Gastrointestinal (GI) cancer develops in the organs
of the alimentary canal, including the esophagus, liver and bile
ducts, gallbladder, pancreas, stomach and small and large
intestines (1). Although some
distinct mutations have been reported in different GI organs, GI
tumors display several key molecular alterations (2,3).
Keratins (KRTs) are a family of fibrous structural
proteins that are present in normal epithelia, however, some are
upregulated in neoplasms (4). The
differential expression of KRTs facilitates the diagnosis of
several tumors, including GI tumors, using molecular techniques and
allows KRTs to be used as biomarkers to discriminate primary from
metastatic adenocarcinoma (5–8). In
addition, KRTs have long been considered epithelial differentiation
markers (9) and the
differentiation of cells within the GI tract is associated with an
increased susceptibility to GI cancer (10,11).
In addition to being involved in conventional
tumorigenesis, epithelial-mesenchymal transition (EMT) plays key
roles in cellular differentiation and cancer progression (12,13).
KRTs have been reported to be aberrant in cells undergoing EMT
(14,15). For example, the detection of
KRT20-positive circulating tumor cells is associated with worse
prognosis in patients with colorectal cancer (CRC) (16). In addition to structural KRTs,
other genes often exhibit dysregulated expression during EMT.
Previously, placenta specific 8 (PLAC8), a gene expressed under
physiological conditions, was reported to play a key role in the
tumorigenic and EMT pathways in a number of types of human cancer,
such as CRC or pancreatic cancer (CaP) (17,18).
PLAC8 was expressed at high levels in the GI tract and KRT20
expression patterns were highly specific (19–21).
In addition, elevated PLAC8 levels are positively associated with
tumor metastasis and recurrence in CRC (18,22).
Understanding the interaction between KRT20 and PLAC8 could have
clinical implications for the treatment of GI cancer. In the
present study, immunostaining was employed to detect the expression
of KRT20 and PLAC8 in the tissues of patients with GI cancer
[gastric cancer (GC), CaP and CRC]. Furthermore, the mRNA levels of
KRT20 in CRC cells displaying differential PLAC8 expression were
quantified.
Archived 4 µm formalin-fixed paraffin-embedded
(FFPE) tissue sections from four GC, four CaP and four CRC patients
who had undergone surgery at the Department of Surgery of Cathay
General Hospital before December, 2000 were obtained and used in
the present study. All procedures were approved by the Cathay
General Hospital Institutional Ethics Committee and a waiver of
consent was approved by the same committee. Patient information was
anonymized.
For each cancer (GC, CaP and CRC), two patients had
well-differentiated cancer [one at American Joint Committee on
Cancer (AJCC) stage II and one at stage III] and two had poorly
differentiated cancer (one at AJCC stage II and one at stage III)
(23). Cancer diagnoses were
performed by a pathologist. The human CRC cell lines SW620 [cat.
no. CRL-1831; AJCC stage III) and Caco-2 (cat. no. HTB-37) were
obtained from the American Type Culture Collection (ATCC) and the
medium suggested by the ATCC for each cell line was used for
culture; Leibovitz's L-15 medium for SW620 cells and the Eagle's
Minimum Essential medium for Caco-2 cells. The two CRC cell lines
were selected due to their high PLAC8 expression levels (SW620
cells) (24) and their
differentiation capacity (Caco-2 cells) (25). SW620 cells were incubated at 37°C
and 100% air (with very low CO2) in a humidified
incubator and subcultured 2 to 3 times per week (25). To induce intestinal
differentiation, Caco-2 cells were cultured to confluence in a
humidified incubator at 37°C with 5% CO2 for 21 days, as
described in a previous study (25).
PLAC8 mRNA levels were knocked down in SW620 cells
using a lentivirus-mediated small hairpin (sh) RNA targeting PLAC8
to obtain shPLAC8-SW620 cells. Control (shLUC-SW620) cells were
obtained using a lentivirus-mediated shRNA targeting luciferase
(25). The lentiviruses and the
protocol for lentivirus infection were acquired from the National
RNAi Core Facility of Academia Sinica. Briefly, 1×106
SW620 cells were grown in a 10 cm plate for 24 h, and then infected
with lentivirus at a multiplicity of infection of 3. Stable
infected cells were selected and maintained in medium containing 2
µg/ml puromycin for 48 h (Thermo Fisher Scientific, Inc.). The
total RNA of the transfected cells was then extracted using
RNAzol® RT (Molecular Research Center) and reverse
transcribed into cDNA using a high-capacity cDNA Reverse
Transcription kit (Catalog No. 4368813; Thermo Fisher Scientific,
Inc.) in the presence of oligo(dT) primers, according to the
manufacturer's instructions. The level of mRNA was considered as
the gene expression level and was measured by PCR in the presence
of specific amplification primers (Table I), a TaqMan probe and TaqMan master
mix (Roche Diagnostics GmbH), according to the manufacturer's
instructions. Cycling conditions were: 2 min at 50°C and 10 min at
95°C, followed by 50 cycles each consisting of 15 sec at 95°C and 1
min at 60°C. mRNA levels were adjusted relative to the level of
GAPDH to estimate the relative levels of gene expression with the
method of the 2−ΔΔCq method (26). LightCycler (version 4.05; Roche
Diagnostics GmbH) was used to analyze the PCR kinetics.
Data were presented as mean ± standard deviation.
The relative expression levels of PLAC8 and KRT20 in cells were
compared between samples using the Student's t-test. All
statistical analyses were performed using SPSS (version 19; IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
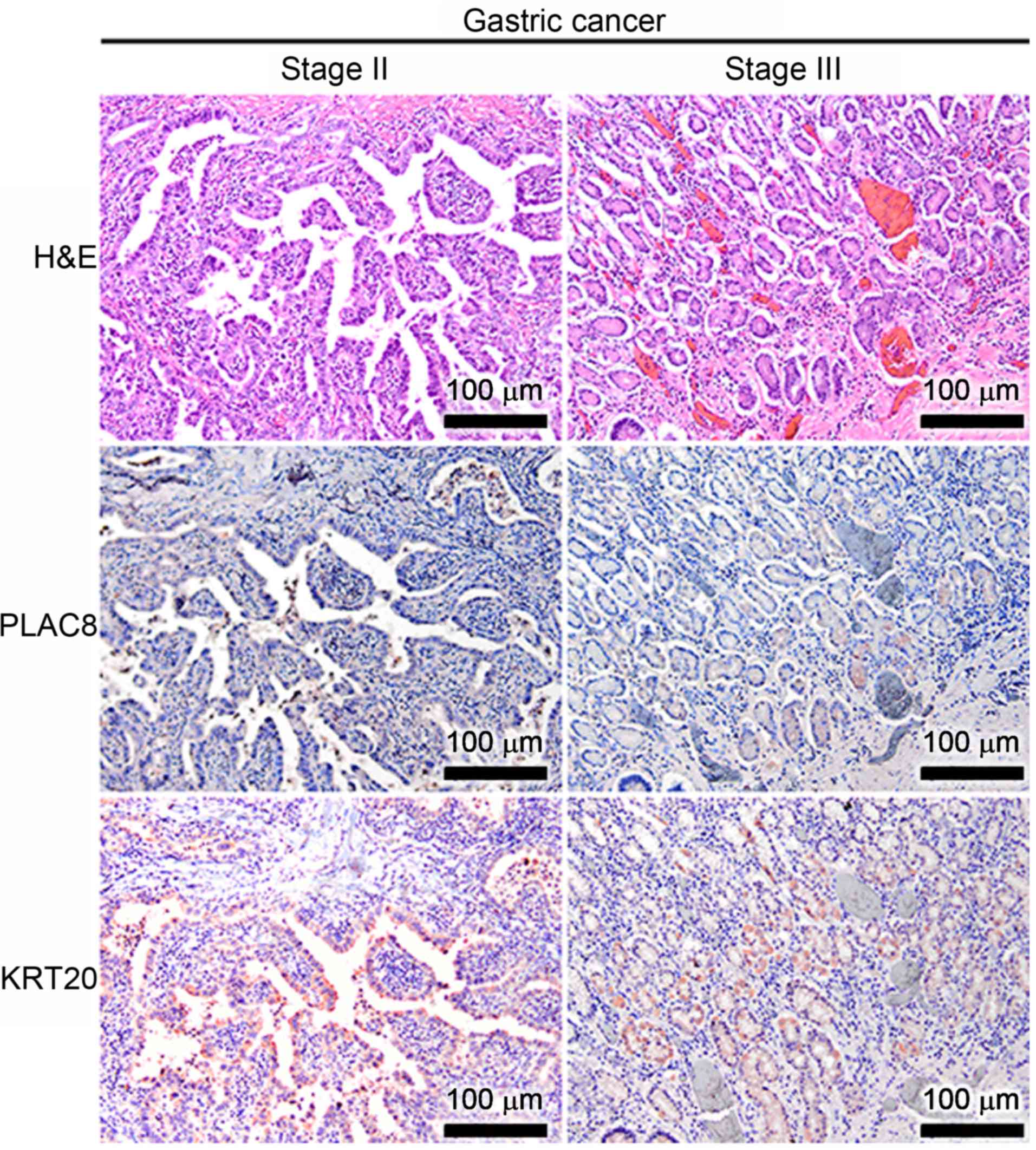
Cellular KRT20 and PLAC8 proteins were detected in
each of the cancer tissues using immunohistochemistry. In the
well-differentiated GC cancer tissues, PLAC8 levels were low and
tissues were KRT20-positive regardless of tumor stage (Fig. 1). Conversely, tissues were positive
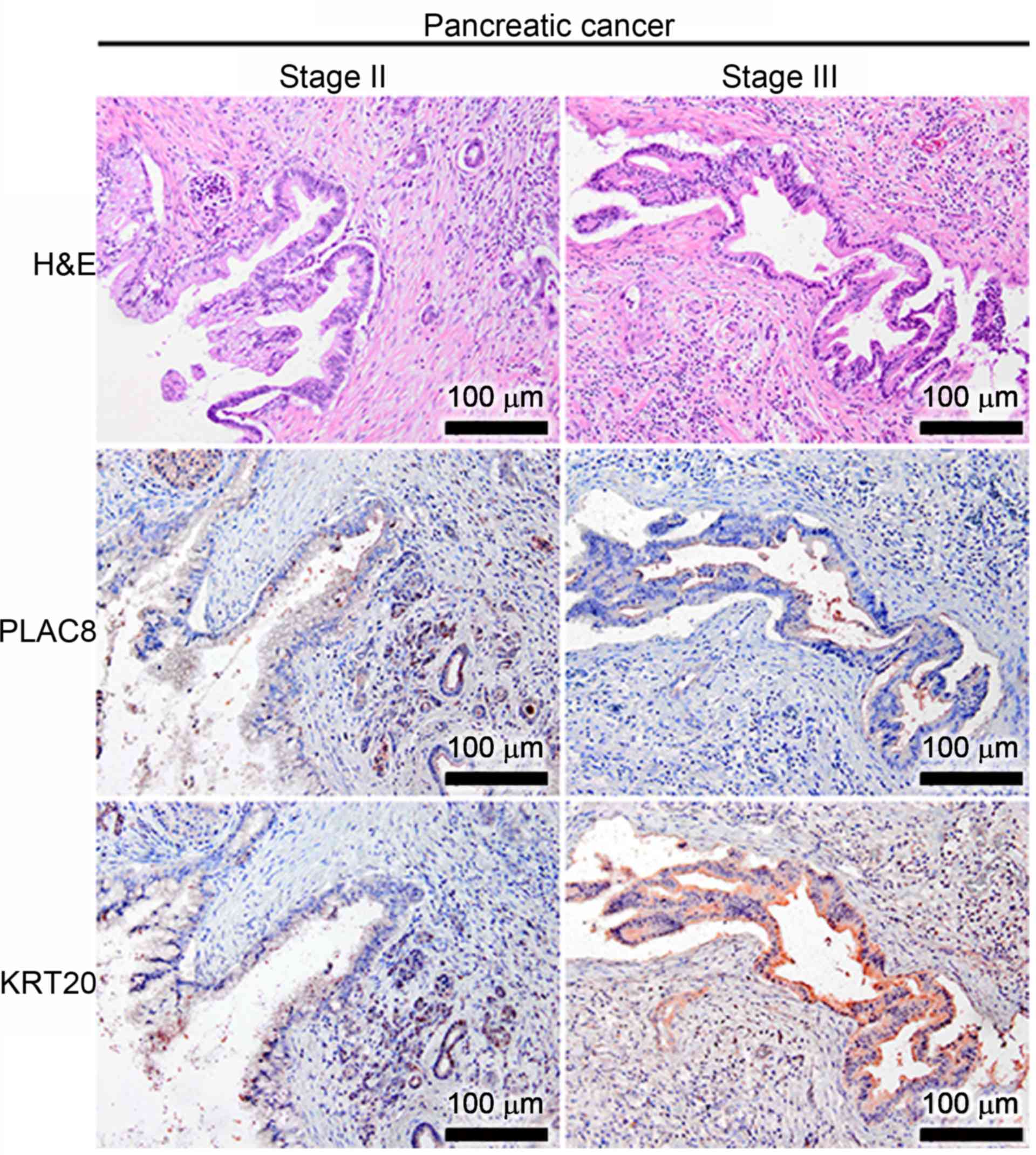
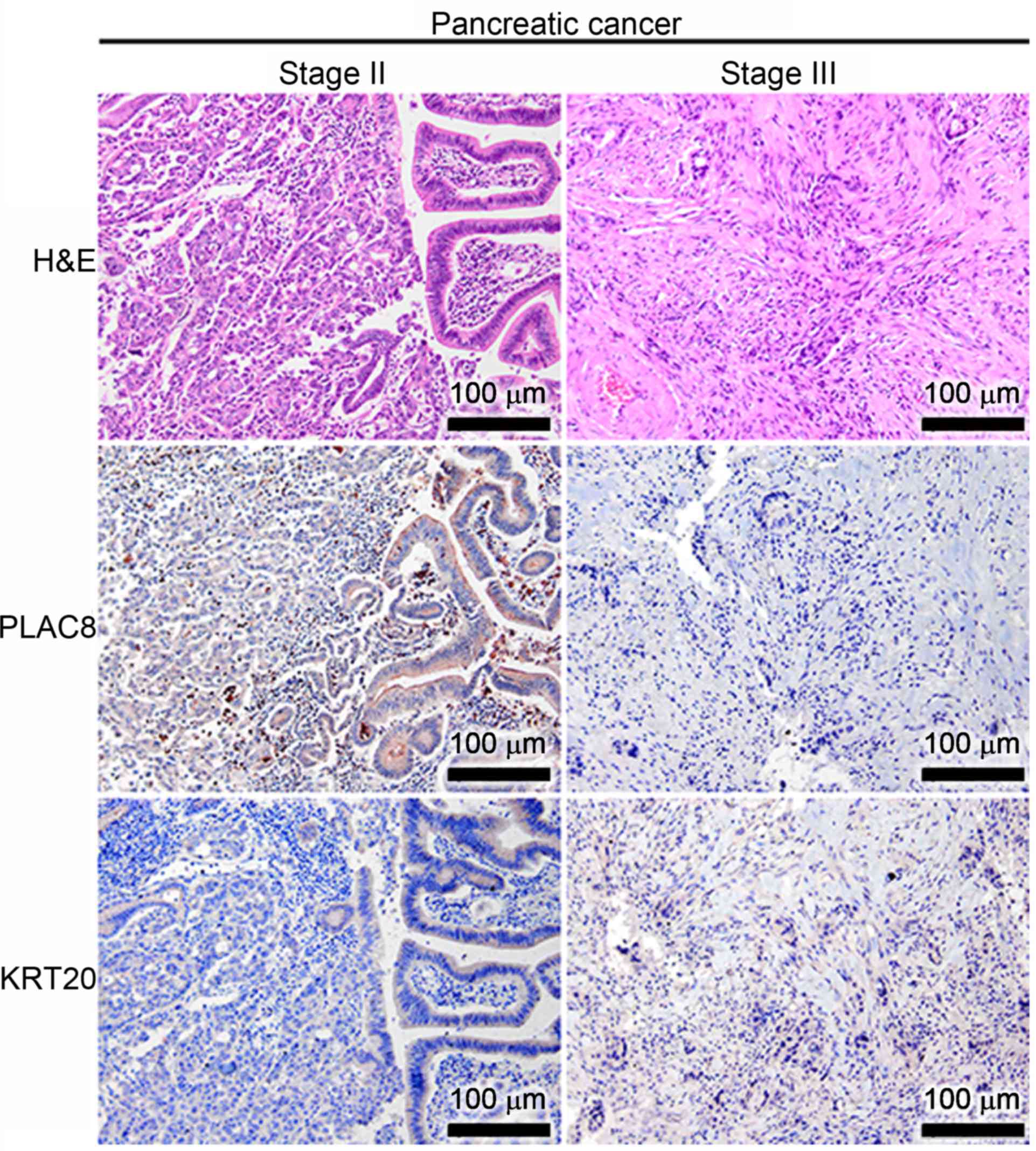
for both PLAC8 and KRT20 in the well-differentiated CaP tissues at
stages II and III, but PLAC8 displayed a luminal staining pattern
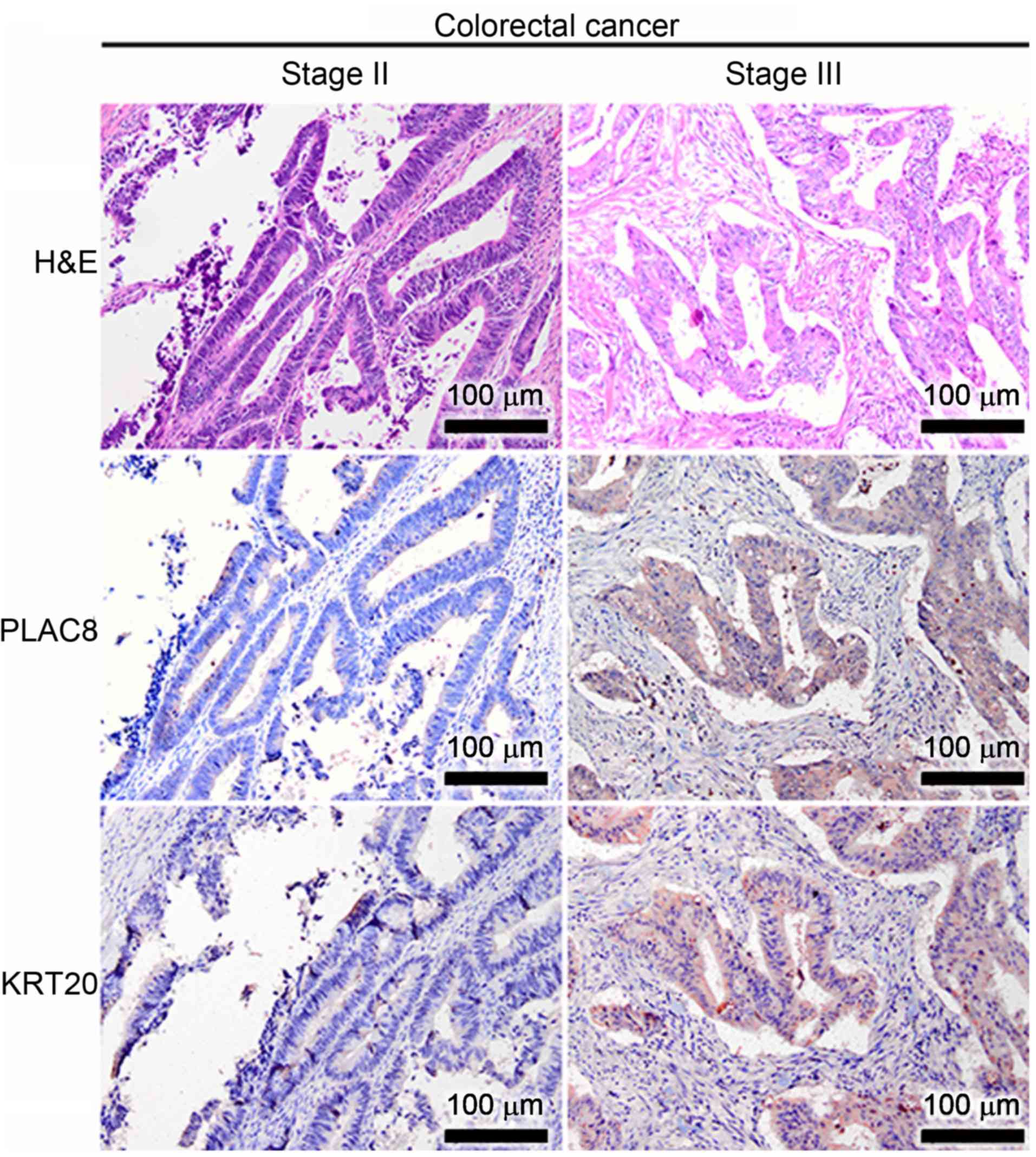
(Fig. 2). In addition to the
expression patterns in the well-differentiated GC and CaP cases,
the expression patterns of PLAC8 and KRT20 were also immunodetected
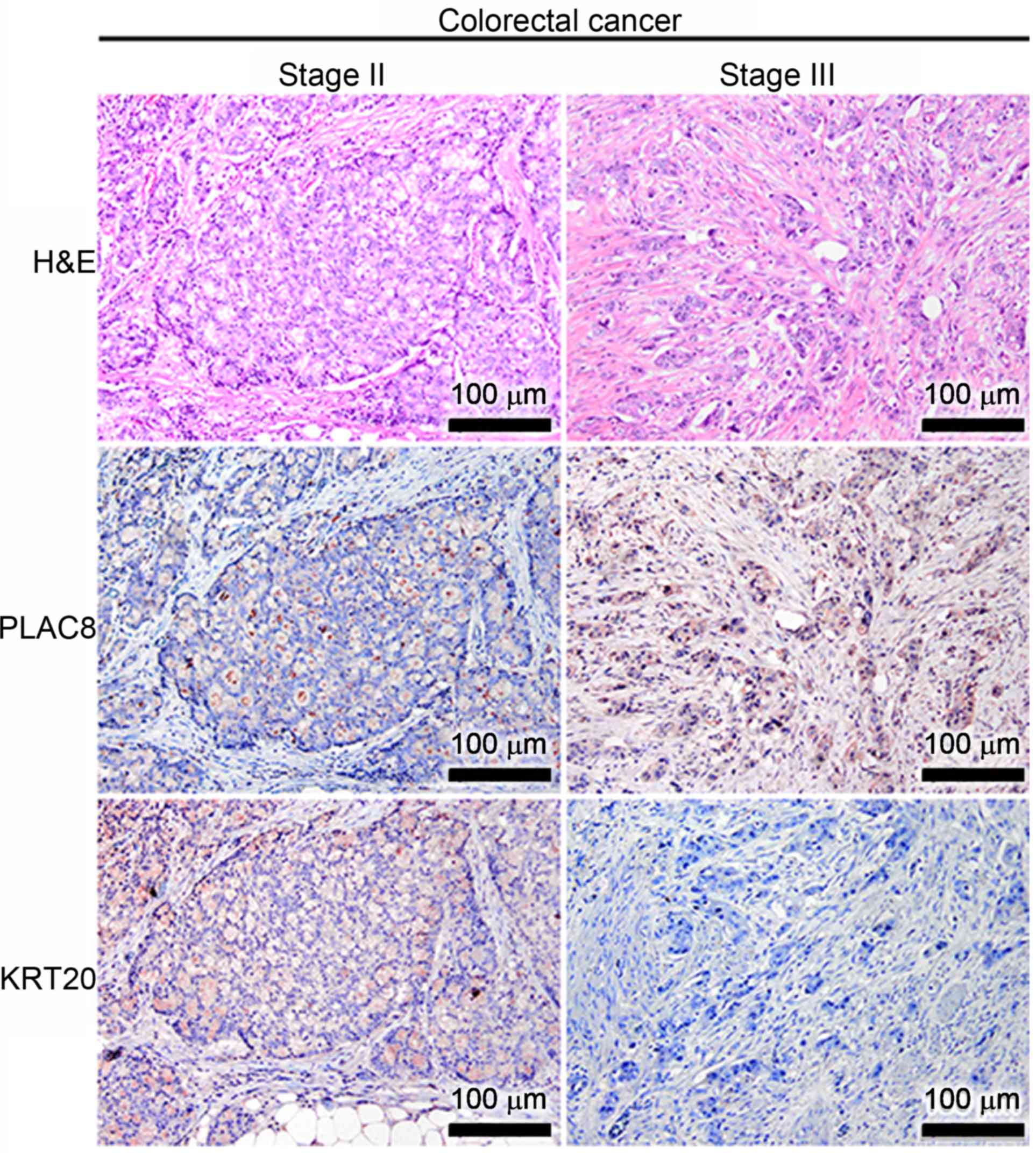
in well-differentiated CRC cases (Fig.
3). PLAC8 signals were consistently low in the
well-differentiated CRC at stage II (the left panel of Fig. 3), but the small number of
PLAC8-positive CRC cells appeared to also express KRT20.
Furthermore, a co-expression of PLAC8 and KRT20 was observed in the
well-differentiated CRC cells at stage III (the right panel of
Fig. 3).
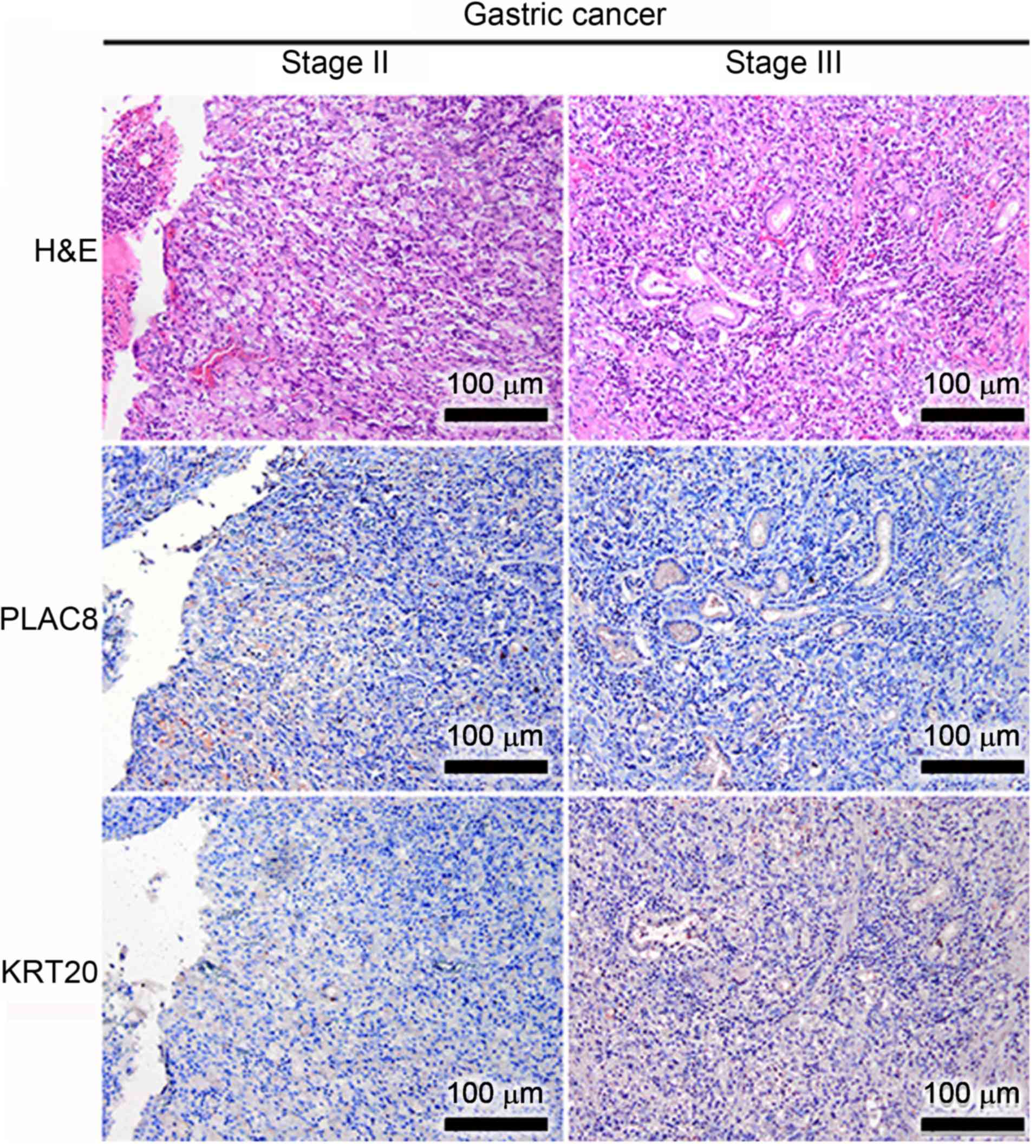
Immunohistochemical staining revealed that although
poorly differentiated GC tissues expressed similar levels of PLAC8
at stage II and III (the middle panel of Fig. 4), these tissues expressed higher
levels of KRT20 at stage III compared to stage II (the bottom panel
of Fig. 4). Conversely, the poorly
differentiated CaP at stage II displayed higher levels of PLAC8
compared with the CaP tissues at stage III, and KRT20 levels were
low in the poorly differentiated CaP at both stage II and III
(Fig. 5). The PLAC8 and KRT20
expression patterns in poorly differentiated CRC were different
from those in well-differentiated CRC (Fig. 6). PLAC8 expression was higher in
the poorly differentiated CRC tissue at stage III than at stage II
(the middle panel of Fig. 6).
However, the late tumor stage (stage III) did not appear to
increase the expression of KRT20 in the poorly differentiated CRC
tissue.
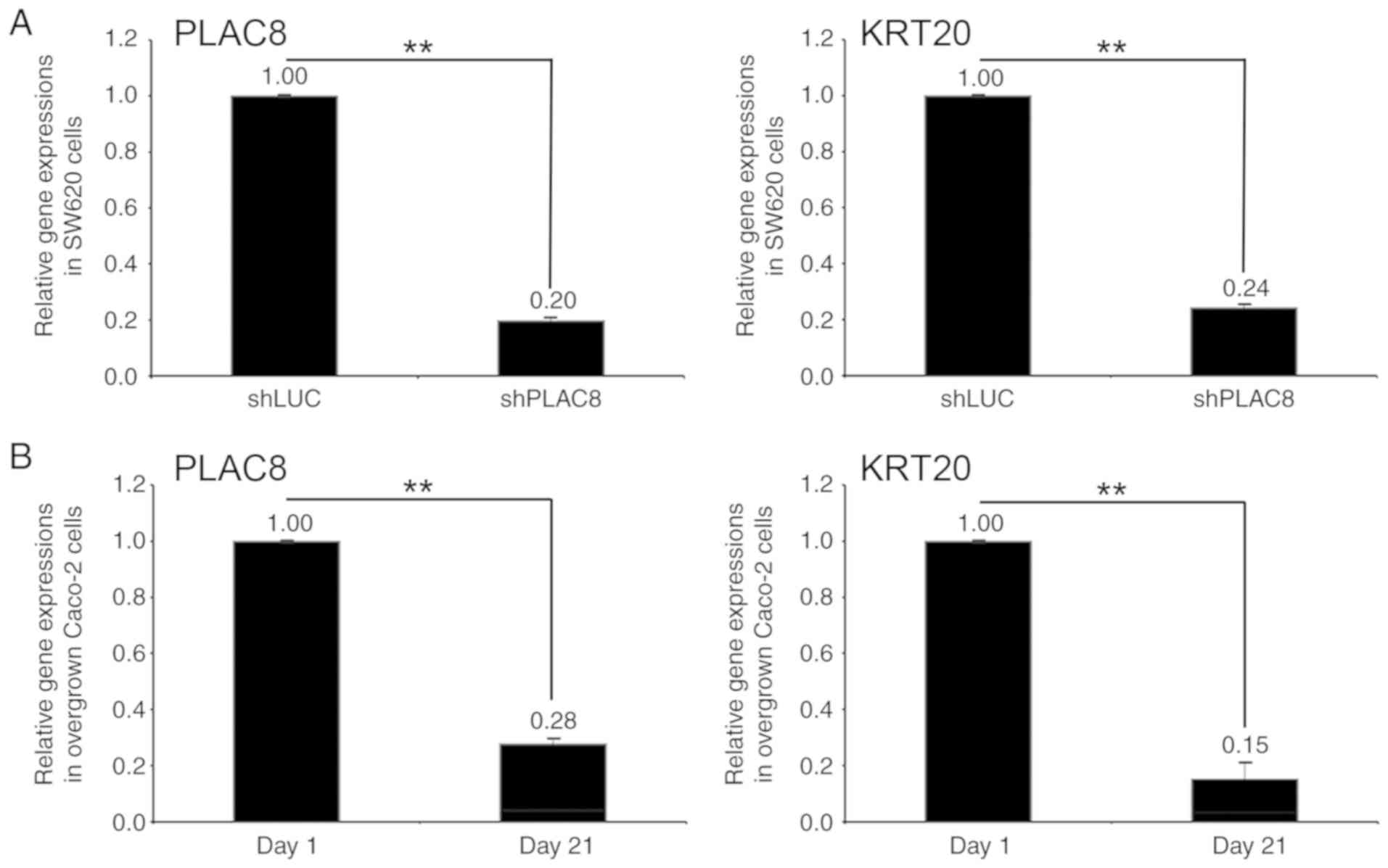
PLAC8 expression was knocked down in SW620 cells,
displayed by an 80% decrease in PLAC8 mRNA levels (shPLAC8-SW620)
compared with the levels in the control cells (shLUC-SW620;
Fig. 7A). The KRT20 mRNA levels in
the shPLAC8-SW620 cells also decreased by 76% compared with the
levels in the control cells (Fig.
7A). The mRNA levels of both PLAC8 and KRT20 in the
differentiated Caco-2 cells (day 21) decreased by 72 and 85%,
respectively, compared with the levels in the day 1 Caco-2 cells
(Fig. 7B).
In clinical settings, patients with different types
of GI cancer should be diagnosed using appropriate biomarkers, even
though gut-derived adenocarcinomas display similar genetic
alterations (28–32). Lukyanchuk et al (33) reported that KRT20 had clinical
significance in GI cancer, including GC, CaP and CRC. Thus, the
present study focused on investigating KRT20 and PLAC8 expression
in these types of GI cancer. In the present study, the aberrant
co-expression of the cytoplasmic protein PLAC8 and the cytokeratin
KRT20 were found in the well-differentiated CRC at stage III, but
this expression pattern was not observed in poorly differentiated
CRC. No such co-expression was observed in the GC and CaP tissue
sections, regardless of tumor stage and differentiation state. CRC
tissues at stages II and III have been frequently studied to
improve prognosis and to avoid the incorrect use of
chemotherapeutic agents (34,35).
Cytoskeletal rearrangement is required for cell
migration and invasion, which are key steps in cancer metastasis
(36,37). Highly dynamic biological processes
of cytoskeletal organization in cancer have been extensively
explored (38–42). Among the different cytoskeletal
molecules, KRTs might be the most examined based on clinical
significance (43,44), and several KRTs have been
previously studied from a tumor progression perspective (45–47).
For example, previous studies have reported that upregulation of
KRT17 and KRT19 may be involved in tumor metastasis (5,48)
and that KRT18 and KRT19 are associated with colorectal malignancy
(49–52). In addition, aberrant KRT20
expression has been observed in generalized GI cancer (16,19,53)
and is recognized as a marker of circulating CRC cells (54). Therefore, KRT20 could be a suitable
marker for the evaluation of the primary origin of GI cancer,
including CRC (19,55).
PLAC8, a novel oncogenic marker that mediates tumor
progression, has also been reported to play a key role in the EMT
of CRC (18,22). In the present study, an association
between KRT20 and PLAC8 expression was observed in CRC cells. The
KRT20 mRNA levels decreased in the PLAC8-knockdown SW620 CRC cells,
which were diagnosed as AJCC stage III. In addition, the intestinal
differentiation of Caco-2 cells was used to evaluate the
well-differentiated state of GI cancer (56,57).
Such spontaneously differentiated Caco-2 cells displayed decreasing
levels of KRT20 and PLAC8 expression upon differentiation. The
Caco-2 cell line, which is applied extensively as an intestinal
epithelial barrier model, displays favorable differentiation in a
continuous culture (58,59). In addition, the positive
association between KRT20 and PLAC8 expression levels in the
well-differentiated CRC was confirmed by immunostaining of archived
FFPE tissue sections. The FFPE tissue sections of other
well-differentiated GI cancer (GC and CaP) at stages II and III did
not display patterns similar to those of CRC and no association
between PLAC8 and KRT20 expression levels were observed in the
three poorly differentiated GI cancer tissues (GC, CaP and CRC).
The results from the present study suggested that understanding the
expression of PLAC8 and KRT20 could be critical for predicting the
prognosis of patients with CRC.
Experiments exploring the molecular heterogeneity of
CRC could facilitate the formulation of effective therapies
(60,61). CRC development and progression is a
complex process involving multiple genetic changes (62–64).
The genes involved in CRC tumorigenesis should therefore be
identified for clinical applications (65). Chemotherapy, target molecule
therapy (with vascular endothelial growth factor or epidermal
growth factor receptor) and immunotherapy (anti-programmed death-1)
lead to increased survival rates and decreased recurrence rates in
CRC (66–68). Imai et al (69) revealed that the KRT20 expression
was closely associated with the invasive histological phenotype in
poorly differentiated colorectal adenocarcinoma. However, in the
present study, it was suggested that the differentiation status of
GI cancer may influence KRT20 expression, particularly in CRC.
A recent animal study reported that PLAC8 expression
might be associated with the gut microbiota (70) and others detected that aberrant
KRT20 expression is induced by altering the gut microbiota
(71). Taken together, these
results implied that KRT20 and PLAC8 might work cooperatively in
different types of GI cancer. The present study suggested that
PLAC8 expression could influence KRT20 expression. Therefore, it
could be hypothesized that a well-differentiated CRC may have poor
prognosis if KRT20 is induced via the upregulation of PLAC8.
Conversely, the downregulation of PLAC8 may reduce the expression
levels of KRT20. These suggested molecular dynamics imply that RNA
interference of PLAC8 expression could be used as a therapeutic
technique for the treatment of GI cancer at stages II and III. A
similar concept that uses small interfering RNA as a cancer
therapeutic agent has been explored extensively (72–74).
In addition, PLAC8 promotes tumor growth, invasion and metastasis
in other tumors, which could explain why patients with
well-differentiated CRC display different clinical outcomes, in
comparison with the patients with poorly-differentiated CRC
(17,22,75,76).
Therefore, the prognostic significance of KRT20 and PLAC8 could be
particularly essential for patients with CRC displaying
well-differentiated phenotypes.
Not applicable.
The present study was supported by the fund (grant
no. 2018 to Chi-Jung Huang) from The Department of Medical Research
of Cathay General Hospital.
All data generated or analyzed during this study are
included in this published article.
CSH, YCW and CYL designed the study. CSH and CYL
wrote the initial version of the manuscript. JWG and CCH performed
the cell studies. JWG, JYY and CYL performed the immunostaining and
pathologic diagnosis. RNY, CLL, MHS and CCH interpreted the patient
data regarding the well-differentiated and poorly-differentiated
CRC. CCH and CJH performed the statistical analyses. CYL provided
supervision throughout the study. All authors discussed, modified
and approved of the final version.
All procedures were approved by The Cathay General
Hospital Institutional Ethics Committee and a waiver of consent was
approved by the same committee.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Keighley MR: Gastrointestinal cancers in
europe. Aliment Pharmacol Ther. 18:7–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Sethi NS, Hinoue T, Schneider BG,
Cherniack AD, Sanchez-Vega F, Seoane JA, Farshidfar F, Bowlby R,
Islam M, et al: Comparative molecular analysis of gastrointestinal
adenocarcinomas. Cancer Cell. 33:721–735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vedeld HM, Andresen K, Eilertsen IA,
Nesbakken A, Seruca R, Gladhaug IP, Thiis-Evensen E, Rognum TO,
Boberg KM and Lind GE: The novel colorectal cancer biomarkers CDO1,
ZSCAN18 and ZNF331 are frequently methylated across
gastrointestinal cancers. Int J Cancer. 136:844–853. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moll R, Levy R, Czernobilsky B,
Hohlweg-Majert P, Dallenbach-Hellweg G and Franke WW: Cytokeratins
of normal epithelia and some neoplasms of the female genital tract.
Lab Invest. 49:599–610. 1983.PubMed/NCBI
|
|
5
|
Kim HS, Lee JJ, Do SI, Kim K, Do IG, Kim
DH, Chae SW and Sohn JH: Overexpression of cytokeratin 17 is
associated with the development of papillary thyroid carcinoma and
the presence of lymph node metastasis. Int J Clin Exp Pathol.
8:5695–5701. 2015.PubMed/NCBI
|
|
6
|
Osborn M, van Lessen G, Weber K, Kloppel G
and Altmannsberger M: Differential diagnosis of gastrointestinal
carcinomas by using monoclonal antibodies specific for individual
keratin polypeptides. Lab Invest. 55:497–504. 1986.PubMed/NCBI
|
|
7
|
Tang KD, Kenny L, Perry C, Frazer I and
Punyadeera C: The overexpression of salivary cytokeratins as
potential diagnostic biomarkers in head and neck squamous cell
carcinomas. Oncotarget. 8:72272–72280. 2017.PubMed/NCBI
|
|
8
|
Tot T: Cytokeratins 20 and 7 as
biomarkers: Usefulness in discriminating primary from metastatic
adenocarcinoma. Eur J Cancer. 38:758–763. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuchs E: Keratins as biochemical markers
of epithelial differentiation. Trends Genet. 4:277–281. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lipkin M: Biomarkers of increased
susceptibility to gastrointestinal cancer: New application to
studies of cancer prevention in human subjects. Cancer Res.
48:235–245. 1988.PubMed/NCBI
|
|
11
|
Mills JC and Sansom OJ: Reserve stem
cells: Differentiated cells reprogram to fuel repair, metaplasia,
and neoplasia in the adult gastrointestinal tract. Sci Signal.
8:re82015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou XZ, Liu T, Gong ZC, Hu CP and Zhang Z:
MicroRNAs-Mediated epithelial-mesenchymal transition in fibrotic
diseases. Eur J Pharmacol. 796:190–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serrano MJ, Ortega FG, Alvarez-Cubero MJ,
Nadal R, Sanchez-Rovira P, Salido M, Rodriguez M, Garcia-Puche JL,
Delgado-Rodriguez M, Sole F, et al: EMT and EGFR in CTCs
cytokeratin negative non-metastatic breast cancer. Oncotarget.
5:7486–7497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hinz S, Hendricks A, Wittig A, Schafmayer
C, Tepel J, Kalthoff H, Becker T and Roder C: Detection of
circulating tumor cells with CK20 RT-PCR is an independent negative
prognostic marker in colon cancer patients-a prospective study. BMC
Cancer. 17:532017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang WL, Liu YW, Dang YL, Jiang XX, Xu H,
Huang X, Wang YL, Wang H, Zhu C, Xue LQ, et al: PLAC8, a new marker
for human interstitial extravillous trophoblast cells, promotes
their invasion and migration. Development. 145:dev1489322018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li C, Ma H, Wang Y, Cao Z, Graves-Deal R,
Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, et al:
Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon
cancer. J Clin Invest. 124:2172–2187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miettinen M: Keratin 20:
Immunohistochemical marker for gastrointestinal, urothelial, and
merkel cell carcinomas. Mod Pathol. 8:384–388. 1995.PubMed/NCBI
|
|
20
|
Moll R, Divo M and Langbein L: The human
keratins: Biology and pathology. Histochem Cell Biol. 129:705–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rogulski K, Li Y, Rothermund K, Pu L,
Watkins S, Yi F and Prochownik EV: Onzin, a c-Myc-repressed target,
promotes survival and transformation by modulating the Akt-Mdm2-p53
pathway. Oncogene. 24:7524–7541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia Y, Ying X, Zhou J, Chen Y, Luo X, Xie
S, Wang QC, Hu W and Wang L: The novel KLF4/PLAC8 signaling pathway
regulates lung cancer growth. Cell Death Dis. 9:6032018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee CL, Huang CJ, Yang SH, Chang CC, Huang
CC, Chien CC and Yang RN: Discovery of genes from feces correlated
with colorectal cancer progression. Oncol Lett. 12:3378–3384. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang CJ, Lee CL, Yang SH, Chien CC, Huang
CC, Yang RN and Chang CC: Upregulation of the growth
arrest-specific-2 in recurrent colorectal cancers, and its
susceptibility to chemotherapy in a model cell system. Biochim
Biophys Acta. 1862:1345–1353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Washington MK, Berlin J, Branton P,
Burgart LJ, Carter DK, Fitzgibbons PL, Halling K, Frankel W, Jessup
J, Kakar S, et al: Protocol for the examination of specimens from
patients with primary carcinoma of the colon and rectum. Arch
Pathol Lab Med. 133:1539–1551. 2009.PubMed/NCBI
|
|
28
|
Duffy MJ, Lamerz R, Haglund C, Nicolini A,
Kalousova M, Holubec L and Sturgeon C: Tumor markers in colorectal
cancer, gastric cancer and gastrointestinal stromal cancers:
European group on tumor markers 2014 guidelines update. Int J
Cancer. 134:2513–2522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dolscheid-Pommerich RC, Manekeller S,
Walgenbach- Brunagel G, Kalff JC, Hartmann G, Wagner BS and
Holdenrieder S: Clinical performance of CEA, CA19-9, CA15-3, CA125
and AFP in gastrointestinal cancer using LOCI-based assays.
Anticancer Res. 37:353–359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar Y, Tapuria N, Kirmani N and Davidson
BR: Tumour M2-pyruvate kinase: A gastrointestinal cancer marker.
Eur J Gastroenterol Hepatol. 19:265–276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Myllykangas S, Bohling T and Knuutila S:
Specificity, selection and significance of gene amplifications in
cancer. Semin Cancer Biol. 17:42–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Myllykangas S, Himberg J, Bohling T, Nagy
B, Hollmen J and Knuutila S: DNA copy number amplification
profiling of human neoplasms. Oncogene. 25:7324–7332. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lukyanchuk VV, Friess H, Kleeff J, Osinsky
SP, Ayuni E, Candinas D and Roggo A: Detection of circulating tumor
cells by cytokeratin 20 and prostate stem cell antigen RT-PCR in
blood of patients with gastrointestinal cancers. Anticancer Res.
23:2711–2716. 2003.PubMed/NCBI
|
|
34
|
Dalerba P, Sahoo D, Paik S, Guo X, Yothers
G, Song N, Wilcox-Fogel N, Forgo E, Rajendran PS, Miranda SP, et
al: CDX2 as a prognostic biomarker in stage II and stage III colon
cancer. N Engl J Med. 374:211–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pilati C, Taieb J, Balogoun R, Marisa L,
de Reynies A and Laurent-Puig P: CDX2 prognostic value in stage
II/III resected colon cancer is related to CMS classification. Ann
Oncol. 28:1032–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh R, Kapur N, Mir H, Singh N, Lillard
JW Jr and Singh S: CXCR6-CXCL16 axis promotes prostate cancer by
mediating cytoskeleton rearrangement via Ezrin activation and
alphavbeta3 integrin clustering. Oncotarget. 7:7343–7353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu K, Zhang X, Li F, Xiao D, Hou Y, Zhu S,
Liu D, Ye X, Ye M, Yang J, et al: Frequent alterations in
cytoskeleton remodelling genes in primary and metastatic lung
adenocarcinomas. Nat Commun. 6:101312015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen NP, Uddin B, Voit R and Schiebel E:
Human phosphatase CDC14A is recruited to the cell leading edge to
regulate cell migration and adhesion. Proc Natl Acad Sci USA.
113:990–995. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou H, Zhang Y, Chen Q and Lin Y: AKT and
JNK signaling pathways increase the metastatic potential of
colorectal cancer cells by altering transgelin expression. Dig Dis
Sci. 61:1091–1097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mills KM, Brocardo MG and Henderson BR:
APC binds the Miro/Milton motor complex to stimulate transport of
mitochondria to the plasma membrane. Mol Biol Cell. 27:466–482.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ivanovska J, Zlobec I, Forster S,
Karamitopoulou E, Dawson H, Koelzer VH, Agaimy A, Garreis F, Soder
S, Laqua W, et al: DAPK loss in colon cancer tumor buds:
Implications for migration capacity of disseminating tumor cells.
Oncotarget. 6:36774–36788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zeng Y, Xie H, Qiao Y, Wang J, Zhu X, He
G, Li Y, Ren X, Wang F, Liang L and Ding Y: Formin-Like2 regulates
Rho/ROCK pathway to promote actin assembly and cell invasion of
colorectal cancer. Cancer Sci. 106:1385–1393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Asfaha S, Hayakawa Y, Muley A, Stokes S,
Graham TA, Ericksen RE, Westphalen CB, von Burstin J, Mastracci TL,
Worthley DL, et al: Krt19(+)/Lgr5(−) cells are radioresistant
cancer-initiating stem cells in the colon and intestine. Cell Stem
Cell. 16:627–638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li WX, Xiao HW, Hong XQ and Niu WX:
Predictive value of CK20 in evaluating the efficacy of treatment
and prognosis after surgery for colorectal cancer. Genet Mol Res.
14:5823–5829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Saad RS, Ismiil N, Dube V, Nofech-Mozes S
and Khalifa MA: CDX-2 expression is a common event in primary
intestinal-type endocervical adenocarcinoma. Am J Clin Pathol.
132:531–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bluemke K, Bilkenroth U, Meye A, Fuessel
S, Lautenschlaeger C, Goebel S, Melchior A, Heynemann H, Fornara P
and Taubert H: Detection of circulating tumor cells in peripheral
blood of patients with renal cell carcinoma correlates with
prognosis. Cancer Epidemiol Biomarkers Prev. 18:2190–2194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mizuuchi E, Semba S, Kodama Y and Yokozaki
H: Down-modulation of keratin 8 phosphorylation levels by PRL-3
contributes to colorectal carcinoma progression. Int J Cancer.
124:1802–1810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ding SJ, Li Y, Tan YX, Jiang MR, Tian B,
Liu YK, Shao XX, Ye SL, Wu JR, Zeng R, et al: From proteomic
analysis to clinical significance: Overexpression of cytokeratin 19
correlates with hepatocellular carcinoma metastasis. Mol Cell
Proteomics. 3:73–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hammoudi A, Song F, Reed KR, Jenkins RE,
Meniel VS, Watson AJ, Pritchard DM, Clarke AR and Jenkins JR:
Proteomic profiling of a mouse model of acute intestinal apc
deletion leads to identification of potential novel biomarkers of
human colorectal cancer (CRC). Biochem Biophys Res Commun.
440:364–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang SH, Huang CJ, Lee CL, Liu CC, Chien
CC and Chen SH: Fecal RNA detection of cytokeratin 19 and ribosomal
protein L19 for colorectal cancer. Hepatogastroenterology.
57:710–715. 2010.PubMed/NCBI
|
|
51
|
Yang RN, Yang SH, Chang CC, Chien CC, Pan
S and Huang CJ: Upregulation of fecal cytokeratin 19 is associated
with prognosis in older colorectal cancer patients. Genet Test Mol
Biomarkers. 14:703–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chang CC, Yang SH, Chien CC, Chen SH, Pan
S, Lee CL, Lin CM, Sun HL, Huang CC, Wu YY, et al: Clinical meaning
of age-related expression of fecal cytokeratin 19 in colorectal
malignancy. BMC Cancer. 9:3762009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kende AI, Carr NJ and Sobin LH: Expression
of cytokeratins 7 and 20 in carcinomas of the gastrointestinal
tract. Histopathology. 42:137–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Samija I, Lukac J, Mubrin MK, Kirac I,
Kovacevic D and Kusic Z: Detection of cytokeratin-20-positive cells
in preoperative and postoperative blood samples from colorectal
cancer patients by real-time RT-PCR. Int J Biol Markers.
28:174–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kojima S, Sakamoto T, Nagai Y, Honda M and
Ogawa F: Metachronous rectal metastasis from primary transverse
colon cancer: A case report. Surg Case Rep. 4:902018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Reisher SR, Hughes TE, Ordovas JM,
Schaefer EJ and Feinstein SI: Increased expression of
apolipoprotein genes accompanies differentiation in the intestinal
cell line Caco-2. Proc Natl Acad Sci USA. 90:5757–5761. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ferruzza S, Rossi C, Scarino ML and Sambuy
Y: A protocol for differentiation of human intestinal Caco-2 cells
in asymmetric serum-containing medium. Toxicol In Vitro.
26:1252–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Meunier V, Bourrie M, Berger Y and Fabre
G: The human intestinal epithelial cell line Caco-2;
pharmacological and pharmacokinetic applications. Cell Biol
Toxicol. 11:187–194. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Buhrke T, Lengler I and Lampen A: Analysis
of proteomic changes induced upon cellular differentiation of the
human intestinal cell line Caco-2. Dev Growth Differ. 53:411–426.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Allegra C and Sargent D: Molecular
diagnostics: Assays, tissues, progress, and pitfalls. J Clin Oncol.
21:395–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bustin SA and Dorudi S: Gene expression
profiling for molecular staging and prognosis prediction in
colorectal cancer. Expert Rev Mol Diagn. 4:599–607. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bardelli A and Velculescu VE: Mutational
analysis of gene families in human cancer. Curr Opin Genet Dev.
15:5–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rozen P: Cancer of the gastrointestinal
tract: Early detection or early prevention? Eur J Cancer Prev.
13:71–75. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vogelstein B, Fearon ER, Hamilton SR, Kern
SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM and Bos
JL: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Imai Y, Yamagishi H, Fukuda K, Okamura T,
Ono Y, Ban S, Inoue T and Ueda Y: Expression of cytokeratin 20
indicates invasive histological phenotype in poorly differentiated
colorectal adenocarcinoma. Anticancer Res. 34:159–167.
2014.PubMed/NCBI
|
|
70
|
Barr T, Sureshchandra S, Ruegger P, Zhang
J, Ma W, Borneman J, Grant K and Messaoudi I: Concurrent gut
transcriptome and microbiota profiling following chronic ethanol
consumption in nonhuman primates. Gut Microbes. 9:338–356.
2018.PubMed/NCBI
|
|
71
|
Turroni S, Vitali B, Candela M, Gionchetti
P, Rizzello F, Campieri M and Brigidi P: Antibiotics and probiotics
in chronic pouchitis: A comparative proteomic approach. World J
Gastroenterol. 16:30–41. 2010.PubMed/NCBI
|
|
72
|
Singh A, Trivedi P and Jain NK: Advances
in siRNA delivery in cancer therapy. Artif Cells Nanomed
Biotechnol. 46:274–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Das M, Musetti S and Huang L: RNA
interference-based cancer drugs: The roadblocks, and the ‘Delivery’
of the promise. Nucleic Acid Ther. 29:61–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng M, Tao W, Zou Y, Farokhzad OC and
Shi B: Nanotechnology-based strategies for siRNA brain delivery for
disease therapy. Trends Biotechnol. 36:562–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bukholm IR, Bondi J, Wiik P, Nesland JM,
Andersen SN, Bakka A and Bukholm G: Presence of isolated tumour
cells in mesenteric lymph nodes predicts poor prognosis in patients
with stage II colon cancer. Eur J Surg Oncol. 29:862–866. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang Y, Hu Q, Li G, Li L, Liang S, Zhang
Y, Liu J, Fan Z, Li L, Zhou B, et al: ONZIN upregulation by mutant
p53 contributes to osteosarcoma metastasis through the CXCL5-MAPK
signaling pathway. Cell Physiol Biochem. 48:1099–1111. 2018.
View Article : Google Scholar : PubMed/NCBI
|