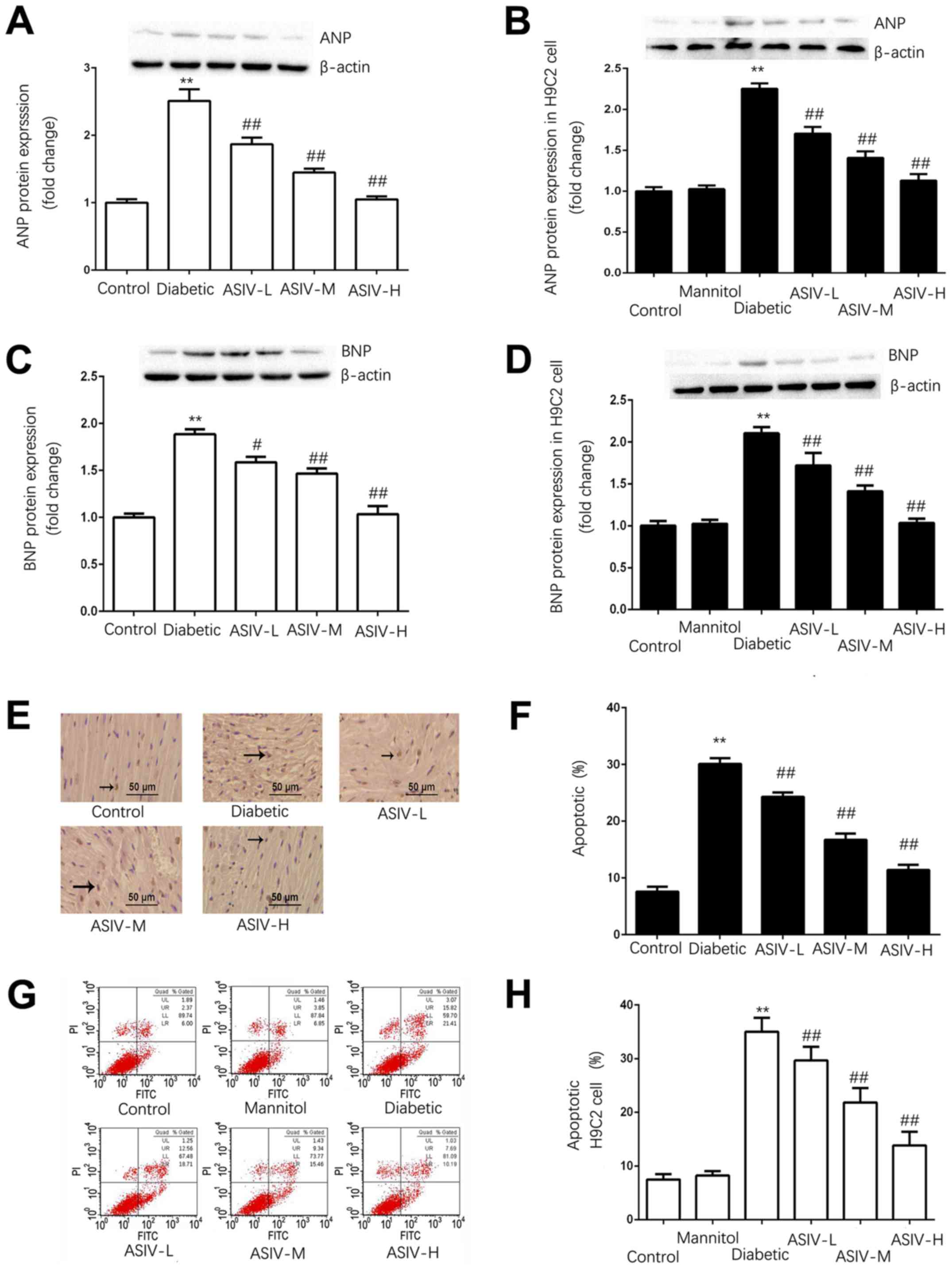 | Figure 3.ASIV decreases the damage of
myocardial tissue of diabetic rats and H9C2 cells in high-glucose
environment. Protein expression of ANP in (A) the myocardium and
(B) H9C2 cells. The protein expression of BNP in (C) the myocardium
and (D) H9C2 cells. (E) Myocardial apoptosis assayed by TUNEL
staining. TUNEL-positive cells were manifested as a marked
appearance of dark brown apoptotic cell nuclei, and (F) the
percentage of apoptotic cells in myocardial tissue. (G) The
detection of apoptosis in H9C2 cells by AnnexinV-FITC and PI double
staining, and (H) the percentage of apoptotic H9C2 cells. The
protein expression of (I) caspase-3, (J) cleaved caspase-3 and (K)
cytoplasmic Cyt C in the myocardium, and (L) caspase-3, (M) cleaved
caspase-3 and (N) cytoplasmic Cyt C in H9C2 cells. The data are
expressed as the means ± SD. n=4. **P<0.01 vs. control group.
#P<0.05 and ##P<0.01 vs. diabetic
group. The black arrows indicate the nucleus of apoptotic
cardiomyocytes. ANP, atrial natriuretic peptide; ASIV,
astragaloside IV; L, low; M, mid; H, high; BNP, brain natriuretic
peptide; PI, propidium iodide; Cyt C, cytochrome c. |
TUNEL staining and FITC/PI double staining were used
to detect the apoptosis of the myocardial tissue and H9C2 cells,
respectively (Fig. 3E-H). In the
myocardial tissue and H9C2 cells, the apoptotic rate of the control
group was <10%. Apoptosis was significantly increased in the
diabetic group and HG group compared with the control in tissue and
cells. The apoptotic rate was dose-dependently decreased by ASIV
compared with the diabetic model groups.
The expression of caspase-3, cleaved caspase-3 and
cytoplasmic Cyt C (Fig. 3I-N),
revealed that the expression of Cyt C in the cytoplasm and cleaved
caspase-3 was significantly increased and that caspase-3 was
decreased in the model group, whereas the expression of Cyt C and
cleaved caspase-3 in the control group was not significantly
different from that in the mannitol group; this excluded the
influence of osmotic pressure on the results. Following ASIV
administration, the protein expression of Cyt C and cleaved
caspase-3 were decreased both in heart tissues and H9C2 cells,
whereas caspase-3 was significantly increased. The results
demonstrated that ASIV can downregulate the increased expression of
Cyt C and cleaved caspase-3 induced by the diabetes model.
ASIV can regulate the expression of
energy metabolism-associated signals and the level of energy
metabolism in myocardial tissue and H9C2 cells
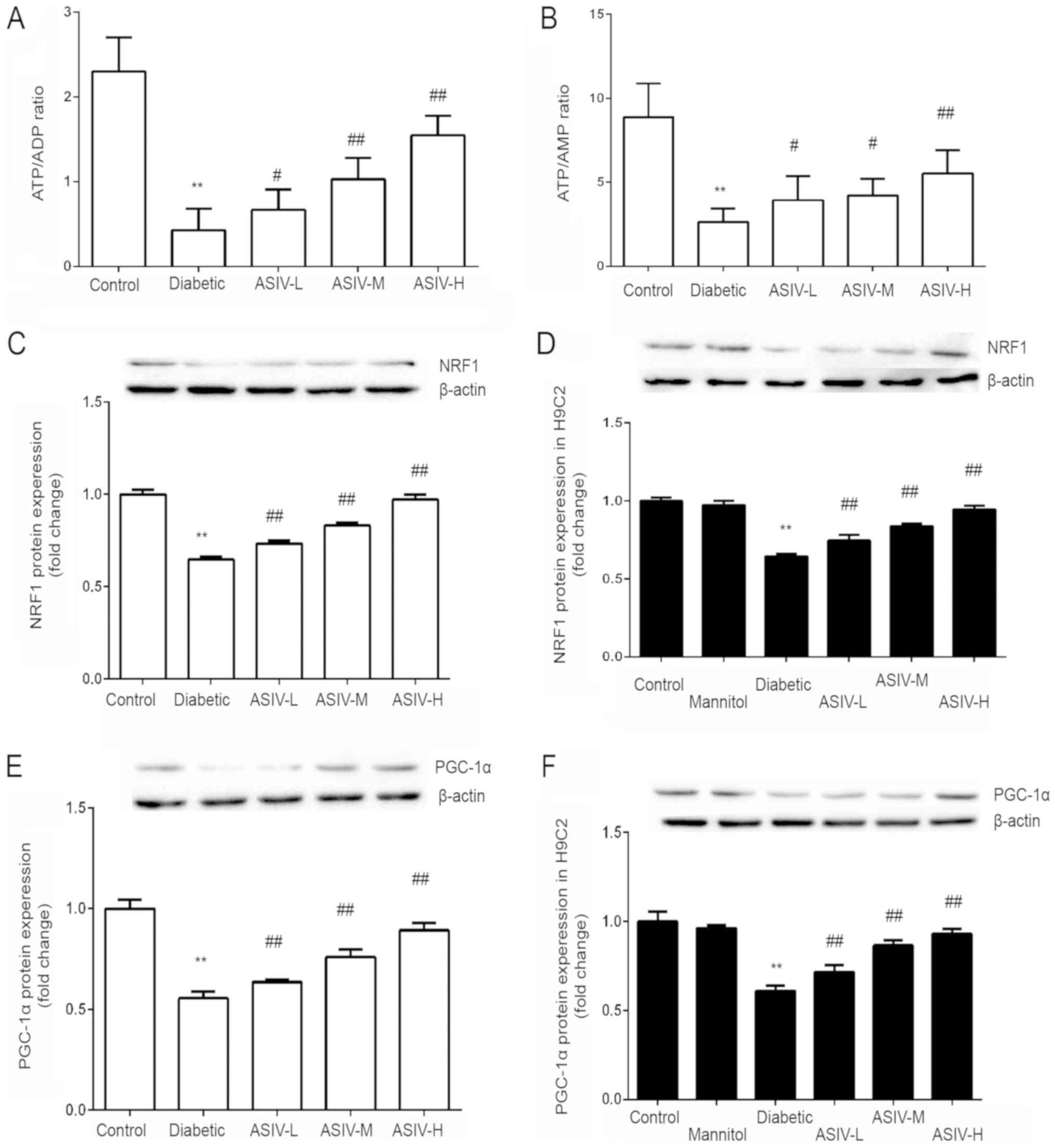
The ATP/ADP and ATP/AMP ratios of each group were
calculated. The results show that, compared with that of the
control group, the ratios were significantly decreased in the
diabetic group. These ratios were increased by the different doses
of ASIV (Fig. 4A and B).
Compared with that in the control group and the
model (diabetic and HG) groups, the expression of PGC-1α and NRF1
was significantly decreased in the model group (Fig. 4C-F). Following ASIV administration,
the expression of PGC-1α and NRF1 was increased, and the expression
increased with increasing ASIV concentration. This indicated that
ASIV may improve myocardial energy metabolism abnormalities caused
by diabetes. By detecting the mRNA expression of PGC-1α and NRF1 in
the H9C2 cells, under high-glucose conditions, the influence of
osmotic pressure was determined and obtained the same result as
that of histone expression, fully demonstrating the function of
ASIV in improving myocardial energy metabolism.
Discussion
The main cause of morbidity and mortality in
diabetic patients is cardiovascular complications, which accounts
for 80% of mortalities in diabetic patients (23). The most notable cardiovascular
complication is diabetic cardiomyopathy, which increases the risk
of future heart failure 5-fold (24–28).
Diabetes has adverse effects on the different cell types of the
heart, including endothelial cells (29,30),
fibroblasts and myocardial cells (31), and the damage to myocardial cells
may be the main cause of heart failure (31–35).
With the development of diabetes mellitus, the heart experiences
cardiac hypertrophy, decreased compliance, decreased contractility
and cell stability damage, leading to cardiac dysfunction (36). The number of apoptotic and necrotic
cardiomyocytes began to increase. When the normal physiological
structure was destroyed to a certain extent, compensatory
adjustment can no longer compensate for myocardial damage,
ultimately leading to heart failure (37).
Damage to the myocardium was mainly manifested by
myocardial hypertrophy and apoptosis. ASIV, as one of the main
active components in Astragalus, is a purified
small-molecule saponin. According to existing studies, ASIV has
anti-inflammatory and antioxidant effects. In previous studies,
ASIV demonstrated efficacious inhibitory effect on apoptosis in
vascular endothelial cells and to regulate the energy metabolism of
vascular endothelial cells (15–20,38,39).
Additionally, in other experiments, it was found that its
anti-inflammatory and antioxidant effects can improve the
hypertrophy and apoptosis of the myocardium due to inflammation.
Therefore, a diabetes model and the administration of ASIV was
conducted, in order to study the effect and mechanism of ASIV in
diabetic cardiomyopathy.
For the choice of index of hypertrophy, ANP and BNP
were measured. Of course, there are other subtypes of natriuretic
peptide, especially C-type natriuretic peptide (CNP) and
N-terminal-proBNP (NT-proBNP) (40). CNP is involved in the regulation of
myocardial changes in myocardial infarction, ischemia-reperfusion
and heart failure, but its expression level was also affected by
blood pressure and vascular endothelial cells, which cannot
specifically respond to myocardial changes. Although NT-proBNP has
no biological activity, it has a longer half-life and is more
stable than BNP, and has been proposed as a biomarker of heart
failure by the American Society of Clinical Chemistry (40–42).
However, NT-proBNP could not reflect the changes in cardiac
myocytes. ANP and BNP, as important biological signals of
myocardial hypertrophy, are mainly synthesized in the atrium and
left ventricle respectively, and in addition to indexes such as
heart weight index (HWI) and left ventricular weight index (LVWI),
ANP and BNP correspond to increased wall tension (43–46).
The plasma level of polypeptides was shown to be positively
correlated with the pressure caused by the filling of the heart,
making it a good marker of LV dysfunction and LV wall stress
abnormalities. There was a 23- to 85-fold increase in BNP,
particularly in tissues with hypertrophy, compared with that in the
control group. Apoptosis is regulated by a series of enzyme-linked
reactions, mainly including two signal transduction pathways. One
is the extrinsic pathway, that is, the death receptor pathway,
which is under the stimulation of external factors; extracellular
signal are transmitted to the intracellular caspase-protease
promoter enzyme (47). The other
is the intrinsic pathway, that is, the mitochondrial pathway
(48). Caspase-3, the core
protease in the apoptosis signal transduction pathway and an
important executive enzyme for apoptosis, acts downstream in both
the external pathway and the internal pathway (48,49).
The protein expression of caspase-3, as a downstream regulator in
the death receptor pathway and mitochondrial apoptosis pathway,
directly affects the apoptosis of myocardial cells. After Cyt C is
transported from the mitochondria to the cytoplasm, it regulates
the activity of the downstream caspase-3 enzyme and promotes the
apoptosis of myocardial cells (50,51).
In the present study, STZ was used to establish a
diabetes model, and gradient ASIV was used to treat diabetes model
animals. The myocardial injury indicators were examined, including
myocardial hypertrophy and myocardial apoptosis. HWI, LVWI, ANP and
BNP increased, and myocardial hypertrophy occurred in the model
group. The expression of cytoplasmic Cyt C and cleaved caspase-3
was increased, and these data combined with the flow cytometry
results and TUNEL staining results suggest that apoptosis was
increased in the model group. Following ASIV treatment, the indexes
of myocardial hypertrophy and myocardial apoptosis recovered to
varying degrees. The same results were obtained in H9C2 cells,
indicating that ASIV has a therapeutic effect on diabetic
myocardial injury.
How does ASIV improve myocardial injury in diabetes?
Thus far, previous studies have established the correlation between
diabetic complications and mitochondrial dysfunction in multiple
tissues (52–54), but the potential mechanism of
mitochondrial dysfunction in DCM was not clarified. Normally, the
heart relies on persistent aerobic metabolism to maintain the ATP
supply and redox balance for optimal systolic function. More than
90% of heart metabolism is aerobic (55,56).
Hyperglycemia is a major driver of mitochondrial dysfunction in
diabetic myocardium (57–59). Due to the high-energy demand of the
heart and the relief of myocardial injury in diabetic
cardiomyopathy following abnormal energy metabolism is corrected,
mitochondrial dysfunction was considered to be the cause of DCM
(60–62). Studies using noninvasive 31p
nuclear magnetic resonance imaging found that left ventricular
hypertrophy and contractile inhibition are associated with
decreased ATP levels, indicating that mitochondrial function was
impaired under high-glucose conditions (63,64).
PGC-1α is the most important coactivator in mitochondrial synthesis
and energy metabolism and has a crucial role in regulating
mitochondrial biosynthesis. PGC-1α regulates mitochondrial
biosynthesis by activating downstream transcription factors, such
as nuclear respiration factor (NRF). NRF is the most important
transcription factor in the regulation of mitochondrial
biosynthesis (65). The nuclear
transcription factor NRF1 regulates the transcription, translation
and replication of mitochondrial DNA and the expression of various
proteins, including enzymes in the respiratory chain that promote
oxidative energy supply, in the mitochondria.
ASIV was postulated to affect mitochondrial
synthesis by regulating PGC-1α and NRF1, thereby improving
mitochondrial energy metabolism and decreasing the myocardial
damage caused by diabetic cardiomyopathy. The expression of PGC-1α
and NRF1 protein in myocardial tissues, and the mRNA expression of
PGC-1α and NRF1 in H9C2 cells were detected in each group. ASIV
upregulated the expression of PGC-1α and NRF1, and the levels of
ATP and ADP in H9C2 cells were also detected. The results showed
that ASIV improved the energy metabolism level.
Based on the findings of the present study, it can
be concluded that ASIV can decrease the damage of myocardial
hypertrophy and apoptosis by improving mitochondrial energy
metabolism via regulating PGC-1α and NRF1 in diabetic
cardiomyopathy. Moreover, the experimental results showed that ASIV
had a hypoglycemic effect, but the underlying mechanism was still
unclear. In addition, based on previous studies, ASIV also has a
protective and therapeutic role in ischemia-reperfusion and
oxidative stress on the heart; therefore, the protective effect of
ASIV on diabetes mellitus on cardiomyopathy is not specific. Thus,
ASIV has a broad protective effect on the heart.
In conclusion, ASIV can ameliorate myocardial
mitochondrial energy metabolism abnormalities induced by diabetes
mellitus via regulating the expression of PGC-1α and NRF1.
Improving abnormal energy metabolism in mitochondria can inhibit
apoptosis and myocardial hypertrophy, thereby reducing myocardial
injury. Thus, ASIV has the potential to alleviate myocardial damage
induced by diabetes, by improving energy metabolism.
Acknowledgements
The authors would like to thank the laboratory
teachers Dr Meili Lu, Dr Yang Liu, Dr Tairan Sun, Mr. Cong Li, Mr.
Kun Zhao, Miss Tong Zhang, Miss Xiaoyao Zhang and Miss Yang Sun
(Key Laboratory of Cardiovascular and Cerebrovascular Drug Researh
of Liaoning Province, Junzhou Medical University) for providing
technical support in the course of the experiment.
Funding
This work was supported by the National Nature
Science Foundation of China (grant nos. 81673632 and 81703793) and
LiaoNing Revitalization Talents Program (grant no.
XLYC1802017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW and ZZ designed the whole experiment, and
contributed to the writing and modification of the article. ZZ, HZ,
YZ and JW were responsible for the collection of data. ZZ and HZ
performed the analysis and interpretaion of the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments and procedures were approved by the
Medical Ethics Committee of Jinzhou Medical University (approval
No. LNMU-2016-121). The raising of animals and the use of drugs are
in accordance with the guidelines for the Care and use of
Experimental Animals (8th Edition, Press of the National Academy of
Sciences).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee CD, Folsom AR, Pankow JS and Brancati
FL; Atherosclerosis Risk in Communities (ARIC) Study Investigators,
: Cardiovascular events in diabetic and nondiabetic adults with or
without history of myocardial infarction. Circulation. 109:855–860.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knapp M, Tu X and Wu R: Vascular
endothelial dysfunction, a major mediator in diabetic
cardiomyopathy. Acta Pharmacol Sin. 40:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escaned J, Colmenárez H, Ferrer MC,
Gutiérrez M, Jiménez-Quevedo P, Hernández R, Alfonso F, Bañuelos C,
Deisla LP, Zamorano JL and Macaya C: Diastolic dysfunction in
diabetic patients assessed with Doppler echocardiography:
Relationship with coronary atherosclerotic burden and
microcirculatory impairment. Rev Esp Cardiol. 62:1395–1403. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng X, Sun T and Wang X: Activation of
type 2 cannabinoid receptors (CB2R) promotes fatty acid oxidation
through the SIRT1/PGC-1α pathway. Biochem Biophys Res Commun.
436:377–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun X, Chen RC, Yang ZH, Sun GB, Wang M,
Ma XJ, Yang LJ and Sun XB: Taxifolin prevents diabetic
cardiomyopathy in vivo and in vitro by inhibition of oxidative
stress and cell apoptosis. Food Chem Toxicol. 63:221–232. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rowe GC, Jiang A and Arany Z: PGC-1
coactivators in cardiac development and disease. Circ Res.
107:825–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arany Z, He H, Lin J, Hoyer K, Handschin
C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al: Transcriptional
coactivator PGC-1 alpha controls the energy state and contractile
function of cardiac muscle. Cell Metab. 1:259–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sebastiani M, Giordano C, Nediani C,
Travaglini C, Borchi E, Zani M, Feccia M, Mancini M, Petrozza V,
Cossarizza A, et al: Induction of mitochondrial biogenesis is a
maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll
Cardiol. 50:1362–1369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koh JH, Hancock CR, Terada S, Higashida K,
Holloszy JO and Han DH: PPARβ is essential for maintaining normal
levels of PGC-1α and mitochondria and for the increase in muscle
mitochondria induced by exercise. Cell Metab. 25:1176–1185.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Z, Puigserver P, Andersson U, Zhang C,
Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC and
Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and
respiration through the thermogenic coactivator PGC-1. Cell.
98:115–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura H, Matoba S, Iwai-Kanai E, Kimata
M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y,
et al: p53 promotes cardiac dysfunction in diabetic mellitus caused
by excessive mitochondrial respiration-mediated reactive oxygen
species generation and lipid accumulation. Circ Heart Fail.
5:106–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anderson EJ, Rodriguez E, Anderson CA,
Thayne K, Chitwood WR and Kypson AP: Increased propensity for cell
death in diabetic human heart is mediated by
mitochondrial-dependent pathways. Am J Physiol Heart Circ Physiol.
300:H118–H124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mei M, Tang F, Lu M, He X, Wang H, Hou X,
Hu J, Xu C and Han R: Astragaloside IV attenuates apoptosis of
hypertrophic cardiomyocyte through inhibiting oxidative stress and
calpain-1 activation. Environ Toxicol Pharmacol. 40:764–773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Tang F, Yang Y, Lu M, Luan A,
Zhang J, Yang J and Wang H: Astragaloside IV protects against
isoproterenol-induced cardiac hypertrophy by regulating
NF-κB/PGC-1α signaling mediated energy biosynthesis. PLoS One.
10:e01187592015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia G, Leng B, Wang H and Dai H:
Inhibition of cardiotrophin-1 overexpression is involved in the
anti-fibrotic effect of Astrogaloside IV. Mol Med Rep.
16:8365–8370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai H, Jia G, Lu M, Liang C, Wang Y and
Wang H: Astragaloside IV inhibits isoprenaline-induced cardiac
fibrosis by targeting the reactive oxygen species/mitogen-activated
protein kinase signaling axis. Mol Med Rep. 15:1765–1770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu C, Tang F, Lu M, Yang J, Han R, Mei M,
Hu J and Wang H: Pretreatment with Astragaloside IV protects human
umbilical vein endothelial cells from hydrogen peroxide induced
oxidative stress and cell dysfunction via inhibiting eNOS
uncoupling and NADPH oxidase-ROS-NF-κB pathway. Can J Physiol
Pharmacol. 94:1132–1140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu C, Tang F, Lu M, Yang J, Han R, Mei M,
Hu J, Zhou M and Wang H: Astragaloside IV improves the
isoproterenol-induced vascular dysfunction via attenuating eNOS
uncoupling-mediated oxidative stress and inhibiting ROS-NF-κB
pathways. Int Immunopharmacol. 33:119–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu M, Tang F, Zhang J, Luan A, Mei M, Xu
C, Zhang S, Wang H and Maslov LN: Astragaloside IV attenuates
injury caused by myocardial ischemia/reperfusion in rats via
regulation of toll-like receptor 4/nuclear factor-κB signaling
pathway. Phytother Res. 29:599–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holly EN, Shimamoto A, Debold JF and
Miczek KA: Sex differences in behavioral and neural
cross-sensitization and escalated cocaine taking as a result of
episodic social defeat stress in rats. Pyschopharmacology (Berl).
224:179–188. 2012. View Article : Google Scholar
|
|
22
|
Walczak M and Błasiak T: Midbrain
dopaminergic neuron activity across alternating brain states of
urethane anaesthetized rat. Eur J Neurosci. 45:1068–1077. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seferović PM, Petrie MC, Filippatos GS,
Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino
F, de Boer RA, et al: Type 2 diabetes mellitus and heart failure: A
position statement from the Heart Failure Association of the
European Society of Cardiology. Eur J Heart Fail. 20:853–872. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amos AF, McCarty DJ and Zimmet P: The
rising global burden of diabetes and its complications: Estimates
and projections to the year 2010. Diabet Med. 14 (Suppl 5):S1–S85.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dries DL, Sweitzer NK, Drazner MH,
Stevenson LW and Gersh BJ: Prognostic impact of diabetes mellitus
in patients with heart failure according to the etiology of left
ventricular systolic dysfunction. J Am Coll Cardiol. 38:421–428.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kannel WB, Hjortland M and Castelli WP:
Role of diabetes in congestive heart failure: The framingham study.
Am J Cardiol. 34:29–34. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alaei Faradonbeh N, Nikaeen F, Akbari M,
Almasi N and Vakhshoori M: Cardiovascular disease risk prediction
among Iranian patients with diabetes mellitus in Isfahan Province,
Iran, in 2014, by using Framingham risk score, atherosclerotic
cardiovascular disease risk score, and high-sensitive C-reactive
protein. ARYA Atheroscler. 14:163–168. 2018.PubMed/NCBI
|
|
28
|
Tadic M and Cuspidi C: Obesity and heart
failure with preserved ejection fraction: A paradox or something
else? Heart Fail Rev. 24:379–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Icks A, Claessen H, Kirchberger I, Heier
M, Peters A, Trentinaglia I, Giani G, von Scheidt W and Meisinger
C: Mortality after first myocardial infarction in diabetic and
non-diabetic people between 1985 and 2009. The MONICA/KORA
registry. Eur J Epidemiol. 29:899–909. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feuvray D: Diabetic cardiomyopathy. Arch
Mal Coeur Vaiss. 97:261–265. 2004.PubMed/NCBI
|
|
31
|
Boudina S and Abel ED: Diabetic
cardiomyopathy revisited. Circulation. 115:3213–3223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fiordaliso F, Li B, Latini R, Sonnenblick
EH, Anversa P, Leri A and Kajstura J: Myocyte death in
streptozotocin-induced diabetes in rats in angiotensin
II-dependent. Lab Invest. 80:513–527. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Carli MF, Janisse J, Grunberger G and
Ager J: Role of chronic hyperglycemia in the pathogenesis of
coronary microvascular dysfunction in diabetes. J Am Coll Cardiol.
41:1387–1393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Riehle C and Bauersachs J: Of mice and
men: Models and mechanisms of diabetic cardiomyopathy. Basic Res
Cardiol. 114:22018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gilca GE, Stefanescu G, Badulescu O,
Tanase DM, Bararu I and Ciocoiu M: Diabetic cardiomyopathy: Current
approach and potential diagnostic and therapeutic targets. J
Diabetes Res. 2017:13102652017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Voulgari C, Papadogiannis D and
Tentolouris N: Diabetic cardiomyopathy: From the pathophysiology of
the cardiac myocytes to current diagnosis and management
strategies. Vasc Health Risk Manag. 6:883–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu M, Leng B, He X, Zhang Z, Wang H and
Tang F: Calcium sensing receptor-related pathway contributes to
cardiac injury and the mechanism of Astragaloside IV on
cardioprotection. Front Pharmacol. 9:11632018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guan FY, Yang SJ, Liu J and Yang SR:
Effect of astragaloside IV against rat myocardial cell apoptosis
induced by oxidative stress via mitochondrial ATP-sensitive
potassium channels. Mol Med Rep. 12:371–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kakoullis L, Giannopoulou E,
Papachristodoulou E, Pantzaris ND, Karamouzos V, Kounis NG, Koniari
I and Velissaris D: The utility of brain natriuretic peptides in
septic shock as markers for mortality and cardiac dysfunction: A
systematic review. Int J Clin Pract. 73:e133742019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pemberton CJ, Johnson ML, Yandle TG and
Espiner EA: Deconvolution analysis of cardiac natriuretic peptides
during acute volume overload. Hypertension. 36:355–359. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang WH, Francis GS, Morrow DA, Newby LK,
Cannon CP, Jesse RL, Storrow AB and Christenson RH; NACB Committee,
: National academy of clinical biochemistry laboratory medicine
practice guidelines: Clinical utilization of cardiac biomarker
testing in heart failure. Clin Biochem. 41:210–221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li S, Xiao Z, Li L, Hu B, Zhou Z, Yi S,
Luo J, Xie L, Nie B, Mo L and Wang S: Establishment of normal
reference values of NT-proBNP and its application in diagnosing
acute heart failure in children with severe hand food and mouth
disease. Medicine. 97:e122182018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Candido R, Forbes JM, Thomas MC, Thallas
V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME
and Burrell LM: A breaker of advanced glycation end products
attenuates diabetes-induced myocardial structural changes. Circ
Res. 92:785–792. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Connelly KA, Kelly DJ, Zhang Y, Prior DL,
Martin J, Cox AJ, Thai K, Feneley MP, Tsoporis J, White KE, et al:
Functional, structural and molecular aspects of diastolic heart
failure in the diabetic (mRen-2)27 rat. Cardiovasc Res. 76:280–291.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ritchie RH, Quinn JM, Cao AH, Drummond GR,
Kaye DM, Favaloro JM, Proietto J and Delbridge LM: The antioxidant
tempol inhibits cardiac hypertrophy in the insulin-resistant
GLUT4-deficient mouse in vivo. J Mol Cell Cardiol. 42:1119–1128.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huynh K, McMullen JR, Julius TL, Tan JW,
Love JE, Cemerlang N, Kiriazis H, Du XJ and Ritchie RH:
Cardiac-specific IGF-1 receptor transgenic expression protects
against cardiac fibrosis and diastolic dysfunction in a mouse model
of diabetic cardiomyopathy. Diabetes. 59:1512–1520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Das DK: Redox regulation of cardiomyocyte
survival and death. Antioxid Redox Signal. 3:23–37. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Webster KA, Graham RM and Bishopric NH:
BNip3 and signal-specific programmed death in the heart. J Mol Cell
Cardiol. 38:35–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Das S, Khan N, Mukherjee S, Bagchi D,
Gurusamy N, Swartz H and Das DK: Redox regulation of
resveratrol-mediated switching of death signal into survival
signal. Free Radic Biol Med. 44:82–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lax NZ, Turnbull DM and Reeve AK:
Mitochondrial mutations: Newly discovered players in neuronal
degeneration. Neuroscientist. 17:645–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li XD, Li XM, Gu JW and Sun XC: MiR-155
regulates lymphoma cell proliferation and apoptosis through
targeting SOCS3/JAK-STAT3 signaling pathway. Eur Rev Med Pharmacol
Sci. 21:5153–5159. 2017.PubMed/NCBI
|
|
52
|
Fetterman JL, Holbrook M, Westbrook DG,
Brown JA, Feeley KP, Bretón-Romero R, Linder EA, Berk BD, Weisbrod
RM, Widlansky ME, et al: Mitochondrial DNA damage and vascular
function in patients with diabetes mellitus and atherosclerotic
cardiovascular disease. Cardiovasc Diabetol. 15:532016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kalvala AK, Khan I, Gundu C and Kumar A:
An overview on ATP dependent and independent proteases including an
anterograde to retrograde control on mitochondrial function; Focus
on diabetes and diabetic complications. Curr Pharm Des. Jul
18–2019.(Epub ahead of print). doi:
10.2174/1381612825666190718153901. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jin H, Zhu B, Liu X, Jin J and Zou H:
Metabolic characterization of diabetic retinopathy: An
1H-NMR-based metabolomic approach using human aqueous
humor. J Pharm Biomed Anal. 174:414–421. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rolfe DF and Brown GC: Cellular energy
utilization and molecular origin of standard metabolic rate in
mammals. Physiol Rev. 77:731–758. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Trost SU, Belke DD, Bluhm WF, Meyer M,
Swanson E and Dillmann WH: Overexpression of the sarcoplasmic
reticulum Ca(2+)-ATPase improves myocardial contractility in
diabetic cardiomyopathy. Diabetes. 51:1166–1171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Camara AK, Bienengraeber M and Stowe DF:
Mitochondrial approaches to protect against cardiac ischemia and
reperfusion injury. Front Physiol. 2:132011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chatham JC and Seymour AM: Cardiac
carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc
Res. 55:104–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dey S, DeMazumder D, Sidor A, Foster DB
and O'Rourke B: Mitochondrial ROS drive sudden cardiac death and
chronic proteome remodeling in heart failure. Circ Res.
123:356–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Y, Gao P, Wei C, Li H, Zhang L, Zhao
Y, Wu B, Tian Y, Zhang W, Wu L, et al: Calcium sensing receptor
protects high glucose-induced energy metabolism disorder via
blocking gp78-ubiquitin proteasome pathway. Cell Death Dis.
8:e27992017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ares-Carrasco S, Picatoste B, Camafeita E,
Carrasco-Navarro S, Zubiri I, Ortiz A, Egido J, López JA, Tuñón J
and Lorenzo O: Proteome changes in the myocardium of experimental
chronic diabetes and hypertension: Role of PPARα in the associated
hypertrophy. J Proteomics. 75:1816–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tsushima K, Bugger H, Wende AR, Soto J,
Jenson GA, Tor AR, McGlauflin R, Kenny HC, Zhang Y, Souvenir R, et
al: Mitochondrial reactive oxygen species in lipotoxic hearts
induce Post-translational modifications of AKAP121, DRP1, and OPA1
that promote mitochondrial fission. Circ Res. 122:58–73. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Knowlton AA, Chen L and Malik ZA: Heart
failure and mitochondrial dysfunction: The role of mitochondrial
fission/fusion abnormalities and new therapeutic strategies. J
Cardiovasc Pharmacol. 63:196–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song M, Mihara K, Chen Y, Scorrano L and
Dorn GW II: Mitochondrial fission and fusion factors reciprocally
orchestrate mitophagic culling in mouse hearts and cultured
fibroblasts. Cell Metab. 21:273–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Recchia FA, McConnell PI, Bernstein RD,
Vogel TR, Xu X and Hintze TH: Reduced nitric oxide production and
altered myocardial metabolism during the decompensation of
pacing-induced heart failure in the conscious dog. Circ Res.
83:969–979. 1998. View Article : Google Scholar : PubMed/NCBI
|