Introduction
Rheumatoid arthritis (RA) is a complicated disease
caused by environmental and genetic factors that involves a
hyperactive immune system and synovial inflammation in multiple
joints, which if left untreated irreversibly destroys joints and
leads to severe disability (1).
The common drugs used to treat RA, including methotrexate, cannot
prevent the disease and cause severe complications in many patients
(2); therefore, the development of
safer, cost-effective therapeutics is required.
RA involves the action of pro-inflammatory cytokines
and chemokines produced by synoviocytes and infiltrating immune
cells (3), including interleukin
(IL)-1, IL-2, IL-3, IL-4, IL-6, IL-8, IL-17, interferon (IFN)-α and
IFN-β, tumor necrosis factor (TNF)-α, transforming growth factor β,
granulocyte-macrophage colony-stimulating factor and macrophage
inflammatory protein (MIP)-3α (2,4–7).
These cytokines and chemokines are able to activate NF-κB,
upregulate the expressions of cyclooxygenase-2 (COX-2) and nitric
oxide synthase, and promote the production of prostaglandin E2
(PGE2) and nitric oxide. Moreover, these changes contribute to
synovial inflammation accompanied by arthrosis, swelling,
hyperplasia, angiogenesis, bone destruction and arthritic decay
(8,9). Natural products that target these
molecules are less likely to induce adverse effects may be used as
therapeutic agents owing to their high therapeutic potential
(2,10–13).
For example, bioactive natural compounds extracted from plants,
such as polyphenolic compounds, commonly exert multiple therapeutic
effects (14–17).
Tripterygium wilfordii hook (also known as
thunder god vine) is a common plant species, which has been used
for a variety of purposes in traditional Chinese medicine (TCM)
(18). Previous studies in the TCM
literature suggest that T. wilfordii can treat several
autoimmune and inflammatory conditions including RA, and
improvements to its efficacy and safety have been made (14,19).
Currently, >380 secondary metabolites have been
isolated from T. wilfordii, and ≥350 of these are
structurally diverse terpenoids with a wide range of
pharmacological activities, including anti-inflammatory,
immunosuppressive and antineoplastic effects (20). It has been shown that two
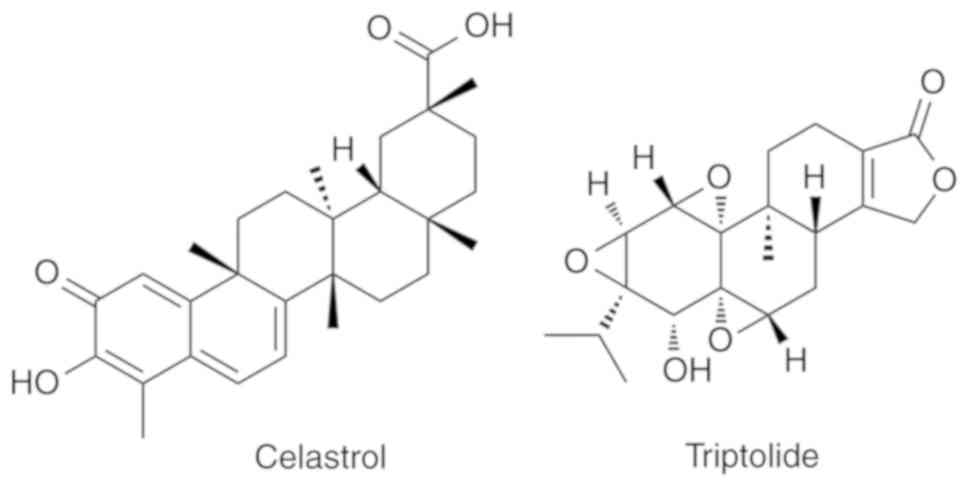
diterpenoid tri-epoxides, celastrol and triptolide (Fig. 1), are primarily responsible for the
anti-inflammatory and immunosuppressive effects of T.
wilfordii preparations (21,22).
Furthermore, these two compounds are the most abundant and most
pharmacologically active of the metabolites found in T.
wilfordii extracts (23).
The present study critically reviews the chemistry,
bioavailability, bioactivities, multi-target toxicities and
molecular targets of celastrol and triptolide for the treatment of
RA (Fig. 2; Table I). The present review also
discussing future directions for research into the aforementioned
promising natural products.
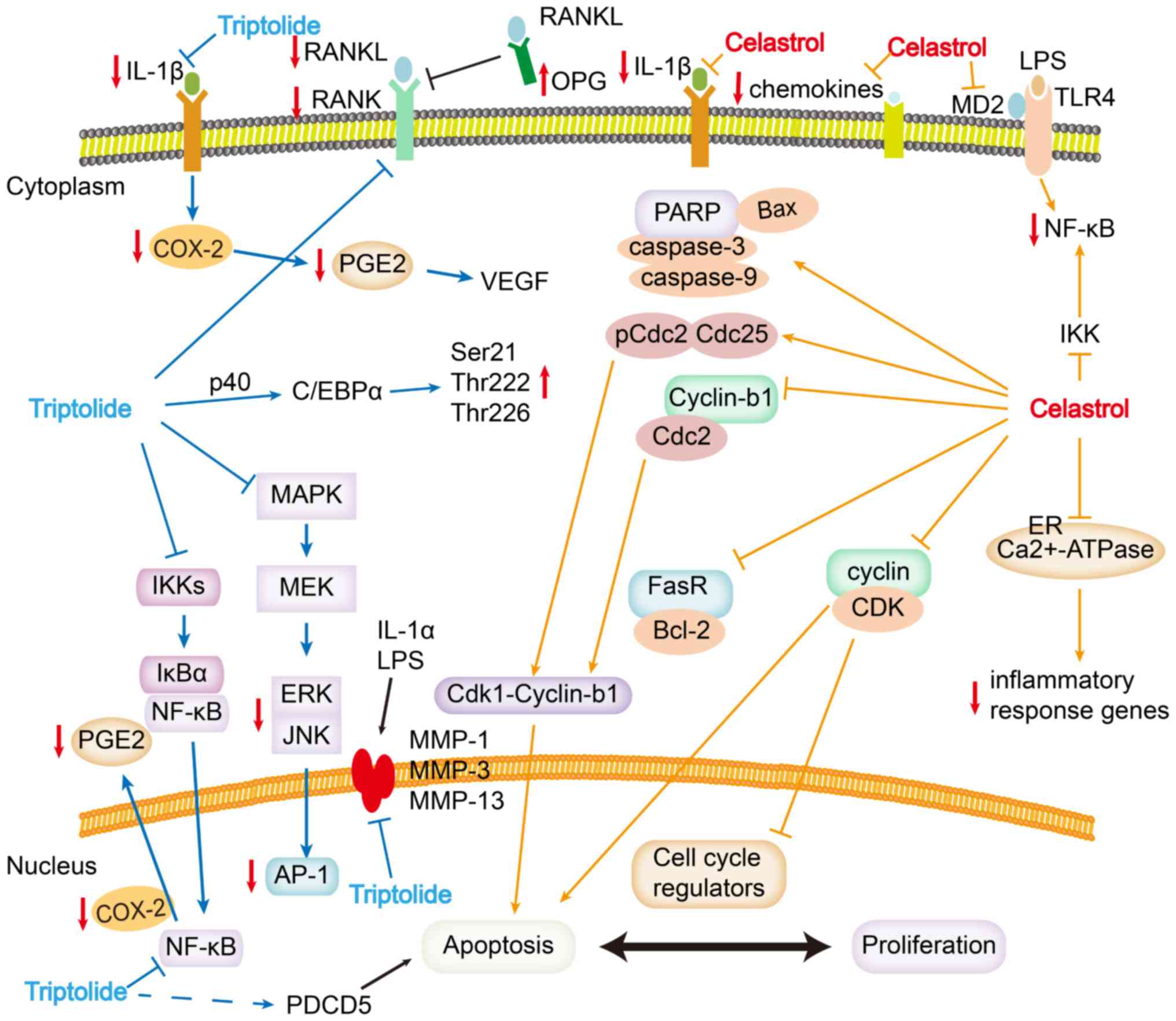 | Figure 2.Schematic of the molecular targets of
celastrol and triptolide in the treatment of RA. Arrows indicate
activating effects; horizontal lines indicate inhibitory effects.
Celastrol targets numerous signaling pathways associated with RA,
including those involving NF-κB, endoplasmic reticulum
Ca2+-ATPase, MD2, TLR4, pro-inflammatory chemokines, DNA
damage, cell cycle arrest and apoptosis. Triptolide inhibits NF-κB,
RANKL/RANK/OPG signaling, COX-2, matrix metalloproteases and
cytokines. AP-1, activating protein-1; C/EBPα, CCAAT/enhancer
binding protein-α; cdc, cell division cycle protein; COX-2,
cyclooxygenase-2; ER, endoplasmic reticulum; IκB, inhibitor of
NF-κB; IKK, IκB kinase; IL, interleukin; LPS, lipopolysaccharide;
MAPK, mitogen-activated protein kinase; MD2, myeloid
differentiation factor 2; MEK, MAPK/ERK kinase; MMP, matrix
metalloproteinase; OPG, osteoprotegerin; p, phosphorylated; PARP,
poly (ADP-ribose) polymerase; PDCD5, programmed cell death protein
5; PGE2, prostaglandin E2; RA, rheumatoid arthritis; RANK, receptor
activator of NF-κB; RANKL, RANK ligand; TLR4, Toll-like receptor 4;
VEGF, vascular endothelial growth factor. |
 | Table I.Molecular targets, signaling pathways
and potential biological effects of celastrol and triptolide in the
treatment of rheumatoid arthritis. |
Table I.
Molecular targets, signaling pathways
and potential biological effects of celastrol and triptolide in the
treatment of rheumatoid arthritis.
| A, Celastrol |
|---|
| Molecular
targets | Signaling
pathways | Potential
biological effects | (Refs.) |
|---|
| IκB kinase | NF-κB | NF-κB function is
regulated via rapid degradation of its endogenous inhibitory
molecule IκB | (32–39) |
|
Ca2+-ATPase | Ca2+
signaling | Alter
Ca2+ signaling pathways to downregulate inflammatory
response genes | (40–44) |
| Cyclins with
CDKs | DNA damage, cell
cycle arrest and apoptosis | Inhibit cell cycle
progression by blocking the association of cyclins with
cyclin-dependent kinases | (33,45–47) |
| MD2 or TLR4 | Interaction between
LPS and the TLR4/MD2 complex | Block the most
upstream step in TLR4 activation, maybe targets MD2. | (48–51) |
| Pro-inflammatory
chemokines | Reduces expression
levels of RANTES, MCP-1, MIP-1α and GRO/KC, as well as the
pro-inflammatory cytokines tumor necrosis factor-α and IL-1β | Inhibit leukocyte
migration into joints | (4,5,51–55) |
|
| B,
Triptolide |
|
| Molecular
targets | Signaling
pathways | Potential
biological effects | (Refs.) |
|
| IκB kinase | NF-κB | Rapid degradation
of its endogenous inhibitory molecule IκB | (56–64) |
| RANKL | RANKL/RANK/OPG
signaling | Reduce the number
of osteoclasts in areas of bone destruction by downregulating RANKL
and RANK, and upregulating OPG | (65–68) |
| COX-2 and matrix
metalloproteinases | NF-κB | Downregulate COX-2
and PGE2, and alleviate LPS-induced inflammation | (69–79) |
| Cytokines | Cytokines
pathway | Inhibit
cytokines | 80–83 |
| VEGF | Infiltration of the
synovial membrane | Inhibit several
downstream effects of IL1-β, including cell adhesion of human FLS,
upregulate several angiogenic activators and activate MAPK
signaling | (5,84–86) |
Metabolism and bioavailability
Oral administration of triptolide is recommended for
to treat inflammation, autoimmune diseases and tumors (24). In rats, triptolide at a dose of 1
mg/kg exhibits a bioavailability of 81.6% after intravenous
injection and 63.9% after oral administration (25). Moreover, it achieves a maximum
concentration of 293.19±24.43 ng/ml within ~10 min. Triptolide
distributes into the liver, followed by plasma, kidney, lung,
spleen, heart and testicular tissue, and is quickly excreted
through the biliary, urinary and fecal routes with a half-life of
0.42 h (26). Moreover, the
metabolism and bioavailability of triptolide is not fully
understood as clinical trials investigating the use of triptolide
to treat RA have not been performed (27,28).
Celastrol has low aqueous solubility (13.25±0.83
µg/ml at 37°C) and poor intestinal absorption, which results in low
oral bioavailability and limits its clinical application (29). To overcome these constraints, the
compound can be delivered using solid lipid nanoparticles,
liposomes, micelles or nanostructured lipid carriers (30,31).
Molecular targets of celastrol
Celastrol inhibits activation of NF-κB
by targeting inhibitor of NF-κB (IκB) kinase
NF-κB regulates the transcription of numerous genes
that are involved in immune, inflammatory and anti-apoptotic
responses (32). Furthermore, the
function of NF-κB is regulated by rapid degradation of its
endogenous inhibitory molecule IκB. Inflammatory stimuli, such as
cytokines, initiate a signaling cascade that leads to the
activation of two IκB kinases (IKK), IKK-1 and IKK-2, which then
phosphorylate IκB at two N-terminal serine residues (33). The IKK complex is expressed in
fibroblast-like synoviocytes (FLS) and is activated by IL-1 and
TNF-α (34–36). These studies demonstrated that
celastrol suppresses NF-κB activation by inhibiting IKK activity,
possibly by targeting cysteine 179 in the activation loop of IKK
(34,35,37).
Moreover, celastrol inhibits the activation of NF-κB by targeting
IκB kinase, which is not specific in the RA (38). It has been revealed that the NF-κB
signaling pathway is involved in a number of diseases, including
RA, systemic lupus erythematosus and ankylosing spondylitis
(32,34–37).
Moreover, myeloid differentiation factor 2 (MD2) and Toll-like
receptor 4 (TLR4) are associated with RA, thus it is likely that
celastrol targets MD2 (39).
Celastrol inhibits endoplasmic
reticulum (ER) Ca2+-ATPase
In RA, Ca2+ signaling mediates the
expression of pro-inflammatory cytokines through autoreactive T-
and B-lymphocytes after autoantigen stimulation (40). Various pumps maintain
Ca2+ homeostasis, among which is the ER
Ca2+-ATPase, which can be inhibited by celastrol
(41). Therefore, celastrol may
alter Ca2+ signaling pathways to downregulate
inflammatory response genes (42)
and promote Ca2+-mediated autophagic cell death
(43,44), as shown by studies in RA-FLS. Thus,
it is speculated that resistance of FLS to apoptosis may be a
characteristic of RA.
Celastrol induces DNA damage, cell
cycle arrest and apoptosis
Celastrol blocks RA-FLS at G2/M phase (45), which may be a potential mechanism
for its ability to inhibit proliferation and induce apoptosis. It
has also been demonstrated that celastrol inhibits cell cycle
progression by blocking the association of cyclins with
cyclin-dependent kinases (33).
Furthermore, celastrol leads to an increase in cell division cycle
protein 2 homolog (Cdc2) phosphorylation and downregulation of Cdc2
and Cyclin-b1, which may reduce the number of Cdk1-Cyclin-b1
complexes and arrest cells at the G2/M phase (45). Celastrol also increases
phosphorylation of Cdc25, which may contribute to G2/M phase arrest
(46).
It has been shown that celastrol activates cleaved
caspases 3 and 9, as well as cleaved poly (ADP-ribose) polymerase,
downregulates FasR and increases the Bax/Bcl-2 ratio (46). Therefore, celastrol may induce
apoptosis in RA-FLS, which express a variety of death-inducing
surface receptors of the TNF receptor family such as Fas/CD95,
TNF-related apoptosis-inducing ligand-receptor (TRAIL-R1), TRAIL-R2
and TNF receptor 1 (47).
Celastrol targets MD2 and inhibits
TLR4 activation
TLR4 exists as a complex with a co-receptor, MD2, in
the plasma membrane of various immune cells (48). Celastrol blocks the most upstream
step in TLR4 activation (49), and
thus it likely targets MD2. Moreover, celastrol may function
similar to the anti-inflammatory phytochemicals sulforaphane and
caffeic acid phenethyl ester, which interfere with the interaction
between lipopolysaccharide (LPS) and the TLR4/MD2 complex (50,51).
Furthermore, it is speculated that celastrol may have intracellular
targets, including MD2 and TLR4 (48,49).
Celastrol modulates pro-inflammatory
chemokines
Chemokines are a superfamily of cytokines that are
associated with cell migration and recruitment to sites of
inflammation (5). Chemokines are
categorized into four groups, CXC, CX3C, CC and C, based on the
location of conserved cysteine residues (21). In inflammatory disorders such as
RA, chemokines bind to their receptors leading to leukocyte
trafficking into the joints, where leukocytes exacerbate
inflammation and lead to pannus formation and tissue damage
(4). Moreover, several chemokines
have been implicated in RA and experimental arthritis, including T
cell specific protein RANTES [RANTES; also known as C-C motif
chemokine 5 (CCL5)], monocyte chemotactic protein-1 (MCP-1; also
known as CCL2), MIP-1α (also known as CCL3) and growth-related
oncogene/keratinocyte chemoattractant (GRO/KC) (51–53).
It has been revealed that treating arthritic rats with celastrol
significantly reduces expression levels of RANTES, MCP-1, MIP-1α
and GRO/KC, as well as the pro-inflammatory cytokines TNF-α and
IL-1β (54), and this may inhibit
leukocyte migration into joints (55).
Molecular targets of triptolide
Triptolide inhibits the NF-κB
pathway
The NF-κB family comprises Rel domain-containing
proteins that regulate inflammatory and immune responses (56). In resting cells, these proteins are
retained in the cytosol by a group of inhibitory proteins, such as
IκBα (57). Upon activation, IKKs,
including IKK-1 and IKK-2, phosphorylate IκBα, which is
subsequently ubiquitinated and destroyed by the proteasome. This
liberates NF-κB to translocate into the nucleus, where it activates
several genes associated with RA (56). It has been shown that triptolide
regulates IKK-1 and IKK-2 activities induced by various stimuli
(57). The purified T.
wilfordii component PG490 inhibits both IKK-1 and IKK2
activities with similar potency (58). As NF-κB transcription factors
upregulate the expression of several genes involved in inflammatory
responses, the targeting of components of NF-κB signaling is a
major therapeutic strategy for treating autoimmune diseases
(59).
Similar to NF-κB, activating protein-1 (AP-1)
transcription factors, comprising Jun and Fos family proteins,
regulate cell proliferation, transformation and death, and may be
potential therapeutic targets for the control of inflammation
(60–62). Triptolide also inhibits
mitogen-activated protein kinase (MAPK)/AP-1 signaling pathways,
effectively suppressing MAP kinases, including JNK, p38 and ERK
activities (59). Therefore,
triptolide is a promising candidate immunomodulatory drug for
autoimmune disorder therapy (63,64).
Triptolide alters RANKL/RANK/OPG
signaling
Osteoclasts are the primary bone resorptive cells,
and are located mainly in the synovial inflammatory tissue; RANKL
stimulates osteoclast-mediated bone destruction in RA by binding to
its receptor RANK (65). Under
physiological conditions, osteoblasts and activated T cells express
RANKL, which binds to RANK on osteoclasts to trigger osteoclast
maturation and bone resorption. Osteoblasts counteract the action
of osteoclasts in the balance between bone formation and
destruction; osteoblasts express osteoprotegerin (OPG), which ‘mops
up’ RANKL and prevents it from binding to RANK, thus inhibiting
bone resorption (66). However,
under pathological conditions such as RA, this balance is shifted
toward bone destruction (67). In
mice with collagen-induced arthritis, triptolide significantly
reduces the number of osteoclasts in areas of bone destruction by
downregulating RANKL and RANK, and upregulating OPG (68).
Triptolide inhibits COX-2 and matrix
metalloproteinases (MMPs)
It has been revealed that injury, tumorigenesis and
invasion from the joint into multiple organs upregulate COX-2 via
NF-κB to produce prostaglandins, which induce inflammation and
increase capillary permeability (69). Moreover, triptolide downregulates
COX-2 and PGE2, thus alleviating LPS-induced inflammation (70).
MMPs participate in tumorigenesis, tumor metastasis
and inflammatory diseases such as RA (57,71–75).
In human synovial fibroblasts and mouse macrophages, triptolide
inhibits IL-1α-induced phosphorylation of MMP-1 and LPS-induced
phosphorylation of MMP-3. By inhibiting MMP-3 and MMP-13,
triptolide slows the degradation of extracellular matrix and
cartilage degeneration in mice with collagen-induced arthritis, as
well as in primary human osteoarthritis and bovine chondrocytes
(26,76–79).
Triptolide inhibits cytokines
Antigen-presenting cells produce IL-12 and IL-23,
which are heterodimeric cytokines sharing a p40 subunit; these
cytokines stimulate the generation and functions of T helper (Th)1
and Th17 cells, respectively. These cytokines are involved in the
pathogenesis of several autoimmune disorders, including RA and
systemic lupus erythematosus (80). Triptolide downregulates p40, in
part, by activating the expression and phosphorylation of
CCAAT/enhancer binding protein-α (C/EBPα) via the kinases ERK1/2
and AKT-glycogen synthase kinase 3β (81). This phosphorylation allows C/EBPα
to bind antagonistically to the p40 promoter (81). Furthermore, programmed cell death 5
enhances the ability of triptolide to induce FLS apoptosis in RA,
and therefore may be a potential therapeutic target in RA (29,82,83).
Triptolide targets vascular
endothelial growth factor (VEGF)
VEGF-driven angiogenesis promotes RA progress by
allowing inflammatory cell infiltration of the synovial membrane
(5). Triptolide prevents the
formation of new blood vessels in vitro and in vivo,
and it inhibits several downstream effects of IL-1β, including cell
adhesion of human FLS in RA and human umbilical vein endothelial
cells (HUVECs) (84). Furthermore,
triptolide upregulates several angiogenic activators, including
TNF-α, IL-17, VEGF, VEGFR, Angiopoietin (Ang)-1, Ang-2 and Tie2,
and activates the MAPK signaling pathway involving phosphorylated
(p)-ERK, p-p38 and p-JNK (85).
Moreover, triptolide disrupts tube formation in HUVECs on Matrigel,
and suppresses VEGF-induced chemotactic migration of HUVECs and
human FLS in RA (86).
Adverse effects of T. wilfordii
T. wilfordii is a Chinese herb that has been
traditionally used in clinics for RA treatment (3). Numerous preclinical studies have
demonstrated that extracts from T. wilfordii roots inhibit
the expression levels of RA-related inflammatory factors secreted
by macrophages, lymphocytes, synovial fibroblasts and chondrocytes
(87–91). Moreover, T. wilfordii
suppresses lymphocytes and synovial fibroblast proliferation by
inducing apoptosis (46). The
anti-angiogenesis property of synovial fibroblasts has been shown
in a previous study (83).
Although T. wilfordii has several promising bioactivities
in vivo and in vitro, its multi-target toxicity has
restricted its clinical application (88). Data from the China Food and Drug
Administration catalogue at least 633 instances of adverse
reactions (53 severe) involving reproductive organ, liver and renal
toxicity. Furthermore, clinical studies have concluded that T.
wilfordii can damage the digestive system, including liver
injury and stomachache, as well as the endocrine and reproductive
systems (30,32,92).
Moreover, 271 patients with RA have reported digestive tract
symptoms and irregular menstruation. As a compound of T.
wilfordii, triptolide-induced toxicity was shown to be
dependent on dosage and administration times (30,93).
To avoid toxicity, previous studies have attempted to alter the
dosage and structure, and to assess its compatibility with other
drugs (34). For example, Freag
et al (94) developed
self-assembled celastrol phytosomal nanocarriers (celastrol-PHY) to
improve celastrol solubility and oral bioavailability; these were
confirmed through an in vitro release study and a
pharmacokinetic study in rabbits (94). Structural modification and
alternation of triptolide can produce derivatives of triptolide
with lower toxicity and relative higher activity. Apart from
structural alterations, the development of novel triptolide
delivery systems is a valuable strategy to improve water
solubility, and the efficiency of absorption and metabolism, and to
reduce toxicity (95).
Conclusions
Celastrol and triptolide from T. wilfordii
are effective against RA; they target numerous signaling pathways,
proteases and cytokines. The present review examined the chemistry
and bioavailability of celastrol and triptolide, and their
molecular targets in treating RA, which may be potential effective
drugs. However, owing to the strong toxicity of T.
wilfordii, novel approaches are required for the safe
application of this TCM. These may include investigating new
triptolide formulations or its combination with other drugs.
Furthermore, defining early toxicity markers, investigating dosage
ranges for different target organs and establishing a toxicity
warning system are required.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. U1804179), The Key
Scientific and Technological Projects in Henan Province (grant no.
202102310190), The Henan Science and Technology Innovation Team,
Investigation on Plant Resources in Dabie Mountains and The Study
and Utilization of Active Components of Special Plants (grant no.
2017083), and The Nanhu Scholars Program for Young Scholars of
Xinyang Normal University (grant no. 2018001).
Availability of data and materials
Not applicable.
Authors' contributions
XS conceived and designed the study, and wrote the
manuscript. YZ and ED analyzed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deane KD and Holers VM: The natural
history of rheumatoid arthritis. Clin Ther. 41:1256–1269. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burmester GR and Pope JE: Novel treatment
strategies in rheumatoid arthritis. Lancet. 389:2338–2348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ridgley LA, Anderson AE and Pratt AG: What
are the dominant cytokines in early rheumatoid arthritis? Curr Opin
Rheumatol. 30:207–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noack M and Miossec P: Selected cytokine
pathways in rheumatoid arthritis. Semin Immunopathol. 39:365–383.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang S, Tanaka T, Narazaki M and Kishimoto
T: Targeting interleukin-6 signaling in clinic. Immunity.
50:1007–1023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siouti E and Andreakos E: The many facets
of macrophages in rheumatoid arthritis. Biochem Pharmacol.
165:152–169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasuda K, Takeuchi Y and Hirota K: The
pathogenicity of Th17 cells in autoimmune diseases. Semin
Immunopathol. 41:283–297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arleevskaya MI, Larionova RV, Brooks WH,
Bettacchioli E and Renaudineau Y: Toll-like receptors, infections,
and rheumatoid arthritis. Clin Rev Allergy Immunol. May
29–2019.(Epub ahead of print).
|
|
9
|
Alam J, Jantan I and Bukhari SNA:
Rheumatoid arthritis: Recent advances on its etiology, role of
cytokines and pharmacotherapy. Biomed Pharmacother. 92:615–633.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silvagni E, Di Battista M, Bonifacio AF,
Zucchi D, Governato G and Scirè CA: One year in review 2019:
Novelties in the treatment of rheumatoid arthritis. Clin Exp
Rheumatol. 37:519–534. 2019.PubMed/NCBI
|
|
11
|
Conigliaro P, Triggianese P, De Martino E,
Fonti GL, Chimenti MS, Sunzini F, Viola A, Canofari C and Perricone
R: Challenges in the treatment of rheumatoid arthritis. Autoimmun
Rev. 18:706–713. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cecchi I, Arias de la Rosa I, Menegatti E,
Roccatello D, Collantes-Estevez E, Lopez-Pedrera C and Barbarroja
N: Neutrophils: Novel key players in rheumatoid arthritis. Current
and future therapeutic targets. Autoimmun Rev. 17:1138–1149. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheung TT and McInnes IB: Future
therapeutic targets in rheumatoid arthritis? Semin Immunopathol.
39:487–500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou W, Liu B and Xu H: Triptolide:
Medicinal chemistry, chemical biology and clinical progress. Eur J
Med Chem. 176:378–392. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong Y, Chen H, Gao J, Liu Y, Li J and
Wang J: Bioactive ingredients in Chinese herbal medicines that
target non-coding RNAs: Promising new choices for disease
treatment. Front Pharmacol. 10:5152019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y, Ma S, Wang Y, Yan R, Wang S, Liu
N, Chen B, Chen J and Liu L: The role of traditional Chinese herbal
medicines and bioactive ingredients on ion channels: A brief review
and prospect. CNS Neurol Disord Drug Targets. 18:257–265. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong Y, Wang P, Feng X, Li B, Wang Z and
Li H: The role of Chinese herbal medicines and bioactive
ingredients targeting myocardial KCa and KATP Channels in
cardiovascular diseases. Curr Pharm Des. 23:1070–1076. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv H, Jiang L, Zhu M, Li Y, Luo M, Jiang
P, Tong S, Zhang H and Yan J: The genus Tripterygium: A
phytochemistry and pharmacological review. Fitoterapia.
137:1041902019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Venkatesha SH, Dudics S, Astry B and
Moudgil KD: Control of autoimmune inflammation by celastrol, a
natural triterpenoid. Pathog Dis. 74(pii): ftw0592016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu L, Su P, Zhang Z, Gao L, Wang J, Hu T,
Zhou J, Zhang Y, Zhao Y, Liu Y, et al: Genome of Tripterygium
wilfordii and identification of cytochrome P450 involved in
triptolide biosynthesis. Nat Commun. 11:9712020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin N, Sato T and Ito A: Triptolide, a
novel diterpenoid triepoxide from Tripterygium wilfordii
Hook. f., suppresses the production and gene expression of
pro-matrix metalloproteinases 1 and 3 and augments those of tissue
inhibitors of metalloproteinases 1 and 2 in human synovial
fibroblasts. Arthritis Rheum. 44:2193–2200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Astry B, Venkatesha SH, Laurence A,
Christensen-Quick A, Garzino-Demo A, Frieman MB, O'Shea JJ and
Moudgil KD: Celastrol, a Chinese herbal compound, controls
autoimmune inflammation by altering the balance of pathogenic and
regulatory T cells in the target organ. Clin Immunol. 157:228–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di YM, Zhou ZW, Guang Li C and Zhou SF:
Current and future therapeutic targets of rheumatoid arthritis.
Antiinflamm Antiallergy Agents Med Chem. 10:92–120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Zhou X, Chen XY and Zhong DF:
Excretion of [3H]triptolide and its metabolites in rats after oral
administration. Acta Pharmacol Sin. 35:549–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Q: Triptolide and its expanding
multiple pharmacological functions. Int Immunopharmacol.
11:377–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li XJ, Jiang ZZ and Zhang LY: Triptolide:
Progress on research in pharmacodynamics and toxicology. J
Ethnopharmacol. 155:67–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng Y, Chen G, Wang L, Kong J, Pan J, Xi
Y, Shen F and Huang Z: Triptolide-induced mitochondrial damage
dysregulates fatty acid metabolism in mouse sertoli cells. Toxicol
Lett. 292:136–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xi C, Peng S, Wu Z, Zhou Q and Zhou J:
Toxicity of triptolide and the molecular mechanisms involved.
Biomed Pharmacother. 90:531–541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song J, Shi F, Zhang Z, Zhu F, Xue J, Tan
X, Zhang L and Jia X: Formulation and evaluation of
celastrol-loaded liposomes. Molecules. 16:7880–7892. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi J, Lu Y and Wu W: Absorption,
disposition and pharmacokinetics of solid lipid nanoparticles. Curr
Drug Metab. 13:418–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng X, Wang J, Song H, Cui D, Li L, Li J,
Lin L, Zhou J and Liu Y: Optimized preparation of celastrol-loaded
polymeric nanomicelles using rotatable central composite design and
response surface methodology. J Biomed Nanotechnol. 8:491–499.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cascao R, Fonseca JE and Moita LF:
Celastrol: A spectrum of treatment opportunities in chronic
diseases. Front Med (Lausanne). 4:692017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Venkatesha SH and Moudgil KD: Celastrol
and its role in controlling chronic diseases. Adv Exp Med Biol.
928:267–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen YF, Zhang X, Wang Y, Cao FF, Uzan G,
Peng B and Zhang DH: Celastrol targets IRAKs to block Toll-like
receptor 4-mediated nuclear factor-κB activation. J Integr Med.
14:203–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JH, Koo TH, Yoon H, Jung HS, Jin HZ,
Lee K, Hong YS and Lee JJ: Inhibition of NF-κB activation through
targeting I kappa B kinase by celastrol, a quinone methide
triterpenoid. Biochem Pharmacol. 72:1311–1321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mercurio F, Zhu H, Murray BW, Shevchenko
A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A and Rao
A: IKK-1 and IKK-2: Cytokine-activated IkappaB kinases essential
for NF-kappaB activation. Science. 278:860–866. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salminen A, Lehtonen M, Paimela T and
Kaarniranta K: Celastrol: Molecular targets of thunder God vine.
Biochem Biophys Res Commun. 394:439–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samarpita S, Kim JY, Rasool MK and Kim KS:
Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a
new potential anti-rheumatoid arthritis drug. Arthritis Res Ther.
22:162020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berridge MJ: Calcium signalling
remodelling and disease. Biochem Soc Trans. 40:297–309. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Clapham DE: Calcium signaling. Cell.
131:1047–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wong VKW, Qiu C, Xu SW, Law BYK, Zeng W,
Wang H, Michelangeli F, Dias IRSR, Qu YQ, Chan TW, et al:
Ca2+ signalling plays a role in celastrol-mediated
suppression of synovial fibroblasts of rheumatoid arthritis
patients and experimental arthritis in rats. Br J Pharmacol.
176:2922–2944. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoo SA, Park BH, Park GS, Koh HS, Lee MS,
Ryu SH, Miyazawa K, Park SH, Cho CS and Kim WU: Calcineurin is
expressed and plays a critical role in inflammatory arthritis. J
Immunol. 177:2681–2690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Villalobo A, Ishida H, Vogel HJ and
Berchtold MW: Calmodulin as a protein linker and a regulator of
adaptor/scaffold proteins. Biochim Biophys Acta Mol Cell Res.
1865:507–521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Z, Wu G, Wei X, Chen X, Wang Y and Chen
L: Celastrol induced DNA damage, cell cycle arrest, and apoptosis
in human rheumatoid fibroblast-like synovial cells. Am J Chin Med.
41:615–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan XX, Li N, Wu JL, Zhou YL, He JX, Liu L
and Leung EL: Celastrol induces apoptosis in gefitinib-resistant
non-small cell lung cancer cells via caspases-dependent pathways
and Hsp90 client protein degradation. Molecules. 19:3508–3522.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu LM, Zheng YJ, Wang Y, Yang Y, Cao FF,
Peng B, Xu XF, An HZ, Zheng AX, Zhang DH, et al: Celastrol inhibits
lung infiltration in differential syndrome animal models by
reducing TNF-α and ICAM-1 levels while preserving differentiation
in ATRA-induced acute promyelocytic leukemia cells. PLoS One.
9:e1051312014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fang Z, He D, Yu B, Liu F, Zuo J, Li Y,
Lin Q, Zhou X and Wang Q: High-throughput study of the effects of
celastrol on activated fibroblast-like synoviocytes from patients
with rheumatoid arthritis. Genes (Basel). 8(pii): E2212017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mukherjee S, Huda S and Sinha Babu SP:
Toll-like receptor polymorphism in host immune response to
infectious diseases: A review. Scand J Immunol. 90:e127712019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang X, Wang Y, Ge HY, Gu YJ, Cao FF,
Yang CX, Uzan G, Peng B and Zhang DH: Celastrol reverses palmitic
acid (PA)-caused TLR4-MD2 activation-dependent insulin resistance
via disrupting MD2-related cellular binding to PA. J Cell Physiol.
233:6814–6824. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Khan MA, Khurana N, Ahmed RS, Umar S, Md G
Sarwar AH, Alam Q, Kamal MA and Ashraf GM: Chemokines: A potential
therapeutic target to suppress autoimmune arthritis. Curr Pharm
Des. 25:2937–2946. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Eustace AD, McNaughton EF, King S, Kehoe
O, Kungl A, Mattey D, Nobbs AH, Williams N and Middleton J: Soluble
syndecan-3 binds chemokines, reduces leukocyte migration in vitro
and ameliorates disease severity in models of rheumatoid arthritis.
Arthritis Res Ther. 21:1722019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bahlas S, Damiati L, Dandachi N, Sait H,
Alsefri M and Pushparaj PN: Rapid immunoprofiling of cytokines,
chemokines and growth factors in patients with active rheumatoid
arthritis using luminex multiple analyte profiling technology for
precision medicine. Clin Exp Rheumatol. 37:112–119. 2019.PubMed/NCBI
|
|
54
|
Lee JY, Lee BH, Kim ND and Lee JY:
Celastrol blocks binding of lipopolysaccharides to a Toll-like
receptor4/myeloid differentiation factor2 complex in a
thiol-dependent manner. J Ethnopharmacol. 172:254–260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li G, Liu D, Zhang Y, Qian Y, Zhang H, Guo
S, Sunagawa M, Hisamitsu T and Liu Y: Celastrol inhibits
lipopolysaccharide-stimulated rheumatoid fibroblast-like
synoviocyte invasion through suppression of TLR4/NF-κB-mediated
matrix metalloproteinase-9 expression. PLoS One. 8:e689052013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Venkatesha SH, Astry B, Nanjundaiah SM, Yu
H and Moudgil KD: Suppression of autoimmune arthritis by
celastrus-derived celastrol through modulation of pro-inflammatory
chemokines. Bioorg Med Chem. 20:5229–5234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li GQ, Liu D, Zhang Y, Qian YY, Zhu YD,
Guo SY, Sunagawa M, Hisamitsu T and Liu YQ: Anti-invasive effects
of celastrol in hypoxia-induced fibroblast-like synoviocyte through
suppressing of HIF-1α/CXCR4 signaling pathway. Int Immunopharmacol.
17:1028–1036. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Park B, Sung B, Yadav VR, Chaturvedi MM
and Aggarwal BB: Triptolide, histone acetyltransferase inhibitor,
suppresses growth and chemosensitizes leukemic cells through
inhibition of gene expression regulated by
TNF-TNFR1-TRADD-TRAF2-NIK-TAK1-IKK pathway. Biochem Pharmacol.
82:1134–1144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang Y, Ye Y, Qiu Q, Xiao Y, Huang M, Shi
M, Liang L, Yang X and Xu H: Triptolide inhibits the migration and
invasion of rheumatoid fibroblast-like synoviocytes by blocking the
activation of the JNK MAPK pathway. Int Immunopharmacol. 41:8–16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fan D, He X, Bian Y, Guo Q, Zheng K, Zhao
Y, Lu C, Liu B, Xu X, Zhang G and Lu A: Triptolide modulates TREM-1
signal pathway to inhibit the inflammatory response in rheumatoid
arthritis. Int J Mol Sci. 17:4982016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ho LJ, Chang WL, Chen A, Chao P and Lai
JH: Differential immunomodulatory effects by Tripterygium
wilfordii Hook f-derived refined extract PG27 and its purified
component PG490 (triptolide) in human peripheral blood T cells:
Potential therapeutics for arthritis and possible mechanisms
explaining in part Chinese herbal theory ‘Junn-Chenn-Zuou-SS’. J
Transl Med. 11:2942013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ruland J: Return to homeostasis:
Downregulation of NF-κB responses. Nat Immunol. 12:709–714. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kanarek N and Ben-Neriah Y: Regulation of
NF-κB by ubiquitination and degradation of the IκBs. Immunol Rev.
246:77–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Criswell LA: Gene discovery in rheumatoid
arthritis highlights the CD40/NF-kappaB signaling pathway in
disease pathogenesis. Immunol Rev. 233:55–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Schonthaler HB, Guinea-Viniegra J and
Wagner EF: Targeting inflammation by modulating the Jun/AP-1
pathway. Ann Rheum Dis. 70 (Suppl 1):i109–i112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xiao C, Zhou J, He Y, Jia H, Zhao L, Zhao
N and Lu A: Effects of triptolide from radix Tripterygium
wilfordii (Leigongteng) on cartilage cytokines and
transcription factor NF-kappaB: A study on induced arthritis in
rats. Chin Med. 4:132009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bezerra MC, Carvalho JF, Prokopowitsch AS
and Pereira RM: RANK, RANKL and osteoprotegerin in arthritic bone
loss. Braz J Med Biol Res. 38:161–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ho TY, Santora K, Chen JC, Frankshun AL
and Bagnell CA: Effects of relaxin and estrogens on bone remodeling
markers, receptor activator of NF-κB ligand (RANKL) and
osteoprotegerin (OPG), in rat adjuvant-induced arthritis. Bone.
48:1346–1353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Geusens P: The role of RANK
ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv
Musculoskelet Dis. 4:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu Q, Chen T, Chen G, Shu X, Sun A, Ma P,
Lu L and Cao X: Triptolide impairs dendritic cell migration by
inhibiting CCR7 and COX-2 expression through PI3-K/Akt and
NF-kappaB pathways. Mol Immunol. 44:2686–2696. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu C, Zhang Y, Kong X, Zhu L, Pang J, Xu
Y, Chen W, Zhan H, Lu A and Lin N: Triptolide prevents bone
destruction in the collagen-induced arthritis model of rheumatoid
arthritis by targeting RANKL/RANK/OPG signal pathway. Evid Based
Complement Alternat Med. 2013:6260382013.PubMed/NCBI
|
|
72
|
Brinker AM, Ma J, Lipsky PE and Raskin I:
Medicinal chemistry and pharmacology of genus Tripterygium
(Celastraceae). Phytochemistry. 68:732–766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xue M, McKelvey K, Shen K, Minhas N, March
L, Park SY and Jackson CJ: Endogenous MMP-9 and not MMP-2 promotes
rheumatoid synovial fibroblast survival, inflammation and cartilage
degradation. Rheumatology (Oxford). 53:2270–2279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Geng Y, Blanco FJ, Cornelisson M and Lotz
M: Regulation of cyclooxygenase-2 expression in normal human
articular chondrocytes. J Immunol. 155:796–801. 1995.PubMed/NCBI
|
|
75
|
Maekawa K, Yoshikawa N, Du J, Nishida S,
Kitasato H, Okamoto K, Tanaka H, Mizushima Y and Kawai S: The
molecular mechanism of inhibition of interleukin-1beta-induced
cyclooxygenase-2 expression in human synovial cells by
Tripterygium wilfordii Hook F extract. Inflamm Res.
48:575–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Flower RJ: The development of COX2
inhibitors. Nat Rev Drug Discov. 2:179–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Geng Y, Fang M, Wang J, Yu H, Hu Z, Yew DT
and Chen W: Triptolide down-regulates COX-2 expression and PGE2
release by suppressing the activity of NF-κB and MAP kinases in
lipopolysaccharide-treated PC12 cells. Phytother Res. 26:337–343.
2012.PubMed/NCBI
|
|
78
|
Ma J, Dey M, Yang H, Poulev A, Pouleva R,
Dorn R, Lipsky PE, Kennelly EJ and Raskin I: Anti-inflammatory and
immunosuppressive compounds from Tripterygium wilfordii.
Phytochemistry. 68:1172–1178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liacini A, Sylvester J and Zafarullah M:
Triptolide suppresses proinflammatory cytokine-induced matrix
metalloproteinase and aggrecanase-1 gene expression in
chondrocytes. Biochem Biophys Res Commun. 327:320–327. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lin N, Liu C, Xiao C, Jia H, Imada K, Wu H
and Ito A: Triptolide, a diterpenoid triepoxide, suppresses
inflammation and cartilage destruction in collagen-induced
arthritis mice. Biochem Pharmacol. 73:136–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang Y and Ma X: Triptolide inhibits
IL-12/IL-23 expression in APCs via CCAAT/enhancer-binding protein
alpha. J Immunol. 184:3866–3877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jiang J, Wang N, Guan Z and Houshan LV:
Programmed cell death 5 factor enhances triptolide-induced
fibroblast-like synoviocyte apoptosis of rheumatoid arthritis.
Artif Cells Blood Substit Immobil Biotechnol. 38:38–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tasneem S, Liu B, Li B, Choudhary MI and
Wang W: Molecular pharmacology of inflammation: Medicinal plants as
anti-inflammatory agents. Pharmacol Res. 139:126–140. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ziaei S and Halaby R: Immunosuppressive,
anti-inflammatory and anti-cancer properties of triptolide: A mini
review. Avicenna J Phytomed. 6:149–164. 2016.PubMed/NCBI
|
|
85
|
Kong X, Zhang Y, Liu C, Guo W, Li X, Su X,
Wan H, Sun Y and Lin N: Anti-angiogenic effect of triptolide in
rheumatoid arthritis by targeting angiogenic cascade. PLoS One.
8:e775132013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang W, Li F and Gao W: Tripterygium
wilfordii inhibiting angiogenesis for rheumatoid arthritis
treatment. J Natl Med Assoc. 109:142–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ramgolam V, Ang SG, Lai YH, Loh CS and Yap
HK: Traditional Chinese medicines as immunosuppressive agents. Ann
Acad Med Singapore. 29:11–16. 2000.PubMed/NCBI
|
|
88
|
Cameron M, Gagnier JJ and Chrubasik S:
Herbal therapy for treating rheumatoid arthritis. Cochrane Database
Syst Rev. CD0029482011.PubMed/NCBI
|
|
89
|
Lipsky PE and Tao XL: A potential new
treatment for rheumatoid arthritis: Thunder god vine. Semin
Arthritis Rheum. 26:713–723. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lv QW, Zhang W, Shi Q, Zheng WJ, Li X,
Chen H, Wu QJ, Jiang WL, Li HB, Gong L, et al: Comparison of
Tripterygium wilfordii Hook F with methotrexate in the
treatment of active rheumatoid arthritis (TRIFRA): A randomised,
controlled clinical trial. Ann Rheum Dis. 74:1078–1086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tao X, Younger J, Fan FZ, Wang B and
Lipsky PE: Benefit of an extract of Tripterygium Wilfordii
Hook F in patients with rheumatoid arthritis: A double-blind,
placebo-controlled study. Arthritis Rheum. 46:1735–1743. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao Q, Liu F, Cheng Y, Xiao XR, Hu DD,
Tang YM, Bao WM, Yang JH, Jiang T, Hu JP, et al: Celastrol protects
from cholestatic liver injury through modulation of SIRT1-FXR
signaling. Mol Cell Proteomics. 18:520–533. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang Y, Jiang Z, Xue M, Zhang S, Wang Y
and Zhang L: Toxicogenomic analysis of the gene expression changes
in rat liver after a 28-day oral Tripterygium wilfordii
multiglycoside exposure. J Ethnopharmacol. 141:170–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Freag MS, Saleh WM and Abdallah OY:
Self-assembled phospholipid-based phytosomal nanocarriers as
promising platforms for improving oral bioavailability of the
anticancer celastrol. Int J Pharm. 535:18–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xu H and Liu B: Triptolide-targeted
delivery methods. Eur J Med Chem. 164:342–351. 2019. View Article : Google Scholar : PubMed/NCBI
|