Introduction
Infertility is a worldwide reproductive health
problem that affects ~15% of couples, with the male responsible for
approximately half of cases (1,2).
Asthenozoospermia, a common cause of male infertility, is
characterized by reduced sperm motility in fresh ejaculate, which
prevents the sperm from moving towards and penetrating the egg and
results in infertility (3,4). In mammals, the generation of
spermatozoa that are capable of fertilization is a highly complex
process that includes spermatogenesis in the testis and sperm
maturation in the epididymis (5).
During these processes, sperm require adenosine triphosphate (ATP)
that is produced by mitochondrial oxidative phosphorylation in the
middle piece of the sperm flagellum and by glycolysis in the
principal piece of the sperm flagellum in order to provide energy
for motility. Glycolysis is essential for sperm motility and male
fertility, rather than mitochondrial metabolism (6). A total of three testis-specific
glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase,
testis-specific (GAPDHS), phosphoglycerate kinase 2 (PGK2) and
lactate dehydrogenase C (LDHC), known to be involved in glycolysis
in spermatozoa (7–9). GAPDHS acts between the ATP-consuming
and ATP-generating segments of glycolysis. PGK2 catalyzes the first
ATP-generating step in the central metabolic pathway of glycolysis.
Conversion of pyruvate to lactate with the concomitant oxidation of
nicotinamide adenine dinucleotide (NADH) to NAD+ is
essential for the continued production of ATP by glycolysis and
this reaction is catalyzed by LDH. LDHC is a unique isozyme of LDH
that is specifically expressed in germ cells (10,11)
and is abundant in spermatids and spermatozoa (12). In studies using knockout models,
these three enzymes have been identified to be associated with
sperm motility and male fertility (13–15).
In the authors' previous study, it wasidentified that PGK2 is
closely associated with sperm quality (9); PGK2 was downregulated in spermatozoa
from elderly adults and asthenozoospermic patients, suggesting that
there is a common mechanism underlying poor sperm quality. However,
this molecular mechanism and the associationwith glycolysis remains
unclear.
Normal semen specimens contain a certain proportion
of abnormal sperm with defective motility and morphology. A high
percentage of abnormal sperm is diagnosed as asthenozoospermia. It
was hypothesized that fertile males also produce poor-quality sperm
(immature sperm) and the underlying mechanism of the poor sperm
quality maybe similar to that in infertile men. Therefore, the
differential expression of GAPDHS, PGK2 and LDHC were analyzed in
the testes of young and elderly males in the present study, and
their expression levels in normal spermatozoa, immature spermatozoa
and asthenozoospermic spermatozoa were evaluated. The results
revealed similar reduced expression patterns of GAPDHS, PGK2 and
LDHC in the poor-quality sperm, which suggested that there may be a
common mechanism responsible for sperm quality that involves
glycolysis. The present study provided novel insights for
understanding sperm motility regulation and the causes of male
infertility.
Materials and methods
Ethics statement
This study was approved by the ethics committee of
Yantai Yuhuangding Hospital (Yantai, China). Written informed
consent was signed by participants or their relatives.
In silico analysis
Gene expression profiles of the GDS3113 dataset were
extracted from the Gene expression Omnibus (GEO) database
(https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS3113),
which included the gene expression values of different human
tissues. The expression values of GAPDHS, PGK2 and LDHC in the main
tissues (heart, kidney, liver, lung, prostate, spleen and testis)
were downloaded and re-compared. The GDS2390 (https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS2390)
dataset included the gene expression values of different male mouse
germ cells (type A and type B spermatogonia, spermatocytes and
round spermatids).
Sample preparation
Human testicular specimens were collected from
Yantai Yuhuangding Hospital between March 2016 and December 2017.
Human testes were obtained from five young adults (age, 27–36
years) who had died in car accidents. None of them exhibited a
history of pathologies that could affect reproductive function and
all had previously indicated a willingness to donate their bodies
to medical research. The donation of their organs for medical
research was approved by their immediate family members. Aged
testes samples were obtained from five elderly adults (age, 78–82
years) with prostate cancer who had undergone testicular
castration. None of them had undergone anti-androgen treatment and
all had normal or mixed spermatogenesis (Johnsen score >6) and
provided written informed consent. Testis score evaluation was
performed according to Bergmann and Kliesch (16). All procedures were approved by the
Ethics Committee of Yantai Yuhuangding Hospital.
Human semen specimens were collected from Yantai
Yuhuangding Hospital between January 2017 and December 2017. Semen
samples were collected from 15 healthy young adults males (age,
28–36 years) and 15 patients with asthenozoospermia (age, 25–36
years; progressive motility, <32%). Semen was obtained by
masturbation following 7 days of sexual abstinence. Collection and
processing of semen samples were conducted in accordance with the
World Health Organization's (WHO) Laboratory Manual for the
Examination and Processing of Human Semen (5th edition, 2010).
Spermatozoa were used for quantitative immunofluorescence
experiments and western blot analysis. All sperm donors gave
written informed consent when donating the sperm ejaculates for the
purposes of the research project. All procedures were approved by
the Ethics Committee of Yantai Yuhuangding Hospital.
For preparing the corresponding 80% Percoll sperm
fraction (17), an aliquot of the
same semen sample was centrifuged through 2.0 ml of an 80%
single-phase Percoll gradient at 500 × g for 20 min at room
temperature to obtain mature sperm. The sperm pellet was
resuspended in 2 ml PBS and re-centrifuged at 600 × g for 10 min at
room temperature to eliminate the residual Percoll. The rest of the
semen sample was used to obtain immature sperm. After diluting in
PBS and centrifuging at 800 × g for 10 min at room temperature, the
supernatant was discarded and the sperm pellet was re-suspended in
PBS by gentle pipetting prior to centrifugation at 500 × g for 10
min at room temperature and washed with PBS three times.
Immunohistochemistry
Immunohistochemistry was performed to detect the
cellular expression of GAPDHS, PGK2 or LDHC in the human testes. As
described previously (18), the
testicular tissues were fixed with Bouin's solution at room
temperature for 12 h and then embedded in paraffin according to
conventional methods. Subsequently, 4-µm-thick paraffin-sections
were prepared. Sections were dewaxed in xylene, rehydrated in
descending ethanol series (100, 95 and 80%) and washed with
ultrapure water. Antigen retrieval was performed for 20 min in
microwave (above 94°C). Following three washes with phosphate
buffer saline (PBS), endogenous peroxidases were inhibited by
incubation with 3% (v/v) H2O2 for 10 min. The
sections were washed with PBS three times and blocked in 3% (v/v)
bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) in PBS at room temperature for 1 h and subsequently
incubated with primary antibodies against GAPDHS (cat. no.
ab153802; 1:100 in blocking solution; Abcam, Cambridge, UK), PGK2
(cat. no. ab183031; 1:100 in blocking solution; Abcam, Cambridge,
UK) or LDHC (cat. no. ab222910; 1:100 in blocking solution; Abcam,
Cambridge, UK) overnight at 4°C. Subsequently, the sections were
washed with PBS three times and incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin (Ig)G
(cat. no. ZB2301; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China) at a final dilution of 1:400 for 1 h at 37°C.
A DAB kit (OriGene Technologies, Inc., Beijing, China) was used to
visualize the positive staining results. Hematoxylin was used to
counterstain the sections at room temperature for 1 min, then the
sections were dehydrated and mounted onto a Leica DM LB2 light
microscope (Leica Microsystems GmbH, Wetzlar, Germany) for
bright-field microscopy. The negative controls were processed using
pre-immune IgG (1 µg/ml; cat. no. I5006; Sigma-Aldrich; Merck KGaA)
as the primary antibody. The sections were examined by bright field
microscopy and the mean density of positive immunostaining was
analyzed with Image-Pro Plus 6.0 image analysis software (Media
Cybernetics, Inc., Rockville, MD, USA).
Immunofluorescence quantitative
evaluation of GAPDHS, PGK2 and LDHC in spermatozoa
Cellular localization and quantitative analysis of
GAPDHS, PGK2 and LDHC in the spermatozoa were performed using
immunofluorescence as previously described (9). The washed spermatozoa were smeared on
coverslips that were pre-coated with 1% (v/v) gelatin. The
air-dried slides were fixed with cold methanol at room temperature
for 10 min, blocked for 1 h at room temperature with 3% (v/v) BSA
in PBS and incubated at 37°C for 1 h with the aforementioned
primary antibodies against GAPDHS, PGK2 or LDHC, which were diluted
1:50 in PBS containing 3% (w/v) BSA. After washing with PBS, the
corresponding secondary antibody [fluorescein
isothiocyanate-labelled anti-rabbit IgG; 1:100 in PBS containing 3%
(w/v) BSA] was applied to the slides and they were incubated at
room temperature for 1 h. Pre-immune IgG was used as the negative
control. Propidium iodide (0.01 mg/ml; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) counterstaining was performed
to visualize the nuclei. The sections were mounted in 80% (v/v)
glycerol and examined with a Zeiss LSM-510 META confocal laser
scanning microscope (Carl Zeiss AG, Oberkochen, Germany).
Quantitative assessment of the protein expression in
the spermatozoa was performed using a Zeiss LSM 510 laser confocal
microscope (Carl Zeiss AG). The slides were systematically examined
at ×400 magnification, according to the WHO manual (2010), until a
total of 200 spermatozoa had been assessed. Using Zeiss LSM 510
Meta software version 3.2 (Carl Zeiss AG), the fluorescence
intensity value per stained cell was calculated automatically by
subtracting the background fluorescence intensity, which was
determined by scanning a sperm free area.
Sperm motility analysis
Following liquefication of the semen at 37°C for 30
min, 3 µl semen was placed into 20 µm deep chambers (2X-CEL;
Hamilton Thorne Research, Beverly, MA, USA). The motility
parameters, including progressive motility and other parameters of
curvilinear velocity, straight-line velocity, average path velocity
and linearity were measured and recorded at 37°C using
computer-aided sperm analyzer (HTM-IVOS; Version 12.3, Hamilton
Thorne Research).
Protein extraction
Sperm samples and testis tissues were collected for
protein extraction. Briefly, the spermatozoa suspensions were
sonicated in radioimmunoprecipitation assay (RIPA) lysis solution
(20 mM Tris, pH 7.5; 150 mM NaCl; 1% Nonidet P-40; 0.5% sodium
deoxycholate; 1 mM EDTA and 0.1% SDS) and kept on ice for 2 h for
protein lysis. The testicular tissues were ground in liquid
nitrogen, solubilized with RIPA lysis buffer and kept on ice for 2
h for protein lysis. Following lysis, the mixtures were centrifuged
at 12,000 × g for 20 min at 4°C. The supernatant was collected and
precipitated with 4 volumes of ice-cold acetone for 2 h and
centrifugated at 12,000 × g for 20 min at 4°C. The precipitate was
collected and dissolved in lysis buffer. The solubilized samples
were divided into aliquots and stored at −80°C for western
blotting.
Western blot analysis
Western blot analysis was used for comparative
analysis of the gene expression of GAPDHS, LDHC and PGK2 in the
different samples. The proteins samples (20 µg each) were separated
by 12% SDS-PAGE and transferred to polyvinylidene difluoride
membranes. After blocking with 5% (w/v) skimmed milk for 1 h at
37°C, the membranes were co-incubated with the aforementioned
primary antibodies used for immunohistochemical analysis under
gentle agitation at 4°C overnight. Subsequently, the membranes were
washed with 0.5% (v/v) Tween-20 in TBS three times and subsequently
incubated with HRP-conjugated goat anti-rabbit or goat anti-mouse
IgG (cat. nos. ZB2301 and ZB2305, respectively; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.) for 1 h at room temperature. The
immune-reactive complexes were detected using an Enhanced
Chemiluminescence kit (GE Healthcare, Chicago, IL, USA). GeneTools
(version 4.02; Syngene Europe, Cambridge, UK) was used to analyze
the density of each reaction band. The results are presented as the
relative quantity of target proteins compared with β-actin (cat.
no. sc-81178; 1:200 in blocking solution; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) or β-tubulin (cat. no. sc-5274; 1:200 in
blocking solution; Santa Cruz Biotechnology, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation.
The means of two groups were analyzed using a Student's t-test and
the means of more than two groups were analyzed by one-way analysis
of variance and Newman-Keuls was used to compare all groups.
Pearson's correlation was performed to identify correlation
coefficient. GraphPad Prism 7 (GraphPad Software, Inc., La Jolla,
CA, USA) was used to perform the statistical analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
In silico bioinformatics analysis of
GAPDHS, PGK2 and LDHC in the testes
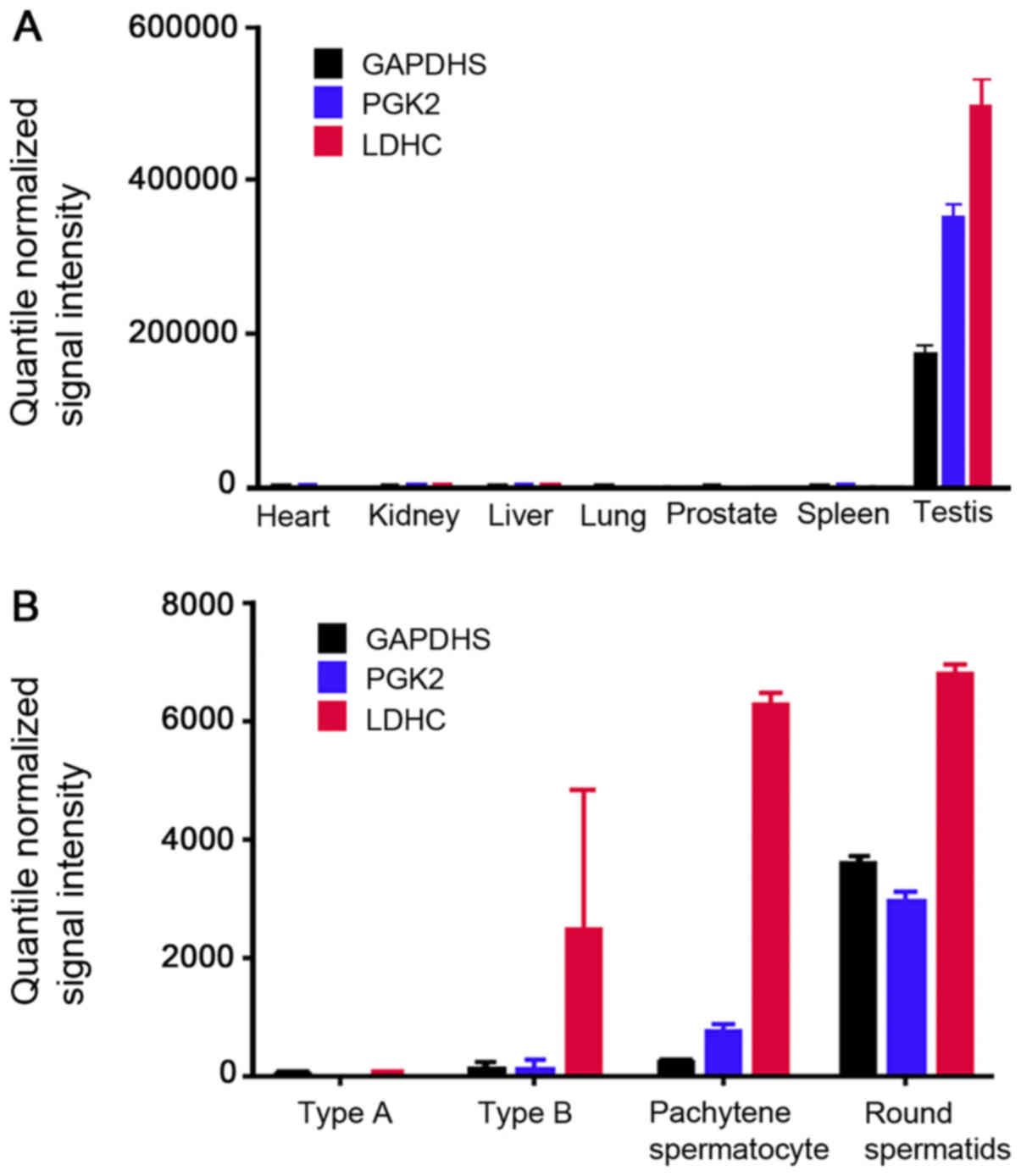
Gene expression profiles of the GDS3113 dataset were
extracted from the GEO Database, which included the gene expression
values of different human tissues. The expression values of GAPDHS,
PGK2 and LDHC in the main tissues (heart, kidney, liver, lung,
prostate, spleen and testis) were downloaded and re-compared. The
results demonstrated that GAPDHS, PGK2 and LDHC were specifically
expressed in the testis (Fig. 1A).
The GDS2390 dataset included murine gene expression information
from different germ cells of type A and type B spermatogonia,
spermatocytes and round spermatids (Fig. 1B). The comparative analysis
demonstrated that GAPDHS and PGK2 were mainly expressed in the
round spermatids, whereas LDHC was mainly expressed in the
spermatocytes and round spermatids. The results indicated that
GAPDHS, PGK2 and LDHC are post-meiotic germ cell-specific genes in
the testes.
Cellular localization of GAPDHS, PGK2
and LDHC in the human testes
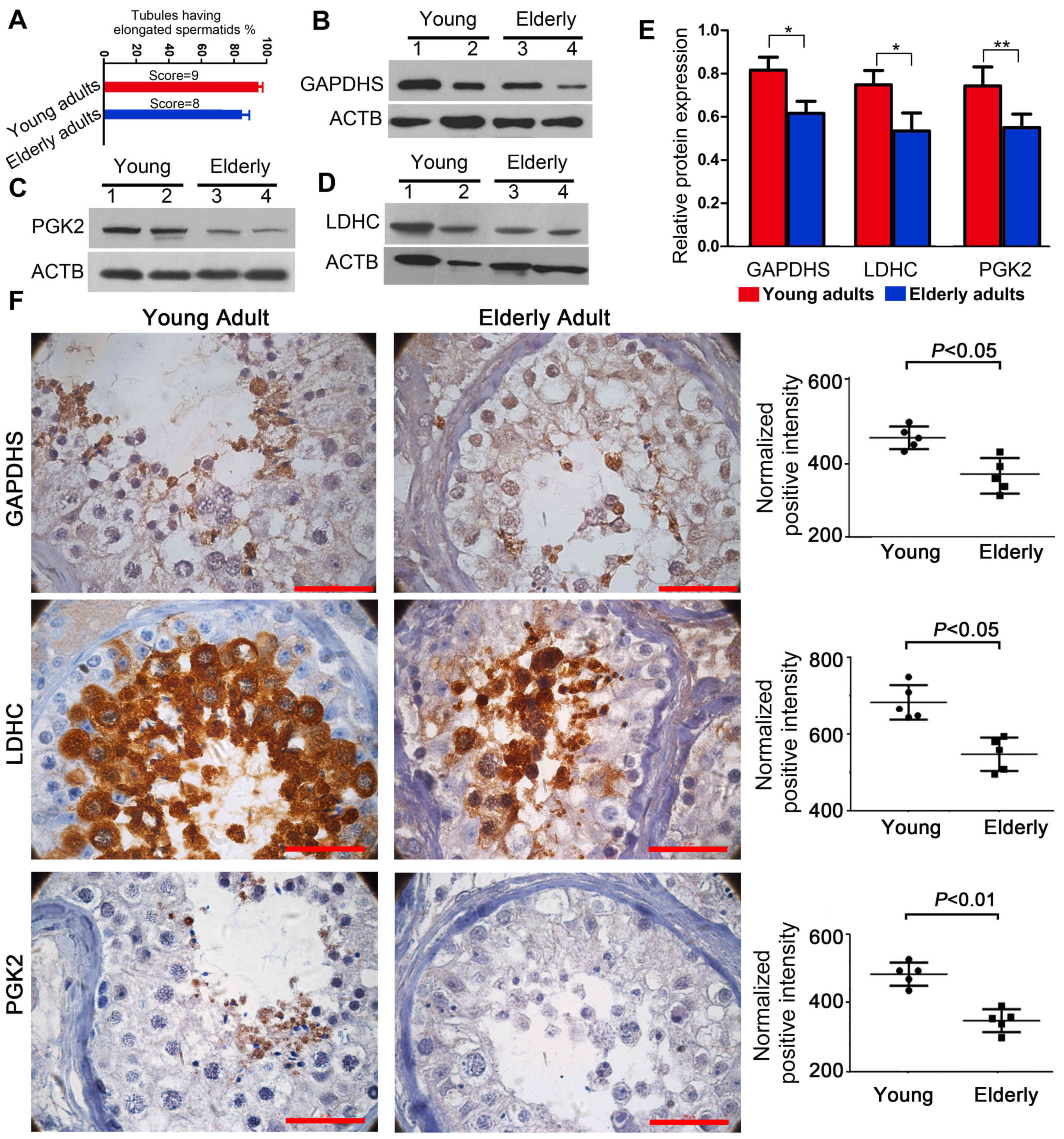
The score evaluation of the testicular sections
demonstrated that the testes of young adults contained normal
spermatogenesis (score=9), whereas the testes from elderly adults
exhibited atrophic morphology (score=8) (Fig. 2A). Western blot analysis
demonstrated that the protein expression levels of GAPDHS, PGK2 and
LDHC were lower in elderly testes compared with young adults
(Fig. 2B-E). Immunohistochemical
analysis results demonstrated that GAPDHS, PGK2 and LDHC were
expressed in specific localizations in the testicular germ cells.
GAPDHS and PGK2 were mainly located in the round spermatids,
whereas LDHC was mainly observed in the spermatocytes and round
spermatids (Fig. 2F). The images
in Fig. 2F also indicated that,
compared with the tests from young adults, there were notable
morphological alterations in testes of the elderly adults,
including an irregular arrangement of the germ cells and an
increase in the mesenchymal cell layers; these morphological
changes agreed well with the score evaluation of the testicular
sections. These cellular localizations were also consistent with
those of the bioinformatics analysis. The results demonstrated an
age-associated reduction in the expression levels of GAPDHS, PGK2
and LDHC in the human testes, which suggested that the expression
levels are closely associated with spermatogenesis.
Quantitative expression of GAPDHS,
PGK2 and LDHC in sperm
The quantified expression levels of GAPDHS, PGK2 and
LDHC in the sperm were closely associated with sperm quality. The
expression of GAPDHS and PGK2 in the sperm was significantly
associated with the concentration of sperm (Table I). PGK2 had higher correlation
coefficients with progressive sperm motility (0.62) and sperm
concentration (0.54) (P<0.05; Table
I).
 | Table I.Association between expression of
GAPDHS, PGK2 and LDHC in ejaculated sperm and progressive sperm
motility and sperm concentration. |
Table I.
Association between expression of
GAPDHS, PGK2 and LDHC in ejaculated sperm and progressive sperm
motility and sperm concentration.
|
| Progressive sperm
motility | Sperm
concentration |
|---|
|
|
|
|
|---|
| Parameter | R | P-value | R | P-value |
|---|
| GAPDHS | 0.53 | <0.05 | 0.49 | <0.05 |
| PGK2 | 0.62 | <0.05 | 0.54 | <0.05 |
| LDHC | 0.51 | <0.05 | 0.39 | NS |
Immature spermatozoa were further isolated from the
spermatozoon populations. Significantly different proportions of
immature spermatozoa were present in the normozoospermia
(23.15±2.54%) and asthenozoospermia (59.14±3.09%) group (P<0.05;
Fig. 3A). The immature spermatozoa
and asthenozoospermic spermatozoa exhibited levels of significantly
lower progressive motility than the normozoospermic spermatozoa
(immature, 24.2±1.68%; asthenozoospermia, 18.9±2.08%;
normozoospermia, 62.3±3.19%; P<0.05; Fig. 3B). Immunofluorescence quantitative
analysis of GAPDHS, PGK2 and LDHC in the spermatozoa indicated that
GAPDHS, PGK2 and LDHC were mainly localized in the principal piece
of the sperm, in which the energy for sperm motility is produced
through glycolysis (Fig. 3C;
representative PGK2 expression, which is similar to GAPDHS and LDHC
expression patterns). Significantly lower percentages of positive
expression and fluorescence intensities were observed in the
immature spermatozoa and asthenozoospermic spermatozoa (P<0.05;
Fig. 3D and E, respectively).
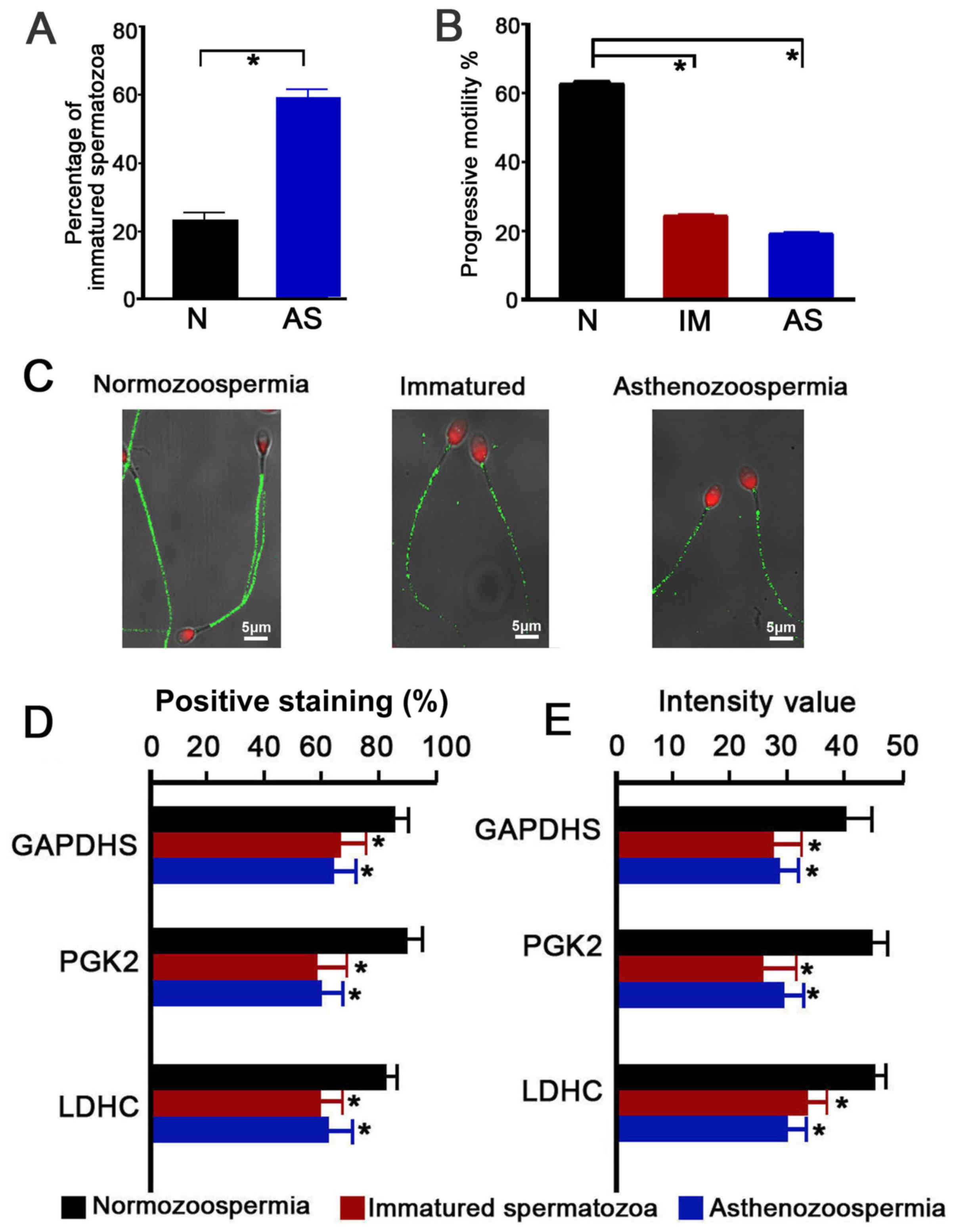 | Figure 3.Immunofluorescence localization and
intensity of GAPDHS, PGK2 and LDHC in ejaculated spermatozoa of
normal, immature and asthenozoospermia samples. (A) Percentage of
immature spermatozoa in normal and asthenozoospermia samples. (B)
Percentage of progressive motility of spermatozoa from normal,
immature and asthenozoospermia samples. (C) Representative images
of PGK2 (green) expression localization in spermatozoa; scale bar,
5 µm; nuclei were stained with propidium iodide (red). (D and E)
Quantitative analysis of (D) percentage of positive-stained cells
and (E) fluorescence intensity of GAPDHS, PGK2 and LDHC on
spermatozoa. Data were compared by one-way analysis of variance;
*P<0.05 vs. N. AS, asthenozoospermia; GAPDHS,
glyceraldehyde-3-phosphate dehydrogenase, testis-specific; IM,
immature spermatozoa; LDHC, lactate dehydrogenase C; N,
normozoospermia; PGK2, phosphoglycerate kinase 2. |
Western blot analysis revealed similarly reduced
expression levels of GAPDHS, PGK2 and LDHC in the asthenozoospermic
spermatozoa and immature sperm from the normal young adults
(Fig. 4).
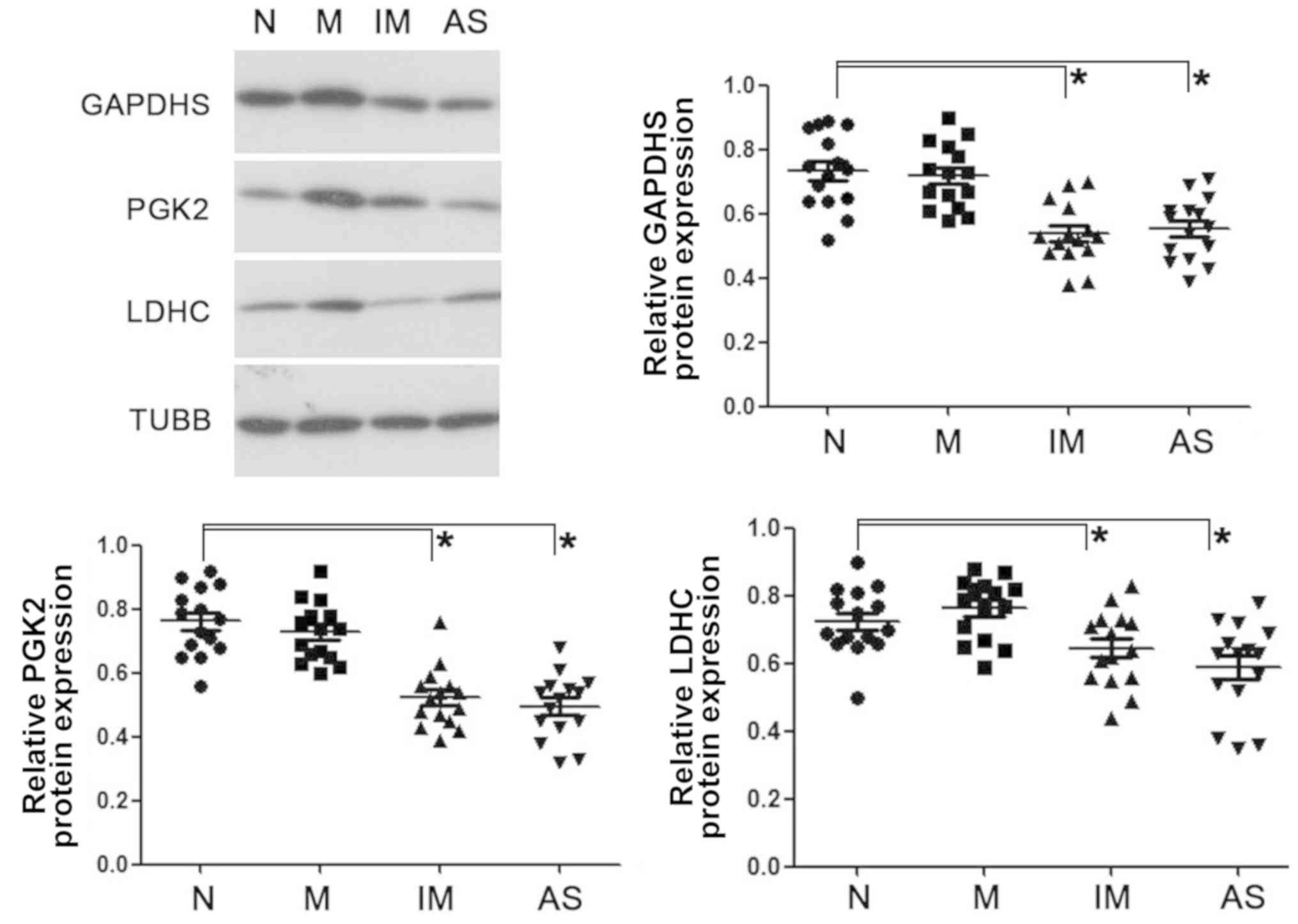 | Figure 4.Western blot analysis of GAPDHS, PGK2
and LDHC on ejaculated spermatozoa in normal, mature, immature and
asthenozoospermia samples. Data were compared by one-way analysis
of variance; *P<0.05. AS, asthenozoospermia; GAPDHS,
glyceraldehyde-3-phosphate dehydrogenase, testis-specific; IM,
immature spermatozoa; M, matured spermatozoa; N, normozoospermia;
PGK2, phosphoglycerate kinase 2; LDHC, lactate dehydrogenase C;
TUBB, β-tubulin. |
Discussion
Spermatozoa are produced in the testes and mature in
the epididymis. The sperm population of each ejaculation is
heterogeneous and includes mature and immature spermatozoa
(19). In the present study it was
hypothesized that different proportions of mature and immature
spermatozoa contribute to the phenotype of poor sperm quality in
patients with asthenozoospermia and elderly males, and therefore
there is a similar mechanism underlying the poor sperm quality of
immature and asthenozoospermic spermatozoa.
Multiple signaling and metabolic molecules are
highly expressed in the testes and are likely to contribute to male
infertility (20). Glycolysis is
the main energy source for sperm motility. GAPDHS, PGK2 and LDHC
are the key enzymes involved in glycolysis. Disruption of these
enzymes leads to aberrant ATP production and sperm motility.
However, their association with sperm quality requires
investigation prior to their clinical application as promising
diagnostic or contraceptive targets (21,22).
In the present study, the key glycolytic enzymes GAPDHS, PGK2 and
LDHC were comprehensively characterized in the human testis and
spermatozoa, which provided novel information for understanding the
regulation of sperm quality.
As revealed by in silico analysis, GAPDHS,
PGK2 and LDHC are specifically expressed in the testes. GAPDHS and
PGK2 were mainly expressed in the post-meiotic germ cells,
particularly in the round spermatids, and LDHC was mainly expressed
in the spermatocytes and round spermatids. The expression pattern
was further confirmed by immunohistochemical analysis of the testes
from young and elderly adults. In addition, GAPDHS, PGK2 and LDHC
were mainly localized in the principal piece of the sperm, in which
the energy is produced for sperm motility by glycolysis. The tail
localization of these proteins in sperm and the importance of the
glycolytic pathway in supplying energy for human sperm motility
suggests that these proteins serve an important role in sperm
motility (23). The comprehensive
characteristics of GAPDHS, PGK2 and LDHC in human testes and sperm
provides information for clinical applications.
Compared with the testes from the young adults,
GAPDHS, PGK2 and LDHC exhibited reduced expression intensities in
the testes from the elderly adults in the present study. Most
prostate cancer patients have normal spermatogenesis (24), in the present study testes were
selected with Johnsen score >6. There may be excess oxidative
stress and a reduction in androgen in the testes of elderly males
that result in clear morphological differences, including reduced
spermatogenesis and loose organization of germ cells (25). The authors' previous study
indicated that aged testes are a good model for the identification
of age-associated spermatogenesis-associated proteins (26). The negative effects of aging on
testes may contribute to altered expression levels of proteins
associated with key biological processes. The present results
indicated that GAPDHS, PGK2 and LDHC are associated with
spermatogenesis. As described in the authors' previous study
(9), there was no statistical
difference of spermatozoa morphology among the young adults,
elderly adults and asthenozoospermia patients; however, similar
lower sperm quality (lower progressive sperm motility) was present
in spermatozoa of elderly adults and asthenozoospermia patients.
Our previous study also revealed that there were lower expression
levels of PGK2 in sperm from males or asthenozoospermic patients
compared with sperm from healthy young males. Sperm functional
analysis in a previous study validated the close association
between the expression of PGK2 and sperm motility (9). In the present study, normal semen
samples were divided into mature and immature sperm. The mature
sperm exhibited good motility and morphology, whereas the immature
population exhibited poor sperm quality that was similar to the
spermatozoa of the asthenozoospermic patients. The sperm intensity
value and localization percentage of GAPDHS, PGK2 and LDHC were
downregulated in the immature and asthenozoospermic spermatozoa.
These results suggested that expression levels of GAPDHS, PGK2 and
LDHC in sperm were associated with sperm quality, and immature and
asthenozoospermic spermatozoa may share similar molecular
mechanisms. The results indicated that asthenozoospermia may
involve an increased percentage of immature sperm, which may lead
to poor sperm quality. The common mechanism underlying poor sperm
quality requires further investigation.
The authors' previous study suggested that a similar
mechanism underlying poor sperm quality may exist in elderly adults
and patients with asthenozoospermia (9). There may be gene expression
variations occurring in elderly adults and asthenozoospermic
patients. In the present study, it was demonstrated that there is
reduced expression of GAPDHS, PGK2 and LDHC in asthenozoospermic
and immature spermatozoa compared with normozoospermic males. The
present study hypothesized that the expression of testis-specific
genes undergo complex and sophisticated regulation in different
spermatogenic cells at specific stages. Suboptimum expression of
certain genes may contribute to poor-quality spermatozoa suffering
from an abnormal process of sperm formation.
The results of the present study indicated that
GAPDHS, PGK2 and LDHC were associated with sperm quality and
function. These enzymes catalyze successive steps in glycolysis and
loss of any will eliminate ATP production via this pathway.
Systematic analysis of GAPDHS, PGK2 and LDHC may improve the
understanding of the metabolism-dependent signaling events required
for fertilization. The present study indicated that a similar
molecular mechanism of poor sperm quality maybe shared by immature
and asthenozoospermic spermatozoa. These results may provide novel
insights into the mechanisms underlying sperm quality and may
contribute to the diagnosis and treatment of asthenozoospermia.
Prior to any clinical application of these molecules as biomarkers
for the diagnosis of male infertility, additional cases should be
used to assess relevance with sperm quality and evaluate
reliability of quantitative methods.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81741027), The
Clinical Research Special Fund of Chinese Medical Association
(grant no. 17020160685) and The Key Research and Development Plan
of Yantai (grant no. 2017YT06000491).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJL conceptualized the study design and drafted the
manuscript. XXL, QL and WJW acquired and analyzed the data, and
revised the manuscript. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qingdao University Affiliated Yuhuangding Hospital
(Yantai, China). All participants provided informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li J, Liu F, Liu X, Liu J, Zhu P, Wan F,
Jin S, Wang W, Li N, Liu J and Wang H: Mapping of the human
testicular proteome and its relationship with that of the
epididymis and spermatozoa. Mol Cell Proteomics. 10:M110.0046302011
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng HL: Molecular biology of male
infertility. Arch Androl. 49:19–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curi SM, Ariagno JI, Chenlo PH, Mendeluk
GR, Pugliese MN, Sardi Segovia LM, Repetto HE and Blanco AM:
Asthenozoospermia: Analysis of a large population. Arch Androl.
49:343–349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ortega C, Verheyen G, Raick D, Camus M,
Devroey P and Tournaye H: Absolute asthenozoospermia and ICSI: What
are the options? Hum Reprod Update. 17:684–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sullivan R and Mieusset R: The human
epididymis: Its function in sperm maturation. Hum Reprod Update.
22:574–587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narisawa S, Hecht NB, Goldberg E,
Boatright KM, Reed JC and Millán JL: Testis-specific cytochrome
c-null mice produce functional sperm but undergo early testicular
atrophy. Mol Cell Biol. 22:5554–5562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Margaryan H, Dorosh A, Capkova J,
Manaskova-Postlerova P, Philimonenko A, Hozak P and Peknicova J:
Characterization and possible function of
glyceraldehyde-3-phosphate dehydrogenase-spermatogenic protein
GAPDHS in mammalian sperm. Reprod Biol Endocrinol. 13:152015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Odet F, Gabel SA, Williams J, London RE,
Goldberg E and Eddy EM: Lactate dehydrogenase C and energy
metabolism in mouse sperm. Biol Reprod. 85:556–564. 2001.
View Article : Google Scholar
|
|
9
|
Liu XX, Zhang H, Shen XF, Liu FJ, Liu J
and Wang WJ: Characteristics of testis-specific phosphoglycerate
kinase 2 and its association with human sperm quality. Hum Reprod.
31:273–279. 2016.PubMed/NCBI
|
|
10
|
Goldberg E: Reproductive implications of
LDH-C4 and other testis-specific isozymes. Exp Clin Immunogenet.
2:120–124. 1985.PubMed/NCBI
|
|
11
|
Coonrod S, Vitale A, Duan C, Bristol-Gould
S, Herr J and Goldberg E: Testis-specific lactate dehydrogenase
(LDH-C4; Ldh3) in murine oocytes and preimplantation embryos. J
Androl. 27:502–509. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei D and Wei L, Li X, Wang Y and Wei L:
Effect of hypoxia on Ldh-c expression in somatic cells of Plateau
Pika. Int J Environ Res Public Health. 13(pii): E7732016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miki K, Qu W, Goulding EH, Willis WD,
Bunch DO, Strader LF, Perreault SD, Eddy EM and O'Brien DA:
Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific
glycolytic enzyme, is required for sperm motility and male
fertility. Proc Natl Acad Sci USA. 101:16501–16506. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Odet F, Duan C, Willis WD, Goulding EH,
Kung A, Eddy EM and Goldberg E: Expression of the gene for mouse
lactate dehydrogenase C (Ldhc) is required for male fertility. Biol
Reprod. 79:26–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danshina PV, Geyer CB, Dai Q, Goulding EH,
Willis WD, Kitto GB, McCarrey JR, Eddy EM and O'Brien DA:
Phosphoglycerate kinase 2 (PGK2) is essential for sperm function
and male fertility in mice. Biol Reprod. 82:136–145. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bergmann M and Kliesch S: Hodenbiopies In:
Krause W, Weidner W (eds) Andrologie. Enke Verlag, Stuttgart.
pp66–71. 1998.
|
|
17
|
Kovanci E, Kovacs T, Moretti E, Vique L,
Bray-Ward P, Ward DC and Huszar G: FISH assessment of aneuploidy
frequencies in mature and immature human spermatozoa classified by
the absence or presence of cytoplasmic retention. Hum Reprod.
16:1209–1217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu XX, Shen XF and Liu FJ: Screening
targeted testis-specific genes for molecular assessment of aberrant
sperm quality. Mol Med Rep. 14:1594–1600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moustafa MH, Sharma RK, Thornton J, Mascha
E, Abdel-Hafez MA, Thomas AJ Jr and Agarwal A: Relationship between
ROS production, apoptosis and DNA denaturation in spermatozoa from
patients examined for infertility. Hum Reprod. 19:129–138. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Z, Danshina PV, Mohr K, Qu W,
Goodson SG, O'Connell TM and O'Brien DA: Sperm function, protein
phosphorylation, and metabolism differ in mice lacking successive
sperm-specific glycolytic enzymes. Biol Reprod. 97:586–597. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danshina PV, Qu W, Temple BR, Rojas RJ,
Miley MJ, Machius M, Betts L and O'Brien DA: Structural analyses to
identify selective inhibitors of glyceraldehyde 3-phosphate
dehydrogenase-S, a sperm-specific glycolytic enzyme. Mol Hum
Reprod. 22:410–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Odet F, Gabel S, London RE, Goldberg E and
Eddy EM: Glycolysis and mitochondrial respiration in mouse
LDHC-null sperm. Biol Reprod. 88:952013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nascimento JM, Shi LZ, Tam J,
Chandsawangbhuwana C, Durrant B, Botvinick EL and Berns MW:
Comparison of glycolysis and oxidative phosphorylation as energy
sources for mammalian sperm motility, using the combination of
fluorescence imaging, laser tweezers, and real-time automated
tracking and trapping. J Cell Physiol. 217:745–751. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moody JA, Ahmed K, Horsfield C, Pedersen
MRV, Yap T and Shabbir M: Fertility preservation in testicular
cancer-predictors of spermatogenesis. BJU Int. 122:236–242. 2008.
View Article : Google Scholar
|
|
25
|
Jankovic Velickovic L and Stefanovic V:
Hypoxia and spermatogenesis. Int Urol Nephrol. 46:887–894. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu FJ, Liu X, Han JL, Wang YW, Jin SH,
Liu XX, Liu J, Wang WT and Wang WJ: Aged men share the sperm
protein PATE1 defect with young asthenozoospermia patients. Hum
Reprod. 30:861–869. 2015. View Article : Google Scholar : PubMed/NCBI
|