Introduction
Osteoarthritis (OA) is known to be associated with
congenital factors including aging, genetic factors, and acquired
factors including trauma and obesity. These factors influence
diverse biochemical signaling pathways in chondrocytes leading to
unbalanced intracellular metabolic activities (1). OA is caused by cartilage
degeneration, which is caused by metabolic imbalance (2). Articular cartilage consists of a
high-density extracellular matrix (ECM) comprised of fibronectin,
sulfated proteoglycan, and collagen (3). The differentiated chondrocyte
phenotype is detected based on type II collagen expression. OA
accompanies dedifferentiation and reduction in the level of type II
collagen expression (4).
Inflammatory reactions play a crucial role in
articular cartilages, and hence, inflammation is a key player in
the pathogenesis of OA. Cyclooxygenases (COXs) appear in two
protein isoforms, COX-1 and COX-2, among which the latter is
produced by inducible enzymes and participates mainly in
inflammatory regulation (5,6).
COX-2 manifestation is controlled during transcription,
post-transcription, and translation (7).
Mitogen-activated protein kinase (MAPK) is the
foundation of intracellular signaling that regulates cellular
functions including cell survival and apoptosis, mitosis, gene
expression, cellular proliferation, inflammatory reaction, and
differentiation. These processes are induced by stimulation by
external signaling molecules such as cytokines, growth factors, and
hormones (8,9). This pathway is found in all
eukaryotic cells and presumed to play a pivotal role in cell
viability (9). Such signal
transduction begins with phosphorylation and activation of MAPK
kinase (MAPKK) by a group of protein kinases known as MAPK kinase
kinase (MAPKKK), followed by the phosphorylation and activation of
MAPKs by MAPKKs (10). These
MAPKKK and MAPKK enzymes phosphorylate three major proteins of the
MAPK family, namely, p38 kinase, extracellular signal-regulated
kinase (ERK-1/-2), and c-Jun N-terminal kinase (JNK) (11).
The endoplasmic reticulum (ER) is a huge and dynamic
organelle that plays a variety of intracellular roles in calcium
preservation, lipid metabolism, and cell signaling. The ER is
mainly responsible for protein synthesis and folding. In addition,
it facilitates various post-translational modifications that are
essential for protein function (12,13).
The disruption of ER homeostasis and accumulation of misfolded or
unfolded proteins inside the cell causes unfolded protein response
(UPR), which is an intracellular signaling system that induces cell
damage and apoptosis (14). The
mechanism of UPR signaling involves binding of a misfolded protein
and glucose-regulated protein 78 kDa (GRP78) to sensor proteins
located on the ER membrane including, inositol-requiring enzyme 1
(IRE1), pancreatic ER kinase (PERK), and activating transcription
factor 6 (ATF6) (15–17). Among these, IRE1 is the most
well-preserved UPR signaling protein in eukaryotes that undergoes
autophosphorylation for activation when the level of unfolded
proteins increases in the ER to trigger the downstream signaling
pathway for X-box binding protein 1 (XBP-1) mRNA splicing. The
XBP-1 mRNA after splicing is designated as XBP-1s, while that
without splicing is designated XBP-1u. XBP-1s mRNA, which is
spliced by IRE1 from XBP-1u mRNA, is expressed as an active XBP-1
protein, which promotes ER-stress-related signaling (18). Persistent ER-stress leads to
diseases such as cancer, diabetes, and obesity (19).
2-Deoxy-D-glucose (2DG) is a glucose derivative with
2′OH replaced by an H, and this derivative inhibits several
metabolic processes, such as glycosylation, leading to ER stress.
Treating chondrocytes with 2DG reduces the expression of type II
collagen and COX-2, while inducing ER-stress-related
unglycosylation (20). Gallotannin
(GT) is a hydrolyzable tannin, i.e., a plant polyphenol, which is
used as a protective agent in a similar manner to a drug, and
exhibits strong activities against cancers, oxidation, viruses,
bacteria, and parasites. Treatment of chondrocytes with GT was
revealed to promote the expression of type II collagen and COX-2
(21). Although previous studies
have examined the independent effects of each of these agents on
chondrocytes, the effects of simultaneous treatment with GT and
2DG, in addition to the signaling pathways regulating their
activities, have not been adequately investigated (20–22).
Thus, the present study aimed to elucidate the effects of treatment
with GT and 2DG on chondrocytes and the mechanisms involved.
Materials and methods
Culture of chondrocytes
The present study was approved by the Ethics
Committee of Kongju National University. Fifty specific
pathogen-free (SPF) rabbits were sacrificed, one or two rabbits
weekly for one year, in compliance with the ethical guidelines, to
obtain articular chondrocytes from healthy, normal rabbits (2-weeks
old; New Zealand white rabbits; Koatech). The SPF rabbits were
supplied from Koatech. Koatech is accredited by the Association for
Assessment and Accreditation of Laboratory Animal Care
International (AAALAC), and also complies with the ‘Guide for the
Care and Use of Laboratory Animals; 8th ed.’ (1996, National
Research Council). After arrival the condition of the rabbits was
monitored every 6 h and they were sacrificed for the experiment 24
h upon arrival. The rabbits were anesthetized using 7% ether and
ethically euthanized via cervical dislocation. Ethyl ether was the
inhalation anesthetic used, and the concentration was decided based
on the contents of a book published in Korea in 1996. Euthanasia of
rabbits was confirmed by cardiac arrest (23). The rabbits were fed separately, and
had free access to unlimited food and water in an undisturbed and
clean environment. The rabbits were housed in a cage that had a
12-h light/dark cycle, and was maintained at a temperature of
~22-25°C and a relative humidity ranging from 40 to 60%.
Chondrocytes were separated from rabbit articular cartilages, as
previously described (24). A
piece of cartilage was melted with 0.1% collagenase for 9 h in a
36.5°C CO2 incubator. Cells (5×104
cells/dish) were cultured in Dulbecco's modified Eagle's medium
(DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) including 10%
(v/v) fetal bovine-calf serum (Tissue Culture Biologicals),
penicillin (50 unit/ml), and streptomycin (50 µg/ml).
Treatment of cells
GT (molecular formula:
C76H52O46; CAS no. 1401-55-4;
Sigma-Aldrich; Merck KGaA) and 2DG (molecular formula:
C6H12O5; CAS no. 154-17-6;
Sigma-Aldrich Merck KGaA) were first dissolved with autoclaved
triple distilled water and filtered. Chondrocytes were treated with
GT 100 µM and 2DG 10 mM for 24 h. The concentration and duration of
GT and 2DG used in the present study, were determined to best
represent the effect of each reagent on chondrocytes based on
previous studies (20–22). In addition, SB (CAS no.
152121-47-6; BIOMOL; Enzo Life Sciences), PD (CAS no. 167869-21-8;
Calbiochem; EMD/Merck KGaA) and Salubrinal (CAS no.
ALX-270-428-M005; Enzo Life Sciences, Inc.) blockers were added 2 h
prior to treatment with GT and 2DG. SB, PD, and Salubrinal inhibit
p38 kinase, ERK-1/-2, phosphorylation and dephosphorylation of
p-eIF2α.
Western blot analysis
Protein quantification and SDS-PAGE for protein
confirmation were conducted from chondrocytes, as previously
described (24). The antibodies
used (1:1,000) were as follows: Type II collagen (cat. no.
sc-52658); COX-2 (cat. no. sc-1745); GRP78 (cat. no. sc-1050; all
from Santa Cruz Biotechnology, Inc.); IRE1α (cat. no. NB100-2324;
Novus Biologicals); p-eIF2α (cat. no. sc-101670; Santa Cruz
Biotechnology, Inc.); ATF6 (cat. no. 70B1413.1; Enzo Life Sciences,
Inc.); GAPDH (cat. no. sc-166545; Santa Cruz Biotechnology, Inc.);
p-p38 (cat. no. 4511; Cell Signaling Technology, Inc.); p-ERK (cat.
no. sc-7383; Santa Cruz Biotechnology, Inc.); anti-rabbit IgG (cat.
no. AP132; Sigma-Aldrich; Merck KGaA); anti-goat IgG (cat. no.
sc-2354; Santa Cruz Biotechnology, Inc.); and anti-mouse IgG (cat.
no. BML-SA204-0100; Enzo Life Sciences, Inc.). An ECL (Daeil Lab
Service Co., Ltd.) reagent was used to saw protein bands using the
LAS4000 (GE Healthcare Life Sciences).
RT-PCR
To confirm the protein expression regulated at the
transcriptional level, RNA was extracted from chondrocytes
utilizing TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.).
Furthermore, mRNA was synthesized and amplified by cDNA, and the
amount of gene expression was confirmed by electrophoresis on 2%
agarose gel. Each sample was incubated at 95°C for 30 sec, 50°C for
30 sec, and 72°C for 30 sec for 28 cycles. The primer sequences
were as follows: Type II collagen sense, 5′-GACCCCATGCAGTACATGCG-3′
and antisense, 5′-AGCCGCCATTGATGGTCTCC-3′; COX-2 sense,
5′-CCCTATGAATCGTTCGAGGA-3′ and antisense,
5′-GGACAGCCCTTCACATTGTT-3′; XBP-1 sense, 5′-TGGATGCCATGGTTACTGAA-3′
and antisense, 5′-CTGGCAGTTTCTGGAGAAGC-3′; GAPDH sense,
5′-TCACCATCTTCCAGGAGCGA-3′ and antisense,
5′-CACAATGCCGAAGTGGTCGT-3′.
Alcian blue staining
Proteoglycans were measured using 0.1% alcian blue
solution (Sigma-Aldrich; Merck KGaA) to determine differentiation
of chondrocytes. Cells were reacted with 3.5% paraformaldehyde for
25 min and stained as previously described (24). The absorbance was assessed using a
microplate reader at 595 nm.
Immunofluorescence staining
Revelation of type II collagen, COX-2 and GRP78
within cells was identified. Cells were fixed and antibodies were
attached as previously described (24). All primary antibodies used were the
same as those used in the western blotting experiment. The
secondary antibodies used were: TRITC conjugated anti-mouse IgG
(cat. no. T5393; Sigma-Aldrich; Merck KGaA) for type II collagen,
FITC conjugated anti-goat IgG (cat. no. ab6881; Abcam) for COX-2
and TRITC conjugated anti-goat IgG (cat. no. ab150130; Abcam) for
GRP78. These secondary antibodies were incubated at room
temperature for 2 h. Then, the cells were counterstained with
4′6′-diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen;
Thermo Fisher Scientific, Inc.). Fluorescent images were captured
using a BX51 fluorescence microscope (Olympus Corporation).
Transfection
IRE1 siRNA was transfected into chondrocytes using a
TurboFect transfection reagent (Thermo Fisher Scientific, Inc.).
The primer sequences were as follows: IRE1 siRNA sense,
5′-GAUGUCCCACUUUGUGUCCTT-3′ and antisense,
5′-GGACACAAAGUGGGACAUCTT-3′; negative control (NC) siRNA sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Statistical analysis
All experimental results were repeated at least 3
times and presented as the mean ± standard deviation (SD). Data
were assayed using one-way analysis of variance (ANOVA) followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
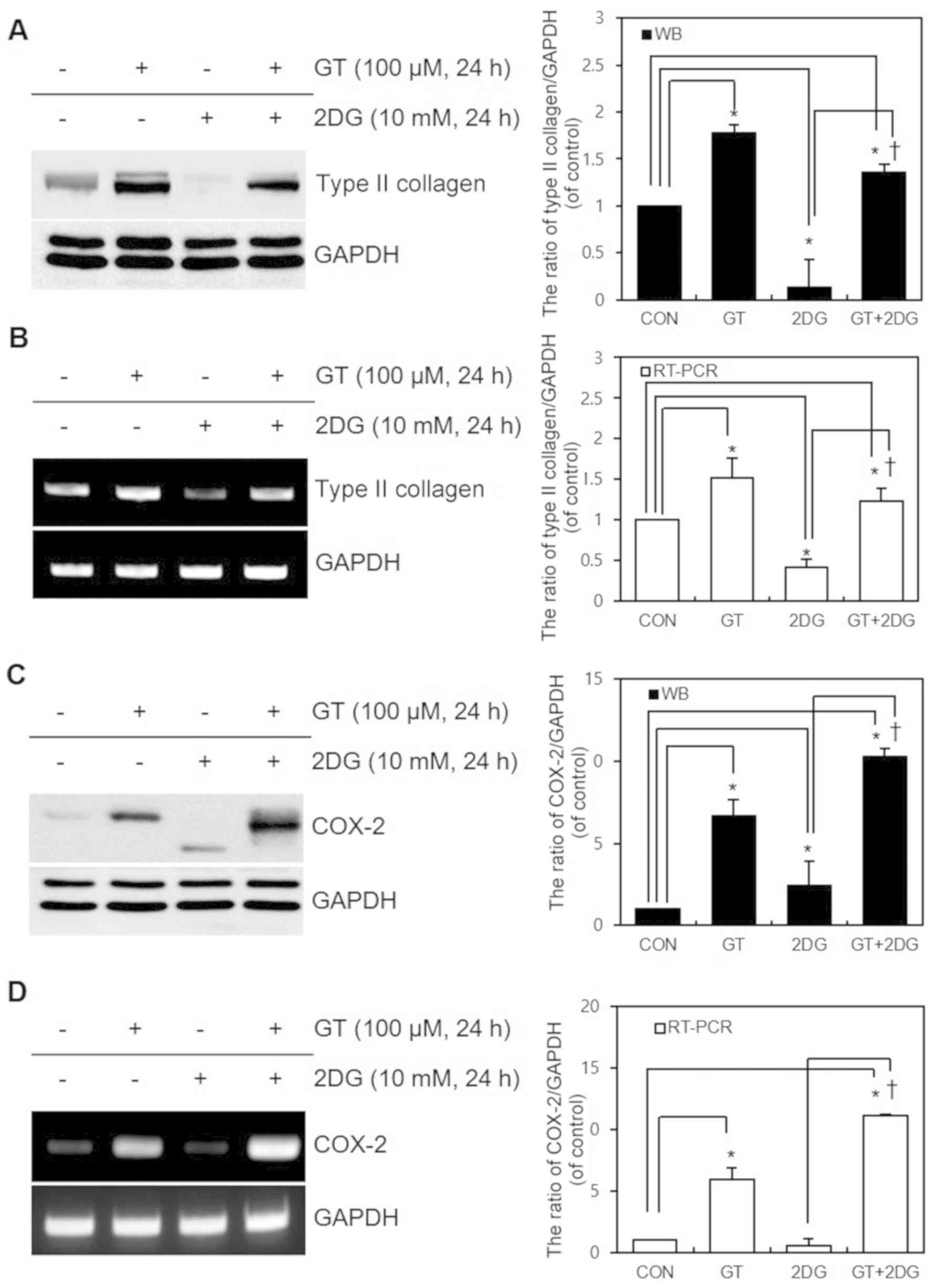
GT regulates 2DG-induced
dedifferentiation, inflammatory reactions, and ER stress
The effects of GT and 2DG treatment for 24 h on
differentiation and inflammatory reactions in cultured chondrocytes
were examined. Following treatment, western blot and RT-PCR
analyses were performed to detect the changes in the expression of
type II collagen (the differentiated phenotype of chondrocytes) and
COX-2 (a major protein involved in inflammatory reactions). The
results revealed that, after GT and 2DG treatment, the 2DG-induced
dedifferentiation was attenuated by GT at the transcriptional level
(Fig. 1A and B). GT also
attenuated the 2DG-triggered decrease in COX-2 expression and
ER-stress-induced unglycosylation, while promoting COX-2
expression. The regulation of ER-stress-induced COX-2
unglycosylation is considered to have occurred at the protein
translational or post-translational regulation level and not at the
transcriptional level (Fig. 1C and
D). The statistical significance of the data from western
blotting and RT-PCR were quantified using ImageJ (Fig. 1A-D). These findings provided
evidence that GT regulates 2DG-driven dedifferentiation,
inflammatory reactions, and ER stress.
GT regulates 2DG-increased
dedifferentiation, inflammatory reactions, and ER stress via the
p38 kinase pathway
To identify the signaling pathway that GT is
involved with in the regulation of 2DG-induced dedifferentiation,
inflammatory reactions, and ER-stress in chondrocytes, SB and PD,
the respective blockers of the p38 kinase and ERK-1/-2 pathways,
and Salubrinal, a blocker of the ER-stress eIF2α pathway, were used
to pre-treat the cells for 2 h. Then, western blotting was
performed to verify the changes in the expression of type II
collagen (a differentiated phenotype of chondrocytes), COX-2 (a
major protein in inflammatory reactions), and GRP78 (an indicator
of ER stress). Treatment of cells with Salubrinal (for blocking
ER-stress eIF2α signaling) resulted in reduced ER stress, while
there were no changes in dedifferentiation or inflammatory
reactions (Figs. 2A and 3A). Furthermore, treatment of cells with
PD to block ERK-1/-2 signaling in fact retriggered COX-2
unglycosylation and increased ER stress. In addition, no changes
related to dedifferentiation occurred (Figs. 2C and 3A). In contrast, treatment of cells with
SB to block p38 kinase signaling caused changes in
dedifferentiation, inflammatory reactions, and ER stress (Figs. 2B and 3A). While these changes were induced
during transcription, COX-2 unglycosylation due to ER stress was
considered to have occurred during protein translation or
post-translational regulation and not during transcription
(Fig. 3B). The statistical
significance of the western blotting and RT-PCR expression data of
type II collagen and COX-2 were quantified using ImageJ (Fig. 3C and D). To verify the results of
the western blot analysis, alcian blue staining was used to
quantify sulfated proteoglycan, another indicator of chondrocyte
differentiation; the results were in conformity with that of the
western blot analysis (Fig. 2D).
Finally, immunofluorescence staining was used to monitor the
cytological changes, and the findings were in agreement with the
other results (Fig. 4). These
results provided evidence that GT regulates 2DG-induced
dedifferentiation, inflammatory responses, and ER stress via the
p38 kinase pathway.
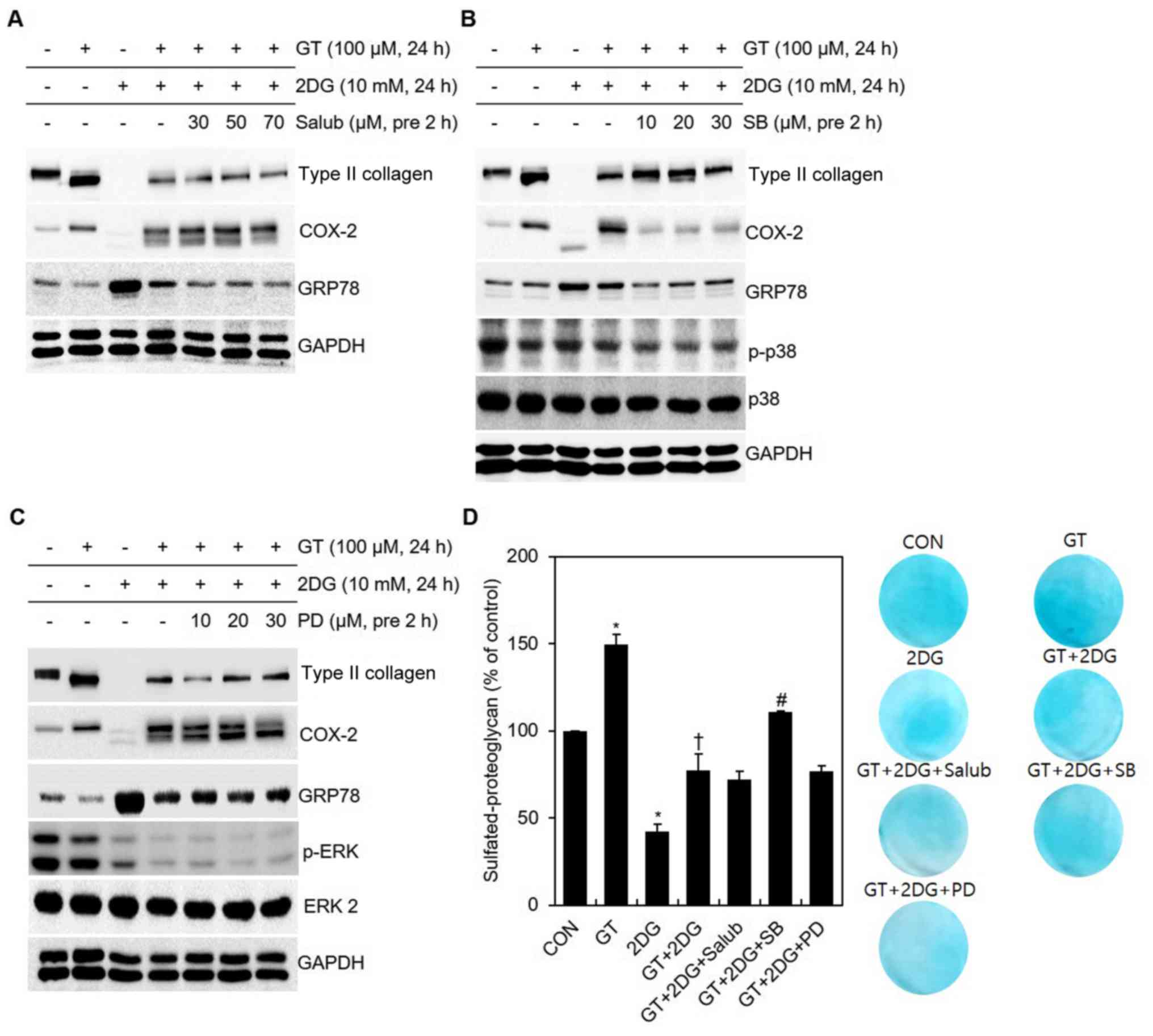 | Figure 2.Identification of signaling pathways
according to the blocker concentration after treatment of
chondrocytes with GT and 2DG. (A-D) After pre-treatment for 2 h
with specific concentrations of Salub, SB, or PD, chondrocytes were
treated with 100 µM GT and 10 mM 2DG for 24 h After pre-treatment
with a specific concentration of (A) Salub, (B) SB, or (C) PD,
western blotting was used to determine the expression of type II
collagen, COX-2, GRP78, p-p38, p-ERK and GAPDH. (D) Sulfated
proteoglycan was examined using alcian blue staining. GAPDH, p38
and ERK 2 were used as the loading controls. Results are presented
as the means ± SD. *P<0.05 compared with untreated cells,
†P<0.05 compared with 2DG-treated cells,
#P<0.05 compared with GT + 2DG-treated cells. GT,
gallotannin; 2DG, 2-deoxy-D-glucose; Salub, salubrinal; COX-2,
cyclooxygenase-2; GRP78, glucose-regulated protein 78 kDa. |
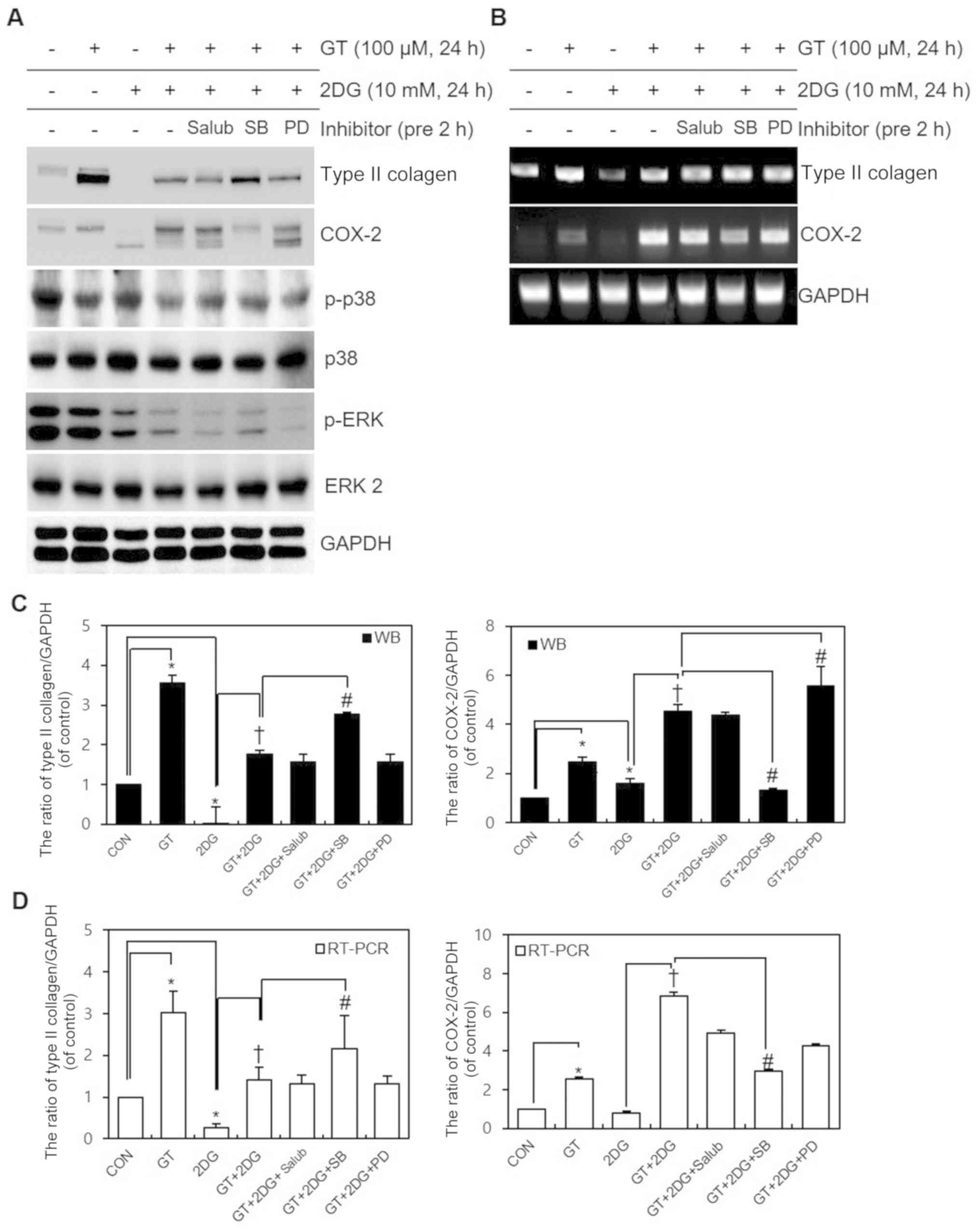 | Figure 3.Identification of signaling pathways
according to the blocker after treatment of chondrocytes with GT
and 2DG. (A and B) After pre-treating chondrocytes for 2 h with
specific concentrations of Salub, SB, or PD, chondrocytes were
treated with 100 µM GT and 10 mM 2DG for 24 h. (A) Western blotting
was used to determine the expression of type II collagen, COX-2,
p-p38, p-ERK, and GAPDH. (B) RT-PCR was used to determine the
expression of type II collagen, COX-2, and GAPDH. p38, ERK 2 and
GAPDH was used as the loading control. (C and D) Relative
expression (% of control) of type II collagen and COX-2. Results
are presented as the means ± SD. *P<0.05 compared with untreated
cells, †P<0.05 compared with 2DG-treated cells,
#P<0.05 compared with GT + 2DG-treated cells. GT,
gallotannin; 2DG, 2-deoxy-D-glucose; Salub, salubrinal; COX-2,
cyclooxygenase-2; GRP78, glucose-regulated protein 78 kDa. |
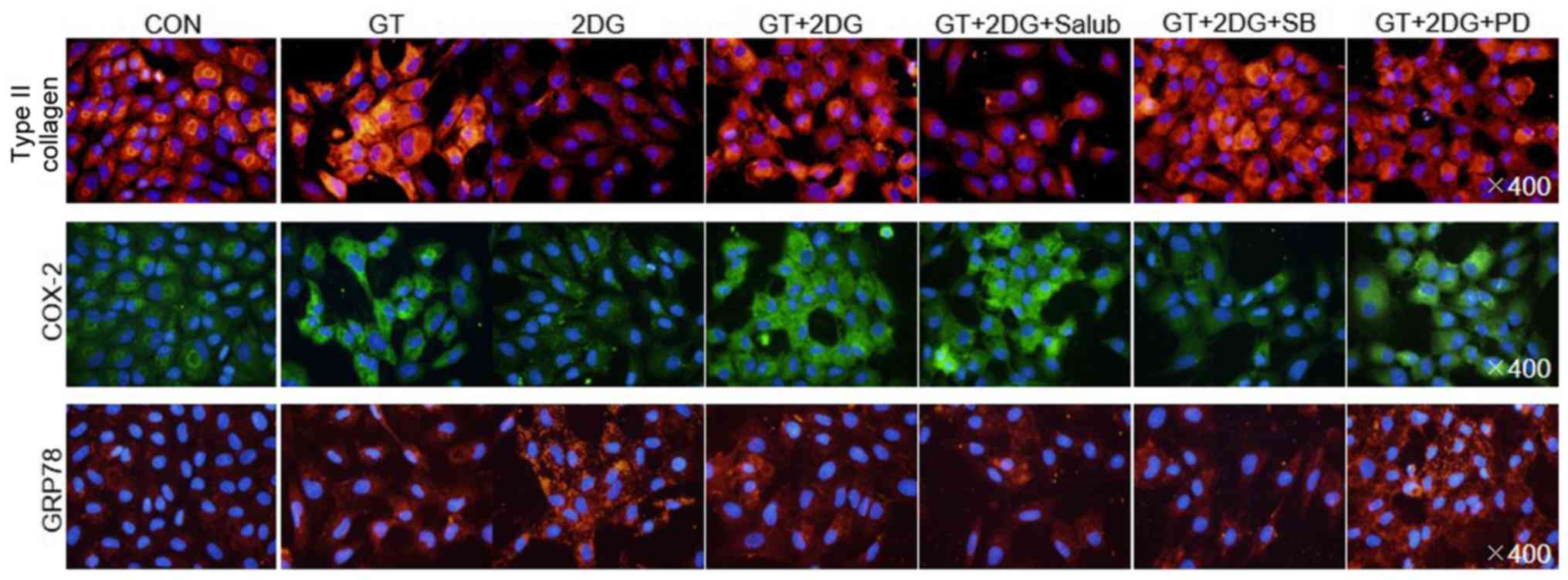 | Figure 4.Identification of signaling pathways
using immunofluorescence after treatment of chondrocytes with GT
and 2DG. After pre-treating chondrocytes for 2 h with specific
concentrations of Salub, SB, or PD, chondrocytes were treated with
100 µM GT and 10 mM 2DG for 24 h. Immunofluorescence microscopy
(magnification, ×400) was used to determine the expression of type
II collagen, COX-2, and GRP78. The cellular nuclei were stained
with DAPI. GT, gallotannin; 2DG, 2-deoxy-D-glucose; Salub,
salubrinal; COX-2, cyclooxygenase-2; GRP78, glucose-regulated
protein 78 kDa. |
GT regulates 2DG-triggered
dedifferentiation, inflammatory reactions, and ER stress via the
ER-stress-induced p38 kinase pathway downstream from the IRE1
pathway
The findings of previous experiments revealed that
GT regulates 2DG-induced dedifferentiation, inflammatory responses,
and ER stress through the p38 kinase pathway. This pathway was
revealed to be a part of the IRE1 downstream signaling pathway
among the three signaling pathways triggered by ER stress. When the
activities of all three signaling pathways were subsequently
assessed, ATF6 and p-eIF2α did not display any change, whereas
IRE1, compared to the control, exhibited increased activity after a
single treatment with a single 2DG dose and decreased activity with
a single GT dose. We attempted to obtain eLF-2α bands as a loading
control to compare the expression of phosphorylated eLF-2α bands
due to reagent treatment. However, eLF-2α bands could not be
obtained due to a lack of immunoreactivity with a number of tested
antibodies. In addition, after concurrent treatment with 2DG and
GT, the IRE1 activity that was increased by 2DG was reduced by GT
(Fig. 5A). This supported that GT
is involved in the IRE1 signaling pathway in the inhibition of
2DG-induced ER stress. Furthermore, the changes in protein
expression when IRE1 expression was reduced through IRE1 siRNA
transfection were in concordance with the results of cells treated
with SB to block the p38 kinase pathway (Fig. 5B). Lastly, it was examined whether
splicing of XBP-1 mRNA was induced by the activated IRE1 downstream
signaling pathway that was related to ER stress. It was revealed
that, when compared to the control whereby no reagent was added,
2DG treatment induced splicing and increased XBP-1s, while GT
treatment decreased XBP-1u. The concurrent treatment with GT and
2DG revealed that GT reduced the increased XBP-1s by 2DG-induced ER
stress. The reduction of IRE1 expression after GT treatment or IRE1
siRNA transfection was accompanied by a decreased expression of
XBP-1 mRNA (Fig. 5C). These
findings provided evidence that GT regulates 2DG-induced
dedifferentiation, inflammatory reactions, and ER stress via the
ER-stress-induced p38 kinase pathway downstream from the IRE1
pathway.
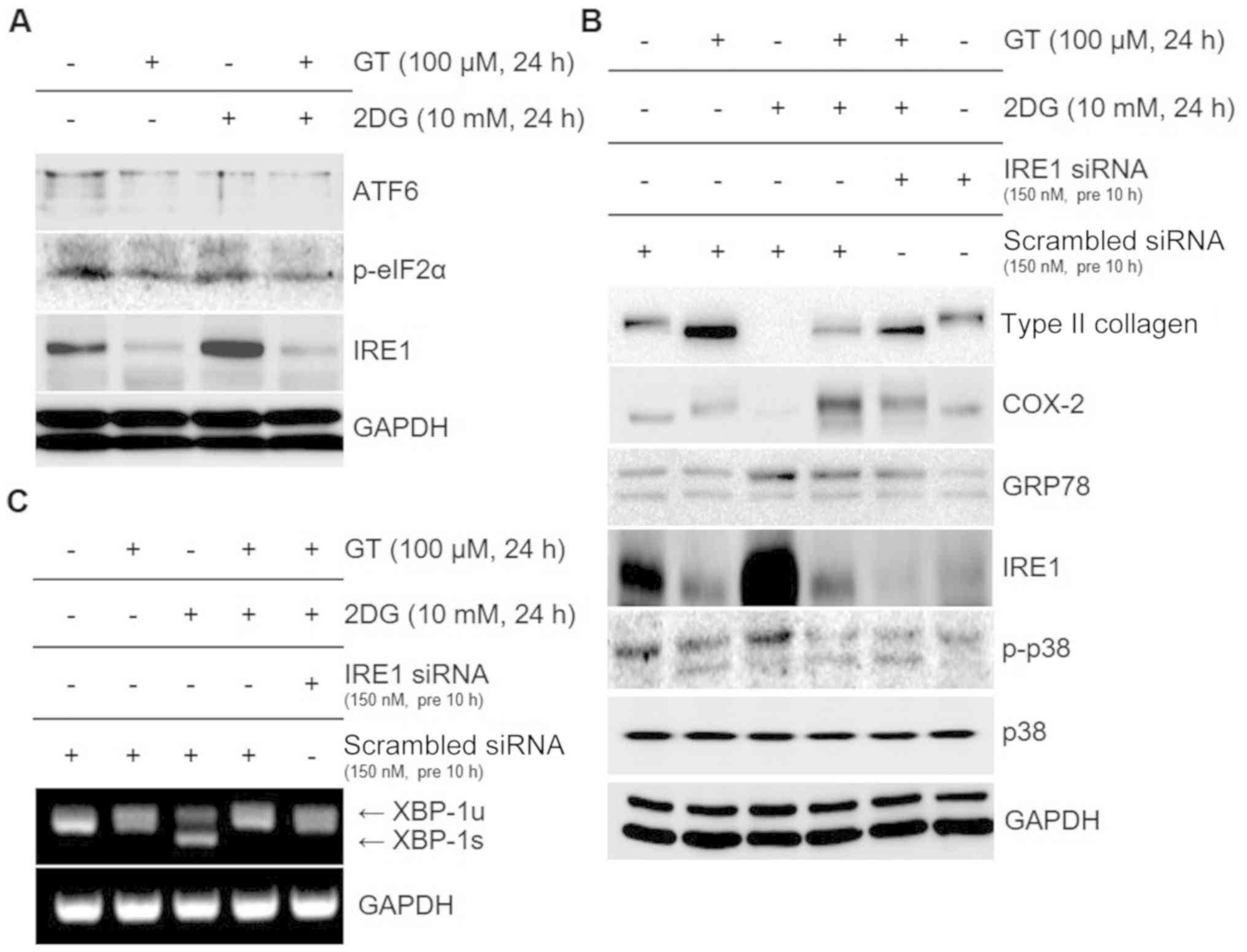 | Figure 5.Identification of signaling pathways
after treatment of chondrocytes with GT and 2DG. (A and B)
Chondrocytes were treated with 100 µM GT and 10 mM 2DG for 24 h.
(A) Western blotting was used to determine the expression of ATF6,
p-eIF2α, and IRE1. (B) Western blot was used to confirm expression
of type II collagen, COX-2, GRP78, IRE1, p-p38, and GAPDH for the
chondrocytes that had undergone transfection with 150 nM IRE1
siRNA. (C) RT-PCR was used to verify the expression of XBP-1 and
GAPDH. p38 and GAPDH were used as the loading controls and
scrambled siRNA was used as the negative control. GT, gallotannin;
2DG, 2-deoxy-D-glucose; ATF6, activating transcription factor 6;
IRE1, inositol-requiring enzyme 1; COX-2, cyclooxygenase-2; GRP78,
glucose-regulated protein 78 kDa; XBP-1, X-box binding protein
1. |
Discussion
The number of patients with OA is continuously
increasing due to aging and prolonged human lifespan. However,
inadequate research and current therapeutic development warrant the
need for more studies. Cartilage degeneration leads to OA. Among
the various causes of OA, the most recognized is metabolic
imbalance (2). Metabolic
imbalance, as a representative cause of OA, may result from the
intracellular accumulation of 2DG, a glucose derivative with 2′OH
replaced by an H, which inhibits normal glycolysis and
glycosylation to perturb the metabolic balance and induce ER
stress, thereby inducing dedifferentiation in chondrocytes
(20,22). In contrast, GT, a hydrolyzable
tannin and a plant polyphenol, is used as a protective agent in a
similar manner to a drug, and exhibits high tolerance against
cancers, oxidation, viruses, bacteria, and parasites by promoting
differentiation of chondrocytes (21). Although the independent effects of
2DG and GT in chondrocytes have been examined in previous studies,
the effects of concurrent treatment with GT and 2DG, as well as
signaling pathways regulating their activities, have not been
adequately investigated. Thus, the present study was performed to
verify whether GT works against 2DG to promote differentiation of
chondrocytes. This study also identified the signaling pathways
involved in this regulation.
When chondrocytes were treated with both GT and 2DG,
type II collagen expression, which was otherwise reduced by 2DG,
was enhanced. The type II collagen band shifts are slightly
different depending on the experimental conditions. Type II
collagen is predominant and consists of approximately 80% of total
vitreous collagen. Possibly it depends on the enzymatic degradation
of extracellular matrix. Similarly, GT promoted COX-2 expression
that was otherwise reduced by 2DG, and prevented unglycosylation
caused by 2DG-induced ER stress (Fig.
1). These findings provide evidence that GT attenuates
2DG-induced dedifferentiation and ER stress and regulates
inflammatory reactions. To demonstrate the role of GT in
attenuating ER stress, the reduced expression of GRP78 as an
indicator of ER stress (15) was
observed (Figs. 2 and 4). To identify the signaling pathway
involved in GT attenuating 2DG-induced dedifferentiation and ER
stress and regulation of inflammatory reactions, chondrocytes were
treated with SB and PD, blockers of the p38 kinase and ERK-1/-2
pathways, respectively, during differentiation and inflammation,
and with Salubrinal, a blocker of the ER-stress eIF2α pathway
(25). Among these pathways,
inhibition of the p38 kinase pathway resulted in an increased
expression of type II collagen and COX-2 expression was further
decreased, indicating that the p38 kinase pathway was influenced by
GT (Figs. 2–4). These findings thus provide evidence
that GT attenuates 2DG-induced dedifferentiation, inflammatory
reactions, and ER stress through the p38 kinase pathway.
Furthermore, after having confirmed that IRE1 downstream signaling
pathways involve the p38 kinase pathway among the three signaling
pathways activated by ER stress, the activities of all three
signaling pathways were assessed. ATF6 and p-eIF2α did not display
changes in their activities, whereas IRE1 activity that was
otherwise increased by 2DG was suppressed by GT. The subsequent
experiment using IRE1 siRNA transfection produced results identical
to those when the p38 kinase pathway was inhibited; type II
collagen expression was further enhanced and COX-2 expression was
further reduced. Moreover, the splicing of XBP-1 mRNA, which was
caused by the ER-stress-related IRE1 downstream signaling pathway,
confirmed that GT reduced the increased XBP-1s due to 2DG-induced
ER stress. In addition, IRE1 expression was reduced after GT
treatment and IRE1 siRNA transfection accompanied the reduced XBP-1
mRNA expression (Fig. 5),
suggesting that IRE1 had an influence on the expression of XBP-1
mRNA; however, further studies are required to confirm these
findings. The present results thus provide evidence that GT
regulates 2DG-induced dedifferentiation, inflammatory reactions,
and ER stress via the ER-stress-induced p38 kinase pathway
downstream from the IRE1 pathway.
The findings aggregately support that GT inhibits
2DG-induced dedifferentiation and ER-stress-induced COX-2
unglycosylation in chondrocytes. GT was also revealed to regulate
differentiation and inflammation via the ER-stress-induced p38
kinase pathway downstream from the IRE1 pathway. These effects of
GT on dedifferentiation and ER stress suggest that GT could be
utilized in the therapeutic treatment of arthritis. Furthermore,
based on our in vitro experimental results, further in
vivo experiments should be performed to provide fundamental
data and generate concrete evidence for treating arthritis using
chondrocytes.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Kongju National University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SMK, YH, SMY and SJK designed the experiments,
conducted the study, analyzed the data and wrote the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The research conducted in the present study was
approved by the Ethics Committee of Kongju National University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bian Q, Wang YJ, Liu SF and Li YP:
Osteoarthritis: Genetic factors, animal models, mechanisms, and
therapies. Front Biosci (Elite Ed). 4:74–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak
JS, Lee G, Rhee J, Ryu JH, Chun CH and Chun JS: Regulation of the
catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis.
Cell. 156:730–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen JL, Duan L, Zhu W, Xiong J and Wang
D: Extracellular matrix production in vitro in cartilage tissue
engineering. J Transl Med. 12:882014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eyre D: Collagen of articular cartilage.
Arthritis Res. 4:30–35. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chandrasekharan NV and Simmons DL: The
cyclooxygenases. Genome Biol. 5:2412004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pang LY, Hurst EA and Argyle DJ:
Cyclooxygenase-2: A role in cancer stem cell survival and
repopulation of cancer cells during therapy. Stem Cells Int.
2016:20487312016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herschman HR, Reddy ST and Xie W: Function
and regulation of prostaglandin synthase-2. Adv Exp Med Biol.
407:61–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gronowski AM and Rotwein P: Rapid changes
in nuclear protein tyrosine phosphorylation after growth hormone
treatment in vivo. Identification of phosphorylated
mitogen-activated protein kinase and STAT91. J Biol Chem.
269:7874–7878. 1994.PubMed/NCBI
|
|
9
|
Pelech SL and Sanghera JS:
Mitogen-activated protein kinases: Versatile transducers for cell
signaling. Trends Biochem Sci. 17:233–238. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwarz DS and Blower MD: The endoplasmic
reticulum: Structure, function and response to cellular signaling.
Cell Mol Life Sci. 73:79–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ellgaard L and Helenius A: Quality control
in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 4:181–191.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang K and Kaufman RJ: Protein folding in
the endoplasmic reticulum and the unfolded protein response. Handb
Exp Pharmacol. 2006:69–91. 2006. View Article : Google Scholar
|
|
15
|
Ni M, Zhang Y and Lee AS: Beyond the
endoplasmic reticulum: Atypical GRP78 in cell viability, signalling
and therapeutic targeting. Biochem J. 434:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hetz C, Martinon F, Rodriguez D and
Glimcher LH: The unfolded protein response: Integrating stress
signals through the stress sensor IRE1α. Physiol Rev. 91:1219–1243.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee AH, Iwakoshi NN and Glimcher LH: XBP-1
regulates a subset of endoplasmic reticulum resident chaperone
genes in the unfolded protein response. Mol Cell Biol.
23:7448–7459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang K and Kaufman RJ: The unfolded
protein response: A stress signaling pathway critical for health
and disease. Neurology. 66 (2 Suppl 1):S102–S109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu SM and Kim SJ: Endoplasmic reticulum
stress (ER-stress) by 2-deoxy-D-glucose (2DG) reduces
cyclooxygenase-2 (COX-2) expression and N-glycosylation and induces
a loss of COX-2 activity via a Src kinase-dependent pathway in
rabbit articular chondrocytes. Exp Mol Med. 42:777–786. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee WK, Chung KW, Kim GH and Kim SJ:
Gallotannin causes differentiation and inflammation via ERK-1/-2
and p38 kinase pathways in rabbit articular chondrocytes. Mol Med
Rep. 7:701–707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu SM, Kim HA and Kim SJ:
2-Deoxy-D-glucose regulates dedifferentiation through beta-catenin
pathway in rabbit articular chondrocytes. Exp Mol Med. 42:503–513.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korean academy of nursing: ‘Ether
anesthesia’, . The great encyclopedia of nursing science. Korea
dictionary research publishing. 1–1951. 1996.
|
|
24
|
Eo SH, Kim DW, Choi SY, Kim HA and Kim SJ:
PEP-1-SIRT2 causes dedifferentiation and COX-2 expression via the
MAPK pathways in rabbit articular chondrocytes. Exp Cell Res.
339:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Darling NJ and Cook SJ: The role of MAPK
signalling pathways in the response to endoplasmic reticulum
stress. Biochim Biophys Acta. 1843:2150–2163. 2014. View Article : Google Scholar : PubMed/NCBI
|