Introduction
Spinal cord ischemia and reperfusion (I/R) injury,
including permanent paraparesis or paraplegia remains a devastating
complication of thoraco-abdominal aortic surgery. As previously
reported, the incidence of spinal cord ischemia is 4–16% in
patients having received thoraco-abdominal aortic surgery (1). Despite numerous advances in
neuroprotective strategies, such as surgical techniques,
hypothermia treatment and cerebrospinal fluid drainage, aiming to
decrease the negative impact of I/R injury, the efficacy of each
intervention has not yet been fully determined (2,3).
Novel methods, such as ischemic pre- and
post-conditioning have recently been demonstrated to provide
protection in several organs, including the spinal cord. Compared
with conventional ischemic pre-and post-conditioning,
pharmacological pre- and post-conditioning, which only requires
drug administration as adjunctive treatment prior to ischemia or at
the time of early reperfusion, has demonstrated improved results in
preventing organ I/R injuries (4–6).
This is due to the fact that drug administration leads to less
adverse effects compared with the mechanical stimulation of vessels
(7,8). If the exact times of onset time for
the organ ischemia can be predicted, pharmacological
pre-conditioning will be more effective in improving the durability
of tissue to ischemic insult. However, this method is not currently
available in numerous clinical settings. From a clinical point of
view, post-conditioning, which is more amenable to unpredicted
ischemia in patients with thoraco-abdominal aortic aneurysm, may
offer greater advantages over pre-conditioning.
Extensive previous studies have suggested that
opioid receptor pre- and post-conditioning can protect tissues
against I/R injury in the central nervous system and other organ
systems (9–11). D-Ala2,
D-Leu5-Enkephalin (DADLE), a selective delta opioid
receptor agonist, has received increasing interest as a link
between hibernation and neuroprotection (12). DADLE has been revealed
experimentally to improve injury to cortical neurons caused by
oxygen-glucose deprivation, and also to decrease neuronal death and
intellectual disability induced by forebrain ischemia (13,14).
A previous study has demonstrated that
administration of 0.05 mg/kg DADLE by regional perfusion into the
clamped aorta during the ischemic period, could induce
neuroprotective efficacy against spinal cord I/R in rabbits
(15). Whether an improved effect
may be acquired when DADLE was delivered prior to ischemia onset or
in the early reperfusion phase remains unclear. Considering the
advantages of pharmacological pre- and post-conditioning in the
clinical setting, the present study further compared the
neuroprotective effects of DADLE administration before, during and
after ischemia, in order to determine the optimal conditioning
strategy.
Materials and methods
Animals and ethics
The animal protocol was approved by the Animal Care
and Use Committee of Shanghai Jiaotong University, and was in
accordance with the Guide for The Care and Use of Laboratory
Animals (16). Efforts were made
to minimize the number of animals and their suffering.
Method of anesthesia
A total of 40 New Zealand white rabbits were
supplied by Animal Research Laboratory at Shanghai General Hospital
Affiliated to Shanghai Jiaotong University School of Medicine. They
were housed in a room under at ambient temperature (20–25°C),
relative humidity 40–70% and a 12-h light/dark cycle, with free
access to food and water. Animals aged 4–6 months with body weight
2.0–3.0 kg (20 male and 20 female) were randomly divided into 5
groups (n=8): Sham-operated group (Sham), normal saline
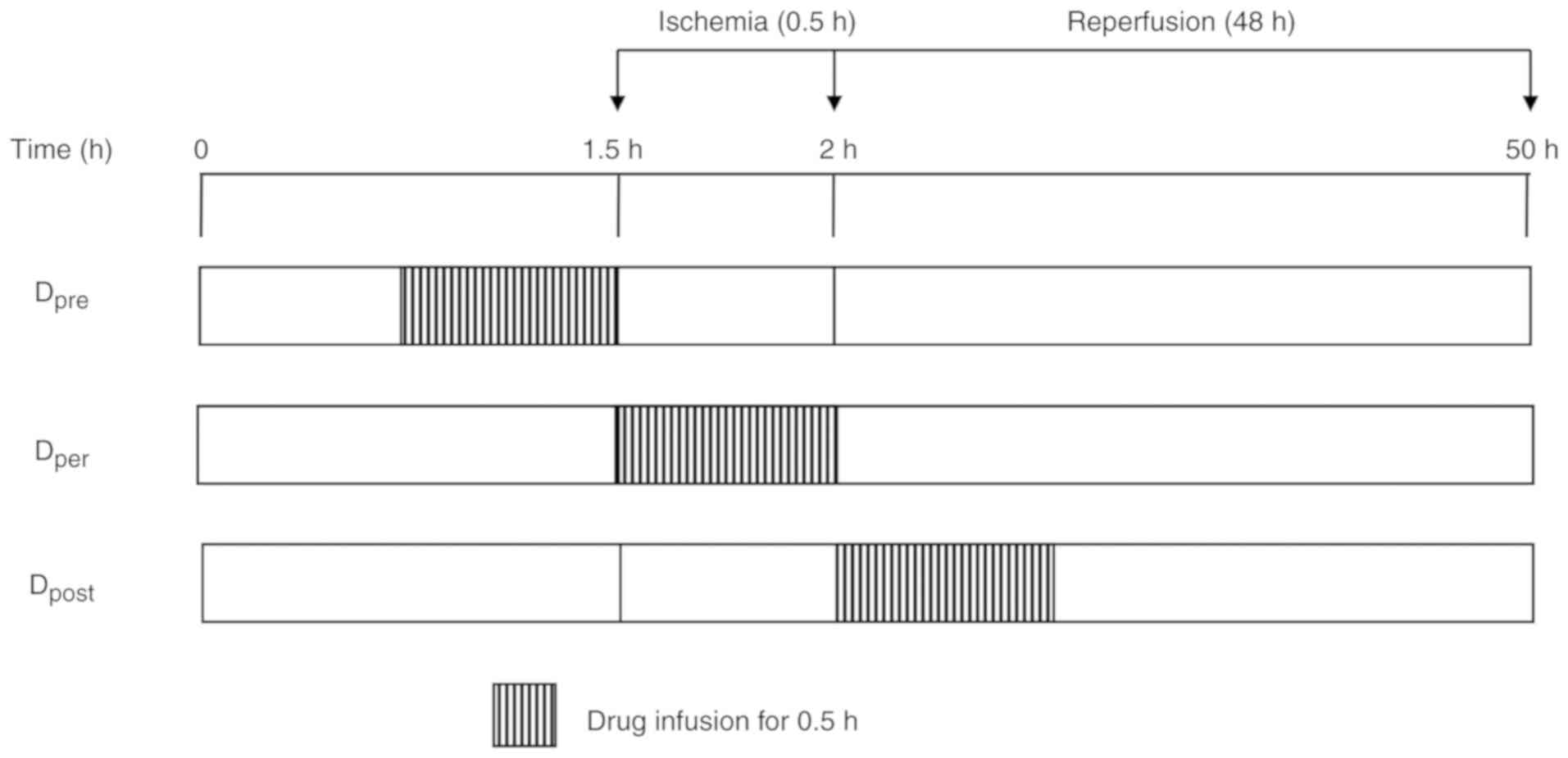
pre-conditioning (NS), DADLE per-conditioning (Dper),
DADLE pre-conditioning (Dpre) and DADLE
post-conditioning (Dpost). DADLE (Sigma-Aldrich; Merck
KGaA) was dissolved in NS and the rabbits received DADLE at a
dosage of 0.05 mg/kg. For the rabbits in the NS and Dper
groups, NS or DADLE were infused, respectively, for 30 min during
the entire spinal cord ischemia period. The rabbits in the
Dpre group received DADLE for 30 min prior to aortic
occlusion and were then immediately subjected to the 30 min
ischemia. The rabbits in the Dpost group were given 30
min DADLE at the immediate onset of reperfusion (Fig. 1). The rabbits in the Sham group
underwent the operation but the aorta was not occluded.
General anesthesia was induced with ketamine (20–25
mg/kg) and atropine (0.06–0.10 mg/kg). A catheter (22-G) was
inserted into the left ear vein for venous administration. The
rabbits were ventilated mechanically with volume-controlled
ventilation. The parameters of mechanical ventilation were adjusted
as follows: Tidal volume, 10 ml/kg; respiratory rate, 30 breaths
per min; ratio of inspiratory time to expiratory time, 1:1.5; and
fraction of inspired oxygen, 1.0. Core body temperature was
monitored and maintained at 37°C with a heating lamp. The right ear
central artery was cannulated with a 22-G catheter for mean
arterial pressure (MAP) and heart rate (HR) monitoring and blood
sampling. NS containing penicillin 40 U was infused continuously
during the operation at a rate of 10 ml/kg/h. Midazolam (0.5
mg/kg), fentanyl (10 µg/kg) and vecuronium (0.25 mg/kg) were
injected intermittently to maintain anesthesia.
Animal model and drug perfusion
protocol
The model of 30 min aortic occlusion in rabbits was
established, as previously described (15,17).
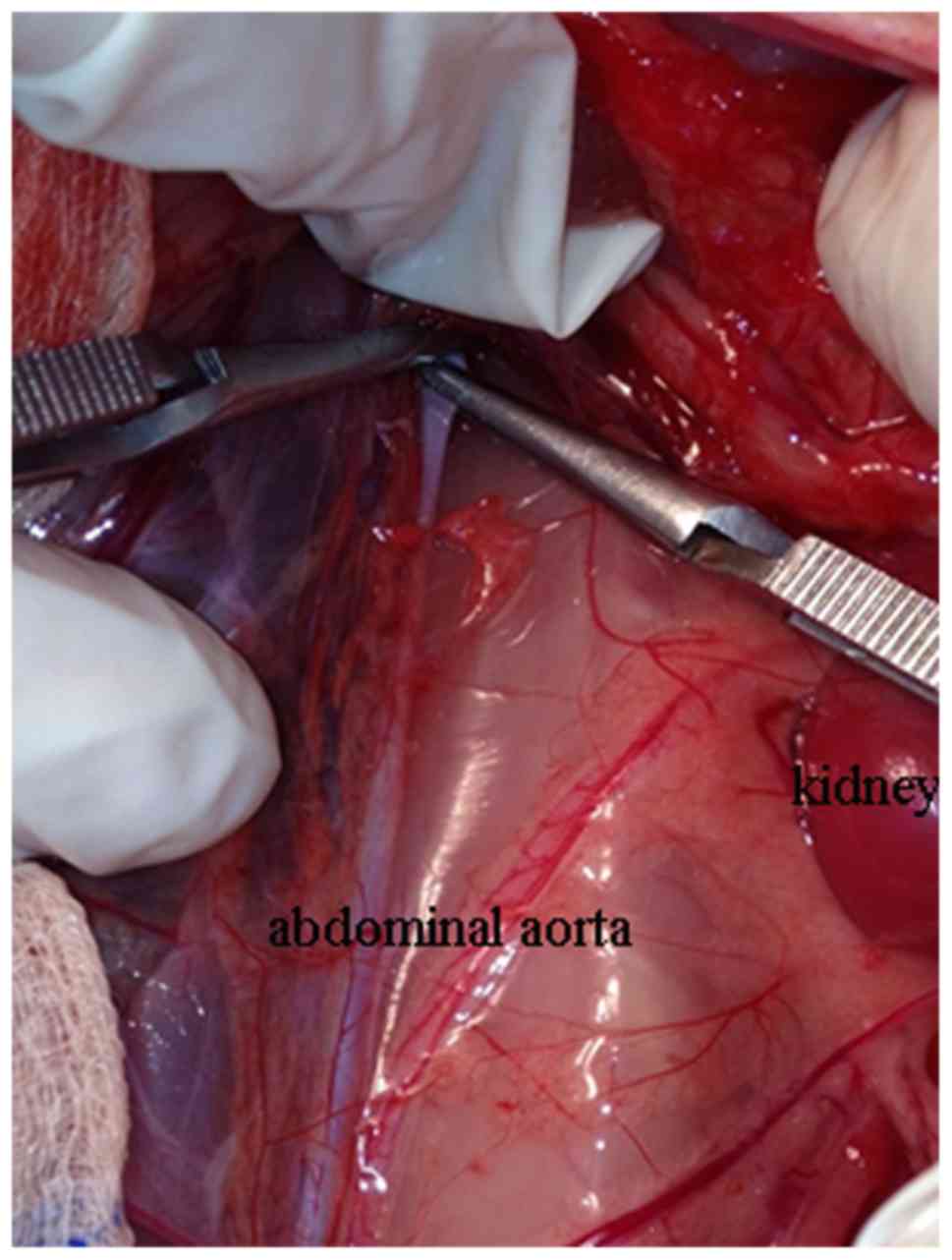
Briefly, under general anesthesia, the femoral arteries of the
rabbits were exposed. The infrarenal abdominal aorta was exposed
via abdominal incision with the ligatures placed loosely around it.
Following systemic heparinization (1 mg/kg), a polycarbonate
catheter (20-G) was inserted into the aorta via femoral artery
incision with the tip reaching 1–2 cm below the left renal artery.
The end of the catheter was connected to a transducer in order to
monitor aortic pressure and infuse drugs. To achieve spinal cord
ischemia, the infrarenal abdominal aorta was blocked with two
artery clips (Fig. 2). The
ischemic period was lasted for 30 min and was confirmed by the
presence of <20 mmHg of distal abdominal aortic pressure. To
regain blood supply, the artery clips were removed and reperfusion
was performed for 48 h. The rabbits in the Sham group underwent the
surgical procedures but the aorta was not occluded. Finally, the
catheter was withdrawn and the abdomen was closed following the
femoral artery ligation. The animals were extubated when normal
spontaneous breathing was restored.
Neurobehavioral evaluation
The animals were scored according to the Tarlov
criterion (18) at the time of
regaining consciousness, 6, 24 and 48 h after reperfusion,
respectively. The behavioral scores were graded in a scale 0–4,
with 4 being the best score: i) 0, Paralysis with no lower-limb
movement; ii) 1, weak lower-limb movement, but unable to work
against gravity; iii) 2, good lower-limb motor function against
gravity, but incapable of dragging legs or hopping; iv) 3, ability
to drag legs and hop, but not normally; and v) 4, normal lower-limb
motor function. The rabbits received a single score. According to
the Tarlov scores analysis, the rabbits that scored 0 or 1 were
defined as paraplegic, while those that scored 0 or 1 or 2 or 3
were defined as neurological dysfunction (18). Behavioral scores were given in a
blinded manner by a laboratory personnel and then the results
compared.
Histopathological examination of
α-motor neuron
The rabbits were intubated and anesthetized 48 h
after reperfusion. Lumbar spinal cords were exposed via incision on
the back at the left lateral position. Spinal cord segments of
L4-L5 were removed and fixed in 10% formalin for 48 h at 4°C. The
rabbits were sacrificed by intravenous injection of sodium
pentobarbital (200 mg/kg). Following dehydration in graded ethanol,
specimens were embedded in paraffin and sliced into 5-µm thick
sections for hematoxylin and eosin staining for ~3 h at room
temperature. A single anterior horn was randomly selected from two
slices of spinal segments from each specimen. The number of viable
α-motor neurons in the anterior spinal cord (three horizons
gathered from the vertex of the anterior horn to the central canal
perpendicular) was counted under a light microscope using a ×40
objective by an investigator blinded to the group assignment.
Viable α-motor neurons were counted based on the following
standard: i) Polygonal perikarya; ii) basophilic stippled cytoplasm
(containing normal Nissl bodies); and iii) round nucleus located
centrally with loosely textured chromatin and prominent nucleoli
(19).
Statistical analysis
Hemodynamic data (MAP/HR), body weight and core
temperature are expressed as the mean ± standard deviation. The
overall difference was compared using one-way analysis of variance
and repeated measures analysis of variance followed by Dunnett's
test. The number of viable α-motor neurons and Tarlov scores were
compared using the Kruskal-Wallis nonparametric rank sum test
followed by the Mann-Whitney U test. To obtain a 95% confidence
interval value, a Bonferroni correction was used to adjust the type
I error rate for multiple comparisons. The incidences of paraplegia
and neurological dysfunction were compared using a Fisher's exact
test followed by Bonferroni correction. The Bonferroni-adjusted
P-value was obtained by multiplying the unadjusted P-value by the
comparisons number (i.e., 3), and was presented as the ‘corrected
P’. Corrected P<0.05 was considered to indicate a statistically
significant result. The number of viable neurons is expressed as
the median (25 and 75th percentiles). Tarlov scores are presented
in absolute numbers. All statistical tests were two-tailed.
Results
DADLE per-conditioning attenuates
spinal cord I/R injury
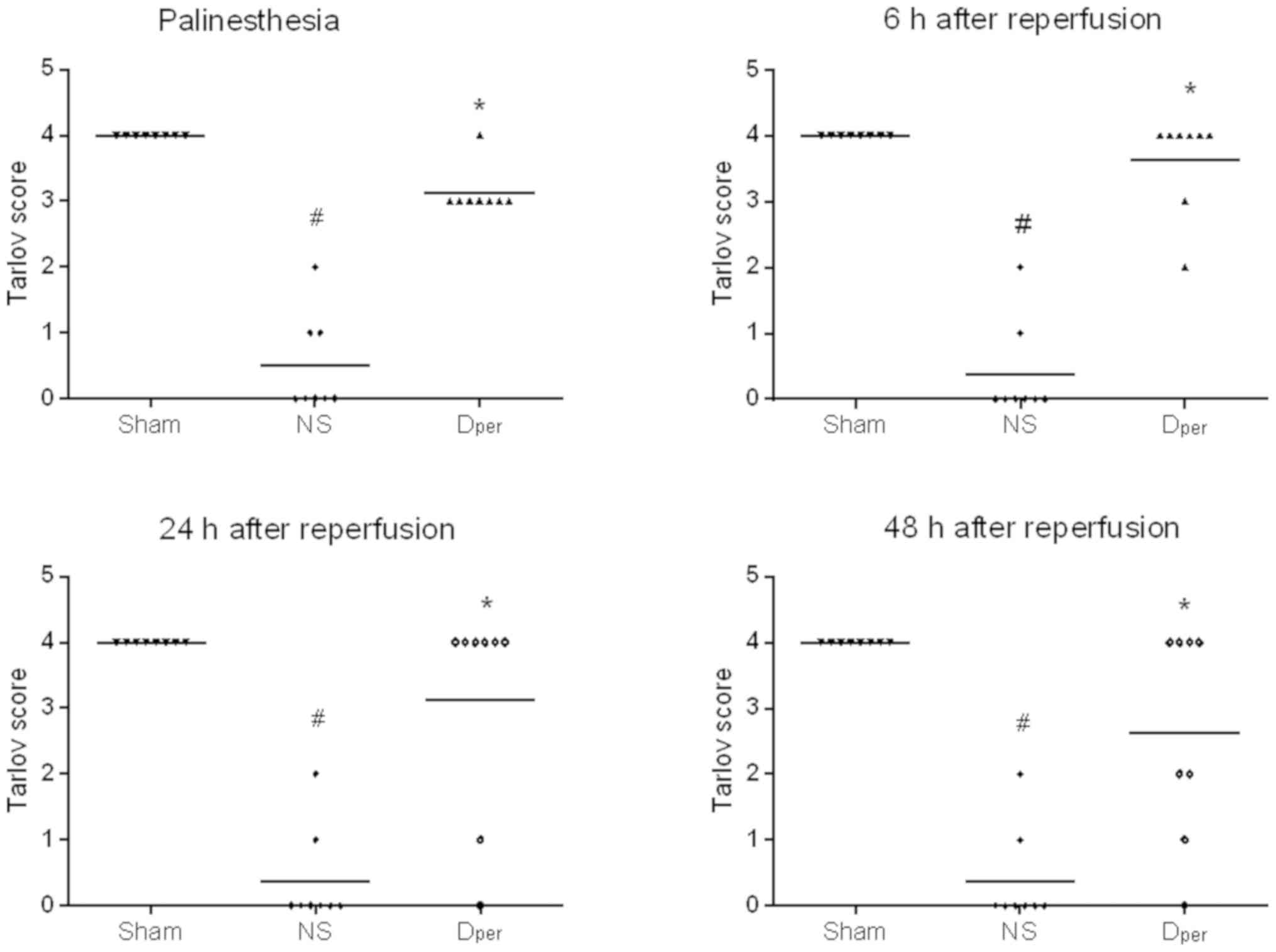
Tarlov scores of the Sham, NS and Dper
groups at different time points following reperfusion are presented
in Fig. 3. All rabbits in the Sham
group retained unimpaired neurological functions; however, spinal
cord I/R injury induced significant neurological dysfunction
[corrected P=0.0006 at all different time points following
reperfusion (palinesthesia, and 6, 24 and 48 h); Fig. 3]. Compared with the NS group, the
animals that received DADLE perfusion demonstrated significantly
improved neurological deficits (corrected P=0.0006 at the time
points of palinesthesia; P=0.0009 at 6 h; P=0.0141 at 24 h; and
P=0.0270 at 48 h after reperfusion). The paraplegia rates were
significantly decreased from 87.5% in the NS group to 25% in the
DADLE per-conditioning group at 48 h after the reperfusion
(P=0.0400; data not shown).
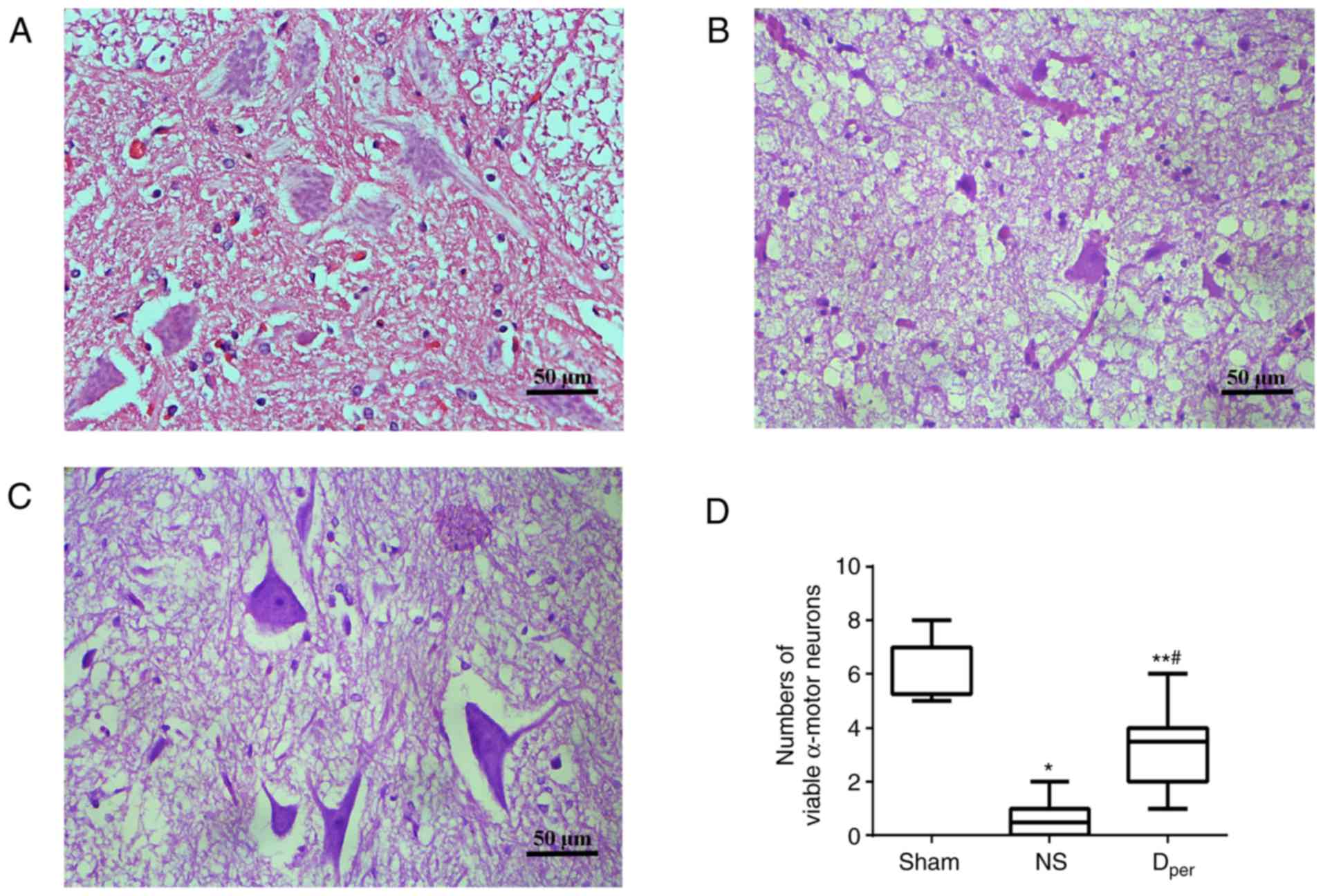
Representative images of sections stained with
hematoxylin and eosin of the Sham, NS and Dper groups
are presented in Fig. 4A-C.
According to the cell count, I/R injuries demonstrated a
significant increase in the amount of damaged neurons compared with
the Sham group (corrected P=0.0006; Fig. 4D). The median number of viable
α-motor neurons at 48 h after reperfusion was 3.5 (range, 2–4) in
the Dper group, which was significantly higher than the
median value of 0.5 (range, 0–1) in the NS group (corrected
P=0.0060), but lower than the median value of 7 (range, 5.25–7) in
the Sham group (corrected P=0.0018). The results of the Tarlov
scores and histopathological examination of the spinal cords of the
rabbits that received DADLE were consistent with a previous study
(15).
Effects of DADLE pre-and
post-conditioning on spinal cord I/R injury
Physiologic parameters
The average weight and the core temperature of the
animals were not significantly different between the three groups,
as presented in Table I. HR and
MAP were observed and maintained in each group, as presented in
Table II.
 | Table I.Body weight and core temperature of
the rabbits. |
Table I.
Body weight and core temperature of
the rabbits.
|
|
|
| Core temperature,
°C |
|---|
|
|
|
|
|
|---|
| Group (n=8 per
group) | Body weight, g | P-value | Pre-ischemia | P-value | Intra-ischemia | P-value | Post-ischemia | P-value |
|---|
|
Dper | 2612±260 |
| 37.3±0.8 |
| 37.1±0.9 |
| 37.2±0.8 |
|
|
Dpre | 2670±280 | 0.83 | 37.4±0.7 | 0.95 | 37.3±0.9 | 0.90 | 37.3±0.7 | 0.95 |
|
Dpost | 2590±265 |
| 37.3±0.6 |
| 37.2±0.8 |
| 37.2±0.6 |
|
 | Table II.Comparison of hemodynamic parameter
at different time points. |
Table II.
Comparison of hemodynamic parameter
at different time points.
| Group (n=8 per
group) | Pre-ischemia, 5
min | P-value | Ischemia, 10
min | P-value | Ischemia, 15
min | P-value | Ischemia, 20
min | P-value | Ischemia, 30
min | P-value | Reperfusion, 15
min | P-value |
|---|
| MAP, mmHg |
|
Dper | 77±11 | 0.68 | 77±14 | 0.93 | 77±15 | 0.88 | 78±12 | 0.94 | 84±14 | 0.96 | 84±9 | 0.27 |
|
Dpre | 75±11 |
| 77±12 |
| 78±10 |
| 80±12 |
| 82±14 |
| 78±11 |
|
|
Dpost | 80±12 |
| 79±11 |
| 80±11 |
| 80±14 |
| 83±12 |
| 76±10 |
|
| HR, bpm |
|
Dper | 241±32 | 0.75 | 247±30 | 0.96 | 246±27 | 0.79 | 236±27 | 0.95 | 243±29 | 0.75 | 254±30 | 0.51 |
|
Dpre | 240±24 |
| 243±27 |
| 237±29 |
| 236±25 |
| 242±26 |
| 255±27 |
|
|
Dpost | 249±21 |
| 245±28 |
| 237±34 |
| 232±33 |
| 234±22 |
| 240±28 |
|
Neurological outcomes
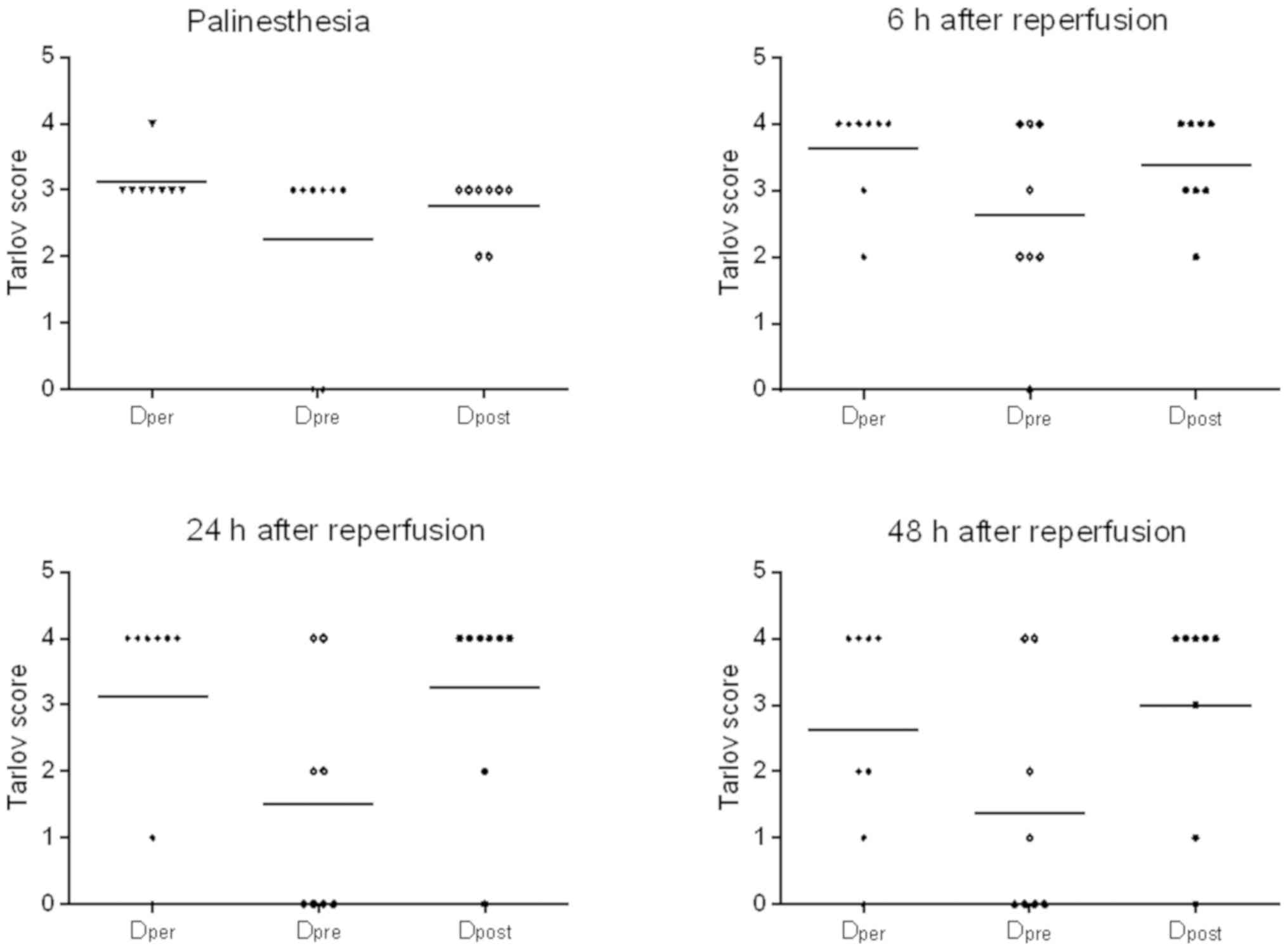
The Tarlov scores of Group Dper, Group
Dpre and Group Dpost are presented in
Fig. 5. DADLE per-conditioning
improved neurological outcome at different time points following
reperfusion. The Tarlov scores of DADLE perfusion were then
compared during ischemia, prior to ischemia onset or at the time of
early reperfusion in order to determine the optimum time point of
administration. The results revealed that there were no significant
differences between the three groups (P>0.05; Fig. 5). However, the Tarlov scores were
higher in the DADLE per- and post-conditioning groups compared with
those in the DADLE pre-conditioning group, but these were not
statistically significant (corrected P>0.05; Fig. 5). The paraplegia rates are
summarized in Table III. The
rates of paraplegia and neurological dysfunction were determined as
above. In the Dper group and the Dpost group,
25% rabbits suffered from hind-limb paraplegia at 48 h after the
reperfusion compared with 62.5% rabbits in the Dpre
group (P>0.05). In addition, the results revealed that there
were no significant differences in the incidences of neurological
dysfunction between the three groups. However, the neurological
dysfunction rates were higher in the Dpre group when
compared with the Dper and Dpost groups at 6,
24 and 48 h after the reperfusion, but these were also not
statistically significant (P>0.05; Table III).
 | Table III.Incidence of paraplegia and
neurological dysfunction at different time points during ischemia
and reperfusion injury. |
Table III.
Incidence of paraplegia and
neurological dysfunction at different time points during ischemia
and reperfusion injury.
|
| Incidence of
paraplegia, % | Incidence of
neurological dysfunction, % |
|---|
|
|
|
|
|---|
| Time point |
Dper |
Dpre |
Dpost | P-value |
Dper |
Dpre |
Dpost | P-value |
|---|
| Palinesthesia |
0.0 | 25.0 |
0.0 | 0.30 | 87.5 | 100.0 | 100.0 | 1.00 |
| 6 h |
0.0 | 12.5 |
0.0 | 1.00 | 25.0 |
62.5 |
50.0 | 0.46 |
| 24 h | 25.0 | 50.0 | 12.5 | 0.40 | 25.0 |
62.5 |
25.0 | 0.36 |
| 48 h | 25.0 | 62.5 | 25.0 | 0.36 | 50.0 |
75.0 |
37.5 | 0.46 |
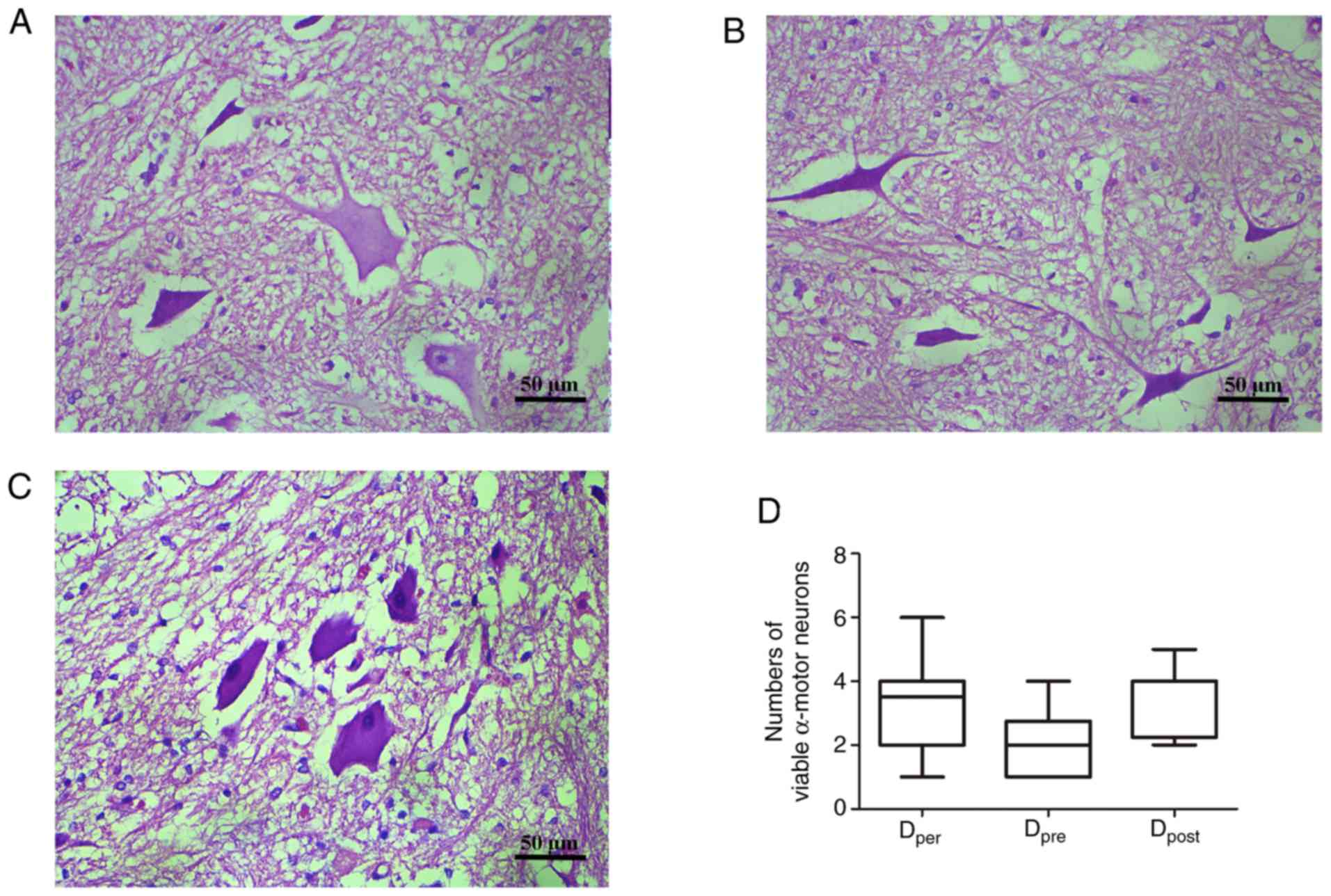
Histopathological changes in the
anterior horn
Representative images of sections stained with
hematoxylin and eosin are presented in Fig. 6A-C. There was a greater number of
viable α-motor neurons observed in the Dper and
Dpost group than the Dpre group, but this was
not statistically significant (P>0.05; Fig. 6D). The number of normal α-motor
neurons appeared largest in the post-conditioning group with DADLE,
and least in the pre-conditioning group. These histopathological
changes were closely associated with the neurological outcomes,
suggesting that DADLE pre- and post-conditioning offer a method of
preservation of normal neurons that is just as effective as
per-conditioning.
Discussion
The rabbit model utilized in the present study was
established based on a classical model and previous research
(20,21). It has been demonstrated that spinal
cord injury is relatively consistent with infrarenal aortic
occlusion for 30 min and the paraplegia rate can approach ~80%. In
contrast to systematic administration, intra-aortic administration
presents clear advantages, such as improved protective efficiency
of the spinal cord and low systemic side effects. The biggest
advantage of this method is that a much higher pharmaceutical
concentration of DADLE can be infused directly to the ischemic
spinal cord segments through the lumbar arteries (22).
A previous study demonstrated that regional
administration of DADLE via the abdominal aorta provided
dose-dependent protection on spinal cord I/R in rabbits (15). After studying the dose-dependent
protective effect, it was revealed that the time of drug
administration was also acritical factor for a neuroprotective
effect. Considering the advantages of pharmacological pre- and
post-conditioning in the prevention and treatment of patients with
ischemic events in the clinical setting, a new experiment was
designed. The purposes of the previous and the present study are
different. In the present study, the neuroprotective effects of
DADLE administration were compared at different time periods, i.e.,
before, during and after ischemia in order to determine which
conditioning strategy was the best.
Similar to previous findings, the results from the
present study revealed that DADLE per-conditioning provided greater
protection than NS. In addition, it was demonstrated that DADLE
perfusion at the other two time points also provided
neuroprotection of the brain against I/R injury. However, the
Tarlov scores and the incidences of paraplegia in the rabbits
treated with DADLE per- and post-conditioning were higher than
those subjected to DADLE pre-conditioning, despite not being
statistically significant. DADLE post-conditioning markedly
decreased normal motor neuron injury and improved the neurologic
deficit scores 48 h after spinal cord ischemia in the present
study. Such effective protection of DADLE post-conditioning in the
spinal cord was in accordance with that reported in the brain
(14) and heart (23). The results from the present study
suggested that DADLE can be applied following ischemia occurrence,
which can not only protect the spinal cord from ischemia, but is
also more practical in the clinical setting compared with
per-conditioning, and provides a better therapeutic option.
Ischemic pre- and post-conditioning have emerged as
useful new strategies for ameliorating organ injuries, preserving
associated functions and potentially improving morbidity and
mortality (24). Ischemic
pre-conditioning, as the first-used form of pre-conditioning, is an
adaptive response triggered by brief ischemia applied prior to
prolonged ischemia, and has been demonstrated to have a powerful
protective effect against I/R injury for the spinal cord (25,26).
Later, ischemic post-conditioning via mechanical interruptions of
reperfusion reported by Zhao et al (27) was as effective as ischemic
pre-conditioning in decreasing infarct size in open-chest dogs.
However, these mechanical approaches are invasive in nature, and
there are inherent risks of thromboembolism and damage with
repeated clamping and declamping of the aorta. Therefore,
pharmacological methods became the focus of the experiments to
assess the anticipated I/R injury of an organ or tissue. In several
previous studies, a variety of diverse pharmacological pre- and
post-conditioning agents, such as adenosine, natriuretic peptide
and bradykinin led to beneficial organ protective effects (4–6).
Among these agents, opioid pre-and post-conditioning as opposed to
transient ischemic stimuli have now been demonstrated to elicit a
satisfactory protective effect against ischemia from the heart to
the brain in animal models (9,11,28).
DADLE, an artificial synthetic delta opioid receptor
agonist, has been demonstrated to prevent the central nervous
system from ischemic injury in cultured cells and animal
experiments (13,29). For example, Su et al
(13) demonstrated that
intracerebroventricular administration of DADLE 45 min before
forebrain ischemia had a protective effect against hippocampal CA1
neurons loses and a dose-dependent improvement of intellectual
disability. In addition, the authors revealed that DADLE
administered at the onset of reperfusion also exhibited a
preservation of CA1 neurons and cognitive benefits in rats with
transient forebrain ischemia (14). However, to the best of our
knowledge, there are no studies currently published that
investigate the effects on the spinal cord. Therefore, the present
study was designed and a positive result was observed. From the
perspective of clinical application, the utilization of drug
post-conditioning is more practical considering the
unpredictability of disease occurrence. Post-conditioning with
DADLE may be more feasible when applying the technique to
post-ischemic spinal cord tissue.
In the present study, the paraplegia rate and loss
of normal motor neurons were higher in the DADLE pre-conditioning
compared with the DADLE per- and post-conditioning groups, although
these results were not statistically significant. Previously, Su
et al (13) demonstrated
that DADLE provided protection when administered 45 min before
ischemia, which was different to what was observed in the present
study. These differences may be due to the different animal models
used. Lee and Amidon (30)
demonstrated that the half-lives of DADLE in distribution and
elimination phases were very short, ~0.5 and 5 min, respectively.
DADLE reached plateau plasma concentration within 15 min of
intravenous administration and was rapidly cleared following
absorption. Therefore, mitigated neuroprotection of DADLE
pre-conditioning might be due to its short half-life.
DADLE treatment is known to result in transient
depression of MAP and HR in spinal cord I/R injury (17). In this study, it was revealed that
the MAP was lower at the point of pre-ischemia compared with the
point of ischemia and reperfusion in the DADLE pre-conditioning
group; however, this was not statistically significant. Similarly,
it was also observed that MAP and HR were lower at the point of
post-ischemia compared with the point of pre-ischemia and during
ischemia in the DADLE post-conditioning group; however, again, this
was not statistically significant. The statistical significances of
HR among the three groups were not determined. Two reasons may
account for this phenomenon. Firstly, a relatively low-dose of
DADLE may lead to fewer hemodynamic changes took place in rabbits.
Secondly, NS was infused intravenously at a rate of 10 ml/kg per h
to maintain fluid requirements in the present study.
Furthermore, whether the results of the present
study could be applied to a clinical setting remains unknown.
American Spinal Injury Association (ASIA) guidelines (31) are used for the assessment of
patient motor, sensory and autonomic dysfunction following spinal
cord injury that should be assessed by clinicians. The present
study used the Tarlov scoring system instead of ASIA for
neurological evaluation, as the source of blood supply to the
spinal cord of rabbits is different from that in humans. The
homosegmental blood supply of spinal cords in rabbits begins at the
abdominal aorta caudally to the origin of renal arteries with
minimal or no intraspinal collateral arterial system. However, the
blood supply to the spinal cord in humans originates from segmental
arteries (lumbar arteries) and the vertebral artery (32). The Tarlov score is a widely
accepted and matched method for evaluation of rabbit neural
dysfunction following spinal cord injury (33). A significant benefit was observed
when DADLE was administered after the start of abdominal aorta
occlusion, even at an early phase of reperfusion in rabbits. The
results from the present study may provide an innovative
therapeutic strategy for a clinical situation.
There are several limitations to the present study.
First, the present study only investigated the effectiveness of the
strategies in question and, therefore, the underlying molecular
mechanisms remain unclear. Tian et al (34) reported that DADLE was able to
inhibit cellular transcription by regulating phosphorylation of RNA
polymerase II in primary cortical neurons, which may provide a
potential insight into the molecular mechanism underlying
neuroprotection. In the present study, it was revealed that the
protective effects of DADLE per-conditioning may be associated with
its anti-oxidant and anti-apoptotic properties in the rabbit model
of spinal cord I/R injury (35).
Future studies may aim to clarify the potential mechanisms
responsible for the different protective effects of DADLE pre- and
post-conditioning.
In summary, the present study suggested that DADLE
administration at three time points, before ischemia onset, during
the ischemic period or at the early reperfusion period for 30 min
exerted preservation effects on neurological function and normal
neurons in a rabbit model of spinal cord I/R. The therapeutic
effects appeared most notable in the post-conditioning group with
DADLE, and was worst in the pre-conditioning group. The results
from the present study may provide new therapeutic potentials in
improving clinical outcomes in patients with thoraco-abdominal
aortic cross-clamping.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Natural
Science Foundation of China (grant. no. 81771269), The Shanghai
Pujiang Program (grant. no. 17PJD035) and The Shanghai Natural
Science Foundation (grant no. 15ZR1433800).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and HuL conceived and designed the experiments.
DF performed the experiments, analyzed the data and wrote the
manuscript. HaL performed the experiments and contributed to the
reagents, materials and analysis tools.
Ethics approval and consent to
participate
The animal protocol was approved by The Animal Care
and Use Committee of Shanghai Jiaotong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zvara DA: Thoracoabdominal aneurysm
surgery and the risk of paraplegia: Contemporary practice and
future directions. J Extra Corpor Technol. 34:11–17.
2002.PubMed/NCBI
|
|
2
|
Ballard JL: Thoracoabdominal aortic
aneurysm repair: Historical review and description of a
re-engineered technique. Perspect Vasc Surg Endovasc Ther.
17:207–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehmedagic I, Resch T and Acosta S:
Complications to cerebrospinal fluid drainage and predictors of
spinal cord ischemia in patients with aortic disease undergoing
advanced endovascular therapy. Vasc Endovascular Surg. 47:415–422.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitakaze M, Asakura M, Kim J, Shintani Y,
Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T,
et al: Human atrial natriuretic peptide and nicorandil as adjuncts
to reperfusion treatment for acute myocardial infarction (J-WIND):
Two randomised trials. Lancet. 370:1483–1493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kloner RA, Forman MB, Gibbons RJ, Ross AM,
Alexander RW and Stone GW: Impact of time to therapy and
reperfusion modality on the efficacy of adenosine in acute
myocardial infarction: The AMISTAD-2 trial. Eur Heart J.
27:2400–2405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Danielisova V, Gottlieb M, Nemethova M,
Kravcuková P, Domoráková I, Mechírová E and Burda J: Bradykinin
postconditioning protects pyramidal CA1 neurons against delayed
neuronal death in rat hippocampus. Cell Mol Neurobiol. 29:871–878.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L and Zuo Z: Isoflurane
postconditioning induces neuroprotection via Akt activation and
attenuation of increased mitochondrial membrane permeability.
Neuroscience. 199:44–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu QJ, Zhou QS, Huang HB, Wang YL, Tian SF
and Duan DM: Protective effect of ketamine on ischemic spinal cord
injury in rabbits. Ann Vasc Surg. 22:432–439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Charron C, Messier C and Plamondon H:
Neuroprotection and functional recovery conferred by administration
of kappa- and delta 1-opioid agonists in a rat model of global
ischemia. Physiol Behav. 93:502–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schultz JE, Rose E, Yao Z and Gross GJ:
Evidence for involvement of opioid receptors in ischemic
preconditioning in rat hearts. Am J Physiol. 268:H2157–H2161.
1995.PubMed/NCBI
|
|
11
|
Zatta AJ, Kin H, Yoshishige D, Jiang R,
Wang N, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ, Caffrey JL and
Vinten-Johansen J: Evidence that cardioprotection by
postconditioning involves preservation of myocardial opioid content
and selective opioid receptor activation. Am J Physiol Heart Circ
Physiol. 294:H1444–H1451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borlongan CV, Wang Y and Su TP: Delta
opioid peptide (D-Ala 2, D-Leu 5) enkephalin: Linking hibernation
and neuroprotection. Front Biosci. 9:3392–3398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su DS, Wang ZH, Zheng YJ, Zhao YH and Wang
XR: Dose-dependent neuroprotection of delta opioid peptide [D-Ala2,
D-Leu5] enkephalin in neuronal death and retarded behavior induced
by forebrain ischemia in rats. Neurosci Lett. 423:113–117. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Duan Y, Su D, Li W, Tan J, Yang D,
Wang W, Zhao Z and Wang X: Delta opioid peptide [D-Ala2, D-Leu5]
enkephalin (DADLE) triggers postconditioning against transient
forebrain ischemia. Eur J Pharmacol. 658:140–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Chen B, Li S and Yao J:
Dose-dependent neuroprotection of delta-opioid peptide [D-Ala(2),
D-Leu(5)] enkephalin on spinal cord ischemia-reperfusion injury by
regional perfusion into the abdominal aorta in rabbits. J Vasc
Surg. 63:1074–1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the care and use of
laboratory animalsWashington (DC): National Academies Press (US);
1996
|
|
17
|
Liu H, Chen B, Zhang Y, Qiu Y, Xia Y, Li S
and Yao J: Protective effect of delta opioid agonist [D-Ala2,
D-Leu5] enkephalin on spinal cord ischemia reperfusion injury by
regional perfusion into abdominal aorta in rabbits. Neurosci Lett.
584:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tarlov IM: Acute spinal cord compression
paralysis. J Neurosurg. 36:10–20. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ehrlich M, Knolle E, Ciovica R, Böck P,
Turkof E, Grabenwöger M, Cartes-Zumelzu F, Kocher A, Pockberger H,
Fang WC, et al: Memantine for prevention of spinal cord injury in a
rabbit model. J Thorac Cardiovasc Surg. 117:285–291. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Apaydin AZ and Buket S: Regional lidocaine
infusion reduces postischemic spinal cord injury in rabbits. Tex
Heart Inst J. 28:172–176. 2001.PubMed/NCBI
|
|
21
|
Yao JY, Weng H, Zhang L, Wang QY, Yuan YQ,
Tang Y and Li JS: The reperfusion injury model improvement and the
tolerance time investigation of rabbit spinal cord ischemia under
normothermia. Sichuan Da Xue Xue Bao Yi Xue Ban. 38:497–500, 542.
2007.(In Chinese). PubMed/NCBI
|
|
22
|
Hamaishi M, Orihashi K, Isaka M, Kumagai
H, Takahashi S, Okada K, Ohtaki M and Sueda T: Low-dose edaravone
injection into the clamped aorta prevents ischemic spinal cord
injury. Ann Vasc Surg. 23:128–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuardo M, Lemoine S, Lo Coco C, Hanouz JL
and Massetti M: [D-Ala2,D-Leu5]-enkephalin (DADLE) and
morphine-induced postconditioning by inhibition of mitochondrial
permeability transition pore, in human myocardium. Exp Biol Med
(Maywood). 238:426–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hausenloy DJ and Yellon DM:
Preconditioning and postconditioning: Underlying mechanisms and
clinical application. Atherosclerosis. 204:334–341. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toumpoulis IK, Papakostas JC, Matsagas MI,
Malamou-Mitsi VD, Pappa LS, Drossos GE, Derose JJ and
Anagnostopoulos CE: Superiority of early relative to late ischemic
preconditioning in spinal cord protection after descending thoracic
aortic occlusion. J Thorac Cardiovasc Surg. 128:724–730. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zvara DA, Colonna DM, Deal DD, Vernon JC,
Gowda M and Lundell JC: Ischemic preconditioning reduces neurologic
injury in a rat model of spinal cord ischemia. Ann Thorac Surg.
68:874–880. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: Comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z, Li T and Zhang B: Morphine
postconditioning protects against reperfusion injury in the
isolated rat hearts. J Surg Res. 145:287–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma MC, Qian H, Ghassemi F, Zhao P and Xia
Y: Oxygen-sensitive {delta}-opioid receptor-regulated survival and
death signals: Novel insights into neuronal preconditioning and
protection. J Biol Chem. 280:16208–16218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HJ and Amidon GL: The effect of enzyme
inhibitor and absorption site following [D-ala2, D-leu5]enkephalin
oral administration in rats. Biopharm Drug Dispos. 23:131–141.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krassioukov AV, Karlsson AK, Wecht JM,
Wuermser LA, Mathias CJ and Marino RJ; Joint Committee of American
Spinal Injury Association and International Spinal Cord Society, :
Assessment of autonomic dysfunction following spinal cord injury:
Rationale for additions to international standards for neurological
assessment. J Rehabil Res Dev. 44:103–112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santillan A, Nacarino V, Greenberg E,
Riina HA, Gobin YP and Patsalides A: Vascular anatomy of the spinal
cord. J Neurointerv Surg. 4:67–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seppälä M, Antinheimo J, Pohjola J and
Hernesniemi J: Acute spinal cord compression. Duodecim.
129:2655–2660. 2013.(In Finnish). PubMed/NCBI
|
|
34
|
Tian J, Gu Y, Sun K, Wang B, Chen J, Wang
X and Su D: [D-Ala2, D-Leu5] encephalin (DADLE) reversibly inhibits
cellular transcription in neurons without causing cell injury.
Brain Res. 1565:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu D, Liu H, Li S, Chen L and Yao J:
Antioxidative and antiapoptotic effects of delta-opioid peptide
[D-Ala2, D-Leu5] enkephalin on spinal cord
ischemia-reperfusion injury in rabbits. Front Neurosci. 11:6032017.
View Article : Google Scholar : PubMed/NCBI
|