Introduction
Osteosarcoma (OA) is a malignant and aggressive bone
tumor prevalent in children and young adults, representing 60% of
all bone tumors globally (1).
Although OA treatment including surgery and systemic chemotherapy
has progressed, local infiltration and distant metastasis are
frequent. For patients lacking tumor spread and metastasis, the
five-year survival rates are 60–80%. For patients with tumor
metastasis, the five-year survival rates decrease to 17% (2). A deeper understanding of the key
mechanisms promoting OA tumorigenesis and effective therapeutic
interventions towards OA are thus essential.
Melatonin is secreted by the pineal gland and plays
a cyto-protective role in the regulation of oxidative stress,
apoptosis-related factors and signaling pathways (3). Melatonin is beneficial during the
treatment of insomnia, obesity, type 2 diabetes and liver fibrosis
(4–6) and can inhibit hormone-dependent or
hormone-independent tumors (7).
Notably, melatonin was found to exert its anticancer activity
through various biological processes including chemosensitivity,
reduced drug resistance and anti-proliferative effects in ovarian,
breast, prostate, oral, gastric and colorectal cancers (8–10).
The detailed mechanisms underlying these effects and its antitumor
activity remain poorly defined. Epithelial-to-mesenchymal
transition (EMT) leads to cytological changes whereby tumor cells
become more invasive during metastasis and progression. According
to Menéndez-Menéndez et al (11), the antitumor effects of melatonin
on cell survival, invasion and the metastasis of breast cancer
cells occur through EMT regulation, as shown by the increased
levels of E-cadherin and loss of vimentin, Snail in cancer stem
cells (CSCs) (12). Research has
demonstrated that EMT transcription factors are key to OA
development (13). Here, we used
TGF-β1-induced EMT in OA cells to confirm the role of melatonin and
to explore new methods for OA treatment.
Materials and methods
Reagents
Melatonin, trypsin, MTT and Triton X-100 were
purchased from Sigma Chemical Co./Merck KGaA. Dulbecco's modified
Eagle's medium (DMEM), penicillin-streptomycin and fetal bovine
serum (FBS) were purchased from Gibco Laboratories (Thermo Fisher
Scientific, Inc.); TGF-β1 was purchased from (R&D); YC-1 (cat.
no. sc-202856) was purchased from Santa Cruz Biotechnology, Inc.
Antibodies against MMP-9 (cat. no. sc-13520), E-cadherin (cat. no.
sc-52327), N-cadherin (cat. no. sc-8424), vimentin (cat. no.
sc-53464), Snail (cat. no. sc-10437), β-actin (cat. no. sc-69879)
and HIF-1α (cat. no. sc-53546) were purchased from Santa Cruz
Biotechnology, Inc. The ECL kit was purchased from Pierce/Thermo
Fisher Scientific, Inc. RIPA buffer and the BCA protein assay kit
were purchased from Beyotime. PVDF membranes were purchased from
Millipore. All reagents used were trace element analysis grade. All
water used was glass distilled.
Cell culture
OS MG-63 cells were purchased from the Shanghai Cell
Bank (Shanghai, China). The cells were treated with DMEM containing
10% FBS and 1% penicillin/streptomycin at 37°C in 5% CO2
with 95% humidity. Cells were passaged at ~80% confluency.
Cell viability assays
MG-63 cells were seeded into 96-well plates at a
density of 2×104 cells/well and exposed to 0–1,000
nmol/l) melatonin for 24 h. MTT reagent (10 µl) was added to each
well and incubated for 4 h at 37°C. Reaction products were
extracted with DMSO (150 µl) and absorbances were recorded at ~450
nm on a microplate reader (Bio-Rad Laboratories, Inc.).
Western blot analysis
MG-63 cells were lysed in RIPA buffer and BCA assays
performed. Proteins (10 µg) were resolved by SDS-PAGE and
transferred to PVDF membranes. Membranes were blocked in 5% milk in
TBS (containing 0.5% Tween-20) and probed with primary antibodies
at 4°C overnight. The antibodies included: Anti-β-actin (dilution
1:400), anti-HIF-1α (dilution 1:400), anti-E-cadherin (dilution
1:400), anti-N-cadherin (dilution 1:400), anti-vimentin (dilution
1:400), anti-Snail (dilution 1:400), and anti-MMP-9 (dilution
1:400). After washing three times with TBS/0.1% Tween 20, the
membranes were labeled with HRP-conjugated secondary antibodies
(cat. no. sc-2030; dilution 1:1,000; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. Immunoreactive bands were
visualized using ECL. The intensity of the bands was quantified
using Image Lab software (version 2.1, Bio-Rad Laboratories, Inc.).
All blots were representative of three independent experiments.
Immunofluorescence
MG-63 cells were fixed in 4% paraformaldehyde,
permeabilized in 0.2% Triton X-100 for 5 min and blocked in 10%
AB-serum in 1% bovine serum albumin (BSA) for 30 min. Cells were
then washed and stained with anti-E-cadherin primary antibodies
(dilution 1:400) for 2 h at 37°C and incubated with
TRITC-conjugated fluorescent secondary antibodies (cat. no. BA1089;
dilution 1:100) for 30 min at room temperature. Nuclei were stained
with Hoechst 33342 for 10 min and cell morphology was examined
under an optical microscopy (magnification, ×400; Olympus
Corporation).
Transient transfections of Snail
cDNA
Snail was cloned into pcDNA3.1 (Genechem Co.) and
transiently transfected into MG-63 cells using Lipofectamine 2000
(Invitrogen/Thermo Fisher Scientific, Inc.). Cells were harvested
48 h post-transfection.
RT-PCR
Total RNA was extracted using TRIzol
(Invitrogen/Thermo Fisher Scientific, Inc.) and reverse transcribed
using SYBR PrimeScript RT-PCR kits (Takara Inc.) according to the
manufacturer's protocol. cDNAs were amplified by polymerase chain
reaction (PCR) using the primers shown in Table I. PCR reactions were performed
using a Gene Amp PCR system 9700 (PerkinElmer). Amplified products
were electrophoresed on 2% agarose gels and visualized by ethidium
bromide staining. Images were quantified using FluoroImager SI (GE
Healthcare). Representative results were shown (n=3).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Forward primer | Reverse primer |
|---|
| Snail |
AAGGCCTTCTCTAGGCCCT |
CGCAGGTTGGAGCGGTCAG |
| HIF-1α |
TTCCTTCTCTTCTCCGCGTG |
ACTTATCTTTTTCTTGTCGTTCGC |
| MMP-9 |
TTGACAGCGACAAGAAGTGG |
CCCTCAGTGAAGCGGTACAT |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
Statistical analysis
All statistical analyses were performed using SPSS
(version 19.0; IBM Corp.). Data are represented as the mean ± SD.
One-way ANOVA test was used for statistical comparisons. If
multigroup comparisons were made, then ANOVA was used together with
Scheffe post-hoc test (n=5). P<0.05 was considered to
indicate a statistically significant difference.
Results
TGF-β1-mediated EMT in MG-63
cells
EMT is key to cancer progression and can be induced
by TGF-β (14). MG-63 cells were
cultured with TGF-β1 (20 ng/ml) to assess its ability to induce EMT
in OA cells (15) through the
expression of known EMT markers including E-cadherin, vimentin and
N-cadherin by western blot analysis (Fig. 1A) and RT-PCR (Fig. 1B). E-cadherin was downregulated,
while N-cadherin and vimentin were significantly induced by TGF-β1
in a time-dependent manner. These data suggested that TGF-β1
triggers EMT in OA cells.
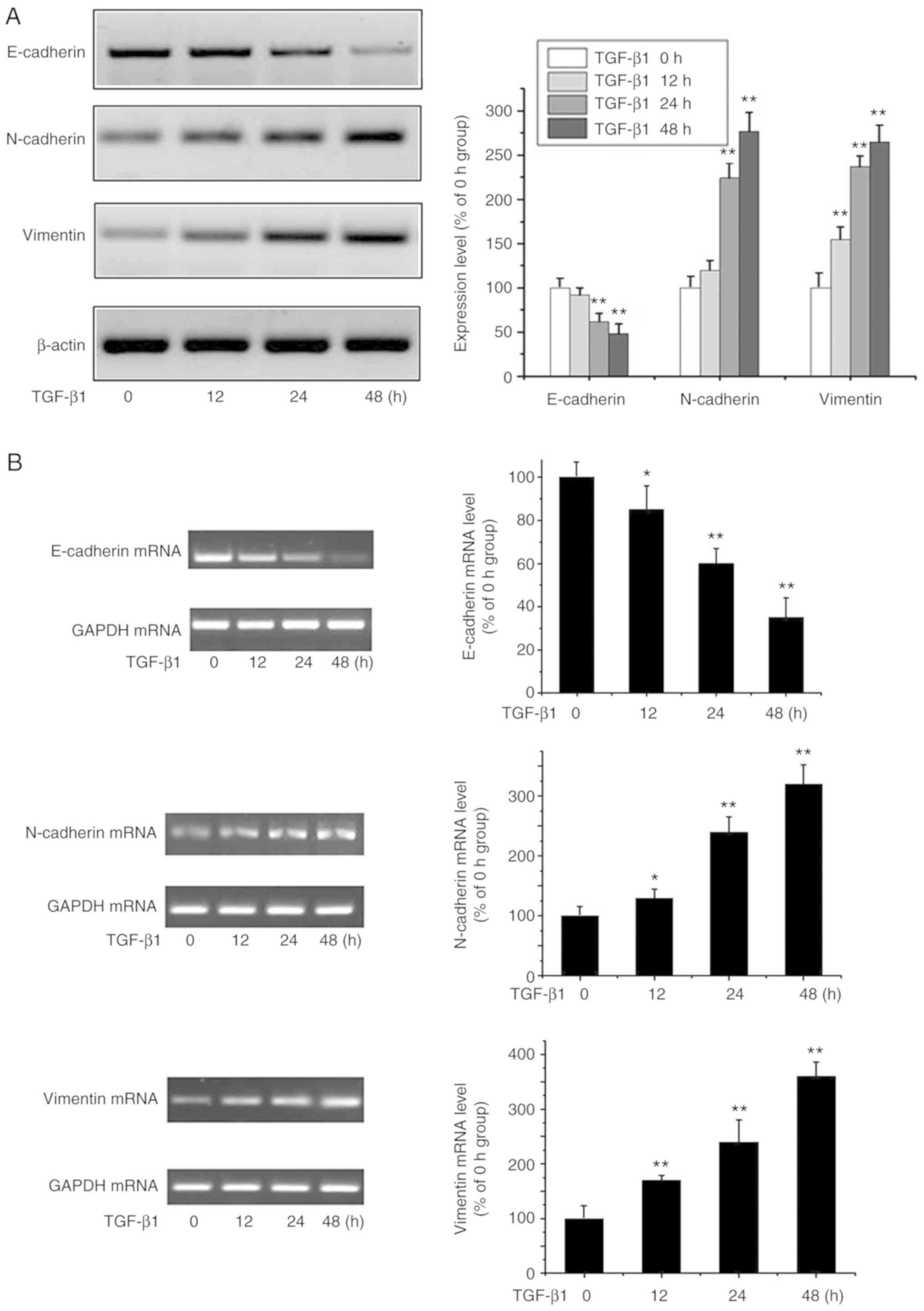 | Figure 1.EMT is triggered by TGF-β1. (A) OA
MG-63 cells were treated with TGF-β1 (20 ng/ml) for 0, 12, 24 and
48 h, and E-cadherin, N-cadherin, vimentin and β-actin were
analyzed by western blot analysis. Data are presented as means ± SD
of 3 independent experiments. β-actin was used as the loading
control. **P<0.01 vs. the 0 h group. (B) OS MG-63 cells were
treated with TGF-β1 (20 ng/ml) for 0, 12, 24 and 48 h, and the
levels of E-cadherin, N-cadherin, vimentin were detected by RT-PCR.
Data are presented as means ± SD (n=3). GAPDH was used as the
loading control. *P<0.05 and **P<0.01 vs. the 0 h group. OA,
osteosarcoma; EMT, epithelial-to-mesenchymal transition; TGF-β1,
transforming growth factor β1. |
Melatonin suppresses EMT in MG-63
cells
Previous studies have reported that melatonin
inhibits tumor invasion through EMT inhibition (16) but its effects on OA cells are
unclear. Through MTT assays, no significant changes were observed
in cell survival rates for the different concentrations (0, 50,
100, 200, 500 and 1,000 nM) of melatonin (Fig. 2A). In melatonin-containing media,
the morphology of the MG-63 cells was unchanged, and no apoptosis
occurred (Fig. 2B). In subsequent
experiments, 200 nM (intermediate concentration) of melatonin was
used which had minimal effect on MG-63 cell survival. However,
immunofluorescence and western blot analysis suggested that
melatonin partially reversed the loss of E-cadherin expression and
increase in N-cadherin and vimentin expression in response to
TGF-β1 (Fig. 2C and D). These
results show for the first time that melatonin can reverse EMT
processes in OA cells.
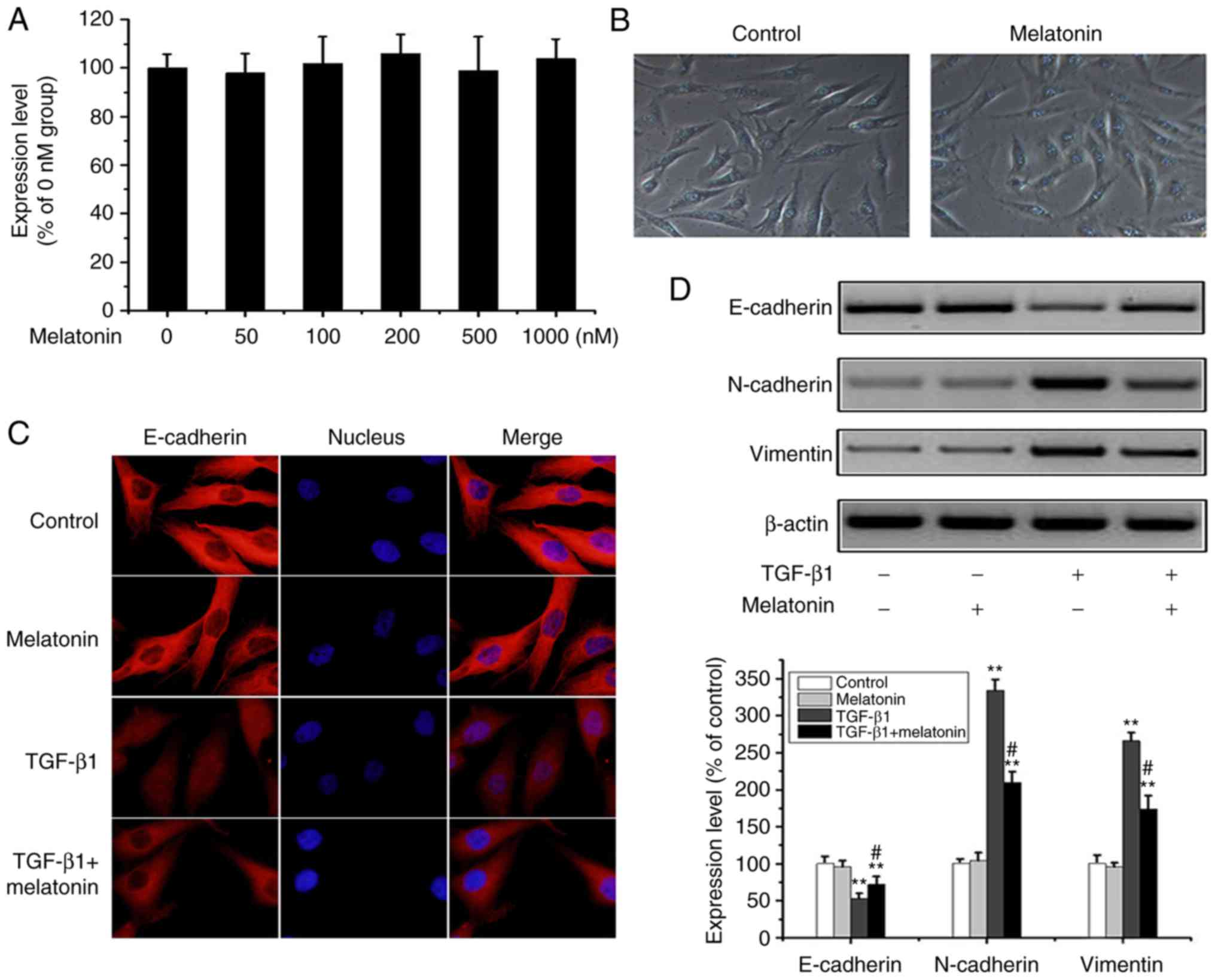 | Figure 2.Melatonin reverts TGF-β1-mediated EMT
in MG-63 cells. (A) OA MG-63 cells were treated with various doses
of melatonin (0–1,000 nM) for 24 h, and cell viability was examined
by MTT assay. Data are presented as means ± SD (n=3). (B) OS MG-63
cells were treated with 200 nM melatonin for 24 h, and cell
morphology was observed under bright-field microscopy
(magnification, ×100). (C) MG-63 cells were cultured with 20 ng/m
TGF-β1 in the presence or absence of 200 nM melatonin for 24 h, and
E-cadherin was detected by fluorescence microscopy (magnification,
×400). (D) Cells were treated as above, and E-cadherin, N-cadherin,
vimentin and β-actin were detected by western-blot analysis. The
results were representatives of three independent experiments.
β-actin was used as loading control. **P<0.01 vs. the control
group; #P<0.01, the TGF-β1 group vs. the TGF-β1 +
melatonin group). OA, osteosarcoma; EMT, epithelial-to-mesenchymal
transition; TGF-β1, transforming growth factor β1. |
Melatonin suppresses TGF-β1-mediated
EMT through the downregulation of Snail/MMP-9 and HIF-1α
Extensive research indicates that the Snail/MMP-9
signaling plays a vital role in EMT and tumor metastasis (17). To further explore the underlying
mechanism of the inhibitory effects of melatonin on TGF-β1-mediated
EMT, Snail/MMP-9 signaling were analyzed using western blot
analysis. Fig. 3A and B shows that
the levels of Snail and MMP-9 were upregulated in response to
TGF-β1 in a time-dependent manner. In addition, TGF-β1 activated
Snail/MMP-9 signaling while melatonin alone had no effects on
Snail/MMP-9 activation. The addition of melatonin to
TGF-β1-stimulated cells reversed the activation of Snail/MMP-9
signaling (Fig. 3C and D).
Similarly, the effects of melatonin on HIF-1α expression suggested
that melatonin attenuated TGF-β1 signaling through HIF-1α (Fig. 3E and F). Snail expression in
response to TGF-β1 was markedly downregulated in cells pretreated
with melatonin. Taken together, these data indicate that melatonin
exerts its inhibitory effects in part by antagonizing Snail/MMP-9
and HIF-1α pathways in OA cells.
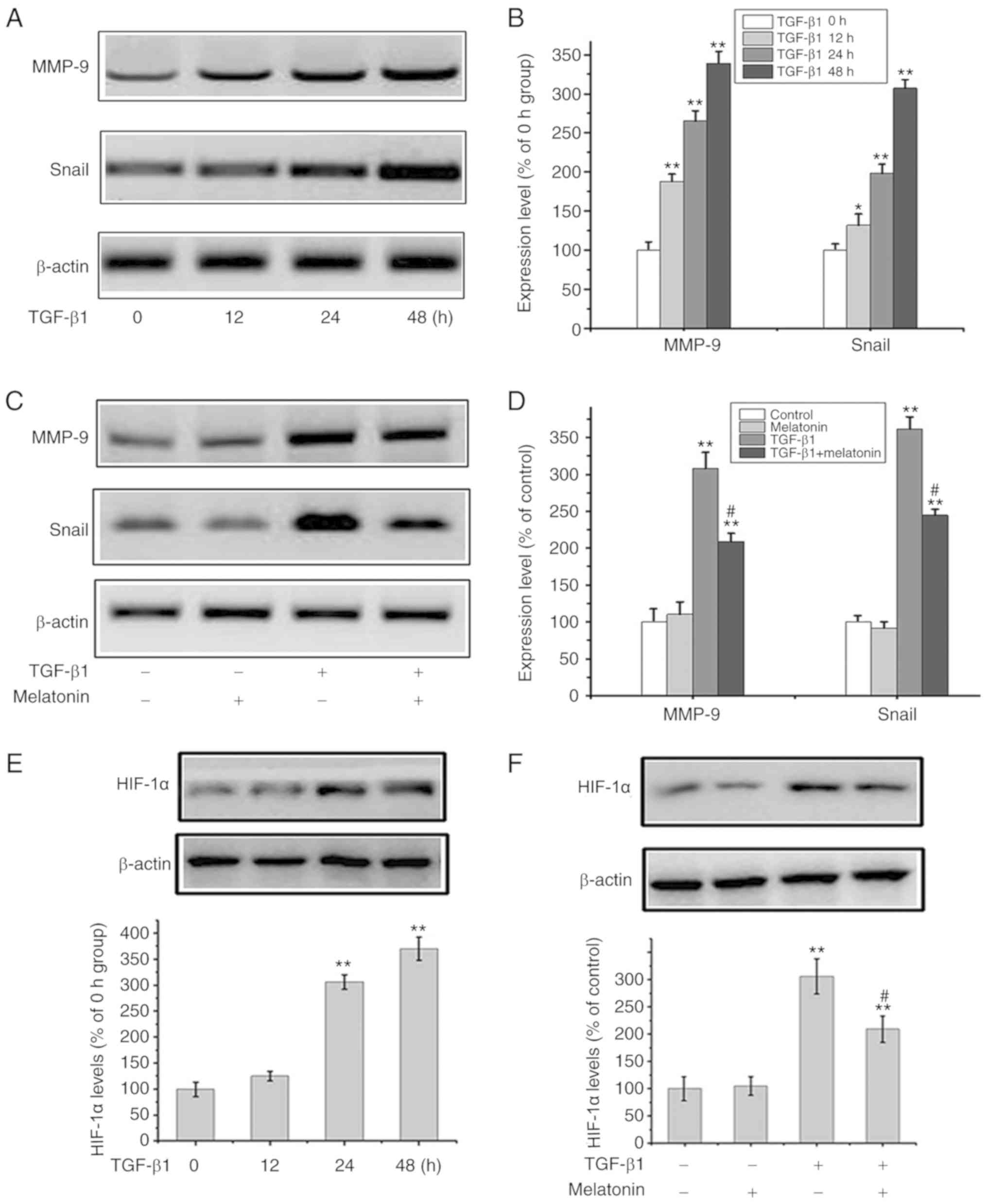 | Figure 3.Melatonin suppresses the Snail/MMP-9
and HIF-1α pathway. (A and B) OA MG-63 cells were exposed to TGF-β1
(20 ng/ml) for 0, 12, 24 and 48 h, and then MMP-9, Snail and
β-actin were assessed by western blot analysis. In B: Data are
presented as means ± SD of 3 independent experiments. β-actin was
used as the loading control. *P<0.05, **P<0.01 vs. the 0 h
group. (C and D) MG-63 cells were cultured with 20 ng/m TGF-β1 in
the presence or absence of 200 nM melatonin for 24 h, and MMP-9,
Snail and β-actin were detected by western blot analysis. In D:
Data are presented as means ± SD of 3 independent experiments.
β-actin was used as the loading control. **P<0.01 vs. the
control group; #P<0.01, TGF-β1 group vs. the TGF-β1 +
melatonin group. (E) MG-63 cells were exposed to TGF-β1 (20 ng/ml)
for 0, 12, 24 and 48 h, and HIF-1α and β-actin were assessed by
western blot analysis. Data are presented as means ± SD of 3
independent experiments. **P<0.01 vs. the 0 h group. (F) MG-63
cells were cultured with 20 ng/m TGF-β1 in the presence or absence
of 200 nM melatonin for 24 h, and HIF-1α and β-actin were detected
by western blot analysis. Data are presented as means ± SD of 3
independent experiments. **P<0.01 vs. the control group;
#P<0.01, TGF-β1 group vs. the TGF-β1 + melatonin
group. OA, osteosarcoma; HIF-1α, hypoxia-inducible factor 1α;
MMP-9, matrix metalloproteinase 9; TGF-β1, transforming growth
factor β1. |
Snail overexpression prevents
melatonin-mediated EMT suppression in MG-63 cells
The data obtained to this point suggested that
Snail/MMP-9 signaling regulates EMT. To further investigate the
effects of melatonin on Snail/MMP-9 signaling, Snail was
overexpressed in MG-63 cells (Fig.
4A). Snail overexpression was coupled to a marked reduction in
E-cadherin and increased expression of vimentin/N-cadherin. The
melatonin-mediated suppression of EMT in MG-63 cells was attenuated
through Snail overexpression (Fig.
4B). These data further confirmed that melatonin suppresses
Snail/MMP-9 signaling to inhibit EMT in OA cells.
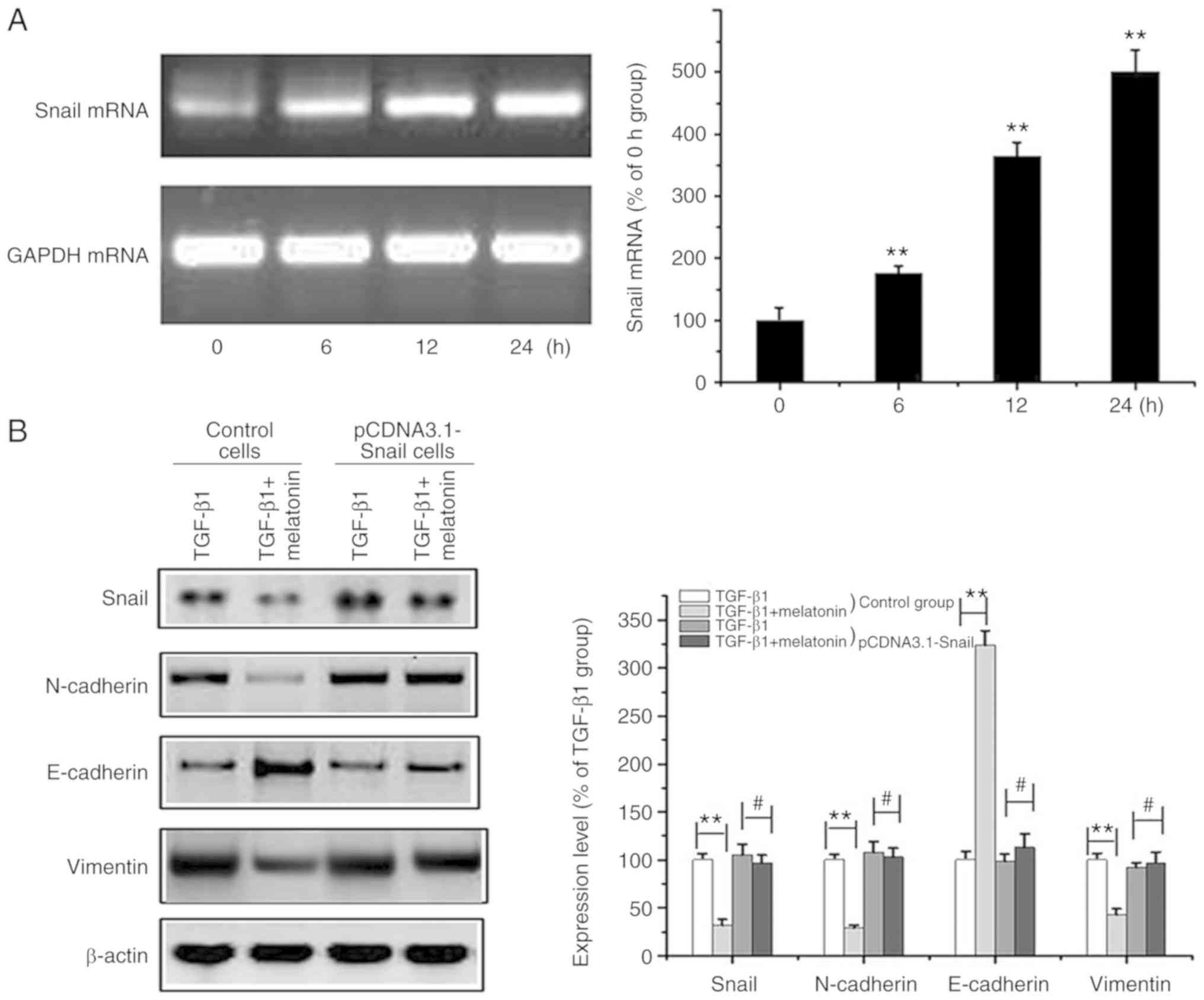 | Figure 4.Overexpression of Snail reverses
melatonin-mediated suppression of EMT in MG-63 cells. (A) OA MG-63
cells were transfected with the Snail-pcDNA3.1 plasmid and the
Snail mRNA level at different time intervals (0, 6, 12, 24 h) was
determined by RT-PCR. GAPDH was used as the loading control. Data
are represented as mean ± SD (n=5). **P<0.01 vs. the 0 h group.
(B) Control and Snail-overexpressing cells (pCDNA3.1-Snail) (after
transfection for 24 h) were exposed to 20 ng/m TGF-β1 in the
presence or absence of 200 nM melatonin for 24 h, and Snail,
E-cadherin, N-cadherin, vimentin and β-actin were measured by
western blot analysis. Data are presented as means ± SD of 3
independent experiments. β-actin was used as the loading control.
**P<0.01 control cells: TGF-β1 group vs. the TGF-β1 + melatonin
group; #P>0.05 pcDNA3.1-Snail cells: TGF-β1 group vs.
the TGF-β1 + melatonin group). OA, osteosarcoma; EMT,
epithelial-to-mesenchymal transition; TGF-β1, transforming growth
factor β1. |
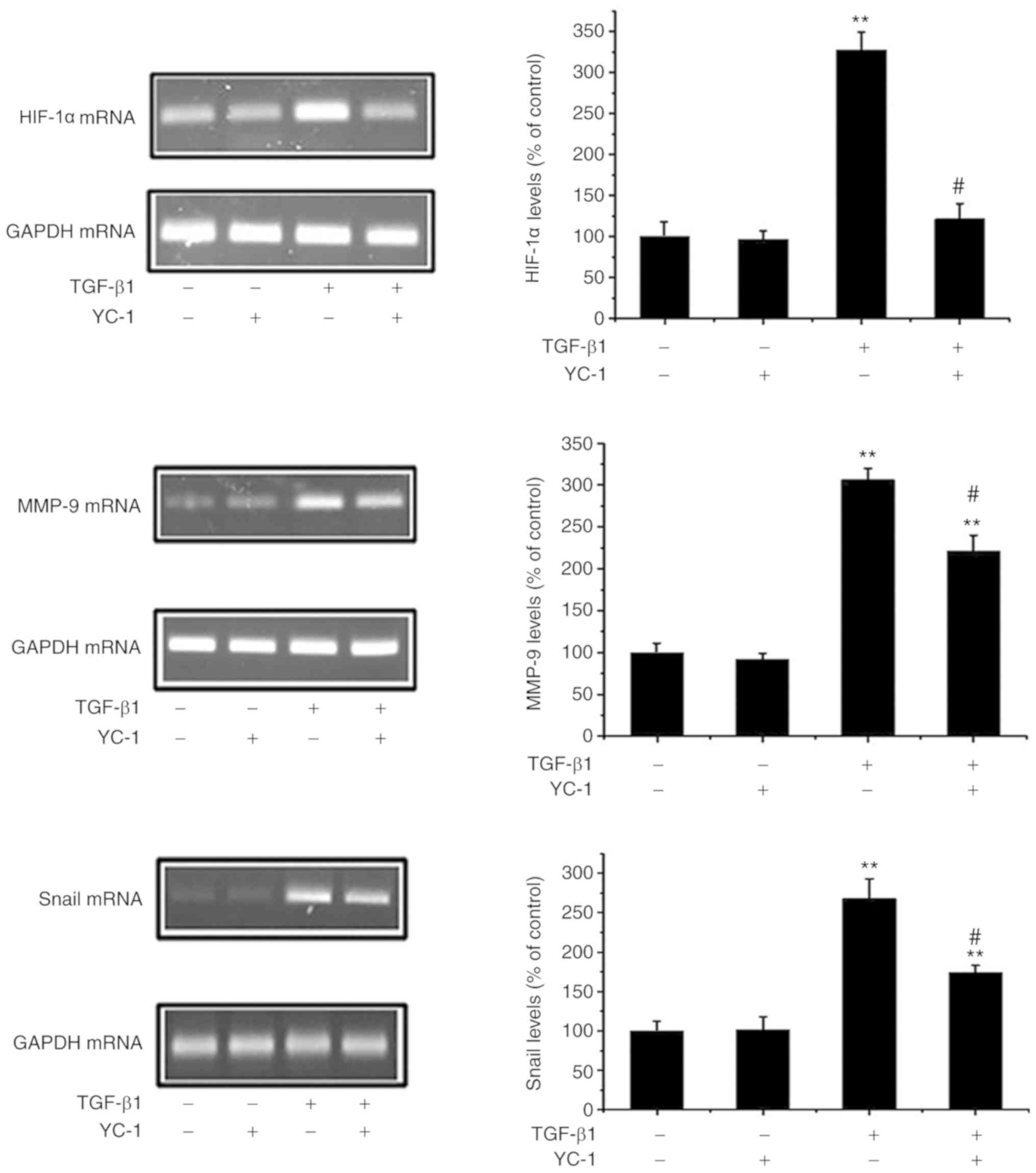
HIF-1α inhibition reverses the
TGF-β1-induced upregulation of Snail/MMP-9
HIF-1α can induce EMT and metastasis in cancer cells
(18). Next, it was ascertained
whether a loss of HIF-1α negatively affects the Snail/MMP pathways.
As shown in Fig. 5, the HIF-1α
inhibitor YC-1 not only downregulated HIF-1α expression, but
markedly inhibited the upregulation of Snail and MMP-9 in response
to TGF-β1. These data provide evidence that HIF-1α activates
Snail/MMP-9 expression and that inhibition of HIF-1α attenuates EMT
in MG-63 cells.
Discussion
Previous studies have confirmed that
epithelial-to-mesenchymal transition (EMT) is a key stage in the
transdifferentiation of epithelial cells and plays a central role
in disease progression, wound healing, fibrosis and cancer
(19,20). It is generally believed that the
EMT phenomenon only occurs in epithelial-derived cells. However,
recent studies have shown that certain mesenchymal cells can also
alter EMT-related protein and enhance the metastasis process
(21,22). Osteosarcoma (OA) is the most common
bone malignant tumor of mesenchymal origin. In an OA cell line, the
cells were found to regulate EMT-related protein expression and
enhance invasion and metastasis, which suggested that EMT is not
only the key step in epithelium-derived tumor cells but also in
mesenchymal cell-derived OA (23,24).
Thus, targeting EMT represents a key therapeutic goal for OA
treatment (25). In recent years,
melatonin has emerged as a key molecule for the prevention and
management of cancer due to its limited cytotoxicity and/or side
effects. The roles of melatonin in OA however, remain largely
uncharacterized.
Melatonin isolated from the bovine pineal has
numerous physiological functions including the control of the
circadian rhythm, sleep-wake rhythms, body temperature, neuronal
protection and immune activation (26–28).
Melatonin has strong therapeutic potential for various cancers
including prostate, breast and ovarian cancer (29,30).
Recent studies have demonstrated that melatonin treatment increases
apoptosis in breast cancer cells (31). It has also been reported that in
thyroid cancer, melatonin inhibits p65 phosphorylation and
subsequent redox stress (32).
Melatonin also exerts anticancer effects by indirectly regulating
the body's immune system (33).
Although an array of mechanisms have been proposed, few studies
have evaluated the role of melatonin on EMT. Similarly, the
anticancer potential of melatonin on OA cells is undefined.
In the present study, the role of melatonin in
inhibiting TGF-β1-mediated EMT was investigated and the signaling
pathways involved in this regulation were explored. Our findings
suggested that melatonin pretreatment provides effective protection
against TGF-β1-mediated EMT as evidenced by the downregulation of
N-cadherin and vimentin and the increased expression of E-cadherin
in MG-63 cells. The mechanisms of these effects were next
explored.
Snail regulates EMT and plays a crucial role in
tumor invasion and metastasis (34,35).
Naber et al reported that TGF-β is pro-invasive through its
activation of transcriptional repressors (including Slug and Snail)
thus inducing EMT (36). In this
study, it was demonstrated that melatonin inhibits TGF-β1-induced
Snail expression in MG-63 cells. Melatonin exerted its inhibitory
effects in part by antagonizing Snail/MMP-9 signaling in OA cells.
Moreover the overexpression of Snail prevented EMT suppression in
response to melatonin. Thus, targeting EMT and inhibiting
Snail/MMP-9 signaling represents a promising strategy to prevent
metastasis and improve the survival of OA patients.
Melatonin suppresses the viability and angiogenesis
of cancer cells through the downregulation of HIF-1α/ROS/VEGF in
solid tumors containing abundant blood vessels (37). HIF-1α also serves an important role
in EMT processes and tumor metastasis (38). Our results demonstrated that
melatonin inhibits HIF-1α expression which is stimulated by TGF-β1
in MG-63 cells. We next studied the effects of HIF-1α on
Snail/MMP-9 signaling. YC-1 inhibited TGF-β1-mediated EMT in MG-63
cells through its ability to inhibit HIF-1α signaling. This
demonstrated that melatonin inhibits Snail/MMP-9 signaling in
response to TGF-β1 via inhibiting HIF-1α expression.
In summary, the present study demonstrated that
melatonin attenuates TGF-β1-mediated EMT in MG-63 cells by
preventing TGF-β1-induced activation of the Snail/MMP-9 and HIF-1α
signaling pathways. These findings provide new insight into the
mechanisms by which melatonin prevents the development and invasion
of OA. These findings also provide experimental evidence for the
development of new strategies for OA treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from the Inner Mongolia Autonomous Region Natural Science Fund
Project (grant nos. 2018MS08145 and 2014MS0812), the Baotou Medical
College Natural Science Fund Sailing Project (grant nos. YF201687
and BYJJ-YF201718) and the Baotou Science and Technology Plan
Project (grant no. wsjj2017027).
Availability of data and materials
The datasets used and/or anlayzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and TZ conceived and designed the study. XL, ZL,
DZ, WX and YC performed the experiments. TZ and ZL wrote the paper.
YC and WX reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Review Board of the Department of Laboratory Animal
Science of Baotou Medical College (Baotou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OA
|
osteosarcoma
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
MMP-9
|
matrix metalloproteinase 9
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
PBS
|
phosphate-buffered saline
|
|
TBS
|
Tris-buffered saline
|
|
TGF
|
transforming growth factor
|
|
FBS
|
fetal bovine serum
|
|
PVDF
|
polyvinylidene fluoride
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Basu-Roy U, Basilico C and Mansukhani A:
Perspectives on cancer stem cells in osteosarcoma. Cancer Lett.
338:158–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hardeland R, Cardinali DP, Srinivasan V,
Spence DW, Brown GM and Pandi-Perumal SR: Melatonin-a pleiotropic,
orchestrating regulator molecule. Prog Neurobiol. 93:350–384. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fernández Vázquez G, Reiter RJ and Agil A:
Melatonin increases brown adipose tissue mass and function in
Zücker diabetic fatty rats: Implications for obesity control. J
Pineal Res. 64:e124722018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karamitri A and Jockers R: Melatonin in
type 2 diabetes mellitus and obesity. Nat Rev Endocrinol.
15:105–125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haeger P, Bouchet A, Ossandon C and Bresky
G: Treatment with melatonin improves cognitive behavior and motor
skills in a rat model of liver fibrosis. Ann Hepatol. 18:101–108.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Li S, Zhou Y, Meng X, Zhang JJ, Xu
DP and Li HB: Melatonin for the prevention and treatment of cancer.
Oncotarget. 8:39896–39921. 2017.PubMed/NCBI
|
|
8
|
Parkin DM: International variation.
Oncogene. 23:6329–6340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jablonska K, Pula B, Zemla A, Kobierzycki
C, Kedzia W, Nowak-Markwitz E, Spaczynski M, Zabel M,
Podhorska-Okolow M and Dziegiel P: Expression of the MT1 melatonin
receptor in ovarian cancer cells. Int J Mol Sci. 15:23074–23089.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reiter RJ, Rosales-Corral SA, Tan DX,
Acuna-Castroviejo D, Qin L, Yang SF and Xu K: Melatonin, a full
service anti-cancer agent: Inhibition of initiation, progression
and metastasis. Int J Mol Sci. 18(pii): E8432017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Menéndez-Menéndez J, Hermida-Prado F,
Granda-Díaz R, González A, García-Pedrero JM, Del-Río-Ibisate N,
González-González A, Cos S, Alonso-González C and Martínez-Campa C:
Deciphering the molecular basis of melatonin protective effects on
breast cells treated with doxorubicin: TWIST1 a transcription
factor involved in EMT and metastasis, a novel target of melatonin.
Cancers (Basel). 11(pii): E10112019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao L, Dauchy RT, Blask DE, Slakey LM,
Xiang S, Yuan L, Dauchy EM, Shan B, Brainard GC, Hanifin JP, et al:
Circadian gating of epithelial-to-mesenchymal transition in breast
cancer cells via melatonin-regulation of GSK3β. Mol Endocrinol.
26:1808–1820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seba V, Silva G, Santos MBD, Baek SJ,
França SC, Fachin AL, Regasini LO and Marins M: Chalcone
derivatives 4′-amino-1-naphthyl-chalcone (D14) and
4′-amino-4-methyl-1-naphthyl-chalcone (D15) suppress migration and
invasion of osteosarcoma cells mediated by p53 regulating
EMT-related genes. Int J Mol Sci. 19(pii): E28382018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki S, Toyoma S, Tsuji T, Kawasaki Y
and Yamada T: CD147 mediates transforming growth factor-β1-induced
epithelial-mesenchymal transition and cell invasion in squamous
cell carcinoma of the tongue. Exp Ther Med. 17:2855–2860.
2019.PubMed/NCBI
|
|
15
|
Li L, Qi L, Liang Z, Song W, Liu Y, Wang
Y, Sun B, Zhang B and Cao W: Transforming growth factor-β1 induces
EMT by the transactivation of epidermal growth factor signaling
through HA/CD44 in lung and breast cancer cells. Int J Mol Med.
36:113–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gonçalves Ndo N, Colombo J, Lopes JR,
Gelaleti GB, Moschetta MG, Sonehara NM, Hellmén E, Zanon Cde F,
Oliani SM and Zuccari DA: Effect of melatonin in epithelial
mesenchymal transition markers and invasive properties of breast
cancer stem cells of canine and human cell lines. PLoS One.
11:e01504072016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moirangthem A, Bondhopadhyay B, Mukherjee
M, Bandyopadhyay A, Mukherjee N, Konar K, Bhattacharya S and Basu
A: Simultaneous knockdown of uPA and MMP9 can reduce breast cancer
progression by increasing cell-cell adhesion and modulating EMT
genes. Sci Rep. 6:219032016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ha JH, Ward JD, Radhakrishnan R, Jayaraman
M, Song YS and Dhanasekaran DN: Lysophosphatidic acid stimulates
epithelial to mesenchymal transition marker Slug/Snail2 in ovarian
cancer cells via Gαi2, Src, and HIF1α signaling nexus. Oncotarget.
7:37664–37679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JH and Yoon J: Schizandrin inhibits
fibrosis and epithelial-mesenchymal transition in transforming
growth factor-β1-stimulated AML12 cells. Int Immunopharmacol.
25:276–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amaar YG and Reeves ME: RASSF1C regulates
miR-33a and EMT marker gene expression in lung cancer cells.
Oncotarget. 10:123–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rubina KA, Surkova EI, Semina EV, Sysoeva
VY, Kalinina NI, Poliakov AA, Treshalina HM and Tkachuk VA:
T-Cadherin expression in melanoma cells stimulates stromal cell
recruitment and invasion by regulating the expression of
chemokines, integrins and adhesion molecules. Cancers (Basel).
7:1349–1370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang H, Nie C, Qin X, Zhou J and Zhang L:
Diosgenin inhibits the epithelial-mesenchymal transition initiation
in osteosarcoma cells via the p38MAPK signaling pathway. Oncol
Lett. 18:4278–4287. 2019.PubMed/NCBI
|
|
23
|
Fan S, Gao X, Chen P and Li X:
Carboxypeptidase E-ΔN promotes migration, invasiveness, and
epithelial-mesenchymal transition of human osteosarcoma cells via
the Wnt-β-catenin pathway. Biochem Cell Biol. 97:446–453. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao H, Peng C, Lu X, Guo M, Yang T, Zhou
J and Hai Y: PDCD5 inhibits osteosarcoma cell metastasis via
targeting TGF-β1/Smad signaling pathway and is associated with good
prognosis. Am J Transl Res. 11:1116–1128. 2019.PubMed/NCBI
|
|
25
|
Sung JY, Park SY, Kim JH, Kang HG, Yoon
JH, Na YS, Kim YN and Park BK: Interferon consensus
sequence-binding protein (ICSBP) promotes epithelial-to-mesenchymal
transition (EMT)-like phenomena, cell-motility, and invasion via
TGF-β signaling in U2OS cells. Cell Death Dis. 5:e12242014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baba K, Davidson AJ and Tosini G:
Melatonin entrains PER2:LUC bioluminescence circadian rhythm in the
mouse cornea. Invest Ophthalmol Vis Sci. 56:4753–4758. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dijk DJ, Duffy JF, Riel E, Shanahan TL and
Czeisler CA: Ageing and the circadian and homeostatic regulation of
human sleep during forced desynchrony of rest, melatonin and
temperature rhythms. J Physiol. 516:611–627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jenwitheesuk A, Nopparat C, Mukda S,
Wongchitrat P and Govitrapong P: Melatonin regulates aging and
neurodegeneration through energy metabolism, epigenetics, autophagy
and circadian rhythm pathways. Int J Mol Sci. 15:16848–16884. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao L, Summers W, Xiang S, Yuan L, Dauchy
RT, Reynolds A, Wren-Dail MA, Pointer D, Frasch T, Blask DE and
Hill SM: Melatonin represses metastasis in Her2-postive human
breast cancer cells by suppressing RSK2 expression. Mol Cancer Res.
14:1159–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tai SY, Huang SP, Bao BY and Wu MT:
Urinary melatonin-sulfate/cortisol ratio and the presence of
prostate cancer: A case-control study. Sci Rep. 6:296062016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sonehara NM, Lacerda JZ, Jardim-Perassi
BV, de Paula Jr R Jr, Moschetta-Pinheiro MG, Souza YST, de Andrade
JCJ and De Campos Zuccari DAP: Melatonin regulates tumor
aggressiveness under acidosis condition in breast cancer cell
lines. Oncol Lett. 17:1635–1645. 2019.PubMed/NCBI
|
|
32
|
Zou ZW, Liu T, Li Y, Chen P, Peng X, Ma C,
Zhang WJ and Li PD: Melatonin suppresses thyroid cancer growth and
overcomes radioresistance via inhibition of p65 phosphorylation and
induction of ROS. Redox Biol. 16:226–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fic M, Gomulkiewicz A, Grzegrzolka J,
Podhorska-Okolow M, Zabel M, Dziegiel P and Jablonska K: The impact
of melatonin on colon cancer cells' resistance to doxorubicin in an
in vitro study. Int J Mol Sci. 18(pii): E13962017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo L, Sun C, Xu S, Xu Y, Dong Q, Zhang L,
Li W, Wang X, Ying G and Guo F: Knockdown of long non-coding RNA
linc-ITGB1 inhibits cancer stemness and epithelial-mesenchymal
transition by reducing the expression of Snail in non-small cell
lung cancer. Thorac Cancer. 10:128–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naber HP, Drabsch Y, Snaar-Jagalska BE,
ten Dijke P and van Laar T: Snail and Slug, key regulators of
TGF-β-induced EMT, are sufficient for the induction of single-cell
invasion. Biochem Biophys Res Commun. 435:58–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng J, Yang HL, Gu CJ, Liu YK, Shao J,
Zhu R, He YY, Zhu XY and Li MQ: Melatonin restricts the viability
and angiogenesis of vascular endothelial cells by suppressing
HIF-1α/ROS/VEGF. Int J Mol Med. 43:945–955. 2019.PubMed/NCBI
|
|
38
|
Singh SK, Mishra MK and Singh R:
Hypoxia-inducible factor-1α induces CX3CR1 expression and promotes
the epithelial to mesenchymal transition (EMT) in ovarian cancer
cells. J Ovarian Res. 12:422019. View Article : Google Scholar : PubMed/NCBI
|