Introduction
Diabetic peripheral neuropathy (DPN) is a common
microvascular complication of diabetes mellitus and can cause pain,
limb disorders and repeated hospitalization, which can reduce
patient quality of life (1,2).
Although DPN is common, it is difficult to cure clinically. Medical
intervention has improved patient symptoms, but there is no
specific neuroprotective agent for DPN treatment (3,4).
Therefore, the treatment of DPN remains a challenge for
clinicians.
Due to the complex pathogenicity of DPN,
investigations into novel injury mechanism interventions for its
treatment are urgently required (5). Some studies have suggested that
long-term hyperglycemia can cause energy metabolism disorders and
vascular imbalance (6,7). Additionally, mitochondrial
ATP-sensitive potassium (Mito-K-ATP) channels function as the
high-fidelity metabolic sensors, which couple the intracellular
metabolic state to membrane excitability and play a homeostatic
role in blood glucose regulation in organisms (8). Moreover, the opening of Mito-K-ATP
channels contributes to the regulation of the mitochondrial
function and the protection of organs from damage induced by
ischemic preconditioning (9).
Moreover, several studies have shown a correlation between
dysfunction in the Mito-K-ATP channels gating and insulin secretory
disorder, which indicated that diabetes and its complications may
be associated with the dysfunction of Mito-K-ATP channels (8,9). In
addition, reactive oxygen species (ROS) and inflammatory cytokines
also serve different roles at a variety of stages, including in the
early and late stages of DPN (7).
This is consistent with previous studies that have demonstrated
that the inhibition of ROS and inflammation may restore energy
metabolism, vascular balance, and improve or reverse neuropathy
(7,10).
In previous years, a number of studies have
indicated that molecular hydrogen (H2) exhibits anti-inflammatory,
anti-antioxidative and anti-apoptotic effects in vivo and
in vitro, and can prevent stroke, sepsis, multiple organ
dysfunction syndrome, ischemia-reperfusion injury and
atherosclerosis (7,11,12).
Previous studies have revealed that H2 serves a therapeutic role in
sepsis, sepsis-associated organ damage and
lipopolysaccharide-induced acute lung injury by reducing
inflammatory mediators in cells and tissues, including tumor
necrosis factor- α (TNF-α), interleukin (IL)-10, IL-1β and high
mobility group box 1 (HMGB1) (13–16).
The use of saline in therapeutic doses of hydrogen [hydrogen-rich
saline (HS)] is a technique used to transfer molecular hydrogen
from blood to the nucleus and mitochondria of cells (7,17).
Therefore, HS may be used as a manageable, safe and portable
clinical hydrogen delivery method. Based on the results of the
aforementioned studies, the present study aimed to indicate that
the use of HS may be a novel strategy to treat DPN by activating
the Mito-K-ATP pathway and reducing oxidative stress, inflammatory
cytokines and apoptosis in diabetic rats.
Materials and methods
Animals
Adult male Sprague Dawley rats (n=80) were purchased
from the Laboratory Animal Center of the Beijing Academy of
Military Medical Sciences. Each rat weighed 240±20 g. A period of 1
week prior to the experiment, all animals were housed in room
temperature (20-22°C) and 30–70% humidity (5 rats per cage) for
12-h light and dark cycles fed with normal food and water ad
libitum. All experiments in the present study were approved by the
Animal Experimental Ethics Committee of Tianjin Medical University
General Hospital (license no. 2014-X6-09). The efforts were made to
minimize the number of animals used in the studies. During the
study, humane endpoints were used in accordance with Tianjin
Medical University General Hospital standard operating protocol. In
case of infection at the surgical site, wound dehiscence, weight
loss (>20%) or if the animal became cachectic, had difficulty
eating, drinking or moving around freely, the animal was euthanized
by inhalation of carbon dioxide. No animal deaths occurred
throughout the experiment.
Drugs and reagents
Streptozotocin (STZ; cat. no. S0130) and
5-hydroxydecanoate (5-HD; cat. no. H135), which is a selective
Mito-K-ATP blocker, were purchased from Sigma-Aldrich; Merck KGaA;
TNF-α (cat. no. RAB0479), IL-6 (cat. no. RAB0311) and IL-10 (cat.
no. RAB0247) ELISA kits were purchased from Sigma-Aldrich; Merck
KGaA. The HMGB1 (cat. no. BOS-14703) ELISA kit was bought from BOSK
(www.bosk-bio.com). A caspase-3 activity kit (cat.
no. 5723S) was purchased from Cell Signaling Technology, Inc. The
superoxide dismutase (SOD) assay kit (cat. no. 7500-100-K) were
purchased from R&D Systems, Inc. The chloramphenicol
acetyltransferase (CAT) assay kit (cat. no. ab238537) and
malondialdehyde (MDA) assay kit (cat. no. ab118970) were purchased
from Abcam.
Preparation of HS
Previous studies have indicated the details of HS
preparation (7,18). At 0.4 MPa, H2 was dissolved in
normal saline for 6 h, until it reached supersaturation. The
saturated HS was sterilized using γ rays and preserved in an
aluminum bag at an atmospheric pressure of 4, as described by
Ohsawa et al (19). The
concentration of hydrogen gas at different time points were
detected by a special microelectrode as described in the authors'
previous article (7). The HS was
replaced weekly to ensure that the hydrogen concentration was
maintained at >0.6 mmol/l, which is the 3/4 of the saturation
concentration (0.8 mmol/l) of hydrogen and is enough to produce
biological effects (10,20).
Induction of experimental
diabetes
A single dose of 55 mg/kg STZ was dissolved in
citrate buffer (pH 4.4; 0.1 mol/l) and injected into the abdominal
cavity of rats to induce diabetes mellitus. Rats in the control
group received an equal amount of citrate buffer. Then, 2 ml of
blood for blood sugar determination were collected from the tail
vein 48 h after STZ injection. The main criterion for inclusion in
the present study was a blood sugar level of ≥13.8 mM (21).
Experimental protocol
In the first experiment, according to the basic
record of injury response during week 4 of STZ injection, rats were
randomized into the control (n=8), diabetic model (DM; n=8) and HS
treatment groups (DM + HS; n=24). From week 4 of STZ injection, the
HS group was injected intraperitoneally for 4 weeks (2.5 ml, 5 ml
and 10 ml/kg/day, respectively).
Rats in the control group and diabetic
model group were intraperitoneally injected with an equal amount of
saline
Behavioral tests were performed to assess damage
thresholds (n=8 rats per group) at weeks 4, 5, 6, 7 and 8.
Detection of motor nerve conduction velocity of the sciatic nerve
was performed following the last behavioral test. The same groups
of rats were used for the mechanical allodynia test and motor nerve
conduction velocity test in the present study. The conduction
velocity of the sciatic and motor nerves was measured following the
last behavioral test. Rats were subsequently decapitated. The MDA
content, SOD and CAT activity, and TNF-α, IL-6, IL-10 and HMGB1
levels were determined in the serum and sciatic nerve in week 8.
Additionally, the caspase-3 activity in the sciatic nerve was also
determined.
In the second experiment, 40 diabetic rats were
randomized into 5 groups (8 rats in each group): The control (n=8),
diabetic model (DM; n=8), HS treatment (DM + HS; n=8), diabetic
model + 5-HD (DM + 5-HD; n=8) and HS treatment + 5-HD group (DM +
5-HD + HS; n=8). According to results of the aforementioned
experiment, the dose of HS used in experiment two was 10 ml/kg. In
the DM + HS and DM + HS + 5-HD groups, HS (10 ml/kg/day) and 5-HD
were intraperitoneally injected daily for 4 weeks starting from
week 4 of STZ injection. 5-HD (selective Mito-K-ATP blocker; 20
ml/kg) was injected intraperitoneally 30 min prior to
administration.
An intraperitoneal injection of 5-HD (a selective
Mito-K-ATP blocker, 20 mg/kg) 30 min prior to administration was
required. The activities of SOD and CAT, and the levels of TNF-α,
IL-6, IL-10 and HMGB1 in the serum and sciatic nerve were
determined in week 8. Furthermore, neuronal apoptosis in the
sciatic nerve was assessed using a caspase-3 activity kit (Cell
Signaling Technology, Inc.).
Assessment of mechanical
allodynia
The mechanical threshold of hind paw retraction
after mechanical stimulation in rats and the abnormal mechanical
pain, was evaluated using an electromechanical pain relief test
machine (BME-404) on weeks 4, 5, 6, 7 and 8. The rats were placed
in a suspension cage on a metal mesh floor for 1 h. A stainless
steel wire (0.6 mm in diameter) and linear incremental force (50 g
cut-off) were used to stimulate the bottom of the rear claw to
avoid tissue damage. The force (g) of the claw withdrawal was
subsequently recorded and this was repeated three times per rat, as
described by Fu et al (11).
Motor nerve conduction velocity
(MNCV)
The nerve conduction velocity was measured at week 8
using a BL-420 biomechanical system (BL-420; Chengdu TaiMeng Co.,
Ltd.). Rats were anesthetized using pentobarbital (30 mg/kg) and
placed on a heating pad in a greenhouse at 25°C. The rectal
temperature was kept at 37°C. Proximal nerve stimulation and distal
action potential were recorded. The nerve was stimulated using
square wave pulses (duration, 0.01 msec; intensity, 1 V)
transmitted by bipolar recording electrodes. The average values of
six potential traces were recorded using electrodes. MNCV
(m/s)=(distance between stimulating electrode and recording
electrode)/latency, as described by Xu et al (22).
Sample collection
At the end of 8 weeks of treatment, the animals were
anesthetized with 30 mg/kg of pentobarbital. After a few minutes,
the rats were gently clamped on their toes with tweezers, if there
were no reactions, the rats were judged to have entered a deep
anesthesia state. Then, the rats were executed by cervical
dislocation as quickly as possible. When the rats stopped
breathing, the head was cut off immediately and a total of 2 ml
blood was collected. The serum was acquired following
centrifugation at 4°C for 15 min at 3,000 × g and stored at −80°C
until assayed. Moreover, the left sciatic nerves of all rats were
fixed with 10% formalin buffer solution for 24 h at 4°C. The right
sciatic nerve of all rats was removed immediately, frozen in liquid
nitrogen and preserved at −80°C. Sciatic nerve samples were
homogenized in frozen PBS and centrifuged for 10 min at a speed of
10,000 × g at 4°C. All the samples were long-term preservation in
liquid nitrogen prior to testing. Standard commercial kits (Bio-Rad
Laboratories, Inc.) were used to determine tissue protein
concentrations.
Measurement of MDA, SOD and CAT
The content of MDA and the activity of SOD and CAT
in serum and sciatic nerve samples were determined using a
commercial kit. All readings were measured using a
spectrophotometer at 490 nm (Beckman 640B).
Measurement of inflammatory
cytokines
Serum and sciatic nerve samples were used to detect
TNF-α, IL-6, IL-10 and HMGB1 levels using specific ELISA kits.
Measurement of apoptosis
The apoptotic neurons evaluation of the sciatic
nerve was performed in week 8. All rats were euthanized following
the last behavioral assessment and apoptosis was detected using a
Caspase-3 activity kit.
Statistical analysis
Each experimental was repeated 3 times. All data
were reported as the mean ± standard deviation. An unpaired t-test
(if the value exhibited a Gauss distribution) or the Man-Whitney U
test (if the value did not exhibit a Gaussian distribution) to
analyze the difference between two groups and a one-way analysis of
variance with Bonferroni post hoc test to analyze the interactions
among all groups. P<0.05 was considered to indicate a
statistically significant result and significance tests were two
tailed. GraphPad prism software (version 5.0; GraphPad Software,
Inc.) and SPSS statistical software (version 16.0; IBM Corp.) was
used for statistical analysis.
Results
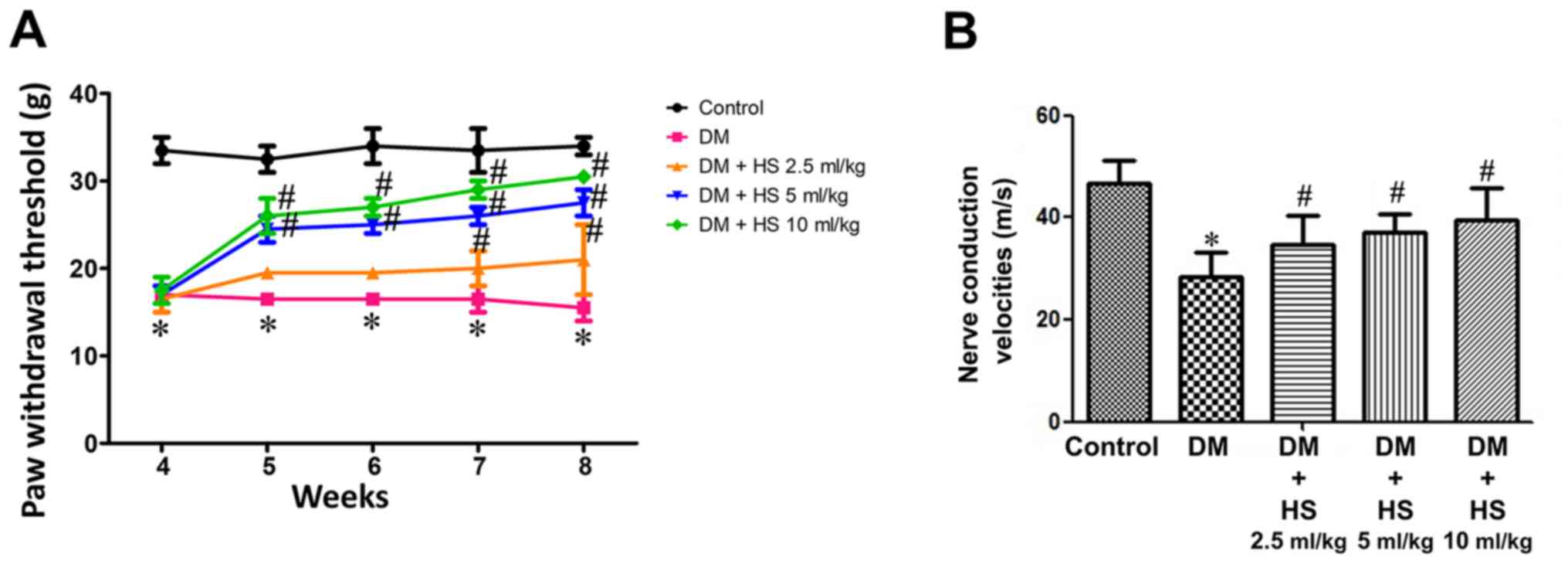
Effect of HS treatment on mechanical
hyperalgesia and MNCV
Compared with the normal control group, the
withdrawal threshold of feet in the diabetic group was
significantly reduced (P<0.05) and this was attenuated in the DM
+ HS groups. Compared with the other DM + HS groups, the withdrawal
threshold of claws in diabetic rats treated with HS (10 ml/kg) was
significantly decreased (P<0.05). HS therapy corrected for the
reduction of the paw withdrawal threshold, which was caused by
diabetes, in a dose-dependent manner (Fig. 1A). MNCV deficiency was also
indicated in diabetic rats. However, the nerve conduction velocity
in the treatment group was significantly decreased compared with
the control group (P<0.05). HS therapy corrected for the
decrease in MNCV, which is caused by diabetes, in a dose-dependent
manner (Fig. 1B).
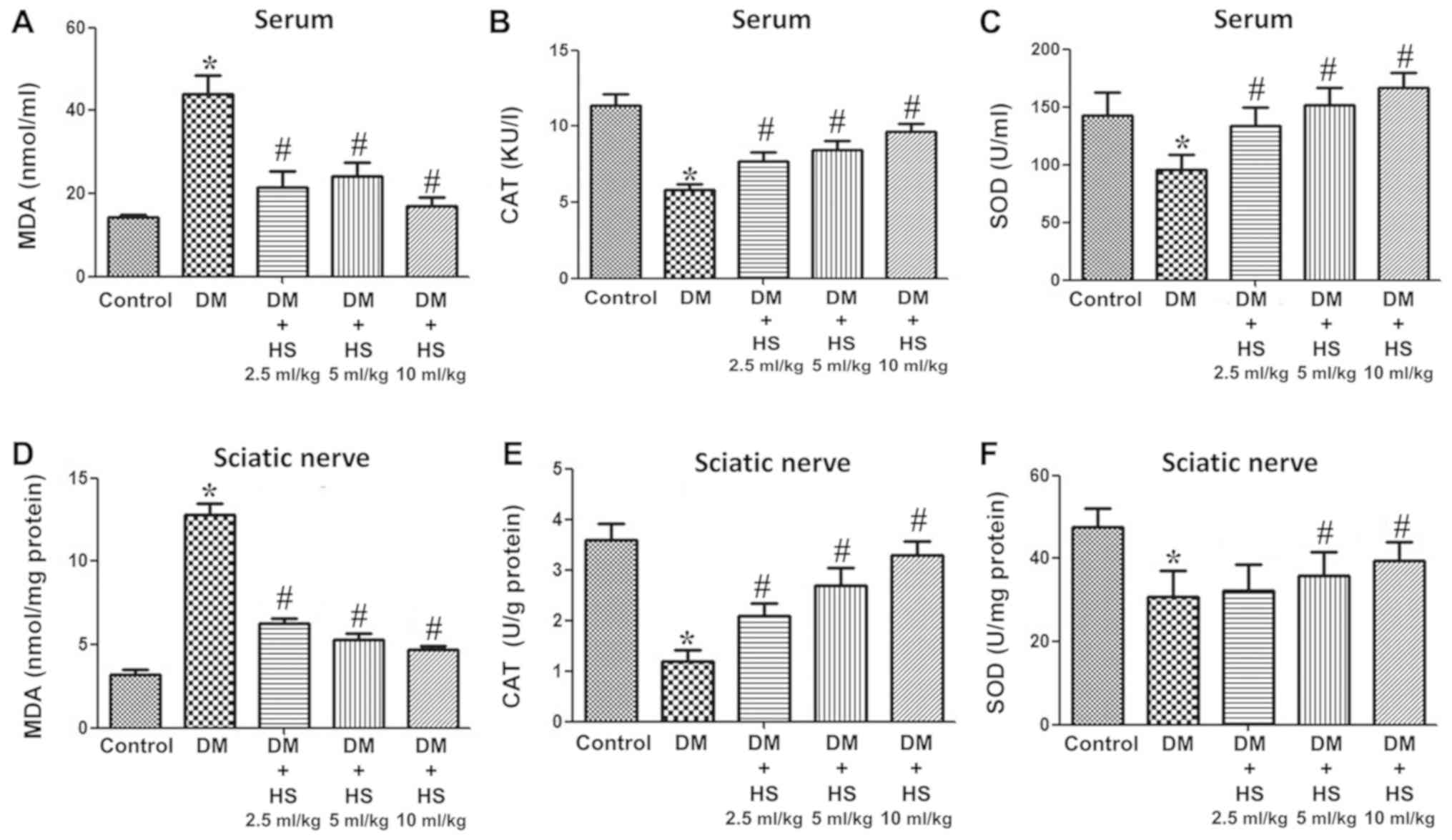
Effect of HS on MDA, SOD and CAT in
the sciatic nerve and serum
As presented in Fig.
2, hyperglycemia caused a significant increase in MDA and a
decrease in SOD and CAT in the serum and sciatic nerve of diabetic
rats at week 8 compared with the control group (P<0.05).
Compared with the DM group, HS treatment led to a significant
decrease in MDA and an increase in the activities of SOD and CAT
(P<0.05). DM + HS treatment, at a dose of 10 ml/kg,
significantly mitigated the previous effects compared with the
other DM + HS groups (P<0.05). The results indicated that
oxidative stress induced by hyperglycemia could increase the
content of MDA in the serum and sciatic nerve, and decrease the
activity of SOD and CAT. HS treatment caused a dose-dependent
decrease in MDA content and an increase in SOD and CAT
activities.
Effect of HS treatment on the levels
of TNF-α, IL-6, IL-10 and HMGB-1 in the sciatic nerve and
serum
As indicated in Fig.
3, TNF-α, IL-6, IL-10 and HMGB1 levels were also measured in
the serum and sciatic nerve. TNF-α and IL-6 are ‘early’
inflammatory factors, HMGB1 is a type of ‘late’ inflammatory factor
and IL-10 is an anti-inflammatory factor. The results showed that
the levels of TNF-α, IL-6, Il-10 and HMGB1 in serum and sciatic
nerve of diabetic rats increased at week 8 (P<0.05; Fig. 3). However, the levels of TNF-α,
IL-6 and HMGB1 in the DM+HS groups (2.5 ml/kg, 5 ml/kg and 10 ml/kg
HS) were significantly decreased compared with those in the DM
groups (P<0.05), but the IL-10 levels in the DM + HS groups were
significantly increased compared with the DM group (P<0.05). The
results demonstrated that hyperglycemia increased the
pro-inflammatory and anti-inflammatory cytokines in the serum and
sciatic nerve Additionally, hyperglycemia decreased
pro-inflammatory but not anti-inflammatory cytokines with
dose-dependent HS treatment.
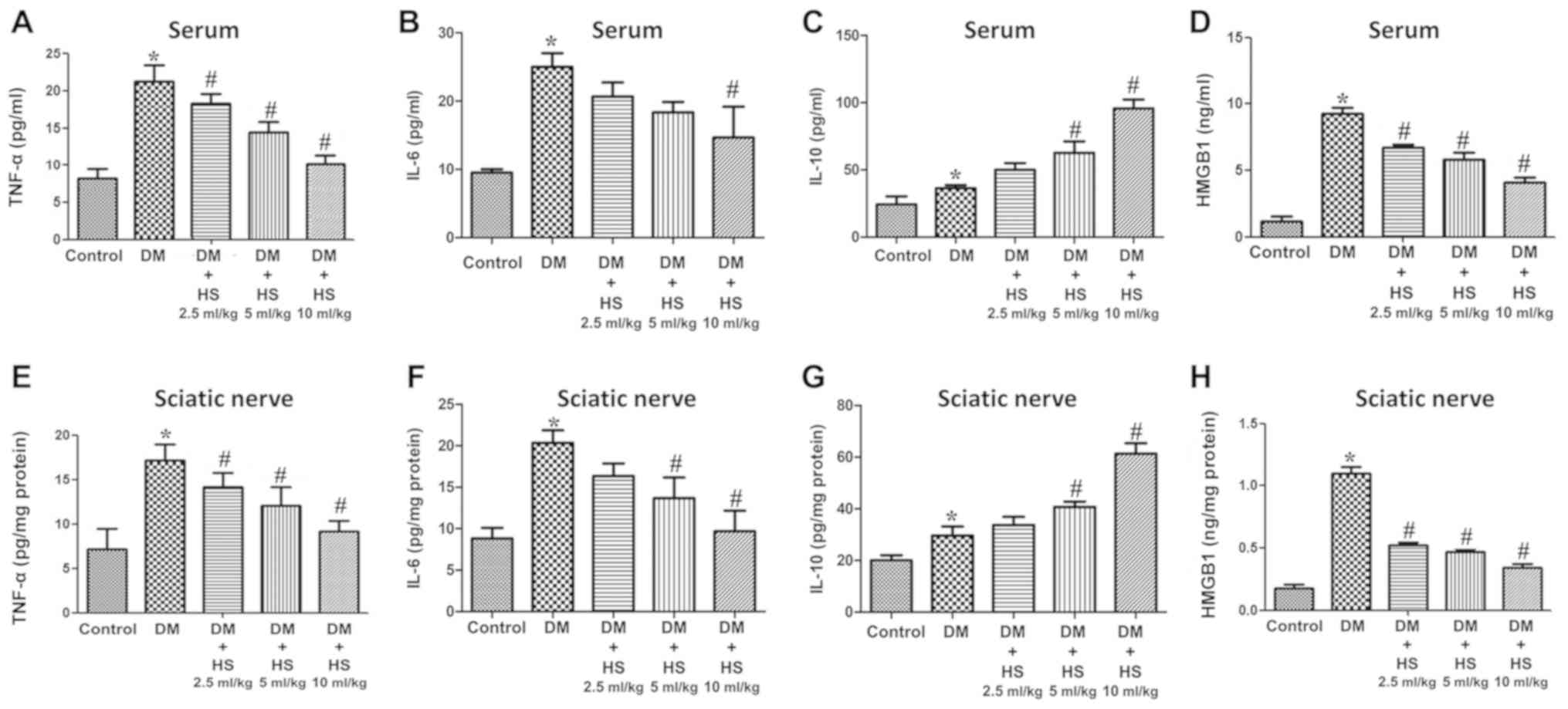 | Figure 3.Effects of HS on the levels of
pro-inflammatory and anti-inflammatory cytokines in the serum and
sciatic nerve of diabetic rats. On week 8 following streptozotocin
injection, serum and sciatic nerve were harvested to detect levels
of (A) TNF-α in serum, (B) IL-6 in serum, (C) IL-10 in serum and
(D) HMGB1 in serum, (E) TNF-α in sciatic nerve, (F) IL-6 in sciatic
nerve, (G) IL-10 in sciatic nerve and (H) HMGB1 in sciatic nerve.
Data are expressed as the mean ± standard deviation (n=6/group).
*P<0.05 vs. the control group; #P<0.05 vs. the DM
group. TNF-α, tumor necrosis factor-α; HMGB1, high mobility group
box 1; DM, diabetic model; IL-interleukin; HS, hydrogen-rich
saline. |
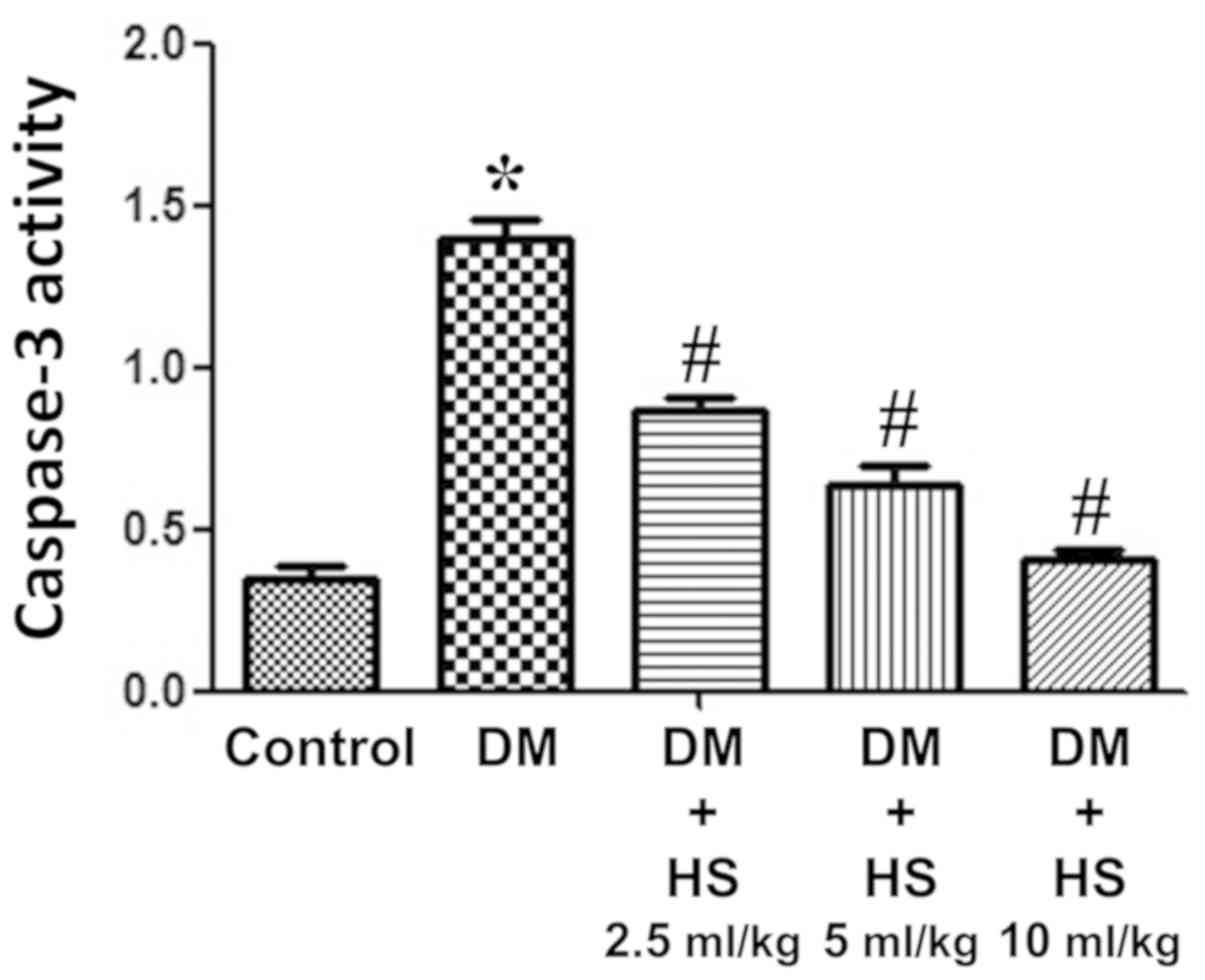
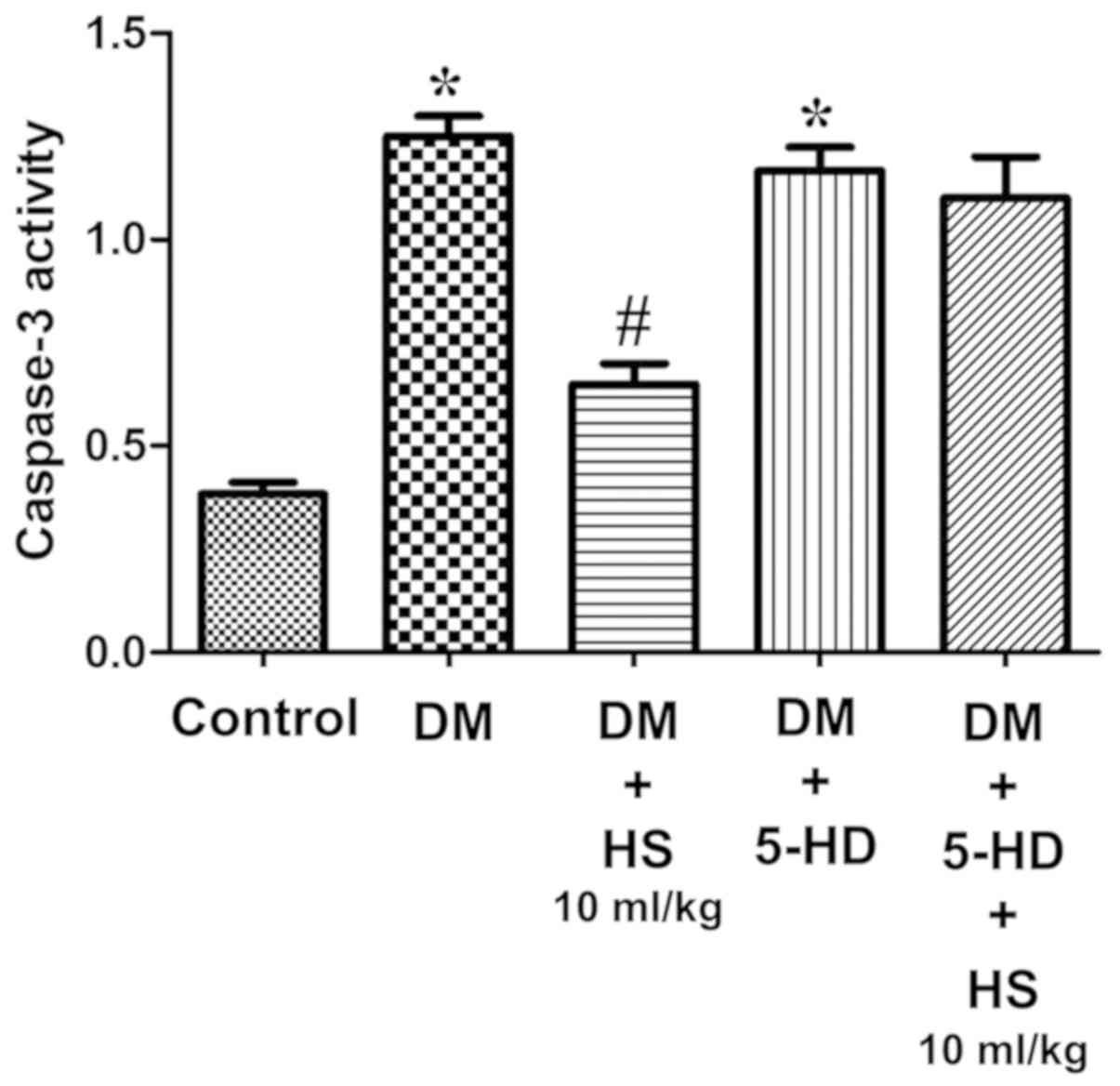
Effect of HS treatment on caspase-3
activity
As presented in Fig.
4, the caspase-3 activity of the sciatic nerve was measured at
week 8. The activity of caspase-3 in the sciatic nerve of diabetic
rats significantly increased in the DM group compared with the
control (P<0.05) and HS significantly decreased this activity in
the sciatic nerve in a dose-dependent manner (P<0.05). The
results indicated that HS reduced the apoptosis of sciatic nerve
cells by reducing caspase-3 activity in experimental patients with
diabetes mellitus.
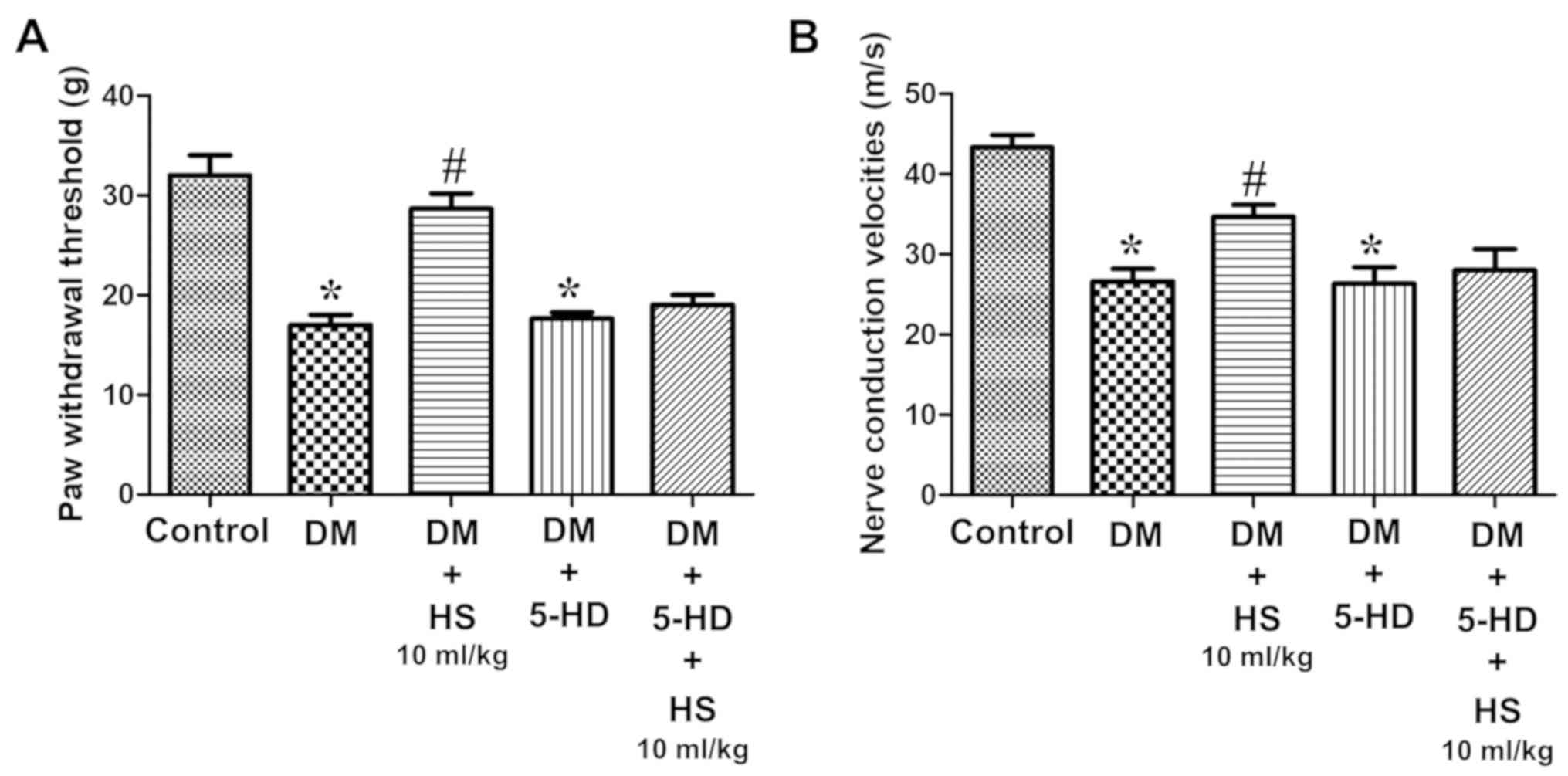
Mito-K-ATP channels
The role of the Mito-K-ATP pathway in HS
neuroprotection was also assessed in the current study. Compared
with the diabetic group, the mechanical withdrawal threshold of the
hind claw and the sciatic nerve cell pressure in the diabetic + HS
group, were significantly increased (P<0.05; Fig. 5). SOD and CAT activity, MDA content
and levels of inflammatory cytokines (TNF-α, IL-6, IL-10 and HMGB1)
in the serum and sciatic nerve were measured at week 8 (Figs. 6 and 7). Compared with the DM group, MDA,
pro-inflammatory cytokines (TNF-α, IL-6, HMGB1) levels in the DM +
HS group decreased significantly, but the IL-10 level, SOD and CAT
activity significantly increased (P<0.05; Figs. 6 and 7). In addition to evaluating the
apoptosis of the sciatic nerve the data revealed that caspase-3
activity in the DM group was significantly increased compared with
the control group (P<0.05). HS treatment significantly reduced
the activity of c aspase-3 in the sciatic nerve of diabetic rats
(P<0.05; Fig. 8). However, the
DM + 5-HD and DM + 5-HD + HS groups exhibited no significant effect
on rat behavior (P>0.05; Fig.
5), SOD, CAT and MDA levels (P>0.05; Fig. 6), TNF-α, IL-6, IL-10 and HMGB1
levels (P>0.05; Fig. 7) and
caspase-3 activity (P>0.05; Fig.
8). The results demonstrated that 5-HD is a selective
Mito-K-ATP channels blocker and that the therapeutic effect of HS
on STZ-induced diabetic rats can be significantly reduced. These
outcomes indicated that activation of the Mito-K-ATP pathway may be
conducive to the therapeutic effect of HS in STZ-induced diabetic
rats.
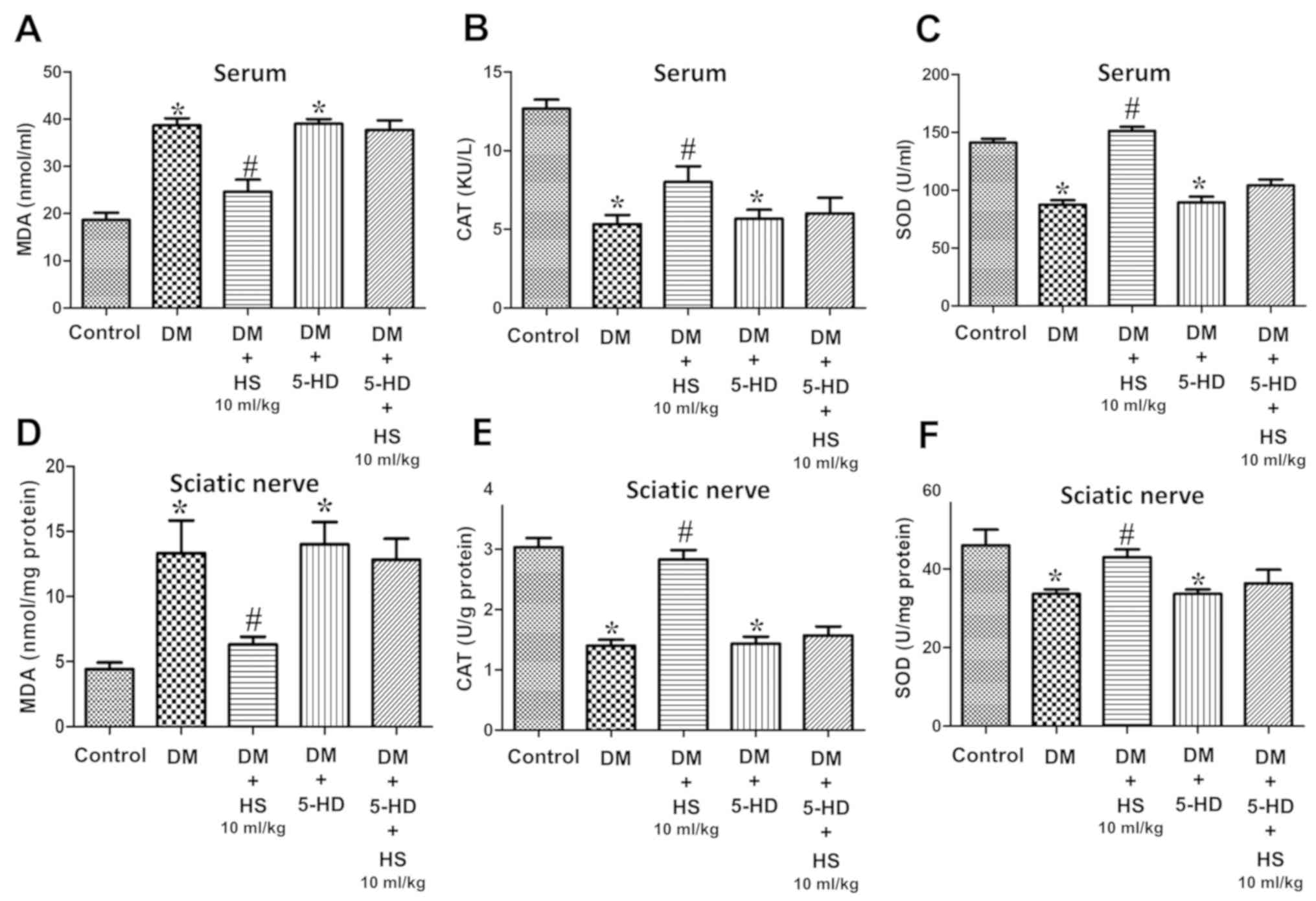 | Figure 6.Effects of HS on changes in
antioxidant enzymes and oxidative products after blockade of
mitochondrial ATP-sensitive potassium channels in the serum and
sciatic nerve of diabetic rats. On week 8 following 5-HD and
streptozotocin injection, serum and sciatic nerve were harvested to
determine the levels of (A) MDA in serum, (B) CAT in serum, (C) SOD
in serum, (D) MDA in the sciatic nerve, (E) CAT in the sciatic
nerve and (F) SOD in the sciatic nerve. Data are expressed as the
mean ± standard deviation (n=6/group). *P<0.05 vs. the control
group; #P<0.05 vs. the DM group. MDA,
malondialdehyde; CAT, chloramphenicol acetyltransferase; SOD,
superoxide dismutase; DM, diabetic model; HS, hydrogen-rich saline;
5-HD, 5-hydroxydecanoate. |
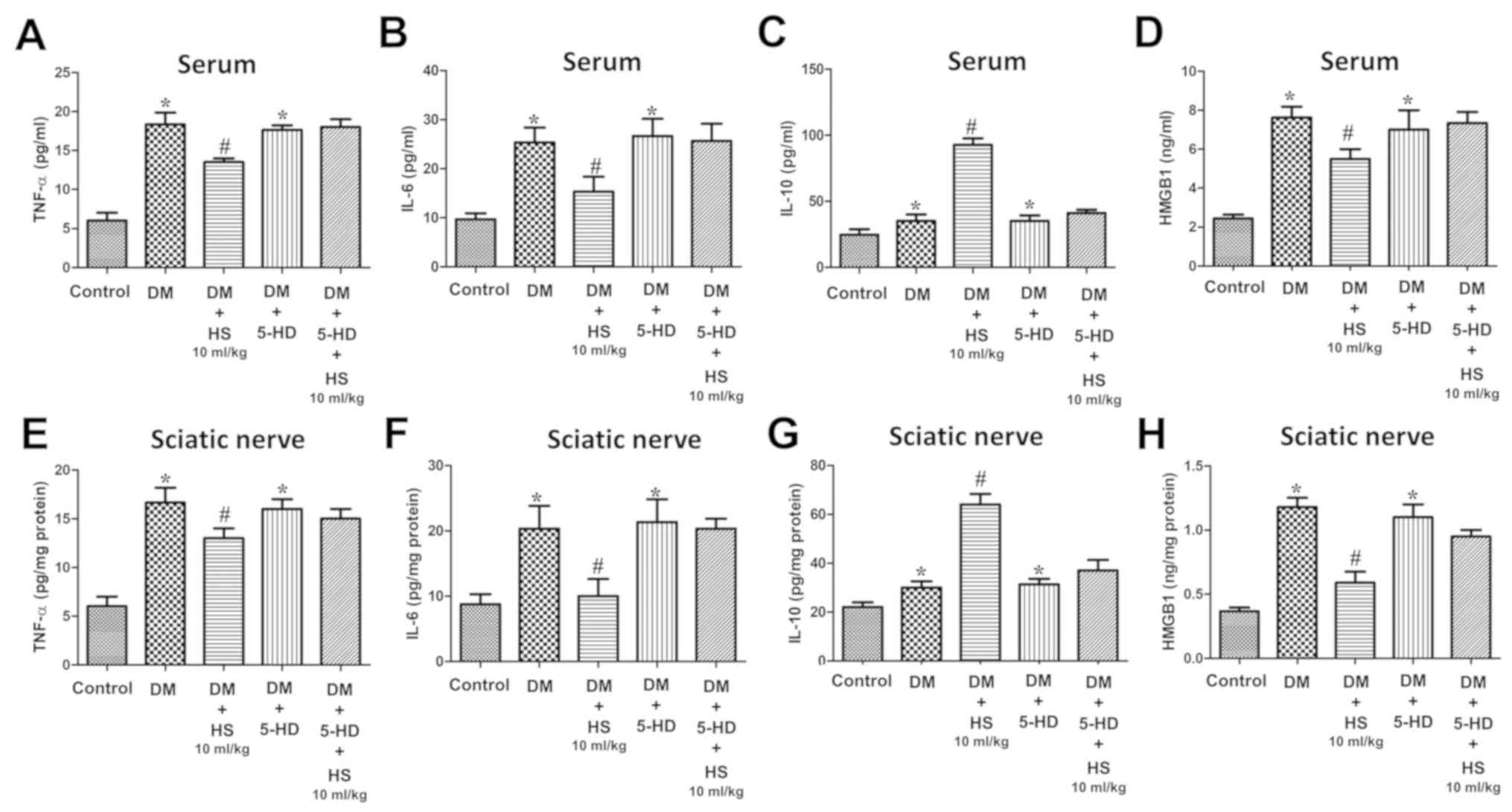 | Figure 7.Effects of HS on the levels of
pro-inflammatory and anti-inflammatory cytokines after blockade of
the mitochondrial ATP-sensitive potassium channels in the serum and
sciatic nerve of diabetic rats. On week 8 following 5-HD and
streptozotocin injection, the serum and sciatic nerve were
harvested to detect the levels of (A) TNF-α, (B) IL-6, (C) IL-10
and (D) HMGB1 in the serum and (E) TNF-α, (F) IL-6, (G) IL-10 and
(H) HMGB1 in the sciatic nerve. Data are expressed as the mean ±
standard deviation (n=6/group). *P<0.05 vs. the control group;
#P<0.05 vs. the DM group. TNF-α, tumor necrosis
factor- α; HMGB1, high mobility group box 1; DM, diabetic model;
HS, hydrogen-rich saline; 5-HD, 5-hydroxydecanoate. |
Discussion
The results of the current study demonstrated that
HS treatment improved the STZ- induced nociceptive threshold
(mechanical and thermal hyperalgesia) and MNCV, but reduced
neuronal apoptosis in diabetic rats. On week 5 of STZ injection,
rats received HS treatment every day for 4 weeks, which produced
beneficial effects in a dose-dependent manner and 10 ml/kg HS
treatment exhibited the most beneficial effect.
The results of the current study also demonstrated
that 5-HD (the Mito-K-ATP channels blocker) eliminated the
neuroprotective effects of HS treatment. Furthermore, the
therapeutic effect of HS was indicated to be closely associated
with the decrease of oxidative products (MDA) and pro-inflammatory
cytokine (TNF-α, IL-6 and HMGB1) levels, the increase of
antioxidant enzyme (SOD and CAT) activities and the
anti-inflammatory cytokine (IL-10) level in the serum and sciatic
nerve. Additionally, the activity of caspase-3 in the sciatic nerve
was downregulated by HS therapy. Therefore, HS may alleviate DPN by
activating the Mito-K-ATP pathway, reducing oxidative stress,
inflammatory cytokines and apoptosis.
STZ-induced DPN in rats is widely used in
neurological, biochemical and histopathological studies (10,21).
In the current study, significant differences were observed between
STZ-injected and non-diabetic rats, suggesting that diabetic rats
exhibited mechanical hyperalgesia. These results are consistent
with other observations. Previous studies have demonstrated that
behavioral changes can be detected after 1 week of STZ injection
and the maximum deterioration time of symptoms in STZ-induced
diabetic rats is between 4 and 8 weeks (23). Therefore, the final assessment of
oxidative stress, inflammatory cytokines and apoptosis was
performed at week 5 of STZ injection and week 8 of the present
study. Behavioral changes were caused by DPN, which is
characterized by the mechanical hyperalgesia of large fibers and
the sensorimotor deficits of larger fibers. In the present study,
HS treatment resulted in a reversal of the lower paw withdrawal
threshold and a reduction of MNCV deficiency in diabetic rats.
Oxidative stress is one of the main causes of the complex
pathophysiology of DPN (1,7). Previous studies have indicated that
hyperglycemia increases the production of free radicals by
increasing intracellular glucose oxidation, which subsequently
results in neurotoxicity (7). MDA
is a common end point of lipid peroxidation, which is induced by
ROS and its concentration is considered to reflect the total level
of lipid peroxidation (24).
Antioxidant enzymes SOD and CAT counteract the harmful effects of
ROS (25). The results of the
current study are consistent with previous experimental results
that have revealed that the reduction of oxidative damage in the
serum and sciatic nerve, and the increase of endogenous antioxidant
enzyme activity, may be attributed to the therapeutic effect of HS
(7). The quenching of free
radicals by HS may be an important mechanism of antioxidant stress
and neurotoxicity reduction. In chronic hyperglycemia, the
production of endogenous TNF-α accelerates microvascular and
neurological problems, and can lead to increased microvascular
permeability, hypercoagulability and neurological damage. These
neurological problems can promote the development of diabetic
microvascular diseases and multiple neuropathies (4,26).
The results of the aforementioned study indicated that TNF-α and
IL-6 serve a major role in pain conduction and neurodegeneration
(27). HMGB1 can activate ischemic
or hypoxic cells to initiate an inflammatory response (28). HMGB1, which is a late cytokine-like
mediator, can mediate chronic neuroinflammation and promote
progressive neurodegeneration, and serves a key role in diabetic
complications (29). In
experimental diabetic models, early and late pro-inflammatory
cytokines can interact and promote peripheral nerve injury. The
results of the current study revealed that diabetic rats exhibited
increased inflammatory cytokine release and HS treatment reduced
the production and release of these inflammatory cytokines in a
dose-dependent manner. Additionally, apoptosis may be an important
mode of neuronal death and neurodegeneration in this experimental
diabetes mellitus model. At the molecular level, caspase cascades,
including caspase-12 and caspase-3, can activate apoptosis.
Caspase-3 which can be activated by an initial caspase is
considered to be the most important initiator of caspase cascades
and its expression is generally regarded as a reliable indicator of
apoptosis (30). In the present
study, caspase-3 activity was indicated to increase in the diabetic
model rats, but decreased markedly during HS treatment.
Mitochondria, the source of ROS and the target of sepsis, are
considered to be very important in DPN (31). The opening of Mito-K-ATP channels
can reduce ROS production in the mitochondria of the heart, brain
and spinal cord, activate ROS scavengers and lead to
neuroprotection (8). The
activation of the Mito-K-ATP pathway can alleviate diabetic
neuropathy by reducing oxidative stress and apoptosis (8,9).
5-HD is considered to be an inhibitor of the Mito-K-ATP channels
(7). Currently, 5-HD is used to
test the participation of the Mito-K-ATP channels and in the
present study, it was demonstrated that the neuroprotection induced
by HS is mediated by the Mito-K-ATP pathway activation in
experimental diabetic models.
The current study has several limitations. First, a
small number of time points were measured and the changes in
inflammatory cytokines, the activities of CAT, MDA and caspase-3
(and the expression of caspase-3) were only recorded at week 8.
Changes in the serum and/or sciatic nerve at different time points
should be assessed in future studies. Second, knockout mice were
not used in the present study. However, the inhibitor of Mito-K-ATP
(5-HD) that was used in the research can also be used for this
purpose. Finally, HS was not added to the control group. However,
the authors' previous studies have already reported that there were
no statistical differences between control and control plus HS
group in different animal models (12,32,33).
In conclusion, the results of the current study
demonstrated that HS was able to reduce oxidative stress, the
release of pro-inflammatory cytokines and apoptosis by activating
the Mito-K-ATP pathway, and may be an effective drug for the
treatment of DPN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Fund of China (grant no. 81671888), the Natural
Science Foundation of Tianjin (grant no. 18JCYBJC94400), the
Science & Technology Development Fund of Tianjin Education
Commission for Higher Education (grant no. 2017KJ194) and the Youth
Incubating Fund of Tianjin Medical University General Hospital
(grant no. ZYYFY2016036).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KX and YoY conceived and designed the study. YJ, BL,
YaY, GW and XG performed the experiments. YJ and YaY wrote the
manuscript. KX and GW reviewed and edited the manuscript. All
authors read and approved the manuscript.
Ethics approval and consent to
participate
All experiments in the present study were approved
by the Animal Experimental Ethics Committee of Tianjin Medical
University General Hospital (license no. 2014-X6-09).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chung YC, Lim JH, Oh HM, Kim HW, Kim MY,
Kim EN, Kim Y, Chang YS, Kim HW and Park CW: Calcimimetic restores
diabetic peripheral neuropathy by ameliorating apoptosis and
improving autophagy. Cell Death Dis. 9:11632018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang LQ, Chen Z, Zhang K, Liang N, Yang
GY, Lai L and Liu JP: Zusanli (ST36) acupoint injection for
diabetic peripheral neuropathy: A systematic review of randomized
controlled trials. J Altern Complement Med. Sep 26–2018.(Epub ahead
of print). View Article : Google Scholar
|
|
3
|
Alam U, Riley DR, Jugdey RS, Azmi S,
Rajbhandari S, D'Août K and Malik RA: Diabetic neuropathy and gait:
A review. Diabetes Ther. 8:1253–1264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vinik AI, Camacho P, Reddy S, Valencia WM,
Trence D, Matsumoto AM and Morley JE: Aging, Diabetes, and Falls.
Endocr Pract. 23:1117–1139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li K, Shi X, Luo M, Inam-U-Llah, Wu P,
Zhang M, Zhang C, Li Q, Wang Y and Piao F: Taurine protects against
myelin damage of sciatic nerve in diabetic peripheral neuropathy
rats by controlling apoptosis of schwann cells via NGF/Akt/GSK3β
pathway. Exp Cell Res. 383:1115572019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montero AR, Dubin JS, Sack P and Magee MF:
Future technology-enabled care for diabetes and hyperglycemia in
the hospital setting. Wold J Diabetes. 10:473–480. 2019. View Article : Google Scholar
|
|
7
|
Li Q, Jiao Y and Yu Y, Wang G and Yu Y:
Hydrogen-rich medium alleviates high glucose-induced oxidative
stress and parthanatos in rat Schwann cells in vitro. Mol
Med Rep. 19:338–344. 2019.PubMed/NCBI
|
|
8
|
Liang W, Chen M, Zheng D, Li J, Song M,
Zhang W, Feng J and Lan J: The opening of ATP-sensitive K+ channels
protects H9c2 cardiac cells against the high glucose-induced injury
and inflammation by inhibiting the ROS-TLR4-necroptosis pathway.
Cell Physiol Biochem. 41:1020–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi N, He J, Guo Q, Liu T and Han J:
Liraglutide protects against diabetes mellitus complicated with
focal cerebral ischemic injury by activating mitochondrial
ATP-sensitive potassium channels. Neuroreport. 30:479–484. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rojas DR, Tegeder I, Kuner R and Agarwal
N: Hypoxia-inducible factor 1α protects peripheral sensory neurons
from diabetic peripheral neuropathy by suppressing accumulation of
reactive oxygen species. J Mol Med (Berl). 96:1395–1405. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu Y, Ito M, Fujita Y, Ito M, Ichihara M,
Masuda A, Suzuki Y, Maesawa S, Kajita Y, Hirayama M, et al:
Molecular hydrogen is protective against 6-hydroxydopamine-induced
nigrostriatal degeneration in a rat model of Parkinson's disease.
Neurosci Lett. 453:81–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Dong Y, Chen H, Han H, Yu Y, Wang G,
Zeng Y and Xie K: Protective effects of hydrogen-rich saline in a
rat model of permanent focal cerebral ischemia via reducing
oxidative stress and inflammatory cytokines. Brain Res.
1486:103–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Xie K, Chen Y, Wang Y, Wang Y,
Lian N, Zhang K and Yu Y: Nrf2/HO-1 signaling pathway participated
in the protection of hydrogen sulfide on neuropathic pain in rats.
Int Immunopharmacol. 75:1057462019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Huo X, Chen H, Li B, Liu J, Ma W,
Wang X, Xie K, Yu Y and Shi K: Hydrogen-rich saline activated
autophagy via HIF-1α pathways in neuropathic pain model. Biomed Res
Int. 2018:46708342018.PubMed/NCBI
|
|
15
|
Xin Y, Liu H, Zhang P, Chang L and Xie K:
Molecular hydrogen inhalation attenuates postoperative cognitive
impairment in rats. Neuroreport. 28:694–700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong A, Yu Y, Wang Y, Li C, Chen H, Bian
Y, Zhang P, Zhao Y, Yu Y and Xie K: Protective effects of hydrogen
gas against sepsis-induced acute lung injury via regulation of
mitochondrial function and dynamics. Int Immunopharmacol.
65:366–372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hedenstierna G: Mechanisms of
postoperative pulmonary dysfunction. Acta Chir Scand Suppl.
550:152–158. 1989.PubMed/NCBI
|
|
18
|
Yang T, Wang L, Sun R, Chen H, Zhang H, Yu
Y, Wang Y, Wang G, Yu Y and Xie K: Hydrogen-rich medium ameliorates
lipopolysaccharide-induced barrier dysfunction via rhoa-Mdia1
signaling in Caco-2 Cells. Shock. 45:228–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Zhang H, Hu J, Gu Y, Shen Z, Xu L,
Jia X, Zhang X and Ding X: Hydrogen-rich saline alleviates kidney
fibrosis following AKI and retains klotho expression. Front
Pharmacol. 8:4992017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang C, Gao J, Wu B, Yan N, Li H, Ren Y,
Kan Y, Liang J, Jiao Y and Yu Y: Minocycline attenuates the
development of diabetic neuropathy by inhibiting spinal cord Notch
signaling in rat. Biomed Pharmacother. 94:380–385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Z, Zhou J, Cai J, Zhu Z, Sun X and
Jiang C: Anti-inflammation effects of hydrogen saline in LPS
activated macrophages and carrageenan induced paw oedema. J Inflamm
(Lond). 9:22012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parashar A, Mehta V and Malairaman U: Type
2 diabetes mellitus is associated with social recognition memory
deficit and altered dopaminergic neurotransmission in the amygdala.
Ann Neurosci. 24:212–220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reis R, Charehsaz M, Sipahi H, Ekici AI,
Macit Ç, Akkaya H and Aydın A: Energy drink induced lipid
peroxidation and oxidative damage in rat liver and brain when used
alone or combined with alcohol. J Food Sci. 82:1037–1043. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barany T, Simon A, Szabo G, Benkő R, Mezei
Z, Molnár L, Becker D, Merkely B, Zima E and Horváth EM: Oxidative
stress-related parthanatos of circulating mononuclear leukocytes in
heart failure. Oxid Med Cell Longev. 2017:12496142017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi M and Zochodne DW: Diabetic
neuropathy and the sensory neuron: New aspects of pathogenesis and
their treatment implications. J Diabetes Investig. 9:1239–1254.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sherchan P, Huang L, Wang Y, Akyol O, Tang
J and Zhang JH: Recombinant Slit2 attenuates neuroinflammation
after surgical brain injury by inhibiting peripheral immune cell
infiltration via Robo1-srGAP1 pathway in a rat model. Neurobiol
Dis. 85:164–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L
and Wang G: Protective effects of hydrogen gas on murine
polymicrobial sepsis via reducing oxidative stress and HMGB1
release. Shock. 34:90–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Feng C, Qiao Y and Zhao X: Sigma 1
receptor mediated HMGB1 expression in spinal cord is involved in
the development of diabetic neuropathic pain. Neurosci Lett.
668:164–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Yao W, Shi H, Liu H, Li Y, Gao Y,
Liu R and Xu L: Paeoniflorin protects Schwann cells against high
glucose induced oxidative injury by activating Nrf2/ARE pathway and
inhibiting apoptosis. J Ethnopharmacol. 185:361–369. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roman-Pintos LM, Villegas-Rivera G,
Rodriguez-Carrizalez AD, Miranda-Diaz AG and Cardona-Munoz EG:
Diabetic polyneuropathy in type 2 diabetes mellitus: Inflammation,
oxidative stress, and mitochondrial function. J Diabetes Res.
2016:34256172016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng X, Chen H, Wang G, Yu Y and Xie K:
Hydrogen-rich saline attenuates chemotherapy-induced ovarian injury
via regulation of oxidative stress. Exp Ther Med. 10:2277–2282.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Hua N, Xie K, Zhao T and Yu Y:
Hydrogen-rich saline reduces cell death through inhibition of DNA
oxidative stress and overactivation of poly (ADP-ribose)
polymerase-1 in retinal ischemia-reperfusion injury. Mol Med Rep.
12:2495–2502. 2015. View Article : Google Scholar : PubMed/NCBI
|