Introduction
Coronary heart disease (CHD) and major depression
are closely related epidemiologically and biologically (1). Epidemiological studies have revealed
that in addition to traditional risk factors (alcoholism,
hyperlipidemia, hypertension and diabetes), depression is another
important concern in patients with coronary artery disease (CAD)
(2). As a risk factor for coronary
disease, in 30–40% of the patients with CHD, emotional stressors of
daily life have led to myocardial ischemia (3), and induced the high morbidity and
mortality rate in patients with CAD (4–6).
Consistently, ~40% of patients with depression succumbed to CHD,
while the rate for the general population without depression was
revealed to be eight times lower (7).
Since it is difficult to examine the impact of
stress on human cardiovascular disease, animal models have been
used to investigate the underlying mechanisms and develop
pharmacotherapy. For example, He et al used chronic mild
stress (CMS) combined with a blocked left anterior descending
artery animal model to verify the effect of Ginseng Fruit Saponins
on the serotonin system (8). The
effects of escitalopram on myocardial apoptosis and the expression
of Bax and Bcl-2 were also examined during myocardial
ischemia/reperfusion in a rat model with depression (9). Furthermore, anti-depressive medicine,
such as escitalopram, fluoxetine, Shuangxinfang, also exhibited a
cardio-protective effect (9–11).
However, the exact mechanisms involved in CHD in combination with
depression are still mostly unknown and effective medicines are
limited.
Matrix metalloproteinases (MMPs) including matrix
metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9)
are involved in cardiac pathophysiology and function as biomarkers
of atherosclerosis, myocardial infarctions (MIs), congestive heart
failure, and non-ischemic or ischemic cardiomyopathy (12–14).
MMPs also play an essential role in brain function. They are
associated with cognitive efficiency, depression (15), and neuroinflammation. However, the
role MMPs play in CHD in combination with depression remains
elusive.
Traditional Chinese medicine (TCM) usually
attenuates physical and mental dysfunctions (16). Kai-Xin-San (KXS), a Chinese herbal
medicine formula from ginseng (Panax ginseng C.A. Meyer),
hoelen (Poria cocos F.A. Wolf), polygala (Polygala
tenuifolia Willd) and acorus (Acorus tatarinowii Schott)
at the ratio of 3:3:2:2, has been revealed to be effective at
treating depression and improve learning and memory (17–19).
It has the potential to balance serotonin and increase the
expression of brain-derived neurotrophic factor (BDNF) (19–21).
Most of its components exhibit protective effects on both the brain
and heart, such as Ginsenoside Rd and Re (22–24),
Methyl 3,4,5-trimethoxycinnamate (M-TMCA) and oligosaccharide ester
(25,26) and extracts of hoelen (27). Recently the effects of Kai-Xin-San
in fluoxetine-resistant depressive rats were revealed to influence
various inflammatory pathways (19).
Based on these previous results, in the present
study, an MI and depression model was applied to i) evaluate
whether pharmacological treatment with KXS exerts
antidepressant-like activity and cardio-protective effects and ii)
explore the molecular mechanisms of KXS in regulating MMP levels
and apoptosis.
Materials and methods
Animals
All animal experiments were conducted following the
Use of Laboratory Animals by the U.S. National Institutes of Health
and approved by the Animal Experimentation Ethics Committee of the
Chinese PLA General Hospital. In the present study, 60 male
Sprague-Dawley (SD) rats weighing approximately 210–230 g at 6
weeks were obtained from Vital River Laboratory Animal Technology
Co., Ltd. [SCXX (Beijing) 2012–0521]. Rats were housed in an animal
laboratory at 22±2°C and 60±3% humidity, under a 12 h dark/light
cycle.
KXS extract preparation
Herbal KXS was purchased from the Yu Ye Group Co.,
Ltd. in 2017, and was authenticated by Professor Ping Liu (PLA
General Hospital). The total extract was prepared and standardized
in accordance with our previous study (28,29).
KXS contains four indigenous medicines: Ginseng, hoelen, polygala
and acorus. Ginseng was refluxed with 60% ethanol, after which it
was heated and refluxed 4 times, for 1 h each time. The extracts
were combined and filtered for use, and the ginseng drug residue
was also used. Acorus was soaked in water 6 times for 12 h, and
volatile oil was extracted for 8 h. The obtained volatile oil was
added to ethanol to achieve a 50% oleyl alcohol solution. The
ginseng and acorus drug residue with hoelen and polygala were added
to 7.4 l of water. They were extracted 3 times for 1 h each time
and combined with liquid medicine, after which they were
concentrated and added to 50% ethanol, and then chilled overnight
and filtered. The filtrate was combined with ginseng extract, and
ethanol was recovered. The mixture was concentrated and dried under
reduced pressure.
Experimental protocol
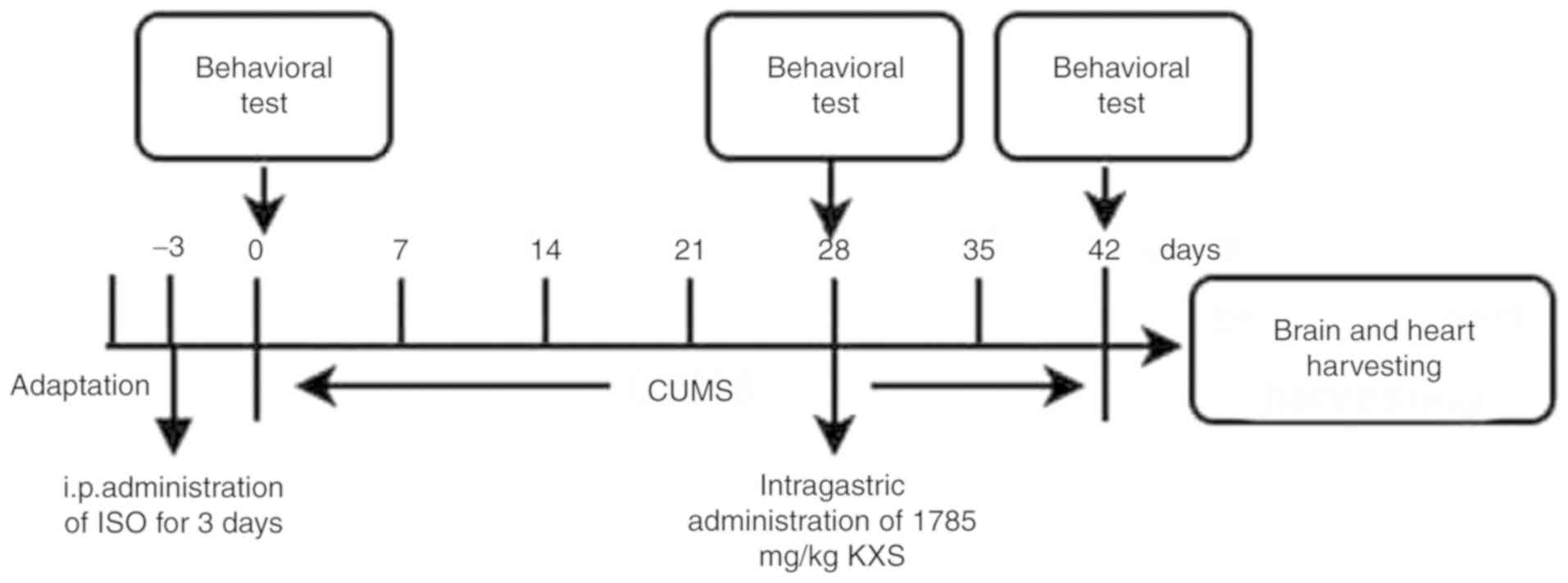
Fig. 1 displays the
timeline of all procedures. After 3 days of adaptation, the rats
were randomly divided into five groups (10 animals each), receiving
the following treatments respectively: Normal control rats treated
with intragastric administration of saline (control group);
celisc-injection of isopropyl adrenaline (ISO)-induced MI rats with
intragastric administration of saline (ISO group); depressive rats
treated with 4 weeks of chronic mild stress (CMS)and intragastric
administration of saline (depression group); ISO-induced MI plus
CMS rats and intragastric administration of saline (model group);
and ISO-induced MI plus CMS rats with intragastric administration
of 1,785 mg/kg KXS daily for 14 days (KXS group). The rats received
a standard diet, and free access to water. All rats were sacrificed
3 days after the last day of behavioral testing, and the levels of
MMPs in the brain and heart were examined.
ISO-induced MI
The ISO-induced MI animal model was obtained by i.p.
administration of ISO (150 mg/kg body weight; Sigma-Aldrich; Merck
KGaA) for 3 days (30).
Depression
Chronic unpredictable mild stress (CUMS) is widely
used to establish depressive animal models, and this experiment
adopted a modified protocol as previously reported (19,26).
Rats received 4 weeks of stress stimulations, which consisted of
water deprivation (24 h), food deprivation (24 h), restraint (1 h),
isolation (24 h), forced cold water swimming (10 min), flashing
light (3 h) and were group-housed in a soiled cage overnight, in a
random and unpredictable order for 42 days.
ISO combined with depression
The ISO + depression group, the group of rats with
ISO-induced MI, and the depression group were then treated by CUMS
for four weeks.
Sucrose-preference test
The tests were performed at 0, 28, and 42 days of
the experiment. Before the sucrose-preference test, rats were
deprived of water and food for 24 h and then fed with two
pre-weighted bottles containing water for 1 h and 1% sucrose
solution. Intake was measured by weighing the bottles before and
after each test. The sucrose preference was calculated as sucrose
intake/total water intake (sucrose intake + water intake) (31).
Forced swim test
An adapted version of the forced swim test
originally described by Porsolt et al (32) was used. Twenty-four hours after the
last KXS treatment, rats were forced to swim individually for 5 min
in a Plexiglas cylinder (height: 40 cm, diameter: 30 cm) filled
with water (temperature: 24±1°C; depth: 30 cm). The behavior of the
rats was videotaped. The overall time spent in (i) immobility
(floating and making only those movements necessary to keep the
head above water), (ii) swimming (active swimming motions that
moved the animal across the center or in circles within the center
of the cylinder), and (iii) climbing (attempts to climb the wall of
the cylinder) was scored by an experienced experimenter blind to
the different treatment groups.
Analysis of cardiac function by
echocardiography
Rats were anesthetized in a chamber with a mixture
of 4.0 to 5.0% isoflurane and oxygen and maintained by a mixture of
1.2 to 2.0% isoflurane and oxygen. After the hairs on their chest
were removed, the rats were fixed in a supine position on the
scanning platform of a high-resolution ultrasound system with a
40-MHz transducer (Vero 770; FUJIFILM VisualSonics, Inc.). LV
end-diastolic and -systolic dimensions (LVID and LVIS,
respectively) were measured on an M-mode obtained from a
parasternal short-axis view at the mid papillary level. The
fractional shortening (FS) was defined as (LVID-LVIS)/LVID). The
ejection fraction (EF) was calculated from the M-mode (LVID3-LVIS3)
(33).
Tissue preparation and ELISA
analyses
After the final behavioral test, all rats were
anesthetized by 10% chloral hydrate (350 mg/kg), and decapitated
with a standard rodent guillotine. Brain and hearts were excised,
rinsed in ice-cold isotonic saline, accurately weighed, and then
stored at −80°C prior to further analysis. For ELISA analyses, 9
times the volume of normal saline with tissue was added and a
homogenate was prepared in an ice water bath which was then
centrifuged at 1509.3 × g for 10 min. The level of MMP-2 and MMP-9
in heart and brain tissues was analyzed by ELISA (Beijing Andy
Huatai Technology Co., Ltd.). Extracted supernatants of each sample
were added into MMP-2 and MMP-9 ELISA Assay Kit plates. The
absorbance value was measured at 450 nm to analyze cardiac fibrosis
responses and each procedure was performed according to the
manufacturer's instructions.
Real-time PCR analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Then, reverse transcription and
real-time PCR reactions were performed with Revert Aid™ first
strand cDNA synthesis kit (Thermo Fisher Scientific, Inc.) and
SYBR® Green PCR Master Mix (ABI; Thermo Fisher
Scientific, Inc.), respectively. All primers were purchased from
TSINGKE Co, Ltd. The primers used in the present study were: Bax
forward, 5′-GCTGATGGCAACTTCAACTGGG-3′ and reverse,
5′-TTCTTCCAGATGGTGAGCGAGG-3′; Bcl-2 forward,
5′-TACCGTCGTGACTTCGCAGAGAT-3′ and reverse,
5′-AGGAGAAATCAAACAGAGGTCGC-3′; and GAPDH forward,
5′-CTGCCTTCTCTTGTGACA-3′ and reverse, 5′-TGTAGACCATGTAGTTGAGG-3′.
The PCR thermocycling conditions were: Pre-denaturation at 95°C for
30 sec, 39 cycles of denaturation at 95°C for 3 sec, annealing at
60°C for 30 sec and extension at 72°C for 15 sec. Quantification of
mRNA levels relative to GAPDH (a housekeeping gene) was performed
with the 2−ΔΔCq method (34).
Western blotting
Proteins were obtained from the whole heart or
brain. The frozen animal tissues were homogenized in ice-cold lysis
radioimmunoprecipitation assay (RIPA) buffer (Applygen
Technologies, Inc.) for 10 min on ice, and then centrifuged at
12,000 × g at 4°C for 15 min. Total amounts of proteins in each
sample were determined by BCA kit, and the protein concentration so
fall samples were adjusted to be the same. Supernatants containing
20 µg proteins were separated on 10% SDS-PAGE and
electrotransferred onto NC membranes. The blots were probed with a
primary antibody overnight after blocking with 5% non-fat milk.
Glyceraldehyde-3-phosphatedehydrogenase (GAPDH) and β-actin were
used as controls. The antibodies and dilutions were as follows:
MMP-2 (1:300, ab51075), TIMP1 (1:300, ab61224), Bcl-2 (1:500,
ab196495) and Bax (1:500; ab32503; all from Abcam). The membranes
were blocked with 5% non-fat dry milk and incubated with primary
antibodies overnight at 4°C, followed by a secondary horseradish
peroxidase-conjugated antibody (1:6,000, ab205718, Abcam) for 2 h.
The blots were developed using a electrochemiluminescence system
and determined using an image analysis system (Bio-Imaging
Analyzer, UVP). Blot quantification was performed with ImageJ
version 1.43u (National Institutes of Health). All western blot
analyses were performed in duplicate.
Statistical analysis
The data were expressed as the mean ± standard
deviation (X ± SD), statistically evaluated by one-way analysis of
variance with Tukey-Kramer's test for post hoc analysis and a
Bonferroni correction for multiple comparisons for each outcome
variable separately. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of KXS on bodyweight, sucrose
preference and immobility
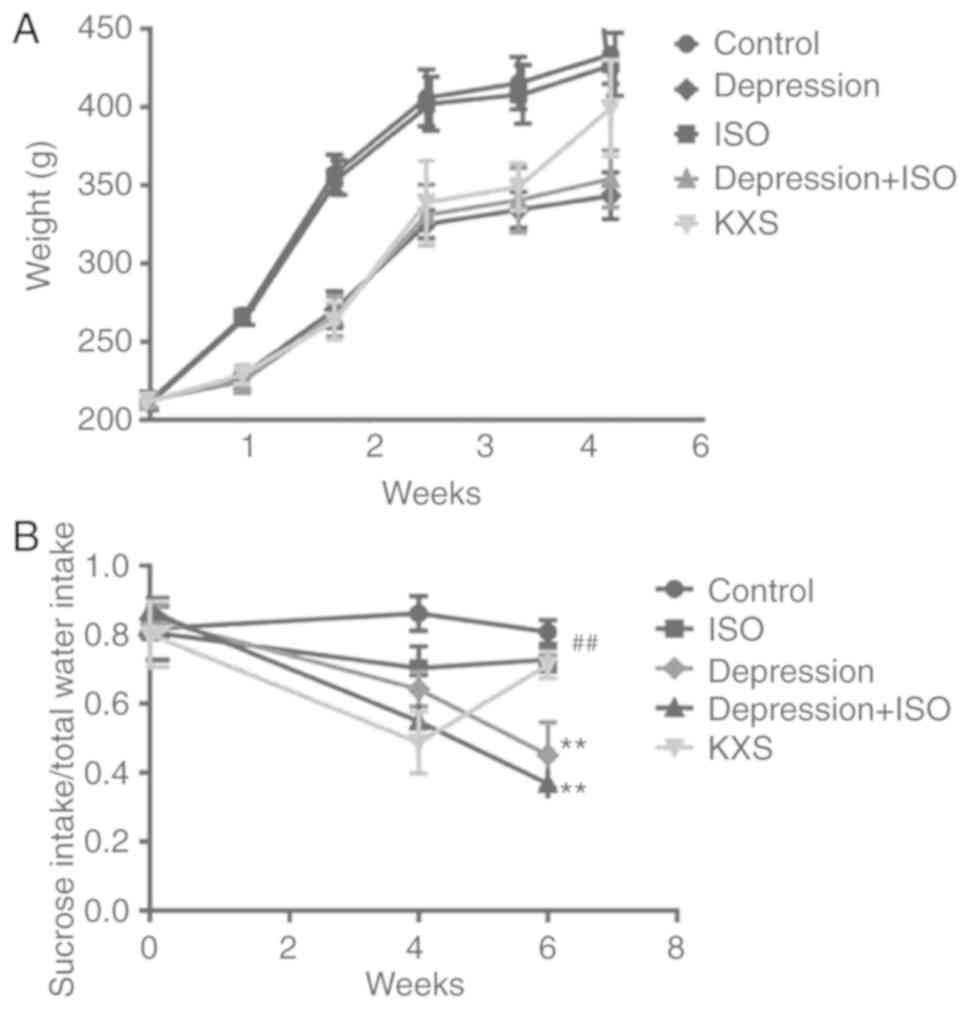
Body weight (BW) was measured before the onset of
the CUMS regimen and then weekly until the end of the procedure and
treatment with KXS weeks later (Fig.
2A). There was no difference observed between the body weight
of the ISO and control group, however, the body weight of the rats
in the depression group and the depression + ISO group was
increasingly decreased. KXS treatment restored the depression +
ISO-induced BW reduction in the rats. The results of the sucrose
preference test are presented in Fig.
2B. After 4 weeks of CUMS, the three model groups all exhibited
a decrease of sucrose intake, and among them, the depression + ISO
group exhibited the most significant decrease of sucrose preference
compared with the control group. Two weeks of KXS treatment
significantly increased sucrose preference up to the baseline.
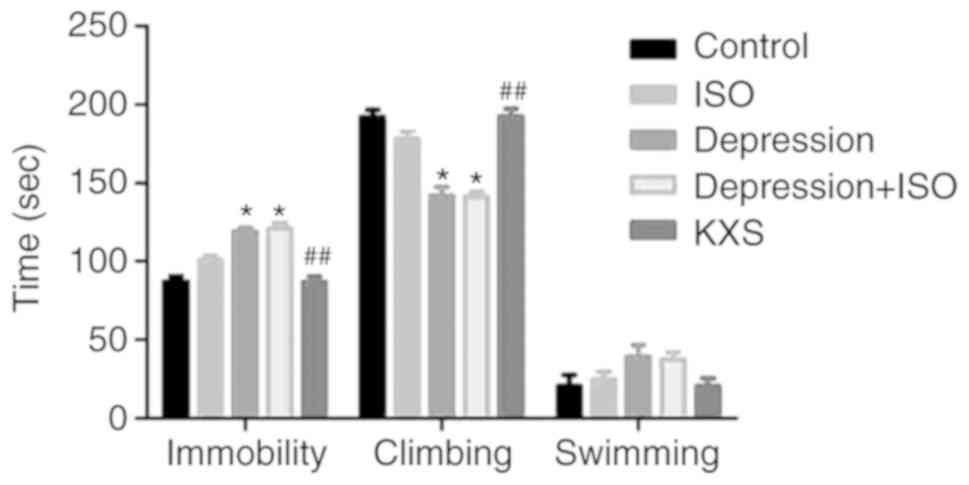
Behavior during the forced swimming test is presented in Fig. 3. The depression + ISO rats
exhibited a greater amount of immobility and a smaller amount of
climbing behavior compared to the control and the KXS-treated
rats.
Effects of KXS on cardiac
function
Echocardiography revealed markedly increased LVIDd
and LVIDs and decreased EF and FS in the treated groups, compared
with the control group. Among them, the depression + ISO group
induced the highest LVIDd and LVIDs and the lowest EF and FS
compared to the other groups. Notably, compared with the depression
+ ISO group, the KXS group increased the EF and FS and decreased
the LVIDd and LVIDs (Table I). The
heart weight (HW) corrected for BW was significantly increased in
the depression + ISO group compared to the control group and was
significantly reduced by KXS compared to the depression + ISO group
(Table I).
 | Table I.KXS effect on cardiac function in
rats with MI and depression. |
Table I.
KXS effect on cardiac function in
rats with MI and depression.
| Groups | LVIDd (mm) | LVIDs (mm) | EF (%) | FS (%) | HW/BW |
|---|
| Control |
3.76±0.25 |
3.69±0.30 |
87.46±4.28 |
52.50±3.94 |
0.0030±0.0001 |
| ISO |
5.54±0.28 |
4.69±0.51 |
50.78±4.78b |
25.59±3.25b |
0.0034±0.0001b |
| Depression |
5.19±0.25 |
5.01±0.28 |
77.31±5.05a |
45.82±6.07a |
0.0037±0.0004b |
| Depression +
ISO |
5.71±0.42 |
5.48±0.33 |
49.97±6.11b |
24.71±3.38b |
0.0039±0.0001b |
| KXS |
4.54±0.33 |
4.36±0.5 |
58.70±7.66c |
31.59±6.09c |
0.0031±0.0002c |
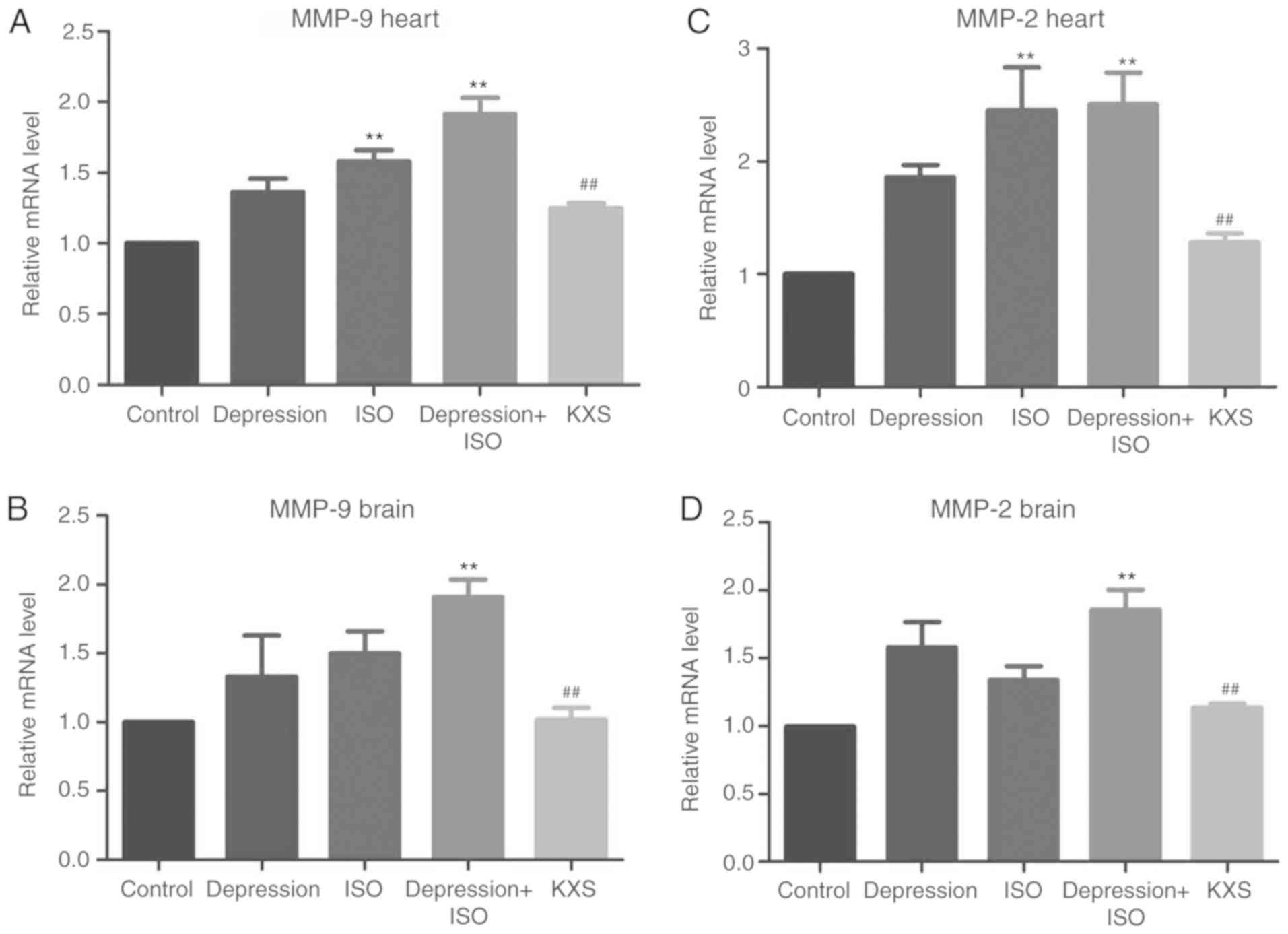
Effects of KXS on the level of MMP-2
and MMP-9 in the brain and heart of rats with MI and
depression
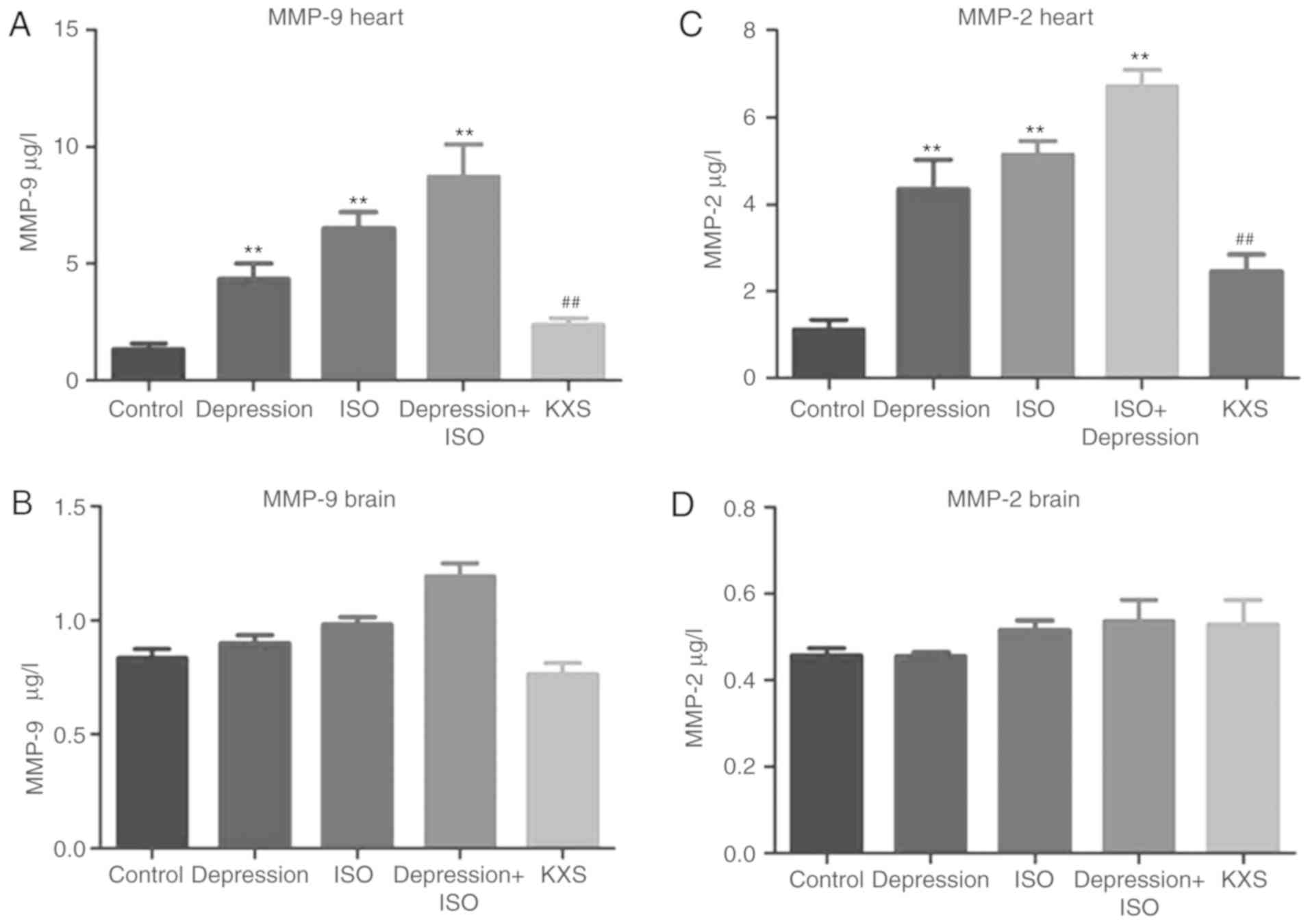
The levels of MMP-2 and MMP-9 mRNA expression were
significantly increased in both the heart and brain in the
depression + ISO group compared with the control group. Among them,
the depression + ISO group induced higher MMP levels compared with
the ISO group alone in the heart. Also, treatment with KXS
significantly alleviated the depression + ISO-induced increase of
MMP-2 and MMP-9 levels in the heart and brain (Fig. 4). The levels of MMP-2 and MMP-9
proteins in the heart were consistent with the mRNA levels,
however, the levels of protein of MMP-2 and MMP-9 in all groups in
the brain were not significantly different (Fig. 5).
Effects of KXS on the level of MMP-2
and TIMP-2 in the heart of rats with MI and depression
As demonstrated in Fig.
6, compared with the control group, MMP-2 and TIMP-2 were both
upregulated in the depression + ISO group at the protein level. The
changes in MMP-2 protein levels in different groups were examined
by western blotting, which was consistent with the results observed
using ELISA. The TIMP-2 expression in the depression group was not
as significantly altered as the ISO or depression + ISO group. KXS
decreased the MMP-2 and TIMP expression compared with the
depression + ISO rats.
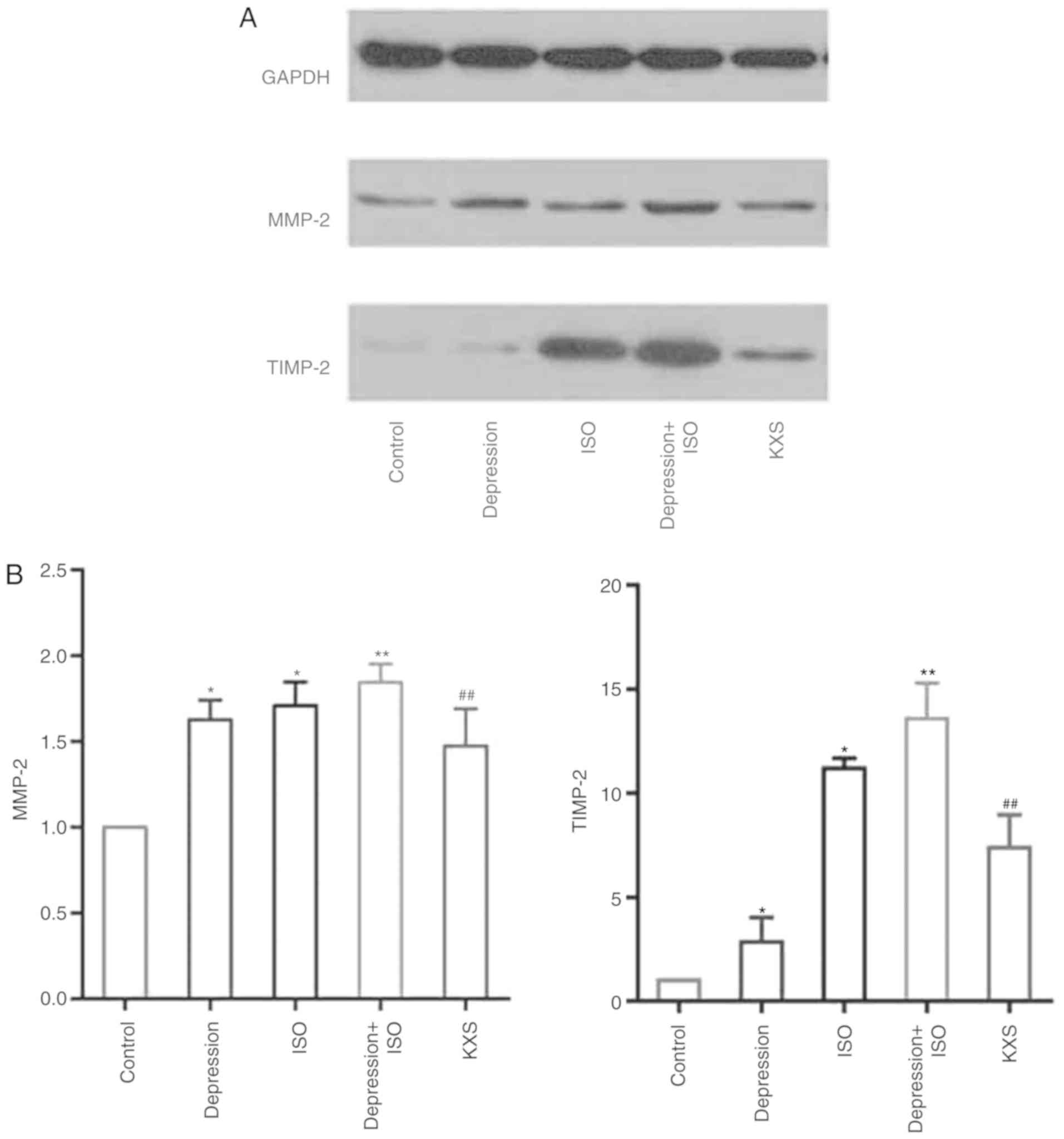 | Figure 6.Effect of KXS on MMP-2 and TIMP-2
detected by western blotting in the heart of the rats with MI and
depression. (A) Representative western blots, revealing bands of
TIMP-2 and MMP-2. (B) Bar graphs, showing the densitometric data
corresponding to A. (n=5 in each group). *P<0.05 and
**P<0.01, vs. the control; ##P<0.01 vs. the
depression + ISO group. Control, normal rats; ISO, injected ISO;
depression, chronic mild stress rats; depression + ISO, depression
with ISO; KXS, KXS + depression with ISO. KXS, Kai-Xin-San; MMP,
matrix metalloproteinase; MI, myocardial infarction; ISO, isopropyl
adrenaline. |
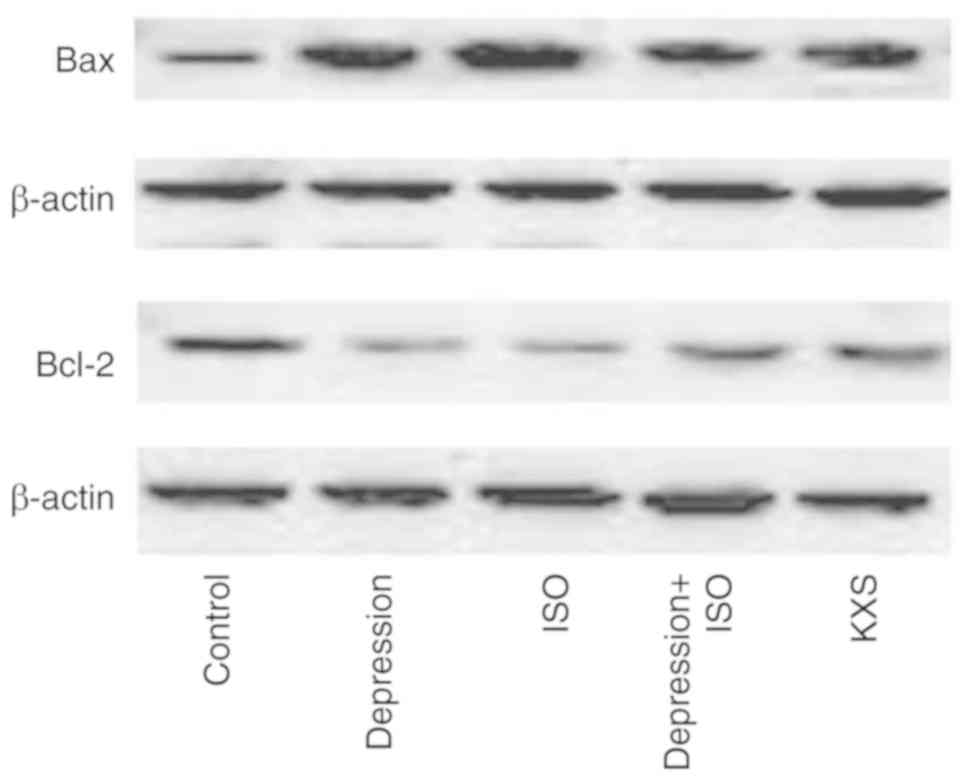
Effect of KXS on Bcl-2, and BAX
expression levels in the heart of rats with MI and depression
Compared with the control group, in the depression +
ISO group, Bax was significantly increased while Bcl-2 was
significantly decreased. Compared with the depression + ISO group,
in the KXS group, Bax expression was significantly decreased, while
Bcl-2 expression was significantly increased, and the ratio of
Bax/Bcl-2 in the KXS group was significantly decreased (Fig. 7 and Table II).
 | Table II.Effect of KXS on the expression of
protein Bax and Bcl-2 in rats with MI and depression (mean ± SEM,
IOD). |
Table II.
Effect of KXS on the expression of
protein Bax and Bcl-2 in rats with MI and depression (mean ± SEM,
IOD).
| Groups | Bax | Bcl-2 | Bax/Bcl-2 |
|---|
| Control |
25.97±6.79 |
116.5±10.09 |
0.22±0.06 |
| ISO |
114.1±16.67a |
56.32±7.35a |
2.05±0.36a |
| Depression |
93.73±3.89a |
83.19±5.76a |
1.13±0.13a |
| Depression +
ISO |
128±10.44a |
52.4±4.62a |
2.47±0.36a |
| KXS |
75.21±4.90b |
90.47±2.96b |
0.93±0.06b |
Discussion
In the present study, the effect of KXS, a
traditional Chinese medicine, was investigated on MI plus
stress-induced depression and cardiac damage. KXS not only
significantly improved depressive behavior, as previously reported
(19,21), but also improved cardiac function,
inhibited MMP-2 and MMP-9 expression, and reduced myocardial
apoptosis.
The complex model mimicking coronary heart disease
complicated by depression is very limited. It is commonly
established with the ligation of the left anterior descending
branch of the rat to induce the MI, plus stress to induce the
depressive behavior (9,10,35).
In the present study, a comprehensive disease model of ISO-induced
MI plus CUMS-induced depression was established. After ISO
injection and 4-weeks of continuous various stresses, MI plus
depression resulted in even worse EF and FS and the decreased
consumption of sucrose, compared with the depression or MI groups
alone, which indicated that MI plus depression induced even worse
cardiac function and depressive behaviors. KXS significantly
improved depression-like symptoms and displayed its
cardio-protective effect.
MMPs are an endogenous family of proteolytic enzymes
implicated in the pathophysiology and function as biomarkers of
atherosclerosis, MIs, congestive heart failure, and non-ischemic or
ischemic cardiomyopathy (12–14).
Previous studies demonstrated that the gene expression and
gelatinolytic activity of MMPs in the left ventricles were
significantly increased in experimental MI in mice (36), and the gene deletion of the MMP-9
gene attenuated cardiac remodeling post-MI by reducing macrophage
infiltration and collagen accumulation through increased apoptosis
and reduced inflammation (37).
In addition, myocardial apoptosis has been causally
linked to the pathogenesis of MI (38,39).
Certain apoptosis-related proteins, including Bcl-2, Bax and
caspase-3, are involved in the development of myocardial apoptosis
(40), and level of caspase-3 and
Bax (as a pro-apoptotic protein) were significantly reduced and
Bcl-2 (as an inhibitor of apoptosis) was enhanced in the myocardial
tissues of MI rats compared to an MI group (41). A decreased Bcl-2/Bax ratio has also
been revealed to increase the probability for myocardial cell
apoptosis (42). Several studies
have demonstrated the association between MMPs and apoptosis. The
downregulation of the expression of MMP-2 has been revealed to be
associated to attenuation of apoptosis in several pharmacotherapies
for cardiac dysfunction (43,44).
Also, the increased MMP-2 expression and imbalance of Bcl-2/Bax
expression may be associated with the development and maintenance
of atrial fibrillation (45).
Collectively, these data indicated that MMPs play a crucial role in
promoting cardiac protection through apoptosis. These results are
consistent with previous research that revealed that
cardio-protection is associated with the modulation of the cardiac
levels of MMP-2, MMP-9 and Bcl-2 or Bax. In the present study, KXS
significantly attenuated the expression of MMP-2 and MMP-9 and
myocardial apoptosis by downregulating Bax expression and
upregulating Bcl-2 expression in cardiocytes.
According to previous studies, KXS improved
depression by influencing various inflammatory pathways (19), and played a neuroprotective role
and enhanced cognitive function by reducing apoptosis and oxidative
stress (46). Proteomic analysis
of the samples of patients demonstrated that the anti-depressive
effect of KXS may also involve the alterations in platelet
activation and inflammatory regulation (47). MMPs/TIMPs (inhibitor of
metalloproteinase) imbalance plays an important role in the
transformation of the vessel wall into pathologic thrombus
formation by the release of platelets or induced inflammation
(48,49). Changes in MMP and TIMP expression
may also be a common element in, or perhaps even a marker for,
recurrent depressive disorders and somatic diseases (50). The effect of KXS on inflammation
and apoptosis has been documented, whereas there are few studies
demonstrating that the activation of MMPs is associated with
inflammation and apoptosis (51,52).
Thus, the observed MMP-2 and TIMP-2 downregulation in cardiac
myocytes may be the result of the anti-inflammatory and
anti-apoptosis effect of KXS. However, more studies are required to
further corroborate this hypothesis.
MMPs are the main interfering agent of the neural
extracellular matrix (nECM), and nECM is usually affected by
central nervous system (CNS) disorders (53), both in chronic dysfunction such as
neurodegenerative diseases and in acute/subacute disorders with
chronic sequelae, such as cerebrovascular and inflammatory
pathology (53–55). The present data did not detect a
significant difference in MMP-2 and MMP-9 protein levels between
the KXS and MI plus depression groups, however, a difference was
observed in the mRNA expression of MMP-2 and MMP-9 in the brain at
the end of the experiment (6 weeks). It is possible that the
observed MMP mRNA in the brain modified the nECM in chronic
modification during MI plus depression.
KXS could work well on the patients with qi-blood
circulation deficiency syndrome (QDS), which is the key point of
the relationship between brain and heart disease in the TCM theory.
In this study, it was further observed that KXS could significantly
decrease the depression-like behavior and effectively protect from
cardiac damage the MI plus depressive rats, which indicates that it
may be more helpful for the patients with MI and are comorbid with
major depressive disorder. The beneficial effect of KXS in animal
models may be mediated at least partially by the inhibition of
increased MMP-2 and MMP-9 activities as well as myocardial
apoptosis. In the future, more stable complex models are required
to be established to understand the exact neurobiological pathways
by which KXS regulates MMPs and how they are related to oxidative
stress, inflammation, or the platelet pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81573876).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, XD and PL contributed to the study design, TZ,
HM, WY and YW performed the experiments and analyzed the data. YH
and XD contributed to the experiments and writing and performed the
secondary data analyses and revising the manuscript for
intellectual and scientific content, and PL and YC contributed to
the conception of the study. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted following the
Use of Laboratory Animals by the U.S. National Institutes of Health
and approved by the Animal Experimentation Ethics Committee of the
Chinese PLA General Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
KXS
|
Kai-Xin-San
|
|
MMP
|
metalloproteinase
|
|
LV
|
left ventricular
|
|
FS
|
fractional shortening
|
|
EF
|
ejection fraction
|
|
CHD
|
coronary heart disease
|
|
CAD
|
coronary artery disease
|
|
HPA
|
hypothalamic-pituitary-adrenal
|
|
BDNF
|
brain-derived neurotrophic factor
|
|
CUMS
|
chronic unpredictable mild stress
|
|
TCM
|
Traditional Chinese Medicine
|
|
M-TMCA
|
Methyl 3,4,5-trimethoxycinnamate
|
|
SD
|
Sprague-Dawley
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
GAPDH
|
glyceraldehyde-3-phosphatedehydrogenase
|
|
ECM
|
extracellular matrix
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
QDS
|
qi-blood circulation deficiency
syndrome
|
|
TIMP
|
inhibitor of metalloproteinase
|
References
|
1
|
Halaris A: Psychocardiology: Moving toward
a new subspecialty. Future Cardiol. 9:635–640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khot UN, Khot MB, Bajzer CT, Sapp SK,
Ohman EM, Brener SJ, Ellis SG, Lincoff AM and Topol EJ: Prevalence
of conventional risk factors in patients with coronary heart
disease. JAMA. 290:898–904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soufer R, Jain H and Yoon AJ: Heart-brain
interactions in mental stress-induced myocardial ischemia. Curr
Cardiol Rep. 11:133–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanner JE, Frazier L and Udtha M: The role
of platelet serotonin and depression in the acute coronary syndrome
population. Yale J Biol Med. 86:5–13. 2013.PubMed/NCBI
|
|
5
|
Ladwig KH, Baumert J, Marten-Mittag B,
Lukaschek K, Johar H, Fang X, Ronel J, Meisinger C, Peters A and
KORA Investigators: Room for depressed and exhausted mood as a risk
predictor for all-cause and cardiovascular mortality beyond the
contribution of the classical somatic risk factors in men.
Atherosclerosis. 257:224–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lichtman JH, Froelicher ES, Blumenthal JA,
Carney RM, Doering LV, Frasure-Smith N, Freedland KE, Jaffe AS,
Leifheit-Limson EC, Sheps DS, et al: Depression as a risk factor
for poor prognosis among patients with acute coronary syndrome:
Systematic review and recommendations: A scientific statement from
the american heart association. Circulation. 129:1350–1369. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roose SP and Spatz E: Treating depression
in patients with ischaemic heart disease: Which agents are best to
use and to avoid? Drug Saf. 20:459–465. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He DF, Ren YP and Liu MY: Effects of
ginseng fruit saponins on serotonin system in sprague-dawley rats
with myocardial infarction, depression, and myocardial infarction
complicated with Depression. Chin Med J (Engl). 129:2913–2919.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Zhang H, Chai F, Liu X and Berk M:
The effects of escitalopram on myocardial apoptosis and the
expression of Bax and Bcl-2 during myocardial ischemia/reperfusion
in a model of rats with depression. BMC Psychiatry. 14:3492014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang J, Yuan X, Shi S, Wang F, Chen Y, Qu
C, Chen J, Hu D and Yang B: Effect and mechanism of fluoxetine on
electrophysiology in vivo in a rat model of postmyocardial
infarction depression. Drug Des Devel Ther. 9:763–772. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Hou J, Du H, Yan S, Yang J, Wang
Y, Zhang X, Zhu L and Zhao H: Anti-depressive effect of
Shuangxinfang on rats with acute myocardial infarction: Promoting
bone marrow mesenchymal stem cells mobilization and alleviating
inflammatory response. Biomed Pharmacother. 111:19–30. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mayer F, Falk M, Huhn R, Behmenburg F and
Ritz-Timme S: Matrixmetalloproteinases and tissue inhibitors of
metalloproteinases: Immunhistochemical markers in the diagnosis of
lethal myocardial infarctions? Forensic Sci Int. 288:181–188. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hojo Y, Ikeda U, Ueno S, Arakawa H and
Shimada K: Expression of matrix metalloproteinases in patients with
acute myocardial infarction. Jpn Cir J. 65:71–75. 2001. View Article : Google Scholar
|
|
14
|
Cadete VJ, Arcand SA, Chaharyn BM,
Doroszko A, Sawicka J, Mousseau DD and Sawicki G: Matrix
metalloproteinase-2 is activated during ischemia/reperfusion in a
model of myocardial infarction. Can J Cardiol. 29:1495–1503. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bobinska K, Szemraj J, Galecki P and
Talarowska M: The role of MMP genes in recurrent depressive
disorders and cognitive functions. Acta Neuropsychiatr. 28:221–231.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang AL, Chen Z, Luo J, Shang QH and Xu H:
Systematic review on randomized controlled trials of coronary heart
disease complicated with depression treated with chinese herbal
medicines. Chin J Integr Med. 22:56–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Y, Liu M, Liu P, Yan JJ, Liu MY, Zhang
GQ, Zhou XJ and Yu BY: Effect of Kai Xin San on learning and memory
in a rat model of paradoxical sleep deprivation. J Med Food.
16:280–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang N, Jia YM, Zhang B, Xue D, Reeju M,
Li Y, Huang SM and Liu XW: Neuroprotective mechanism of Kai Xin
San: Upregulation of hippocampal insulin-degrading enzyme protein
expression and acceleration of amyloid-beta degradation. Neural
Regen Res. 12:654–659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong XZ, Wang DX, Lu YP, Yuan S, Liu P and
Hu Y: Antidepressant effects of Kai-Xin-San in fluoxetine-resistant
depression rats. Braz J Med Biol Res. 50:e61612017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu Y, Zhou XJ, Liu P, Dong XZ, Mu LH, Chen
YB, Liu MY and Yu BY: Antidepressant and neuroprotective effect of
the chinese herb kaixinsan against lentiviral shRNA knockdown
brain-derived neurotrophic factor-induced injury in vitro and in
vivo. Neuropsychobiology. 69:129–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Chao C, Duan X, Cheng X, Liu P, Su
S, Duan J, Dong TT and Tsim KW: Kai-Xin-San series formulae
alleviate depressive-like behaviors on chronic mild stressed mice
via regulating neurotrophic factor system on hippocampus. Sci Rep.
7:14672017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu XY, Zhou XY, Hou JC, Zhu H, Wang Z,
Liu JX and Zheng YQ: Ginsenoside Rd promotes neurogenesis in rat
brain after transient focal cerebral ischemia via activation of
PI3K/Akt pathway. Acta Pharmacol Sin. 36:421–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung JY, Seong KJ, Moon IO, Cho JH, Kim SH
and Kim WJ: Ginsenosides have a suppressive effect on c-Fos
expression in brain and reduce cardiovascular responses increased
by noxious stimulation to the rat tooth. Korean J Physiol
Pharmacol. 17:121–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang QW, Yu XF, Xu HL, Jiang YC, Zhao XZ
and Sui DY: Ginsenoside re attenuates isoproterenol-induced
myocardial injury in rats. Evid Based Complement Alternat Med.
2018:86371342018.PubMed/NCBI
|
|
25
|
Zhao Z, Fang M, Xiao D, Liu M, Fefelova N,
Huang C, Zang WJ and Xie LH: Potential antiarrhythmic effect of
methyl 3,4,5-trimethoxycinnamate, a bioactive substance from roots
of polygalae radix: Suppression of triggered activities in rabbit
myocytes. Biol Pharm Bull. 36:238–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu Y, Liu M, Liu P, Guo DH, Wei RB and
Rahman K: Possible mechanism of the antidepressant effect of
3,6′-disinapoyl sucrose from polygala tenuifolia willd. J Pharm
Pharmacol. 63:869–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Gao Y, Dong J, Wang S, Yao B,
Zhang J, Hu S, Xu X, Zuo H, Wang L, et al: The compound chinese
medicine ‘Kang Fu Ling’ protects against high power
microwave-induced myocardial injury. PLoS One. 9:e1015322014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Y, Cao Y, Liu M, Liu P, Cui H and
Dai-Hong G: Behavioral and biochemical effects of a formulation of
the traditional chinese medicine, Kai-Xin-San, in fatigued rats.
Exp Ther Med. 6:973–976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Liu P, Dai-Hong G, Rahman K, Wang
DX, Chen ML and Xie TT: Behavioral and biochemical effects of
kaixin-san, a traditional Chinese medicinal empirical formula. Drug
Dev Res. 69:267–271. 2008. View Article : Google Scholar
|
|
30
|
Lin Y, Wang LN, Xi YH, Li HZ, Xiao FG,
Zhao YJ, Tian Y, Yang BF and Xu CQ: L-arginine inhibits
isoproterenol-induced cardiac hypertrophy through nitric oxide and
polyamine pathways. Basic Clin Pharmacol Toxicol. 103:124–130.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi HS, Zhu WL, Liu JF, Luo YX, Si JJ,
Wang SJ, Xue YX, Ding ZB, Shi J and Lu L: PI3K/Akt signaling
pathway in the basolateral amygdala mediates the rapid
antidepressant-like effects of trefoil factor 3.
Neuropsychopharmacology. 37:2671–2683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Porsolt RD, Le Pichon M and Jalfre M:
Depression: A new animal model sensitive to antidepressant
treatments. Nature. 266:730–732. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng Y, Zhao H, Xu X, Buys ES, Raher MJ,
Bopassa JC, Thibault H, Scherrer-Crosbie M, Schmidt U and Chao W:
Innate immune adaptor MyD88 mediates neutrophil recruitment and
myocardial injury after ischemia-reperfusion in mice. Am J Physiol
Heart Circ Physiol. 295:H1311–H1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arseneault-Breard J, Rondeau I, Gilbert K,
Girard SA, Tompkins TA, Godbout R and Rousseau G: Combination of
lactobacillus helveticus R0052 and bifidobacterium longum R0175
reduces post-myocardial infarction depression symptoms and restores
intestinal permeability in a rat model. Br J Nutr. 107:1793–1799.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rohde LE, Ducharme A, Arroyo LH, Aikawa M,
Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P and Lee
RT: Matrix metalloproteinase inhibition attenuates early left
ventricular enlargement after experimental myocardial infarction in
mice. Circulation. 99:3063–3070. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iyer RP, de Castro Bras LE, Patterson NL,
Bhowmick M, Flynn ER, Asher M, Cannon PL, Deleon-Pennell KY, Fields
GB and Lindsey ML: Early matrix metalloproteinase-9 inhibition
post-myocardial infarction worsens cardiac dysfunction by delaying
inflammation resolution. J Mol Cell Cardiol. 100:109–117. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Frangogiannis NG: Pathophysiology of
myocardial infarction. Compr Physiol. 5:1841–1875. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raish M: Momordica charantia
polysaccharides ameliorate oxidative stress, hyperlipidemia,
inflammation, and apoptosis during myocardial infarction by
inhibiting the NF-κB signaling pathway. Int J Biol Macromol.
97:544–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crow MT: The mitochondrial death pathway
and cardiac myocyte apoptosis. Circ Res. 95:957–970. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang W, Li Y and Ge Z: Cardiaprotective
effect of crocetin by attenuating apoptosis in isoproterenol
induced myocardial infarction rat model. Biomed Pharmacother.
93:376–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Condorelli G, Morisco C, Stassi G, Notte
A, Farina F, Sgaramella G, de Rienzo A, Roncarati R, Trimarco B and
Lembo G: Increased cardiomyocyte apoptosis and changes in
proapoptotic and antiapoptotic genes bax and bcl-2 during left
ventricular adaptations to chronic pressure overload in the rat.
Circulation. 99:3071–3078. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song YH, Cai H, Gu N, Qian CF, Cao SP and
Zhao ZM: Icariin attenuates cardiac remodelling through
down-regulating myocardial apoptosis and matrix metalloproteinase
activity in rats with congestive heart failure. J Pharm Pharmacol.
63:541–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen SL, Hu ZY, Zuo GF, Li MH and Li B:
I(f) current channel inhibitor (ivabradine) deserves
cardioprotective effect via down-regulating the expression of
matrix metalloproteinase (MMP)-2 and attenuating apoptosis in
diabetic mice. BMC Cardiovasc Disord. 14:1502014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diao SL, Xu HP, Zhang B, Ma BX and Liu XL:
Associations of MMP-2, BAX, and Bcl-2 mRNA and protein expressions
with development of atrial fibrillation. Med Sci Monit.
22:1497–1507. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu YM, Wang XC, Xu TT, Li HY, Hei SY, Luo
NC, Wang H, Zhao W, Fang SH, Chen YB, et al: Kai xin san
ameliorates scopolamine-induced cognitive dysfunction. Neural Regen
Res. 14:794–804. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen C, Hu Y, Dong XZ, Zhou XJ, Mu LH and
Liu P: Proteomic analysis of the antidepressant effects of
shen-zhi-ling in depressed patients: Identification of proteins
associated with platelet activation and lipid metabolism. Cell Mol
Neurobiol. 38:1123–1135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Robert S, Gicquel T, Bodin A, Lagente V
and Boichot E: Characterization of the MMP/TIMP imbalance and
collagen production induced by IL-1β or TNF-α release from human
hepatic stellate cells. PLoS One. 11:e01531182016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gresele P, Falcinelli E, Sebastiano M and
Momi S: Matrix metalloproteinases and platelet function. Prog Mol
Biol Transl Sci. 147:133–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bobinska K, Szemraj J, Czarny P and
Galecki P: Expression and activity of metalloproteinases in
depression. Med Sci Monit. 22:1334–1341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
2013:9283152013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu S, Webb SE, Lau TCK and Cheng SH:
Matrix metalloproteinases (MMPs) mediate leukocyte recruitment
during the inflammatory phase of zebrafish heart regeneration. Sci
Rep. 8:71992018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
De Luca C and Papa M: Matrix
metalloproteinases, neural extracellular matrix, and central
nervous system pathology. Prog Mol Biol Transl Sci. 148:167–202.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tai SH, Chen HY, Lee EJ, Chen TY, Lin HW,
Hung YC, Huang SY, Chen YH, Lee WT and Wu TS: Melatonin inhibits
postischemic matrix metalloproteinase-9 (MMP-9) activation via dual
modulation of plasminogen/plasmin system and endogenous MMP
inhibitor in mice subjected to transient focal cerebral ischemia. J
Pineal Res. 49:332–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nakaji K, Ihara M, Takahashi C, Itohara S,
Noda M, Takahashi R and Tomimoto H: Matrix metalloproteinase-2
plays a critical role in the pathogenesis of white matter lesions
after chronic cerebral hypoperfusion in rodents. Stroke.
37:2816–2823. 2006. View Article : Google Scholar : PubMed/NCBI
|