Introduction
Ovarian cancer is one of the most common
gynecological cancers (1,2). Ovarian cancer patients have a very
high death rate because they do not exhibit initial symptoms, and
the cancer is usually found only after metastasis. Between 1999 and
2014, there were 2,413 cases reported with 1,021 deaths from
ovarian cancer, showing a 57.7% survival rate in Korea (3). Current cancer treatments involve
various methods including surgery, chemotherapy, radiation, and
immune therapy. However, the rate of mortality remains high due to
drug resistance and undesired side effects (1). Therefore, it is necessary to
understand the mechanisms of ovarian cancer cell death and find new
and safer agents.
Resveratrol (3,5,4′-trans-trihydroxystilbene) is a
polyphenolic phytoalexin. It is abundantly found in a variety of
food sources including grapes, berries and peanuts. Resveratrol is
known to have cardioprotective, anti-oxidant and anti-inflammatory
effects (4). It also provides
antitumor activities in various cancers such as breast, prostate,
lung, colon, and liver (5–10). Its antitumor activities have been
suggested to be closely related with generation of reactive oxygen
species (ROS) and Akt signaling (11,12).
Notch signaling is a cell contact-dependent pathway
involved in various differentiation processes. Recent studies have
suggested that Notch signaling and related transcriptional factors
act as upregulators in the death of invasive human cancer cells
(13,14). However, the role of Notch signaling
in resveratrol-induced death in human ovarian cancer cells is not
clear.
In the present study, we demonstrated that
resveratrol induced human ovarian cancer cell death through
ROS/Notch1/PTEN/Akt signaling. The results suggest that resveratrol
can be considered as a potential therapeutic agent for treating
human ovarian cancer.
Materials and methods
Reagents
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide (MTT), SF1670 and resveratrol were purchased from
Sigma-Aldrich Chemical (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). EF.hlCN1.CMV.GFP was purchased from Addgene (Cambridge,
MA, UK). Z-DEVD-FMK was purchased from Calbiochem (San Diego, CA,
USA). Antibodies (anti-cleavde-Notch1, anti-total-Notch1,
anti-phospho-PTEN, anti-phospho-Akt, anti-total-Akt,
anti-cleaved-caspase-3) were obtained from Cell Signaling
Technology (Beverly, MA, USA). Anti-GAPDH was procured from Santa
Cruz Biotechnology Inc. (Dallas, TX, USA). All other chemicals were
of the highest commercial grade available.
Cell culture
A2780 and SKOV3 cells were obtained from the
American Type Culture Collection (Rockville, MD, USA) and
maintained by serial passages in 75-cm2 culture flasks
(Costar, Cambridge, MA, USA). The cells were grown in Roswell Park
Memorial Institute (RPMI)-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% heat inactivated fetal
bovine serum (HyClone, Logan, UT, USA) at 37°C in a humidified
incubator filled with 95% air and 5% CO2. When cells
were grown to reach confluence, they were detached using 0.02%
EDTA-0.05% trypsin solution and subculture was performed.
Measurement of cell viability
MTT assay was performed to determine cell viability.
After washing out the culture media bathing the cells, fresh
culture media containing 0.5 mg/ml of MTT was added to each well.
After incubating for 2 h, the media was removed by aspiration and
the formazan crystals produced by viable cells in each well were
solubilized in dimethyl sulfoxide. A 0.1 ml aliquot of each sample
was then transferred to 96-well plates and the absorbance of each
well was measured with ELISA Reader (FLUOstar OPTIMA; BMG Labtech
GmbH, Ortenberg, Germany) at 550 nm.
Measurement of apoptosis
Cell apoptosis was evaluated using FITC Annexin V
Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA).
Changes in FITC Annexin V fluorescence was measured using a FACSort
Becton Dickinson Flow Cytometer and data were analyzed with
CELLQuest Software (FACSCalibur™ and Cellquest™; BD Biosciences,
Franklin Lakes, NJ, USA). The Annexin V binding assay was carried
out according to the manual provided by the manufacturer. After
exposure to experimental protocols, cells were washed twice with
physiological buffer solution (PBS). Cells were then detached by
treatment with 0.025% trypsin and harvested by washing and
centrifugation with cold PBS. Cells were then resuspended in
Annexin V binding buffer and incubated for 15 min with binding
solution containing FITC Annexin V and propidium iodide in the
dark. Flow cytometric analysis was performed with the excitation
filter at 488 nm. The proportion of apoptotic cells was estimated
as the quadrant statistics of the early and late apoptotic region
to the entire cell population.
Measurement of reactive oxygen species
(ROS)
The changes in cellular ROS level were measured
using DCFH-DA. DCFH-DA itself is a non-fluorescent ester. As it is
highly permeable to the cell membrane, it readily accumulates in
the intracellular space. Within the cells, it is hydrolyzed to DCFH
by the cellular esterases. In the presence of cellular peroxidase
and ROS, DCFH is then rapidly oxidized to a highly fluorescent DCF.
Thus, cellular DCF fluorescence is an excellent indicator that
reflects the change in the intracellular ROS level. Changes in DCF
fluorescence was assayed using FACSort Becton Dickinson Flow
Cytometer (BD Biosciences) and data were analyzed with CellQuest
software.
Western blot analysis
After exposure to experimental protocols, cells were
collected and disrupted in lysis buffer composed of 1% Triton
X-100, 1 mM EGTA, 1 mM EDTA, 10 mM Tris-HCl, pH 7.4. After removal
of cell debris by centrifugation, the resulting supernatants were
resolved on a 10% SDS-PAGE under denatured reducing conditions and
transferred to nitrocellulose membranes. The membranes were blocked
with 5% non-fat dried milk at room temperature for 30 min and
incubated with different primary antibodies. The membranes were
washed and incubated with horseradish peroxidase-conjugated
secondary antibodies (goat Anti-Rabbit IgG, goat anti-mouse IgG;
Santa Cruz Biotechnology). The signal was visualized using an
enhanced chemiluminescence (ECL; Bio-Rad, Hercules, CA, USA).
Transfection
To modulate the activity of Akt, cells were
transfected transiently with the constitutively active form of Akt.
Cells were grown on 6-well plastic plates to reach 70% of
confluency. Using Lipofectamine (Invitrogen; Thermo Fisher
Scientific), 2 µg cDNA was transiently transfected according to
manufacturer's guidelines. After 4-h incubation at 37°C, cells were
maintained in normal culture media for 24 h. To overexpress
intracellular Notch1, we transferred EF.hlCN1.CMV.GFP (Addgene,
Cambridge, MA, USA) according to the manufacturer's
instructions.
Statistical analysis
The data are expressed as means ± SEM and the
difference between two groups was evaluated using Student's t-test.
Multiple group comparison was done using one-way analysis of
variance followed by the Tukey post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Resveratrol inhibits cell viability
and induces apoptosis
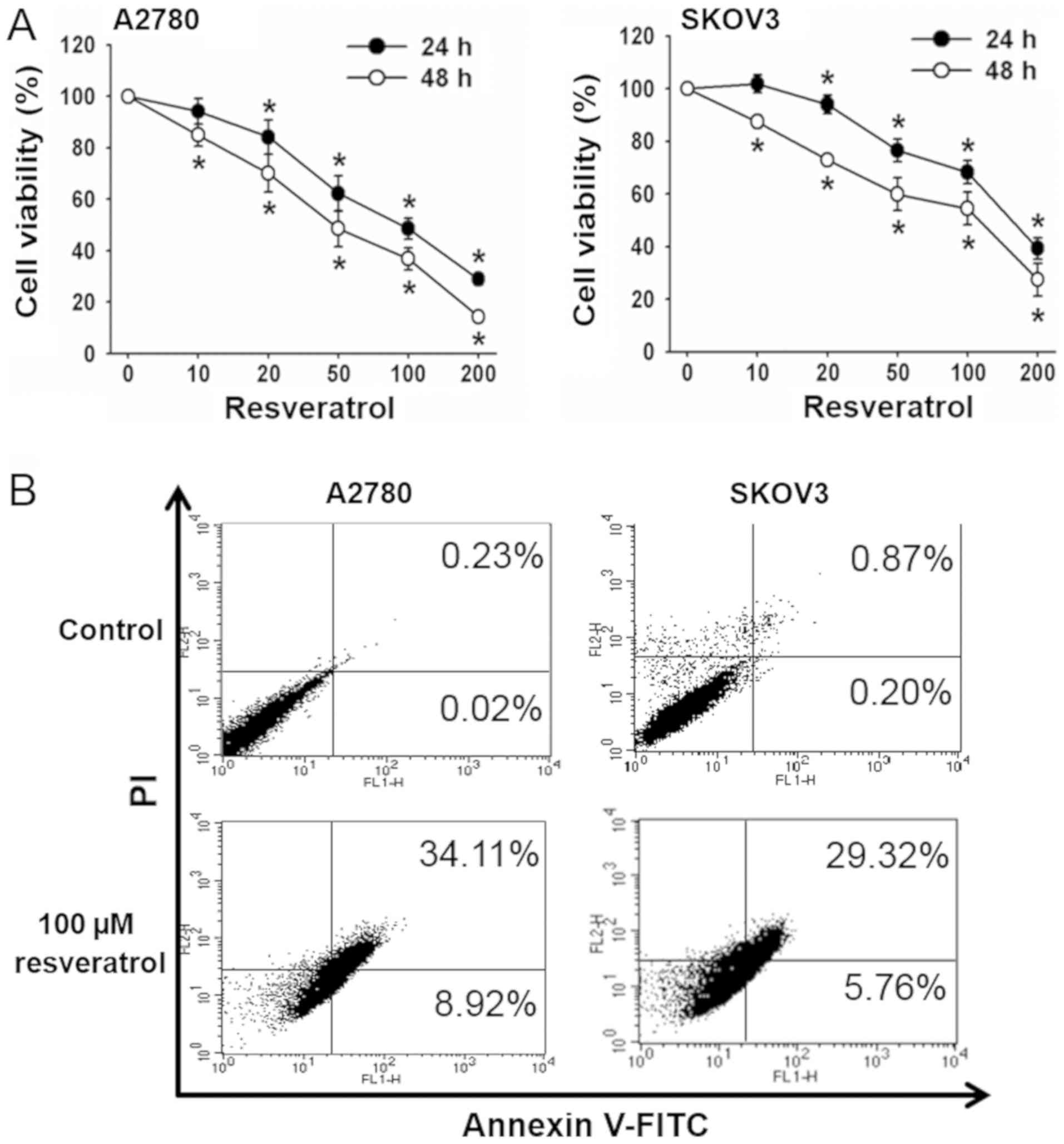
We investigated the effect of resveratrol on the
viability of the human ovarian cancer cells, A2780 and SKOV3. After
exposure of cells to 0–200 µM of resveratrol for 24 and 48 h, MTT
assay was performed to examine the cell viability. Resveratrol
significantly decreased the viability of both cell lines in time-
and dose-dependent manners. The concentrations of resveratrol to
show 50% inhibition of cell viability (IC50) were 46.6±6.2 and
116.6±12.8 µM in A2780 and SKOV3 cells, respectively. Cell
viability was 42–58% with 100 µM resveratrol after 48 h of exposure
(Fig. 1A). Therefore, 100 µM
resveratrol for 48 h was used in subsequent experiments.
To examine whether the resveratrol-induced reduction
of cell viability was caused by apoptotic cell death, Annexin V/PI
staining was performed. Resveratrol treatment increased apoptotic
cell population from 0.25% in the control to 43.0% in A2780 cells,
and from 1.1 to 35.1% in SKOV3 cells (Fig. 1B). These results suggest that
resveratrol-induced cell death of these ovarian cancer cells
occurred mainly through apoptosis.
Resveratrol stimulates ROS production
in ovarian cancer cells
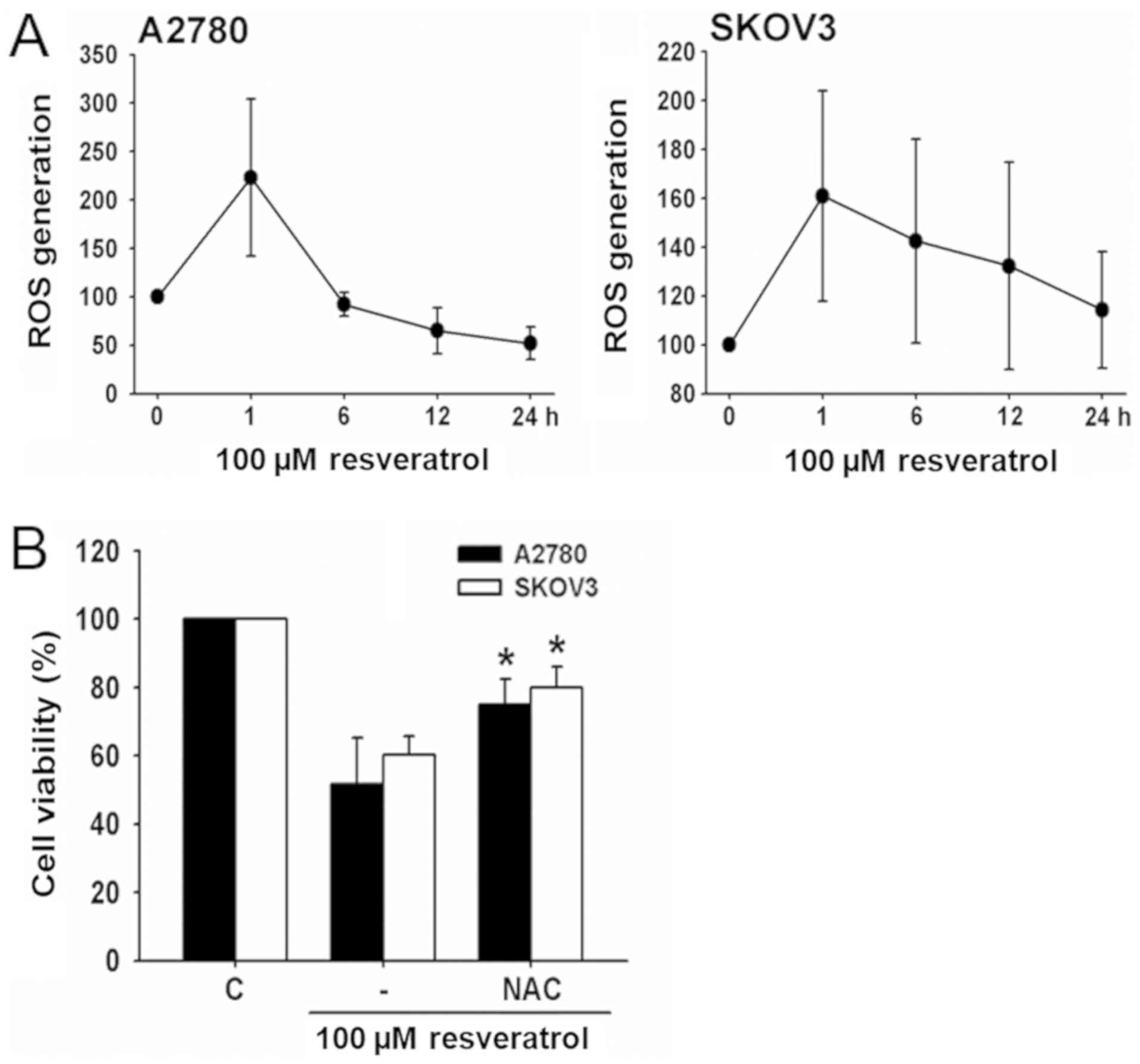
To determine whether resveratrol stimulated ROS
production in ovarian cancer cell lines, A2780 and SKOV3 cells were
exposed to resveratrol and changes in DCF fluorescence were
measured using flow cytometry. Resveratrol caused a transient
increase in ROS generation with a maximum rise after 1 h of
treatment (Fig. 2A).
To examine the role of ROS production in
resveratrol-induced cell death, the effect of the antioxidant, NAC,
on cell viability was determined. Resveratrol-induced cell death
was significantly decreased by NAC (Fig. 2B), indicating that this process was
associated with ROS generation.
Resveratrol decreases Notch1
expression in ovarian cancer cells
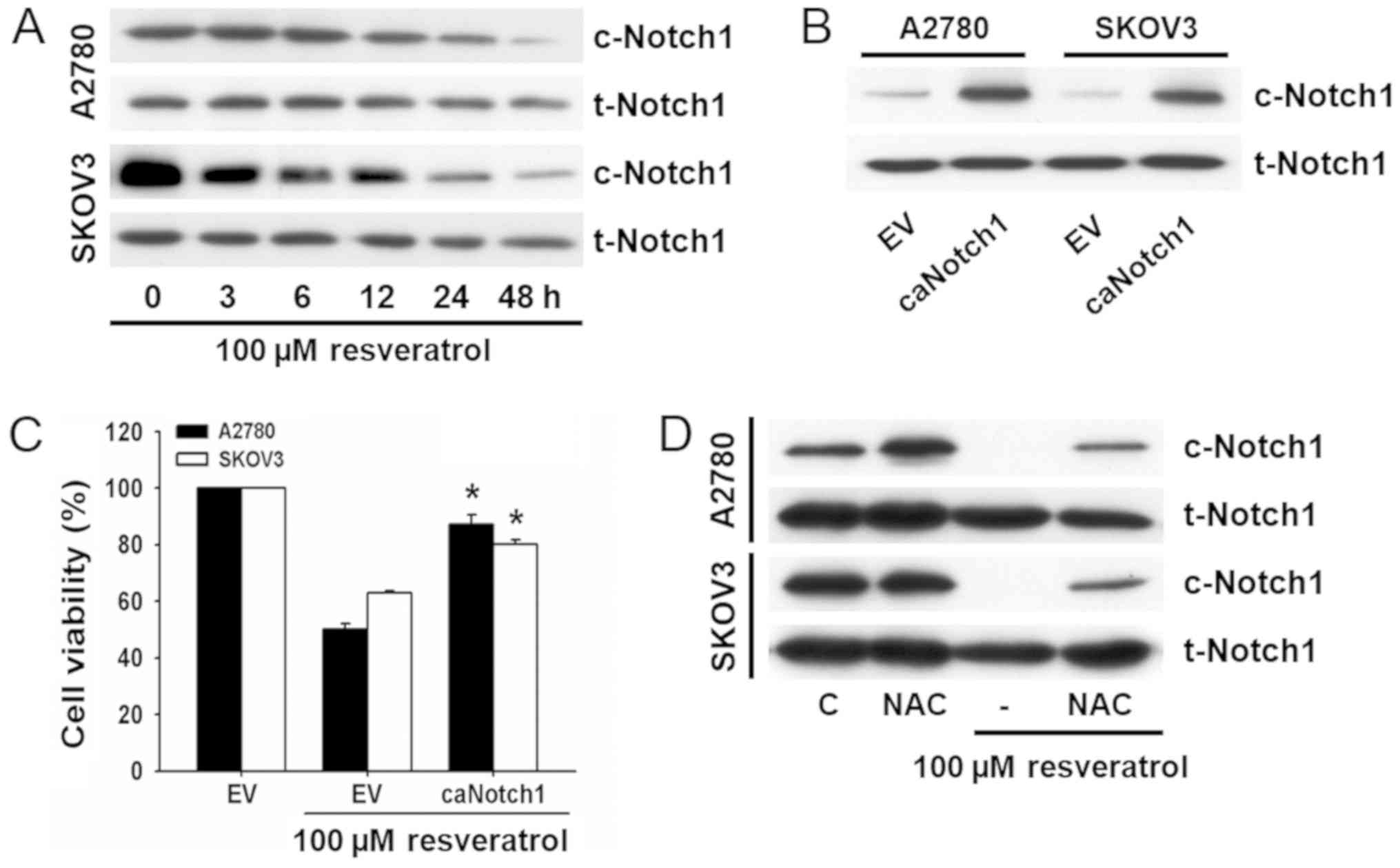
Notch1 signaling has been reported as an important
signaling cascade that determines cancer cell fate. Thus, we
evaluated the role of Notch1 expression in resveratrol-induced cell
death. The expression of Notch1 in resveratrol-treated cells was
assessed by western blot analysis using the primary antibody for
cleaved notch1 which detects Notch1 intracellular domain (NICD1).
As shown in Fig. 3A, resveratrol
decreased the expression of Notch1 in a time-dependent manner in
both ovarian cancer cell lines.
To determine whether Notch1 signaling was involved
in resveratrol-induced cell death, viability was determined in
cells transfected with EF.hlCN1.CMV.GFP. The overexpression of
Notch1 was assessed by western blot analysis (Fig. 3B) and cell viability was analyzed
by the MTT assay. Both ovarian cancer cell lines overexpressing
Notch1 exhibited resistance to resveratrol-induced cell death
(Fig. 3C).
To examine whether resveratrol suppressed Notch1
expression through the stimulation of ROS production, cells were
pretreated with NAC before exposure to resveratrol, and changes in
Notch1 expression were examined by western blot analysis. As shown
in Fig. 3D, the
resveratrol-induced decrease in Notch1 expression was prevented by
treatment with NAC. These results suggest that the decrease of
Notch1 is associated with ROS production and critically implicated
in resveratrol-induced cell death.
Resveratrol induces cell death through
PTEN/Akt signaling
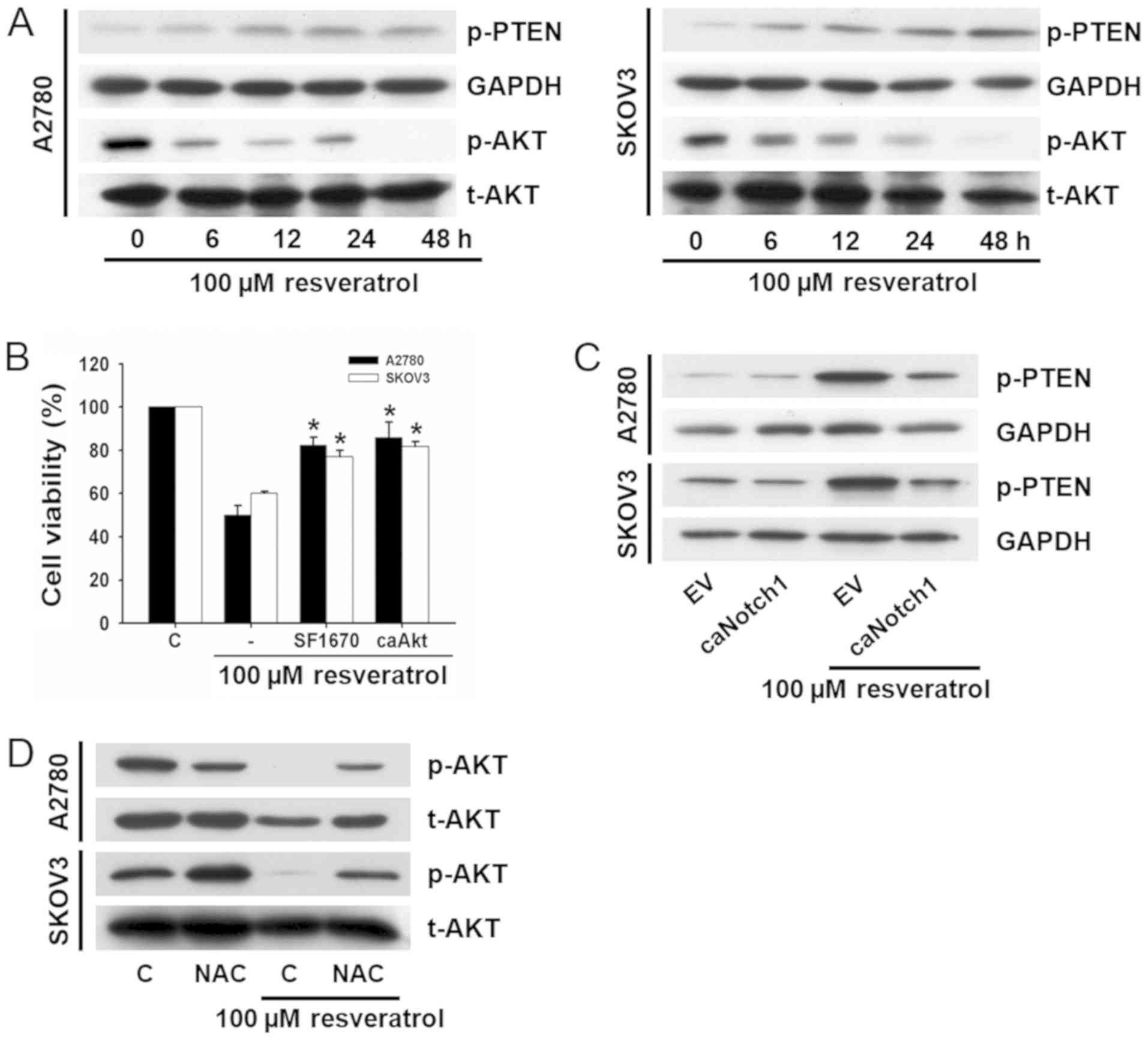
PTEN/Akt signaling plays important roles in cell
proliferation, survival, and differentiation. ROS generation and
Notch1 are intimately related with PTEN/Akt signaling. Therefore,
we evaluated whether resveratrol-induced cell death was associated
with this signaling. After exposure of cells to 100 µM resveratrol
for different time periods, phosphorylation of PTEN and Akt were
analyzed by western blot analyses. Resveratrol induced upregulation
of p-PTEN and downregulation of p-Akt in a time-dependent manner
(Fig. 4A).
To confirm whether PTEN/Akt signaling was involved
in resveratrol-induced cell death, cells were pretreated with a
PTEN inhibitor, SF1670, or transfected with a constitutively active
form of Akt (caAkt), and cell viability was measured. The
transfection efficiency was estimated to be >70% by
immunofluorescence, and the expression of p-Akt was increased
compared with cells transfected with the empty vector (data not
shown). Resveratrol-induced cell death was significantly suppressed
both by treatment with SF1670 and transfection with caAkt (Fig. 4B).
To investigate whether resveratrol-induced
downregulation of p-Akt was attributable to Notch1 signaling, cells
were transfected with EF.hlCN1.CMV.GFP before exposure to
resveratrol and changes in PTEN phosphorylation were determined by
western blot analysis. Resveratrol induced upregulation of p-PTEN
was prevented by transfection with EF.hlCN1.CMV.GFP (Fig. 4C).
To determine whether resveratrol-induced
downregulation of p-Akt through the stimulation of ROS generation,
cells were pretreated with NAC before exposure to resveratrol, and
changes of p-Akt expression were examined by western blot analysis.
As shown in Fig. 4D, the
resveratrol-induced downregulation of p-Akt was prevented by
treatment with NAC. These results suggest that resveratrol-induced
cell death is closely related to the Notch1-dependent upregulation
of p-PTEN and downregulation of p-Akt. In addition, it is mediated
by increased ROS generation.
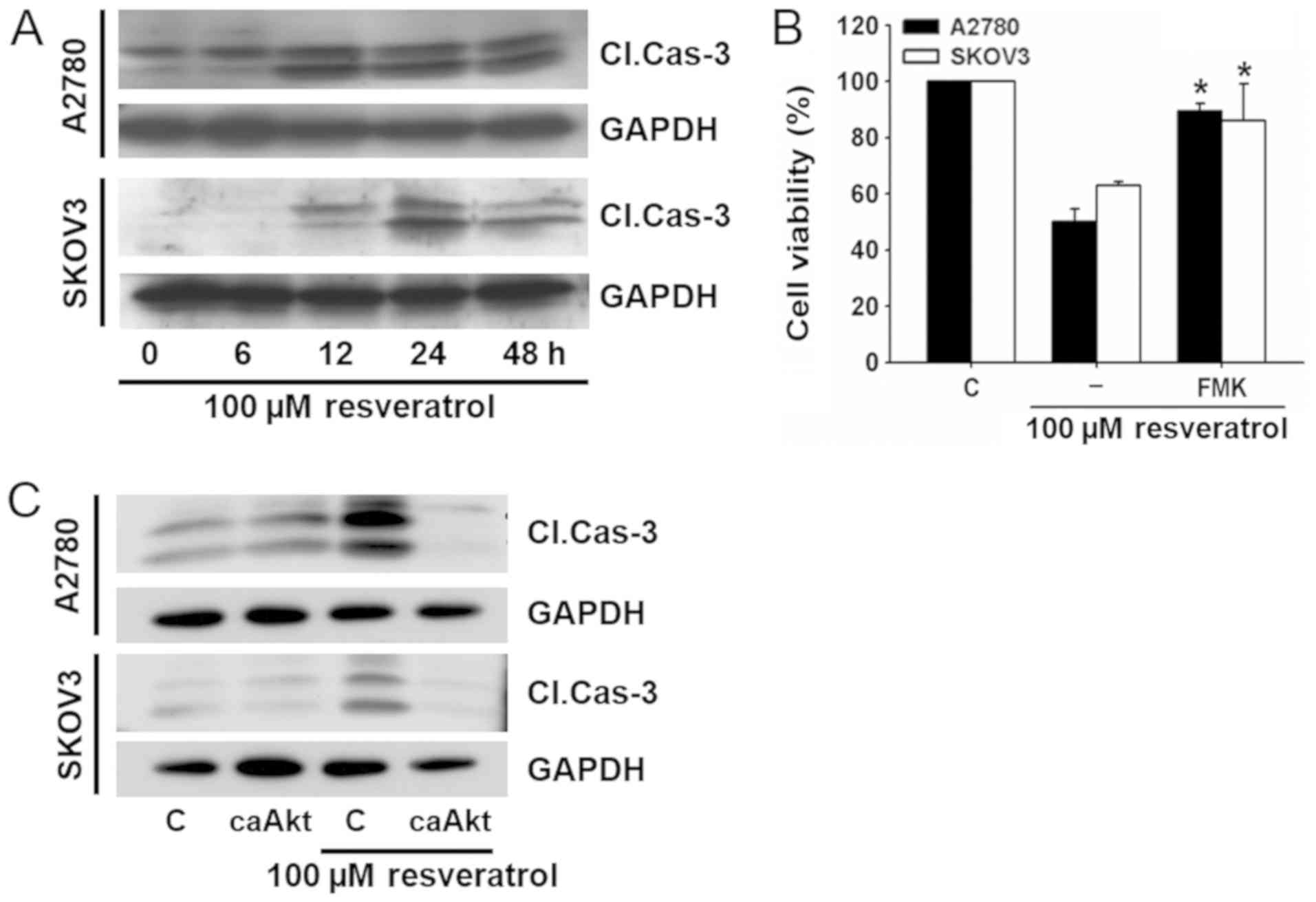
Resveratrol induces caspase-dependent
cell death
The caspase family of enzymes is essential in the
apoptotic pathway. In the process, caspase-3 plays a critical role
in the execution phase of apoptosis. We examined the role of
caspase-3 in resveratrol-induced cell death.
Resveratrol increased the cleaved caspase-3 level in
a time-dependent manner in both ovarian cancer cell lines (Fig. 5A). To examine further the role of
activated caspase-3 in resveratrol-induced cell death, the effect
of a caspase inhibitor (Z-DEVD-FMK) was examined. When cells were
pretreated with Z-DEVD-FMK, the effect of resveratrol on cell
viability was significantly attenuated (Fig. 5B).
To investigate whether resveratrol-induced cleavage
of caspase-3 was attributable to Akt signaling, cells were
transfected with caAkt before exposure to resveratrol, and the
change of caspage-3 cleavage was measured by western blotting. As
shown in Fig. 5C,
resveratrol-induced cleavage of caspage-3 was significantly
decreased by transfection with caAkt. These results suggest that
resveratrol-induced cell death is associated with Akt
signaling-dependent cleavage of caspase-3.
Discussion
Resveratrol is a type of polyphenol found in many
plants, including berries, peanuts, raspberries, and grapes
(15). Resveratrol has
wide-ranging effects such as antioxidant, anti-inflammatory, and
antitumor properties in various cancers models. It was reported
that ovarian cancer cell is more susceptible to ROS when compared
with normal epithelial cells (16–18).
However, the precise cellular mechanism of resveratrol-induced cell
death has not yet been clarified. The present study provided us
with clues to understand the molecular mechanisms of the
resveratrol-induced antitumor activity in human ovarian cancer
cells.
Intracellular ROS play a pivotal role in cell
signaling and homeostasis (19).
Although resveratrol is well-known for its antioxidant activity, it
may also behave as a pro-oxidant that are responsible for antitumor
activity in some cancer cells. We consider that resveratrol has
both antioxidant and pro-oxidant properties, depending on the cell
type, drug concentration, and other experimental conditions
(20–23). In the present study, resveratrol
stimulated ROS production, and the antioxidant, NAC, prevented
resveratrol-induced cell death (Fig.
2). These results strongly suggest that resveratrol-induced
cell death is associated with ROS production. Similar results have
been reported in other ovarian cancer cells exposed to resveratrol
(24).
Notch signaling promotes cell growth, migration,
invasion, and apoptosis in various cancer cells (25,26).
Notch receptors are assembled as a large extracellular
ligand-binding domain, a single-pass transmembrane domain, and
Notch intracellular domain (NICD). When γ-secretase bind to Notch
receptors NICD is released. Released NICD translocates to the
nucleus and activates transcription of downstream target genes.
Notch signaling has emerged as a potential target for cancer
therapies. However, the effect of resveratrol on Notch signaling is
not clear yet.
In the present study, we used the cleaved-Notch1
primary antibody to detect NICD1. Resveratrol decreased the
c-Notch1 protein level (Fig. 3A).
In addition, resveratrol-induced cell death was prevented by
overexpression of Notch1 (Fig.
3C). The correlation between ROS generation and Notch1
signaling is very intricate and involves many steps. Notch1
suppresses ROS generation (27)
and, conversely, ROS generation regulates Notch1 signaling
(28). In our study, resveratrol
seemed to suppress Notch1 signaling through ROS generation
(Fig. 3D).
PTEN/Akt signaling works to regulate cellular
responses to various extracellular stimuli (29). This signaling is associated with
cancer cell proliferation, invasion, tumorigenesis, and drug
resistance in many different cell types (30–32).
Activated Akt can inhibit the release of cytochrome c and
thus the cleavage of caspase-3, thereby inhibiting apoptosis and
promoting cancer cell survival. Resveratrol inhibits Akt signaling
thereby inducing apoptosis in several types of cancer cells. The
cleavage of Notch by γ-secretase releases the Notch intracellular
domain into the cytoplasm. The released domain can suppress PTEN,
an inhibitor of Akt. Hence, downregulation of Notch signaling
inhibits Akt signaling by PTEN (33,34).
In the present study, resveratrol upregulated p-PTEN and
downregulated p-Akt in time-dependent manners (Fig. 4A). Resveratrol-induced cell death
was prevented by a PTEN inhibitor, SF1670, or transfection with
caAkt (Fig. 4B). In addition,
resveratrol-induced upregulation of p-PTEN was prevented by
overexpression of Notch1 (Fig.
4C). Resveratrol-induced downregulation of p-Akt was prevented
by treatment with NAC (Fig. 4D).
Although data were not presented in our results pretreatment with
SF1670 and/or transfection of caAkt did not affect ROS generation.
These results suggest that ROS generation resides on upstream of
resveratrol-induced changes in Notch1/PTEN/Akt signaling
pathway.
Caspase-3 plays a critical role during the execution
phase in various forms of apoptosis. Caspase-3 is present as an
inactive pro-enzyme and is activated by proteolytic cleavage. This
cleavage is initiated by ligands of many cell surface receptors in
a complex associated with the cytoplasmic death domain that
triggers the release of cytochrome c from mitochondria.
Cytochrome c binds with apoptotic protease activation factor
1, which then activates caspase-9 that, in turn, cleaves caspase-3
(35,36). There is also an induction of
caspase-3 (37). In the present
study, resveratrol increased the cleavage of caspase-3 as
demonstrated by western blotting (Fig.
5A). Resveratrol-induced cell death was prevented by a
selective caspase-3 inhibitor, Z-DEVD-FMK (Fig. 5B). In addition, resveratrol-induced
cleavage of caspase-3 was blocked by transfection with caAkt
(Fig. 5C).
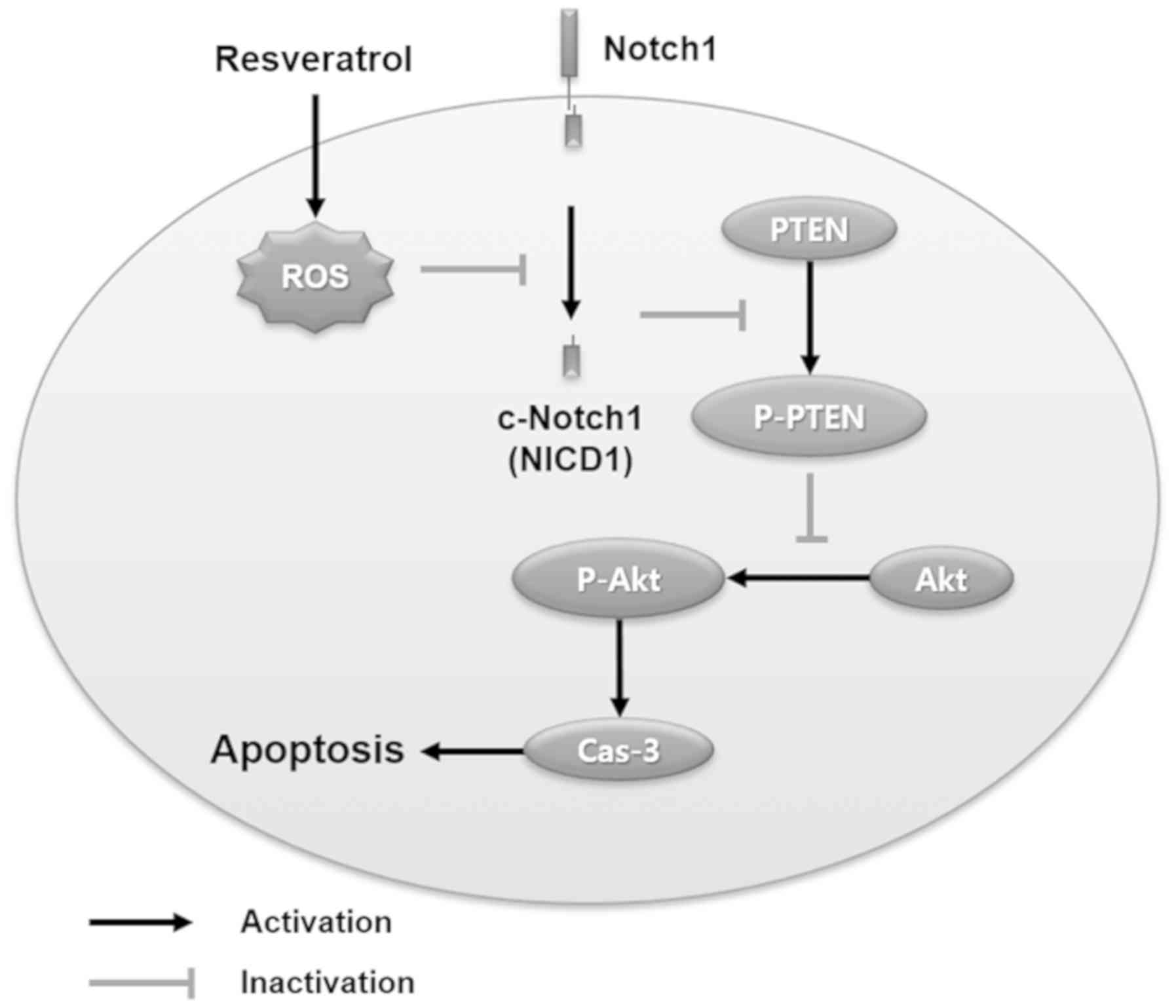
In conclusion, the present study demonstrated that
resveratrol induced human ovarian cancer cell death through
ROS-dependent Notch1/PTEN/Akt signaling. Related signaling
mechanisms are summarized in Fig.
6. Our results suggest that resveratrol could be considered as
a potential candidate for treating human ovarian cancer, and Notch1
signaling could be a potential target for further
investigation.
Acknowledgements
Not applicable.
Funding
The current study was supported by a 2-Year Research
Grant of Pusan National University.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JHP carried out western blotting and collected data.
THK carried out assays for cell viability, apoptosis and ROS
generation. THK and JSW participated in experiment design and the
draft preparation. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansen JM, Coleman RL and Sood AK:
Targeting the tumour microenvironment in ovarian cancer. Eur J
Cancer. 56:131–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH
and Lee KH; Community of Population-Based Regional Cancer
Registries, : Cancer statistics in Korea: Incidence, mortality,
survival, and prevalence in 2014. Cancer Res Treat. 49:292–305.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smoliga JM, Baur JA and Hausenblas HA:
Resveratrol and health-a comprehensive review of human clinical
trials. Mol Nutr Food Res. 55:1129–1141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riba A, Deres L, Sumegi B, Toth K,
Szabados E and Halmosi R: Cardioprotective effect of resveratrol in
a postinfarction heart failure model. Oxid Med Cell Longev.
2017:68192812017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chatterjee A, Ronghe A, Padhye SB, Spade
DA, Bhat NK and Bhat HK: Antioxidant activities of novel
resveratrol analogs in breast cancer. J Biochem Mol Toxicol.
322018.
|
|
7
|
Singh SK, Banerjee S, Acosta EP, Lillard
JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis
with docetaxel in prostate cancer cells via a
p53/p21WAF1/CIP1 and p27KIP1 pathway.
Oncotarget. 8:17216–17228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Wang D and Zhao Y: Effect and
mechanism of resveratrol on the apoptosis of lung adenocarcinoma
cell line A549. Cell Biochem Biophys. 73:527–531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong WH, Zhao N, Zhang ZM, Zhang YX, Yan L
and Li JB: The inhibitory effect of resveratrol on COX-2 expression
in human colorectal cancer: A promising therapeutic strategy. Eur
Rev Med Pharmacol Sci. 21:1136–1143. 2017.PubMed/NCBI
|
|
10
|
Alobaedi OH, Talib WH and Basheti IA:
Antitumor effect of thymoquinone combined with resveratrol on mice
transplanted with breast cancer. Asian Pac J Trop Med. 10:400–408.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li P, Xu J, Wu X, Wu X, Guo Z, Fan L, Song
R, Wang J, Wei L and Teng H: Resveratrol attenuates high
glucose-induced nucleus pulposus cell apoptosis and senescence
through activating the ROS-mediated PI3K/Akt pathway. Biosci Rep.
38(pii): BSR201714542018.PubMed/NCBI
|
|
12
|
Hui Y, Chengyong T, Cheng L, Haixia H,
Yuanda Z and Weihua Y: Resveratrol attenuates the cytotoxicity
induced by amyloid-β1–42 in PC12 cells by upregulating heme
oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem Res.
43:297–305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou Y, Feng S, Wang L, Zhao Z, Su J, Yin
X, Zheng N, Zhou X, Xia J and Wang Z: Inhibition of Notch-1 pathway
is involved in rottlerin-induced tumor suppressive function in
nasopharyngeal carcinoma cells. Oncotarget. 8:62120–62130. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Sha J, Yang G, Huang X, Bo J and
Huang Y: Activation of Notch pathway is linked with
epithelial-mesenchymal transition in prostate cancer cells. Cell
Cycle. 16:999–1007. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bertelli AA and Das DK: Grapes, wines,
resveratrol, and heart health. J Cardiovasc Pharmacol. 54:468–476.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piotrowska H, Myszkowski K, Ziolkowska A,
Kulcenty K, Wierzchowski M, Kaczmarek M, Murias M,
Kwiatkowska-Borowczyk E and Jodynis-Liebert J: Resveratrol analogue
3,4,4′,5-tetramethoxystilbene inhibits growth, arrests cell cycle
and induces apoptosis in ovarian SKOV-3 and A-2780 cancer cells.
Toxicol Appl Pharmacol. 263:53–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vergara D, De Domenico S, Tinelli A,
Stanca E, Del Mercato LL, Giudetti AM, Simeone P, Guazzelli N,
Lessi M, Manzini C, et al: Anticancer effects of novel resveratrol
analogues on human ovarian cancer cells. Mol Biosyst. 13:1131–1141.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seino M, Okada M, Shibuya K, Seino S,
Suzuki S, Takeda H, Ohta T, Kurachi H and Kitanaka C: Differential
contribution of ROS to resveratrol-induced cell death and loss of
self-renewal capacity of ovarian cancer stem cells. Anticancer Res.
35:85–96. 2015.PubMed/NCBI
|
|
19
|
Sena LA and Chandel NS: Physiological
roles of mitochondrial reactive oxygen species. Mol Cell.
48:158–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia N, Daiber A, Förstermann U and Li H:
Antioxidant effects of resveratrol in the cardiovascular system. Br
J Pharmacol. 174:1633–1646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heiss EH, Schilder YD and Dirsch VM:
Chronic treatment with resveratrol induces redox stress- and ataxia
telangiectasia-mutated (ATM)-dependent senescence in p53-positive
cancer cells. J Biol Chem. 282:26759–26766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schilder YD, Heiss EH, Schachner D,
Ziegler J, Reznicek G, Sorescu D and Dirsch VM: NADPH oxidases 1
and 4 mediate cellular senescence induced by resveratrol in human
endothelial cells. Free Radic Biol Med. 46:1598–1606. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de la Lastra CA and Villegas I:
Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and
clinical implications. Biochem Soc Trans. 35:1156–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lang F, Qin Z, Li F, Zhang H, Fang Z and
Hao E: Apoptotic cell death induced by resveratrol is partially
mediated by the autophagy pathway in human ovarian cancer cells.
PLoS One. 10:e01291962015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang P, Li H, Yang B, Yang F, Zhang LL,
Kong QY, Chen XY, Wu ML and Liu J: Biological significance and
therapeutic implication of resveratrol-inhibited Wnt, Notch and
STAT3 signaling in cervical cancer cells. Genes Cancer. 5:154–164.
2014.PubMed/NCBI
|
|
26
|
Zheng XJ, Yang ZX, Dong YJ, Zhang GY, Sun
MF, An XK, Pan LH and Zhang SL: Downregulation of leptin inhibits
growth and induces apoptosis of lung cancer cells via the Notch and
JAK/STAT3 signaling pathways. Biol Open. 5:794–800. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SY, Kim MY, Mo JS and Park HS: Notch1
intracellular domain suppresses APP intracellular domain-Tip60-Fe65
complex mediated signaling through physical interaction. Biochim
Biophys Acta. 1773:736–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coant N, Ben Mkaddem S, Pedruzzi E,
Guichard C, Tréton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik
Y, Woerther PL, et al: NADPH oxidase 1 modulates WNT and NOTCH1
signaling to control the fate of proliferative progenitor cells in
the colon. Mol Cell Biol. 30:2636–2650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging (Albany NY).
3:192–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z, Liu Y, Shi C, Zhang Y, Lv R, Zhang
R, Wang Q and Wang Y: Suppression of PTEN/AKT signaling decreases
the expression of TUBB3 and TOP2A with subsequent inhibition of
cell growth and induction of apoptosis in human breast cancer MCF-7
cells via ATP and caspase-3 signaling pathways. Oncol Rep.
37:1011–1019. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rana C, Piplani H, Vaish V, Nehru B and
Sanyal SN: Downregulation of PI3-K/Akt/PTEN pathway and activation
of mitochondrial intrinsic apoptosis by diclofenac and curcumin in
colon cancer. Mol Cell Biochem. 402:225–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim DH, Kim MJ, Sung B, Suh H, Jung JH,
Chung HY and Kim ND: Resveratrol analogue, HS-1793, induces
apoptotic cell death and cell cycle arrest through downregulation
of AKT in human colon cancer cells. Oncol Rep. 37:281–288. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo D, Teng Q and Ji C: NOTCH and
phosphatidylinositide 3-kinase/phosphatase and tensin homolog
deleted on chromosome ten/AKT/mammalian target of rapamycin (mTOR)
signaling in T-cell development and T-cell acute lymphoblastic
leukemia. Leuk Lymphoma. 52:1200–1210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cornejo MG, Mabialah V, Sykes SM, Khandan
T, Lo Celso C, Lopez CK, Rivera-Muñoz P, Rameau P, Tothova Z, Aster
JC, et al: Crosstalk between NOTCH and AKT signaling during murine
megakaryocyte lineage specification. Blood. 118:1264–1273. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Jin F, Qin A, Hao Y, Dong Y, Ge S
and Dai K: Targeting Notch1 signaling pathway positively affects
the sensitivity of osteosarcoma to cisplatin by regulating the
expression and/or activity of Caspase family. Mol Cancer.
13:1392014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Lei J, Liu J, Ma F and Ju H: In
situ activation and monitoring of the evolution of the
intracellular caspase family. Chem Sci. 6:3365–3372. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mondal A and Bennett LL: Resveratrol
enhances the efficacy of sorafenib mediated apoptosis in human
breast cancer MCF7 cells through ROS, cell cycle inhibition,
caspase 3 and PARP cleavage. Biomed Pharmacother. 84:1906–1914.
2016. View Article : Google Scholar : PubMed/NCBI
|