Introduction
The extensive epidural fibrosis that may occur
following lumbar surgery may lead to the development of adverse
effects, such as nerve radicular pain or lower back pain (1). This process is associated with a 24%
rate of failed back surgery syndrome (2). Epidural fibrosis is associated with
fibroblast hyperplasia and the development of epidural scar tissue.
Fibroblasts proliferate at the operative site following stimulation
by growth factors and inflammatory cytokines. Local defects of the
vertebral plate are repaired by collagen fibers that are produced
by these cells. Fibroblasts transform into fibrocytes, and scar
tissue replaces the fibrous connective tissue, owing to the
production of collagen fibers. The nerve roots in the vertebral
canals or dura mater are subsequently constrained by the epidural
fibrotic tissue, which may cause restriction of nerve root
mobility, nerve root entrapment and dural compression (3).
A number of strategies aiming to prevent epidural
fibrosis by inducing fibroblast apoptosis have been introduced and
successful outcomes have been reported (4–6). The
antitumor agent 10-hydroxycamptothecin (HCPT) has been demonstrated
to restrain cell proliferation or induce cell apoptosis; HCPT is a
cell cycle-specific agent that mainly acts during DNA synthesis (S
phase) (7). HCPT not only
restrains the proliferation of several types of tumor cells, but
also can inhibit the proliferation of non-cancerous cells (8–10).
Although HCPT is known to exhibit antifibrotic properties, the
specific underlying mechanisms have not yet been fully
elucidated.
MicroRNAs (miRNAs) are short, highly conserved
non-coding RNA molecules that regulate gene expression by targeting
the 3′ untranslated region of target genes during various
physiological processes, including cell differentiation, apoptosis
and proliferation (11). Each
miRNA targets numerous genes; thus, miRNAs serve important roles in
physiological processes in several types of cells, including cancer
cells (12) and fibroblasts
(13). miRNA (miR)-23b is an
epidermal differentiation marker and it has several unknown
functions in the skin (14).
miR-23b belongs to the miR-23b/24/27b cluster, which has been
verified to participate in a number of physiological processes,
such as cell migration, differentiation and proliferation (15–17).
The miR-23b/24/27b cluster serves a cancer-inhibitory role in
colorectal, bladder, ovarian and prostate malignancies (18–21),
whereas it has been reported to promote breast cancer (22). The aim of the present study was to
elucidate the effects of HCPT on fibroblast apoptosis and to
determine whether this effect is mediated by the regulation of
miR-23b-3p expression.
Materials and methods
Ethics statement
The present study protocol was approved by the
Research Ethics Committee of Northern Jiangsu People's Hospital
(Yangzhou, China), and written informed consent was obtained from
all the participants for their tissues to be used for the purposes
of this research.
Fibroblast culture and treatment
Fibroblasts were acquired from scar tissue resected
from patients undergoing reoperation laminectomy in Northern
Jiangsu People's Hospital of Yangzhou City in October 2017; patient
information is provided in Table
I. Under sterile conditions, the epidural scars were dissected
into 5×5 mm pieces and dissociated in 0.25% trypsin (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 6 min at 37°C, and
the cell suspension was centrifuged at 240 × g for 5 min. Cells
were maintained in Dulbecco's modified Eagle's Medium (Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and penicillin (100
U/ml)/streptomycin (100 mg/l) (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere of 5% CO2 and
95% air. Cells in the exponential growth phase were selected for
used in all the experiments. The fibroblasts were incubated in
Petri dishes of different specifications until they reached 60–80%
confluence, and subsequently washed with PBS (pH 7.4) and treated
with or without HCPT at 1 µg/ml for 24 h following previous studies
(23–25).
 | Table I.Clinicopathological characteristics of
the six patients used in the study. |
Table I.
Clinicopathological characteristics of
the six patients used in the study.
| Patient no. | Sex | Age (years) | Biopsy
sitea | Duration of the
lesion (months) | Etiology |
|---|
| 1 | Male | 64 | S: Leg | 12 | Trauma |
|
|
|
| NS: Leg |
|
|
| 2 | Male | 46 | S: Leg | 12 | Trauma |
|
|
|
| NS: Leg |
|
|
| 3 | Female | 65 | S: Leg | 12 | Trauma |
|
|
|
| NS: Leg |
|
|
| 4 | Female | 40 | S: Leg | 12 | Trauma |
|
|
|
| NS: Leg |
|
|
| 5 | Male | 63 | S: Leg | 12 | Trauma |
|
|
|
| NS: Leg |
|
|
| 6 | Female | 21 | S: Shoulder | 12 | Trauma |
|
|
|
| NS: Shoulder |
|
|
Lentiviral infection
Lentiviruses containing the Lv-miR-23b-3p,
Lv-anti-miR-23b-3p or their respective control were purchased from
Shanghai Genechem Co. Ltd. (Shanghai, China). Lentiviral
transfection was performed according to the manufacturer's
protocols. Briefly, when fibroblasts reached 60–80% confluence,
fibroblasts were incubated for 72 h at 37°C in the lentiviral
vector (multiplicity of infection=200) in the presence of Polybrene
Infection/Transfection Reagent (2 mg/ml; Gibco; Thermo Fisher
Scientific, Inc.). The medium was removed and replaced by newDMEM.
Transfected fibroblasts were screened by culturing for 72 h in
puromycin (2 mg/ml; Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA).
When the screening is completed, it may be regarded as the stable
expression of the target gene in the cell. Cells with stable
expression were used in the subsequent experiments.
Flow cytometry
Fibroblasts were seeded in 6 cm plates and incubated
for 24 h in 5% CO2 at 37°C. When the fibroblasts reached
60–80% confluence, following treatment under the various
experimental conditions aforementioned, adherent and detached
fibroblasts (1×106 cells/ml) were centrifuged at 13,000
× g for 5 min at 4°C, resuspended in 500 µl 1X binding buffer and
then double-stained with propidium iodide (PI) and Annexin
V-allophycocyanin (APC; BD Biosciences, San Jose, CA, USA),
according to the manufacturer's protocol. Each sample was detected
using FACSDiva Software 6.0 (BD Biosciences). All experiments were
performed in triplicate.
Western blot analysis
Total protein was extracted from the fibroblasts
when they reached 60–80% confluence and protein concentrations were
measured using a Bicinchoninic Protein Assay kit (Thermo Fisher
Scientific, Inc.). A total of 10 µg protein was separated by 10%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes.
Following the blocking of non-specific binding with 5% non-fat milk
dissolved in TBS + 0.05% Tween-20 at room temperature for 2 h, the
membranes were incubated with the following antibodies:
Anti-cleaved poly [ADP-ribose] polymerase [PARP; 1:1,000; catalog
no. 5625; Cell Signaling Technology, Inc. (CST), Danvers, MA, USA],
anti-GAPDH (1:1,000; catalog no. 8884; CST), anti-B cell lymphoma 2
(Bcl-2; 1:1,000; catalog no. 4223; CST), anti-Bax (1:1,000; catalog
no. 5023; CST) and anti-mothers against decapentaplegic homolog 3
(Smad3; 1:1,000; catalog no. ab40854; Abcam, Cambridge, UK) at 4°C
overnight. Following washing with TBST, the membranes were
incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit
secondary antibodies (1:1,000; catalog no. 7074; CST) and the
protein expressions were visualized using the Enhanced
Chemiluminescence System (EMD Millipore, Billerica, MA, USA). The
bands were quantified by densitometry using Image J 1.46r software
(National Institutes of Health, Bethesda, MD, USA). All reactions
were performed in triplicate.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from the treated fibroblasts
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions. The IQ
SYBR Green Supermix kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used to determine the expression levels of miR-23b-3p.
Briefly, a special looped RT primer was used for each miRNA and the
RevertAid First-Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) was used to reverse transcribe 3 µg total RNA,
following the manufacturer's protocol. Subsequent amplification was
performed using IQ SYBR Green Supermix in a CFX connect Real-Time
PCR System (Bio-Rad Laboratories, Inc.). U6 was used as internal
reference, U6 forward, 5′-CGGCGGTAGCTTATCAGACTGATG-3′ and reverse,
5′-CCAGTCGAGGGTCCGAGGTATT-3′. The primers used were as follows:
miR-23b-3p, forward 5′-GCGGCGGATCACATTGCCAGGG-3′; and we use
Universal Primer (catalog no. 1046471; Qiagen GmbH, Hilden,
Germany) as miR-23b-3p as reverse primer. The 2−ΔΔCq
method was performed to calculate the relative expression (26). All reactions were performed in
triplicate.
Terminal deoxynucleotidyl-transferase-mediated dUTP
nick-end labeling (TUNEL) staining of fibroblasts. The apoptotic
rate of HCPT-treated fibroblasts and Lv-transfected fibroblasts was
evaluated using the TUNEL assay (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China), following the protocol recommended by the
manufacturer. Following TUNEL and DAPI nuclear staining, images
were captured using fluorescence microscopy (Zeiss AG, Oberkochen,
Germany). TUNEL-stained fibroblasts were considered to be
apoptotic; DAPI staining was performed to count the total number of
fibroblasts. All reactions were performed in triplicate.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Using GraphPad Prism 6.0 software (GraphPad Software, Inc., La
Jolla, CA, USA), comparisons between two groups were performed
using Student's t-test and comparisons between multiple groups were
performed using one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-23b-3p is overexpressed in
traumatic scar tissues
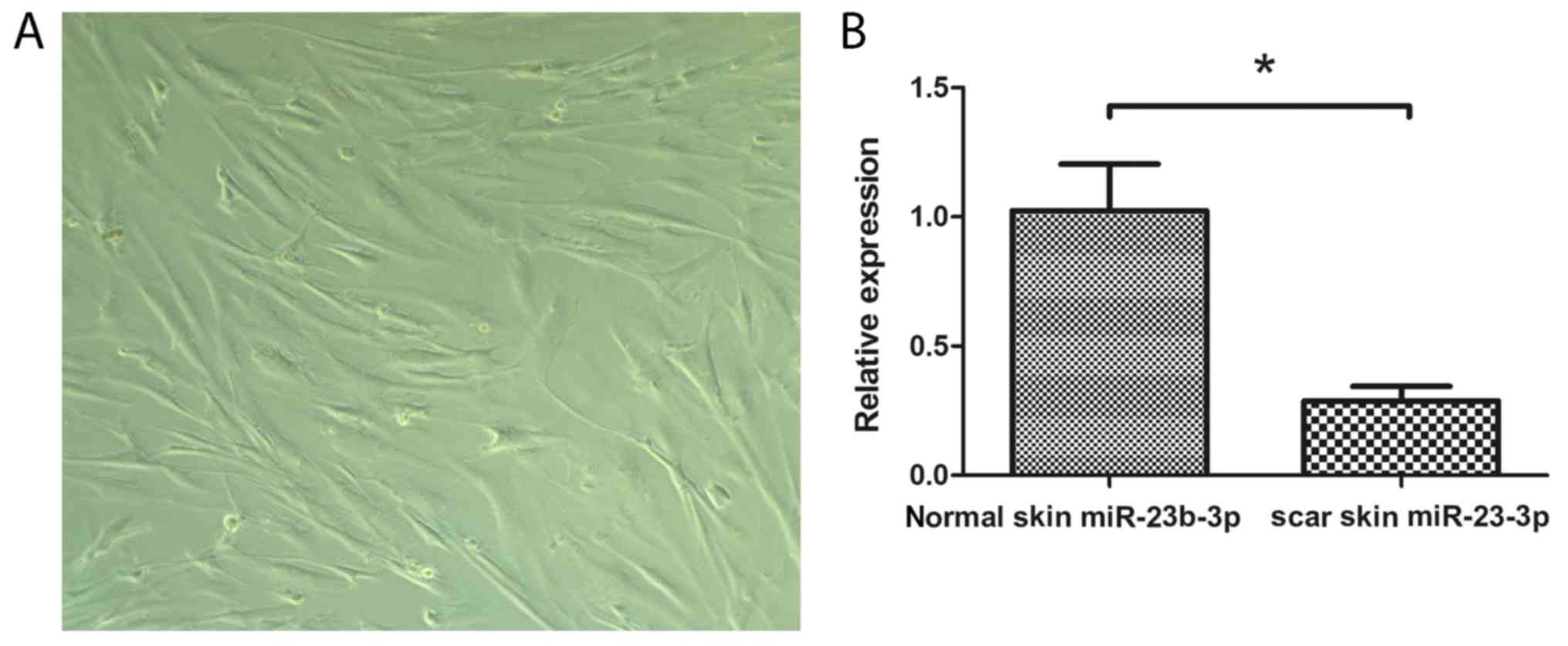
The morphological characteristics of fibroblasts
obtained from patients identified by phase contrast microscopy
(Fig. 1A). Skin tissues were
collected from six patients with traumatic scars and the expression
levels of miR-23b-3p were examined using RT-qPCR. The results
demonstrated that miR-23b-3p expression levels in traumatic scar
tissues was significantly lower compared with expression in the
normal skin tissues (P<0.05; Fig.
1B). These results indicated that miR-23b-3p is associated with
epidural fibrosis.
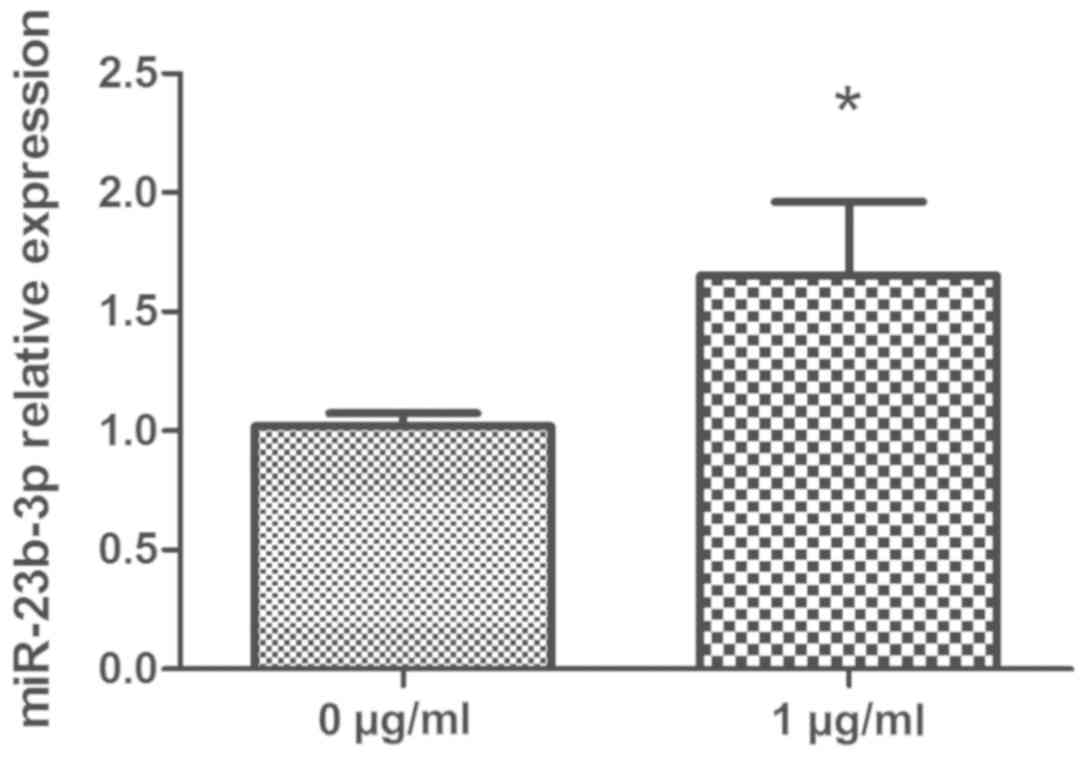
HCPT upregulates miR-23b-3p expression
in human fibroblasts
To determine the effects of HCPT on miR-23b-3p
expression, human fibroblasts were treated with or without 1 µg/ml
HCPT for 24 h and miR-23b-3p expression levels were determined by
RT-qPCR. The results revealed that miR-23b-3p expression in the
HCPT-treated fibroblasts was significantly higher compared with the
expression levels in the untreated control group (P<0.05;
Fig. 2). These data suggested that
HCPT may upregulate miR-23b-3p expression in human fibroblasts.
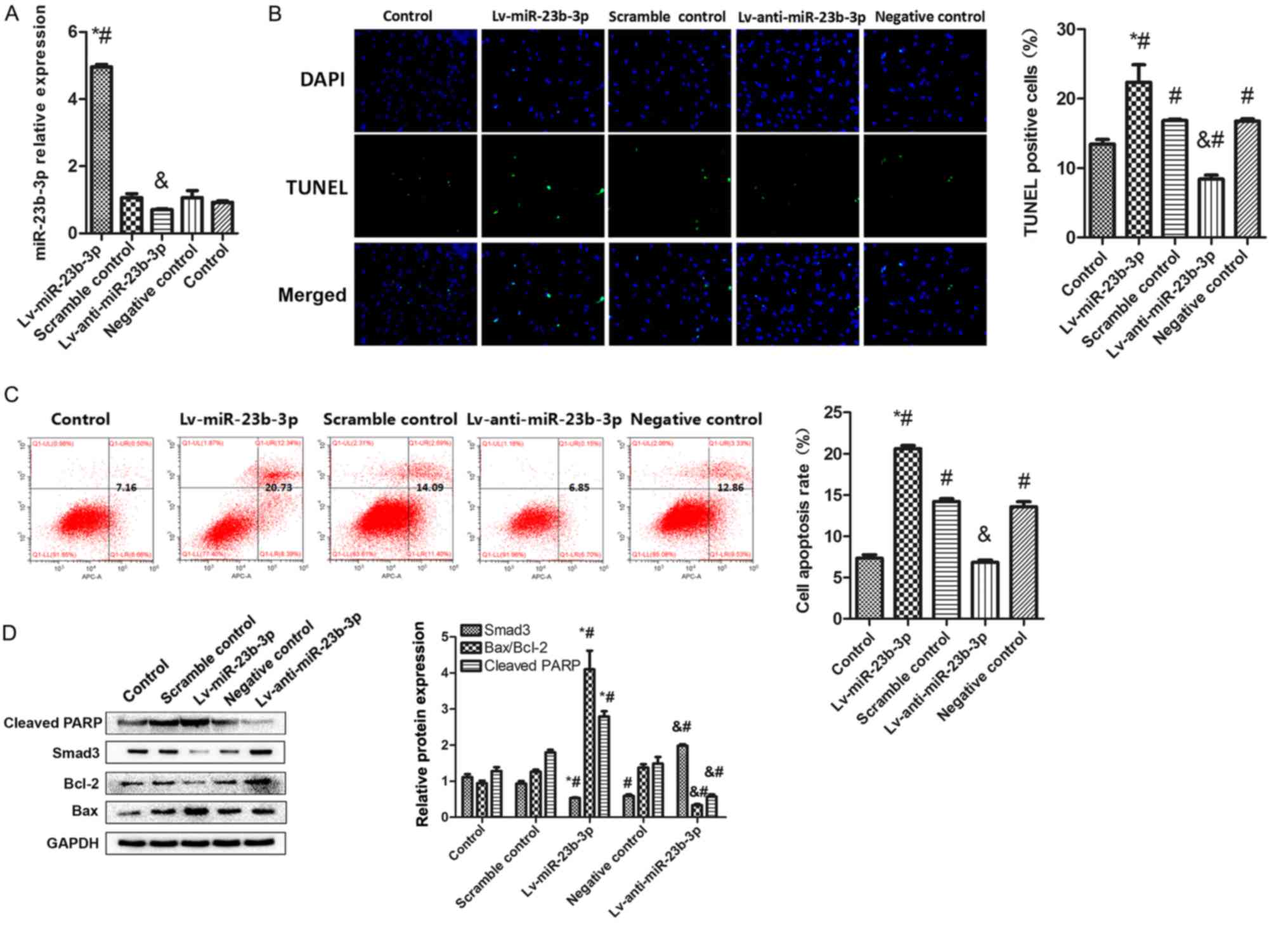
miR-23b-3p promotes fibroblast
apoptosis
To determine the role of miR-23b-3p in fibroblast
apoptosis, human fibroblasts were successfully transfected with
Lv-miR-23b-3p, Lv-anti-miR-23b-3p or their respective controls
(Fig. 3A). Cell apoptosis was
measured using TUNEL and flow cytometry. TUNEL staining data
revealed that miR-23b-3p overexpression induced a significant
increase in the number of TUNEL-staining cells compared with
untreated and Scramble control-transfected cells, indicating an
increase in human fibroblast apoptosis. However, miR-23b-3p
inhibition induced a decrease in the number of TUNEL-staining cells
compared with untreated and Negative control-transfected cells,
which exhibited an decrease in human fibroblast apoptosis. The
apoptotic rate of the Negative control group and the Scramble
control group was significantly increased compared with the control
group, and it was hypothesized that the apoptosis was caused by the
transfection virus operation methods, reagents and viral vectors
(Fig. 3B). Similar results were
observed from flow cytometric analysis, which demonstrated that
miR-23b-3p upregulation resulted in a significantly higher cell
apoptosis compare with Scramble control, while miR-23b-3p
inhibition reduced cell apoptosis rate compared with Negative
control (Fig. 3C).
Western blot analysis revealed that miR-23b-3p
promoted fibroblast apoptosis, while inhibition of miR-23b-3p
exerted the opposite effect. It was also observed that Smad3 and
Bcl-2 decreased significantly in the overexpression group compared
with the Scramble control group, while Smad3, Bcl-2 was
significantly upregulated in the knockout group compared with
Negative control (Fig. 3D). The
apoptotic proteins PARP and Bax demonstrated the opposite; PARP and
Bax increased significantly in the miR-23b-3p upregulation group
compared with Scramble control and PARP and Bax decreased
significantly in miR-23b-3p inhibition group compared with Negative
control These results indicated that miR-23b-3p upregulation may
induce fibroblast apoptosis.
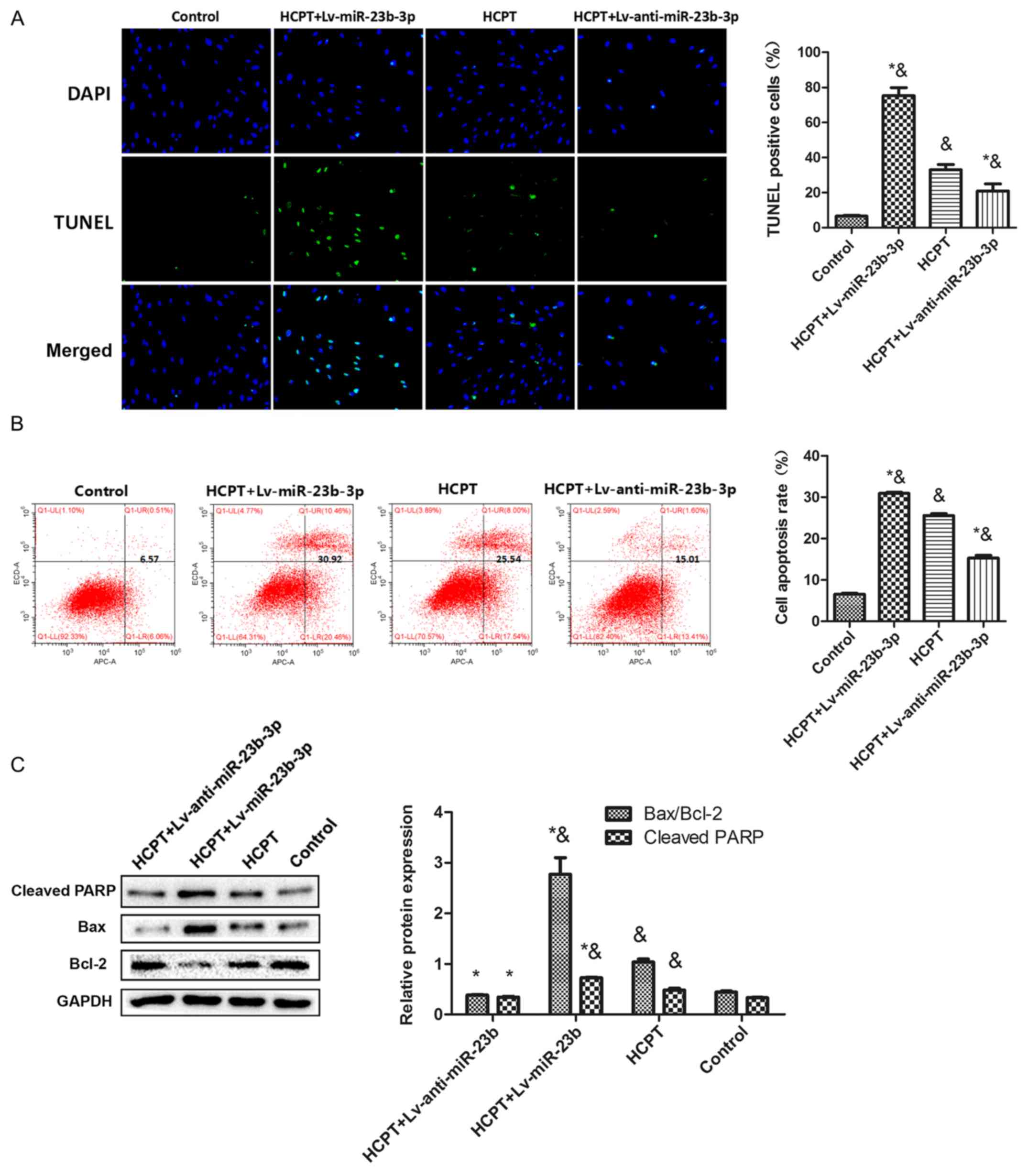
HCPT induces fibroblast apoptosis by
upregulating miR-23b-3p
To verify the role of miR-23b-3p in HCPT-induced
fibroblast apoptosis, human fibroblasts were transfected with
Lv-miR-23b-3p and Lv-anti-miR-23b-3p, followed by co-treatment with
HCPT. TUNEL assays revealed that miR-23b-3p increased HCPT-induced
fibroblast apoptosis (Fig. 4A).
Flow cytometric analysis confirmed the TUNEL assay results; HCPT
treatment increased the fibroblast apoptotic rate compare to the
control group, and transfection with Lv-anti-miR-23b-3p partially
reversed the increased apoptosis caused by HCPT, whereas
transfection with Lv-miR-23b-3p significantly increased cell
apoptosis caused by HCPT (Fig.
4B). Consistent with these apoptosis data, treatment with HCPT
alone or with Lv-miR-23b-3p co-transfection increased the
expression of apoptosis-related proteins cleaved-PARP and BAX, and
decreased the expression of Bcl-2, compared with the Control group
(Fig. 4C). Transfecting
Lv-anti-miR-23b-3p into the fibroblasts partially attenuated the
HCPT-induced increased expression of these proteins. These data
indicated that upregulation of miR-23b-3p expression by HCPT
treatment increases human fibroblast apoptosis.
Discussion
Unsatisfactory outcomes following back surgery,
including persistent lower back pain, radiculopathy and even
disability, is often attributed to extensive epidural fibrosis
(27,28). The development and progression of
epidural fibrosis are affected by several factors, such as
postoperative chronic inflammation, lumbar instability and the
degree of hemostasis during surgery (29,30),
which likely promote the proliferation of fibroblasts and
ultimately leads to epidural fibrosis (31). Thus, research has been focused on
inducing fibroblast apoptosis as a measure of preventing epidural
fibrosis. Owing to the target-specific DNA-damaging ability of
HCPT, this agent has achieved remarkable results in the inhibition
of the proliferation of a number of tumor cells, and has been used
in the treatment of several types of malignant tumors (32,33).
However, as an antitumor agent, HCPT does not only inhibit
fibroblast proliferation, but also may exert an inhibitory effect
on the proliferation of other types of cells.
In our previous studies, fibroblasts treated with 1
µg/ml HCPT for 24 h exhibited typical morphological changes in
chromatin condensation and apoptosis in the nucleus, and a
significant increase in cell apoptotic rate (23,24).
A previous study (25) indicated
that fibroblasts exposed to HCPT at a concentration of 1 µg/ml for
12 h did not cause significant cytotoxicity, but the effects of
exposure became significant after 24 and 48 h, and cell growth was
arrested in S phase. At the same time, treating fibroblasts with
various concentrations of HCPT for 24 h. Active-caspase 3 and
cleaved PARP, which are markers of apoptosis, can only begin to
detected in 1 µg/ml of HCPT. This indicates that under this
condition, HCPT successfully inhibited fibroblast proliferation by
S phase arrest and promotion of apoptosis. Therefore, 24 h and 1
µg/ml was used as the processing time and processing
concentration.
In the present study, cells were transfected with
Lv-miR-23b-3p, Lv-anti-miR-23b-3p or their respective controls for
72 h, and cell apoptosis was examined by staining with PI and APC
Annexin V. miR-23b-3p overexpression caused an increase in the
percentage of total apoptotic fibroblasts. Previous studies have
reported that the upregulation of miR-23b-3p in airway smooth
muscle cells, hypoxia-induced cardiomyocytes and heat-denatured
fibroblasts induces cell apoptosis (34–37),
which strongly suggested that miR-23b-3p may be a key factor in the
apoptotic process. In the present study, the expression of
miR-23b-3p was lower in traumatic scar samples compared with normal
skin, and the use of HCPT increased the expression of miR-23b-3p
compared with normal fibroblasts. These data suggested that HCPT
may induce human fibroblast apoptosis by upregulating miR-23b-3p
expression. Transfection of fibroblasts with a lentivirus to
inhibit miR-23b-3p expression resulted in enhanced fibroblast
proliferation, whereas upregulation of miR-23b-3p resulted in
ectopic apoptosis. Furthermore, transfection of Lv-miR-23b-3p
enhanced the cytostatic effect of HCPT, and transfection of
Lv-anti-miR-23b-3p reduced the apoptotic effect of HCPT. These data
suggest that decreasing miR-23b-3p expression may suppress cell
apoptosis. A previous study demonstrated that downregulation of
miR-23b increased Smad3 protein expression levels, which
facilitated the proliferation of heat-denatured fibroblasts
(34). Furthermore, a number of
other studies have suggested that Smad3 is the target gene of
miR-23b-3p in rat fibroblasts and other cells (34–39);
thus, it was hypothesized that miR-23b-3p may induce human
fibroblast apoptosis by targeting Smad3. The exact effects of
miR-23b-3p on fibroblast apoptosis requires further investigation.
Results from the present study indicated that the apoptosis of
fibroblasts depends on the upregulation of miR-23b-3p. In
conclusion, the results of the present study demonstrated that HCPT
induces fibroblast apoptosis by regulating miR-23b-3p expression.
These data raise the possibility of using miR-23b-3p as a novel
therapeutic intervention to prevent epidural fibrosis.
Acknowledgements
The authors wish to record their appreciation for
the help afforded by the researchers of Orthopedic Institute of
Clinical Medical College of Yangzhou University, Northern Jiangsu
People's Hospital.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81371971 and
81271994) and by the Six Talent Peaks Project of Jiangsu Province
(grant no. 2015-WSN-110).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ conceived and designed the experiments, performed
the experiments, wrote the paper. YS analyzed the data. LY prepared
figures and tables. JW contributed reagents, materials and analysis
tools, reviewed drafts of the paper and collected tissue samples.
XL performed the experiments.
Ethics approval and consent to
participate
The present study protocol was approved by the
Research Ethics Committee of Northern Jiangsu People's Hospital
(Yangzhou, China), and written informed consent was obtained from
all the participants for their tissues to be used for the purposes
of this research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Songer MN, Rauschning W, Carson EW and
Pandit SM: Analysis of peridural scar formation and its prevention
after lumbar laminotomy and discectomy in dogs. Spine (Phila Pa
1976). 20:571–580. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burton CV, Kirkaldy-Willis WH, Yong-Hing K
and Heithoff KB: Causes of failure of surgery on the lumbar spine.
Clin Orthop Relat Res. 191–199. 1981.PubMed/NCBI
|
|
3
|
North RB, Campbell JN, James CS,
Conover-Walker MK, Wang H, Piantadosi S, Rybock JD and Long DM:
Failed back surgery syndrome: 5-year follow-up in 102 patients
undergoing repeated operation. Neurosurgery. 28:685–691. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HM, Yang KH, Han DY and Kim NH: An
experimental study on prevention of postlaminectomy scar formation.
Yonsei Med J. 31:359–366. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abitbol JJ, Lincoln TL, Lind BI, Amiel D,
Akeson WH and Garfin SR: Preventing postlaminectomy adhesion. A new
experimental model. Spine (Phila Pa 1976). 19:1809–1814. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Preul MC, Campbell PK, Garlick DS and
Spetzler RF: Application of a new hydrogel dural sealant that
reduces epidural adhesion formation: Evaluation in a large animal
laminectomy model. J Neurosurg Spine. 12:381–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Darzynkiewicz Z, Bruno S, Del Bino G and
Traganos F: The cell cycle effects of camptothecin. Ann N Y Acad
Sci. 803:93–100. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang R, Li Y, Cai Q, Liu T, Sun H and
Chambless B: Preclinical pharmacology of the natural product
anticancer agent 10-hydroxycamptothecin, an inhibitor of
topoisomerase I. Cancer Chemother Pharmacol. 41:257–267. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beretta GL, Perego P and Zunino F:
Mechanisms of cellular resistance to camptothecins. Curr Med Chem.
13:3291–3305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang SL, Lin SY, Hsieh TF and Chan SA:
Thermal behavior and thermal decarboxylation of
10-hydroxycamptothecin in the solid state. J Pharm Biomed Anal.
43:457–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mori M, Triboulet R, Mohseni M,
Schlegelmilch K, Shrestha K, Camargo FD and Gregory R: Hippo
signaling regulates microprocessor and links cell-density-dependent
miRNA biogenesis to cancer. Cell. 156:893–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi R, O'Carroll D, Pasolli HA, Zhang Z,
Dietrich FS, Tarakhovsky A and Fuchs E: Morphogenesis in skin is
governed by discrete sets of differentially expressed microRNAs.
Nat Genet. 38:356–362. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hildebrand J, Rütze M, Walz N, Gallinat S,
Wenck H, Deppert W, Grundhoff A and Knott A: A comprehensive
analysis of microRNA expression during human keratinocyte
differentiation in vitro and in vivo. J Invest Dermatol. 131:20–29.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ham O, Song BW, Lee SY, Choi E, Cha MJ,
Lee CY, Park JH, Kim IK, Chang W, Lim S, et al: The role of
microRNA-23b in the differentiation of MSC into chondrocyte by
targeting protein kinase A signaling. Biomaterials. 33:4500–4507.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang KC, Garmire LX, Young A, Nguyen P,
Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS and Chien S: Role of
microRNA-23b in flow-regulation of Rb phosphorylation and
endothelial cell growth. Proc Natl Acad Sci USA. 107:3234–3239.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salvi A, Sabelli C, Moncini S, Venturin M,
Arici B, Riva P, Portolani N, Giulini SM, De Petro G and Barlati S:
MicroRNA-23b mediates urokinase and c-met downmodulation and a
decreased migration of human hepatocellular carcinoma cells. FEBS
J. 276:2966–2982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Liu Z, Chen L, Zhou L and Yao Y:
MicroRNA-23b is an independent prognostic marker and suppresses
ovarian cancer progression by targeting runt-related transcription
factor-2. FEBS Lett. 588:1608–1615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chiyomaru T, Seki N, Inoguchi S, Ishihara
T, Mataki H, Matsushita R, Goto Y, Nishikawa R, Tatarano S, Itesako
T, et al: Dual regulation of receptor tyrosine kinase genes EGFR
and c-Met by the tumor-suppressive microRNA-23b/27b cluster in
bladder cancer. Int J Oncol. 46:487–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou X, Xu X, Wang J, Lin J and Chen W:
Identifying miRNA/mRNA negative regulation pairs in colorectal
cancer. Sci Rep. 5:129955015. View Article : Google Scholar
|
|
22
|
Ell B, Qiu Q, Wei Y, Mercatali L, Ibrahim
T, Amadori D and Kang Y: The microRNA-23b/27b/24 cluster promotes
breast cancer lung metastasis by targeting metastasis-suppressive
gene prosaposin. J Biol Chem. 289:21888–21895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Sun Y, Chen H, Zhu G, Liang Y, Wang
Q, Wang J and Yan L: Hydroxycamptothecin induces apoptosis of
fibroblasts and prevents intraarticular scar adhesion in rabbits by
activating the IRE-1 signal pathway. Eur J Pharmacol. 781:139–147.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Chen H, Sun Y, Dai J, Wang S, Wang J
and Yan L: Hydroxycamptothecin prevents intraarticular scar
adhesion by activating the PERK signal pathway. Eur J Pharmacol.
810:36–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin X, Sun H, Yu D, Liang Y, Yuan Z and Ge
Y: Hydroxycamptothecin induces apoptosis of human tenon's capsule
fibroblasts by activating the PERK signaling pathway. Invest
Ophthalmol Vis Sci. 54:4749–4758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JY, Stenzel W, Löhr M, Stützer H,
Ernestus RI and Klug N: The role of mitomycin C in reducing
recurrence of epidural fibrosis after repeated operation in a
laminectomy model in rats. J Neurosurg Spine. 4:329–333. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yildiz KH, Gezen F, Is M, Cukur S and
Dosoglu M: Mitomycin C, 5-fluorouracil, and cyclosporin A prevent
epidural fibrosis in an experimental laminectomy model. Eur Spine
J. 16:1525–1530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sandoval MA and Hernandez-Vaquero D:
Preventing peridural fibrosis with nonsteroidal anti-inflammatory
drugs. Eur Spine J. 17:451–455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tao H and Fan H: Implantation of amniotic
membrane to reduce postlaminectomy epidural adhesions. Eur Spine J.
18:1202–1212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Wang L, Sun S, Liu B, Wu N and Cao
X: The effect of 10-hydroxycamptothecine in preventing fibroblast
proliferation and epidural scar adhesion after laminectomy in rats.
Eur J Pharmacol. 593:44–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zunino F and Pratesi G: Camptothecins in
clinical development. Expert Opin Investig Drugs. 13:269–284. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ulukan H and Swaan PW: Camptothecins: A
review of their chemotherapeutic potential. Drugs. 62:2039–2057.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gammell P: MicroRNAs: Recently discovered
key regulators of proliferation and apoptosis in animal cells:
Identification of miRNAs regulating growth and survival.
Cytotechnology. 53:55–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen M, Shi J, Zhang W, Huang L, Lin X, Lv
Z, Zhang W, Liang R and Jiang S: MiR-23b controls TGF-β1 induced
airway smooth muscle cell proliferation via direct targeting of
Smad3. Pulm Pharmacol Ther. 42:33–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Hao W, Wang X and Su H: miR-23b
targets Smad 3 and ameliorates the LPS-inhibited osteogenic
differentiation in preosteoblast MC3T3-E1 cells. J Toxicol Sci.
41:185–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Yang J, Zhao J, Zhang P and Huang
X: MicroRNA-23b inhibits the proliferation and migration of
heat-denatured fibroblasts by targeting Smad3. PLoS One.
10:e01318672015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leone V, D'Angelo D, Pallante P, Croce CM
and Fusco A: Thyrotropin regulates thyroid cell proliferation by
up-regulating miR-23b and miR-29b that target SMAD3. J Clin
Endocrinol Metab. 97:3292–3301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He W, Che H, Jin C and Ge S: Effects of
miR-23b on hypoxia-induced cardiomyocytes apoptosis. Biomed
Pharmacother. 96:812–817. 2017. View Article : Google Scholar : PubMed/NCBI
|