Introduction
As a metabolic disease characterized by
hyperglycemia, diabetes mellitus (DM) results from deficient
insulin secretion or insulin resistance. The American Diabetes
Association indicates that chronic hyperglycemia causes dysfunction
in a number of organs, including the eyes, kidneys, nerves, heart
and blood vessels (1). It is
reported that DM also adversely affects human bones and is related
to an increased risk of developing osteoporosis (2). Osteoporosis is associated with
fractures, which lead to morbidity and mortality, particularly in
the developed countries (3,4).
Children with type 1 diabetes (T1D) have a 5–21% loss of bone mass,
and clinical surveys indicated that patients with DM have a high
risk of suffering fractures, including hip, vertebral and tibia
fractures, due to insufficient bone mineral density (BMD) (5–8).
Lower BMD was reported to be caused by hyperglycemia in patients
with T1D (9). High glucose (HG)
and MC3T3-E1 cells have been used as an in vitro model to
investigate the effects of DM on bone (10,11).
P2X purinoceptor 7 (P2X7) is a member of the P2X
receptor family, a group of trimeric ligand-gated ion channels
(12). The P2X receptors are
assembled from seven possible subunits (P2X1-7) (13,14).
Extracellular ATP activates P2X family receptors to regulate the
passage of Na+, Ca2+ and other cations
(15). P2X subunits share similar
membrane topology: Intracellular N- and C-termini, two
membrane-spanning domains, and an extracellular loop containing an
ATP binding site (16,17). P2X7 is involved in various
physiological and pathophysiological processes, including
activation of the inflammasome and inflammatory factors (18), stimulation of metalloproteases
(19), and the generation of
reactive oxygen and nitrogen species (20). Previous studies reported that P2X7
may be a susceptibility gene in non-obese diabetic mice (21,22).
P2X7 has also been reported to be involved in the development of
diabetic complications; Portillo et al (23) found that P2X7 was involved in
diabetic retinopathy. The part purinergic system reactivity of P2X7
was identified to be involved in the pathogenesis of diabetic
nephropathy, and antagonizing the P2X7 receptor may alleviate
kidney damage caused by diabetes (24). Wesselius et al (25) demonstrated that the aberrant
function of P2X7 was associated with a low BMD and an increased
risk of osteoporosis. P2X7 has been reported to regulate osteoblast
activity by potentiating the Wnt/β-catenin pathway (26). Therefore, the aim of the present
study was to investigate the effect of P2X7 on HG-induced
pre-osteoblastic MC3T3-E1 cells.
Materials and methods
Cell culture
MC-3T3-E1 cells, derived from the bone of a newborn
mouse, were purchased from The American Type Culture Collection.
MC-3T3-E1 cells were cultured with αMEM (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 u/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.), in an incubator with 5%
CO2 at 37°C. For the HG group, the dose of 30 mmol/l
glucose was selected; since αMEM already contains 5.5 mmol/l
D-glucose, the media was supplemented with 24.5 mmol/l D-glucose
powder (Gibco; Thermo Fisher Scientific, Inc.) in order to generate
the HG conditions. For the mannitol (Man) group, which acted as a
control for osmotic pressure, an equal amount of mannitol, 24.5
mmol/l, was added to αMEM containing 5.5 mmol/l D-glucose. The
cells were cultured for 48 h with 0, 1, 2, 5, 10, 20 µM Brilliant
blue G (BBG; Shanghai Aladdin Bio-Chem Technology., Co., Ltd.).
Reagents
The P2X7 agonist (4-benzoyl-benzoyl)-ATP (BzATP)
(27) was purchased from
MedChemExpress. The P2X7 antagonist BBG (28) was obtained from Aladdin. Cells were
stimulated with BzATP (300 µM) or BBG (10 µM) at a final
concentration 300 or 10 µM for 2 h at room temperature. Application
of PBS was used as the control.
Cell transfection
Cells were seeded in 35 mm plates 24 h prior to
transfection experiments. Then, 50 nmol of P2X7 and mock (empty
vector) plasmids were purchased from Cobioer Biosciences Co., Ltd.
Lipofectamine® (Invitrogen; Thermo Fisher Scientific,
Inc.) was diluted in Opti-MEM (Invitrogen; Thermo Fisher
Scientific, Inc.). The plasmids and Lipofectamine®
solution were added to tubes containing FBS-free αMEM. The mixed
solution was added to the cells for 2 h, after which the medium was
removed and replaced with fresh complete culture medium. The cells
were cultured for a further 24 or 48 h.
Cell viability
MTT was used to determine the levels of cell
viability. MTT was dissolved in PBS to a concentration of 5 mg/ml.
The cells (5×103) were seeded in 96-well plates for 24 h
in a 5% CO2 incubator at 37°C. After the cells were
treated for 24 or 48 h, the culture medium was removed. MTT
solution (5 mg/ml) was diluted to a concentration of 0.5 mg/ml with
FBS-free αMEM. MTT working solution (200 µl) was added to each well
and incubated for 2 h in a 5% CO2 incubator at 37°C. The
MTT working solution was discarded, and 100 µl DMSO was added to
each well for 10 min at 37°C. The absorbance was determined using a
microplate reader at a wavelength of 570 nm.
Colorimetric assay for alkaline
phosphatase (ALP)
After the cells had been treated with the reagents
as aforementioned for 48 h, protein was extracted using cell lysis
buffer (Thermo Fisher Scientific, Inc.) on ice, followed by
centrifugation at 20,000 × g for 15 min at 4°C. The concentration
of total protein was determined using the bicinchoninic acid (BCA)
method. A nitrophenyl phosphate kit (Beijing Leagene Biotech Co.,
Ltd.) was used to determine ALP activity, according to the
manufacturer's instructions. The absorbance was detected using a
spectrophotometer at a wavelength of 405 nm.
Alizarin Red S assay
The cells (2×104 cells/cm2)
were seeded in 6-well plates and treated for 48 h, as
aforementioned. The cells were cultured using complete culture
medium supplemented with vitamin C (50 µg/ml; Sigma-Aldrich; Merck
KGaA) and β-phosphoglycerol (10 mmol/l; Sigma-Aldrich; Merck KGaA)
for 20 days. After removing the medium, PBS was used to wash the
cells three times. The cells were fixed using 0.05% glutaraldehyde
in H2O for 10 min at room temperature and washed three
times with PBS. Alizarin Red S solution (0.4%; Beijing Solarbio
Science and Technology Co., Ltd.) was used to stain the cells for 5
min at room temperature. After washing with PBS, images of the
cells were captured using a light microscope (magnification, ×200;
Olympus Corporation).
Western blot analysis
Total protein was extracted from MC-3T3-E1 cells
using cell lysis as aforementioned, and the concentration was
determined using the BCA method. A protein ladder (Thermo Fisher
Scientific, Inc.) and (20 µg/lane) protein samples were separated
using 10% SDS-PAGE. The proteins were transferred to PVDF
membranes, which were blocked with 5% BSA for 2 h at room
temperature. The following primary antibodies were incubated with
the membranes overnight at 4°C: P2X7 (cat. no. ab109054; 1:1,000;
Abcam) and β-actin (cat. no. ab8227; 1:1,000; Abcam). The membranes
were washed three times with TBS with 0.05% Tween-20 (TBST),
incubated with a horseradish peroxidase-conjugated anti-rabbit
secondary antibody (cat. no. ab7090; 1:1,000; Abcam) at room
temperature for 2 h, and then washed three times with TBST. Protein
bands were visualized using ECL (Thermo Fisher Scientific, Inc.).
An E-gel imager (Thermo Fisher Scientific, Inc.) was used to
capture images and ImageJ (version 1.8, National Institutes of
Health) was used for densitometry analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from MC-3T3-E1 cells using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), followed by
centrifugation for 10 min at 16,000 × g at 4°C. Total RNA was
dissolved in ddH2O and a spectrophotometer was used to detect the
RNA concentration at a wavelength of 260 nm. Total RNA was reverse
transcribed using a cDNA reverse transcription kit (High-Capacity
cDNA reverse transcription kit, Applied Biosystems; Thermo Fisher
Scientific, Inc.) at 42°C for 15 min and 95°C for 3 min. RT-qPCR
was conducted on ABI 7500 Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using SYBR green (Invitrogen;
Thermo Fisher Scientific, Inc.). The following primers were used
for qPCR: P2X7 forward, 5′-CTTCGGCGTGCGTTTTG-3′ and reverse,
5′-AGGACAGGGTGGATCCAATG-3′; ALP forward,
5′-CAGTGGTATTGTAGGTGCTGTG-3′ and reverse,
5′-TTTCTGCTTGAGGTTGAGGTTAC-3′; osteocalcin (Ocn) forward,
5′-GGCGTCCTGGAAGCCAATGTG-3′ and reverse,
5′-GACCAGGAGGACCAGGAAGTCCACGT-3′; and β-actin forward,
5′-AGCAGAGAATGGAAAGTCAAA-3′ and reverse,
5′-ATGCTGCTTACATGTCTCGAT-3′. The conditions of the qPCR were as
follows: 95°C for 1 min, followed by 40 cycles of 95°C for 30 sec,
62°C for 60 sec and 72°C for 30 sec, with a final extension at 72°C
for 90 sec. The relative mRNA expression levels were analyzed using
the 2−ΔΔCq method (29).
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments and were analyzed using one-way ANOVA
followed by Turkey's multiple comparison test. Data were analyzed
using SPSS 21.0 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
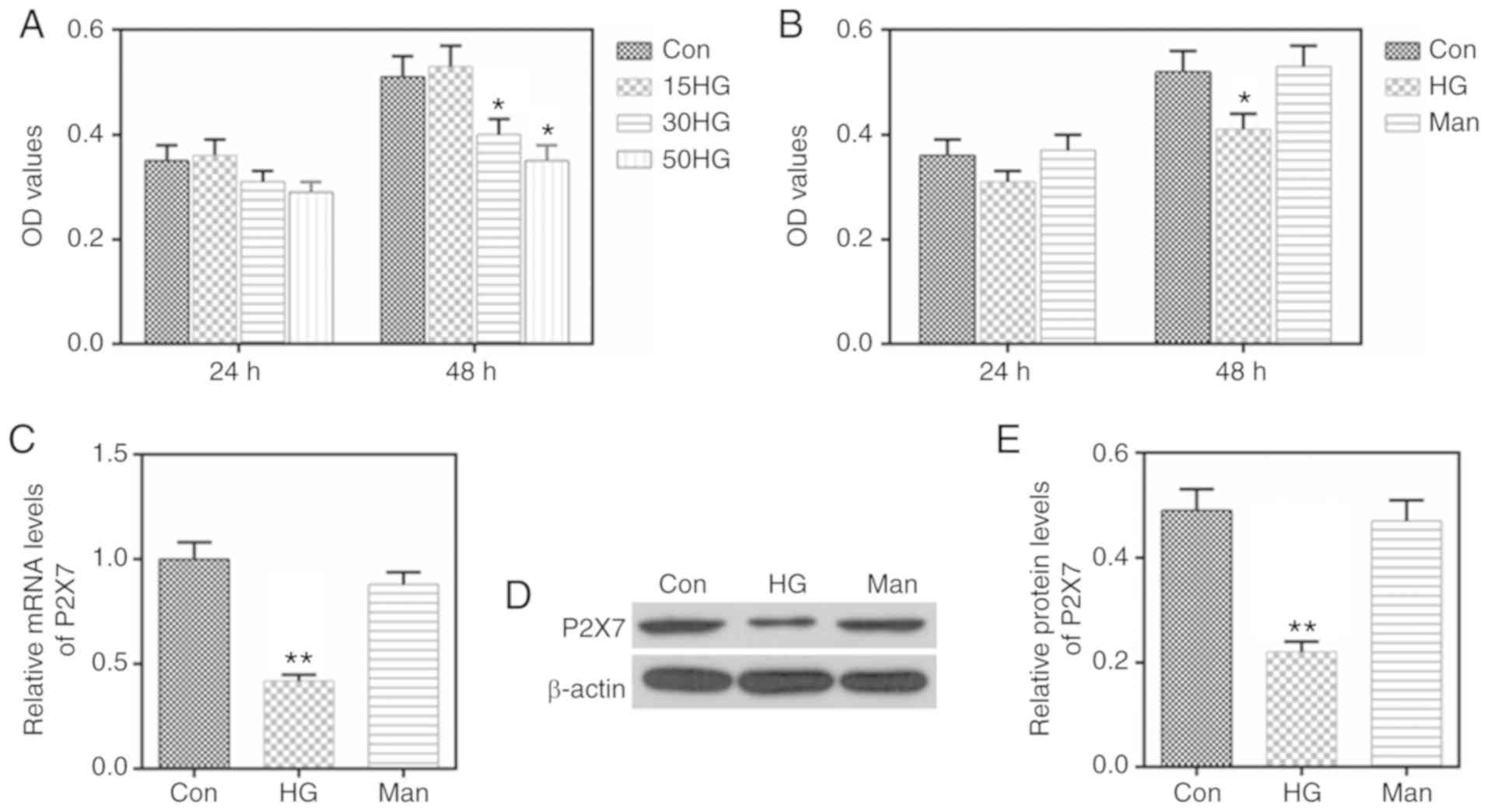
HG reduces MC-3T3-E1 proliferation and
the expression of P2X
Stimulation with 30 and 50 mmol/l glucose
significantly inhibited the proliferation of MC-3T3-E1 cells
(Fig. 1A); therefore, the dose of
30 mmol/l glucose was selected as HG conditions to treat MC-3T3-E1
cells in the subsequent experiments. To control for the effect of
osmotic pressure, Man was used as a control group (Fig. 1B). The osmotic pressure created by
Man, that would be equivalent to the HG conditions, did not affect
cell proliferation (Fig. 1B).
Furthermore, HG was found to inhibit the expression of P2X7, both
at the mRNA and the protein level (Fig. 1C-E).
HG inhibits calcification and the
levels of ALP and Ocn in MC-3T3-E1 cells
Next, the present study investigated whether HG
affected calcification, and the expression levels of ALP and Ocn.
The results indicated that HG stimulation downregulated the mRNA
expression levels of ALP and Ocn (Fig.
2F). In addition, the Alizarin Red S staining in the HG group
was markedly lighter compared with the control group (Fig. 2G), which suggested that HG
stimulation resulted in reduced calcification in MC-3T3-E1
cells.
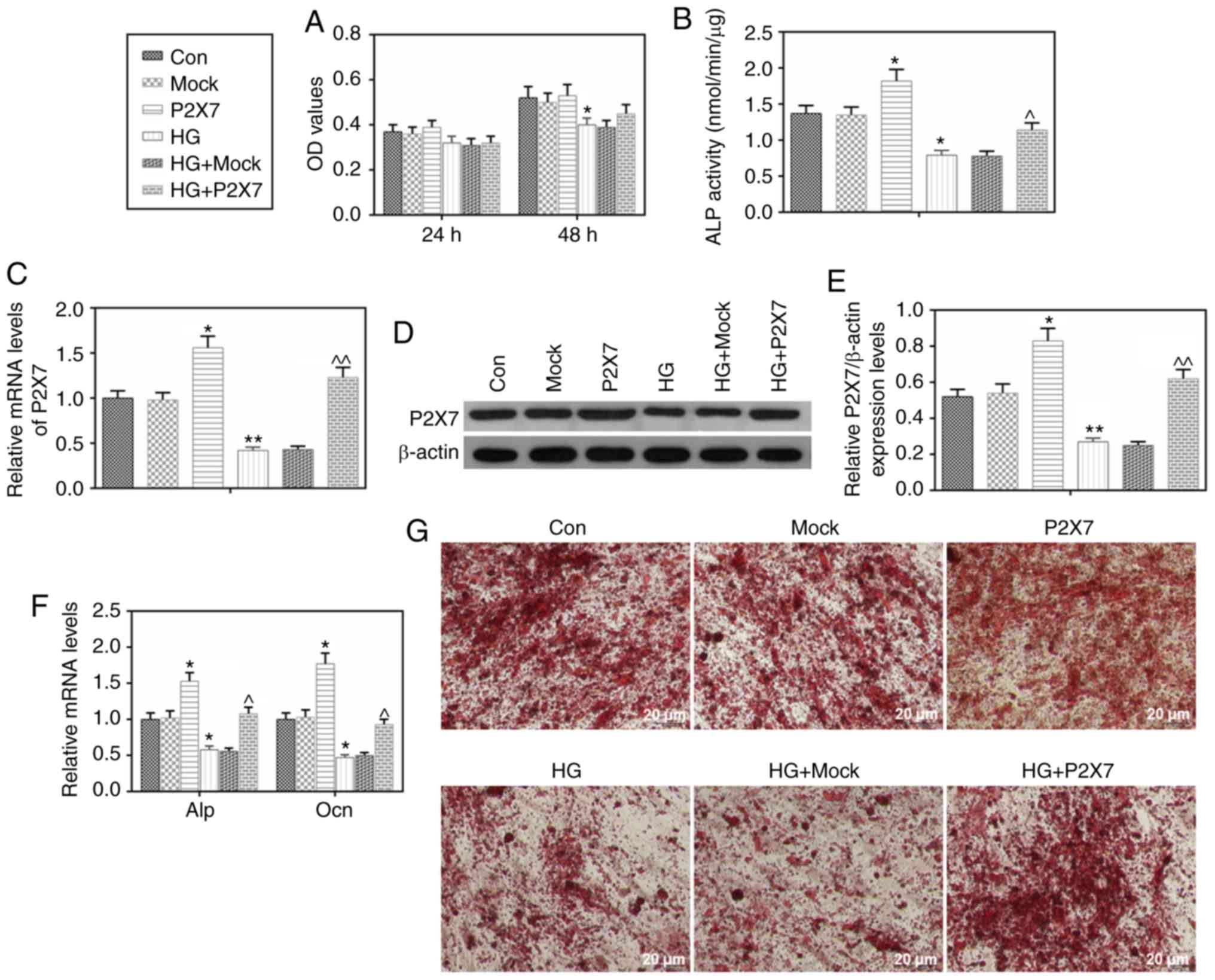 | Figure 2.Effects of HG and P2X7 on
calcification and the expression levels of ALP and Ocn in MC3T3-E1
cells. MC3T3-E1 cells were transfected with mock or
P2X7-overexpressing plasmids, under normal or HG (30 mmol/l)
conditions. (A) After 24 or 48 h of treatments, MTT assay was used
to determine cell viability. (B) After treatments as indicated for
8 h, a nitrophenyl phosphate kit was used to determine ALP
activity. (C) After treatments as indicated for 48 h, mRNA
expression levels of P2X7 were determined by RT-qPCR. (D) After
treatments for 48 h, P2X7 protein expression levels were determined
by western blot analysis. (E) Quantification of the western blot
analysis. (F) After treatments as indicated for 48 h, mRNA
expression levels of ALP and Ocn were determined by RT-qPCR. (G)
Alizarin Red S staining was used to assess calcification in the
MC3T3-E1 cells. Data are presented as the mean ± SD. *P<0.05 and
**P<0.001 vs. Con; ^P<0.05 and
^^P<0.001 vs. HG. HG, high glucose; P2X7, P2X
purinoceptor 7; ALP, alkaline phosphatase; Ocn, osteocalcin;
RT-qPCR, reverse transcription-quantitative PCR; Con, control. |
Overexpression of P2X7 increases
calcification, proliferation and the levels of ALP and Ocn
expression in MC-3T3-E1 cells under HG conditions
First, successful P2X7 overexpression was confirmed,
both at the mRNA and the protein level, in MC-3T3-E1 cells
(Fig. 2C-E). Overexpression of
P2X7 had no significant promotion effect on the cell proliferation
of MC-3T3-E1 cells under HG conditions (Fig. 2A). In addition, P2X7 overexpression
enhanced ALP activity and upregulated the expression of ALP, under
both normal glucose or HG conditions (Fig. 2B and F). P2X7 overexpression also
increased the mRNA expression levels of Ocn, under both normal
glucose or HG conditions (Fig.
2F). The Alizarin Red S staining analysis revealed no
difference between the P2X7 overexpression group and the control
group; however, the HG+P2X7 group had a darker Alizarin Red S
staining than the HG alone group (Fig.
2G), suggesting that P2X7 overexpression improved calcification
in MC-3T3-E1 cells.
Inhibition of P2X7 reduces the levels
of ALP and Ocn in MC-3T3-E1 cells under HG conditions
To investigate the effect of P2X7 on MC-3T3-E1 cells
in HG conditions, the P2X7 antagonist BBG was used. The results
demonstrated that 10 µM BBG had no significant effect on cell
viability (Fig. 3A). BBG treatment
(10 µM) inhibited the activity of ALP (Fig. 3B), the mRNA and protein expression
of P2X7 (Fig. 3C-E), and the mRNA
expression levels of ALP and Ocn (Fig.
3F). By contrast, overexpression of P2X7 partially reversed the
effects of BBG on above the gene expression with or without HG
treatment (Fig. 3C and F).
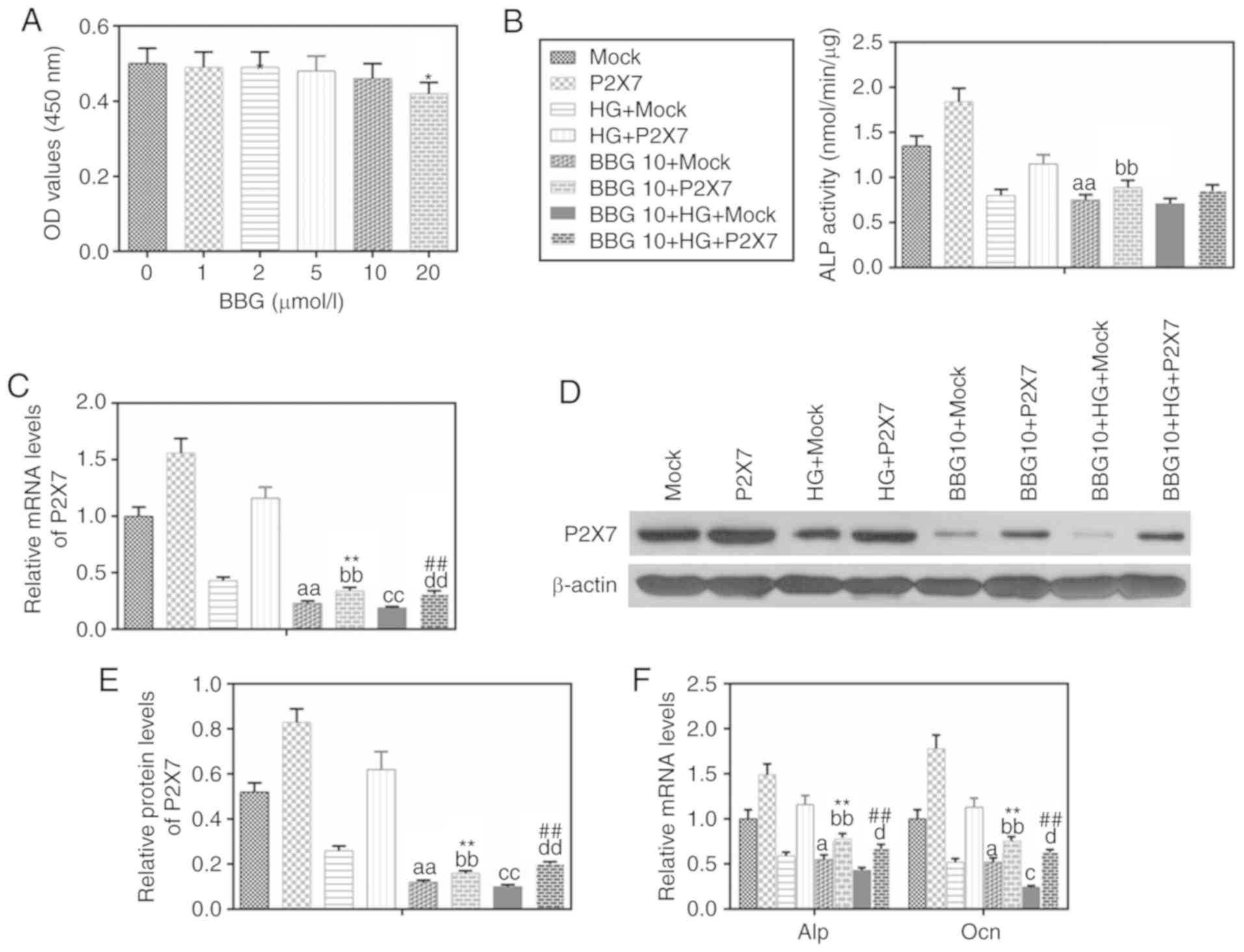 | Figure 3.BBG inhibits the expressions of P2X7,
ALP and Ocn in MC3T3-E1 cells under HG conditions. BBG (10 µmol/l),
P2X7 overexpression, mock or their combination were used to treat
MC3T3-E1 cells for 48 h under normal or HG (30 mmol/l) conditions.
(A) MTT was used to determine cell viability at different BBG
concentrations. (B) A nitrophenyl phosphate kit was used to
determine ALP activity. (C) mRNA expression levels of P2X7 were
determined by RT-qPCR. (D) P2X7 protein expression levels were
analyzed by western blot analysis. (E) Quantification of the
western blot analysis. (F) mRNA expression levels of ALP and Ocn
were determined by RT-qPCR. Data are presented as the mean ± SD.
*P<0.05 vs. 0 µmol/l BBG; aP<0.05 and
aaP<0.001 vs. Mock; bbP<0.001 vs. P2X7;
cP<0.05 and ccP<0.001 vs. HG+Mock;
dP<0.05 and ddP<0.001 vs. HG+P2X7;
**P<0.001 vs. BBG10+Mock; ##P<0.001 vs.
BBG10+HG+Mock. BBG, Brilliant blue G; P2X7, P2X purinoceptor 7;
ALP, alkaline phosphatase; Ocn, osteocalcin; HG, high glucose;
RT-qPCR, reverse transcription-quantitative PCR; Con, control; OD,
density. |
Activation of P2X7 increases the
expression of ALP and Ocn in MC-3T3-E1 cells under HG
conditions
To further investigate the role of P2X7, cells were
treated with the P2X7 agonist BzATP. BzATP treatment promoted ALP
activity (Fig. 4A) and upregulated
the expression of ALP and Ocn (Fig.
4E). Overexpression of P2X7 increased the effects of BzATP
(Fig. 4A-E). BzATP in combination
with the overexpression of P2X7 promoted ALP activity and further
upregulated the expression of ALP and Ocn in the HG group.
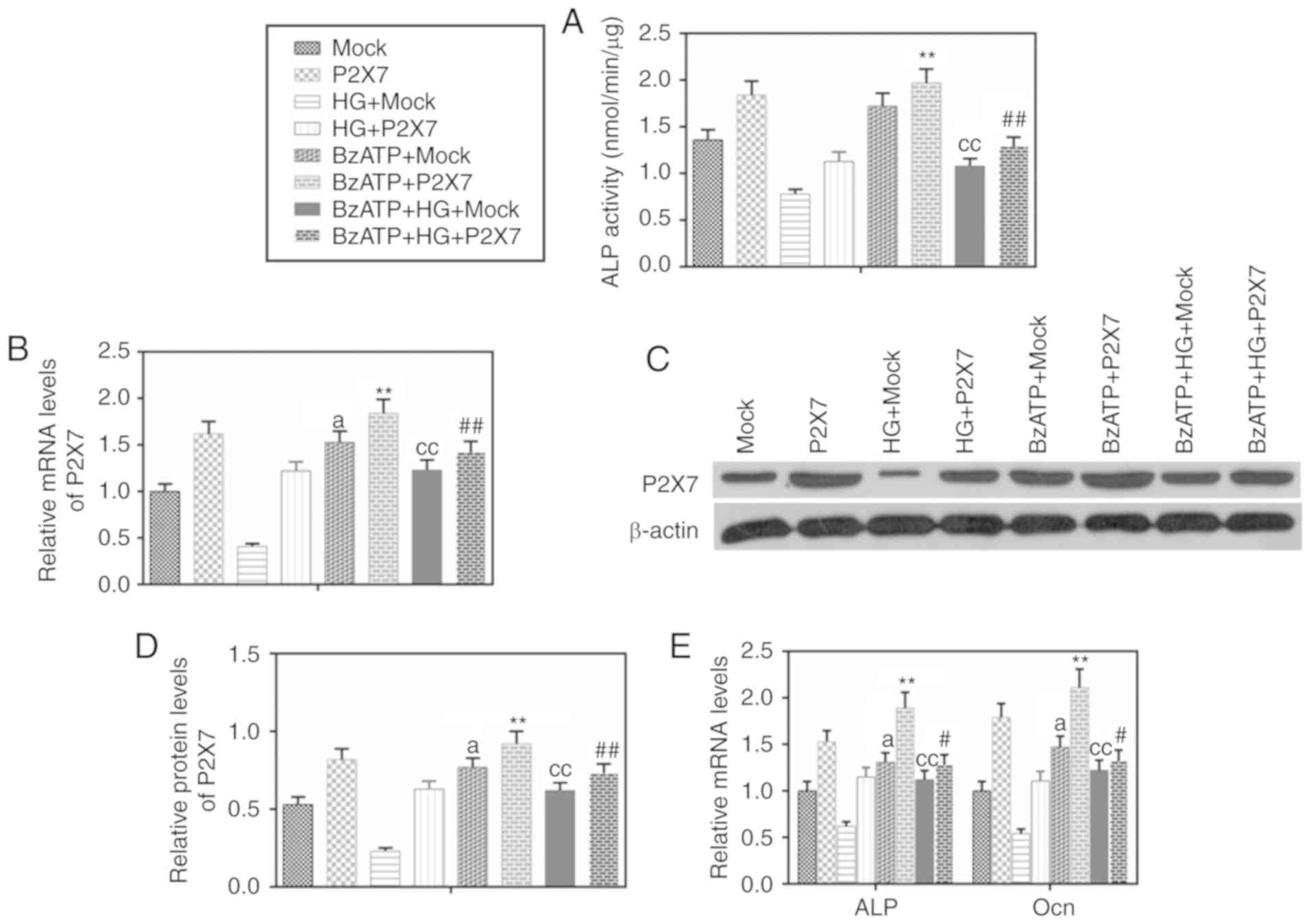 | Figure 4.BzATP promotes the expression of
P2X7, ALP and Ocn in MC3T3-E1 cells under HG conditions. BzATP (300
µmol/l), P2X7 overexpression, mock or their combination were used
to treat MC3T3-E1 cells for 48 h under normal or HG (30 mmol/l)
conditions. (A) A nitrophenyl phosphate kit was used to determine
ALP activity. (B) mRNA expression levels of P2X7 were determined by
RT-qPCR. (C) P2X7 protein expression levels were determined by
western blot analysis. (D) Quantification of the western blot
analysis. (E) mRNA expression levels of ALP and Ocn were determined
by RT-qPCR. Data are presented as the mean ± SD.
aP<0.05 vs. Mock; ccP<0.001 vs.
HG+Mock; **P<0.001 vs. BzATP+Mock; #P<0.05 and
##P<0.001 vs. BzATP+HG+Mock. BzATP,
(4-benzoyl-benzoyl)-ATP; P2X7, P2X purinoceptor 7; ALP, alkaline
phosphatase; Ocn, osteocalcin; HG, high glucose; RT-qPCR, reverse
transcription-quantitative PCR; Con, control. |
Discussion
The present study demonstrated that HG stimulation
caused damage to osteogenic MC3T3-E1 cells, with the damage
becoming more serious if glucose at a high concentration was used
to treat MC3T3-E1 cells for an extended period of time. HG resulted
in the reduced expression of P2X7 in osteogenic MC3T3-E1 cells.
ALP is a plasma membrane-bound glycoprotein
(30). The mammalian ALP is a
zinc-containing metalloenzyme encoded for by a multigene family.
ALP is expressed in the placenta, intestine, liver, kidneys and
bone (30). Romagnoli et al
(31) reported that the expression
of ALP in bones is useful in clinical practice. ALP can be used as
a marker to assess osteoblast activity and decreased serum ALP
activity reduces osteogenesis (32,33).
In the present study, HG stimulation decreased the activity and
expression of ALP, suggesting that HG reduced osteogenesis.
Furthermore, HG inhibited the calcification of osteoblasts.
However, overexpression of P2X7 in osteogenic MC3T3-E1 cells
attenuated the effects of HG and promoted osteogenesis and
calcification. A previous study has found that osteoblasts
regulated glucose homeostasis, which is an aspect of energy
metabolism, by conducting studies on an animal model and conducting
cell biology experiments (34).
Another previous study demonstrated that osteoblasts stimulate
insulin secretion and that this function was regulated by Ocn,
suggesting that osteoblasts act as endocrine cells (35). Genetic evidence from mice suggest
that G-protein coupled receptor family (GPRC6A) is important for
Ocn in regulating insulin secretion and expression (36). GPRC6A is a master regulator of
complex endocrine networks and metabolic processes, including
insulin, bone, liver metabolism and fat metabolism, and immune
function (37). In the present
study, HG stimulation decreased the expression of Ocn, with the
overexpression of P2X7 in osteogenic MC3T3-E1 cells increasing Ocn
expression under HG conditions, indicating that HG may attenuate
the ability of osteoblasts to secrete insulin and that the
overexpression of P2X7 may improve insulin secretion.
To further analyze the role of P2X7 in osteoblasts
under HG conditions, BBG and BzATP were used to antagonize and
activate P2X7 function, respectively. The present results
demonstrated that overexpression of P2X7 promoted the
differentiation of MC3T3-E1. BBG had no significant effect on cell
viability, indicating that BBG had no obvious toxicity to cells. In
addition, BBG and BzATP participated in the differentiation of
osteoblast MC3T3-E1 by regulating the function of P2X7. In
addition, the overexpression of P2X7 partially reversed the effects
of BBG with or without HG treatment. Wu et al (38) used a hyperglycemic mice model to
investigate the effect of HG on bone formation and bone resorption.
Nicke et al (39) describe
a novel P2X7 isoform with distinct functional properties that
contributes to the diversity of P2X7 receptor signaling. In the
present study, it was demonstrated that HG caused damage to
osteogenic MC3T3-E1 cells and that the overexpression of P2X7
increased osteogenic differentiation and proliferation of MC3T3-E1
cells. It was also found that inhibiting P2X7 decreased
osteogenesis under HG conditions. Therefore, the findings of the
present study suggested that P2X7 may be a regulator of the action
of HG on osteogenic MC3T3-E1 cells. Future studies will be required
to provide a more in-depth validation experiments of P2X7 in
osteoblasts under HG conditions.
In conclusion, the present study indicated that HG
stimulation inhibited the proliferation and differentiation of
osteoblasts by downregulating the expression of P2X7, and that
activating P2X7 could significantly improve the function of
osteoblasts under HG conditions. However, the role of P2X7 in
insulin and glucose metabolism requires further investigation,
since the present study did not examine the relationship between
P2X7 and GPRC6A. In addition, the culture of cells in HG conditions
could affect cell viability, which may also affect osteoblast
differentiation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and MZ made substantial contributions to the
conception and design of the present study. JY and CM were involved
in data acquisition, data analysis and interpretation All authors
were involved in drafting the article or critically revising it for
important intellectual content and gave final approval of the
version to be published. All authors agree to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 35 (Suppl
1):S64–S71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamann C, Kirschner S, Günther KP and
Hofbauer LC: Bone, sweet bone-osteoporotic fractures in diabetes
mellitus. Nat Rev Endocrinol. 8:297–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurra S, Fink DA and Siris ES:
Osteoporosis-associated fracture and diabetes. Endocrinol Metab
Clin North Am. 43:233–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnell O and Kanis JA: An estimate of the
worldwide prevalence and disability associated with osteoporotic
fractures. Osteoporosis Int. 17:1726–1733. 2006. View Article : Google Scholar
|
|
5
|
Loureiro MB, Ururahy MA, Freire-Neto FP,
Oliveira GH, Duarte VM, Luchessi AD, Brandão-Neto J, Hirata RD,
Hirata MH, Maciel-Neto JJ, et al: Low bone mineral density is
associated to poor glycemic control and increased OPG expression in
children and adolescents with type 1 diabetes. Diabetes Res Clin
Pract. 103:452–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janghorbani M, Feskanich D, Willett WC and
Hu F: Prospective study of diabetes and risk of hip fracture: The
nurses' health study. Diabetes Care. 29:1573–1578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gunczler P, Lanes R, Paoli M, Martinis R,
Villaroel O and Weisinger JR: Decreased bone mineral density and
bone formation markers shortly after diagnosis of clinical type 1
diabetes mellitus. J Pediatr Endocrinol Metab. 14:525–528. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kemink SA, Hermus AR, Swinkels LM,
Lutterman JA and Smals AG: Osteopenia in insulin-dependent diabetes
mellitus; prevalence and aspects of pathophysiology. J Endocrinol
Invest. 23:295–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vestergaard P: Discrepancies in bone
mineral density and fracture risk in patients with type 1 and type
2 diabetes-a meta-analysis. Osteoporosis Int. 18:427–444. 2007.
View Article : Google Scholar
|
|
10
|
Cunha JS, Ferreira VM, Maquigussa E, Naves
MA and Boim MA: Effects of high glucose and high insulin
concentrations on osteoblast function in vitro. Cell Tissue Res.
358:249–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong K, Qu B, Wang C, Zhou J, Liao D,
Zheng W and Pan X: Peroxisome proliferator-activated receptor a
facilitates osteogenic differentiation in MC3T3-E1 cells via the
Sirtuin 1-dependent signaling pathway. Mol Cells. 40:393–400.
2017.PubMed/NCBI
|
|
12
|
North RA: P2X receptors. Philos Trans R
Soc Lond B Biol Sci. 371(pii): 201504272016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicke A, Bäumert HG, Rettinger J, Eichele
A, Lambrecht G, Mutschler E and Schmalzing G: P2X1 and P2X3
receptors form stable trimers: A novel structural motif of
ligand-gated ion channels. EMBO J. 17:3016–3028. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aschrafi A, Sadtler S, Niculescu C,
Rettinger J and Schmalzing G: Trimeric architecture of homomeric
P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol.
342:333–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burnstock G and Kennedy C: P2X receptors
in health and disease. Adv Pharmacol. 61:333–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baconguis I, Hattori M and Gouaux E:
Unanticipated parallels in architecture and mechanism between
ATP-gated P2X receptors and acid sensing ion channels. Curr Opin
Struct Biol. 23:277–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneider M, Prudic K, Pippel A,
Klapperstück M, Braam U, Müller CE, Schmalzing G and Markwardt F:
Interaction of Purinergic P2X4 and P2X7 receptor subunits. Front
Pharmacol. 8:8602017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dubyak GR: P2X7 receptor regulation of
non-classical secretion from immune effector cells. Cell Microbiol.
14:1697–1706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pupovac A and Sluyter R: Roles of
extracellular nucleotides and P2 receptors in ectodomain shedding.
Cell Mol Life Sci. 73:4159–4173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller CM, Boulter NR, Fuller SJ,
Zakrzewski AM, Lees MP, Saunders BM, Wiley JS and Smith NC: The
role of the P2X7 receptor in infectious diseases. PLoS
Pathog. 7:e10022122011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coutinho-Silva R, Robson T, Beales PE and
Burnstock G: Changes in expression of P2X7 receptors in NOD mouse
pancreas during the development of diabetes. Autoimmunity.
40:108–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menzies RI, Booth JWR, Mullins JJ, Bailey
MA, Tam FWK, Norman JT and Unwin RJ: Hyperglycemia-induced renal
P2X7 receptor activation enhances diabetes-related injury.
EBioMedicine. 19:73–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Portillo JC, Lopez Corcino Y, Miao Y, Tang
J, Sheibani N, Kern TS, Dubyak GR and Subauste CS: CD40 in retinal
Muller cells induces P2X7-dependent cytokine expression in
macrophages/microglia in diabetic mice and development of early
experimental diabetic retinopathy. Diabetes. 66:483–493. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kreft E, Kowalski R, Jankowski M and
Szczepanska-Konkel M: Renal vasculature reactivity to agonist of
P2X7 receptor is increased in streptozotocin-induced diabetes.
Pharmacol Rep. 68:71–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wesselius A, Bours MJ, Henriksen Z, Syberg
S, Petersen S, Schwarz P, Jørgensen NR, van Helden S and Dagnelie
PC: Association of P2X7 receptor polymorphisms with bone mineral
density and osteoporosis risk in a cohort of Dutch fracture
patients. Osteoporos Int. 24:1235–1246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grol MW, Brooks PJ, Pereverzev A and Dixon
SJ: P2X7 nucleotide receptor signaling potentiates the
Wnt/β-catenin pathway in cells of the osteoblast lineage.
Purinergic Signal. 12:509–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu W, Albalawi F, Beckel JM, Lim JC,
Laties AM and Mitchell CH: The P2X7 receptor links mechanical
strain to cytokine IL-6 up-regulation and release in neurons and
astrocytes. J Neurochem. 141:436–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geraghty NJ, Belfiore L, Ly D, Adhikary
SR, Fuller SJ, Varikatt W, Sanderson-Smith ML, Sluyter V, Alexander
SI, Sluyter R and Watson D: The P2X7 receptor antagonist Brilliant
Blue G reduces serum human interferon-γ in a humanized mouse model
of graft-versus-host disease. Clin Exp Immunol. 190:79–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma U, Pal D and Prasad R: Alkaline
phosphatase: An overview. Indian J Clin Biochem. 29:269–278. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Romagnoli E, Minisola G, Carnevale V,
Scillitani A, Frusciante V, Aliberti G and Minisola S: Assessment
of serum total and bone alkaline phosphatase measurement in
clinical practice. Clin Chem Lab Med. 36:163–168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fauran-Clavel MJ and Oustrin J: Alkaline
phosphatase and bone calcium parameters. Bone. 7:95–99. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Langlois MR, Delanghe JR, Kaufman JM, De
Buyzere ML, Van Hoecke MJ and Leroux-Roels GG: Posttranslational
heterogeneity of bone alkaline phosphatase in metabolic bone
disease. Eur J Clin Chem Clin Biochem. 32:675–680. 1994.PubMed/NCBI
|
|
34
|
Wei J and Karsenty G: An overview of the
metabolic functions of osteocalcin. Rev Endocr Metab Disord.
16:93–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD,
Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al:
Endocrine regulation of energy metabolism by the skeleton. Cell.
130:456–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei J, Hanna T, Suda N, Karsenty G and
Ducy P: Osteocalcin promotes β-cell proliferation during
development and adulthood through Gprc6a. Diabetes. 63:1021–1031.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pi M, Nishimoto SK and Quarles LD: GPRC6A:
Jack of all metabolism (or master of none). Mol Metab. 6:185–193.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu M, Ai W, Chen L, Zhao S and Liu E:
Bradykinin receptors and EphB2/EphrinB2 pathway in response to high
glucose-induced osteoblast dysfunction and hyperglycemia-induced
bone deterioration in mice. Int J Mol Med. 37:565–574. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nicke A, Kuan YH, Masin M, Rettinger J,
Marquez-Klaka B, Bender O, Górecki DC, Murrell-Lagnado RD and Soto
F: A functional P2X7 splice variant with an alternative
transmembrane domain 1 escapes gene inactivation in P2X7 knock-out
mice. J Biol Chem. 284:25813–25822. 2009. View Article : Google Scholar : PubMed/NCBI
|