Introduction
Lung cancer is the biggest cause of
cancer-associated mortality in men and is the second biggest cause
of cancer-associated mortality in women worldwide (1). In the USA, there were ~121,680 new
cases of lung cancer in men and ~112,350 in women in 2018 (2). There are two main histological types
of lung cancer: Non-small cell lung cancer (NSCLC) and small cell
lung cancer (SCLC). Overall, 85–90% of all lung cancer cases are
NSCLC (3). The 5-year survival
rate of NSCLC is 55% at an initial stage; however, this reduces to
<4% with distant metastasis (2). Therefore, it is necessary to
characterize the mechanisms involved in NSCLC progression.
NF-κB is a pro-inflammatory transcription factor,
which binds with the inhibitory molecule nuclear factor of κ light
polypeptide gene enhancer in B cells inhibitor (IκB) a in the
majority of cells. NF-κB can be activated by stimulating factors,
including carcinogens and inflammatory proteins, which results in
IκBa phosphorylation and degradation, leading to the release and
nuclear translocation of NF-κB dimers (4). Subsequently, the activated NF-κB
promotes tumor cell division, proliferation, angiogenesis and
metastasis, and also prevents tumor cell apoptosis, which leads to
the development of carcinomas (5).
Therefore, activated NF-κB should suppressed in cancer treatment.
The PI3K/Akt pathway serves a key role in carcinogenesis and has
been identified as constitutively activated in different types of
human cancer (6). The PI3K/Akt
cell survival pathway also plays a key role in the regulation of
apoptotic cell death via NF-κB.
Natural products with potential anticancer effects
are an important source for the identification of novel therapeutic
drugs (7). As a traditional
Chinese medicine with heat-clearing and detoxicating functions
(8,9), Paris polyphylla (Chonglou in
Chinese) is a medicinal herb listed in the pharmacopoeia in China
(10), which has been used for the
treatment of inflammation and cancer, particularly lung cancer.
Polyphyllin VII is an active steroid saponin isolated from P.
polyphylla (11).
The present study was designed to investigate the
cell growth inhibitory effects of Polyphyllin VII on A549 cells and
the mechanisms of Polyphyllin VII-induced apoptotic cell death. The
mechanisms involved were investigated by phase-contrast microscopy,
fluorescence microscopy, flow cytometry and western blot
analysis.
Materials and methods
Chemicals and reagents
Fetal bovine serum (FBS) and RPMI-1640 medium were
obtained from HyClone (GE Healthcare Life Sciences). Acridine
orange (AO), Hoechst 33258, ammonium pyrrolidinedithiocarbamate
(PDTC) and rhodamine 123 were purchased from Sigma-Aldrich (Merck
KGaA). Wortmannin was purchased from MedChem Express. Polyclonal
antibodies against PI3K (cat. no. 20584-1-AP), Akt (cat. no.
10176-2-AP), NF-κB p65 (cat. no. 10745-1-AP), IκB (cat. no.
10268-1-AP), GAPDH (cat. no. 10494-1-AP), lamin B (cat. no.
12987-1-AP), caspase-3 (cat. no. 19677-1-AP), poly-(ADP-ribose)
polymerase (PARP; cat. no. 13371-1-AP) and inhibitor of
caspase-activated DNase (ICAD; cat. no. 10191-2-AP) were obtained
from ProteinTech Group, Inc. Phosphorylated (p)-PI3K (cat. no.
4228), p-Akt (cat. no. 4060) and p-NF-κB p65 (cat. no. 3033)
antibodies were from Cell Signaling Technology, Inc. Horseradish
peroxidase (HRP)-conjugated secondary antibodies (whole IgG
affinity-purified antibodies, cat. no. 115-035-003) were obtained
from Jackson ImmunoResearch Laboratories, Inc. Enhanced
chemiluminescence (ECL) western blotting substrate was from Thermo
Fisher Scientific, Inc., and the Annexin V-FITC/propidium iodide
(PI) staining kit was from 7 Sea Biotech, Inc.
Plant material
Polyphyllin VII was purchased from Chengdu Pufei De
Biotech Co., Ltd. (cat. no. 76296-75-8). Polyphyllin VII was
dissolved in DMSO and diluted with RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences). The DMSO concentration was kept
<0.05% in all cell cultures and did not exert any detectable
effect on cell growth.
Cell culture
Human lung cancer A549 cells were provided by Stem
Cell Bank, Chinese Academy of Sciences (batch no. SCSP-503). Cells
were cultured in RPMI-1640 medium supplemented with 10% FBS, 100
U/ml penicillin and 100 µg/ml streptomycin in a humidified
atmosphere with 5% CO2 at 37°C. Cells in the exponential
phase of growth were used in the experiments.
Cell viability assay
A549 cells were seeded in 96-well flat bottom cell
culture clusters (Corning, Inc.) with 100 µl per well at a density
of 6 ×104 cells/ml. Following culture for 24 h, the
cells were treated with increasing concentrations (0, 0.1, 0.2,
0.4, 0.8 and 1.6 µM) of Polyphyllin VII for 24 h at 37°C.
Alternatively, the cells were treated with 0.41 µM Polyphyllin VII
in the presence or absence of 2 µM wortmannin and 30 µM PDTC for 24
h at 37°C. Subsequently, the cells were washed twice with ice-cold
PBS and incubated with 5 mg/ml MTT solution at 37°C for 4 h. The
medium was then removed and 150 µl DMSO was added to dissolve the
resulting crystals. The optical density was measured using a
microplate reader at 492 nm (Multiskan GO; Thermo Fisher
Scientific, Inc.). The percentage of cell viability was calculated
as follows:
Cell viability
(%)=(A492sample-A492blank)/(A492control-A492blank)
×100.
Observation of morphological changes
and fluorescence microscopy of apoptosis with AO and Hoechst 33258
staining
A549 cells were seeded into 24-well culture plates
(Corning, Inc.) at a density of 2×104 cells/well with or
without Polyphyllin VII (0.41 µM) for 24 h at 37°C. The cellular
morphology changes were observed using a phase-contrast microscope
(Olympus Corporation). Following treatment with Polyphyllin VII,
the cells were stained with 20 µg/ml AO and incubated in the dark
for 15 min at room temperature. For Hoechst 33258 staining, the
medium was removed following treatment with Polyphyllin VII for 24
h, the cells were fixed with 0.5 ml 70% ethanol at 4°C for 30 min,
then washed twice with PBS and incubated with 0.5 ml 10 µg/ml
Hoechst 33258 solution for 5 min at room temperature. All changes
in fluorescence were observed using an Olympus IX73 inverted
fluorescence microscope (Olympus Corporation).
Nuclear protein extracts
preparation
A549 cells were treated with 0.41 µM Polyphyllin VII
under the indicated conditions, then they were harvested and
suspended in cold PBS. Following centrifugation at 1,000 × g for 15
min at 4°C, the cells were resuspended in lysis buffer A (20 mM
HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1
mM DTT and 1 mM PMSF) at 4°C for 60 min. Following centrifugation
at 28,000 × g for 20 min at 4°C, the pellet was resuspended in
lysis buffer B (20 mM HEPES, 25% glycerol, 420 mM NaCl, 1.5 mM
MgCl2, 0.2 mM EDTA, 0.5 mM PMSF, 0.5 mM DTT and 5 µg/ml
leupeptin) at 4°C for 15 min. Finally, after centrifugation at
16,000 × g for 10 min at 4°C, the nuclear proteins were identified
in the supernatant.
Western blot analysis
A549 cells were treated with 0.41 µM Polyphyllin VII
for 0, 12, 24, and 48 h, or were treated with Polyphyllin VII in
the presence or absence of 2 µM wortmannin and 30 µM PDTC for 24 h
at 37°C. Subsequently, the cells were harvested and lysed in RIPA
lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 0.1% SDS, 2 mM EDTA and 100 mM sodium
fluoride) at 4°C for 60 min. Lysates were centrifuged at 12,000 × g
for 15 min at 4°C, the supernatant was pipetted and the protein
content was determined using a Bio-Rad protein assay (Bio-Rad
Laboratories, Inc.). Equal amounts of total protein (30 µg) were
separated by 12% SDS-PAGE and then transferred to an
Immobilon®-P transfer membrane (EMD Millipore). The
membrane was then incubated with blocking buffer (5% skimmed milk
in 0.5% Tween-20) for 2 h at room temperature. Proteins were
detected with primary antibodies against PI3K (1:500), p-PI3K
(1:500), Akt (1:500), p-Akt (1:500), NF-κB p65 (1:500), p-NF-κB p65
(1:500), IκB (1:500), GAPDH (1:500), lamin B (1:500), caspase-3
(1:500), PARP (1:500) and ICAD (1:500), incubated at 4°C for 12 h,
followed by HRP-conjugated secondary antibody (1:20,000), incubated
at room temperature for 2 h. The proteins were visualized using
enhanced chemiluminescence reagent (Thermo Fisher Scientific, Inc.)
with Image Lab software (version 5.2.1, Bio-Rad Laboratories,
Inc.).
Flow cytometric analysis of
apoptosis
Human A549 lung cancer cells were cultured in a
25-cm2 culture bottle (Corning Inc.) at a density of
4×105 cells/bottle for 24 h. Subsequently, the cells
were treated with 0.41 µM Polyphyllin VII for 24 h in the presence
or absence of 2 µM wortmannin or 30 µM PDTC at 37°C. Thereafter,
the cells were harvested using trypsin (without EDTA), and rinsed
with PBS. The cells were then stained with Annexin V-FITC/PI using
a commercial kit, following the manufacturer's protocol. Finally,
cells were analyzed using an Accuri C6 FACScan flow cytometer
(Becton, Dickinson and Company) with CFlow Plus software (version
1.0.264.15, Accuri Cytometers, Inc.).
Determination of mitochondrial
membrane potential
The mitochondrial membrane potential (Δψm) was
examined using the mitochondrial dye rhodamine 123. Following
incubation with 0.41 µM Polyphyllin VII for 24 h in the presence or
absence of 2 µM wortmannin or 30 µM PDTC, A549 cells were collected
and stained with 1 µg/ml rhodamine 123 at 37°C for 30 min.
Subsequently, the fluorescence intensity was measured by flow
cytometric analysis and the results were analyzed by CFlow Plus
software (version 1.0.264.15, Accuri Cytometers, Inc.).
Statistical analysis
All the presented data and results were confirmed in
at least three independent experiments and are presented as the
mean ± standard deviation. Statistical comparisons were made by
Student's t-test or one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
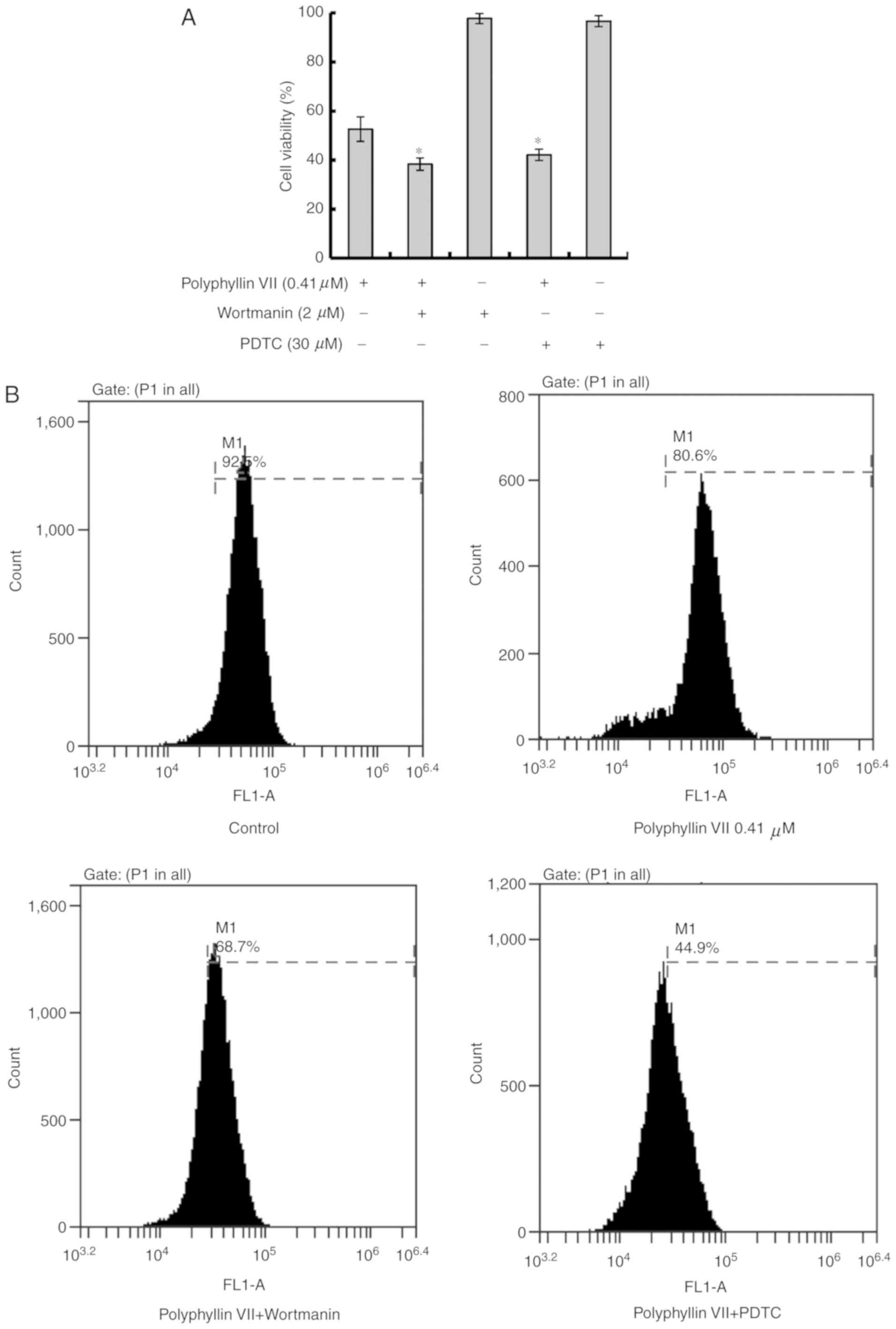
Polyphyllin VII suppresses the
viability of human lung cancer A549 cells
A549 cells were cultured with 0, 0.1, 0.2, 0.4, 0.8,
1.6 µM Polyphyllin VII for 24 h, and the cell viability was
analyzed by MTT assay. Polyphyllin VII decreased the numbers of
viable cells in a concentration-dependent manner, with an
IC50 value at 24 h of 0.41±0.10 µM (Fig. 1A). The morphological changes of
A549 cells were examined to evaluate the features of the decreased
cell viability. The Polyphyllin VII-treated group exhibited
membrane blebbing and granular apoptotic bodies (Fig. 1B). Fluorescence microscopy with AO
staining also showed granular apoptotic bodies (Fig. 1C), and Hoechst 33258 staining
revealed significant nuclear condensation or nuclear fragmentation
(Fig. 1D) following Polyphyllin
VII treatment. These results indicated that Polyphyllin VII
suppressed human lung cancer A549 cell proliferation and that it
may have induced apoptosis.
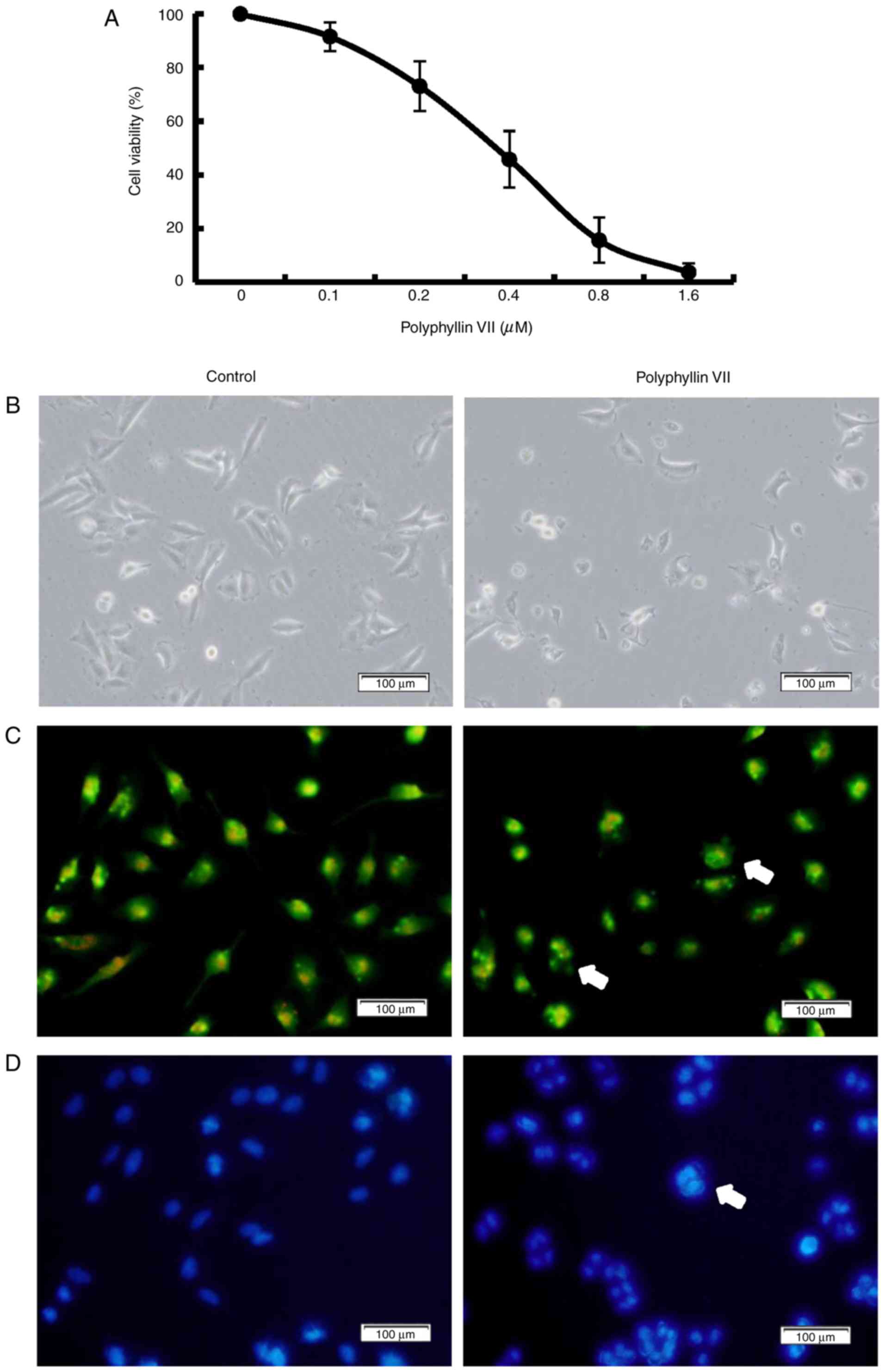 | Figure 1.Polyphyllin VII decreases the
viability of A549 Cells. (A) Cells were treated with 0, 0.1, 0.2,
0.4, 0.8, 1.6 µM Polyphyllin VII for 24 h, and the cell viability
was measured by MTT assay (mean ± standard deviation of 3
independent experiments). (B) A549 cells were incubated with 0.41
µM Polyphyllin VII for 24 h, and the cellular morphological changes
were observed by phase-contrast microscopy (magnification ×100;
scale bar, 100 µm). (C) Fluorescence microscopy following AO
staining (magnification ×100; scale bar, 100 µm) or (D) Hoechst
33258 staining (magnification ×100; scale bar, 100 µm). Arrows
indicate apoptotic bodies in AO staining and DNA condensation in
Hoechst 33258 staining. The experiments were performed in
triplicate. AO, acridine orange. |
Polyphyllin VII induces apoptotic
death in A549 cells
Subsequently, the mechanism of Polyphyllin
VII-induced cell inhibition was investigated. A549 cells were
pretreated with 2 µM wortmannin (a PI3K inhibitor) or 30 µM PDTC (a
NF-κB inhibitor) prior to treatment with Polyphyllin VII. The
concentrations of these inhibitors were determined according to
previous studies and were screened (12,13).
The cell viability of the Polyphyllin VII-treated group was
markedly decreased by co-incubation with wortmannin or PDTC
(Fig. 2A).
It is understood that the integrity of mitochondrial
membranes is associated with apoptotic cell death; therefore, the
mitochondrial membrane potential (Δψm) was detected by flow
cytometric analysis following rhodamine 123 staining. Polyphyllin
VII-treated A549 cells exhibited a decreased fluorescence intensity
due a decrease in Δψm. In addition, this effect was enhanced by
both wortmannin and PDTC combination treatments (Fig. 2B; Table I).
 | Table I.Δψm measurements in A549 cells
following treatments. |
Table I.
Δψm measurements in A549 cells
following treatments.
| Group | Δψm |
|---|
| Control | 93.30±2.21 |
| Polyphyllin VII | 76.60±3.67 |
| Polyphyllin VII +
Wortmanin |
64.23±4.15a |
| Polyphyllin VII +
PDTC |
49.53±5.16a |
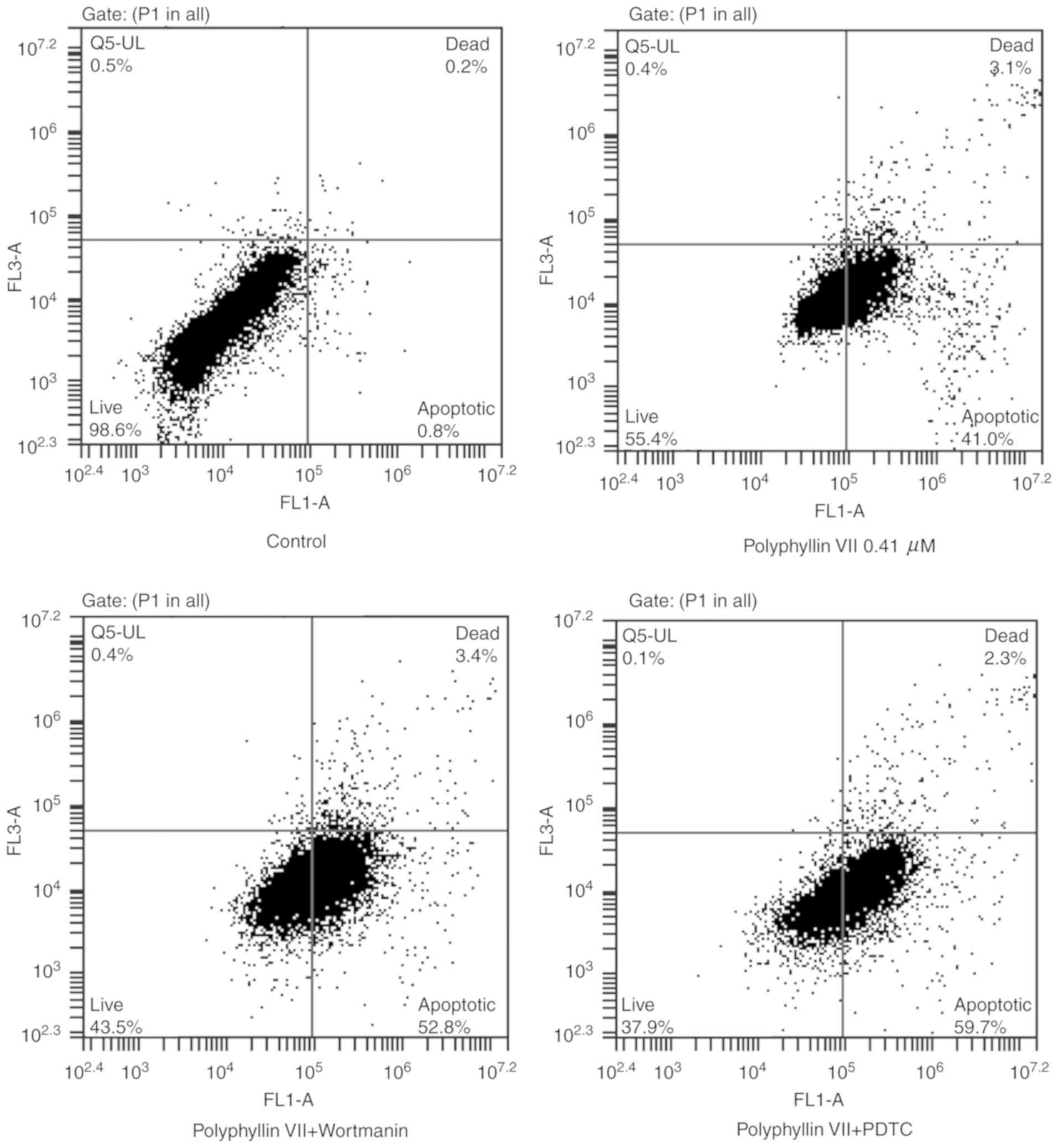
An Annexin V-FITC/PI staining assay was used to
further explore the mechanism underlying the Polyphyllin
VII-induced inhibition of A549 cells. Flow cytometric analysis
revealed a significant increase in the percentage of early
apoptotic cells (Annexin V staining only; lower right panels) and
late apoptotic cells (Annexin V and PI staining; upper right
panels) in the Polyphyllin VII-treated group compared with the
control group (Fig. 3; Table II). The ratio of apoptotic cells
in the Polyphyllin VII-treated group was further increased
following incubation with wortmannin or PDTC (Fig. 3; Table II). In summary, these results
demonstrated that Polyphyllin VII induced apoptotic death in A549
cells.
 | Table II.Apoptotic ratios in A549 cells
following treatments. |
Table II.
Apoptotic ratios in A549 cells
following treatments.
| Group | Annexin
V-FITC+/PI− | Annexin
V-FITC+/PI+ |
|---|
| Control | 0.50±0.36 | 0.10±0.10 |
| Polyphyllin
VII | 41.20±2.81 | 3.70±1.49 |
| Polyphyllin VII +
Wortmanin |
52.93±2.70a | 3.03±1.00 |
| Polyphyllin VII +
PDTC |
61.40±4.40a | 3.30±2.09 |
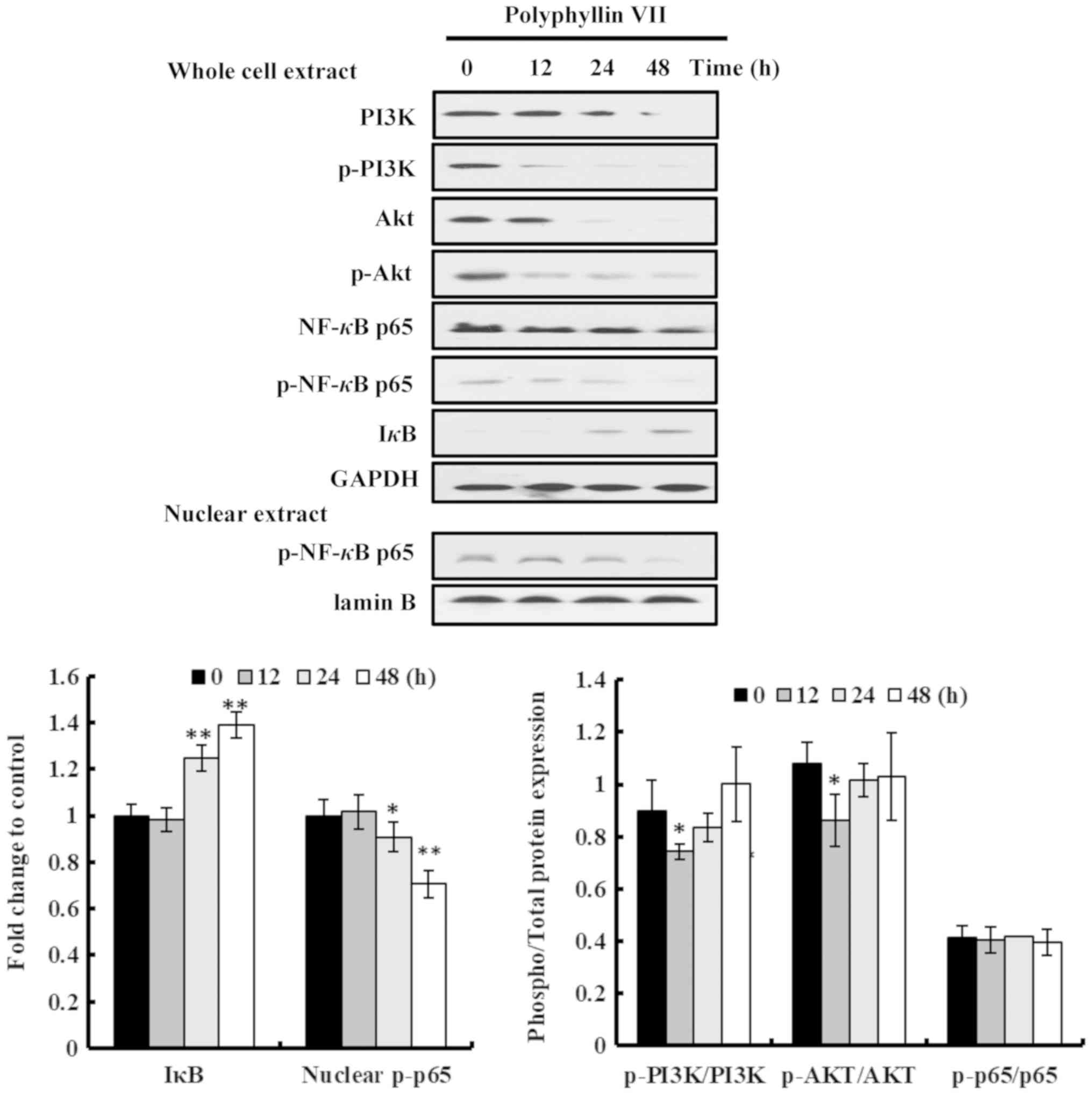
Polyphyllin VII induces apoptotic
death via the PI3K/Akt and NF-κB pathways in A549 cells
Wortmannin and PDTC demonstrated the same phenotypic
effects when incubated with Polyphyllin VII in A549 cells,
suggesting that the PI3K/Akt and NF-κB pathways may be involved in
the role of Polyphyllin VII. The protein expression levels in
Polyphyllin VII-treated A549 cells were measured by western blot
analysis. The results indicated that Polyphyllin VII treatment
induced marked changes in the protein expression of A549 cells.
PI3K, p-PI3K, Akt, p-Akt, NF-κB p65 and p-NF-κB p65 were obviously
downregulated, while IκB was upregulated, in a time-dependent
manner in Polyphyllin VII-treated A549 cells (Fig. 4). Of note, there were no
significant changes in the p-PI3K/PI3K, p-Akt/Akt, p-NF-κB
p65/NF-κB p65 ratios between most of the treatment groups (Fig. 4), suggesting that Polyphyllin VII
downregulated the total expression levels of these proteins rather
than their phosphorylation. The p-NF-κB p65 levels in the nuclear
extract of A549 cells were also downregulated following Polyphyllin
VII-treatment (Fig. 4).
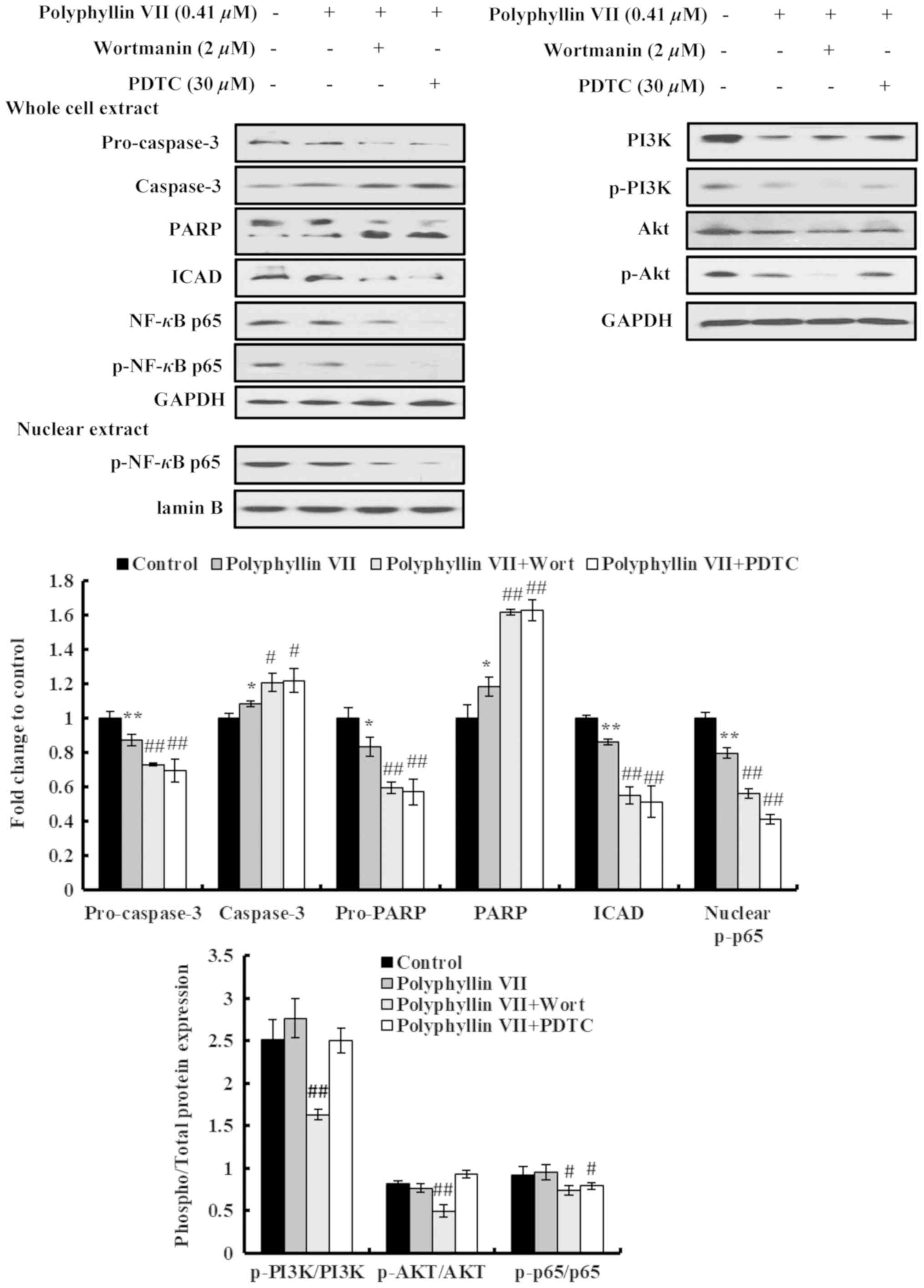
Furthermore, the effects of Polyphyllin VII on the expression
levels of apoptosis-related proteins in A549 cells were also
examined by western blot analysis. Polyphyllin VII treatment
induced significant changes in the expression levels of
apoptosis-related proteins, including increased activation of
cleaved pro-caspase-3 and PARP, and a downregulation of ICAD, which
causes a release of caspase-activated DNase and triggers DNA
fragmentation in nuclei (Fig.
5).
Next, the effects of the combination treatments were
investigated in the protein expression levels of the A549 cells.
Cleavage of pro-caspase-3 and PARP in Polyphyllin VII-treated A549
cells was further increased following cotreatment with wortmannin
or PDTC (Fig. 5). In addition, the
inhibitory effect of Polyphyllin VII on ICAD expression was further
enhanced by wortmannin and PDTC (Fig.
5). Although no significant differences were observed in the
ratios of p-PI3K/PI3K and p-Akt/Akt following Polyphyllin VII
treatment, these p-PI3K/PI3K and p-Akt/Akt ratios were
significantly suppressed by wortmannin pretreatment; however, no
significant effect was observed by PDTC pre-treatment (Fig. 5). The p-NF-κB p65/NF-κB p65, ratio
was significantly decreased by both PDTC and wortmannin
pretreatments (Fig. 5). Similarly,
the levels of nuclear p-NF-κB p65 were significantly decreased by
both the PDTC and wortmannin pretreatments, compared with the
Polyphyllin VII treatment alone (Fig.
5). In summary, these data indicated that Polyphyllin VII
induced apoptotic death in A549 cells via downregulation of the
proteins in the PI3K/Akt and NF-κB pathways.
Discussion
Our previous study demonstrated that P.
polyphylla steroidal saponins induce apoptotic death in A549
cells (14). Polyphyllin VII, a
type of steroidal saponin, was initially isolated from P.
polyphylla. The present study demonstrated that Polyphyllin VII
induced apoptosis in A549 human lung cancer cells via the PI3K/Akt
and NF-κB pathways.
In total, >50% of anticancer drugs are designed
based on natural products. Numerous natural products have been
reported to exert anticancer effects by inducing apoptosis of
cancer cells (15). For example,
Polyphyllin VII, a natural product from P. polyphylla, has
been reported to exhibit anticancer effects (16). The present study identified that
Polyphyllin VII was able to induce apoptotic death in A549 human
lung cancer cells via downregulation of PI3K and Akt and inhibition
of NF-κB. A previous study reported that Polyphyllin VII induced
autophagic cell death by activation of the JNK pathway and
inhibition of the PI3K/Akt/mTOR pathway in human liver cancer HepG2
cells (16). Additionally, it has
been suggested that Polyphyllin VII exerts anticancer effects by
inducing cell cycle arrest and apoptosis in A549 cells; however,
the underlying mechanism remains to be elucidated (17). Polyphyllin VII also induces
apoptosis and autophagy via activation of ERK, Akt, p38 and JNK in
oral cancer cells, which demonstrates that the biological effect of
Polyphyllin VII is associated with Akt activation (18,19).
The present study revealed that the Polyphyllin VII-induced
apoptotic cell death of A549 cells was associated with a
downregulation of the PI3K/Akt pathway.
The PI3K/Akt signaling pathway is closely associated
with programmed cell death, cell proliferation, migration and
differentiation, and serves central roles in tumorigenesis and cell
survival (20). The overexpression
of PI3K and Akt in numerous types of human cancer is significantly
associated with poor overall survival. Akt inhibits cell apoptosis
and activates effector molecules, including NF-κB. Furthermore,
PI3K/Akt signaling affects the expression of Bcl-2 protein, which
is associated with the mitochondrial membrane potential and the
mitochondrial apoptotic pathway (21,22).
In the present study, it was identified that in A549 cells,
Polyphyllin VII reduced the expression levels of PI3K and Akt,
causing a decrease in the mitochondrial membrane potential, and
inducing apoptotic death.
Polyphyllin VII also exerts anti-inflammatory
activities in vitro and in vivo, via a downregulation
of the MAPK and NF-κB pathways (23), which indicates that the effects of
Polyphyllin VII may be associated with the NF-κB pathway. The NF-κB
transcription factor family is extensively involved in numerous
biological processes, including inflammation, immunity, cellular
proliferation and the suppression of apoptosis (24,25).
Phosphorylation of the NF-κB p65 subunit promotes transcriptional
activity of NF-κB, followed by a regulation of the release of
pro-inflammatory cytokines (26).
NF-κB p65 regulates Bcl-2 and Bcl-xl, which localize to the outer
membrane of mitochondria, suppressing the expression of Bax and
Bak. Bax and Bak facilitate a decrease of the mitochondrial
membrane potential and the release of cytochrome c, which triggers
apoptotic cell death (22,27).
The current study identified that Polyphyllin
VII-treatment inhibited the expression of proteins associated with
PI3K/Akt signaling, which resulted in the suppression of NF-κB p65.
Wortmannin, a PI3K inhibitor, combined with Polyphyllin VII,
significantly enhanced the suppression of NF-κB p65 and increased
the apoptosis of A549 cells. Similarly, PDTC, a NF-κB inhibitor,
enhanced the suppression of NF-κB p65 and increased apoptosis;
however, PDTC exhibited no prominent effects on PI3K/Akt signaling
in Polyphyllin VII-treated A549 cells. These results demonstrated
that Polyphyllin VII may be effective against lung cancer A549
cells. Of note, when the NCI-H460 and SK-MES-1 lung cancer cell
lines were used in the present study, different mechanisms were
obvious for the Polyphyllin VII treatment in the different cell
lines (data not shown); therefore, the exact mechanism of
Polyphyllin VII in lung cancer remains under research and will be
further explored in future studies. Polyphyllin VII also exhibits
its effects in combination with other drugs in research on cell
lines. Polyphyllin VII has been reported to increase sensitization
to gefitinib in gefitinib-resistant NSCLC cells via G1 phase arrest
and modulation of the p21 signaling pathway (28). In addition, formosanin C and
Polyphyllin VII have demonstrated a synergistic antitumor effect on
lung cancer cells (29). These
findings may provide a potential strategy to overcome drug
resistance in NSCLC by using a combination of drugs with
Polyphyllin VII.
In summary, it can be concluded that treatment with
Polyphyllin VII was effective against lung cancer A549 cells via
PI3K/Akt-mediated suppression of NF-κB p65 activity, which resulted
in mitochondrial dysfunction and apoptosis. In the future, with
further understanding of its mechanism, Polyphyllin VII might serve
as a potential candidate for the treatment of NSCLC, either alone
or in combination with other compounds.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81603265), the
Innovative Talents Promotion Plan in Shaanxi Province (grant no.
2019KJXX-057), the Shaanxi Natural Science Basic Research Project
(grant no. 2017JQ8042) and the foundation of Xi'an medical
university (grant nos. 2017PT03 and 05041904).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH conceived and designed the study, and also
reviewed and edited the manuscript. CX performed experiments and
wrote the paper. LZ performed data analysis. KW, MJ, YS and ZY
performed experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
Cancer Statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajasinghe LD, Pindiprolu RH and Gupta SV:
Delta-tocotrienol inhibits non-small-cell lung cancer cell invasion
via the inhibition of NF-κB, uPA activator, and MMP-9. Onco Targets
Ther. 11:4301–4314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rasheduzzaman M, Jeong JK and Park SY:
Resveratrol sensitizes lung cancer cell to TRAIL by p53 independent
and suppression of Akt/NF-κB signaling. Life Sci. 208:208–220.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang J, Xu Y, Ren H, Wudu M, Wang Q, Song
X, Su H, Jiang X, Jiang L and Qiu X: MKRN2 inhibits migration and
invasion of non-small-cell lung cancer by negatively regulating the
PI3K/Akt pathway. J Exp Clin Cancer Res. 37:1892018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu X, Deng Y, Chen J, Hu Q, He C, Jordan
JB and Zhong S: Screening study on the anti-angiogenic effects of
traditional Chinese medicine-part I: Heat-clearing and detoxicating
TCM. J Ethnopharmacol. 194:280–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo J, Gao Y, Wang Y, Liu WJ, Zhou J and
Wang Z: Application of herbal medicines with heat-clearing property
to anti-microinflammation in the treatment of diabetic kidney
disease. Evid Based Complement Alternat Med. 2019:61743502019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Committee CP: Pharmacopoeia of The
People's Republic of China China Medical Science Press; Beijing:
2015, PubMed/NCBI
|
|
11
|
Yin X, Qu C, Li Z, Zhai Y, Cao S, Lin L,
Feng L, Yan L and Ni J: Simultaneous determination and
pharmacokinetic study of polyphyllin I, polyphyllin II, polyphyllin
VI and polyphyllin VII in beagle dog plasma after oral
administration of Rhizoma Paridis extracts by LC-MS-MS. Biomed
Chromatogr. 27:343–348. 2013.PubMed/NCBI
|
|
12
|
He H, Zang LH, Feng YS, Chen LX, Kang N,
Tashiro S, Onodera S, Qiu F and Ikejima T: Physalin A induces
apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays
a protective role against apoptosis through p38-NF-κB survival
pathway in A375-S2 cells. J Ethnopharmacol. 148:544–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Otkur W, Li L, Wang Q, He H, Ye Y,
Zhang Y, Hayashi T, Tashiro S, Onodera S and Ikejima T: Autophagy
induced by silibinin protects human epidermoid carcinoma A431 cells
from UVB-induced apoptosis. J Photochem Photobiol B. 123:23–31.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He H, Sun YP, Zheng L and Yue ZG:
Steroidal saponins from Paris polyphylla induce apoptotic cell
death and autophagy in A549 human lung cancer cells. Asian Pac J
Cancer Prev. 16:1169–1173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Zhong J, Bai J, Tong R, An F, Jiao
P, He L, Zeng D, Long E, Yan J, et al: The application of natural
products in cancer therapy by targeting apoptosis pathways. Curr
Drug Metab. 19:739–749. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang C, Jia X, Wang K, Bao J, Li P, Chen
M, Wan JB, Su H, Mei Z and He C: Polyphyllin VII induces an
autophagic cell death by activation of the JNK pathway and
inhibition of PI3K/AKT/mTOR pathway in HepG2 cells. PLoS One.
11:e01474052016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin Z, Liu Y, Li F, Wu J, Zhang G, Wang Y,
Lu L and Liu Z: Anti-lung cancer effects of polyphyllin VI and VII
potentially correlate with apoptosis in vitro and in vivo.
Phytother Res. 29:1568–1576. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen JC, Hsieh MJ, Chen CJ, Lin JT, Lo YS,
Chuang YC, Chien SY and Chen MK: Polyphyllin G induce apoptosis and
autophagy in human nasopharyngeal cancer cells by modulation of AKT
and mitogen-activated protein kinase pathways in vitro and in vivo.
Oncotarget. 7:70276–70289. 2016.PubMed/NCBI
|
|
19
|
Hsieh MJ, Chien SY, Lin JT, Yang SF and
Chen MK: Polyphyllin G induces apoptosis and autophagy cell death
in human oral cancer cells. Phytomedicine. 23:1545–1554. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarris EG, Saif MW and Syrigos KN: The
biological role of PI3K pathway in lung cancer. Pharmaceuticals
(Basel). 5:1236–1264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Catz SD and Johnson JL: Transcriptional
regulation of bcl-2 by nuclear factor kappa B and its significance
in prostate cancer. Oncogene. 20:7342–7351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang C, Li C, Jia X, Wang K, Tu Y, Wang
R, Liu K, Lu T and He C: In vitro and in vivo anti-inflammatory
effects of polyphyllin VII through downregulating MAPK and NF-κB
pathways. Molecules. 24:E8752019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Panday A, Inda ME, Bagam P, Sahoo MK,
Osorio D and Batra S: Transcription Factor NF-κB: An Update on
Intervention Strategies. Arch Immunol Ther Exp (Warsz). 64:463–483.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schuliga M: NF-kappaB signaling in chronic
inflammatory airway disease. Biomolecules. 5:1266–1283. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen T, Wang R, Jiang W, Wang H, Xu A, Lu
G, Ren Y, Xu Y, Song Y, Yong S, et al: Protective effect of
astragaloside IV against paraquat-induced lung injury in mice by
suppressing Rho signaling. Inflammation. 39:483–492. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zamora M, Meroño C, Viñas O and Mampel T:
Recruitment of NF-kappaB into mitochondria is involved in adenine
nucleotide translocase 1 (ANT1)-induced apoptosis. J Biol Chem.
279:38415–38423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Fei Z and Jiang H: Polyphyllin VII
increases sensitivity to gefitinib by modulating the elevation of
P21 in acquired gefitinib resistant non-small cell lung cancer. J
Pharmacol Sci. 134:190–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui J, Man S, Cui N, Yang L, Guo Q, Ma L
and Gao W: The synergistic anticancer effect of formosanin C and
polyphyllin VII based on caspase-mediated cleavage of Beclin1
inhibiting autophagy and promoting apoptosis. Cell Prolif.
52:e125202019. View Article : Google Scholar : PubMed/NCBI
|