Introduction
Solar ultraviolet (UV) radiation is harmful to the
skin (1). Previous studies have
demonstrated that sustained or excessive exposure to UV radiation
leads to the development of several skin pathologies, including
skin inflammation, aging and cancer (2–4). In
addition, UV-induced skin inflammation and damage, such as
wrinkles, are closely associated with altered expression of
inflammation-related enzymes and proteins, such as cyclooxygenase
(COX)-2, matrix metallopeptidases (MMPs) and type 1 collagen
(5–7). Thus, any reagent that alters the
expression of COX-2, MMPs and type 1 collagen induced by UV
radiation in skin cells may be used as a potential
anti-inflammatory agent.
COX-2 is an inducible enzyme that is mainly involved
in the hyperproduction of prostaglandins (PGs) from arachidonic
acid (8). COX-2-derived PGs
mediate a number of inflammatory diseases (9), thus supporting the role of COX-2 and
PGs in inflammation. Previous studies have suggested that COX-2
expression is significantly increased in multiple types of cells
and tissues that have been exposed to internal or external stimuli,
such as inflammatory cytokines, mitogenic factors, tumor promoters
and UV rays (10–13). In addition, stimuli-induced
transcription of COX-2 activates signaling proteins that lead to
the activation of transcription factors, such as activator
protein-1 (AP-1) and nuclear factor-κB (NF-κB), which bind to the
COX-2 promoter (14–16). The COX-2 promoter contains multiple
cis-acting elements, including AP-1, NF-κB and cAMP response
element (CRE), that are crucial for the transcriptional
upregulation of COX-2 (15–17).
The role of AP-1 and NF-κB in the UVB-induced COX-2 expression in
HaCaT human keratinocytes has also been reported (18,19).
Type 1 collagen is a major component of the dermal
extracellular matrix (ECM) (20)
that accounts for 90% of the dermis (21) and provides hydration, resilience
and structural integrity to the skin (22). A reduction in the content of type 1
collagen in the skin, due to decreased expression (synthesis) or
increased degradation, is considered to be the most common cause of
skin damage associated with normal aging or photoaging (6,23,24).
UVB exposure results in the downregulation of type 1 procollagen by
regulating the expression and/or phosphorylation levels of
mitogen-activated protein kinase (MAPK), AMP-activated protein
kinase (AMPK), AP-1 and NF-κB in HaCaT cells and/or human dermal
fibroblasts (HDFs) (25,26). MMPs are zinc-containing
endopeptidases that can degrade dermal ECM proteins such as type 1
collagen and elastin (7,27). MMP-1 (also known as fibroblast
collagenase) and MMP-3 (also known as stromelysin 1) are actively
involved in the degradation of type 1 procollagen and other ECM
components in the dermis associated with normal aging and
photoaging (27,28). The expression of MMP-1 and MMP-3
can be stimulated by UVB, which leads to the production of skin
wrinkles (29).
Palmitoleic acid (C16:1), also known as Omega-7
fatty acid, has been reported to exhibit several potential health
benefits, such as maintaining the health of the heart,
anti-inflammation, anti-aging and accelerated wound healing
(30,31). AlaskOmega® Omega 7 500
(7-MEGA™) is a highly concentrated Omega-7 fatty acid (50%
palmitoleic acid). A recent study has demonstrated that 7-MEGA may
protect against H2O2-induced damage in HaCaT
human keratinocytes by inhibiting cellular oxidative stress and
inflammation (32). At present,
little is known about the role of 7-MEGA in the regulation of
UVB-induced skin inflammation. The present study aimed to
investigate the effects of 7-MEGA on the expression of COX-2, MMP-3
and type 1 procollagen in UVB-irradiated human keratinocytes
(HaCaT) and human dermal fibroblasts (HDFs).
Materials and methods
Cell culture
HaCaT cells (American Type Culture Collection), an
immortalized human keratinocyte cell line, were cultured in
Dulbecco's modified Eagle medium (DMEM)/F12 (cat. no. LM 002-04;
Welgene, Inc.) supplemented with 10% heat-inactivated fetal bovine
serum (FBS; cat. no. S001-01; Welgene, Inc.), 2 mM glutamine (cat.
no. LS 002-01; Welgene, Inc.), 100 U/ml penicillin and 100 mg/ml
streptomycin (cat. no. LS202-02; Welgene, Inc.). Human dermal
fibroblasts (HDFs; ATCC) were cultured in DMEM (cat. no. LM 001-05;
Welgene, Inc.) supplemented with 10% FBS, 2 mM glutamine, 100 U/ml
penicillin and 100 mg/ml streptomycin. HaCaT cells and HDFs were
cultured at 37°C in a humidified atmosphere containing 95% air and
5% CO2.
7-MEGA preparation
7-MEGA purified concentrates containing >500 mg/g
palmitoleic acid were obtained from Organic Technologies. 7-MEGA
was diluted 10-fold with 70% ethanol and the resultant solution was
stored overnight at room temperature (RT; 25–27°C). Subsequently,
the solution was diluted 10-fold with PBS for use in
experiments.
UVB irradiation
Cells were irradiated in cell culture plates using a
Bio-Link BLX-312 UVB lamp (Vilber Lourmat Sté) with an emission
wavelength peak at 312 nm. Prior to UVB irradiation, the culture
medium was replaced with 1 ml/well PBS. The plate lid was removed,
and the cells were irradiated at 10 or 60 mJ/cm2 UVB.
Subsequently, PBS was replaced with complete culture medium
containing either 7-MEGA or vehicle control for the indicated times
and concentrations prior to harvesting.
Cell count analysis
HaCaT cells and HDFs were seeded into 24-well plates
(1×105 cells in 500 µl/well) and cultured overnight. The
cells were treated with 0, 0.5, 1, 2.5 or 5 µl/ml 7-MEGA. At each
time point, cells were stained with trypan blue, and the viable
(unstained) cells were counted under a Nikon Eclipse TS100
phase-contrast microscope (Nikon Corporation). The cell count assay
was performed in triplicate, and ~100 cells were counted in each
evaluation. The survival rate was expressed as a percentage
relative to the untreated control group.
Preparation of whole cell lysates
Following the indicated treatments, HaCaT cells and
HDFs were washed twice with PBS supplemented with 1 mM
Na3VO4 and 1 mM NaF and subsequently exposed
to cell lysis buffer [20 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1 mM
EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 mg/ml leupeptin, 1 mM
phenylmethylsulfonyl fluoride (Sigma-Aldrich; Merck KGaA)]. The
cells were then harvested and centrifuged for 15 min at 4°C and
12,074 × g. The supernatant was extracted, and protein
concentrations were determined by bicinchoninic acid assay at 560
nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Western blotting
Proteins (50 µg/lane) were separated by SDS-PAGE
(10%) and transferred onto polyvinylidene fluoride membranes (EMD
Millipore). The membranes were washed using TBS (10 mM Tris, 150 mM
NaCl) supplemented with 0.05% (v/v) Tween-20 (TBST) and blocked
with TBST containing 5% (w/v) non-fat dried milk. The membranes
were incubated overnight with primary antibodies specific for COX-2
(1:2,000; cat. no. 155745; Cayman Chemical Company), type 1
procollagen (1:2,000; cat. no. ab34170; Abcam), phosphorylated (p-)
c-Jun (1:1,000; cat. no. 2361; Cell Signaling Technology, Inc.),
total (T-) c-Jun (1:1,000; cat. no. 9165; Cell Signaling
Technology, Inc.), c-Fos (1:500; cat. no. sc-8047; Santa Cruz
Biotechnology, Inc.) or β-actin (1:10,000; cat. no. A5441;
Sigma-Aldrich; Merck KGaA) at 4°C. The membranes were then
incubated with horseradish peroxidase-conjugated secondary
antibodies goat anti-rabbit immunoglobulin G (IgG; H+L; 1:2,000;
cat. no. 111-035-045; Jackson ImmunoResearch Laboratories, Inc.)
and goat anti-mouse IgG (H+L; 1:2,000; cat. no. 115-035-062;
Jackson ImmunoResearch Laboratories, Inc.) specific to the primary
antibody for 2 h at RT, followed by washing with TBST at RT. The
immunoreactivity of the membranes was detected using enhanced
chemiluminescence reagents (cat. no. K12045-D50; Advansta, Inc.).
β-actin was used as a loading control.
Reverse-transcription polymerase chain
reaction (RT-PCR)
Following the indicated treatments, total RNA was
isolated from HaCaT cells using TRIzol® (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Total
RNA (3 µg) was reverse-transcribed using a 40 µl reaction mixture
comprising 8 µl Molony Murine Leukemia Virus Reverse Transcriptase
(M-MLV RT; Promega Corporation), 5X buffer, 3 µl 10 mM dNTPs, 0.45
µl 40 U/µl RNase inhibitor, 0.3 µl 200 U/µl M-MLV RT and 3.75 µl 20
µM oligo dT (Bioneer Corporation). Single-stranded cDNA was
amplified using PCR with 4 µl 5X Green Go-Taq® Flexi
reaction buffer, 0.4 µM 10 mM dNTPs, 0.1 µl 5 U/µl Taq polymerase,
1.2 µl 25 mM MgCl2 (all Promega Corporation) and 0.4 µl
primer (20 pM/µl). The sequences of primers used in PCR were as
follows: COX-2 forward, 5′-CCGGACAGGATTCTATGGAGA-3′ and reverse,
5′-CAATCATCAGGCACAGGAGG-3′; MMP-3 forward,
5′-CCTCTGATGGCCCAGAATTGA-3′ and reverse,
5′-GAAATTGGCCACTCCCTGGGT-3′; β-actin forward,
5′-GGTGAAGGTCGGTGTGAACG-3′ and reverse, 5′-GGTAGGAACACGGAAGGCCA-3′.
The PCR conditions for COX-2 were as follows: 30 cycles of
denaturation at 95°C for 30 sec, annealing at 59°C for 30 sec and
extension at 72°C for 30 sec. The PCR conditions for MMP-3 were as
follows: 30 cycles of denaturation at 95°C for 60 sec, annealing at
60°C for 120 sec and extension at 72°C for 180 sec. The PCR
conditions for β-actin were as follows: 25 cycles of denaturation
at 95°C for 30 sec, annealing at 56°C for 30 sec and extension at
72°C for 30 sec. PCR products were separated on a 1% agarose gel,
and visualized and photographed in the Gel Doc XR+ system (Bio-Rad
Laboratories, Inc.). β-actin was used as an internal control to
evaluate the relative expression of COX-2 and MMP-3.
Quantitative PCR (qPCR)
Total cellular RNA was isolated from the treated
HaCaT cells using the RNAiso Plus (Takara Bio, Inc.) according to
the manufacturer's protocol. Total RNA (3 µg) was
reverse-transcribed using a 40 µl reaction mixture containing 8 µl
Molony Murine Leukemia Virus Reverse Transcriptase (M-MLV RT;
Promega Corporation) 5X buffer, 3 µl 10 mM dNTPs, 0.45 µl 40 U/µl
RNase inhibitor, 0.3 µl 200 U/µl M-MLV RT and 3.75 µl 20 µM oligo
dT (Bioneer Corporation). Single-stranded cDNA was amplified using
PCR with the primers listed above. The thermocycling conditions
were as follows: 40 cycles of denaturation at 95°C for 30 sec,
annealing at 60°C for 30 sec and extension at 72°C for 30 sec. The
SYBR Green PCR Master Mix (Takara Bio, Inc.) was used with the
LightCycler 96 Machine (Roche Diagnostics GmbH). The reactions were
performed in triplicate for each sample. The results were
quantified using the 2−ΔΔCq method (33). The Cq value for each sample was
normalized to the value for β-actin.
Statistical analysis
Data were expressed as the mean ± SEM. Differences
between values were analyzed using one-way ANOVA followed by
Dunnett's post hoc test (SPSS software, version 11.5; SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
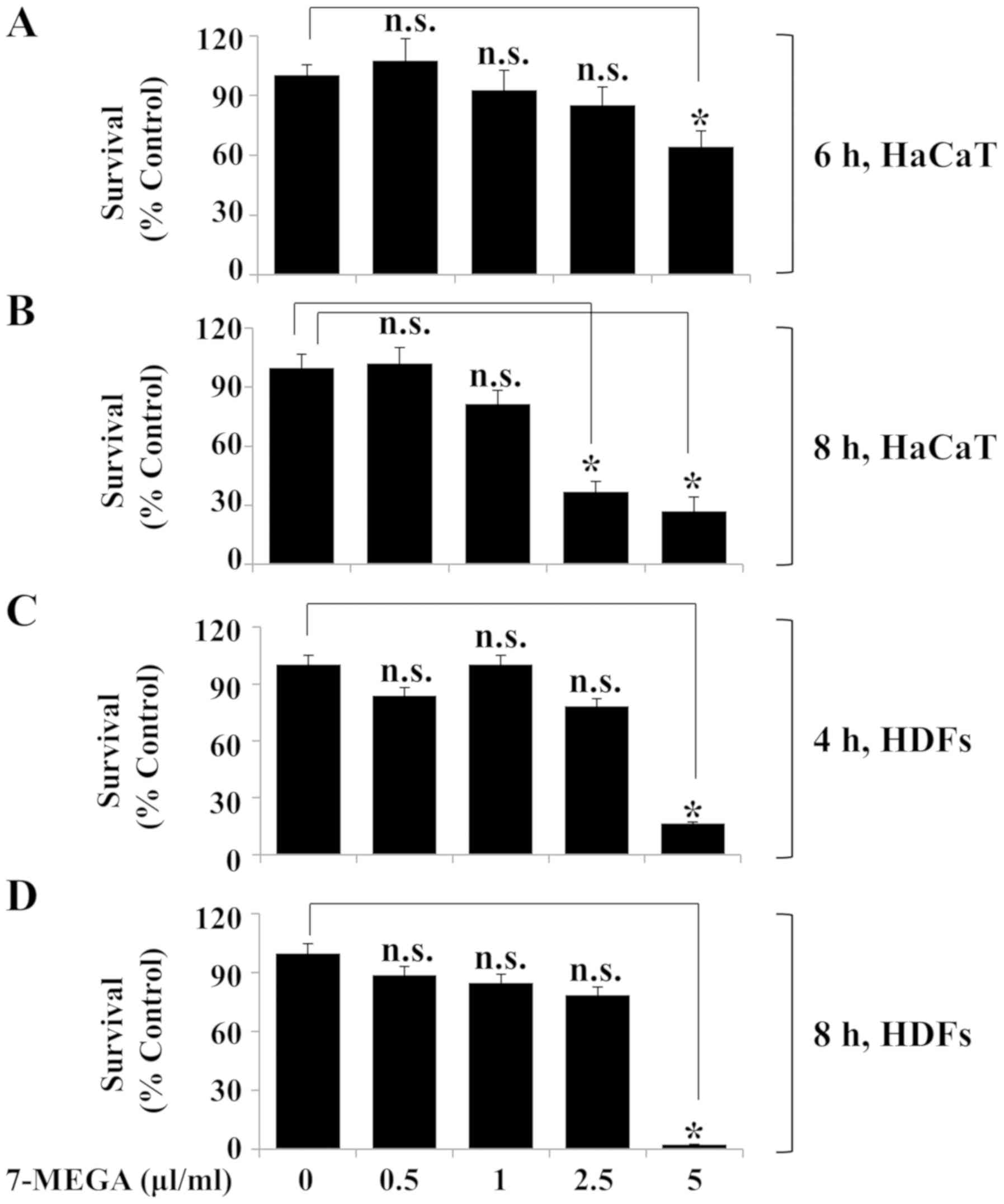
Treatment with 0.5–2.5 µl/ml 7-MEGA
for 4 and 6 h has low to no cytotoxic effect HDFs and HaCaT
keratinocytes
The effects of various concentrations and treatment
durations of 7-MEGA on the viability of HaCaT cells and HDFs were
determined using Trypan blue staining and cell count analysis.
Compared with the untreated control group, treatment with 0.5–2.5
µl/ml 7-MEGA for 6 h did not significantly affect the survival rate
of HaCaT cells; however, treatment with 5 µl/ml 7-MEGA for 6 h was
cytotoxic to HaCaT cells (Fig.
1A). In addition, treatment with 0.5 or 1 µl/ml 7-MEGA for 8 h
did not significantly affect the survival of HaCaT cells, whereas
treatment with 2.5 or 5 µl/ml 7-MEGA for the same duration resulted
in a significant decrease in cell survival rate (Fig. 1B). Treatment with 0.5–2.5 µl/ml
7-MEGA for 4 and 8 h exhibited no effect on HDF survival; however,
treatment with 5 µl/ml 7-MEGA for the same durations resulted in a
significant decrease in the cell survival rate (Fig. 1C and D). These results suggested
that treatment with 0.5–2.5 µl/ml 7-MEGA for 4 or 6 h is not toxic
to HDFs or HaCaT cells.
Exposure of HaCaT keratinocytes to 10
mJ/cm2 UVB for 6 h increases the expression levels of
COX-2 and MMP-3
Since upregulation of COX-2, MMP-1 and MMP-3 is
linked to skin inflammation (5,7), the
effects of exposure to different doses of UVB irradiation for 6 h
on the protein and mRNA expression levels of COX-2 and MMP-3 were
investigated in HaCaT cells. Western blotting experiment results
demonstrated that compared with the non-irradiated control group,
10 mJ/cm2 UVB irradiation for 6 h significantly induced
the expression of COX-2 protein in HaCaT cells (Fig. 2A and B). Similarly, the results of
the RT-PCR analysis revealed increased expression of COX-2 in HaCaT
cells irradiated with 10 mJ/cm2 UVB for 6 h (Fig. 2C). Exposure of HaCaT cells to 10
mJ/cm2 UVB for 6 h strongly induced MMP-3 mRNA
expression in these cells (Fig.
2C), whereas exposure to the tested doses of UVB for 6 h
resulted in only a slight increase in MMP-1 mRNA expression (data
not shown). Subsequent qPCR analysis demonstrated that peak
induction of COX-2 and MMP-3 mRNA expression levels occurred in
HaCaT cells irradiated with 10 mJ/cm2 UVB for 6 h
(Fig. 2D). However, there was
little or no induction of the expression of MMP-1 mRNA under the
same experimental conditions (data not shown). Time course
experiments were performed to evaluate the time at which the
expression levels of COX-2 and MMP-3 protein and/or mRNA were
induced in HaCaT cells irradiated with 10 mJ/cm2 UVB.
Western blotting results demonstrated that whereas COX-2 protein
expression was not detected in HaCaT cells exposed to 10
mJ/cm2 UVB for 0.5 or 1 h, strong COX-2 protein
expression was observed in the cells exposed to UVB for 6 h
(Fig. 2E and F). In addition, the
RT-PCR results demonstrated that the mRNA expression levels of
COX-2 and MMP-3 were higher in HaCaT cells exposed to 10
mJ/cm2 UVB for 3 or 6 h compared with those in the
non-irradiated control (Fig. 2G).
The peak expression levels of COX-2 and MMP-3 mRNA were observed in
HaCaT cells exposed to 10 mJ/cm2 UVB for 6 h. The
protein and mRNA expression levels of the control β-actin remained
largely unchanged under these experimental conditions (Fig. 2A, C, E and G). The results of the
qPCR confirmed that exposure to 10 mJ/cm2 UVB
irradiation for 6 h maximally induced the expression levels of
COX-2 and MMP-3 mRNA in HaCaT cells (Fig. 2H). Based on these results, 10
mJ/cm2 UVB for 6 h was selected for use in subsequent
experiments.
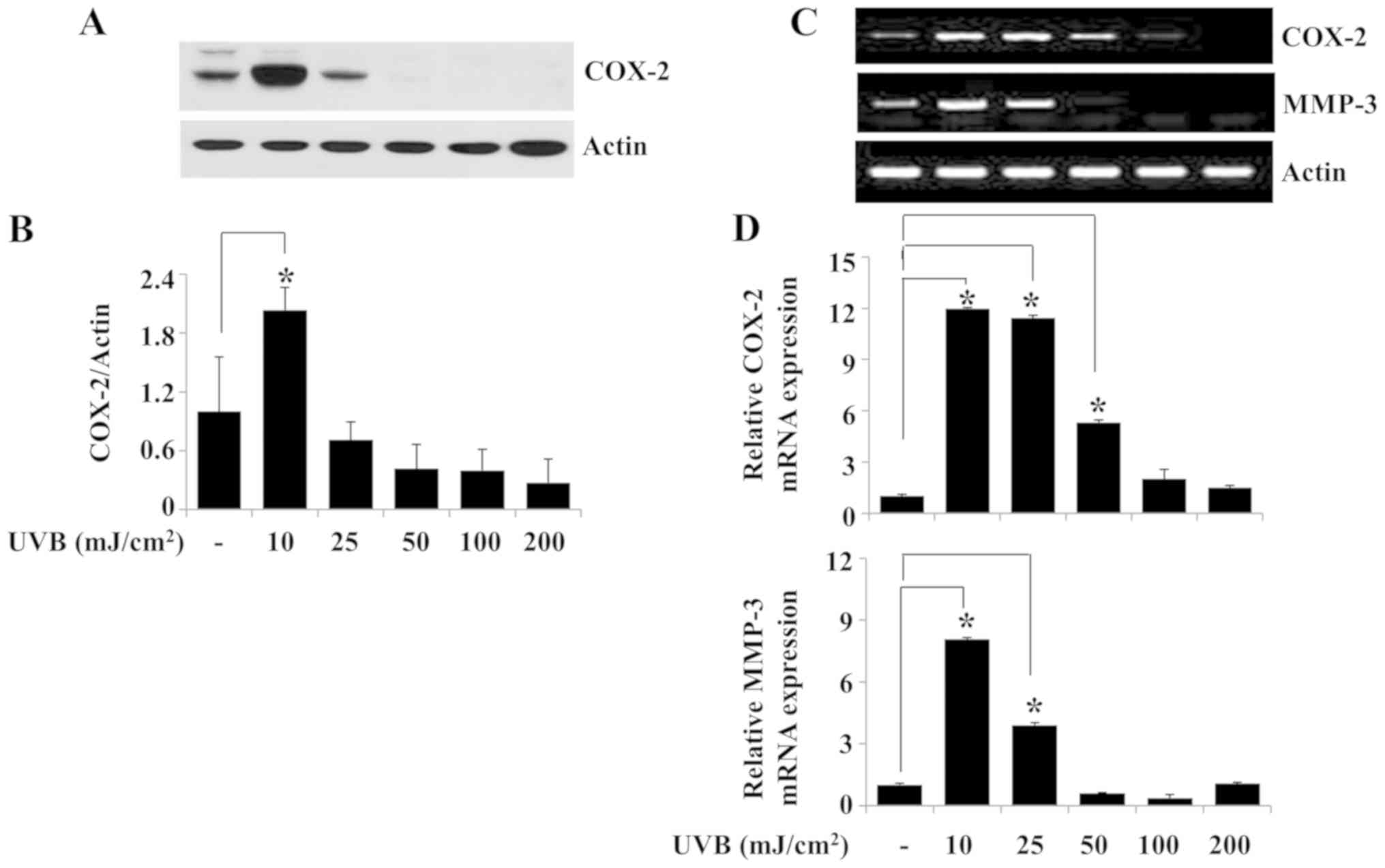 | Figure 2.Effects of UVB irradiation on the
expression levels of COX-2 and MMP-3 in HaCaT human keratinocytes.
(A) HaCaT cells were irradiated with UVB at 0, 10, 25, 50, 100 or
200 mJ/cm2 for 6 h. Whole cell lysates were extracted
from the irradiated cells and analyzed by western blotting to
determine the protein expression levels of COX-2 and β-actin. (B)
Densitometry analysis results of panel A. (C) RT-PCR and (D) qPCR
analysis of the mRNA expression levels of COX-2, MMP-3 and β-actin.
(E) HaCaT cells were irradiated with 10 mJ/cm2 UVB for
0, 0.5, 1, 3 or 6 h. At each time point, whole cell lysates were
extracted from the conditioned cells and analyzed by western
blotting to determine the protein expression levels of COX-2 and
β-actin. (F) Densitometry analysis results of panel E. (G) RT-PCR
and (H) qPCR analysis of the mRNA expression levels of COX-2, MMP-3
and β-actin. *P<0.05 vs. non-irradiated control. COX-2,
cyclooxygenase-2; MMP-3, stromelysin-1; UVB, ultraviolet B; RT-PCR,
reverse transcription-PCR; qPCR, quantitative PCR. |
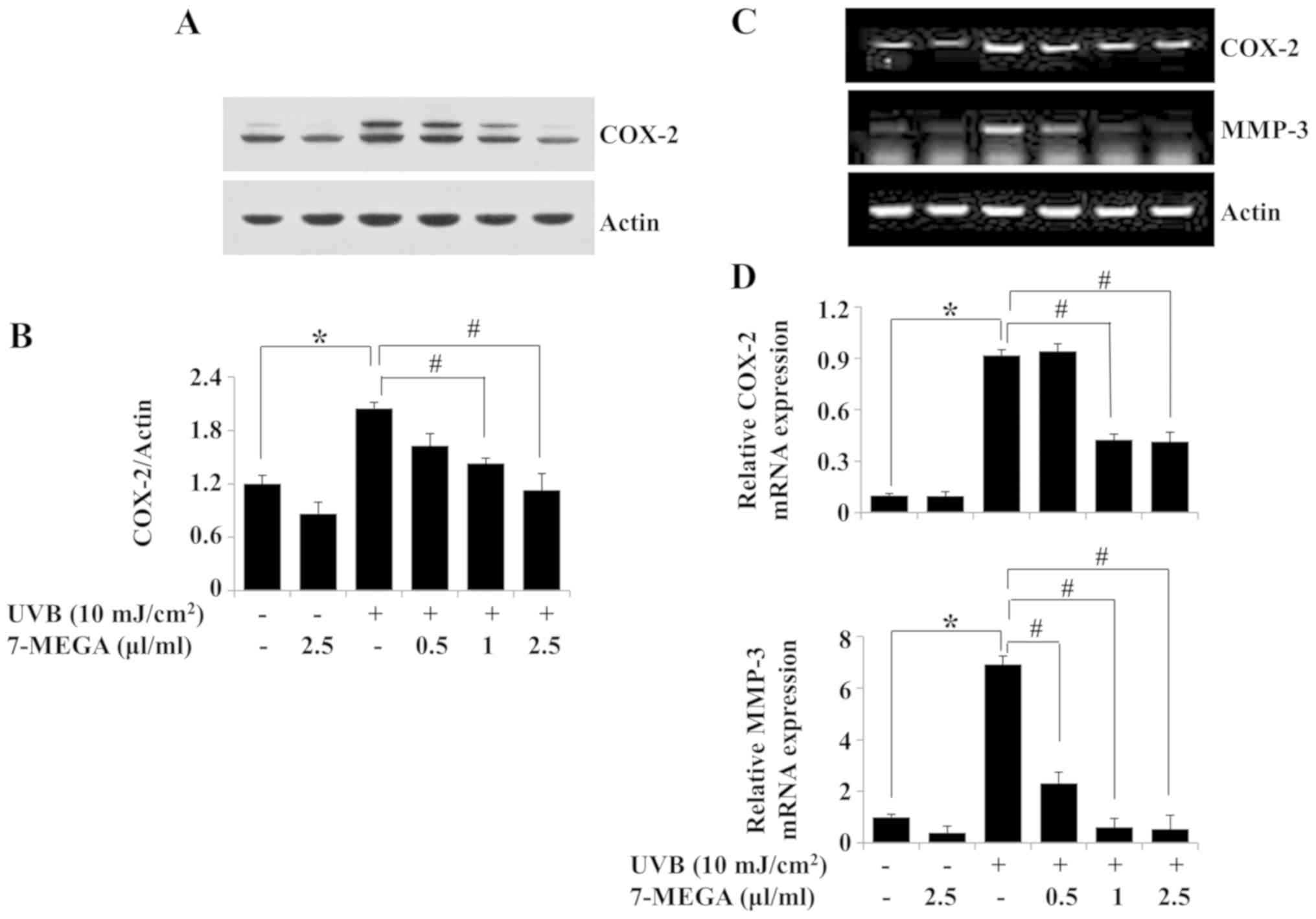
Treatment with 7-MEGA inhibits
UVB-induced expression of COX-2 and MMP-3 in HaCaT
keratinocytes
The effects of 0.5–2.5 µl/ml 7-MEGA on the protein
and mRNA expression levels of COX-2 and MMP-3 in HaCaT cells
exposed to 10 mJ/cm2 of UVB for 6 h were evaluated.
Treatment with 7-MEGA blocked the UVB-induced COX-2 protein
expression in HaCaT cells; 2.5 µl/ml 7-MEGA exhibited the strongest
effect (Fig. 3A and B). Similarly,
the RT-PCR results demonstrated that 7-MEGA treatment inhibited
UVB-induced COX-2 mRNA expression in HaCaT cells (Fig. 3C). Treatment with 7-MEGA also
suppressed UVB-induced MMP-3 mRNA expression in HaCaT cells.
β-actin protein and mRNA expression levels remained constant under
these experimental conditions (Fig. 3A
and C). The results of the qPCR analysis also demonstrated the
ability of 1 and 2.5 µl/ml 7-MEGA to significantly suppress
UVB-induced COX-2 and MMP-3 mRNA expression levels in HaCaT cells
(Fig. 3D). Due to the strong
suppressive effects on UVB-induced COX-2 and MMP-3 expressions in
HaCaT cells, 2.5 µl/ml 7-MEGA was selected as the optimal
concentration for subsequent experiments.
Treatment with 2.5 µl/ml 7-MEGA
inhibits the UVB-induced increase of c-Fos expression and c-Jun
phosphorylation in HaCaT keratinocytes
UVB-induced expression of COX-2 and MMP-3 in HaCaT
cells is affected by AP-1 (18,25,26),
which is composed of c-Fos and c-Jun (34). Therefore, the effects of exposure
to 10 mJ/cm2 UVB irradiation on the protein expression
and/or phosphorylation of c-Fos and c-Jun in HaCaT cells were
determined in the present study. Compared with the non-irradiated
control group, an induction of c-Fos protein expression was
observed in HaCaT cells exposed to UVB for 1, 3 or 6 h (Fig. 4A). In addition, compared with the
non-irradiated control group, the levels of c-Jun phosphorylation
and expression were upregulated in HaCaT cells exposed to UVB at
the tested time points (Fig. 4A and
B). The peak expression and/or phosphorylation of c-Fos and
c-Jun was observed in HaCaT cells exposed to UVB for 3 h (Fig. 4A and B).
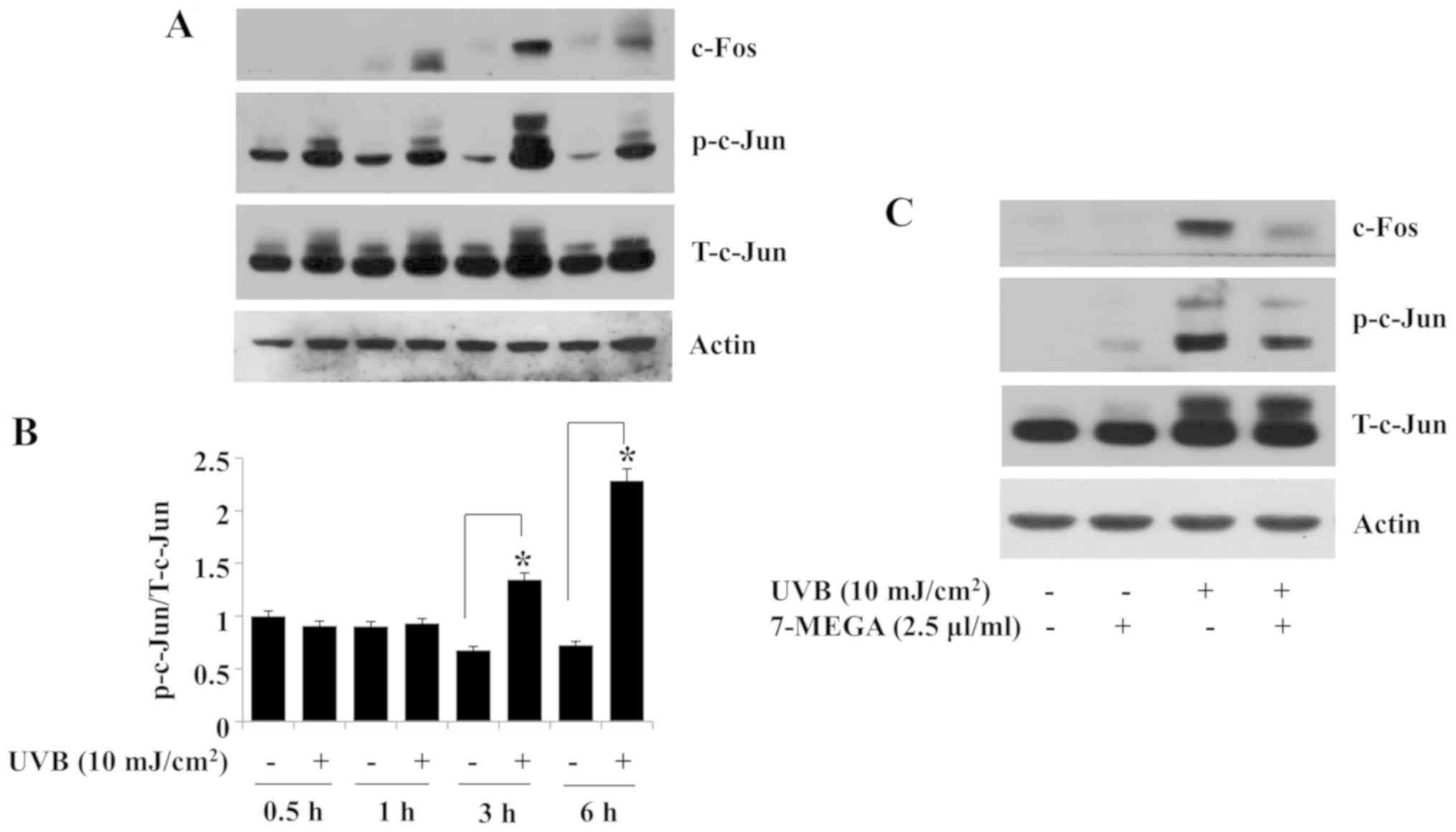 | Figure 4.Effects of UVB and 7-MEGA on the
expression and phosphorylation of c-Fos and c-Jun in HaCaT human
keratinocytes. (A) HaCaT cells were irradiated with 10
mJ/cm2 UVB for 0, 0.5, 1, 3, or 6 h. At each time point,
whole cell lysates were extracted from the conditioned cells and
analyzed by western blotting to determine the protein expression
and phosphorylation levels of c-Fos, c-Jun and β-actin. (B)
Densitometry analysis results of panel A. *P<0.05 vs.
non-irradiated control. (C) HaCaT cells were irradiated with 10
mJ/cm2 UVB for 3 h either in the absence or presence of
2.5 µl/ml 7-MEGA. Whole cell lysates were extracted from the cells
and analyzed by western blotting. 7-MEGA, AlaskOmega®
Omega 7 500; UVB, ultraviolet B; p-c-Jun, phosphorylated c-Jun;
T-c-Jun, total c-Jun. |
The effects of treatment with 2.5 µl/ml 7-MEGA on
the expression or phosphorylation of c-Fos and c-Jun in HaCaT cells
exposed to UVB for 3 h were examined next; treatment with 2.5 µl/ml
of 7-MEGA largely blocked the UVB-induced expression of c-Fos
protein in HaCaT cells (Fig. 4C).
Of note, treatment with 2.5 µl/ml 7-MEGA further partly inhibited
the UVB-induced phosphorylation of c-Jun without affecting its
total protein expression in HaCaT cells. No significant changes
were observed in the control β-actin protein expression levels
under these experimental conditions (Fig. 4A and C).
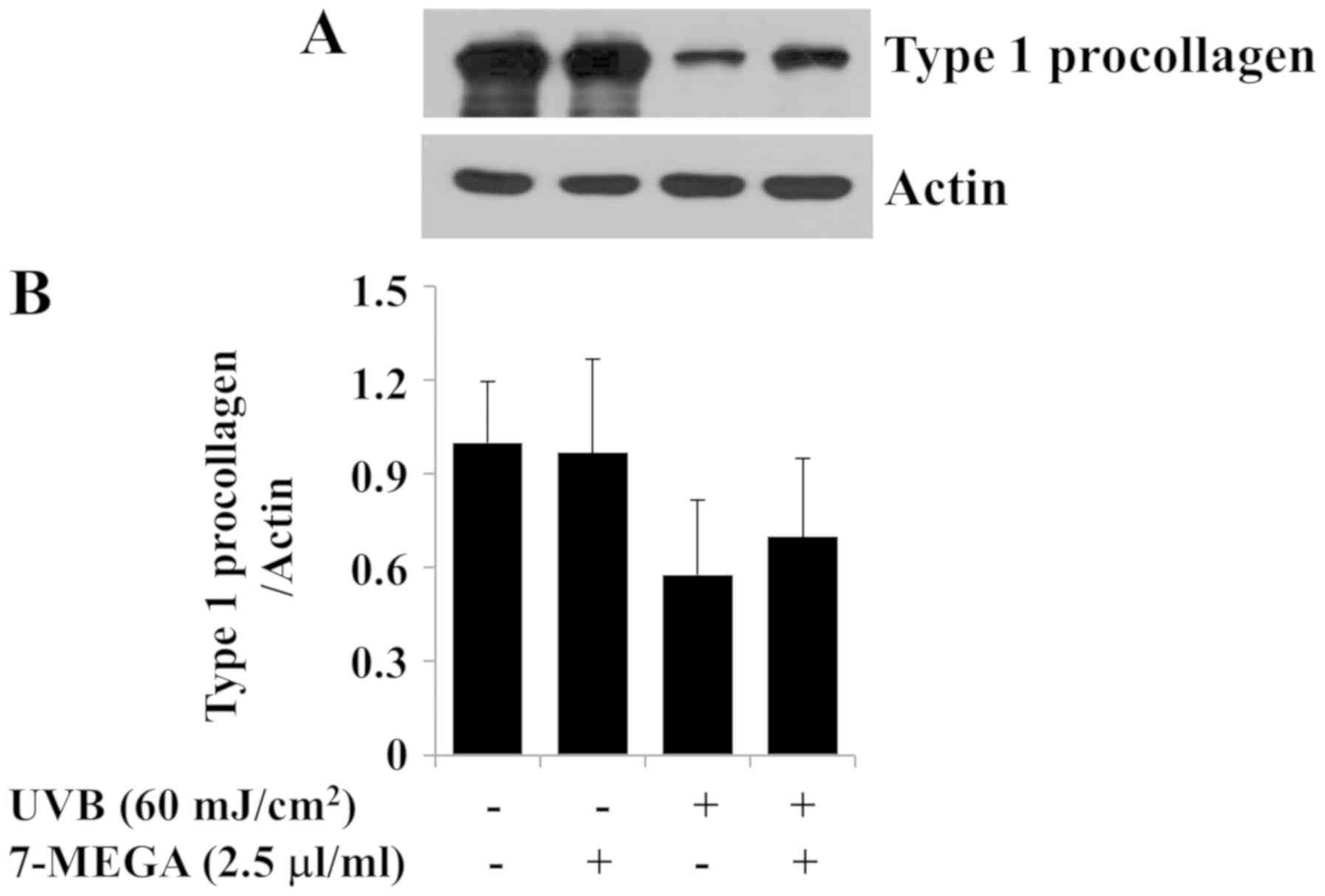
Treatment with 2.5 µl/ml 7-MEGA
inhibits UVB-induced reduction of type 1 collagen expression in
HDFs
The loss or reduction of type 1 collagen contents is
associated with the production of skin wrinkles in photoaging
(6,24). To investigate the role of 7-MEGA on
collagen production in vitro, the effects of UVB irradiation
(60 mJ/cm2) and treatment with 7-MEGA (2.5 µl/ml) on the
protein expression levels of type 1 procollagen were determined in
HDFs. In preliminary experiments, we have analyzed the protein
expression levels of type 1 procollagen in HDFs after exposure of
different doses (30, 60, and 90 mJ/cm2) and times (2, 4,
and 8 h) of UVB and found that 60 mJ/cm2 of UVB
irradiation for 4 h most strongly decreases the protein expression
levels of type 1 procollagen in these cells compared to no UVB
treatment (data not shown). As anticipated, a strong decrease in
the expression levels of type 1 procollagen was observed in HDFs
exposed to 60 mJ/cm2 UVB irradiation for 4 h (Fig. 5A and B). However, treatment with
2.5 µl/ml 7-MEGA partially blocked the UVB-induced reduction of
expression of type 1 procollagen in HDFs. The expression levels of
β-actin remained constant under these experimental conditions.
Discussion
AlaskOmega® Omega 7 500 (7-MEGA) is a
highly concentrated Omega-7 fatty acid (30,31).
The mechanism of 7-MEGA regulation on UVB-induced skin
inflammation, damage and wrinkles is largely unknown. The present
study investigated the effects of 7-MEGA on the expression levels
of COX-2, MMP-1, MMP-3 and type 1 procollagen, which are markers of
skin inflammation, damage and wrinkle formation, in UVB-irradiated
HDFs and HaCaT keratinocytes. To the best of our knowledge, this is
the first study to demonstrate that 7-MEGA may inhibit not only the
UVB-induced upregulation of COX-2 and MMP-3 in HaCaT cells, but
also the UVB-induced reduction of type 1 procollagen expression in
HDFs. These results suggested that 7-MEGA may exhibit
anti-inflammatory, anti-photodamage and anti-wrinkle formation
effects in UVB-irradiated skin cells.
A recent study has reported that 7-MEGA exhibits a
protective effect on H2O2-induced damage in
HaCaT cells and that the effect is mediated through the inhibition
of cellular reactive oxygen species production and downregulation
of the expression of inflammatory mediators, such as COX-2,
interleukin-1β and tumor necrosis factor-α (32). The results of the present study
demonstrated that exposure of HaCaT cells to 10 mJ/cm2
UVB for 6 h significantly increased the expression of COX-2 at the
protein and mRNA levels, whereas treatment with 2.5 µl/ml 7-MEGA
significantly suppressed these effects. Since COX-2 and the
associated PGs mediate UVB-induced cutaneous inflammation (5,35),
these results indicate that 7-MEGA may exhibit an anti-inflammatory
effect on UVB-irradiated human keratinocytes by downregulating
COX-2. UVB induces the upregulation of MMP-1 and MMP-3 in skin
cells and tissues (27,28,36),
which causes UV-mediated skin inflammation and damage (7,37). A
recent study has demonstrated the skin regenerative effects of
7-MEGA on H2O2-treated HaCaT cells through
the downregulation of MMP-1 and upregulation of type 1 procollagen
(32). RT-PCR and qPCR experiments
in the present study revealed that the exposure of HaCaT cells to
10 mJ/cm2 UVB for 6 h resulted in a strong induction of
MMP-3, but not MMP-1 mRNA expression. However, treatment with
7-MEGA inhibited the UVB-induced MMP-3 mRNA upregulation in HaCaT
cells. These results further suggested that 7-MEGA may exhibit
anti-inflammatory and photoprotective effects on UVB-irradiated
HaCaT keratinocytes by downregulating the expression of MMP-3.
However, considering that the skin inflammation and damage pathways
induced by UV irradiation involve multiple pathways other than the
COX-2 (eicosanoids) and MMP-3, it should be noted that the
suppressive effects of 7-MEGA on UVB-induced COX-2 and MMP-3
expression levels in HaCaT cells demonstrated in the present study
may be limited to the cell lines used. Thus, it is currently not
possible to conclude that 7-MEGA has preventive and/or therapeutic
effects on UVB-induced inflammation and photodamage in the skin. A
number of previous studies have demonstrated the induction of COX-2
or MMP-3 expression in HaCaT cells irradiated with UVB at the
intensity of 20, 30 or 60 mJ/cm2 (38–40).
In the present study, irradiation with the lowest tested intensity
of UVB (10 mJ/cm2) for 6 h induced the peak expression
levels of COX-2 and MMP-3 in HaCaT cells; this may be due to the
use of different passage of HaCaT cells, different duration and
devices of UVB-irradiation, different culture conditions
(serum-containing or serum-free media) and different cell lysis
buffers used in previous studies.
Emerging evidence suggests that the AP-1
transcription factor, composed of c-Fos and c-Jun, is important for
the UVB-induced expression of COX-2, MMP-1 and MMP-3 in HaCaT cells
(16,18,25,26).
To date, 7-MEGA regulation of AP-1 in UVB-irradiated skin cells has
not been reported. Previously, UVB has been demonstrated to induce
the activation of AP-1, which is associated with increased c-Fos
expression in HaCaT cells (41).
Consistent with this result, the present study demonstrated that
the exposure of HaCaT cells to 10 mJ/cm2 UVB for 3 h
upregulated c-Fos protein expression. In addition, the expression
and phosphorylation levels of c-Jun were upregulated in the
UVB-irradiated HaCaT cells. These results supported the UVB-induced
AP-1 activation in HaCaT cells. In addition, the results of the
present study demonstrated that 7-MEGA inhibited the UVB-induced
c-Fos expression and c-Jun phosphorylation in HaCaT cells. Assuming
that the promoters of COX-2 and MMP-3 contain the AP-1 cis-acting
element and that 7-MEGA inhibits UVB-induced expression of COX-2
and MMP-3 at their transcript levels in HaCaT cells, the inhibitory
effects of 7-MEGA on UVB-induced expression of COX-2 and MMP-3 in
HaCaT cells may be attributable to AP-1 inhibition.
UVB-induced reduction of type 1 procollagen
expression in HDFs has been previously reported (25,29).
Consistent with this result, the present study demonstrated that
the expression of type 1 procollagen protein was diminished in HDFs
irradiated with 60 mJ/cm2 UVB for 4 h. However,
treatment with 7-MEGA partially blocked the UVB-induced reduction
of type 1 procollagen expression in HDFs, thus supporting the
anti-photoaging effects of 7-MEGA in culture. Additionally, the
family of MMPs expressed and secreted from skin keratinocytes are
involved in the degradation of type 1 procollagen and other ECM
components in the dermis (27–29).
Thus, it may be speculated that 7-MEGA inhibition of UVB-induced
MMP-3 expression in HaCaT keratinocytes may mediate the
anti-wrinkle effects (enhanced expression of type 1 procollagen on
UVB-exposed dermal fibroblasts) of 7-MEGA. Future studies using a
co-culture system of HaCaT keratinocytes and HDFs irradiated with
UVB in the presence or absence of 7-MEGA are required to clarify
this speculation.
The main compounds in 7-MEGA are palmitoleic acid
(C16:1 n-7, 53.5%), palmitic acid (C16:0, 25.7%), eicosapentaenoic
acid (C20:5 n-3, 0.06%) and myristic acid (C14:0, 0.04%) (32). Treatment of HaCaT cells with 7-MEGA
or palmitoleic acid at the same concentration (100 nl/ml) exhibits
similar inhibitory effects on H2O2-induced
expression of COX-2 and MMP-1 (32). At present, it is unclear whether
7-MEGA as a whole or its certain constituent(s) exerts the
regulatory effects on UVB-induced COX-2, MMP-3 and type 1
procollagen expression in HaCaT cells and HDFs. In the present
study, the efficacy of 7-MEGA™ 500 and its major compound
palmitoleic acid on the UVB-induced COX-2, MMP-3 or type 1
procollagen expression was compared in HaCaT cells and HDFs;
however, while treatment with 2.5 µl/ml of 7-MEGA is not cytotoxic
to UVB-exposed HaCaT cells or HDFs, similar or lower concentrations
(0.625, 1.25 and 2.5 µl/ml) of palmitoleic acid were largely
cytotoxic to these cells (data not shown). Since 0.625, 1.25 and
2.5 µl/ml palmitoleic acid are equal to 2.2, 4.4 and 8.8 mM
palmitoleic acid and that treatment with high concentrations of
fatty acids often lead to lipotoxicity-mediated growth inhibition
and cell death in numerous cell types, it is likely that the
cytotoxicity of UVB-exposed HaCaT cells or HDFs triggered by
palmitoleic acid was due to lipotoxicity. It should be emphasized
that palmitoleic acid up to 500 µM was not cytotoxic to UVB-exposed
HaCaT cells or HDFs, but it was ineffective in counteracting the
UVB-induced COX-2, MMP-3 or type 1 procollagen expression in HaCaT
cells and HDFs (data not shown). Considering that although 7-MEGA
contains 53.5% palmitoleic acid, it exhibited little cytotoxicity
to HaCaT and HDFs, it may be speculated that 7-MEGA as a whole, or
other components, in it may serve the function of weakening the
cytotoxicity of palmitoleic acid. In the future, the effects of
7-MEGA and each of its constituent compounds (with the exception of
palmitoleic acid) on the regulation of COX-2, MMP-3 and type 1
procollagen expression levels in HaCaT cells and HDFs exposed to
UVB may be tested and compared. This may clarify the modulatory
effects of 7-MEGA and its constituent compounds on UVB-induced
expression of COX-2 MMP-3 and type 1 procollagen in skin cells.
The results of the present study demonstrated that
7-MEGA regulated the expression levels of inflammation-,
photodamage- and/or wrinkle formation-related proteins and enzymes,
such as COX-2, MMP-3 and type 1 procollagen, in 10 or 60
mJ/cm2 UVB-irradiated HaCaT cells and HDFs. However,
although the doses of UVB irradiation administered to HaCaT cells
and HDFs in the present study were similar to those used previously
in cell culture (42), they are
different from those used in in vivo studies (200–500
mJ/cm2) (43,44). Considering that the HaCaT cells and
HDFs used in the present study are cultured cell lines, the damage
caused by UVB-irradiation in the human skin is not limited to these
cell types and that the effective doses of 7-MEGA may be
significantly different in in vivo dermal cells, future
studies are warranted to evaluate the anti-inflammatory and
anti-wrinkle effects of 7-MEGA on animals and their sera. This
should be accomplished not only by measuring the expression and
secretion of multiple pro-inflammatory, anti-inflammatory and
wrinkle formation-related factors, including COX-2, type 1
procollagen, MMP-3 and immunoglobulins, but also using the direct
microscopic examination of skin improvement or anti-wrinkle effects
in the skin exposed to UVB. This may help establish the
anti-inflammatory, anti-photodamaging and anti-wrinkle effects of
7-MEGA.
In conclusion, the results of the present study
demonstrated that 7-MEGA regulated the expression levels of COX-2,
MMP-3 and type 1 procollagen in UVB-irradiated human keratinocytes
and dermal fibroblasts. These results suggested that 7-MEGA may be
used as an agent to protect against UVB-induced skin inflammation
and potentially wrinkle-formation caused by the dysregulation of
the expression of COX-2, MMP-3 and type 1 procollagen.
Acknowledgements
The authors would like to thank Ms. Kyung-Ran Shin
(KIST Gangneung Institute of Natural Products, Gangneung, Republic
of Korea) for providing technical assistance.
Funding
The present study was funded by Vitech Co., Ltd.
(grant no. 20170406).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YKP, AKY and AR performed the experiments. YWR, JYC,
YKS, BHK, NYL and BCJ designed the experiments and analysed the
data. BCJ wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohania D, Chandel S, Kumar P, Verma V,
Digvijay K, Tripathi D, Choudhury K, Mitten SK and Shah D:
Ultraviolet radiations: Skin defense-damage mechanism. Adv Exp Med
Biol. 996:71–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopes DM and McMahon SB: Ultraviolet
radiation on the skin: A painful experience? CNS Neurosci Ther.
22:118–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Panich U, Sittithumcharee G, Rathviboon N
and Jirawatnotai S: Ultraviolet radiation-induced skin aging: The
role of DNA damage and oxidative stress in epidermal stem cell
damage mediated skin aging. Stem Cells Int. 2016:73706422016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cadet J and Douki T: Formation of
UV-induced DNA damage contributing to skin cancer development.
Photochem Photobiol Sci. 17:1816–1841. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilgus TA, Ross MS, Parrett ML and
Oberyszyn TM: Topical application of a selective cyclooxygenase
inhibitor suppresses UVB mediated cutaneous inflammation.
Prostaglandins Other Lipid Mediat. 62:367–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varani J, Perone P, Fligiel SE, Fisher GJ
and Voorhees JJ: Inhibition of type I procollagen production in
photodamage: Correlation between presence of high molecular weight
collagen fragments and reduced procollagen synthesis. J Invest
Dermatol. 119:122–129. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci. 17(pii):
E8682016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ricciotti E and FitzGerald GA:
Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol.
31:986–1000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hla T, Ristimäki A, Appleby S and
Barriocanal JG: Cyclooxygenase gene expression in inflammation and
angiogenesis. Ann N Y Acad Sci. 696:197–204. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashemi Goradel N, Najafi M, Salehi E,
Farhood B and Mortezaee K: Cyclooxygenase-2 in cancer: A review. J
Cell Physiol. 234:5683–5699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tripp CS, Blomme EA, Chinn KS, Hardy MM,
LaCelle P and Pentland AP: Epidermal COX-2 induction following
ultraviolet irradiation: Suggested mechanism for the role of COX-2
inhibition in photoprotection. J Invest Dermatol. 121:853–861.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashida M, Bito T, Budiyanto A, Ichihashi M
and Ueda M: Involvement of EGF receptor activation in the induction
of cyclooxygenase-2 in HaCaT keratinocytes after UVB. Exp Dermatol.
12:445–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang YJ, Wingerd BA, Arakawa T and Smith
WL: Cyclooxygenase-2 gene transcription in a macrophage model of
inflammation. J Immunol. 177:8111–8122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Newton R, Kuitert LM, Bergmann M, Adcock
IM and Barnes PJ: Evidence for involvement of NF-kappaB in the
transcriptional control of COX-2 gene expression by IL-1beta.
Biochem Biophys Res Commun. 237:28–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santos RC, Rico MA, Bartrons R, Pujol FV,
Rosa JL and de Oliveira JR: The transcriptional activation of the
cyclooxygenase-2 gene in zymosan-activated macrophages is dependent
on NF-kappa B, C/EBP, AP-1, and CRE sites. Inflammation.
34:653–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inoue H, Yokoyama C, Hara S, Tone Y and
Tanabe T: Transcriptional regulation of human
prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide
and phorbol ester in vascular endothelial cells. Involvement of
both nuclear factor for interleukin-6 expression site and cAMP
response element. J Biol Chem. 270:24965–24971. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho JW, Park K, Kweon GR, Jang BC, Baek
WK, Suh MH, Kim CW, Lee KS and Suh SI: Curcumin inhibits the
expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT)
by inhibiting activation of AP-1: p38 MAP kinase and JNK as
potential upstream targets. Exp Mol Med. 37:186–192. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Engel K, Schmidt U, Reuter J, Weckesser S,
Simon-Haarhaus B and Schempp CM: Usnea barbata extract prevents
ultraviolet-B induced prostaglandin E2 synthesis and COX-2
expression in HaCaT keratinocytes. J Photochem Photobiol B.
89:9–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watt FM and Fujiwara H: Cell-extracellular
matrix interactions in normal and diseased skin. Cold Spring Harb
Perspect Biol. 3(pii): a0051242011.PubMed/NCBI
|
|
21
|
Meigel WN, Gay S and Weber L: Dermal
architecture and collagen type distribution. Arch Dermatol Res.
259:1–10. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borumand M and Sibilla S: Effects of a
nutritional supplement containing collagen peptides on skin
elasticity, hydration and wrinkles. J Med Nutr Nutraceut. 4:47–53.
2015. View Article : Google Scholar
|
|
23
|
Varani J, Dame MK, Rittie L, Fligiel SE,
Kang S, Fisher GJ and Voorhees JJ: Decreased collagen production in
chronologically aged skin: Roles of age-dependent alteration in
fibroblast function and defective mechanical stimulation. Am J
Pathol. 168:1861–1868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Varani J, Spearman D, Perone P, Fligiel
SE, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ and Voorhees JJ:
Inhibition of type I procollagen synthesis by damaged collagen in
photoaged skin and by collagenase-degraded collagen in vitro. Am J
Pathol. 158:931–942. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim M, Park YG, Lee HJ, Lim SJ and Nho CW:
Youngiasides A and C isolated from youngia denticulatum inhibit
UVB-induced MMP expression and promote type I procollagen
production via repression of MAPK/AP-1/NF-κB and activation of
AMPK/Nrf2 in HaCaT cells and human dermal fibroblasts. J Agric Food
Chem. 63:5428–5438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HJ, Hwang E, Park B, Zhang M, Sun ZW,
Lee DG, Park SY and Yi TH: Methanol extract of bitter melon
alleviates UVB-induced MMPs expression via MAP kinase and AP-1
signaling in human dermal fibroblasts in vitro. Phytother Res.
30:1519–1526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brenneisen P, Sies H and
Scharffetter-Kochanek K: Ultraviolet-B irradiation and matrix
metalloproteinases: From induction via signaling to initial events.
Ann N Y Acad Sci. 973:31–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buechner N, Schroeder P, Jakob S, Kunze K,
Maresch T, Calles C, Krutmann J and Haendeler J: Changes of MMP-1
and collagen type I alpha1 by UVA, UVB and IRA are differentially
regulated by Trx-1. Exp Gerontol. 43:633–637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging. J
Investig Dermatol Symp Proc. 14:20–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frigolet ME and Gutiérrez-Aguilar R: The
role of the novel lipokine palmitoleic acid in health and disease.
Adv Nutr. 17:173S–181S. 2017. View Article : Google Scholar
|
|
31
|
Weimann E, Silva MBB, Murata GM, Bortolon
JR, Dermargos A, Curi R and Hatanaka E: Topical anti-inflammatory
activity of palmitoleic acid improves wound healing. PLoS One.
13:e02053382018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song IB, Gu H, Han HJ, Lee NY, Cha JY, Son
YK and Kwon J: Effects of 7-MEGA™ 500 on oxidative stress,
inflammation, and skin regeneration in
H2O2-treated skin cells. Toxicol Res.
34:103–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karin M, Liu Zg and Zandi E: AP-1 function
and regulation. Curr Opin Cell Biol. 9:240–246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilgus TA, Parrett ML, Ross MS, Tober KL,
Robertson FM and Oberyszyn TM: Inhibition of ultraviolet light
B-induced cutaneous inflammation by a specific cyclooxygenase-2
inhibitor. Adv Exp Med Biol. 507:85–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cho YH, Bahuguna A, Kim HH, Kim DI, Kim
HJ, Yu JM, Jung HG, Jang JY, Kwak JH, Park GH, et al: Potential
effect of compounds isolated from Coffea arabica against UV-B
induced skin damage by protecting fibroblast cells. J Photochem
Photobiol B. 174:323–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Järvinen TM, Kanninen P, Jeskanen L,
Koskenmies S, Panelius J, Hasan T, Ranki A and Saarialho-Kere U:
Matrix metalloproteinases as mediators of tissue injury in
different forms of cutaneous lupus erythematosus. Br J Dermatol.
157:970–980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aoki R, Aoki-Yoshida A, Suzuki C and
Takayama Y: Protective effect of indole-3-pyruvate against
ultraviolet β-induced damage to cultured HaCaT keratinocytes and
the skin of hairless mice. PLoS One. 9:e968042014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ren X, Shi Y, Zhao D, Xu M, Li X, Dang Y
and Ye X: Naringin protects ultraviolet B-induced skin damage by
regulating p38 MAPK signal pathway. J Dermatol Sci. 82:106–114.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park JH, Mohamed MA, Jung YJ, Shrestha S,
Lee TH, Lee CH, Han D, Kim J and Baek N: Germacrane sesquiterpenes
isolated from the rhizome of Curcuma xanthorrhiza Roxb. inhibit
UVB-induced upregulation of MMP-1, −2, and −3 expression in human
keratinocytes. Arch Pharm Res. 38:1752–1760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen W, Borchers AH, Dong Z, Powell MB and
Bowden GT: UVB irradiation-induced activator protein-1 activation
correlates with increased c-fos gene expression in a human
keratinocyte cell line. J Biol Chem. 273:32176–32181. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jung SK, Ha SJ, Kim YA, Lee J, Lim TG, Kim
YT, Lee NH, Park JS, Yeom MH, Lee HJ and Lee KW: MLK3 is a novel
target of dehydroglyasperin D for the reduction in UVB-induced
COX-2 expression in vitro and in vivo. J Cell Mol Med. 19:135–142.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Choi KS, Kundu JK, Chun KS, Na HK and Surh
YJ: Rutin inhibits UVB radiation-induced expression of COX-2 and
iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential
targets. Arch Biochem Biophys. 559:38–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu S, You L, Zhao Y and Chang X: Hawthorn
polyphenol extract inhibits UVB-induced skin photoaging by
regulating MMP expression and type I procollagen production in
mice. J Agric Food Chem. 66:8537–8546. 2018. View Article : Google Scholar : PubMed/NCBI
|