Introduction
Recurrent miscarriage is a highly heterogeneous
disease, which is defined as at least three pregnancy losses before
22 weeks of gestation, and affects approximately 0.5–3% of fertile
couples (1,2). The pathogenesis of recurrent
spontaneous miscarriage is complicated and remains mostly unknown.
Immunological factors and inflammation have been reported to
participate in the pathological process of recurrent miscarriage
(3–9).
A disintegrin and metalloproteinase with
thrombospondin motifs 7 (ADAMTS-7) is a proteolytic member of the
ADAMTS family (a disintegrin and metalloproteinase with
thrombospondin-like motifs) and has been identified to exert
important effects on inflammatory diseases, such as arthritis and
atherosclerosis (10–12). Previous studies have demonstrated
that systematic inflammation is correlated with recurrent
implantation failure and recurrent spontaneous abortion (SA) in
humans (13). Over the past few
decades, Th17 cells have been reported to be involved in women with
reproductive failure. As a secreted factor of Th17 cells, the
expression of IL-17 in decidua is clearly higher in inevitable
abortion and in unexplained recurrent SA individuals than in the
control group (14,15). In addition, IL-17A, an important
regulatory factor participating in the pathological process of
recurrent SA, has been found to enhance the expression of ADAMTS-7
in human nucleus pulposus cells (16). Therefore, a potential role of
ADAMTS-7 in SA was proposed.
Accumulative evidence has indicated that women with
adverse pregnancy outcomes are associated with a higher risk of
suffering from cardiovascular disease, with preeclampsia as the
most documented (17,18). It is also reported that women with
a history of recurrent miscarriage are more likely to experience
cardiovascular disease (19–21).
Therefore, an underlying association was proposed between the
molecular mechanism of recurrent miscarriage and cardiovascular
disease. A recent study found that circulating ADAMTS-7 levels
could reflect the degree of ventricle remodeling after acute
myocardial infarction in a rat model (22). Moreover, it was also reported that
ADMATS-7 participates in the pathological process of
atherosclerosis through degradation of cartilage oligomeric matrix
protein (COMP) (23–25), which has been confirmed to be
involved in some pathophysiological pathways, including vascular
calcification, atherosclerosis and neointima formation post-injury
(26,27).
Our study aimed to detect the expression of ADMATS-7
and its substrate COMP in the decidua of both LPS-treated mice and
humans with a history of SA as well as the corresponding control
group. We aimed to find a potential association between the
expression of ADAMTS-7 and COMP in the decidua of LPS-treated mice
and SA humans, which would be helpful to further understand the
pathogenesis of recurrent SA in order to find effective measures
for treating this disease.
Materials and methods
Animals
Eight- to ten-week-old C57/BL mice were purchased
from Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China). The mice were housed in an environment controlled
for temperature (22~24°C) and conditions of light (12 h light and
12 h darkness), with free access to standard mouse food and water.
2 female mice were caged with one male mouse every other day, and
vaginal plugs were checked the next morning as a sign of mating
behavior. The day of plug detection was defined as embryonic day
0.5 (E 0.5). The pregnant mice were randomly divided into
lipopolysaccharide (LPS) group and control group, with 10 mice in
each group. LPS (Sigma, Saint Louis, MO, USA) at a dose of 2.5 µg
dissolved in saline was intraperitoneally injected in pregnant
C57BL/6 female mice at E 7.5 to induce abortion. Mice in control
group received the same volume of saline as the vehicle. At E10.5,
the mice were sacrificed and the embryo resorption rate were
calculated by dividing the number of resorbed embryos by the number
of implantations, and the decidua were collected for further
experiments. All of the experiments involving animals in this study
were carried out in accordance with the protocols of the Guidelines
for the Care and Use of Laboratory Animals published by the United
States National Institutes of Health (NIH Publication, revised
2011) and the Guidelines for the Care and Use of Laboratory Animals
of the Chinese Animal Welfare Committee. The procedures were
approved by the Animal Use Committees of Renmin Hospital of Wuhan
University (Wuhan, China).
Human study
The procedures involving human experiments were
performed in accordance with the Declaration of Helsinki and were
approved by the Human Research Ethics Committees of Renmin Hospital
of Wuhan University (Wuhan, China). All participants were patients
who went to the department of obstetrics and gynecology for
treatment, and informed consent was signed by each participant.
People with a history of SA before 22 weeks of gestation were
divided into the SA group. People who went to the hospital for
artificial abortion were placed into the control group. Subjects
who had a history of autoimmune- or thyroid-related disease,
uterine malformation, ultrasonographic evidence of hydrosalpinx,
and hormone therapy within three months were excluded from this
study. All participants shared similar baseline demographics.
Finally, a total of 36 people were involved in this study, with 15
people in the control group and 21 people in the SA group.
Immunohistochemistry
Decidua tissue was collected and washed by PBS three
times. The decidua tissues were then put into 10% formaldehyde at
room temperature for 24 h, followed by deparaffinization and
dehydration. Then, the samples were cut into 5-µm sections for
further immunohistochemistry detection. Endogenous peroxidase was
blocked with 3% H2O2. Non-specific staining
was blocked using 5% bovine serum albumin for 20 min. Subsequently,
the sections were incubated with primary antibody targeted to
ADAMTS-7 (1:200) and COMP (1:200) at 4°C overnight. Thereafter, the
decidua sections were exposed to biotinylated sheep anti-rabbit
immunoglobulin G solution (Proteintech Group, Inc.) at 37°C for 30
min followed by incubation with horseradish peroxidase-labeled
streptavidin (Proteintech Group, Inc.) at 37°C for 30 min. After
washing with PBS three times, the sections were counterstained with
hematoxylin and dehydrated. Then, the sections were observed under
a microscope (Nikon E100; Nikon Corporation, Tokyo, Japan), and the
photomicrographs were obtained by Photo Imaging System (Canon 600D;
Canon, Inc., Tokyo, Japan). A brown color was regarded as a
positive signal. The optic density was detected by Image-Pro Plus
6.0, and the mean of the integrated optical density was obtained to
represent the expression of ADAMTS-7 and COMP.
Western blot and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Briefly, the decidua were weighed, cut and
homogenized in a 1:10 (w/v) RIPA buffer using a homogenizer. After
chilling on ice for 30 min, the suspension was centrifuged at 1,409
× g at 4°C for 15 min and the supernatants were collected. Then,
the protein concentration was determined using a BCA Protein Assay
kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
total of 20 µg protein were separated on SDS-PAGE gels and then
transferred to a PVDF membrane. Subsequently, the membrane was
blocked with 5% non-fat milk dissolved in TBST at room temperature
for 2 h. Then, the membrane was incubated with the following
primary antibodies at 4°C overnight: Rabbit anti-GAPDH antibody
(1:5,000), rabbit anti-ADAMTS-7 antibody (1:1,000, ab28557; Abcam,
Cambridge, UK) and rabbit anti-COMP antibody (1:1,000, ab42225;
Abcam). After washing the membrane with TBST three times, the
membranes were incubated with secondary antibody for 2 h at room
temperature. Finally, the bands were scanned using a two-color
infrared imaging system (Odyssey; LI-COR, Lincoln, NE, USA). Band
intensity was quantified using Odyssey, as described previously,
and the densities of the bands were normalized to GAPDH.
Total RNA was extracted using the Trizol
(Invitrogen; Thermo Fisher Scientific, Inc.) method according to
the manufacturer's instructions. RNA concentration was detected
using an absorbance at 260 and 280 nm (A260/280). Total RNA was
reverse-transcribed into cDNAs using a PrimeScript™ RT-PCR Kit
(04896866001; Roche Diagnostics, Indianapolis, IN, USA).
Quantitative RT-PCR analysis was conducted using LightCycler 480
SYBR Green 1 Master Mix (04707516001; Roche Diagnostics). The
primer sequences for Adamts-7 were as follows: Forward:
5′-TCACCAGGTTCCTTGACCGTG-3′ and reverse:
5′-CCAGCTTGGAGTGACAGGTGGT-3′. The primer sequences for Comp
were as follows: Forward: 5′-GCGCCAGTGTCGCAAGGACAA-3′ and reverse:
5′-TGGGTTTCGAACCAGCGGGC-3′. The primer sequences for Gapdh
were as follows: Forward: 5′-TCATCAACGGGAAGCCCATC-3′ and reverse:
5′-CTCGTGGTTCACACCCATCA-3′. Expression of Adamts-7 and Comp was
determined using the 2−ΔΔCq method and normalized to
Gapdh (28).
Statistical analysis
All data were expressed as the mean ± standard
deviation. The group sizes of the experiments were estimated based
on power analysis of ADAMTS-7 mRNA expression, with an α error of
5% and a power of 80%, which is consistent with a published article
(29). To detect a 10% change in
ADAMTS-7 mRNA expression with an expected SD of 5%, we needed 5
animals per group. We had 10 mice in each group, and for humans,
the sample size in the SA and control groups was 21 and 15,
respectively, which fulfilled the requirement. Statistical analysis
was performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA). All the
data in the present study were normally distributed (P<0.05), as
determined by the Kolmogorov-Smirnov's test. The difference between
two groups was then evaluated using independent samples t-tests
(two-sided). The correlation between ADMATS-7 and COMP was assessed
by Spearman's analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
The expression of ADAMTS-7 and COMP in
mouse decidua of the LPS-induced abortion group and the control
group
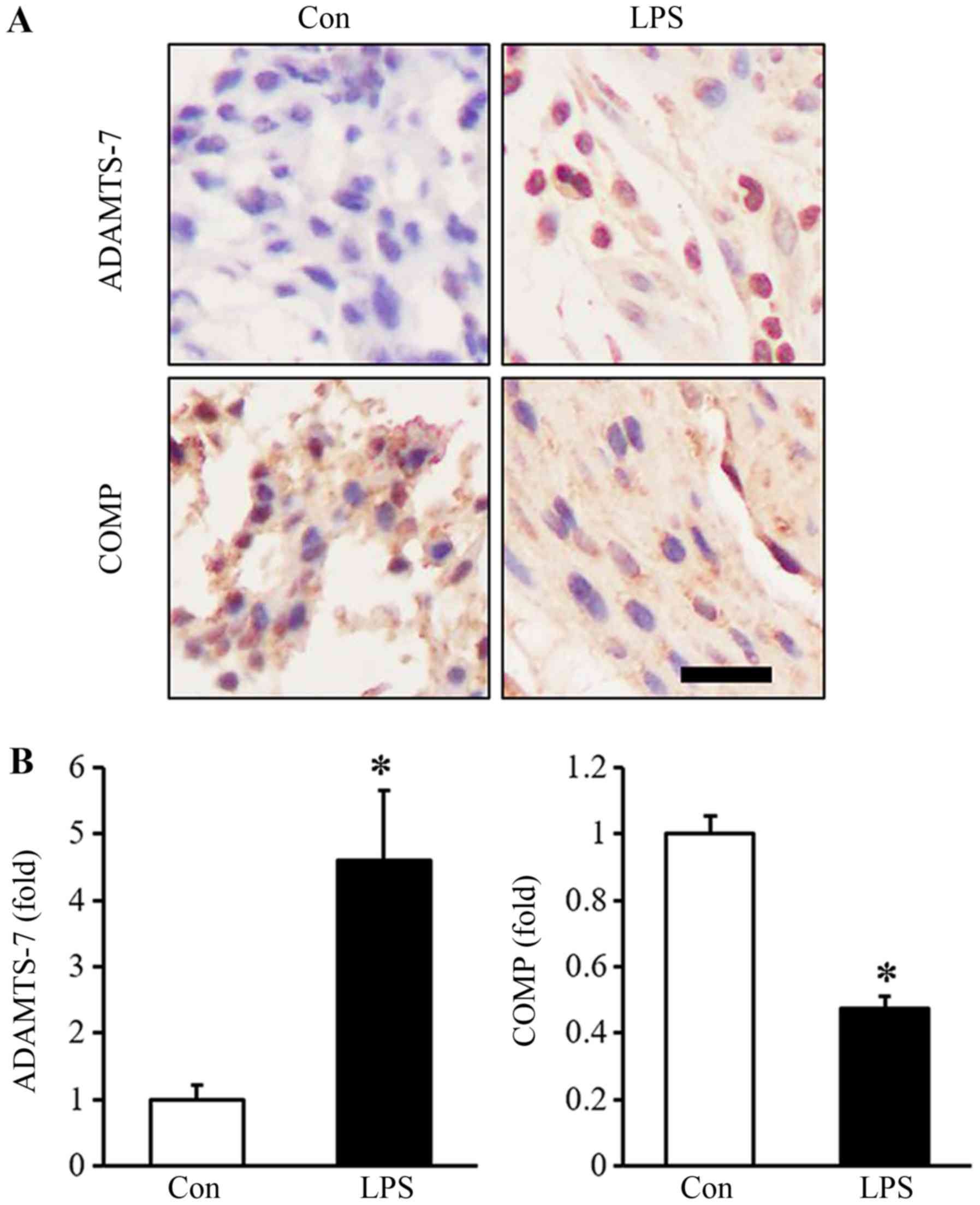
Embryo resorption was 100% after LPS treatment. As
shown in Fig. 1, the expression of
ADAMTS-7 was clearly increased in the mouse decidua of the
LPS-induced abortion group (Fig.
1). As a downstream substrate of ADAMTS-7, the expression of
COMP was clearly decreased in the mouse decidua of LPS group
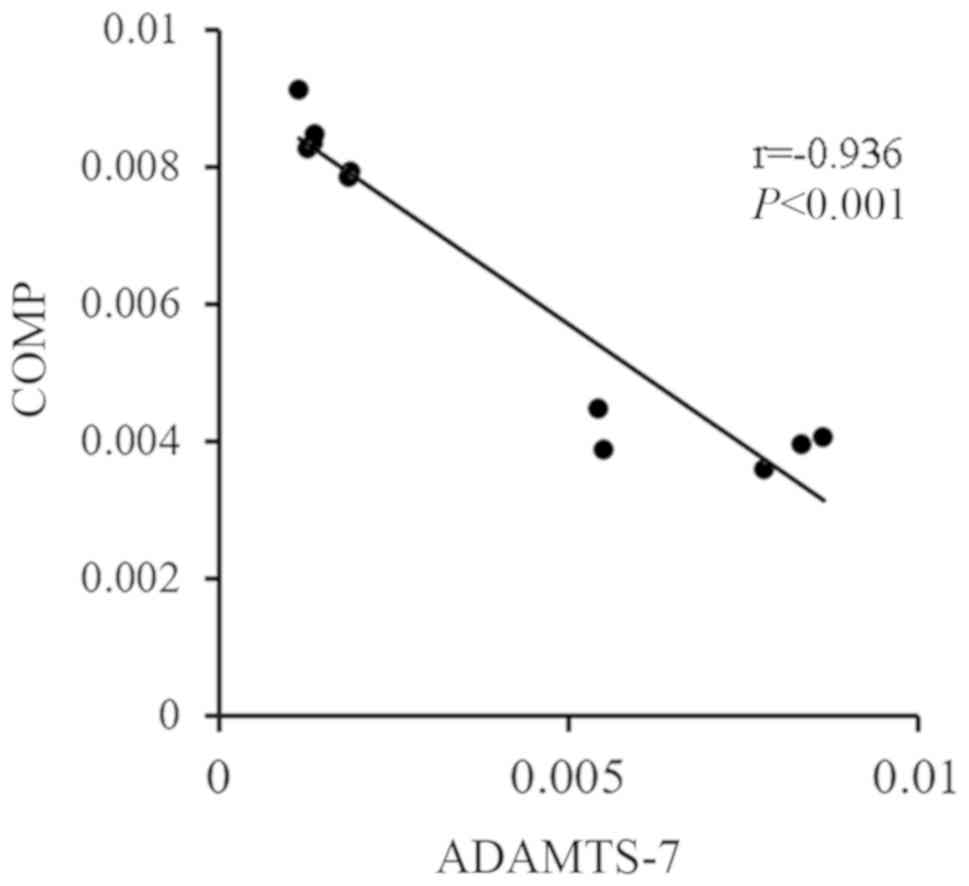
compared with that of the control group (Fig. 1). Spearman's analysis showed that
the expression of ADMATS-7 was negatively correlated with the
expression of COMP in mouse decidual tissue, which had a
correlation coefficient of −0.936 (P<0.001; Fig. 2).
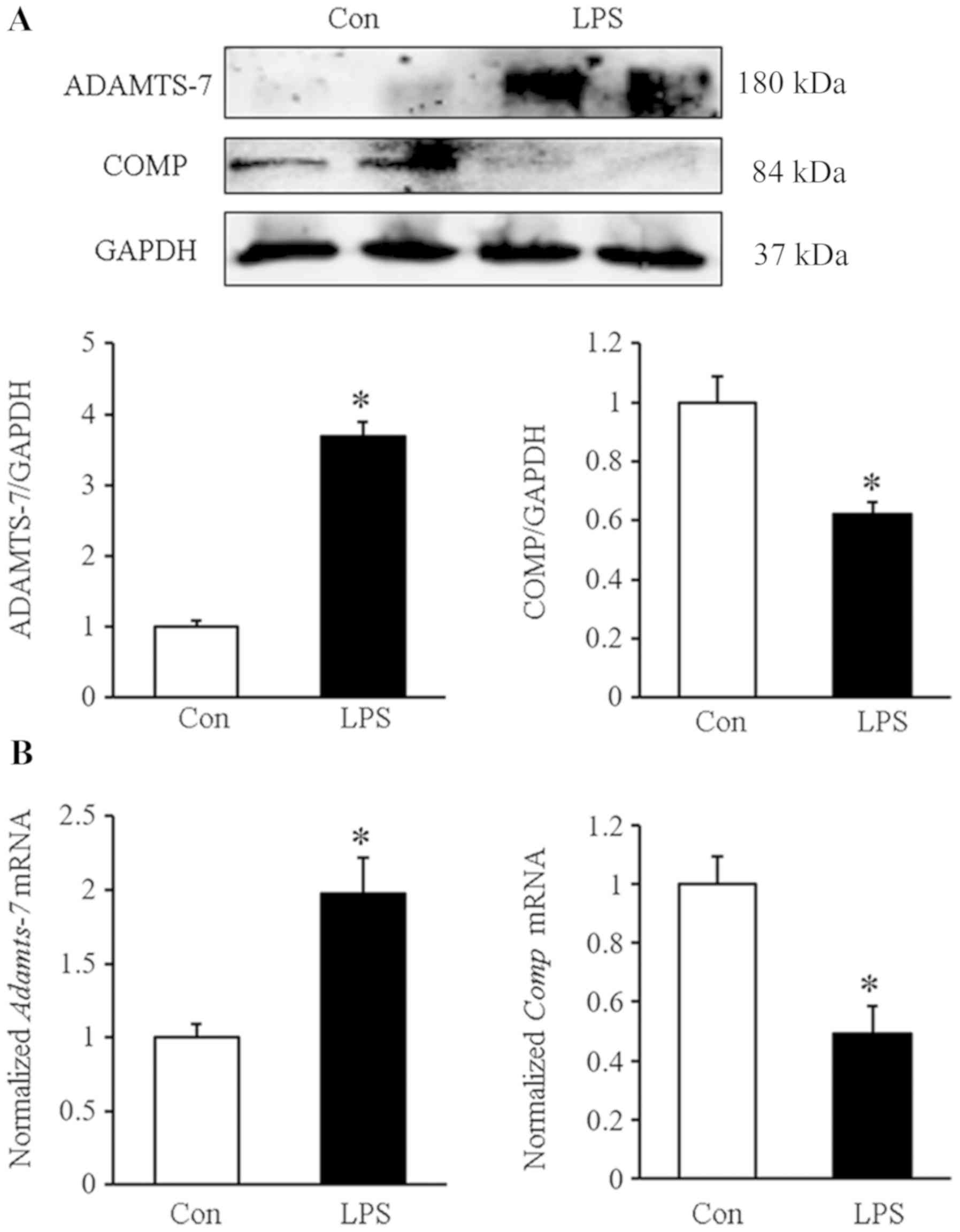
Western blot analysis revealed that the protein
level of ADAMTS-7 was increased in the LPS group compared with the
control group. Additionally, the expression of COMP was decreased
in the decidual tissue of the LPS group (Fig. 3A). The mRNA levels of
Adamts-7 and Comp showed similar results, as
demonstrated by an increased Adamts-7 mRNA level and
decreased Comp mRNA level (Fig.
3B).
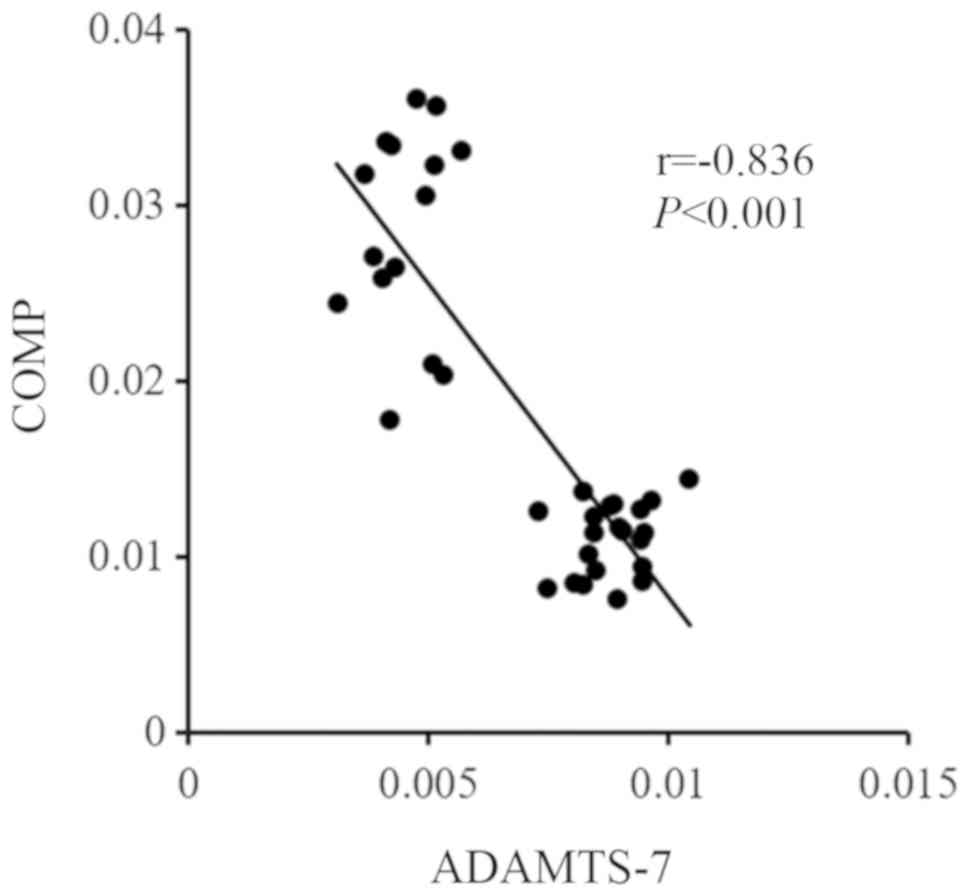
The expression of ADAMTS-7 and COMP in
the human decidua of the SA group and the control group
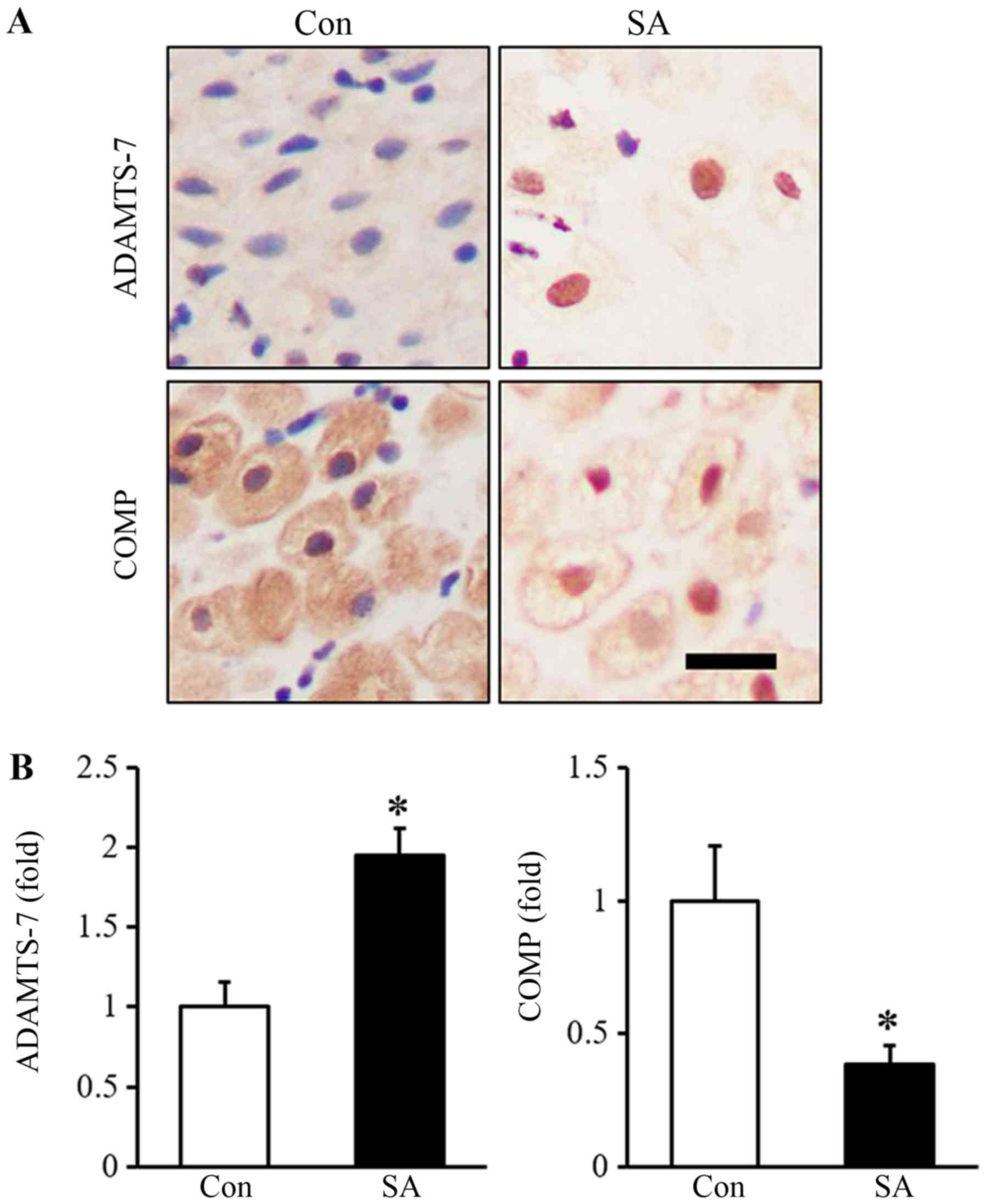
As shown in Fig. 4,
the expression of ADAMTS-7 was clearly increased in the human
decidual tissue of the SA group. The expression of COMP was
decreased in the human decidua of the SA group compared with that
of the control group. Spearman's analysis showed that the
expression of ADMATS-7 was negatively correlated with the
expression of COMP in human decidual tissue, which had a
correlation coefficient of −0.836 (P<0.001; Fig. 5).
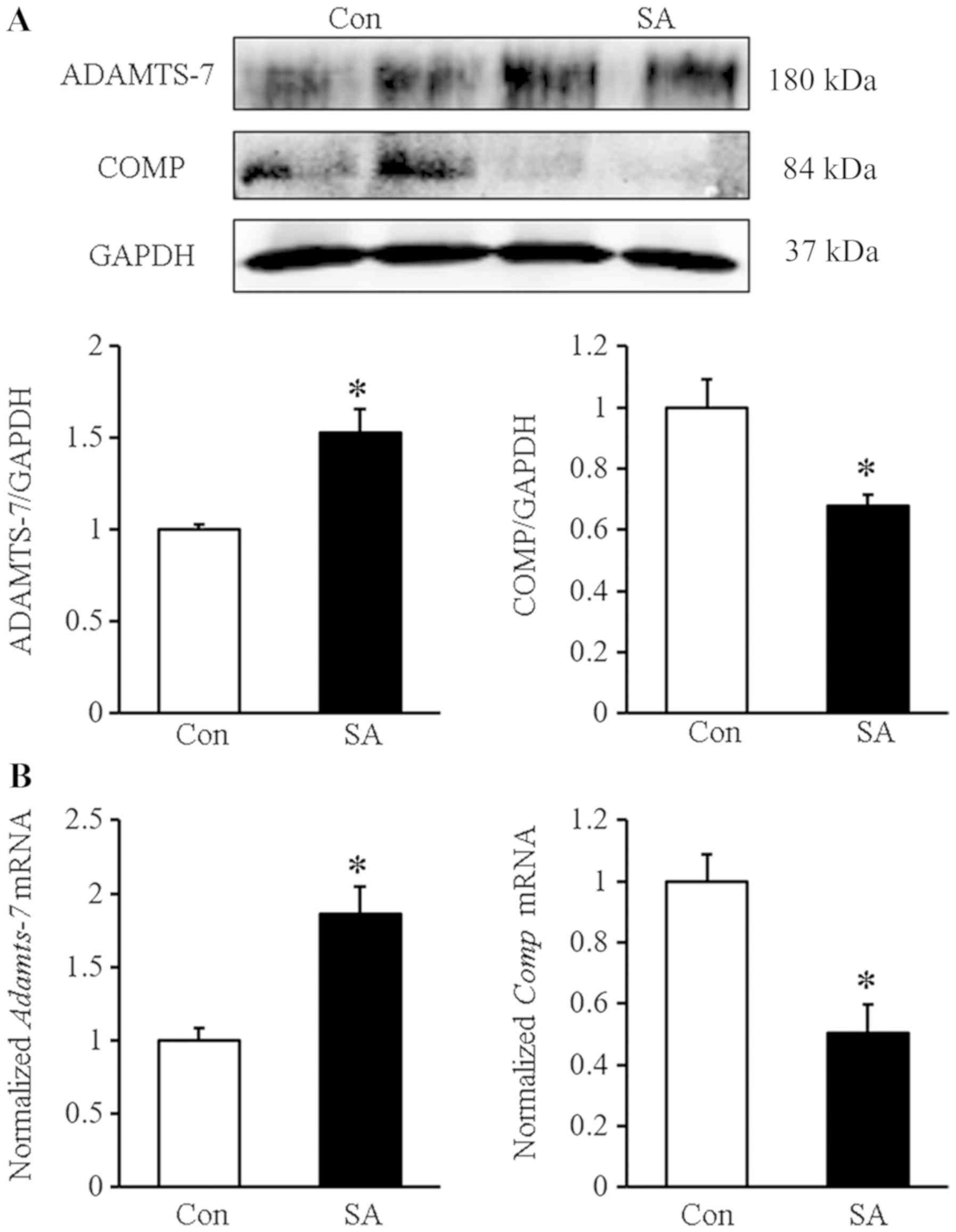
The protein levels of ADAMTS-7 and COMP showed
similar results the mice model, manifested as increased ADMATS-7
and decreased COMP levels (Fig.
6A). The mRNA level of Adamts-7 was increased, and the mRNA
level of COMP was decreased in the SA group compared with the
control group (Fig. 6B).
Discussion
In the present study, we first detected the
expression of ADAMTS-7 and COMP in the decidual tissues of both
LPS-treated mice and human subjects with SA. As demonstrated,
ADAMTS-7 was increased, and COMP was decreased in the decidual
tissue of the LPS-treated mice model compared with that of the
control group, as depicted by immunohistochemistry, western blot
and RT-qPCR. Moreover, we also revealed that ADAMTS-7 was
negatively correlated with COMP in the decidual tissue of mice. The
results in humans were similar to the results in mice. ADAMTS-7 was
upregulated, and COMP was downregulated in the decidual tissue of
the human subjects with SA compared with the corresponding control
group, as demonstrated by immunohistochemistry, western blot and
RT-qPCR. We also found that ADAMTS-7 was negatively correlated with
COMP in human decidual tissue. Thus, in both mice and humans,
ADAMTS-7 and its substrate COMP were proposed to play an important
role in the pathological process of SA.
The ADAMTS family consists of 19 secreted
multidomain proteolytic enzymes and participates in a variety of
pathophysiological activities, including extracellular assembly and
degradation, hemostasis, organogenesis, angiogenesis, cancer and
arthritis (30). ADAMTS-7, a
member of the ADAMTS family, is mainly expressed in tendons,
ligaments, cartilage and skeletal muscle (31). Our study suggested that ADAMTS-7
could also be detected in the uterus. Previous studies focused on
the role of ADAMTS-7 in cartilage degradation, osteoarthritis,
collagen-induced arthritis and cardiovascular disease (10,22,23,32,33).
The expression of ADAMTS-7 was influenced by a series of factors,
including mechanical stimulation, proinflammatory cytokines and
oxidative stress (34). The
expression of ADAMTS-7 was increased by inflammatory cytokines and
decreased by anti-inflammatory cytokines (23,24).
It was also reported that ADAMTS-7 and tumor necrosis factor alpha
(TNF-a) form a positive feedback loop during the regulation of
cartilage degradation and the progression of osteoarthritis
(33,35). As demonstrated by previous studies,
SA was closely associated with inflammation. Elevated TNF-α was
also found to participate in the occurrence of recurrent SA
(36). In this study, we found
that ADAMTS-7 was increased in the decidual tissues of both mice
and humans suffering from SA, which implied that ADAMTS-7 was
involved in the pathological process of SA. Moreover, is was
reported that subjects suffering from SA were more likely to
experience cardiovascular disease later in life. Our findings
suggested that ADAMTS-7, which is an important factor involved in
cardiovascular disease, also participates in the pathological
process of SA. Thus, this study will be helpful toward a better
understanding of the etiology of SA.
ADAMTS-7 used to be regarded as a novel locus
associated with vascular atherosclerosis by stimulating VSMC
migration through the degradation of COMP (24,26,37–41).
COMP, an extracellular matrix protein mainly expressed in cartilage
and bone tissue (42,43), was also reported as a substrate of
ADAMTS-7 and plays a pivotal role in multiple epiphyseal dysplasia
and cancer (44–46). To assess whether COMP is associated
with ADAMTS-7, the expression of COMP was also determined in the
decidual tissues of mice and humans. As demonstrated by
immunohistochemistry, COMP was decreased in the LPS-treated group
and SA group compared with the corresponding control group. Thus,
it was hypothesized that the downregulation of COMP was associated
with the upregulation of ADAMTS-7. Spearman's analysis further
confirmed our hypothesis and revealed that COMP was negatively
correlated with ADAMTS-7. Thus, upregulation of ADAMTS-7 and
downregulation of COMP were associated with SA.
However, there are also limitations in this study.
First, we only described the expression of ADAMTS-7 and COMP in the
decidua of both the LPS-treated mice and human subjects with SA. A
functional study of ADAMTS-7 and COMP in SA as well as a
determination of the potential regulatory molecular mechanism of
ADAMTS-7 and COMP in the pathological process of SA still remain to
be performed.
In conclusion, the present study, for the first
time, presents the expression levels of ADAMTS-7 and COMP in the
decidual tissues of mice and humans suffering from SA. The findings
suggest that upregulation of ADAMTS-7 and downregulation of COMP
are associated with SA. In addition, a negative correlation was
found between ADAMTS-7 and COMP in both mice and humans. Further
studies are required to elucidate the potential mechanisms
underlying the role of ADAMTS-7 and COMP in SA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Project
Grant of Natural Science Foundation of Hubei Province (grant no.
2015CFA074).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM and JY were involved in the study design and
preparation of the manuscript. YM, DZ and NY carried out the
experiments. JD and YM analyzed the data, drafted the manuscript
and critically discussed the results with JY.
Ethics approval and consent to
participate
Animal procedures were approved by the Animal Use
Committees of Renmin Hospital of Wuhan University (Wuhan, China).
The studies involving patients were approved by the Human Research
Ethics Committees of Renmin Hospital of Wuhan University (Wuhan,
China). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SA
|
spontaneous abortion
|
|
COMP
|
cartilage oligomeric matrix
protein
|
|
LPS
|
lipopolysaccharide
|
|
TNF-α
|
tumor necrosis factor α
|
References
|
1
|
Stirrat GM: Recurrent miscarriage. Lancet.
336:673–675. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jivraj S, Anstie B, Cheong YC, Fairlie FM,
Laird SM and Li TC: Obstetric and neonatal outcome in women with a
history of recurrent miscarriage: A cohort study. Hum Reprod.
16:102–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hughes GR: Thrombosis, abortion, cerebral
disease, and the lupus anticoagulant. Br Med J (Clin Res Ed).
287:1088–1089. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geva E, Lerner-Geva L, Burke M, Vardinon
N, Lessing JB and Amit A: Undiagnosed systemic lupus erythematosus
in a cohort of infertile women. Am J Reprod Immunol. 51:336–340.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hefler-Frischmuth K, Walch K, Hefler L,
Tempfer C and Grimm C: Serologic markers of autoimmunity in women
with recurrent pregnancy loss. Am J Reprod Immunol. 77:e126352017.
View Article : Google Scholar
|
|
6
|
Vitagliano A, Noventa M and Gizzo S:
Autoimmunity, systemic inflammation, and their correlation with
repeated implantation failure and recurrent miscarriage: Is chronic
endometritis the missing piece of the jigsaw? Am J Reprod Immunol.
77:e125972017. View Article : Google Scholar
|
|
7
|
Mekinian A, Cohen J, Alijotas-Reig J,
Carbillon L, Nicaise-Roland P, Kayem G, Daraï E, Fain O and Bornes
M: Unexplained recurrent miscarriage and recurrent implantation
failure: Is there a place for immunomodulation? Am J Reprod
Immunol. 76:8–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Comba C, Bastu E, Dural O, Yasa C, Keskin
G, Ozsurmeli M, Buyru F and Serdaroglu H: Role of inflammatory
mediators in patients with recurrent pregnancy loss. Fertil Steril.
104:1467–1474.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmed SK, Mahmood N, Malalla ZH, Alsobyani
FM, Al-Kiyumi IS and Almawi WY: C-reactive protein gene variants
associated with recurrent pregnancy loss independent of CRP serum
levels: A case-control study. Gene. 569:136–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Wei F and Liu CJ: Overexpression
of ADAMTS-7 leads to accelerated initiation and progression of
collagen-induced arthritis in mice. Mol Cell Biochem. 404:171–179.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bengtsson E, Hultman K, Dunér P, Asciutto
G, Almgren P, Orho-Melander M, Melander O, Nilsson J,
Hultgårdh-Nilsson A and Gonçalves I: ADAMTS-7 is associated with a
high-risk plaque phenotype in human atherosclerosis. Sci Rep.
7:37532017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu CJ: The role of ADAMTS-7 and ADAMTS-12
in the pathogenesis of arthritis. Nat Clin Pract Rheumatol.
5:38–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galgani M, Insabato L, Calì G, Della Gatta
AN, Mirra P, Papaccio F, Santopaolo M, Alviggi C, Mollo A, Strina
I, et al: Regulatory T cells, inflammation, and endoplasmic
reticulum stress in women with defective endometrial receptivity.
Fertil Steril. 103:1579–1586.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakashima A, Ito M, Shima T, Bac ND,
Hidaka T and Saito S: Accumulation of IL-17-positive cells in
decidua of inevitable abortion cases. Am J Reprod Immunol. 64:4–11.
2010.PubMed/NCBI
|
|
15
|
Wang WJ, Hao CF, Yi-Lin, Yin GJ, Bao SH,
Qiu LH and Lin QD: Increased prevalence of T helper 17 (Th17) cells
in peripheral blood and decidua in unexplained recurrent
spontaneous abortion patients. J Reprod Immunol. 84:164–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SS, Zhang W, Zhang YQ, Zhao Y, Liu Y,
Li JK, Zhang HX, Cheng L and Nie L: IL-17A enhances ADAMTS-7
expression through regulation of TNF-α in human nucleus pulposus
cells. J Mol Histol. 46:475–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mosca L, Benjamin EJ, Berra K, Bezanson
JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ,
et al: Effectiveness-based guidelines for the prevention of
cardiovascular disease in women-2011 update: A guideline from the
American Heart Association. J Am Coll Cardiol. 57:1404–1423. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu P, Haththotuwa R, Kwok CS, Babu A,
Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA
and Mamas MA: Preeclampsia and future cardiovascular health: A
systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes.
10(pii): e0034972017.PubMed/NCBI
|
|
19
|
Ranthe MF, Andersen EA, Wohlfahrt J,
Bundgaard H, Melbye M and Boyd HA: Pregnancy loss and later risk of
atherosclerotic disease. Circulation. 127:1775–1782. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oliver-Williams CT, Heydon EE, Smith GC
and Wood AM: Miscarriage and future maternal cardiovascular
disease: A systematic review and meta-analysis. Heart.
99:1636–1644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wagner MM, Bhattacharya S, Visser J,
Hannaford PC and Bloemenkamp KW: Association between miscarriage
and cardiovascular disease in a Scottish cohort. Heart.
101:1954–1960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu W, Wang H, Yu C, Li J, Gao Y, Ke Y,
Wang Y, Zhou Y and Zheng J: Association of ADAMTS-7 levels with
cardiac function in a rat model of acute myocardial infarction.
Cell Physiol Biochem. 38:950–958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kessler T, Zhang L, Liu Z, Yin X, Huang Y,
Wang Y, Fu Y, Mayr M, Ge Q, Xu Q, et al: ADAMTS-7 inhibits
re-endothelialization of injured arteries and promotes vascular
remodeling through cleavage of thrombospondin-1. Circulation.
131:1191–1201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu
Q, Zhu Y, Wang N, Kong W and Wang X: ADAMTS-7 mediates vascular
smooth muscle cell migration and neointima formation in
balloon-injured rat arteries. Circ Res. 104:688–698. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riessen R, Fenchel M, Chen H, Axel DI,
Karsch KR and Lawler J: Cartilage oligomeric matrix protein
(thrombospondin-5) is expressed by human vascular smooth muscle
cells. Arterioscler Thromb Vasc Biol. 21:47–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du Y, Wang Y, Wang L, Liu B, Tian Q, Liu
CJ, Zhang T, Xu Q, Zhu Y, Ake O, et al: Cartilage oligomeric matrix
protein inhibits vascular smooth muscle calcification by
interacting with bone morphogenetic protein-2. Circ Res.
108:917–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bond AR, Hultgårdh-Nilsson A, Knutsson A,
Jackson CL and Rauch U: Cartilage oligomeric matrix protein (COMP)
in murine brachiocephalic and carotid atherosclerotic lesions.
Atherosclerosis. 236:366–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Puhl SL, Kazakov A, Müller A, Fries P,
Wagner DR, Böhm M, Maack C and Devaux Y: Adenosine A1 receptor
activation attenuates cardiac hypertrophy and fibrosis in response
to α1-adrenoceptor stimulation in vivo. Br J Pharmacol. 173:88–102.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wagstaff L, Kelwick R, Decock J and
Edwards DR: The roles of ADAMTS metalloproteinases in tumorigenesis
and metastasis. Front Biosci (Landmark Ed). 16:1861–1872. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu CJ, Kong W, Ilalov K, Yu S, Xu K,
Prazak L, Fajardo M, Sehgal B and Di Cesare PE: ADAMTS-7: A
metalloproteinase that directly binds to and degrades cartilage
oligomeric matrix protein. FASEB J. 20:988–990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo F, Lai Y, Tian Q, Lin EA, Kong L and
Liu C: Granulin-epithelin precursor binds directly to ADAMTS-7 and
ADAMTS-12 and inhibits their degradation of cartilage oligomeric
matrix protein. Arthritis Rheum. 62:2023–2036. 2010.PubMed/NCBI
|
|
33
|
Lai Y, Bai X, Zhao Y, Tian Q, Liu B, Lin
EA, Chen Y, Lee B, Appleton CT, Beier F, et al: ADAMTS-7 forms a
positive feedback loop with TNF-α in the pathogenesis of
osteoarthritis. Ann Rheum Dis. 73:1575–1584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jones GC and Riley GP: ADAMTS proteinases:
A multi-domain, multi-functional family with roles in extracellular
matrix turnover and arthritis. Arthritis Res Ther. 7:160–169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Buckland J: Osteoarthritis: Positive
feedback between ADAMTS-7 and TNF in OA. Nat Rev Rheumatol.
9:5662013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li S, Wang L, Xing Z, Huang Y and Miao Z:
Expression level of TNF-α in decidual tissue and peripheral blood
of patients with recurrent spontaneous abortion. Cent Eur J
Immunol. 42:156–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bauer RC, Tohyama J, Cui J, Cheng L, Yang
J, Zhang X, Ou K, Paschos GK, Zheng XL, Parmacek MS, et al:
Knockout of Adamts7, a novel coronary artery disease locus in
humans, reduces atherosclerosis in mice. Circulation.
131:1202–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patel RS and Ye S: ADAMTS7: A promising
new therapeutic target in coronary heart disease. Expert Opin Ther
Targets. 17:863–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanby HA and Zheng XL: Biochemistry and
physiological functions of ADAMTS7 metalloprotease. Adv Biochem.
1:2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
You L, Tan L, Liu L, Shen R, Chaugai S,
Wang DW and Cui W: ADAMTS7 locus confers high cross-race risk for
development of coronary atheromatous plaque. Mol Genet Genomics.
291:121–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Du Y, Gao C, Liu Z, Wang L, Liu B, He F,
Zhang T, Wang Y, Wang X, Xu M, et al: Upregulation of a disintegrin
and metalloproteinase with thrombospondin motifs-7 by miR-29
repression mediates vascular smooth muscle calcification.
Arterioscler Thromb Vasc Biol. 32:2580–2588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Müller G, Michel A and Altenburg E: COMP
(cartilage oligomeric matrix protein) is synthesized in ligament,
tendon, meniscus, and articular cartilage. Connect Tissue Res.
39:233–244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan K and Lawler J: The interaction of
Thrombospondins with extracellular matrix proteins. J Cell Commun
Signal. 3:177–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin WD, Chou IC, Wang CH and Tsai FJ:
Novel mutations in the cartilage oligomeric matrix protein gene
identified in two Taiwanese patients with pseudoachondroplasia and
multiple epiphyseal dysplasia. Pediatr Neonatol. 1–3.
2017.PubMed/NCBI
|
|
45
|
Englund E, Bartoschek M, Reitsma B,
Jacobsson L, Escudero-Esparza A, Orimo A, Leandersson K, Hagerling
C, Aspberg A, Storm P, et al: Cartilage oligomeric matrix protein
contributes to the development and metastasis of breast cancer.
Oncogene. 35:5585–5596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Englund E, Canesin G, Papadakos KS, Vishnu
N, Persson E, Reitsma B, Anand A, Jacobsson L, Helczynski L, Mulder
H, et al: Cartilage oligomeric matrix protein promotes prostate
cancer progression by enhancing invasion and disrupting
intracellular calcium homeostasis. Oncotarget. 8:98298–98311. 2017.
View Article : Google Scholar : PubMed/NCBI
|