Introduction
Renal interstitial fibrosis is one of the main
pathological features of chronic kidney disease (1). As the disease progresses, patients
may suffer glomerular or tubulointerstitial lesions, which may
result in end-stage renal failure and even mortality (2). At present, there are limited
treatments available for alleviating renal fibrosis. Therefore, the
identification of more effective methods for preventing or
decelerating the progression of renal fibrosis is urgently
required.
Loss of peritubular capillaries has been regarded as
the distinguishing feature of progressive interstitial fibrosis
(3–5). Increasing evidence has demonstrated
that renal fibrosis is associated with peritubular capillary loss
in experimental animals and clinical patients (6,7). It
has been demonstrated that renal injury may block peritubular
capillaries and then cause hypoxic injury, which stimulates
interstitial fibrosis (8–10). In addition, the loss of capillaries
has also been confirmed to induce fibrosis under certain
conditions, such as glomerulonephritis and unilateral ureteral
obstruction (UUO) (11,12). Thus, replacing damaged capillary
vessels is an important approach to mitigating interstitial
fibrosis, and angiogenesis serves a crucial role in this process
(12–14). Vascular endothelial growth factor
(VEGF) is important in promoting the formation of tubule-like
structures by endothelial cells and is an essential mediator of
angiogenesis (15). A previous
study demonstrated that thrombospondin 1 (TSP-1), an
anti-angiogenic molecule, could further worsen progressive renal
disease (16). Downregulated VEGF
expression and increased TSP-1 expression are closely associated
with renal interstitial fibrosis (4). In addition, TSP-1 has been confirmed
to induce the activation of transforming growth factor (TGF)-β1,
which is a key cytokine in the induction of renal interstitial
fibrosis (17).
Astaxanthin (ASX) is a natural carotenoid and a
major source of red pigments in marine animals. Growing evidence
suggests that ASX has various biological activities, including
antioxidant, anti-inflammatory, ultraviolet ray-resistance,
anti-tumor and immune regulatory effects (18–21).
A previous study revealed that ASX could prevent capillary
regression in atrophied soleus muscles by upregulating VEGF and
downregulating TSP-1 (22).
However, whether ASX has a protective effect against renal
interstitial fibrosis via regulation of VEGF and TSP-1 remains
unclear.
In the present study, a renal interstitial fibrosis
model was established by UUO in mice. The effects of ASX on
UUO-induced renal interstitial fibrosis and its potential
mechanisms were investigated.
Materials and methods
Animals and experimental protocol
Adult male C57BL/6J mice (n=30; 6–8 weeks) weighing
20–22 g were purchased from Beijing HFK Bioscience Co., Ltd.
(Beijing, China) and maintained at 21–23°C with 45–55% humidity in
a 12 h light/dark cycle, with ad libitum access to food and
water. Mice were randomly divided into five groups (n=6/group):
Sham, ASX 100 mg/kg, UUO, UUO + ASX 50 mg/kg and UUO + ASX 100
mg/kg. The doses of ASX were selected according to previous studies
(23,24). Renal interstitial fibrosis was
induced by UUO, as previously described (25). Briefly, the mice were anesthetized
by intraperitoneal injection of pentobarbital sodium (50 mg/kg).
The right ureter was exposed and ligated. The mice in the sham
group were subjected to the same operation, but without ureter
ligation. Following surgery, the mice in the ASX groups were
treated with 50 or 100 mg/kg ASX (cat. no. A141428; Shanghai
Aladdin Biochemical Technology Co., Ltd., Shanghai, China) once
daily by oral gavage for 7 or 14 days. The mice in the other groups
were treated with the same volume of normal saline. Blood samples
were collected from the mouse eye socket 7 or 14 days after the
operation, and the mice were then sacrificed by cervical
dislocation. Kidney tissues were frozen in liquid nitrogen and
stored at −80°C or fixed in 4% paraformaldehyde at room temperature
until use. The animals were treated in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (26) guidelines. All
animal protocols used in the present study were approved by the
Institutional Animal Care and Use Committee of Xi'an No. 4 Hospital
(Xi'an, China).
Biochemical determinations
The levels of blood urea nitrogen (BUN; cat. no.
C013-2) and serum creatinine (Cr) were detected with commercial
kits (BUN; cat. no. C011-2; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China), according to the manufacturer's
instructions.
Histological examination
Kidney tissues fixed in 4% paraformaldehyde were
washed with water, dehydrated by a graded ethanol series (70, 80,
90 and 100%) and embedded in paraffin. Then, the paraffin-embedded
specimens were cut into 5 µm-thick sections. To observe the
pathological changes in renal tissues, the sections were subjected
to periodic acid-Schiff (PAS) staining for 15 min at room
temperature and scored on a scale from 0 to 4 (0, no changes; 1,
changes affecting <25% of the section; 2, changes affecting
25–50% of the section; 3, changes affecting 50–75% of the section;
and 4, changes affecting 75–100% of the section) (27). Collagen deposition in renal tissues
was evaluated by Masson's trichrome staining and graded as follows:
0, no staining; 1, <25% staining of the section; 2, 25–50%
staining of the section; 3, 50–75% staining of the section; and 4,
75–100% staining of the section (27). The tissue sections were visualized
and photographed under a light microscope (Olympus Corporation,
Tokyo, Japan) at ×200 magnification.
Immunohistochemical staining
The 5 µm-thick paraffin- embedded renal tissue
sections were subjected to immuno-histochemical staining. Following
deparaffinization with xylene and rehydration in a graded ethanol
series (95, 85 and 75%), the sections were heated at 100°C in the
presence of sodium citrate antigen retrieval solution in a
microwave oven for 10 min. Then, the sections were incubated with
10% H2O2 for 15 min at room temperature and
blocked with 10% goat serum (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) for 15 min at room
temperature. Subsequently, the sections were incubated with primary
antibodies against collagen I (1:100; cat. no. BA0325; Boster
Biological Technology, Pleasanton, CA, USA), TSP-1 (1:50; cat. no.
18304-1-AP; ProteinTech Group, Inc., Chicago, IL, USA), VEGF-A
(1:50; cat. no. 19003-1-AP; ProteinTech Group, Inc.) and cluster of
differentiation 34 (CD34) (1:50; cat. no. 14486-1-AP; ProteinTech
Group, Inc.) overnight at 4°C, followed by incubation with
biotin-labeled goat anti-rabbit immunoglobulin G (IgG) (1:200; cat.
no. A0277; Beyotime Institute of Biotechnology, Haimen, China) at
37°C for 30 min. Then, the sections were incubated with horseradish
peroxidase (HRP)-labeled streptavidin (Beyotime Institute of
Biotechnology), stained with a DAB Substrate kit (cat. no. DA1010;
Beijing Solarbio Science & Technology Co., Ltd.) and
counterstained with hematoxylin for 3 min at room temperature. The
stained sections were observed under a light microscope and
photographed at magnification ×400. The results were analyzed by
ImageJ 1.8.0 software (National Institutes of Health, Bethesda, MD,
USA).
Cell culture and treatment
Rat NRK-52E cells were purchased from Procell
(Wuhan, China) and cultured in Dulbecco's modified Eagle's medium
supplemented with 5% fetal bovine serum (Biological Industries,
Kibbutz Beit-Haemek, Israel) at 37°C in 5% CO2. To
investigate the beneficial effect of ASX in vitro, NRK-52E
cells at 70% confluence were treated with ASX (10 µM) in
combination with recombinant TGF-β1 (5 ng/ml, Wuhan USCN Business
Co., Ltd., Wuhan, China) for 72 h at 37°C. The dose of ASX was
selected according to a previous study (28).
Western blotting
Protein was extracted from renal tissues and NRK-52E
cells using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology) containing 1% phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology). The Enhanced BCA
Protein Assay kit (Beyotime Institute of Biotechnology) was used to
determine protein concentration. A total of 40 µg protein was
subjected to SDS-PAGE (8 or 10% gel) and transferred onto
polyvinylidene fluoride membranes. After blocking with 5% skimmed
milk for 1 h at room temperature, the membranes were probed with
primary antibodies against collagen I (1:2,000; cat. no.
14695-1-AP; ProteinTech Group, Inc.), TSP-1 (1:500; cat. no.
18304-1-AP; ProteinTech Group, Inc.), VEGF-A (1:1,000; cat. no.
19003-1-AP; ProteinTech Group, Inc.), TGF-β1 (1:500; cat. no.
21898-1-AP; ProteinTech Group, Inc.), phosphorylated (p)-Smad2
(1:1,000; cat. no. 3108; Cell Signaling Technology, Inc., Danvers,
MA, USA), Smad2 (1:3,000; cat. no. 12570-1-AP; ProteinTech Group,
Inc.), fibronectin (1:1,000; cat. no. 15613-1-AP; ProteinTech
Group, Inc.), α-smooth muscle actin (α-SMA) (1:500; cat. no.
55135-1-AP; ProteinTech Group, Inc.), Smad3 (1:1,000; cat. no.
bs-3484R; BIOSS, Beijing, China), p-Smad3 (1:1,000; cat. no.
bsm-52205R; BIOSS), Smad4 (1:1,000; cat. no. bs-0585R; BIOSS),
Smad7 (1:1,000; cat. no. bs-23328R; BIOSS) and β-actin (1:500; cat.
no. bsm-33036M; BIOSS) overnight at 4°C. Then, the membrane was
incubated with HRP-labeled goat anti-rabbit IgG (cat. no. A0208) or
goat anti-mouse IgG (cat. no. A0216; both 1:5,000; Beyotime
Institute of Biotechnology) at 37°C for 45 min. Proteins were
visualized using BeyoECL Plus (Beyotime Institute of
Biotechnology). Densitometric analysis was performed using Gel-Pro
Analyzer 4 software (Media Cybernetics, Inc., Rockville, MD,
USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from renal tissues using a
Total RNA Isolation kit (cat. no. RP1001; BioTeke Corporation,
Beijing, China). RT was carried out using Super M-MLV Reverse
Transcriptase with buffer (cat. no. PR6502; BioTeke Corporation),
oligo (dT)15 primers (cat. no. C1101-20; Promega Corporation,
Madison, WI, USA), dNTP (cat. no. PR3001, BioTeke Corporation) at
25°C for 10 min, 42°C for 50 min, and then 80°C for 10 min. qPCR
was performed on an Exicycler™ 96 Real-Time Quantitative
Thermal Block (Bioneer Corporation, Daejeon, Korea) using the 2X
Power Taq PCR Master mix (cat. no. PR1702; BioTeke Corporation) and
SYBR Green (cat. no. SY1020; Beijing Solarbio Science &
Technology Co., Ltd.). The following primers were used: Collagen I
(forward, 5′-GGACGCCATCAAGGTCTACT-3′ and reverse,
5′-GAATCCATCGGTCATGCTCT-3′); TSP-1 (forward,
5′-GACCAGAGGGACACGGACAT-3′ and reverse,
5′-TGGCATTAGGCACATAGGGA-3′); VEGF-A (forward,
5′-CGTGAGCCCTCCCCCTTG-3′ and reverse, 5′-GCCCAGAAGTTGGACGAAAA-3′);
and β-actin (forward, 5′-CTGTGCCCATCTACGAGGGCTAT-3′ and reverse,
5′-TTTGATGTCACGCACGATTTCC-3′). The thermocycling conditions were as
follows: Denaturation for 5 min at 95°C, followed by 40 cycles of
10 sec at 94°C, 20 sec at 60°C and 30 sec at 72°C. The relative
mRNA levels were calculated using the 2−ΔΔCq method
(29).
Statistical analysis
All experimental data are presented as the means ±
standard deviation of three experimental repeats. Comparisons among
different experimental groups were performed using one-way analysis
of variance followed by Bonferroni multiple comparisons test using
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
ASX alleviates UUO-induced renal
injury in mice
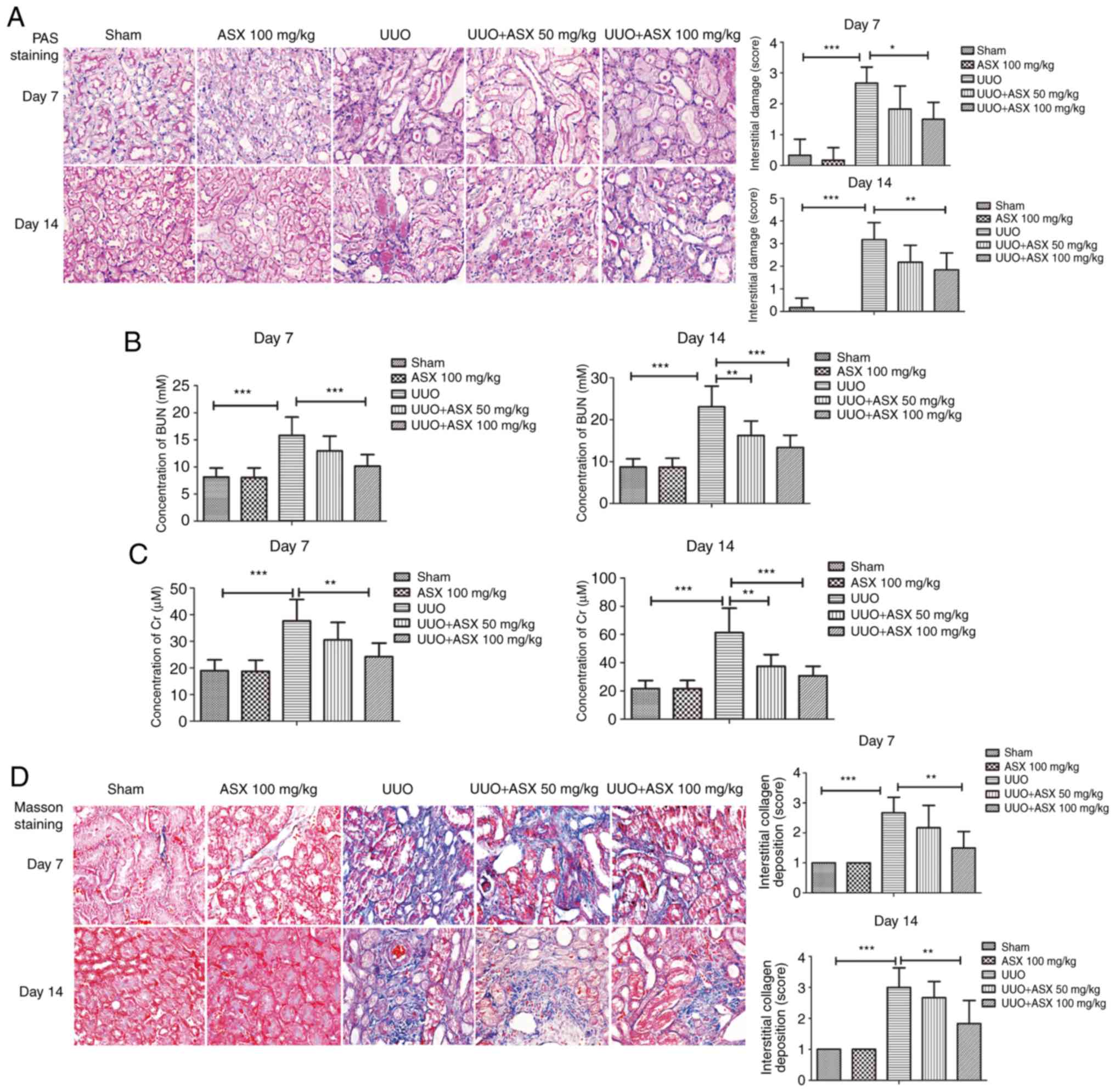
Histopathological changes in the kidneys were
determined by PAS staining. As shown in Fig. 1A, UUO induced significant
interstitial damage in mouse kidneys on day 7 and 14, which could
be relieved by treatment with ASX. Furthermore, the concentration
of BUN and serum Cr were significantly increased in the UUO group
on day 7 and 14 (Fig. 1B and C),
which was consistent with previous studies and indicated a
deterioration of renal function (30,31).
However, ASX treatment markedly reduced the UUO-induced BUN and
serum Cr levels (Fig. 1B and C).
These results indicated that treatment with ASX alleviated
UUO-induced renal injury and improved renal function.
ASX suppresses collagen formation and
renal fibrosis in mice
To observe collagen deposition and renal fibrosis,
Masson's trichrome staining was performed. As presented in Fig. 1D, UUO treatment resulted in
significantly increased interstitial collagen deposition on day 7
and 14 and obvious renal fibrosis, which could be suppressed by ASX
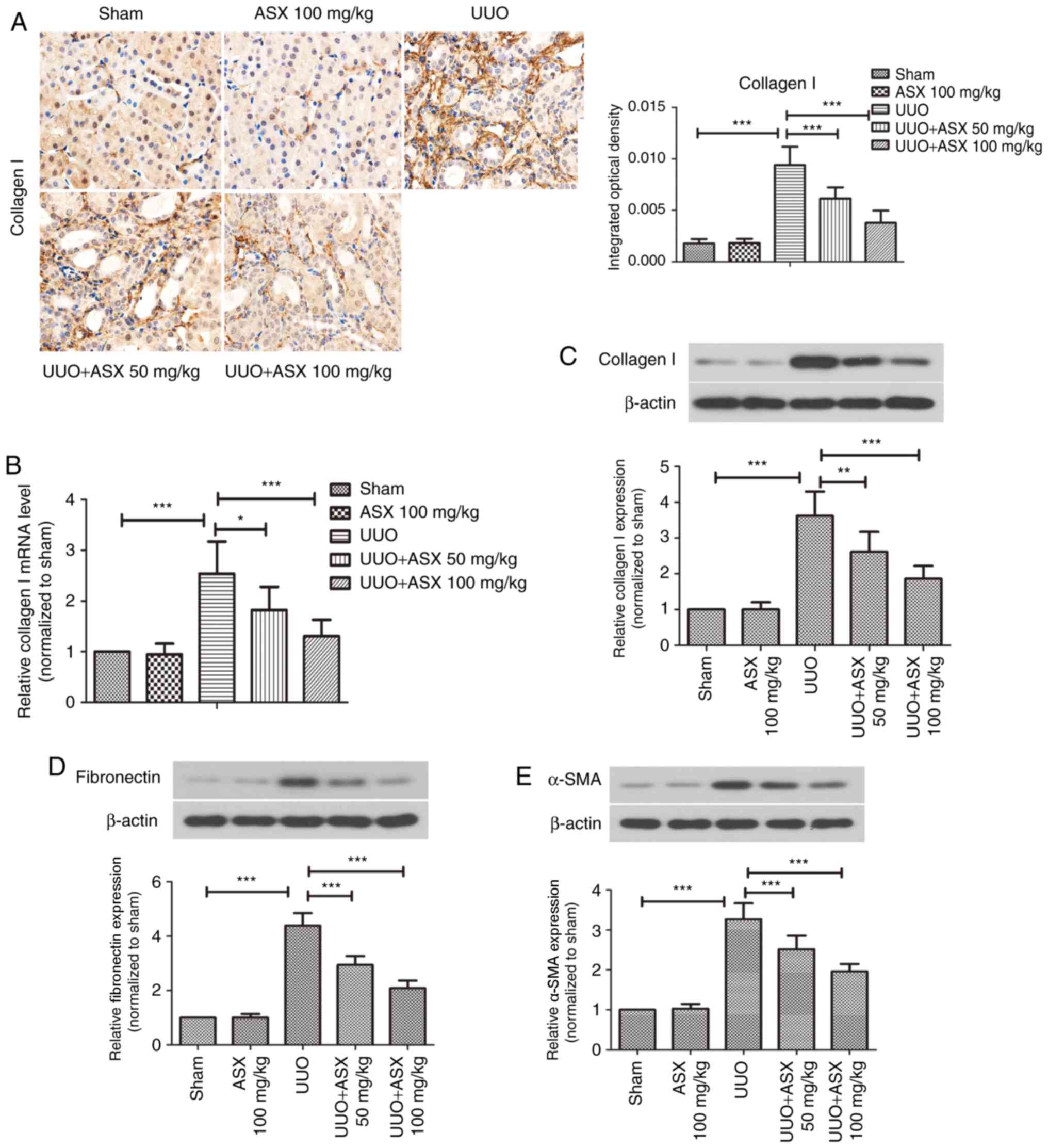
administration. In addition, the expression of collagen I in renal
tissues was evaluated by immunohistochemical staining. As shown in
Fig. 2A, a significant increase in
collagen I staining in renal tissues was observed in the UUO group
on day 14, whereas ASX treatment effectively inhibited UUO-induced
collagen I expression. RT-qPCR and western blot analysis further
demonstrated that ASX prevented the UUO-mediated increase in mRNA
and protein levels of collagen I in renal tissues on day 14, in a
dose-dependent manner (Fig. 2B and
C). In addition, the protein levels of fibronectin and α-SMA
were increased in the renal tissues of UUO-treated mice, and were
suppressed by ASX administration (Fig.
2D and E). These findings suggested that ASX mitigated
UUO-induced renal interstitial fibrosis in mice.
ASX enhances the density of
peritubular capillaries and VEGF-A expression
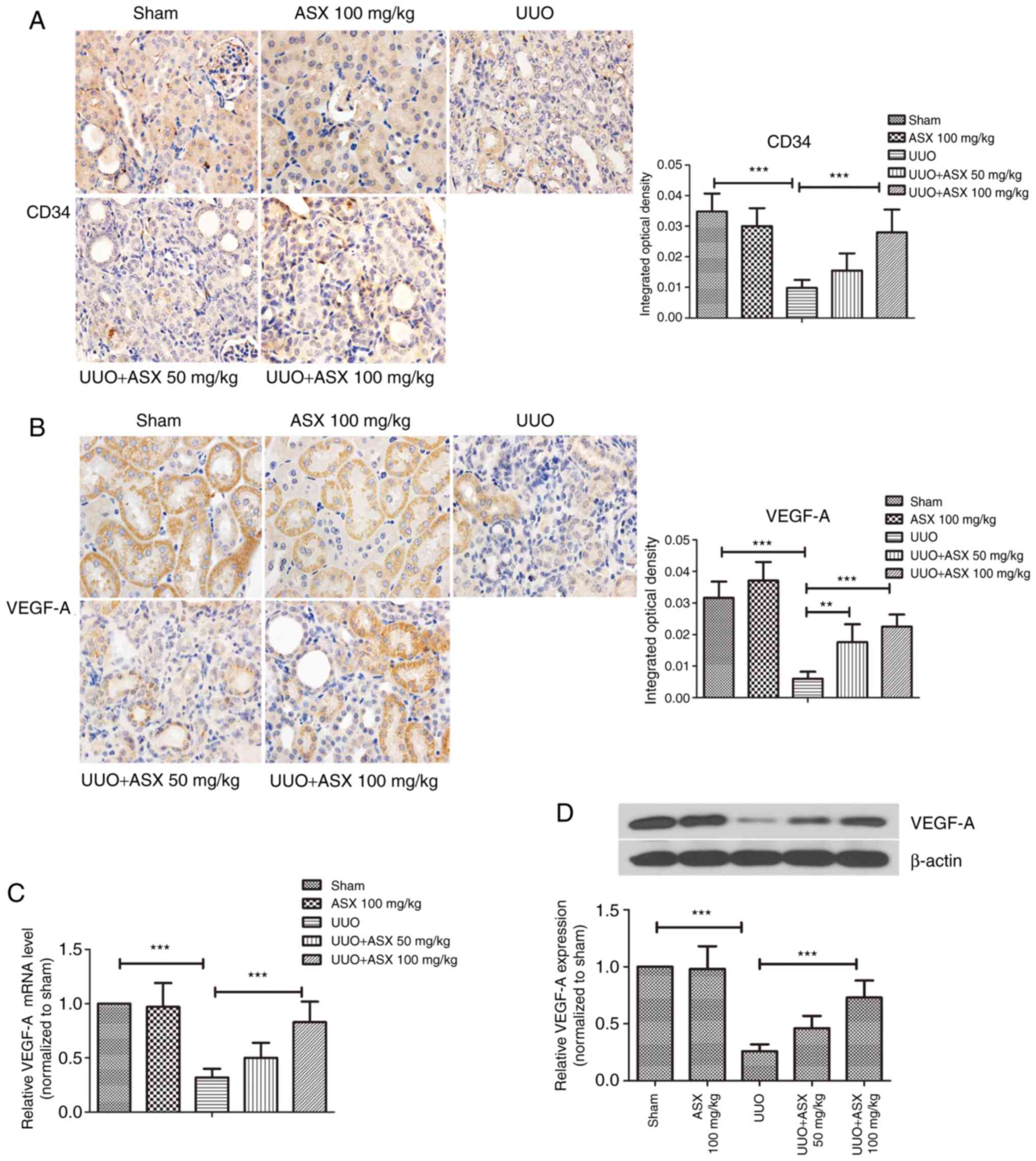
Peritubular capillaries were observed by
immunohistochemical staining of CD34, a typical marker of
endothelial cells. As presented in Fig. 3A, peritubular capillaries could be
easily recognized in the sham and ASX control groups due to CD34
immunostaining. However, UUO treatment led to a decrease in
CD34-positive capillaries in renal tissues on day 14, which was
mitigated when ASX was administered. The expression of VEGF-A, an
important mediator of angiogenesis, was next studied. As shown in
Fig. 3B, the positive
immunohistochemical staining of VEGF-A was markedly reduced by UUO
on day 14. Renal tissues exhibited significantly stronger staining
of VEGF-A in the ASX treatment groups compared with the UUO group.
In addition, the mRNA and protein levels of VEGF-A in renal tissues
were decreased by ~70% in the UUO group, and this was reversed by
ASX in a dose-dependent manner (Fig.
3C and D). These results suggested that ASX prevented the
UUO-induced decrease in density of peritubular capillaries by
upregulating VEGF-A.
ASX inhibits TSP-1 expression in renal
fibrotic tissues
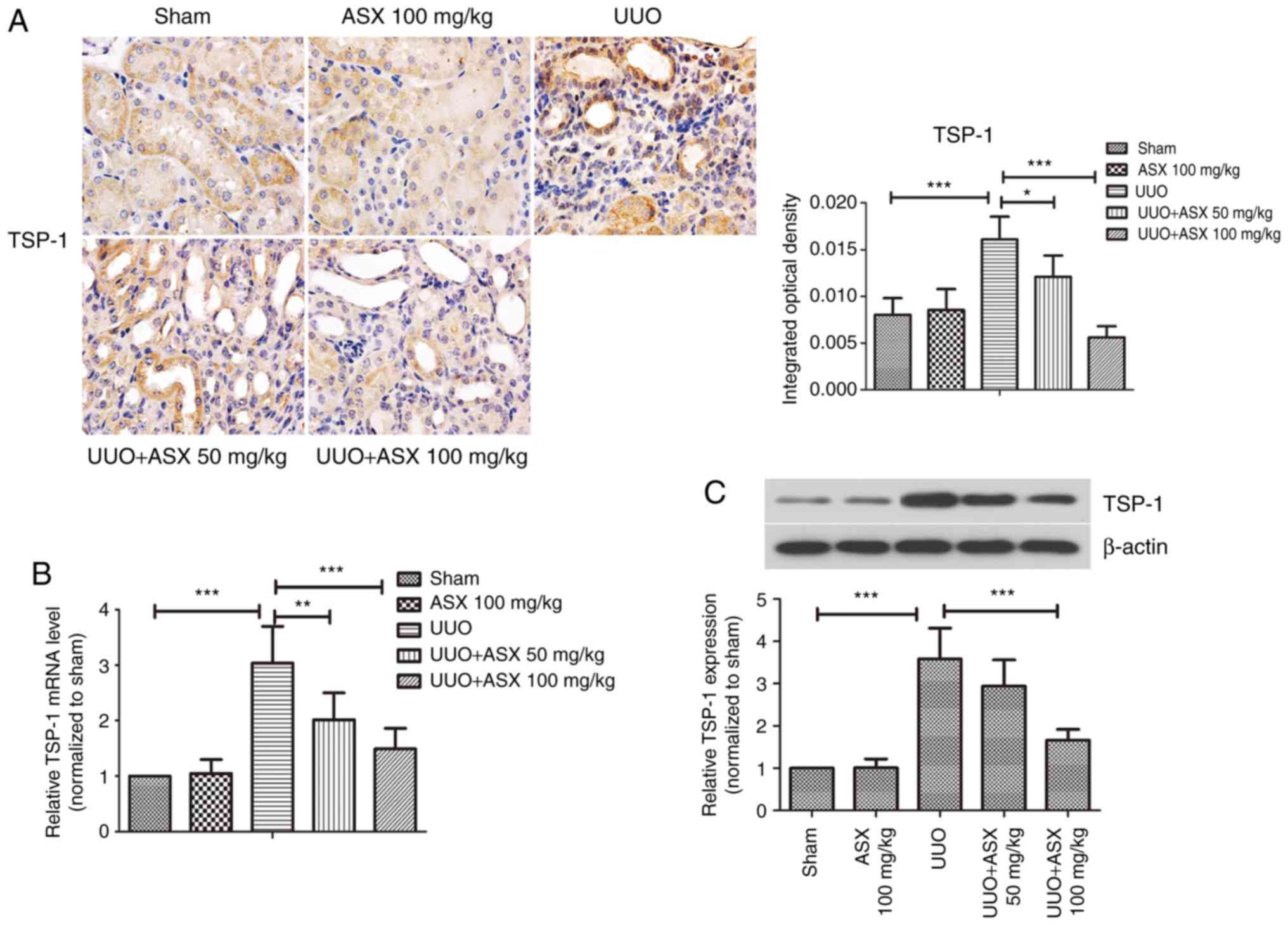
The expression of the anti-angiogenic factor TSP-1
was detected by immunohistochemical staining, as shown in Fig. 4A. Positive staining for TSP-1 was
most pronounced in the UUO group on day 14, which was significantly
reduced by ASX administration. Consistently, the mRNA and protein
expression levels of TSP-1 in renal tissues were increased by
~3-fold in the UUO group on day 14, while they were markedly
repressed by treatment with ASX (Fig.
4B and C). Thus, regulation of TSP-1 expression may be a
potential mechanism underlying the beneficial effects of ASX and
targeting this protein could be useful for treating renal
fibrosis.
ASX prevents UUO-induced activation of
the TGF-β1/Smad signaling pathway
The TGF-β1/Smad signaling pathway serves pivotal
roles in renal fibrosis (32).
Thus, the present study investigated the effect of ASX on
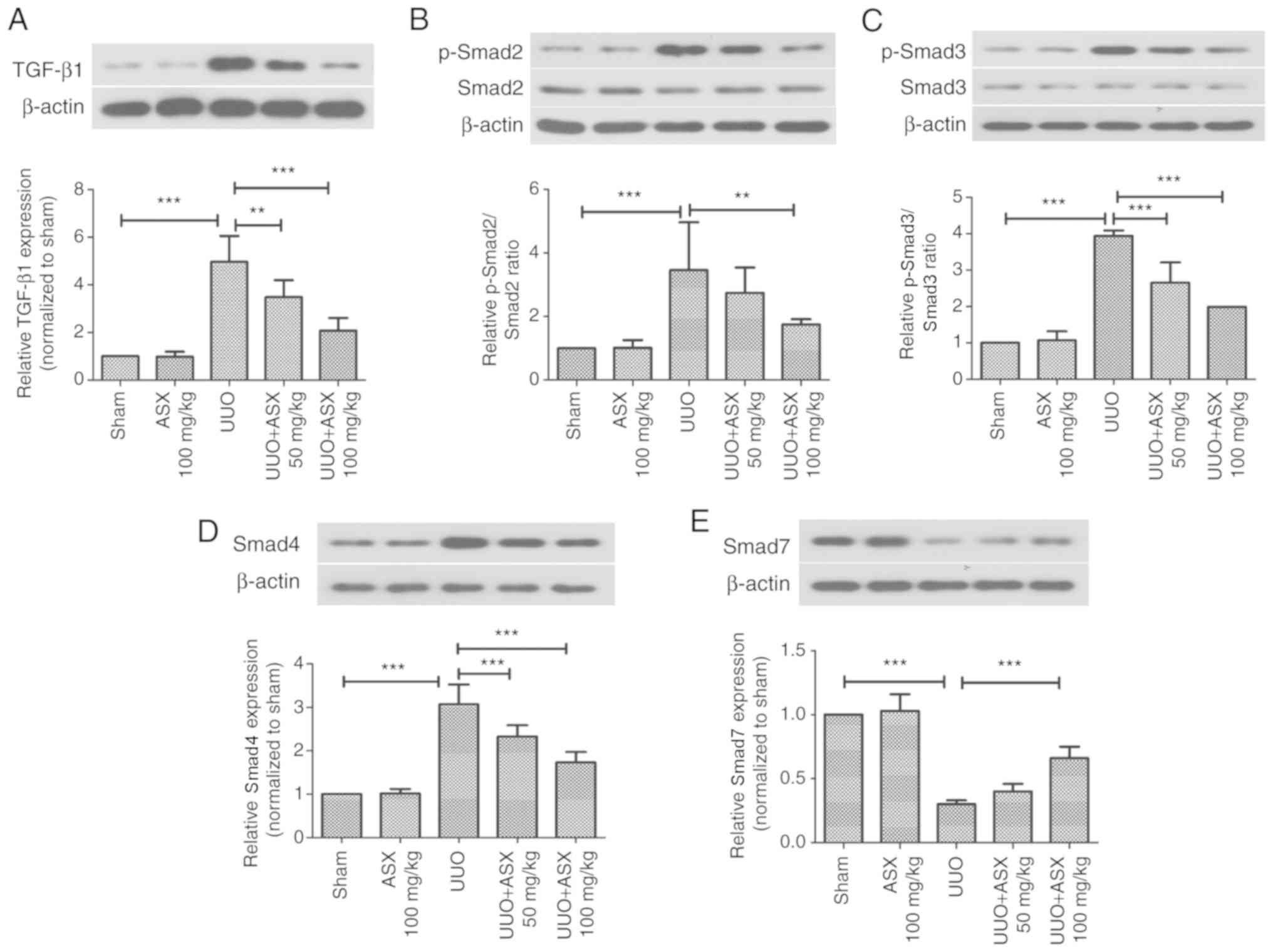
activation of TGF-β1 and Smad2/3/4/7. As illustrated in Fig. 5A-D, the protein expression levels
of TGF-β1, p-Smad2, p-Smad3 as well as Smad4, were significantly
increased by UUO on day 14, while they could be significantly
suppressed by ASX treatment. The protein expression level of Smad7,
which is inhibitory, was decreased by UUO, which was enhanced by
ASX administration (Fig. 5E).
Thus, inactivation of the TGF-β1/Smad signaling pathway appeared to
be involved in the protective mechanism of ASX.
ASX suppresses TGF-β1-induced
expression of profibrogenic factors via inactivation of the Smad
signaling pathway
The present study next investigated the in
vitro effects of ASX on the expression of pro-fibrotic factors
in NRK-52E cells. As shown in Fig. 6A
and B, the protein expression levels of collagen I and α-SMA
were significantly increased upon stimulation with TGF-β1 in
NRK-52E cells. However, this increase in α-SMA and collagen I
levels was suppressed by ASX. Furthermore, the present study
examined whether ASX treatment affected the Smad signaling pathway
in vitro. As illustrated in Fig. 6C and D, treatment with ASX
attenuated the phosphorylation of Smad2 and Smad3 in
TGF-β1-stimulated NRK-52E cells. However, TGF-β1 and ASX treatment
had no effect on the expression of Smad4 or Smad7 proteins in
NRK-52E cells (Fig. 6E and F).
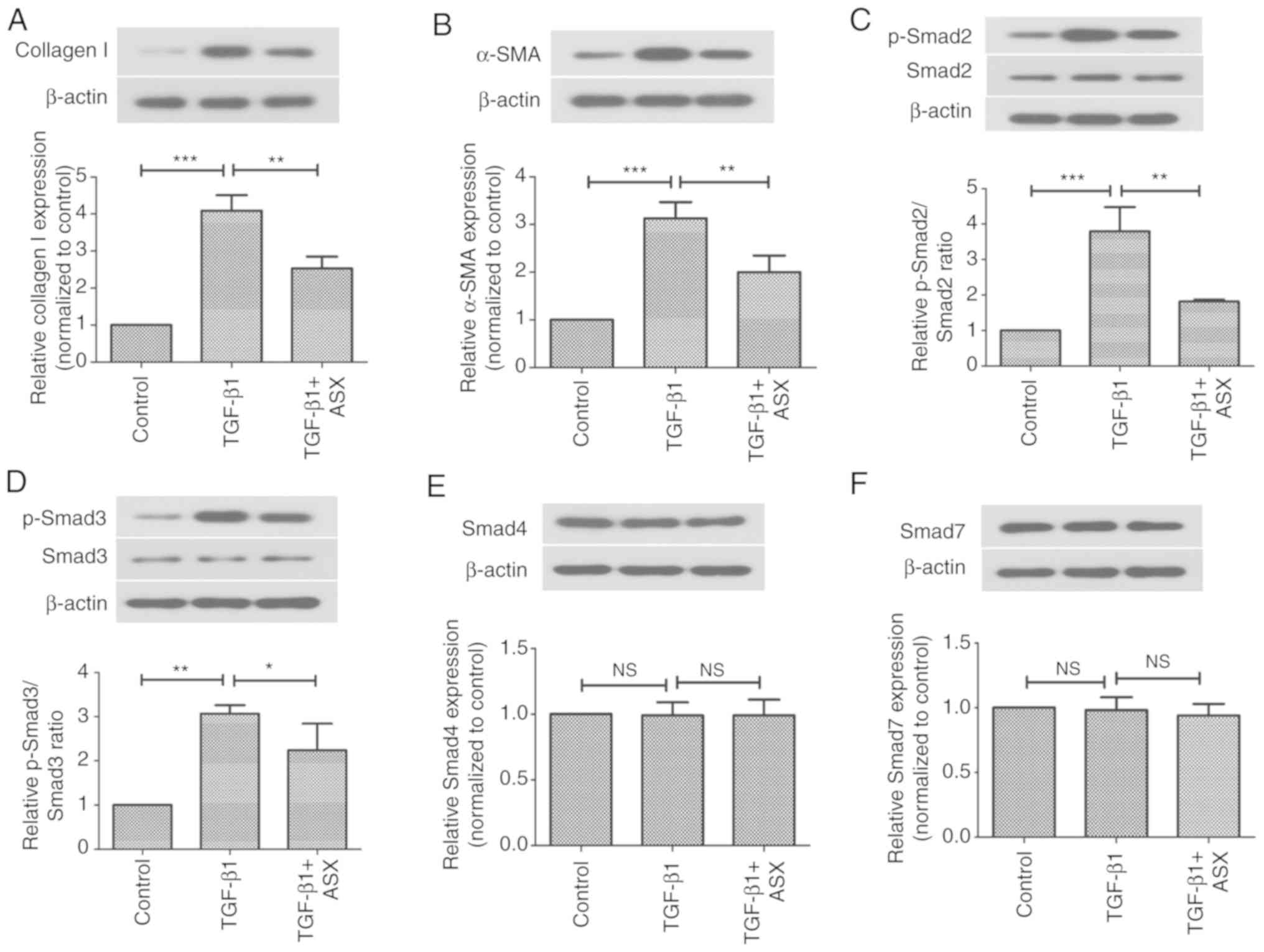 | Figure 6.ASX suppresses TGF-β1-induced
expression of profibrogenic factors via inactivation of the Smad
signaling pathway. Protein expression levels of (A) collagen I, (B)
α-SMA, (C) Smad2, (D) Smad3, (E) Smad4 and (F) Smad7 in NRK-52E
cells were assessed by western blotting. The data are presented as
the means ± standard deviation (n=6). *P<0.05, **P<0.01,
***P<0.001. ASX, Astaxanthin; NS, not significant; p,
phosphorylated; TGF-β1, transforming growth factor β1; UUO,
unilateral ureteral obstruction. |
Discussion
The present study investigated the role of ASX in
UUO-induced renal fibrosis in mice. The results revealed that ASX
treatment effectively ameliorated UUO-induced renal injury and
dysfunction, inhibited renal fibrosis and collagen deposition, and
enhanced the density of peritubular capillaries by up-regulating
VEGF-A and downregulating TSP-1 expression levels. Inactivation of
the TGF-β1/Smad signaling pathway appeared to be involved in the
protective effect of ASX.
Renal injury was determined on day 7 and day 14
following UUO in the present study. Previous studies which
evaluated renal injury on different days post-UUO, indicated that
kidney fibrosis tends to get worse with time (33,34).
Consistently, the present results revealed that UUO-induced renal
damage was exacerbated with the prolonging of time, as evidenced by
PAS staining and increased BUN and serum Cr levels. However, there
was very little difference in the collagen deposition assessed by
Masson's trichrome staining between day 7 and day 14. ASX treatment
for both 7 and 14 days could alleviate UUO-induced renal damage,
but longer treatment seemed to be more effective.
Renal fibrosis is a pathological process that
contributes to chronic renal failure (2,35).
Accumulation of excessive extracellular matrix is an important
feature of renal interstitial fibrosis (36). Mammals have a high collagen
content, with over 27 types of collagen that make up ~30% of the
overall protein (37). Collagen
type I (also known as collagen I) is regarded as a matrix component
and one of the fibril-forming collagens (38). Previous studies revealed that
inhibition of collagen I expression could attenuate fibrosis
formation in various organs, including the liver and kidney
(39,40). Fibronectin and collagen I are
extracellular matrix components, and serve crucial roles in
fibrosis of the kidney and eventual nephropathy (41,42).
α-SMA is a marker of activated myofibroblasts, which is upregulated
by UUO (43). According to the
present results, ASX significantly alleviated UUO-induced renal
fibrosis and collagen deposition, as confirmed by downregulation of
the protein expression levels of collagen I, fibronectin and
α-SMA.
It has been well documented that peritubular
capillaries transport oxygen and nutrients to renal tubular and
interstitial cells, serving a pivotal role in sustaining normal
hemodynamics and kidney function (44). Therefore, chronic tubular hypoxia
may occur due to decreased density of peritubular capillaries,
which further promotes extracellular matrix synthesis and
contributes to the progression of tubulointerstitial fibrosis
(44). The loss of peritubular
capillaries has been verified in patients with interstitial
fibrosis and animal models (5). In
the present study, reduced density of peritubular capillaries was
observed in UUO-treated mice, which was significantly increased by
ASX treatment. Furthermore, the potential mechanisms of ASX in
regulating the density of peritubular capillaries were explored.
Since angiogenesis serves a key role in this process, the present
study investigated whether the expression of angiogenic or
anti-angiogenic factors was altered. VEGF is an important
angiogenic factor. A previous study reported that VEGF was markedly
downregulated in the tubules of rats with interstitial fibrosis
(45). The loss of VEGF has also
been verified in human patients with renal interstitial fibrosis
(46). Thus, reduced VEGF
expression appears to be involved in the pathological mechanism of
renal interstitial fibrosis. The present study also observed that
UUO treatment led to decreased VEGF-A expression, which was
reversed by ASX treatment.
Next, the present study focused on TSP-1, which is
an anti-angiogenic factor and an endogenous activator of TGF-β1
(47,48). It has been previously demonstrated
that TSP-1 is associated with the loss of microvascular endothelium
(49). In addition, TSP-1 can
exert anti-angiogenic effects by preventing VEGF-induced
proliferation of endothelial cells and inducing endothelial cell
apoptosis (50,51). Sun et al (52) suggested that suppression of TSP-1
expression enhances the density of peritubular capillaries and
ameliorates tubulointerstitial fibrosis. Apart from its role in
reducing peritubular capillary density, TSP-1-mediated activation
of TGF-β1 serves crucial roles in promoting fibrosis in multiple
organs (53–55). Thus, TSP-1 may be used as a
therapeutic target for renal fibrosis. In the present study, as
expected, administration of ASX inhibited the UUO-induced increase
in TSP-1 expression. Therefore, it is likely that ASX attenuated
the UUO-induced loss of peritubular capillaries by upregulating
VEGF-A and downregulating TSP-1.
To investigate the underlying mechanisms of ASX in
renal fibrosis, the TGF-β/Smad signaling pathway was explored. The
activation of the TGF-β1 signaling pathway has been demonstrated to
serve key roles in the progression of renal fibrosis (32,56).
TGF-β1 facilitates the protein synthesis of collagen I and promotes
extracellular matrix accumulation via the Smad signaling pathway
(57,58). The most important Smad proteins for
TGF-β1 signal transduction are Smad2, Smad3 and Smad4 as well as
the inhibitor, Smad7. The activation of Smad2 and Smad3 is induced
by TGF-β1. Subsequently, the Smad complex is formed by the binding
of p-Smad2, p-Smad3 and Smad4, which in turn migrates to the
nucleus and regulates the transcription of target genes (59). Smad7, an inhibitory Smad protein,
suppresses the activation and phosphorylation of Smad2/3. A
previous study demonstrated the role of Smad2 in the pro-fibrotic
TGF-β1 signaling pathway (60).
Smad2/3 was confirmed to be activated in the context of renal
interstitial fibrosis in various chronic kidney diseases (61). In addition, silencing of Smad3
suppressed the progression of renal fibrosis in mice (62,63).
In the present study, ASX effectively suppressed the UUO-induced
upregulation of TGF-β1 and p-Smad2/3 protein expression levels in
renal tissues. Furthermore, TGF-β1-induced activation and
phosphorylation of Smad2/3 was also repressed by ASX in NRK-52E
cells. These results indicated that the TGF-β1/Smad signaling
pathway may be involved in the protective mechanism of ASX against
renal fibrosis.
In conclusion, the present study demonstrated that
ASX ameliorated UUO-induced renal interstitial fibrosis in mice by
increasing the number of peritubular capillaries via regulation of
VEGF-A and TSP-1 expression. In addition, ASX exerted its
anti-fibrotic effect by inhibiting activation of the TGF-β1/Smad
signaling pathway. Although the detailed mechanisms must be further
investigated, the results suggested that ASX could be a potential
drug for alleviating renal fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Science and Technology Development Project of Xi'an Science and
Technology Bureau (grant no. 2016046SF/YX02).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JZ and MG were responsible for the experimental
design. MM, JZ and LL performed the experiments. JZ, XZ, LZ and CW
contributed to data analysis and drafted the manuscript. JZ wrote
the manuscript. XZ, LZ, CW, and MG revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal protocols used in the present study were
approved by the Institutional Animal Care and Use Committee of
Xi'an No. 4 Hospital (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fang Y, Yu X, Liu Y, Kriegel AJ, Heng Y,
Xu X, Liang M and Ding X: miR-29c is downregulated in renal
interstitial fibrosis in humans and rats and restored by HIF-alpha
activation. Am J Physiol Renal Physiol. 304:F1274–F1282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boor P, Ostendorf T and Floege J: Renal
fibrosis: novel insights into mechanisms and therapeutic targets.
Nat Rev Nephrol. 6:643–656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun D, Feng J, Dai C, Sun L, Jin T, Ma J
and Wang L: Role of peritubular capillary loss and hypoxia in
progressive tubulointerstitial fibrosis in a rat model of
aristolochic acid nephropathy. Am J Nephrol. 26:363–371. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang DH, Hughes J, Mazzali M, Schreiner GF
and Johnson RJ: Impaired angiogenesis in the remnant kidney model:
II. Vascular endothelial growth factor administration reduces renal
fibrosis and stabilizes renal function. J Am Soc Nephrol.
12:1448–1457. 2001.PubMed/NCBI
|
|
5
|
Babickova J, Klinkhammer BM, Buhl EM,
Djudjaj S, Hoss M, Heymann F, Tacke F, Floege J, Becker JU and Boor
P: Regardless of etiology, progressive renal disease causes
ultrastructural and functional alterations of peritubular
capillaries. Kidney Int. 91:70–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JY, Song SH, Kim YS, Lim BJ, Kim SI,
Kim MS and Jeong HJ: Tubuloreticular inclusions in peritubular
capillaries of renal allografts. Pathol Res Pract. 213:1185–1190.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boor P, Babickova J, Steegh F, Hautvast P,
Martin IV, Djudjaj S, Nakagawa T, Ehling J, Gremse F, Bücher E, et
al: Role of platelet-derived growth factor-CC in capillary
rarefaction in renal fibrosis. Am J Pathol. 185:2132–2142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bohle A, von Gise H, Mackensen-Haen S and
Stark-Jakob B: The obliteration of the postglomerular capillaries
and its influence upon the function of both glomeruli and tubuli.
Functional interpretation of morphologic findings. Klin Wochenschr.
59:1043–1051. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fine LG, Orphanides C and Norman JT:
Progressive renal disease: the chronic hypoxia hypothesis. Kidney
Int. (Suppl 65):S74–S78. 1998.
|
|
10
|
Venkatachalam MA, Weinberg JM, Kriz W and
Bidani AK: Failed tubule recovery, AKI-CKD transition, and kidney
disease progression. J Am Soc Nephrol. 26:1765–1776. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka T and Nangaku M: Angiogenesis and
hypoxia in the kidney. Nat Rev Nephrol. 9:211–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kida Y, Tchao BN and Yamaguchi I:
Peritubular capillary rarefaction: a new therapeutic target in
chronic kidney disease. Pediatr Nephrol. 29:333–342. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reinders ME, Rabelink TJ and Briscoe DM:
Angiogenesis and endothelial cell repair in renal disease and
allograft rejection. J Am Soc Nephrol. 17:932–942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mayer G: Capillary rarefaction, hypoxia,
VEGF and angiogenesis in chronic renal disease. Nephrol Dial
Transplant. 26:1132–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Failla CM, Carbo M and Morea V: Positive
and negative regulation of angiogenesis by soluble vascular
endothelial growth factor receptor-1. Int J Mol Sci. 19(pii):
E13062018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nangaku M and Eckardt KU: Hypoxia and the
HIF system in kidney disease. J Mol Med (Berl). 85:1325–1330. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hugo C: The thrombospondin 1-TGF-beta axis
in fibrotic renal disease. Nephrol Dial Transplant. 18:1241–1245.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue Y, Qu Z, Fu J, Zhen J, Wang W and Cai
Y: The protective effect of astaxanthin on learning and memory
deficits and oxidative stress in a mouse model of repeated cerebral
ischemia/reperfusion. Brain Res Bull. 131:221–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JS, Chyun JH, Kim YK, Line LL and
Chew BP: Astaxanthin decreased oxidative stress and inflammation
and enhanced immune response in humans. Nutr Metab (Lond).
7:182010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni X, Yu H, Wang S, Zhang C and Shen S:
Astaxanthin inhibits PC-3 ×enograft prostate tumor growth in nude
mice. Mar Drugs. 15(pii): E662017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Komatsu T, Sasaki S, Manabe Y, Hirata T
and Sugawara T: Preventive effect of dietary astaxanthin on
UVA-induced skin photoaging in hairless mice. PLoS One.
12:e01711782017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanazashi M, Okumura Y, Al-Nassan S,
Murakami S, Kondo H, Nagatomo F, Fujita N, Ishihara A, Roy RR and
Fujino H: Protective effects of astaxanthin on capillary regression
in atrophied soleus muscle of rats. Acta Physiol (Oxf).
207:405–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu G, Shi Y, Peng X, Liu H, Peng Y and He
L: Astaxanthin attenuates adriamycin-induced focal segmental
glomerulosclerosis. Pharmacology. 95:193–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie C, Meng M, Yin X, He F, Ye H and Xie
D: Effects of astaxanthin on renal fibrosis and cell apoptosis
induced by partial unilateral ureteral obstruction in rats. Nan
Fang Yi Ke Da Xue Xue Bao. 33:305–308. 2013.(In Chinese).
PubMed/NCBI
|
|
25
|
Stroo I, Emal D, Butter LM, Teske GJ,
Claessen N, Dessing MC, Girardin SE, Florquin S and Leemans JC: No
difference in renal injury and fibrosis between wild-type and
NOD1/NOD2 double knockout mice with chronic kidney disease induced
by ureteral obstruction. BMC Nephrol. 19:782018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao YN, Xu GJ and Yang P: GBP1 exerts
inhibitory effects on acute viral myocarditis through the
inhibition of inflammatory response of macrophage in mice. Biochem
Cell Biol. Dec 17–2018.(Epub ahead of print). View Article : Google Scholar :
|
|
27
|
Chiang CK, Sheu ML, Lin YW, Wu CT, Yang
CC, Chen MW, Hung KY, Wu KD and Liu SH: Honokiol ameliorates renal
fibrosis by inhibiting extracellular matrix and pro-inflammatory
factors in vivo and in vitro. Br J Pharmacol. 163:586–597. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Q, Tao J, Li G, Zheng D, Tan Y, Li R,
Tian L, Li Z, Cheng H and Xie X: Astaxanthin ameliorates
experimental diabetes-induced renal oxidative stress and
fibronectin by upregulating connexin43 in glomerular mesangial
cells and diabetic mice. Eur J Pharmacol. 840:33–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu B, Ding F, Hu D, Zhou Y, Long C, Shen
L, Zhang Y, Zhang D and Wei G: Human umbilical cord mesenchymal
stem cell conditioned medium attenuates renal fibrosis by reducing
inflammation and epithelial-to-mesenchymal transition via the
TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res
Ther. 9:72018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu N, Duan J, Li H, Wang Y, Wang F, Chu J,
Sun J, Liu M, Wang C, Lu C and Wen A: Hydroxysafflor yellow a
ameliorates renal fibrosis by suppressing tgf-beta1-induced
epithelial-to-mesenchymal transition. PLoS One. 11:e01534092016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang G, Kang Y, Zhou C, Cui R, Jia M, Hu
S, Ji X, Yuan J, Cui H and Shi G: Amelioratory effects of
testosterone propionate on age-related renal fibrosis via
suppression of TGF-β1/smad signaling and activation of Nrf2-ARE
signaling. Sci Rep. 8:107262018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roberts V, Lu B, Chia J, Cowan PJ and
Dwyer KM: CD39 overexpression does not attenuate renal fibrosis in
the unilateral ureteric obstructive model of chronic kidney
disease. Purinergic Signal. 12:653–660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stefanska A, Eng D, Kaverina N, Pippin JW,
Gross KW, Duffield JS and Shankland SJ: Cells of renin lineage
express hypoxia inducible factor 2alpha following experimental
ureteral obstruction. BMC Nephrol. 17:52016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y: Renal fibrosis: New insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Farris AB and Colvin RB: Renal
interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol
Hypertens. 21:289–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Myllyharju J and Kivirikko KI: Collagens,
modifying enzymes and their mutations in humans, flies and worms.
Trends Genet. 20:33–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kadler K: Extracellular matrix 1:
Fibril-forming collagens. Protein Profile. 2:491–619.
1995.PubMed/NCBI
|
|
39
|
Hasegawa D, Fujii R, Yagishita N,
Matsumoto N, Aratani S, Izumi T, Azakami K, Nakazawa M, Fujita H,
Sato T, et al: E3 ubiquitin ligase synoviolin is involved in liver
fibrogenesis. PLoS One. 5:e135902010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Holopainen I and Kontro P: Uptake and
release of glycine in cerebellar granule cells and astrocytes in
primary culture: Potassium-stimulated release from granule cells is
calcium-dependent. J Neurosci Res. 24:374–383. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lopez-Hernandez FJ and Lopez-Novoa JM:
Role of TGF-beta in chronic kidney disease: An integration of
tubular, glomerular and vascular effects. Cell Tissue Res.
347:141–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen SJ, Wu P, Sun LJ, Zhou B, Niu W, Liu
S, Lin FJ and Jiang GR: miR-204 regulates epithelial-mesenchymal
transition by targeting SP1 in the tubular epithelial cells after
acute kidney injury induced by ischemia-reperfusion. Oncol Rep.
37:1148–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Choi HS, Song JH, Kim IJ, Joo SY1, Eom GH,
Kim I, Cha H, Cho JM, Ma SK, Kim SW and Bae EH: Histone deacetylase
inhibitor, CG200745 attenuates renal fibrosis in obstructive kidney
disease. Sci Rep. 8:115462018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nangaku M: Chronic hypoxia and
tubulointerstitial injury: A final common pathway to end-stage
renal failure. J Am Soc Nephrol. 17:17–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang DH, Anderson S, Kim YG, Mazzalli M,
Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J and Hugo C:
Impaired angiogenesis in the aging kidney: Vascular endothelial
growth factor and thrombospondin-1 in renal disease. Am J Kidney
Dis. 37:601–611. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rudnicki M, Perco P, Enrich J, Eder S,
Heininger D, Bernthaler A, Wiesinger M, Sarközi R, Noppert SJ,
Schramek H, et al: Hypoxia response and VEGF-A expression in human
proximal tubular epithelial cells in stable and progressive renal
disease. Lab Invest. 89:337–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Isenberg JS, Martin-Manso G, Maxhimer JB
and Roberts DD: Regulation of nitric oxide signalling by
thrombospondin 1: implications for anti-angiogenic therapies. Nat
Rev Cancer. 9:182–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Crawford SE, Stellmach V, Murphy-Ullrich
JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP and Bouck N:
Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell.
93:1159–1170. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kang DH, Kanellis J, Hugo C, Truong L,
Anderson S, Kerjaschki D, Schreiner GF and Johnson RJ: Role of the
microvascular endothelium in progressive renal disease. J Am Soc
Nephrol. 13:806–816. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Iruela-Arispe ML, Bornstein P and Sage H:
Thrombospondin exerts an antiangiogenic effect on cord formation by
endothelial cells in vitro. Proc Natl Acad Sci USA. 88:5026–5030.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X and Lawler J: Thrombospondin-based
antiangiogenic therapy. Microvasc Res. 74:90–99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun D, Ma Y, Han H, Yin Z, Liu C, Feng J,
Zhou X, Li X, Xiao A and Yu R: Thrombospondin-1 short hairpin RNA
suppresses tubulointerstitial fibrosis in the kidney of ureteral
obstruction by ameliorating peritubular capillary injury. Kidney
Blood Press Res. 35:35–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liao F, Li G, Yuan W, Chen Y, Zuo Y,
Rashid K, Zhang JH, Feng H and Liu F: LSKL peptide alleviates
subarachnoid fibrosis and hydrocephalus by inhibiting TSP1-mediated
TGF-β1 signaling activity following subarachnoid hemorrhage in
rats. Exp Ther Med. 12:2537–2543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun H, Zhao Y, Bi X, Li S, Su G, Miao Y,
Ma X, Zhang Y, Zhang W and Zhong M: Valsartan blocks
thrombospondin/transforming growth factor/Smads to inhibit aortic
remodeling in diabetic rats. Diagn Pathol. 10:182015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zeisberg M, Tampe B, LeBleu V, Tampe D,
Zeisberg EM and Kalluri R: Thrombospondin-1 deficiency causes a
shift from fibroproliferative to inflammatory kidney disease and
delays onset of renal failure. Am J Pathol. 184:2687–2698. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ma L, Li H, Zhang S, Xiong X, Chen K,
Jiang P, Jiang K and Deng G: Emodin ameliorates renal fibrosis in
rats via TGF-β1/Smad signaling pathway and function study of Smurf
2. Int Urol Nephrol. 50:373–382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Loeffler I and Wolf G:
Epithelial-to-mesenchymal transition in diabetic nephropathy: Fact
or Fiction? Cells. 4:631–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schiller M, Javelaud D and Mauviel A:
TGF-beta-induced SMAD signaling and gene regulation: Consequences
for extracellular matrix remodeling and wound healing. J Dermatol
Sci. 35:83–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lan HY: Smads as therapeutic targets for
chronic kidney disease. Kidney Res Clin Pract. 31:4–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Loeffler I, Liebisch M, Allert S, Kunisch
E, Kinne RW and Wolf G: FSP1-specific SMAD2 knockout in renal
tubular, endothelial, and interstitial cells reduces fibrosis and
epithelial-to-mesenchymal transition in murine STZ-induced diabetic
nephropathy. Cell Tissue Res. 372:115–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chung AC, Zhang H, Kong YZ, Tan JJ, Huang
XR, Kopp JB and Lan HY: Advanced glycation end-products induce
tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc
Nephrol. 21:249–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fujimoto M, Maezawa Y, Yokote K, Joh K,
Kobayashi K, Kawamura H, Nishimura M, Roberts AB, Saito Y and Mori
S: Mice lacking Smad3 are protected against streptozotocin-induced
diabetic glomerulopathy. Biochem Biophys Res Commun. 305:1002–1007.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li J, Qu X, Yao J, Caruana G, Ricardo SD,
Yamamoto Y, Yamamoto H and Bertram JF: Blockade of
endothelial-mesenchymal transition by a Smad3 inhibitor delays the
early development of streptozotocin-induced diabetic nephropathy.
Diabetes. 59:2612–2624. 2010. View Article : Google Scholar : PubMed/NCBI
|