Introduction
Preeclampsia (PE) is a pregnancy-specific
complication with a global incidence of 4–5% (1). PE is characterized by the development
of hypertension with or without proteinuria, after 20 weeks of
gestation (2). While, PE leads to
the morbidity and mortality of mothers and perinatal infants, its
pathogenesis has not been fully elucidated (1,3). The
placenta is crucial for the development of PE (4). It has been shown that placental
hypoxia is considered to be the main factor contributing to the
pathogenesis of PE, and is associated with excessive apoptosis of
trophoblasts, which results in decreased trophoblast invasion and
insufficient spiral artery remodeling (5,6).
Hypoxia-inducible factor 1 (HIF-1) is a
transcriptional factor that helps maintain oxygen homeostasis and
can react quickly to low oxygen tension (7). Furthermore, it is a heterodimer
containing two subunits, α and β (8). HIF-1α is an oxygen-regulated subunit
that responds to changes in cellular oxygen, while HIF-1β is a
constitutively expressed subunit (8). Under normal levels oxygen HIF-1α is
degraded, and under hypoxic conditions degradation of HIF-1α is
inhibited, thus resulting in rapid accumulation of the protein
(9). Moreover, HIF-1α is critical
for placental development, and prolonged expression of HIF-1α
causes pregnancy-associated disorders in placental trophoblasts
(10). In PE, placental
hypoperfusion and ischemia can result in a hypoxic
microenvironment, which induces the expression of HIF-1α (11). Previous studies have demonstrated
that HIF-1α can participate in cell apoptosis by regulating the
expression of Forkhead box O transcription factor 3a (FOXO3a)
(12). FOXO3a is a member of the
FOXO transcription factor family, and four FOXO family members have
been identified in humans: FOXO1, FOXO3a, FOXO4 and FOXO6 (13). Together these transcription factors
control various biological functions such as cellular metabolism,
cell cycle regulation, apoptosis and regulation of stress response
(14). Moreover, previous studies
have found that hypoxia significantly increases the expression of
FOXO3a, and decreases the phosphorylation of Akt and FOXO3a, thus
resulting in increased nuclear accumulation (14,15).
Hu et al (16) revealed
that FOXO3a is a downstream effector of HIF-1α and is activated by
hypoxia. Furthermore, it has been shown that knockdown of FOXO3a
increases apoptosis of human umbilical vein endothelial cells
(HUVECs) cells under hypoxia (16).
The present study investigated the expression levels
of HIF-1α and FOXO3a in placental tissues of patients with early
onset severe PE, and examined its effect on trophoblastic apoptosis
under hypoxia.
Materials and methods
Case selection
Patients were recruited for the study between May
2017 and December 2018 at The Third Affiliated Hospital of
Zhengzhou University. In total, 30 women (mean age, 32.90±5.41
years) with early onset severe PE were chosen as the experimental
group and 30 women (mean age, 32.45±4.66 years) with a normal
pregnancy constituted the control group. Women who were from the
Chinese Han population selected cesarean sections. Inclusion and
exclusion criteria for early onset severe PE were strictly based on
guidelines of the American College of Gynecologists, Task Force on
Hypertension, published in 2013 (17). The exclusion criteria included
multi-fetal pregnancies, gestational diabetes mellitus, chronic
hypertension, connective tissue diseases and smoking. The study was
approved by The Ethics Committee of The Third Affiliated Hospital
of Zhengzhou University, and informed consent was obtained from all
the patients. Detailed clinical information of patients in the two
groups is shown in Table I.
 | Table I.Clinical characteristics of control
and early onset PE group. |
Table I.
Clinical characteristics of control
and early onset PE group.
| Variables | Control (n=30) | Preeclampsia
(n=30) | P-value |
|---|
| Delivery age,
years | 32.45±4.66 | 32.90±5.41 | 0.73 |
| Gestational age,
weeks | 39.00±0.50 | 32.43±1.59 | <0.01 |
| Systolic blood
pressure, mmHg | 114.72±7.26 | 162.29±13.79 | <0.01 |
| Diastolic blood
pressure, mmHg | 72.52±8.22 | 102.81±9.53 | <0.01 |
| Proteinuria, g/24
h | 0.08±0.04 | 4.99±2.96 | <0.01 |
| Newborn birth
weight, g | 3518.28±350.87 | 1457.74±376.13 | <0.01 |
| Maternal body mass
index kg/m2 | 28.01±2.40 | 30.01±2.92 | 0.27 |
| Delivery way | Cesarean
sections | Cesarean
sections |
|
| Parity | Singles | Singles |
|
| Smoking | No | No |
|
| Ethnicity | Ethnic han | Ethnic han |
|
Sample collection
The biopsies were separated from the maternal aspect
of the placenta after delivery. Regions with calcification,
necrosis and infarction were not collected. Blood in the tissues
was removed using sterile filter paper. Specimens were fixed with
10% buffered formalin for 24 h at room temperature and embedded in
paraffin at room temperature to be used for immunohistochemistry
(IHC). The remaining samples were immediately stored at −80°C for
RNA and protein extraction.
IHC staining
Placental tissues were cut into 4 µm sections for
IHC analysis. The tissue sections were heated to 60°C for 2 h and
deparaffinized using xylene, and sequentially rehydrated using a
series of graded ethanol (100, 95, 85 and 75%) for 5 min at room
temperature. This was followed by microwave oven heating to a boil
in 10 mM citrate buffer (pH 6.0; Invitrogen; Thermo Fisher
Scientific, Inc.) for 15 min to achieve antigen retrieval. Tissues
were incubated with 3% H2O2 for 15 min at
37°C to suppressed endogenous peroxidase activity. Then, sections
were incubated with a rabbit anti-human FOXO3a monoclonal antibody
(1:800; cat. no. 12829S; Cell Signaling Technology, Inc.),
overnight at 4°C. Negative controls were treated for 2 h with 10 mM
PBS following the same method. Then, tissues were incubated with a
biotin-conjugated secondary antibody (1:200; cat. no. SP-9001;
OriGene Technologies, Inc.) for 1 h at room temperature. The
product obtained using a 3,3′-diaminobenzidine tetrahydrochloride
substrate kit (ZSGB-BIO) was observed for 2–5 min at room
temperature. Counterstaining of the sections were performed using
0.1% hematoxylin for 5 min at room temperature. The staining of the
sections were independently evaluated by two pathologists, and was
based on the estimated staining intensity scale (18) of 0–3: i) 0, No staining and 0–5%,
positive staining; ii) 1, buff staining and 6–25% positive
staining; iii) 2, pale brown staining and 26–75% positive staining;
and iv) 3, sepia staining and 75–100% positive staining. Light
microscopy images were captured at ×200 magnification. The
immunohistochemical score was the positive percentage multiplied by
staining intensity, and was defined as: 0, negative; <4, weakly
positive; 4–8, positive; >8, strong positive.
Cell culture and treatment
The HTR8/SVneo cell line (American Type Culture
Culture) was incubated with DMEM at high glucose (HyClone; GE
Healthcare Life Sciences), supplemented with 10% FBS (Biological
Industries), 100 U/ml ampicillin and 100 U/ml streptomycin at 37°C
in 5% CO2-humidified incubators. HTR8/SVneo cells were
inoculated into 6-well plates (1×105 cells/well). When
cells had grown to reach a fusion of 60%, they were treated with 0,
125, 250 and 500 µmol/l cobalt chloride (cat. no. c8661;
Sigma-Aldrich; Merck KGaA) for 0, 24, 48 and 72 h at 37°C. After
hypoxia treatment, cellular proteins were extracted using RIPA
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.)
and protease inhibitor, and the optimal concentration and time were
determined using western blot analysis, as described below. In the
present study, 250 µmol/l cobalt chloride was selected as the
concentration and 48 h as the duration of hypoxic condition for
follow-up experiments.
Cell viability assay
HTR8/SVneo cells were inoculated into 96-well plates
(5×103 cells/well) and a Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc.) assay was used to determine
cell viability, according to the manufacturer's protocol. Briefly,
when the cells had grown to reach a fusion of 60%, they were
treated with 250 µmol/l cobalt chloride for 0, 24, 48 and 72 h at
37°C. Subsequently, 10 µl CCK-8 solution was added to each well and
incubated at 37°C for 1 h. Absorption values were obtained using a
microplate reader (Bio-Rad Laboratories, Inc.) at 450 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA of tissues and HTR8/SVneo cells was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and RT into cDNA using a ReverTra Ace RT-
qPCR kit (cat. no. 651600; Toyobo Life Sciences) under the
following conditions: 37°C for 15 min, 50°C for 5 min and 98°C for
5 min. SYBR-Green Realtime PCR Master mix (cat. no. 722100; Toyobo
Life Sciences) was used for specific gene amplification on a
StepOnePlus RT PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were
used: Initial denaturation at 95°C for 60 sec, followed by 40
cycles of amplification at 60°C for 15 sec and a final extension
step at 72°C for 45 sec. The primers used for HIF-1α were: Forward,
5′-GCCGCTGGAGACACACAATCAT-3′ and reverse,
5′-TCCATCGGAAGGACTAGGTGT-3′. The primers used for FOXO3a were:
Forward, 5′-GGTGCTAAGCAGGCCTCATCTC-3′ and reverse,
5′-AATGGCGTGGGATTCACAAAG-3′. The primers used for β-actin were:
Forward, 5′-GGGAAATCGTGCGTGACATTAAGG-3′ and reverse,
5′-CAGGAAGGAAGGCTGGAAGAGTC-3′. All results were normalized to the
expression of β-actin. The 2−ΔΔCq method was used to
calculate the relative change of all the target genes (19).
Western blotting
RIPA lysis buffer and protease inhibitor (Beijing
Solarbio Science & Technology Co., Ltd.) were used at a ratio
of 100:1 to lyse the tissues and cells to collect the supernatant.
Protein concentrations were measured using a bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). Soluble
proteins (40 µg) were separated using 8 and 12% SDS-PAGE gel and
transferred onto PVDF membranes. Membranes were then blocked with
5% non-fat milk in TBS-0.1% Tween-20 (TBST) for 2 h at room
temperature and incubated with a rabbit anti-human HIF-1α (1:1,000;
cat. no. ab51608; Abcam), rabbit monoclonal anti-FOXO3a (1:1,000;
cat. no. 12829S; Cell Signaling Technology, Inc.) and rabbit
polyclonal anti-β-actin (1:2,000; cat. no. ab8227; Abcam) overnight
at 4°C. The membranes were incubated at room temperature for 1 h
and washed three times for 10 min with TBST. Subsequently,
membranes were incubated with a horseradish peroxidase-conjugated
secondary antibody (1:10,000; cat. no. IH-0012; Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) for 1 h at room temperature.
The protein bands were visualized using an enhanced
chemiluminescence Plus kit (Beyotime Institute of Biotechnology)
and an AI600 imaging system (GE Healthcare Life Sciences), and the
relative band density was calculated using Adobe Photoshop 13.0
software (Adobe Systems, Inc.) (20).
Small interfering RNA (siRNA)
transfection
Specific siRNAs for HIF-1α (siHIF-1α;
5′-CAATCAAGAAGTTGCATTA-3′) and FOXO3a (siFOXO3a;
5′-GCACAGAGTTGGATGAAGT-3′) were purchased from Guangzhou RiboBio
Co., Ltd. Then, 250 µl RNase-free water was used to the synthesize
siRNAs until they reached a storage concentration of 20 µM.
HTR8/SVneo cells were cultured in 6-well plates
(1×105/well). Cells with 60% confluence were replaced
with fresh culture serum-free medium 2 h prior to transient
transfection. Using a final concentration of 50 nM siHIF-1α,
siFOXO3a and negative control (siNC), 5 µl siRNAs were transfected
into cells using 5 µl Lipofectamine 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h of transfection,
extraction of total RNA or protein of cells was assessed to confirm
efficiency for subsequent experiments.
Cell immunofluorescence
HTR8/SVneo cells were grown in 12-well plates
(5×104 cells/well) and incubated overnight at 37°C.
Then, cells were transfected using the specific siRNA and exposed
to hypoxic conditions for 48 h at 37°C. Cells were rinsed twice for
3 min with ice-cold PBS and fixed in 4% paraformaldehyde for 15 min
at room temperature. Membranal permeabilization was measured using
0.1% Triton X-100 for 15 min at room temperature, prior to
staining. Subsequently, the cells were incubated with a rabbit
antibody HIF-1α (1:100; cat. no. ab51608; Abcam) or rabbit
monoclonal anti-FOXO3a antibody (1:100; cat. no. 12829S; Cell
Signaling Technology, Inc.) for 1 h at 37°C. Slides were washed
with PBS and incubated with the FITC-conjugated secondary antibody
(1:20; cat. no. 65-6111; Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at 37°C. Slides were washed with PBS and stained with
5 µg/ml DAPI (Sigma-Aldrich; Merck KGaA) for 5 min at room
temperature to observe the nuclear translocation of the
transcription factors HIF-1α and FOXO3a. Images were captured with
a NIKON Eclipse Ci fluorescent microscope (magnification,
×400).
Cell apoptosis
HTR8/SVneo cells were grown in a 6-well plate
(5×104 cells/well) and cultured overnight. Cells were
then transfected with 50 nM siRNA-HIF-1α, siFOXO3a or siNC using
Lipofectamine 3000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and incubated at 37°C for 24 h. Cells were digested using 1X
TrypLE Express (Gibco; Thermo Fisher Scientific, Inc.) and
resuspended in a 1X binding buffer (BD Biosciences). Then, the
cells were double-stained with 20 µg/ml Annexin V-FITC and PI (BD
Biosciences) for 15 min at room temperature. Cell apoptosis was
determined using an Epics XL. MCL flow cytometer (Beckman Coulter,
Inc.) and analyzed using FlowJo version 10.6 software (FlowJo LLC)
(21).
Statistical analysis
All statistical analyses were performed using the
SPSS 21.0 (IBM Corp) and GraphPad Prism 6.0 software (GraphPad
Software, Inc.) (22). Data are
presented as the mean ± SD, or the medians and interquartile
ranges. Data between two of groups with normal distribution were
compared using independent samples t-test, while data between two
groups with a skewed distribution were compared using a Mann
Whitney U test. The immunohistochemical staining intensity of
FOXO3a expression levels were determined using a χ2
test. A Kruskal Wallis test with a Bonferroni's correction post hoc
test was used to analyze multiple groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
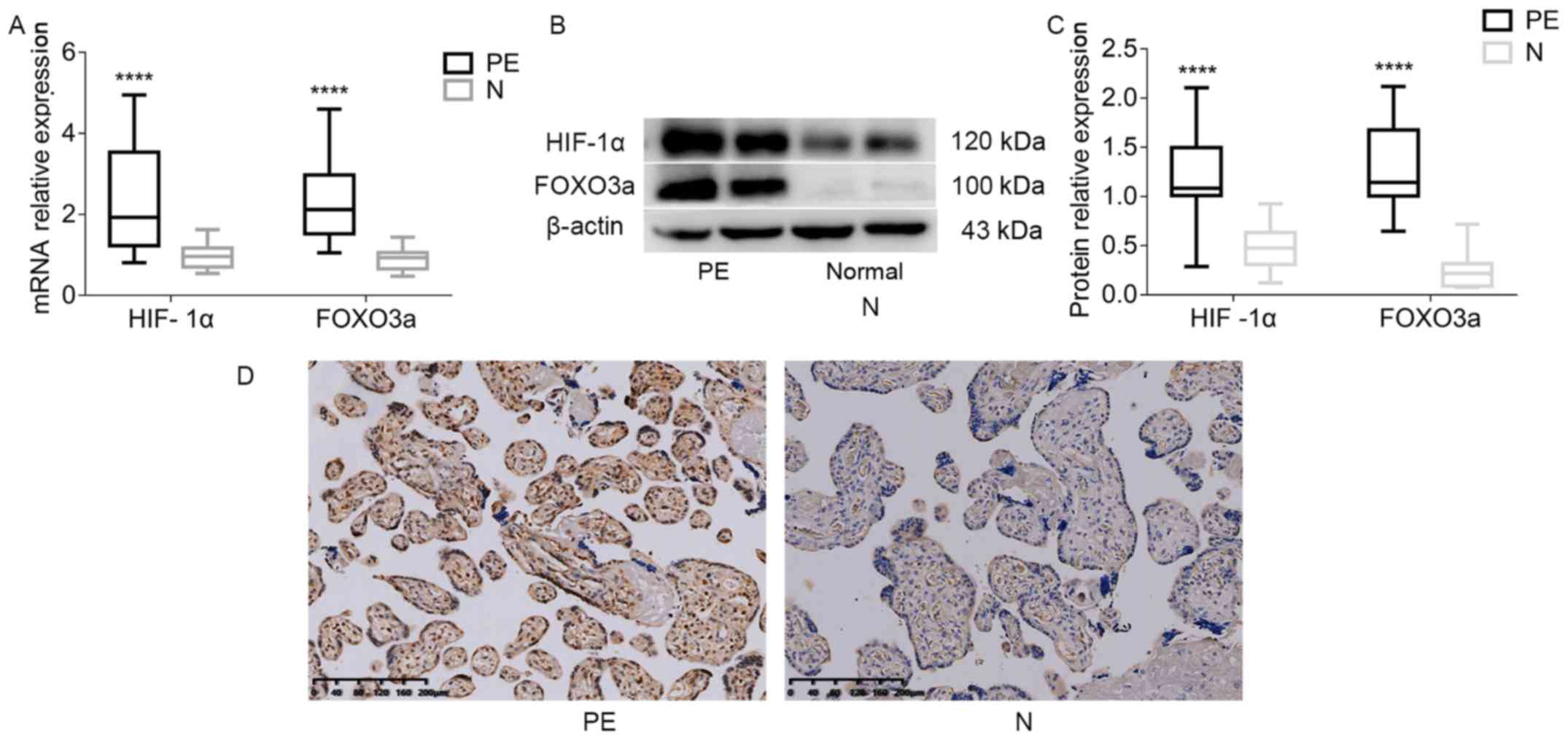
HIF-1α and FOXO3a expression in the
placental tissues of patients with PE is higher compared with
healthy pregnant women
HIF-1α and FOXO3a at mRNA level in placental tissues
were detected using RT-qPCR. It was found that the mRNA expression
levels of HIF-1α and FOXO3a in the PE group were significantly
increased compared with the healthy control group (Fig. 1A). HIF-1α and FOXO3a protein levels
were analyzed using western blotting, and it was demonstrated that
the protein expression levels of both HIF-1α and FOXO3a were
increased in the PE group (Fig. 1B and
C). Subsequently, the location of FOXO3a expression in the
placenta was assessed using IHC staining. The results suggested
that the trophoblast expression of FOXO3a was located in the
cytoplasm and nucleus (Fig. 1D).
Moreover, the staining intensity of FOXO3a was higher in the PE
placental tissues compared with healthy controls
(χ2=22.31; P<0.001; Table II).
 | Table II.Immunostaining of Forkhead box O
transcription factor 3a in control and early onset preeclampsia
groups. |
Table II.
Immunostaining of Forkhead box O
transcription factor 3a in control and early onset preeclampsia
groups.
| Immunohistochemical
staining | Group | None | Weak | Moderate | Strong | χ2 | P-value |
|---|
| FOXO3a | Control (n=30) | 7 | 19 | 3 | 1 | 22.31 | <0.001 |
|
| PE (n=30) | 2 | 5 | 13 | 10 |
|
|
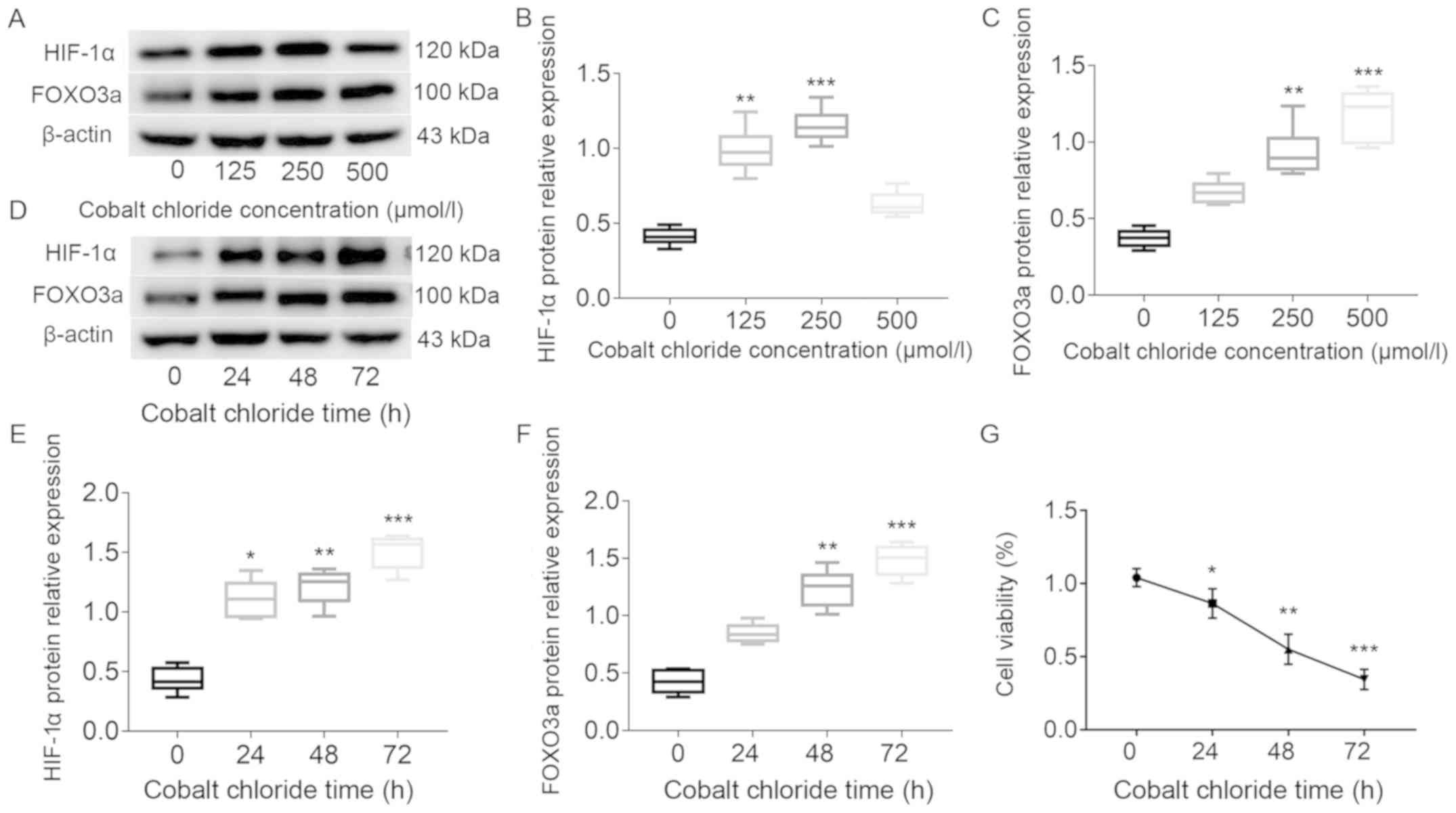
Cobalt chloride-induced hypoxia leads
to increased protein expression levels of HIF-1α and FOXO3a in
HTR8/SVneo cells
HTR8/SVneo cells were cultured in 0, 125, 250 and
500 µmol/l cobalt chloride. Western blot analysis results
identified that the expression levels of HIF-1α and FOXO3a were
significantly elevated after treatment with cobalt chloride
(Fig. 2A-C). However, it was found
that the expression of HIF-1α was decreased in 500 µmol/l cobalt
chloride. Moreover, the protein expression of both HIF-1α and
FOXO3a gradually increased with longer treatment periods with 250
µmol/l cobalt chloride. (Fig.
2D-F). The present results also indicated that cell viability
was significantly decreased with increasing treatment duration,
especially when treatment time was >24 h (Fig. 2G). Therefore, based on the cell
viability at ~50%, 250 µmol/l cobalt chloride for 48 h was used to
induce hypoxic conditions.
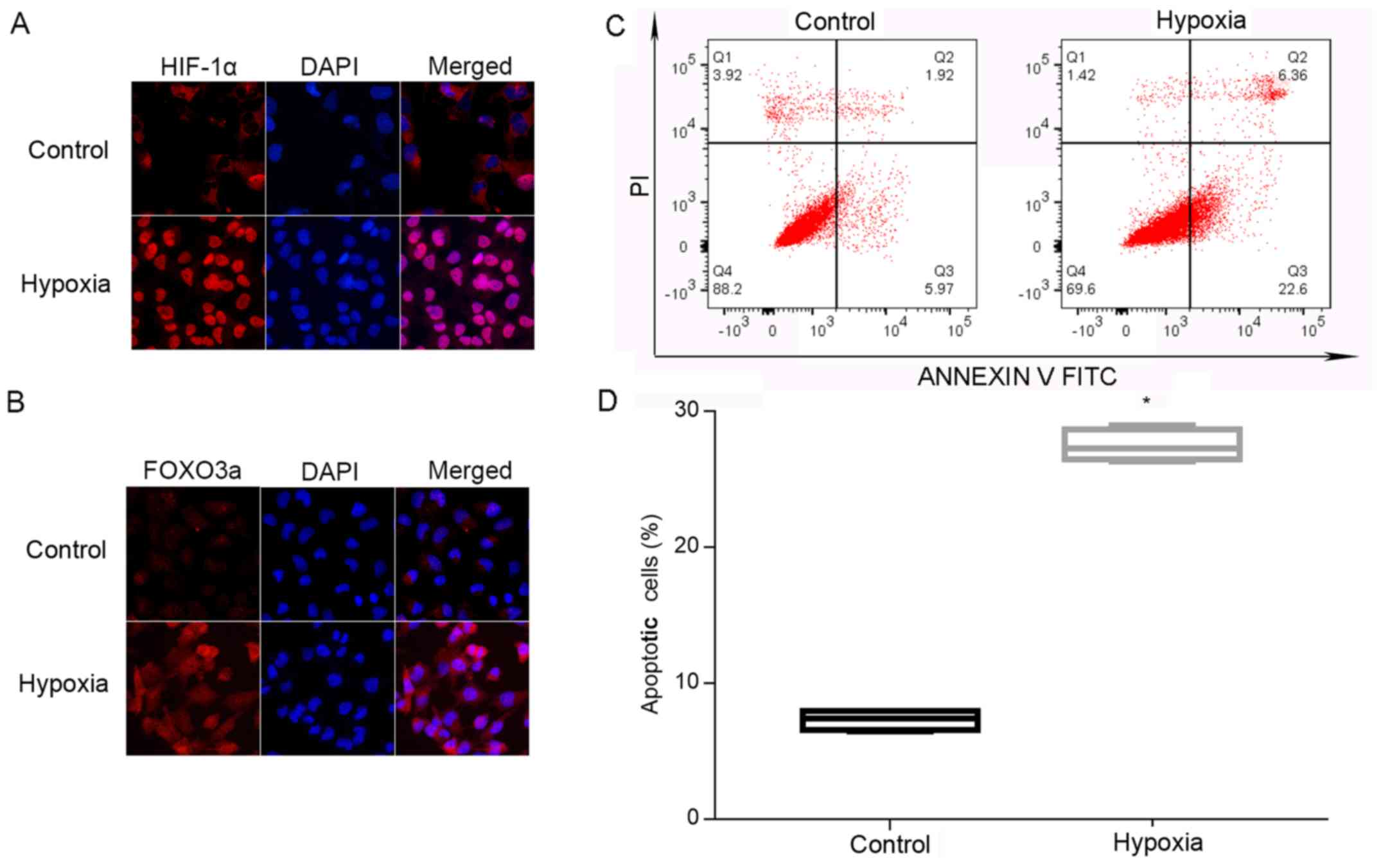
Hypoxia induces nuclear translocation
of HIF-1α and FOXO3a, and increases the rate of apoptosis in
HTR8/SVneo cells
In order to observe intracellular localization of
HIF-1α and FOXO3a, HTR8/SVneo cells were cultured under normal and
hypoxic conditions. Using cell immunofluorescence analysis, it was
demonstrated that the expression of HIF-1α and FOXO3a was at low
levels in the cytoplasm under normal conditions. Furthermore,
nuclear translocation of these factors was significantly increased
during hypoxia (Fig. 3A and B).
Using flow cytometry analysis, it was identified that the rate of
apoptosis of HTR8/SVneo cells was significantly enhanced during
hypoxia (Fig. 3C and D).
Therefore, the present results suggested that hypoxia may cause
nuclear translocation of HIF-1α and FOXO3a in HTR8/SVneo cells, and
augments apoptosis of trophoblasts.
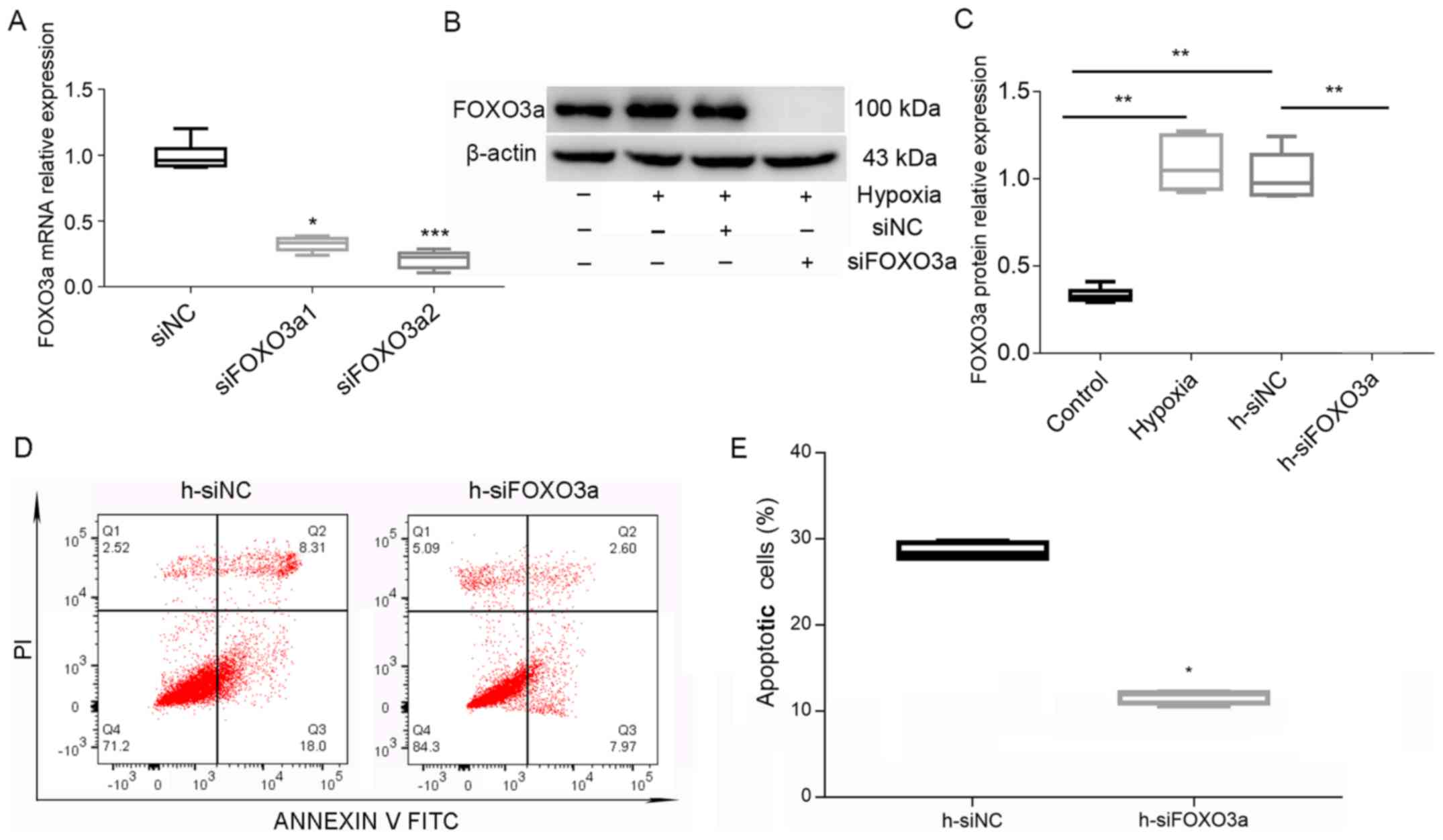
Knockdown of FOXO3a suppresses
hypoxia-induced apoptosis in HTR8/SVneo cells
In order to verify the knockdown efficiency of
FOXO3a, the mRNA expression of FOXO3a after transfection with a
siRNA was measured. Based on the results, siFOXO3a2 was used in
follow-up experiments (Fig. 4A) to
knockdown FOXO3a in HTR8/SVneo cells. Subsequently, western blot
analysis results identified a decrease in the expression of FOXO3a
after transfection with siRNA (Fig. 4B
and C). However, flow cytometry analysis demonstrated that
knockdown of FOXO3a suppressed trophoblastic apoptosis (Fig. 4D and E). Collectively, the present
results suggested that knockdown of FOXO3a could suppress
hypoxia-induced apoptosis of HTR8/SVneo cells.
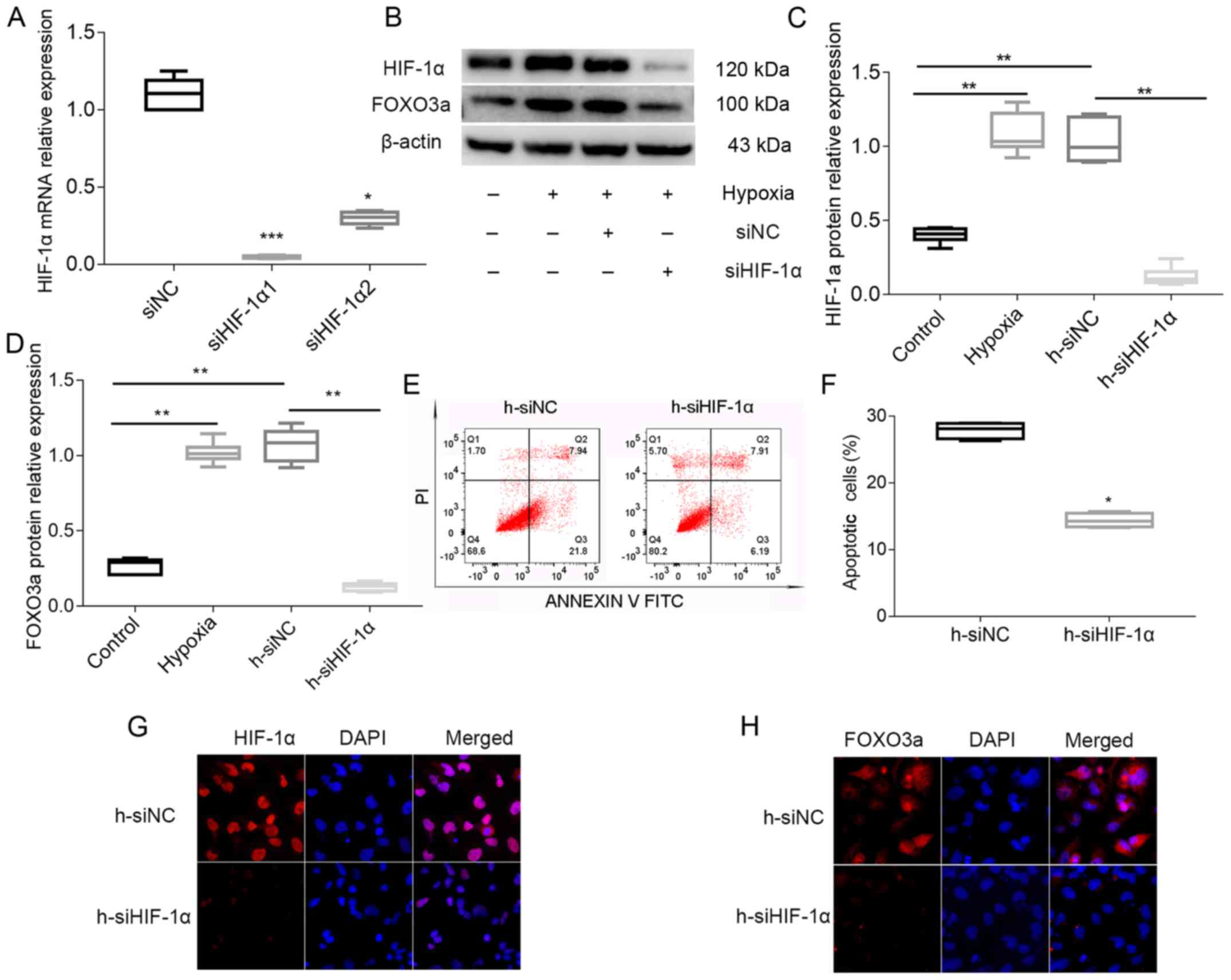
Under hypoxia, HIF-1α affects the
apoptosis of trophoblast cells by regulating the expression of
FOXO3a in HTR8/SVneo cells
In order to measure the knockdown efficiency of
HIF-1α, the mRNA expression of HIF-1α was detected after
transfection with siRNA. Based on the results, siHIF-1α1 was used
in follow-up experiments (Fig. 5A)
to knockdown HIF-1α in HTR8/SVneo cells. It was found that the
protein expression levels of HIF-1α and FOXO3a were significantly
decreased following the transfection with siHIF-1α1 (Fig. 5B-D). Flow cytometry analysis
indicated that knockdown of HIF-1α suppressed trophoblastic
apoptosis (Fig. 5E and F).
Furthermore, cell immunofluorescence results demonstrated that
knockdown of HIF-1α decreased the expression and nuclear
translocation of FOXO3a under hypoxic conditions (Fig. 5G and H). Therefore, the present
results suggested that knockdown of HIF-1α repressed the expression
of FOXO3a and the apoptosis of trophoblasts. Thus, it is speculated
that HIF-1α affects apoptosis of trophoblasts by regulating the
expression of FOXO3a under hypoxia in HTR8/SVneo cells.
Discussion
Previous findings have shown that oxygen tension may
regulate cytotrophoblast proliferation and differentiation, which
affects the development of the placenta (23). At 8–10 weeks of gestation, the
placenta undergoes a physiological hypoxic phase in which
trophoblasts continue proliferating and are poorly differentiated
(24,25). Afterwards, along with an increase
in oxygen pressure, trophoblast cells begin to differentiate
normally and invasion increases (25). When spiral artery remodeling is
complete, adequate maternal blood supply can be provided to the
placenta (26). However, long-term
hypoxia causes an increase in the apoptosis of trophoblasts and a
shallow cell invasion of the uterus, which can result in the
development of PE (27). HIF-1α is
a master regulator of oxygen homeostasis and can regulate diverse
cellular functions (28). It has
been revealed that the expression of HIF-1α increases in placental
tissues in PE (29). Previous
findings have also shown that under hypoxic conditions, the
increase in HIF-1α expression initiates hypoxia-mediated apoptosis
by affecting the expression of downstream molecules (30). This is consistent with the present
results, in which the expression of HIF-1α was found to be
elevated. The FOXO transcription factor family are thought to be
tumor suppressors that mainly regulate the cell cycle, apoptosis,
DNA-damage repair and response to oxidative stress (31,32).
FOXO3a is a member of the FOXO family, and its expression is
enhanced under hypoxia (33). The
present results suggested that the expression of FOXO3a was
significantly enhanced in placental tissues in PE. Moreover,
previous studies have found that HIF-1α can affect the apoptosis of
cardiomyocytes and neurons by regulating the expression of FOXO3a
(33,34). Therefore, it is hypothesized that
HIF-1α may influence the involvement of hypoxia-induced apoptosis
in the development of PE by regulating the expression of
FOXO3a.
The present study used cobalt chloride to construct
a hypoxic model. Furthermore, cobalt chloride-induced hypoxia is
able to cause a high expression level of HIF-1α (35). It has also been shown that the
expression of HIF-1α is increased in HTR8/SVneo cells under hypoxia
and in placental tissues in PE (36). Therefore, the present study
investigated the effect of HIF-1α on downstream gene expression and
cell functions under hypoxic conditions.
FOXO3a is upregulated under hypoxic conditions, and
translocation of FOXO3a from the cytoplasm to the nucleus is
induced by serum starvation and hypoxia (33). Hypoxia-activated FOXO3a can also
promote the apoptosis of cardiomyocytes (37). A previous study showed that the
siRNA knockdown of FOXO3a reduces the protein expression levels of
FOXO3a and Bim, as well as inhibiting the apoptosis of HUVECs
(16). In the present study, it
was also found that hypoxia could increase the expression levels of
FOXO3a and the rate of apoptosis in HTR8/SVneo cells, whereas
inhibiting the expression levels of FOXO3a with siRNA reduced the
rate of hypoxia-induced trophoblast apoptosis. Under hypoxic
conditions, the HIF-1α subunit becomes stable and interacts with
coactivators, such as p300/CREB binding protein, to promote its
nuclear transcriptional activity (38). Moreover, the present results
suggested that the nuclear expression of HIF-1α was elevated under
hypoxia. In addition, suppression of HIF-1α using siRNA is able to
decrease the expression of FOXO3a, hypoxia-induced reactive oxygen
species accumulation and apoptosis of HUVECs (16). Based on the present result that
knockdown of FOXO3a attenuated the apoptosis rate in HTR8/SVneo
cells, it is speculated that the hypoxia-induced increase in the
expression of HIF-1α and the decrease in trophoblast apoptosis
caused by HIF-1α knockdown may be regulated by the expression of
FOXO3a.
In conclusion, under hypoxia, elevated expression of
HIF-1α leads to an increase in trophoblastic apoptosis via the
regulation of FOXO3a, which may be involved in the decrease
infiltration ability observed in the pathogenesis of PE.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Fund for Innovation Leading Research Team of Zhengzhou
City (grant no. 131PCXTD624).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CH and ZZ conceived and designed the study. CH, PW,
JG and YS performed the experiments and analyzed the data. XL, YL
and SY performed sample and data acquisition. CH and ZZ wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics Review
Committee of the Third Affiliated Hospital of Zhengzhou University
and informed consent was obtained from all the patients (ID no.
2015023).
Patient consent for publication
All patients within the present study provided
consent for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Verma S, Pillay P, Naicker T, Moodley J
and Mackraj I: Placental hypoxia inducible factor-1α and CHOP
immuno-histochemical expression relative to maternal circulatory
syncytiotrophoblast micro-vesicles in preeclamptic and normotensive
pregnancies. Eur J Obstet Gynecol Reprod Biol. 220:18–24. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fox R, Kitt J, Leeson P, Aye CYL and
Lewandowski AJ: Preeclampsia: Risk factors, diagnosis, management,
and the cardiovascular impact on the offspring. J Clin Med.
8:E16252019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Travaglino A, Raffone A, Saccone G,
Migliorini S, Maruotti GM, Esposito G, Mollo A, Martinelli P, Zullo
F and D'Armiento M: Placental morphology, apoptosis, angiogenesis
and epithelial mechanisms in early-onset preeclampsia. Eur J Obstet
Gynecol Reprod Biol. 234:200–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kedia K, Smith SF, Wright AH, Barnes JM,
Tolley HD, Esplin MS and Graves SW: Global ‘omics’ evaluation of
human placental responses to preeclamptic conditions. Am J Obstet
Gynecol. 215:238.e1–238.e20. 2016. View Article : Google Scholar
|
|
5
|
Kadyrov M, Kingdom JC and Huppertz B:
Divergent trophoblast invasion and apoptosis in placental bed
spiral arteries from pregnancies complicated by maternal anemia and
early-onset preeclampsia/intrauterine growth restriction. Am J
Obstet Gynecol. 194:557–563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tal R: The role of hypoxia and
hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol
Reprod. 87:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caniggia I and Winter JL: Adriana and
Luisa castellucci award lecture 2001. Hypoxia inducible factor-1:
Oxygen regulation of trophoblast differentiation in normal and
pre-eclamptic pregnancies-a review. Placenta. 23 (Suppl A):S47–S57.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Hypoxia-inducible factor 1:
Control of oxygen homeostasis in health and disease. Pediatr Res.
49:614–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albers RE, Kaufman MR, Natale BV, Keoni C,
Kulkarni-Datar K, Min S, Williams CR, Natale DRC and Brown TL:
Trophoblast-specific expression of hif-1α results in
preeclampsia-like symptoms and fetal growth restriction. Sci Rep.
9:27422019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Depoix CL, de Selliers I, Hubinont C and
Debieve F: HIF1A and EPAS1 potentiate hypoxia-induced upregulation
of inhibin alpha chain expression in human term cytotrophoblasts in
vitro. Mol Hum Reprod. 23:199–209. 2017.PubMed/NCBI
|
|
12
|
Bakker WJ, Harris IS and Mak TW: FOXO3a is
activated in response to hypoxic stress and inhibits HIF1-induced
apoptosis via regulation of CITED2. Mol Cell. 28:941–953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fasano C, Disciglio V, Bertora S, Lepore
Signorile M and Simone C: FOXO3a from the nucleus to the
mitochondria: A round trip in cellular stress response. Cells.
8(pii): E11102019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brucker DP, Maurer GD, Harter PN, Rieger J
and Steinbach JP: FOXO3a orchestrates glioma cell responses to
starvation conditions and promotes hypoxia-induced cell death. Int
J Oncol. 49:2399–2410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Zhao Y, Xu M, Yu L, Zhao Y, Chen
J, Yuan Y, Zheng Q and Niu X: FoxO3a modulates hypoxia stress
induced oxidative stress and apoptosis in cardiac microvascular
endothelial cells. PLoS One. 8:e803422013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Z, Wang F, Wu Z, Gu H, Dong N, Jiang X,
Xu J, Wu Z, Wechsler DS and Zheng D: FOXO3a-dependent up-regulation
of Mxi1-0 promotes hypoxia-induced apoptosis in endothelial cells.
Cell Signal. 51:233–242. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kallela J, Jääskeläinen T, Kortelainen E,
Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A and Laivuori H:
The diagnosis of pre-eclampsia using two revised classifications in
the Finnish Pre-eclampsia Consortium (FINNPEC) cohort. BMC
Pregnancy Childbirth. 16:2212016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yilmaz E, Gul M, Melekoglu R and Koleli I:
Immunhistochemical analysis of nuclear factor kappa beta expression
in etiopathogenesis of ovarian tumors1. Acta Cir Bras. 33:641–650.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rajão MD, Leite CS, Nogueira K, Godoy RF
and Lima EMM: The bone response in endurance long distance horse.
Open Vet J. 9:58–64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El Yazouli L, Seghrouchni F, Hejaji H,
Bouazza M, Alami AA, Dakka N and Radouani F: Cell-mediated immune
response associated with Chlamydia pneumoniae infection in
atherosclerotic patients. Microb Pathog. 139:1038602020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue JJ, Wang TQ, Jia YQ, Xiao Y, Tian MH,
Guan DW, Zhang GH, Wu X, Li RB, Zhao R, et al: Statistical analysis
of the heart and lung mass in forensic anatomical cases and its
forensic significance. Fa Yi Xue Za Zhi. 35:651–656. 2019.(In
Chinese). PubMed/NCBI
|
|
23
|
Genbacev O, Zhou Y, Ludlow JW and Fisher
SJ: Regulation of human placental development by oxygen tension.
Science. 277:1669–1672. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Li P, Wang Y and Yan H:
Hypoxia-induced expression of CXCR4 favors trophoblast cell
migration and invasion via the activation of HIF-1α. Int J Mol Med.
42:1508–1516. 2018.PubMed/NCBI
|
|
25
|
Szpilbarg N, Seyahian A, Paola MD,
Castro-Parodi M, Martinez N, Farina M and Damiano AE: Oxygen
regulation of aquaporin-4 in human placenta. Reprod Biomed Online.
37:601–612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huppertz B, Kadyrov M and Kingdom JC:
Apoptosis and its role in the trophoblast. Am J Obstet Gynecol.
195:29–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DiFederico E, Genbacev O and Fisher SJ:
Preeclampsia is associated with widespread apoptosis of placental
cytotrophoblasts within the uterine wall. Am J Pathol. 155:293–301.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park MJ, Lee DH, Joo BS, Lee YJ, Joo JK,
An BS, Kim SC and Lee KS: Leptin, leptin receptors and
hypoxia-induced factor-1alpha expression in the placental bed of
patients with and without preeclampsia during pregnancy. Mol Med
Rep. 17:5292–5299. 2018.PubMed/NCBI
|
|
30
|
Greijer AE and van der Wall E: The role of
hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J
Clin Pathol. 57:1009–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dansen TB and Burgering BM: Unravelling
the tumor-suppressive functions of FOXO proteins. Trends Cell Biol.
18:421–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferber EC, Peck B, Delpuech O, Bell GP,
East P and Schulze A: FOXO3a regulates reactive oxygen metabolism
by inhibiting mitochondrial gene expression. Cell Death Differ.
19:968–979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YF, Pandey S, Day CH, Chen YF, Jiang
AZ, Ho TJ, Chen RJ, Padma VV, Kuo WW and Huang CY: Synergistic
effect of HIF-1α and FoxO3a trigger cardiomyocyte apoptosis under
hyperglycemic ischemia condition. J Cell Physiol. 233:3660–3671.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero
D and Mu D: Involvement of the PTEN-AKT-FOXO3a pathway in neuronal
apoptosis in developing rat brain after hypoxia-ischemia. J Cereb
Blood Flow Metab. 29:1903–1913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
An WG, Kanekal M, Simon MC, Maltepe E,
Blagosklonny MV and Neckers LM: Stabilization of wild-type p53 by
hypoxia-inducible factor 1alpha. Nature. 392:405–408. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhi Z, Yang W, Liu L, Jiang X and Pang L:
Early missed abortion is associated with villous angiogenesis via
the HIF-1α/VEGF signaling pathway. Arch Gynecol Obstet.
298:537–543. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng CC, Lin CC, Lai YP, Chen TS,
Marthandam Asokan S, Lin JY, Lin KH, Viswanadha VP, Kuo WW and
Huang CY: Hypoxia suppresses myocardial survival pathway through
HIF-1α-IGFBP-3-dependent signaling and enhances cardiomyocyte
autophagic and apoptotic effects mainly via FoxO3a-induced BNIP3
expression. Growth Factors. 34:73–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|