Introduction
Lung cancer is a malignant carcinoma, and a leading
cause of morbidity and mortality worldwide (1,2). The
disease can be divided into two main types, according to
clinicopathological features, namely, small-cell lung cancer and
non-small-cell lung cancer (NSCLC) (3). NSCLC accounts for 80–90% of all cases
of lung cancer (4,5). Although the diagnostic and clinical
therapeutic targets of NSCLC have significantly improved in the
last decade, the high recurrence rate and poor 5-year survival rate
remain (6). Thus, the molecular
mechanism underlying NSCLC tumorigenesis requires further
investigation.
Previous studies have confirmed the involvement of
microRNAs (miRNAs/miRs) in the development of NSCLC (4,7).
miRNAs are a series of short (~22 nucleotides), single-stranded,
non-coding RNAs, which are mainly transcribed from introns or exons
of protein-coding genes (8).
miRNAs are involved in the post-transcriptional regulation of gene
expression in multicellular organisms; they regulate genes by
binding to the 3′-untranslated regions (3′-UTRs) of target mRNAs,
thus decreasing the level of oncoprotein translation (5,9).
Previous studies have reported that miRNA dysregulation occurs in
numerous types of human carcinoma, and that miRNAs are involved in
tumor cell proliferation, apoptosis and metastasis (10,11).
According to the functional roles of downstream genes, miRNAs can
act as oncogenes or tumor suppressors in different types of human
cancer, including NSCLC (12,13).
For example, aberrant miR-28 expression has been detected in
gastric cancer (14), ovarian
cancer (15), B-cell lymphoma
(16), colorectal cancer (17), hepatocellular carcinoma (18) and renal cell carcinoma (19). However, the detailed molecular
mechanism of miR-28 in NSCLC has not been fully elucidated.
The present study aimed to identify the expression
levels, functional roles and molecular mechanisms of miR-28, and to
investigate the target genes associated with tumor cell
proliferation, in NSCLC progression. The present study suggested
that miR-28 acted as a promoter in the process of NSCLC cell
proliferation by targeting PTEN. Furthermore, it was proposed that
the miR-28/PTEN axis may serve as a novel clinical therapeutic
target for NSCLC.
Materials and methods
NSCLC tissues and cell lines
Tumor tissues and matched adjacent non-tumor tissues
(<3 cm from the tumor margin) were harvested from 33 patients
with NSCLC (21 men and 12 women; mean age, 48.9 years; age range,
35–72 years) at Jingzhou Central Hospital between October 2015 and
December 2018. Regarding TNM staging (20), 15 (45.45%) patients were classified
as TNM stage I or II, whereas 18 (54.55%) patients were classified
as TNM stage III or IV. Total tissues were stored at −80°C for
tissue repository establishment. The present study was approved by
the Ethical Committee of the Jingzhou Central Hospital. Written
informed consent was obtained from all patients.
The cell lines, including BEAS-2b, A549, H1650,
H292, H1944 and H1299, were purchased from the Institute of
Biochemistry and Cell Biology of the Chinese Academy of Sciences.
All cell lines were cultured using RPMI-1640 medium supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin at 37°C with 5% CO2.
Prediction of target genes of
miR-28
TargetScan (version 3.1; www.targetscan.org/mamm_31) software was used to
determine the candidate downstream target genes of miR-28.
Reverse transcription-quantitative PCR
(RT-qPCR)
The NSCLC tumor and matched adjacent normal tissues
(100 mg) were ground in liquid nitrogen and the resulting cells
underwent RNA extraction. The aforementioned cell lines were also
used for RT-qPCR. Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using the iScript cDNA synthesis kit
(cat. no. 1708890; Bio-Rad Laboratories, Inc.), according to the
manufacturer's protocol. Subsequently, TaqMan® MicroRNA
assays (Applied Biosystems; Thermo Fisher Scientific, Inc.) were
used to detect the expression of miR-28 (miR-28-5p, forward,
5′-CGGATCCAGGCCCTTCAAGGACTTTCT-3′ and reverse,
5′-CGAATTCACAGAGCTCCTGCTGTGTCA-3′), according to the manufacturer's
protocol. miR-28 expression levels were normalized to the internal
reference gene U6 (forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and
reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′). SYBR Premix Ex Taq II
(Takara Bio, Inc.) was used to determine PTEN mRNA expression
levels. The following primer pairs were used for qPCR: PTEN
forward, 5′-TGGATTCGACTTAGACTTGACCT-3′ and reverse,
5′-GGTGGGTTATGGTCTTCAAAAGG-3′; and GAPDH forward,
5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′. PTEN mRNA expression levels were
normalized to the internal reference gene GADPH. PCR was performed
using the 7500 Realtime PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with the following thermocycling
conditions: 12 min at 96°C; followed by 35 cycles of 15 sec at 96°C
and 1 min at 58°C; and a final extension at 72°C for 5 min.
Relative expression levels were quantified using the
2−∆∆Cq method (21).
Cell transfection and infection
The miR-28 mimic (mimic-miR-28;
GAGUUAUCUGACACUCGAGGAA) and its negative control (mimic-NC;
UUCUCCGAACGUGUCACGU) were purchased from Shanghai GenePharma Co.,
Ltd. A549 cells (2×105 cells/well) were transfected with
mimic-miR-28 or mimic-NC (50 nmol/l) to investigate the interaction
between miR-28 and PTEN. Lentivirus (LV)-short hairpin RNA
(sh)-PTEN (CCACAGCUAGAACUUAUCAAA), LV-anti-miR-28
(CUCAAUAGACUGUGAGCUCCUU), LV-anti-miR-28 + sh-PTEN or the LV-NC
(UUCUCCGAACGUGUCACGU) were infected (MOI=3) into A549 and H292
cells (2×105 cells/well). The lentiviruses used in the
present study were purchased from Shanghai GenePharma Co., Ltd.
Mimic transfection was performed using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.). Following
incubation for 6 h at 37°C, the cell culture medium was replaced
with fresh medium. At 24 h post-transfection, cells were used for
subsequent experiments.
Luciferase activity assay
The luciferase reporter plasmid pGL3 (Shanghai
GenePharma Co., Ltd.) was used to perform the luciferase activity
assay. Renilla luciferase was used as the normalization
control. A549 cells (5×105 cells/well) were seeded into
a 6-well plate. The sequences of the wild-type (WT) PTEN 3′-UTR
(UCCCAAGUCCUUUGUAGCUCCUC) and the mutant (MUT) PTEN 3′-UTR
(UCCCAAGUCCUUUGUUCGAGGAC) were obtained from Shanghai GenePharma
Co., Ltd. and were cloned into the pGL3 vector. Subsequently, A549
cells were transfected with pGL3-WT-PTEN-3′-UTR or
pGL3-WUT-PTEN-3′-UTR (1 µg/ml) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated for 6 h
at 37°C. A549 cells were also transfected with mimic-miR-28 or
mimic-NC (50 nmol/l) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated for 6 h
at 37°C. Subsequently, the cell culture medium was replaced with
fresh medium. After 48 h, the Dual-Luciferase Reporter assay system
(Promega Corporation) was used to measure the luciferase activity,
according to the manufacturer's instructions.
Western blotting
Total protein was extracted from A549 and H292 cells
using ~1 ml RIPA buffer (Beyotime Institute of Biotechnology).
Total protein was quantified using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). Protein (25 µg)
was separated by SDS-PAGE using 10% gels and transferred onto PVDF
membranes. The membranes were blocked with 5% non-fat milk at room
temperature for 2 h. Subsequently, the membranes were incubated
with primary antibodies against PTEN (cat. no. 60300-1-Ig; 1:1,000;
Proteintech Group, Inc.) and GAPDH (cat. no. 60004-1-Ig; 1:20,000;
Proteintech Group, Inc.) overnight at 4°C. The membranes were then
washed with TBS-0.5% Tween 20 and incubated with a horseradish
peroxidase-conjugated secondary antibody (cat. no. ab205719;
1:20,000; Abcam) for 2 h at room temperature. Protein bands were
visualized using an ECL kit (EMD Millipore) and were imaged using a
Bioshine ChemiQ 4600 Mini Chemiluminescence Imaging system
(Ouxiang). Protein expression was quantified using Image J software
(version 1.49; National Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay was used to determine the
proliferation of A549 and H292 cells (5×103 cells/well).
The CCK-8 reagent (10 µl; APExBIO Technology) was added to each
well and subsequently, the cells were counted every 24 h for 5 days
using a Fluoroskan Ascent microplate fluorometer (Thermo Fisher
Scientific, Inc.). The optical density at a wavelength of 450 nm
was measured. After 5 days, the growth curve was generated.
Statistical analysis
All experiments were performed in triplicate and
repeated three times. The data are presented as the mean ± SD. A
paired Student's t-test was used for the comparison of miR-28
expression between NSCLC tumor and matched adjacent normal tissues.
One-way ANOVA followed by Tukey's post hoc test was used to measure
the differences between quantitative variables. P<0.05 was
considered to indicate a statistically significant difference.
GraphPad Prism (version 7.0; GraphPad Software, Inc.) and R studio
(version 3.5.1; www.r-project.org) software were used to perform the
statistical analyses.
Results
Upregulation of miR-28 expression in
NSCLC tissues and cell lines
To investigate whether miR-28 was associated with
NSCLC development, RT-qPCR was performed to measure the expression
levels of miR-28 in NSCLC tumor tissues and cell lines. miR-28
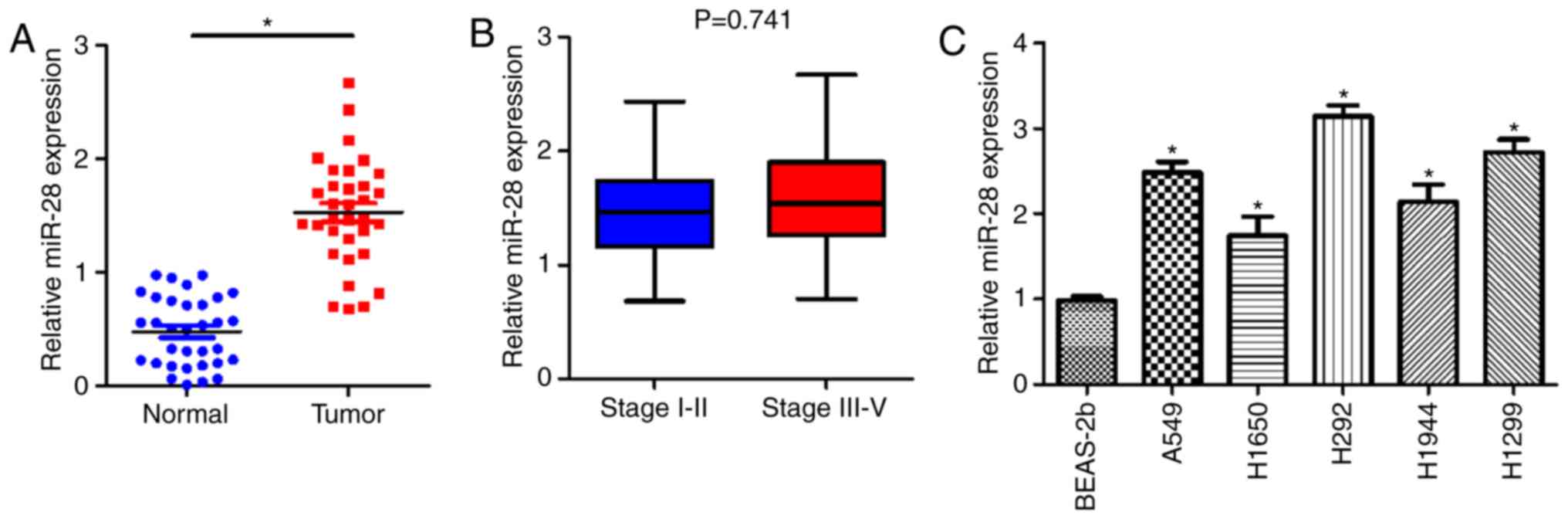
expression was significantly upregulated in NSCLC tissues compared
with matched adjacent non-tumor tissues (Fig. 1A). There was no significant
difference between the expression levels of miR-28 in early (1.497
in stage I/II) and late TNM stage tumors (1.553 in stage III/IV;
P>0.05; Fig. 1B). The
expression levels of miR-28 in A549, H1650, H292, H1944 and H1299
cell lines were significantly higher than in the BEAS-2b cell line
(Fig. 1C), which is a normal lung
bronchus epithelial cell line and acted as the control group. The
results suggested that miR-28 was not only involved in the
progression of NSCLC, but was also upregulated in NSCLC tumor
tissues and cell lines.
miR-28 knockdown reduces proliferation
of A549 and H292 cells
To determine the biological functions of miR-28 in
the development of NSCLC, A549 and H292 cells were infected with
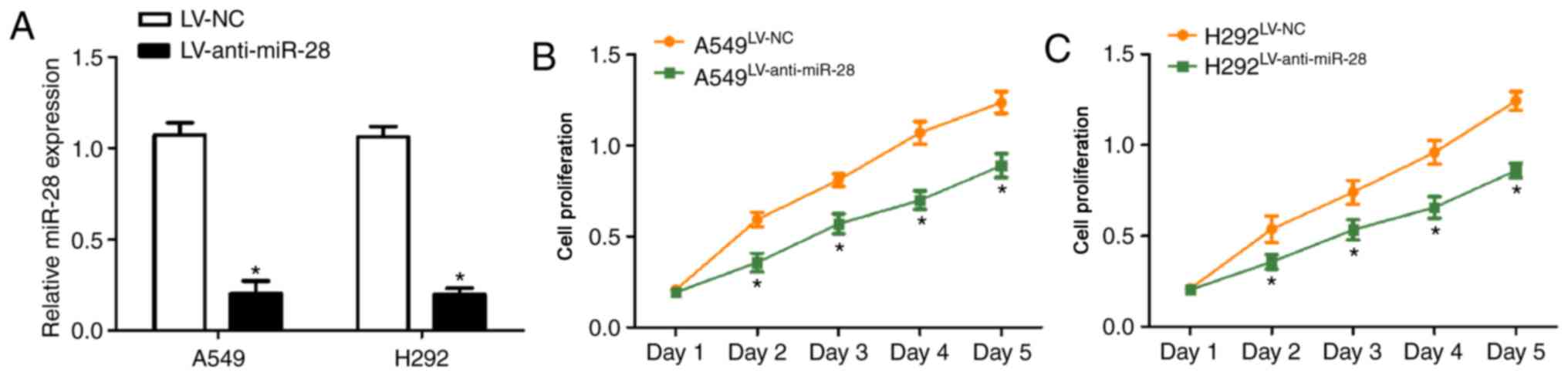
LV-anti-miR-28. miR-28 expression levels were significantly
decreased in both cell lines following infection with
LV-anti-miR-28 compared with those infected with LV-NC (Fig. 2A), indicating that the infection
was successful. The cell proliferation curves generated by
performing the CCK-8 assay suggested that miR-28 knockdown
inhibited proliferation of A549 and H292 cells compared with the
LV-NC group (Fig. 2B and C). The
results indicated that miR-28 might act as a promoter in NSCLC
progression.
PTEN is the direct target gene of
miR-28 and is negatively regulated by miR-28
PTEN was predicted as the downstream gene of miR-28
using TargetScan (www.targetscan.org). To verify whether miR-28 directly
targeted PTEN in NSCLC, a dual-luciferase activity assay was
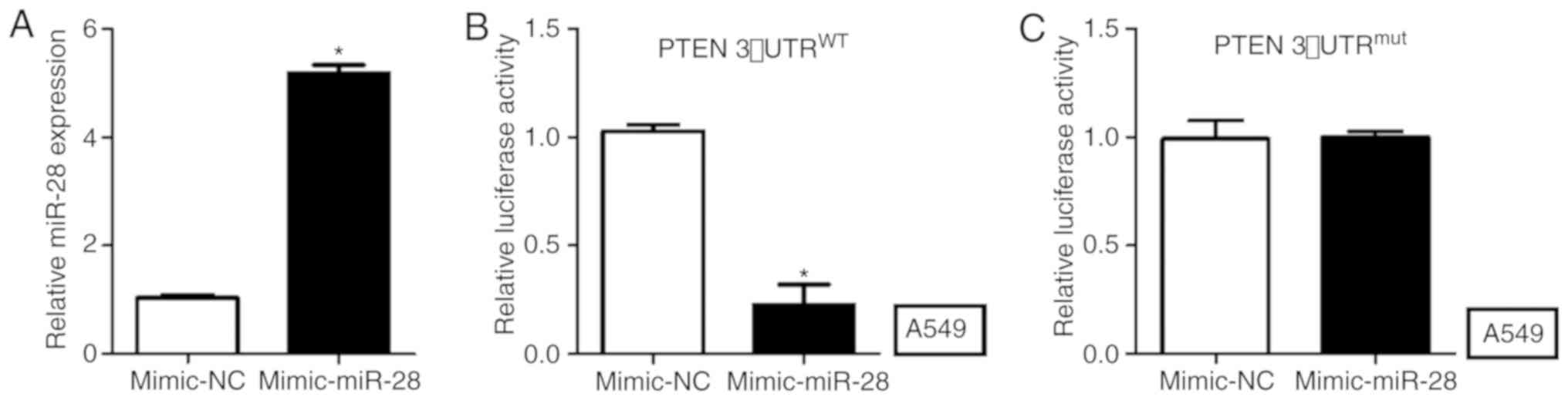
performed in A549 cells. miR-28 expression levels were
significantly increased in A549 cells transfected with mimic-miR-28
compared with those transfected with mimic-NC (Fig. 3A). The luciferase activity in A549
cells co-transfected with WT-PTEN-3′-UTR and mimic-miR-28 was
significantly decreased compared with the mimic-NC group (Fig. 3B). The luciferase activity of A549
cells transfected with MUT-PTEN-3′-UTR was not significantly
altered between cells co-transfected with mimic-miR-28 or mimic-NC
(Fig. 3C).
To explore the direct effect of miR-28 on PTEN,
RT-qPCR and western blotting were performed to measure the mRNA and
protein expression levels of PTEN in A549 and H292 cells infected
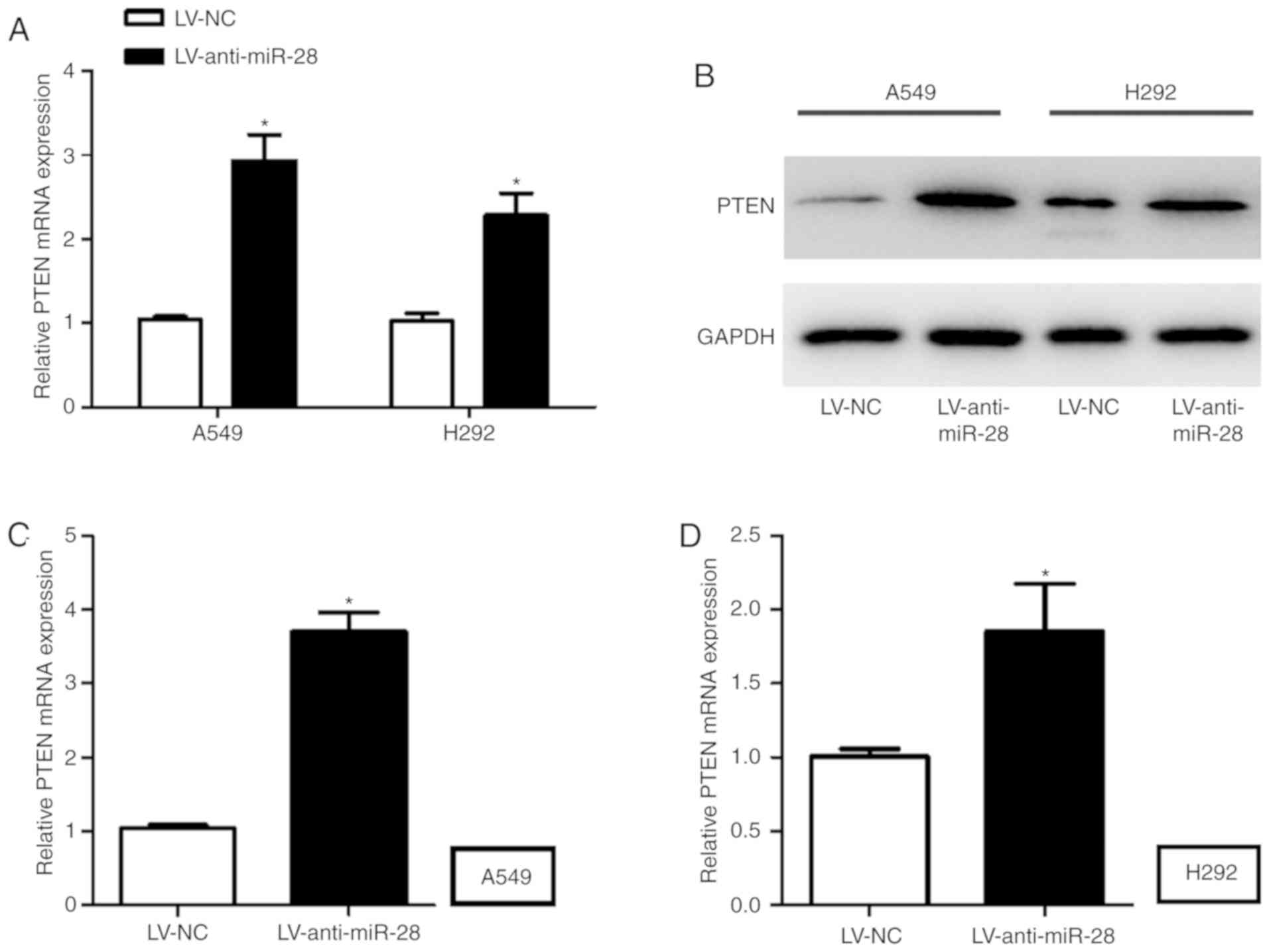
with LV-anti-miR-28. A549 and H292 cells infected with
LV-anti-miR-28 displayed significantly higher PTEN mRNA and protein
expression levels compared with A549 and H292 cells infected with
LV-NC (Fig. 4A-D).
Collectively, these results suggested that PTEN may
be the downstream gene of miR-28 in NSCLC; therefore, miR-28
knockdown promoted the expression of PTEN.
PTEN knockdown reverses the effect of
anti-miR-28 on PTEN expression and cell proliferation in NSCLC
sh-PTEN was used to investigate whether PTEN
affected the progression of NSCLC. PTEN expression levels were
significantly decreased in A549 and H292 cells infected with
LV-sh-PTEN compared with those infected with LV-NC (Fig. 5A and E). The mRNA and protein
expression levels of PTEN in A549 and H292 cells co-infected with
LV-anti-miR-28 + sh-PTEN were significantly lower compared with
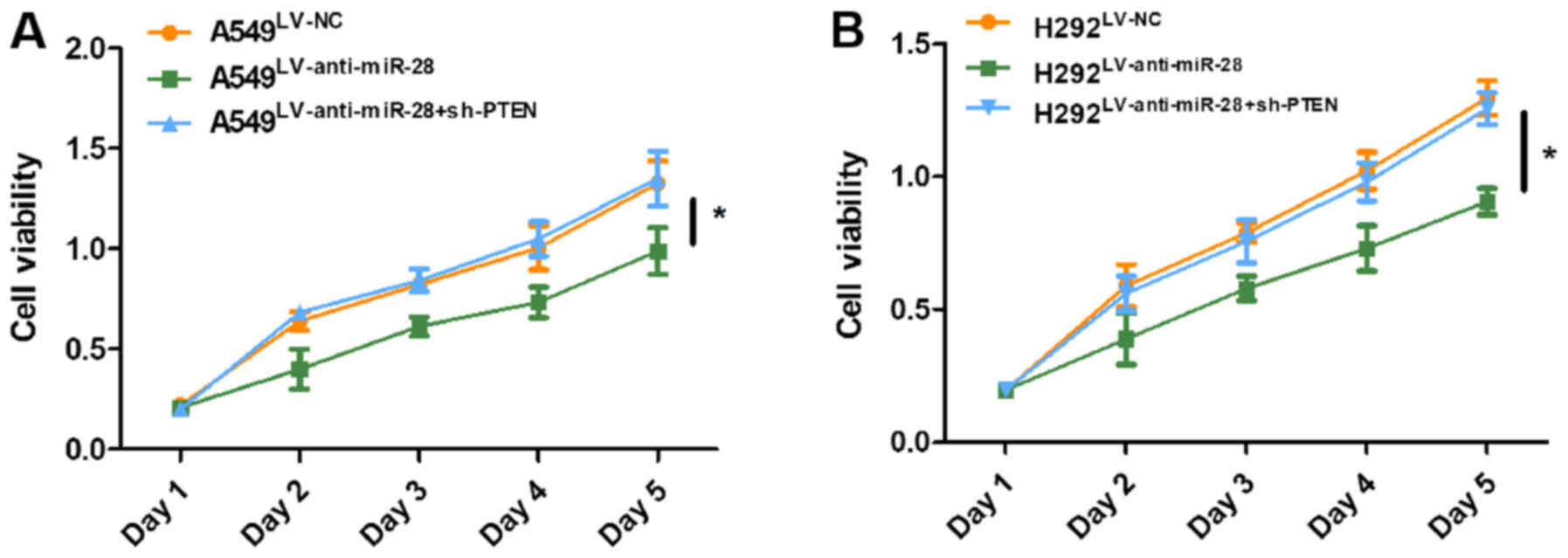
cells infected with LV-anti-miR-28 (Fig. 5B-D and F-H). The CCK-8 assay
suggested that A549 and H292 cells co-infected with LV-anti-miR-28
+ sh-PTEN displayed higher levels of cell proliferation compared
with A549 and H292 cells infected with LV-anti-miR-28 (Fig. 6). The results indicated that PTEN
knockdown reversed the inhibitory effect of LV-anti-miR-28 on cell
proliferation. Furthermore, the results suggested that miR-28 might
play a role as an oncogene in NSCLC by targeting PTEN.
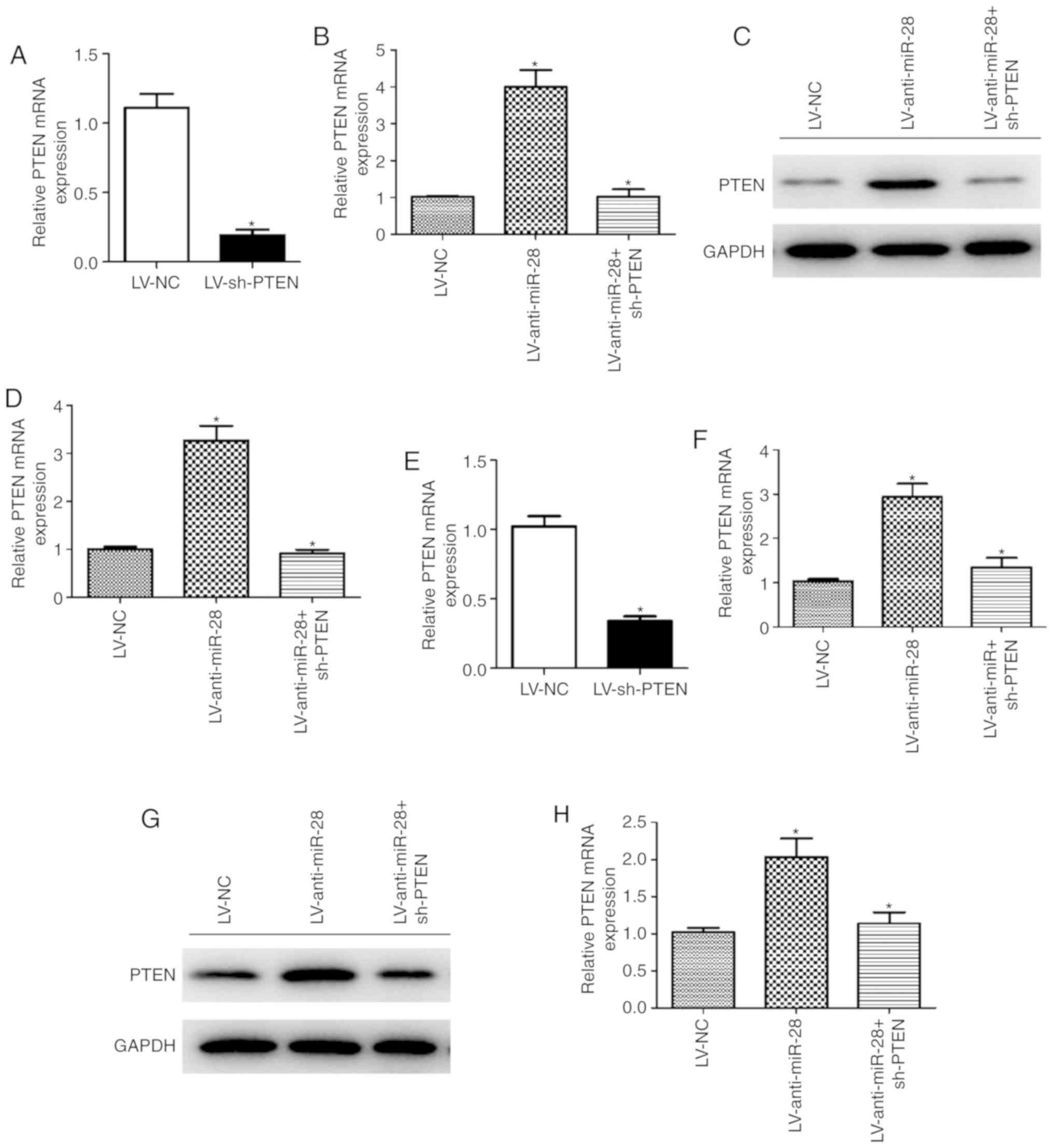 | Figure 5.PTEN knockdown reverses the effect of
miR-28 on PTEN expression in A549 and H292 cells. mRNA expression
levels of PTEN in A549 cells infected with (A) LV-NC or LV-sh-PTEN,
and (B) LV-anti-miR-28, LV-anti-miR-28 + sh-PTEN or LV-NC. Protein
expression levels of PTEN in A549 cells infected with
LV-anti-miR-28, LV-anti-miR-28 + sh-PTEN or LV-NC were (C)
determined by western blot analysis and (D) semi-quantified. (E)
mRNA expression levels of PTEN in H292 cells infected with (E)
LV-NC or LV-sh-PTEN, and (F) LV-anti-miR-28, LV-anti-miR-28 +
sh-PTEN or LV-NC. Protein expression levels of PTEN in H292 cells
infected with LV-anti-miR-28, LV-anti-miR-28 + sh-PTEN or LV-NC (G)
determined by western blot analysis and (H) semi-quantified. All
experiments were performed in triplicate. *P<0.05 vs. LV-NC. LV,
lentivirus; miR, microRNA; NC, negative control; sh, short hairpin
RNA. |
Discussion
In the present study, the underlying functional
roles of miR-28 in NSCLC tumorigenesis were investigated. miR-28
expression levels were significantly upregulated in NSCLC tumor
tissues and cell lines compared with the matched adjacent non-tumor
tissues and the control cell line, respectively. Additionally, PTEN
was identified as the downstream gene of miR-28 during NSCLC
development. miR-28 knockdown increased PTEN expression levels and
reduced tumor cell proliferation in vitro. PTEN knockdown
reduced the effect of miR-28 on NSCLC tumor cell proliferation.
Overall, it could be suggested that miR-28 acted as a promoter in
NSCLC by targeting PTEN. Therefore, the miR-28/PTEN axis may serve
as a potential clinical target for NSCLC diagnosis, treatment and
prognosis.
miR-28, which is located at chromosome 3q28, has two
main subtypes, miR-28-3p and miR-28-5p (22). In previous studies, miR-28 and its
two subtypes have been reported to be aberrantly expressed by
certain pathological mechanisms. Zhou et al (23) reported that miR-28-3p expression
levels were increased in the plasma of patients with pulmonary
embolism. With regards to lymphocytic leukemia, miR-28-5p
expression was significantly increased in patients compared with
healthy controls (24). Platelet
miR-28 expression was also identified as being upregulated in
patients with myeloproliferative neoplasm (25). The aforementioned results indicated
that miR-28 upregulation commonly occurs in diseases, which is
consistent with the findings of the present study.
miR-28 can act as an oncogene or a tumor suppressor
in various types of malignant carcinoma. Schneider et al
(16) reported that overexpression
of miR-28 inhibited cell proliferation in B-cell lymphoma. Xu et
al (15) demonstrated that
miR-28-5p induced the proliferation of ovarian cancer cells, as
well as their migration and invasion, by targeting NEDD4-binding
protein 1. Wu et al (17)
reported that miR-28-5p had a suppressive effect on colorectal
cancer progression by interacting with the downstream gene
structure specific recognition protein 1. However, another similar
study suggested that miR-28-3p acted as a tumor promoter in
colorectal cancer cell migration and invasion (22). Therefore, the aforementioned
studies illustrate that the effects of miR-28 are not always
identical in different types of cancer and that the different
subtypes of miR-28 may have opposite functions in the same cancer.
The present study suggested that miR-28 served as an oncogene in
NSCLC cell proliferation, which is supported by Wang et al
(26) who reported that miR-28 is
one of the potential oncogenes in lung cancer.
PTEN, located at chromosome 10q23.31, has been
identified as a tumor suppressor gene via the PI3K/AKT pathway in a
number of different forms of cancer (27), including renal cancer (28), gastric cancer (29), endometrial cancer (30), breast cancer (31) and malignant melanoma (32). Previously, emerging miRNAs have
been identified as promoters of tumor cell growth, metastasis or
apoptosis by targeting PTEN, including miR-1297 (33), miR-200 (34), miR-130a (35), miR-26a (36) and miR-17 (37). In the present study, PTEN was also
identified as the target gene of miR-28. Additionally, the
oncogenic role of miR-28 in NSCLC proposed in the present study was
the same as that of miRNAs reported previously (33–37).
These results suggested that miR-28 directly targeted PTEN and may
promote tumorigenesis in NSCLC.
A previous study investigated the interaction of the
miR-28/PTEN axis in carcinoma. Li et al (14) reported that miR-28 acts as an
oncogene in gastric cancer growth and invasion by targeting PTEN,
via the PI3K/AKT signaling pathway, which strengthened the present
findings. Moreover, the function of the miR-28/PTEN axis in other
types of human cancer requires further investigation and could
identify additional biomarkers for cancer research.
In conclusion, the present study suggested that
miR-28 was upregulated in NSCLC tumor tissues and cell lines.
Moreover, miR-28 promoted NSCLC cell proliferation by targeting
PTEN, which serves as a suppressor of pathogenesis in numerous
diseases (27). The present study
identified a potential biomarker for the clinical diagnosis,
treatment and prognosis of NSCLC. Further studies investigating
whether there are other target genes of miR-28 in NSCLC and whether
the miR-28/PTEN axis functions in other types of cancer are
required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HQ conceived and designed the present study. FC and
QZ performed the experiments and analyzed the data. FC, QZ and KX
helped to design the study and interpreted the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the Jingzhou Central Hospital. Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castro D, Moreira M, Gouveia AM, Pozza DH
and De Mello RA: MicroRNAs in lung cancer. Oncotarget.
8:81679–81685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu KL, Tsai YM, Lien CT, Kuo PL and Hung
AJ: The roles of MicroRNA in lung cancer. Int J Mol Sci.
20:E16112019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vannini I, Fanini F and Fabbri M:
MicroRNAs as lung cancer biomarkers and key players in lung
carcinogenesis. Clin Biochem. 46:918–925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang WC, Liu J, Xu X and Wang G: The role
of microRNAs in lung cancer progression. Med Oncol. 30:6752013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Angulo M, Lecuona E and Sznajder JI: Role
of MicroRNAs in lung disease. Arch Bronconeumol (Spanish).
48:325–330. 2012. View Article : Google Scholar
|
|
7
|
Del Vescovo V, Grasso M, Barbareschi M and
Denti MA: MicroRNAs as lung cancer biomarkers. World J Clin Oncol.
5:604–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alipoor SD, Adcock IM, Garssen J, Mortaz
E, Varahram M, Mirsaeidi M and Velayati A: The roles of miRNAs as
potential biomarkers in lung diseases. Eur J Pharmacol.
791:395–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guz M, Rivero-Muller A, Okon E,
Stenzel-Bembenek A, Polberg K, Slomka M and Stepulak A:
MicroRNAs-role in lung cancer. Dis Markers. 2014:2181692014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang SM and Lee HJ: MicroRNAs in human
lung cancer. Exp Biol Med (Maywood). 239:1505–1513. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu SG, Chang TH, Liu YN and Shih JY:
MicroRNA in lung cancer metastasis. Cancers (Basl). 11:E2652019.
View Article : Google Scholar
|
|
12
|
Markou A, Sourvinou I, Vorkas PA, Yousef
GM and Lianidou E: Clinical evaluation of microRNA expression
profiling in non small cell lung cancer. Lung Cancer. 81:388–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen J, Liu Z, Todd NW, Zhang H, Liao J,
Yu L, Guarnera MA, Li R, Cai L, Zhan M and Jiang F: Diagnosis of
lung cancer in individuals with solitary pulmonary nodules by
plasma microRNA biomarkers. BMC Cancer. 11:3742011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Zhu X, Shou T, Yang L, Cheng X, Wang
J, Deng L and Zheng Y: MicroRNA-28 promotes cell proliferation and
invasion in gastric cancer via the PTEN/PI3K/AKT signalling
pathway. Mol Med Rep. 17:4003–4010. 2018.PubMed/NCBI
|
|
15
|
Xu J, Jiang N, Shi H, Zhao S, Yao S and
Shen H: miR-28-5p promotes the development and progression of
ovarian cancer through inhibition of N4BP1. Int J Oncol. Mar
16–2017.(Epub ahead of print). View Article : Google Scholar
|
|
16
|
Schneider C, Setty M, Holmes AB, Maute RL,
Leslie CS, Mussolin L, Rosolen A, Dalla-Favera R and Basso K:
MicroRNA 28 controls cell proliferation and is down-regulated in
B-cell lymphomas. Proc Natl Acad Sci USA. 111:8185–8190. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu W, He K, Guo Q, Chen J, Zhang M, Huang
K, Yang D, Wu L, Deng Y, Luo X, et al: SSRP1 promotes colorectal
cancer progression and is negatively regulated by miR-28-5p. J Cell
Mol Med. 23:3118–3129. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z,
Cao Y, Fan J, Huang XW and Zhou J: miR-28-5p-IL-34-macrophage
feedback loop modulates hepatocellular carcinoma metastasis.
Hepatology. 63:1560–1575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Wu C, Yang Q, Ding M, Zhong J,
Zhang CY, Ge J, Wang J and Zhang C: miR-28-5p acts as a tumor
suppressor in renal cell carcinoma for multiple antitumor effects
by targeting RAP1B. Oncotarget. 7:73888–73902. 2016.PubMed/NCBI
|
|
20
|
Nicholson AG, Chansky K, Crowley J,
Beyruti R, Kubota K, Turrisi A, Eberhardt WE and van Meerbeeck J;
Staging and Prognostic Factors Committee, Advisory Boards, and
Participating Institutions; Staging and Prognostic Factors
Committee Advisory Boards and Participating Institutions, . The
international association for the study of lung cancer lung cancer
staging project: Proposals for the revision of the clinical and
pathologic staging of small cell lung cancer in the forthcoming
eighth edition of the TNM classification for lung cancer. J
Thoracic Oncol. 11:300–311. 2016. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Almeida MI, Nicoloso MS, Zeng L, Ivan C,
Spizzo R, Gafà R, Xiao L, Zhang X, Vannini I, Fanini F, et al:
Strand-specific miR-28-5p and miR-28-3p have distinct effects in
colorectal cancer cells. Gastroenterology. 142:886–896.e9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou X, Wen W, Shan X, Qian J, Li H, Jiang
T, Wang W, Cheng W, Wang F, Qi L, et al: MiR-28-3p as a potential
plasma marker in diagnosis of pulmonary embolism. Thromb Res.
138:91–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang YQ, Tian T, Zhu HY, Liang JH, Wu W,
Wu JZ, Xia Y, Wang L, Fan L, Li JY and Xu W: NDRG2 mRNA levels and
miR-28-5p and miR-650 activity in chronic lymphocytic leukemia. BMC
Cancer. 18:10092018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Girardot M, Pecquet C, Boukour S, Knoops
L, Ferrant A, Vainchenker W, Giraudier S and Constantinescu SN:
miR-28 is a thrombopoietin receptor targeting microRNA detected in
a fraction of myeloproliferative neoplasm patient platelets. Blood.
116:437–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang QZ, Xu W, Habib N and Xu R: Potential
uses of microRNA in lung cancer diagnosis, prognosis, and therapy.
Curr Cancer Drug Targets. 9:572–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hopkins BD and Parsons RE: Molecular
pathways: Intercellular PTEN and the potential of PTEN restoration
therapy. Clin Cancer Res. 20:5379–5383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zaman MS, Thamminana S, Shahryari V,
Chiyomaru T, Deng G, Saini S, Majid S, Fukuhara S, Chang I, Arora
S, et al: Inhibition of PTEN gene expression by oncogenic
miR-23b-3p in renal cancer. PLoS One. 7:e502032012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z, Yuan XG, Chen J, Luo SW, Luo ZJ
and Lu NH: Reduced expression of PTEN and increased PTEN
phosphorylation at residue Ser380 in gastric cancer tissues: A
novel mechanism of PTEN inactivation. Clin Res Hepatol
Gastroenterol. 37:72–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoneyama K, Ishibashi O, Kawase R, Kurose
K and Takeshita T: miR-200a, miR-200b and miR-429 are onco-miRs
that target the PTEN gene in endometrioid endometrial carcinoma.
Anticancer Res. 35:1401–1410. 2015.PubMed/NCBI
|
|
31
|
Zhang WL and Zhang JH: miR-181c promotes
proliferation via suppressing PTEN expression in inflammatory
breast cancer. Int J Oncol. 46:2011–2020. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou XP, Gimm O, Hampel H, Niemann T,
Walker MJ and Eng C: Epigenetic PTEN silencing in malignant
melanomas without PTEN mutation. Am J Pathol. 157:1123–1128. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Wang HL, Peng X, Zhou HF and Wang X:
miR-1297 mediates PTEN expression and contributes to cell
progression in LSCC. Biochem Biophys Res Commun. 427:254–260. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Q, Lu RL, Li JX and Rong LJ: MiR-200a
and miR-200b target PTEN to regulate the endometrial cancer cell
growth in vitro. Asian Pac J Trop Med. 10:498–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei H, Cui R, Bahr J, Zanesi N, Luo Z,
Meng W, Liang G and Croce CM: miR-130a deregulates PTEN and
stimulates tumor growth. Cancer Res. 77:6168–6178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li B and Sun H: MiR-26a promotes neurite
outgrowth by repressing PTEN expression. Mol Med Rep. 8:676–680.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao Y, Luo LH, Li S and Yang C: miR-17
inhibitor suppressed osteosarcoma tumor growth and metastasis via
increasing PTEN expression. Biochem Biophys Res Commun.
444:230–234. 2014. View Article : Google Scholar : PubMed/NCBI
|