Introduction
The brain is the most easily injured organ of the
human body when ischemia/reperfusion (I/R) occurs. I/R-induced
injury is defined as the process in which tissue injury occurs
during initial hypoxia, followed by oxygen supply recovery. This
common injury may be caused by organ transplantation, resection,
and other clinical conditions, including iatrogenic processes
(1). Although the underlying
pathogenesis of I/R injury is not fully understood, studies have
shown that oxidative stress may be involved. Oxidative stress is
frequently the result accumulated reactive oxygen species (ROS) and
is defined as an imbalance between the production and elimination
of free radicals (2). Studies
revealed that oxidative stress may cause secondary injury to
existing organ injuries by promoting apoptosis, suggesting it has
an important role in therapies for neuronal injuries (3). The body developed various antioxidant
mechanisms to counterbalance oxidative stress, such as the nuclear
factor erythroid 2-related factor 2 (Nrf2)/antioxidant response
element (ARE) signaling pathway.
Nrf2 is a critical transcription factor responsible
for alleviating cellular oxidative stress and protecting cells from
oxidative stress damage. When exposed to stressors, Nrf2
dissociates from its endogenous suppressor, Kelch-like epoxy
chloropropane-associated protein-1 (Keap1). Subsequently, the Nrf2
nuclear localization signal induces rapid translocation to the
nucleus for activation (4). Upon
entry into the nucleus, Nrf2 binds to the ARE signaling pathway and
initiates the transcription of cytoprotective and antioxidant
genes, thereby promoting cell survival. Among these genes, heme
oxygenase 1 (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1)
represent essential endogenous antioxidants and cytoprotective
enzymes. During the onset of I/R-induced injury, HO-1 and NQO1
expression in neuronal cells increases. In addition, several
studies have found that the Nrf2/ARE signaling pathway can inhibit
neurotoxicity by limiting oxidative stress activation and
suppressing the apoptosis signaling pathway (5,6).
Therefore, therapeutic strategies for regulating antioxidant
capacity and neuroprotective properties may be effective in
treating neuronal injury.
Adenosine monophosphate-activated protein kinase
(AMPK) exerts a crucial role in brain protection regulation and is
involved in neuronal injury in I/R. Recent data suggest that AMPK
does not play an exclusive role in the I/R injury response; rather,
it is an endogenous defensive molecule that mediates
neuroprotective effects in I/R injury (7,8). A
previous study reported that activation of the AMPK signaling
pathway inhibits abnormal oxidative stress, thereby reducing ROS
production and exerting neuroprotective effects. Previous research
suggests that AMPK induces neuroprotection of neuronal cells by
targeting Nrf2/ARE. AMPK may also indirectly regulate Nrf2
activation via glycogen synthase kinase (GSK)-3β phosphorylation
(9,10), which induces the ARE-mediated
neuroprotective response. Furthermore, the AMPK/Nrf2 signaling
pathway was reported to mediate reducing effects on I/R injury in
neuronal cells (10,11).
Recently, phytochemicals that can elicit
improvements in neurodegenerative diseases have received extensive
attention. Many phytochemicals can play valuable roles in
preventing and treating neurodegenerative diseases because of their
strong antioxidant properties (12). P. japonicus has many
pharmacologically active constituents, including petatewalide B,
kaempferol, peteasitin, and quercetin. Petatewalide B is a novel
sesquiterpene compound and is the most important active constituent
of P. japonicus (13,14).
Furthermore, sesquiterpenoids from P. japonicus play a key
role in aging-related disorders, such as atherosclerosis,
hyperlipidemia, Parkinson's disease, Alzheimer's disease, and
memory disorders (15,16). However, petatewalide B's
neuroprotective properties and mechanisms have not been fully
elucidated. In this study, we attempted to explore petatewalide B's
potential neuroprotective mechanisms in SH-SY5Y cells exposed to
OGD/R.
Materials and methods
Cell culture and establishment of the
cell model of I/R injury
The human-derived neuroblastoma SH-SY5Y cells were
obtained from the American Type Culture Collection and maintained
in Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% heat-inactivated fetal
bovine serum and 1% penicillin/streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.), under a humidified atmosphere (95% air,
5% CO2) at 37°C. The I/R injury cell model was used to
perform oxygen and glucose deprivation/reoxygenation (OGD/R). The
cells were washed twice with phosphate-buffered saline (PBS) and
then cultured with OGD medium (glucose-free and serum-free DMEM)
under hypoxic conditions (1% O2, 95% N2, and 5%
CO2) for 8 h at 37°C, followed by quick reoxygenation
(95% air and 5% CO2). The medium was substituted with
regular medium for 24 h. Petatewalide B was purified from P.
japonicus leaves as previously described in another study
(13). Petatewalide B was
solubilized with dimethyl sulfoxide (DMSO) and pretreated with
petatewalide B (5–40 µM) for 1 h before OGD/R treatment.
MTT and lactate dehydrogenase (LDH)
assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and LDH assays were performed to detect the treated SH-SY5Y cells'
viability and cytotoxicity. SH-SY5Y cells were seeded into 24-well
plates at the density of 1×105 cells/ml. The SH-SY5Y
cells were treated with petatewalide B (5–40 µM) or OGD/R after 24
h of culture. In brief, cells were incubated with MTT reagent (50
µg/ml) and maintained for a further 6 h. Thereafter, cells were
treated with DMSO for 5 min. Each sample was analyzed using a
microplate reader (Perkin-Elmer) at 570 nm. The LDH assay was
performed using the cytotoxicity detection kit (LDH; Roche Applied
Science), according to the manufacturer's instructions. Readings
were recorded using a microplate reader (Perkin-Elmer) at an
absorbance of 490 nm.
Measurement of intracellular ROS
The treated cells' intracellular ROS levels were
evaluated using the ROS assay kit (CM-H2DCFDA; Thermo
Fisher Scientific, Inc.) according to manufacturer's instructions.
In brief, SH-SY5Y cells were seeded into 6-well plates at a density
of 1×105 cells/ml. After 24 h of culture, the SH-SY5Y
cells were treated with petatewalide B (20 µM) or OGD/R. The
SH-SY5Y cells were rinsed with PBS and incubated with
CM-H2DCFDA for 30 min in the dark. The fluorescent
intensity was proportional to the intracellular ROS levels.
Thereafter, intracellular ROS levels were determined according to
the fluorescent intensity, which was recorded using a Flow
Cytometer (Beckman Coulter FC500; Beckman Coulter).
Apoptosis assay
Flow cytometric analysis was conducted to
investigate apoptosis in the treated cells. The SH-SY5Y cells were
collected by trypsinization and washed thrice with PBS.
Subsequently, cells were stained with Alexa Fluor® 488
dye-labeled anti-BrdU antibody (APO-BrdU™ TUNEL assay kit;
Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Finally, the cells were subjected to
apoptosis analysis using the Flow Cytometer Cytomics FC 500
(Beckman Coulter).
Preparation of protein extracts
Total protein was extracted from cells using a RIPA
Lysis and Extraction Buffer (Thermo Fisher Scientific, Inc.)
supplemented with protease and phosphatase inhibitors (Roche). The
nuclear extraction of SH-SY5Y cells was carried out using Thermo
Scientific™ NE-PER™ Nuclear and Cytoplasmic Extraction kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The protein concentration was evaluated using the Bio-Rad protein
assay kit (Bio-Rad Laboratories, Inc.).
Western blot analysis
The protein levels of cleaved caspase-3, cleaved
caspase-9, cleaved PARP, p21, p53, Bax, Bcl-2, HO-1, NQO1, Nrf2,
AMPK, GSK-3β, p-AMPK, and p-GSK-3β in the SH-SY5Y cells were
verified via western blot analysis. Equal quantities of protein
samples resolved on 7.5–3% acrylamide sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels were
transferred to polyvinylidene fluoride (PVDF) membranes. The
antibody dilution ratios were as follows: α-tubulin (1:2,000), TBP
(1:500), Nrf2 (1:500), NQO1 (1:500), and HO-1 (1:1,000; Santa Cruz
Biotechnology, Inc.); cleaved caspase-3 (1:1,000), cleaved PARP
(1:1,000), cleaved caspase-9 (1:1,000), p53 (1:1,000), p21
(1:1,000), Bax (1:1,000), AMPK (1:1,000), p-AMPK (1:1,000), GSK-3β
(1:1,000), and p-GSK-3β (1:1,000; Cell Signaling Technology Inc.).
Thereafter, the membranes were incubated with a 1:5,000 dilution of
goat anti-rabbit IgG-HRP secondary antibody (Cell Signaling
Technology Inc.). Data were scanned using an imaging system
(Amersham Biosciences) and analyzed using the ImageQuant 350
analyzer.
Small interfering RNA (siRNA)
transfection
The knockdown of Nrf2, HO-1, and NQO1 in the SH-SY5Y
cells was performed using a commercially available siRNA (Santa
Cruz Biotechnology, Inc.). The cells were transfected using X-treme
GENE siRNA Transfection Reagent (Roche Diagnostics), following the
manufacturer's instructions.
Luciferase activity assay
ARE reporter plasmid assay was performed, as
described in the FuGENE-HD transfection reagent manufacturer's
protocol. Briefly, the cells were transfected with the ARE reporter
plasmid diluted in Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.)
using FuGENE-HD reagent. The cells were gathered and lysed after
transfection, and luciferase activity was verified using the
Dual-Glo® Luciferase Assay System (Promega Corporation)
with a luminometer (Victor3; Perkin-Elmer).
Statistical analysis
Statistical analyses were performed using SPSS 18.0
software (SPSS, Inc.). The experiments were repeated at least three
times. Statistically significant differences were assessed using
one-way analysis of variance (ANOVA) followed by Tukey's post-hoc
test. Data are shown as the mean ± standard deviation (SD). A
P<0.01 or P<0.05 were considered to indicate a statistically
significant difference.
Results
Petatewalide B ameliorates the
OGD/R-exposed SH-SY5Y cell injury
Before evaluating petatewalide B's role, cell
viability and cytotoxicity were investigated using the MTT and LDH
assays. The results showed that petatewalide B concentrations up to
40 µM did not alter cell viability and cytotoxicity. Our
experimental goal was to examine petatewalide B's effect on I/R
injury by constructing an OGD/R-induced SH-SY5Y cell model. In cell
viability and cytotoxicity analysis, OGD/R-exposed SH-SY5Y cells
exhibited a significant downregulation of cell viability and marked
upregulation of cell cytotoxicity. However, these effects were
eliminated by petatewalide B application. The high-dose group (40
µM) exhibited much better effects than the low-dose group (5 µM)
(Fig. 1A-D). Since ROS is an
important indicator of oxidative stress and neuronal injury, the
level of ROS in the SH-SY5Y cells was determined. The results
indicated that, compared with the control group, the ROS production
level was markedly higher after OGD/R in the experimental group.
However, treatment with petatewalide B remarkably reduced ROS
production levels (Fig. 1E). We
further examined the TUNEL-staining levels in each group because
DNA fragments play a central role in apoptosis. Importantly, DNA
fragment levels are elevated after OGD/R. Therefore, an increase in
TUNEL-staining levels indicated that apoptosis was induced. The
TUNEL-staining levels were remarkably higher in the OGD/R group
than in the control group. However, petatewalide B could inhibit
the increase in TUNEL-staining levels after OGD/R, indicating that
petatewalide B prevents apoptosis in OGD/R-exposed SH-SY5Y cells
(Fig. 1F). Western blotting was
performed to analyze gene expression downstream of apoptosis. OGD/R
led to notable increases pro-apoptosis gene expressions (cleaved
PARP, cleaved caspase-9, cleaved caspase-3, p53, p21, and Bax) and
decreases in anti-apoptotic protein Bcl-2 expression in cells,
compared to the control cells. As expected, genes related to
pro-apoptosis and anti-apoptosis were significantly eliminated in
the OGD/R + petatewalide B, compared to the OGD/R group (Fig. 1G and H). This indicated that
petatewalide B may function as an inhibitor of apoptosis-related
genes.
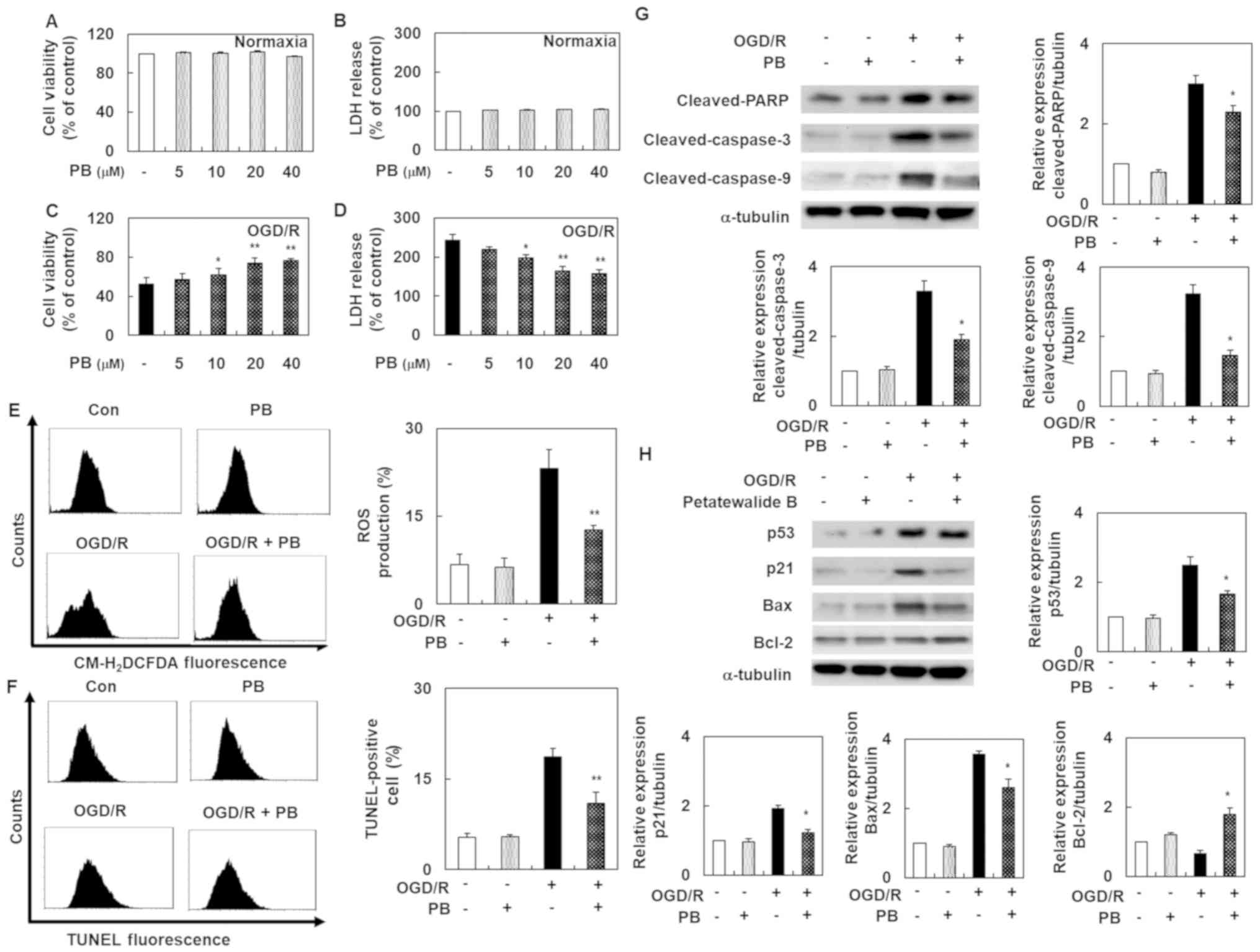 | Figure 1.Cell death, ROS production and
apoptosis in SH-SY5Y cells after OGD/R, with or without PB
treatment. (A) MTT assays were used to examine the viability of
SH-SY5Y cells treated with PB. (B) LDH assays were used to examine
the cytotoxicity of SH-SY5Y cells treated with PB. (C) MTT assays
were used to examine the viability of SH-SY5Y cells pretreated with
PB and co-treated with OGD/R. (D) LDH assays were used to examine
the cytotoxicity of SH-SY5Y cells pretreated with PB and co-treated
with OGD/R. In an intracellular ROS assay, TUNEL staining and
western blotting, cells were pretreated with PB (20 µM) for 1 h,
followed by OGD/R for 8 h and then reoxygenated for a further 24 h.
(E) Representative images of ROS production measured by flow
cytometry. (F) Demonstrative images of TUNEL-staining level
measured by flow cytometry. (G) Western blot analysis was used to
distinguish the protein expression levels of cleaved PARP, cleaved
caspase-9 and cleaved caspase-3. (H) The protein expression levels
of p53, Bax, and p21 were detected via western blotting. The values
are presented as the mean ± SD (n=3). *P<0.05 and **P<0.01
vs. OGD/R group. Con, control; LDH, lactate dehydrogenase; OGD/R,
oxygen-glucose deprivation/reoxygenation; PB, petatewalide B; ROS,
reactive oxygen species. |
Petatewalide B-mediated inhibition of
neuronal injury is inhibited by HO-1 and NQO1 knockdown in
OGD/R-induced SH-SY5Y cells
To elucidate the molecular mechanism by which
petatewalide B induced neuroprotection, our experiment's first goal
was to evaluate petatewalide B's effect on HO-1 and NQO1 expression
in the SH-SY5Y cells exposed to OGD/R. The results indicated that
petatewalide B elevated the protein levels of HO-1 and NQO1
(Fig. 2A and B). To confirm that
the protective regulators, HO-1 and NQO1, are responsible for the
neuroprotective properties of petatewalide B, the cells were
transfected with si-HO-1 or si-NQO1 and then administered
petatewalide B, followed by exposure to OGD/R. The results showed
that the petatewalide B-induced expression of HO-1 and NQO1 was
blocked by si-HO-1 or si-NQO1 (Fig. 2C
and D). Furthermore, the increase of TUNEL-staining levels,
induced by OGD/R, was significantly eliminated by petatewalide B;
however, knockdown of HO-1 and NQO1 significantly attenuated
petatewalide B's effects (Fig. 2E and
F). In addition, knockdown of HO-1 and NQO1 could significantly
reverse the upregulation of cell viability, as well as the
downregulation of cell cytotoxicity, exerted by petatewalide B
(data not shown). These results showed that petatewalide B could
inhibit the neurotoxic response by inducing HO-1 and NQO1
expression.
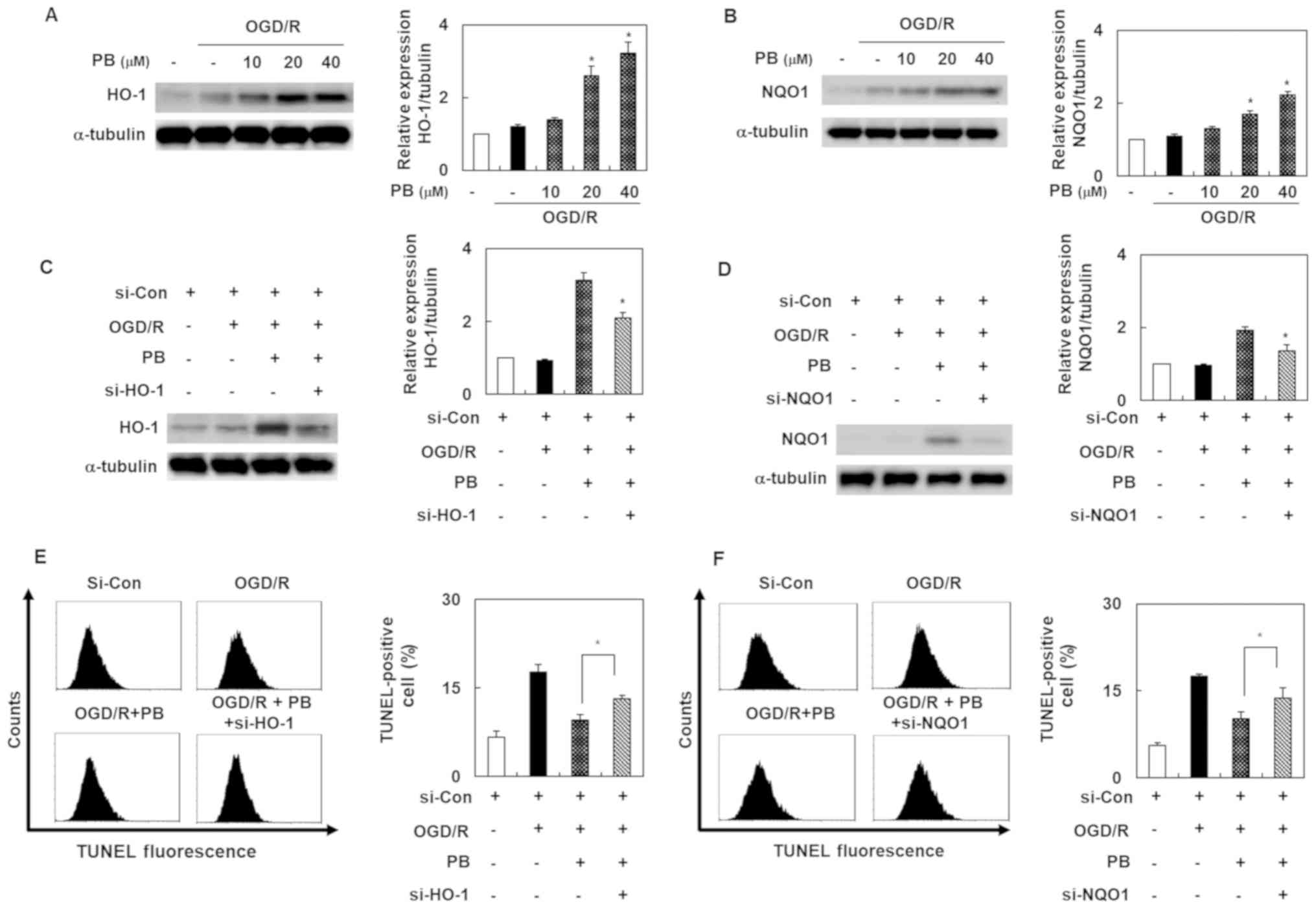 | Figure 2.Expression levels of HO-1 and NQO1 in
SH-SY5Y cells after OGD/R, with or without PB treatment. The
relative protein expression levels of (A) HO-1 and (B) NQO1 were
detected by western blot analysis (C) Cells were pretreated with PB
(20 µM), followed by OGD/R. Western blot analysis was used to
verify the si-HO-1 transfection effects. (D) Western blot analysis
was used to verify the si-NQO1 transfection effects. (E) Effect of
si-HO-1-transfection on the TUNEL staining level in cells after
OGD/R, with or without PB treatment (20 µM). (F) Effect of si-NQO1
transfection on the TUNE-staining level in cells after OGD/R, with
or without PB treatment (20 µM). Values are presented as the mean ±
SD (n=3). *P<0.05 vs. OGD/R group. Con, control; HO-1, heme
oxygenase 1; NQO1, NAD(P)H quinone dehydrogenase 1; OGD/R,
oxygen-glucose deprivation/reoxygenation; PB, petatewalide B; si,
small interfering RNA. |
Petatewalide B ameliorates
neurotoxicity via the Nrf2/ARE signaling pathway in OGD/R-exposed
SH-SY5Y cells
Our experiment's second goal was to determine
Nrf2/ARE's role in petatewalide B's neuroprotective properties. We
assessed the Nrf2/ARE signaling pathway activation by different
means, including immunofluorescence staining, promoter assays,
Western blotting, and siRNA transfection. The nuclear Nrf2 levels
were evaluated using Western blot analysis to unravel
transcriptional factor Nrf2's potential role following petatewalide
B treatment. Nuclear Nrf2 levels were markedly higher in the OGD/R
+ petatewalide B group than in the OGD/R group (Fig. 3A). We further investigated nuclear
Nrf2 distribution in SH-SY5Y cells using immunofluorescence
double-staining of Nrf2 and PI. Petatewalide B increased the level
of Nrf2 in the OGD/R-exposed cells, particularly in the nuclei
(Fig. 3B). The target ARE sites of
Nrf2 binding to the corresponding HO-1 and NQO1 were evaluated
using the luciferase activity assay. The luciferase activity of
cells transfected with the ARE reporter plasmids showed that
petatewalide B elevated luciferase activity after OGD/R (Fig. 3C). Although it was demonstrated
that petatewalide B could activate the Nrf2/ARE signal, it is still
unknown whether the Nrf2/ARE signal was involved in petatewalide
B's neuroprotective effects in the OGD/R-induced SH-SY5Y cells.
Therefore, SH-SY5Y cells were transfected with si-Nrf2 and then
treated with petatewalide B, followed by OGD/R. Thereafter, HO-1
and NQO1 expression in the si-Nrf2 + petatewalide B group was
downregulated compared to the si-con + petatewalide B group
(Fig. 3D). Furthermore, compared
to the OGD/R group, the TUNEL-staining levels of the SH-SY5Y cells
in the OGD/R + petatewalide B decreased. The TUNEL-staining levels
of the SH-SY5Y cells in the si-Nrf2 group were higher than those of
the si-con group cells in the OGD/R + petatewalide B group
(Fig. 3E).
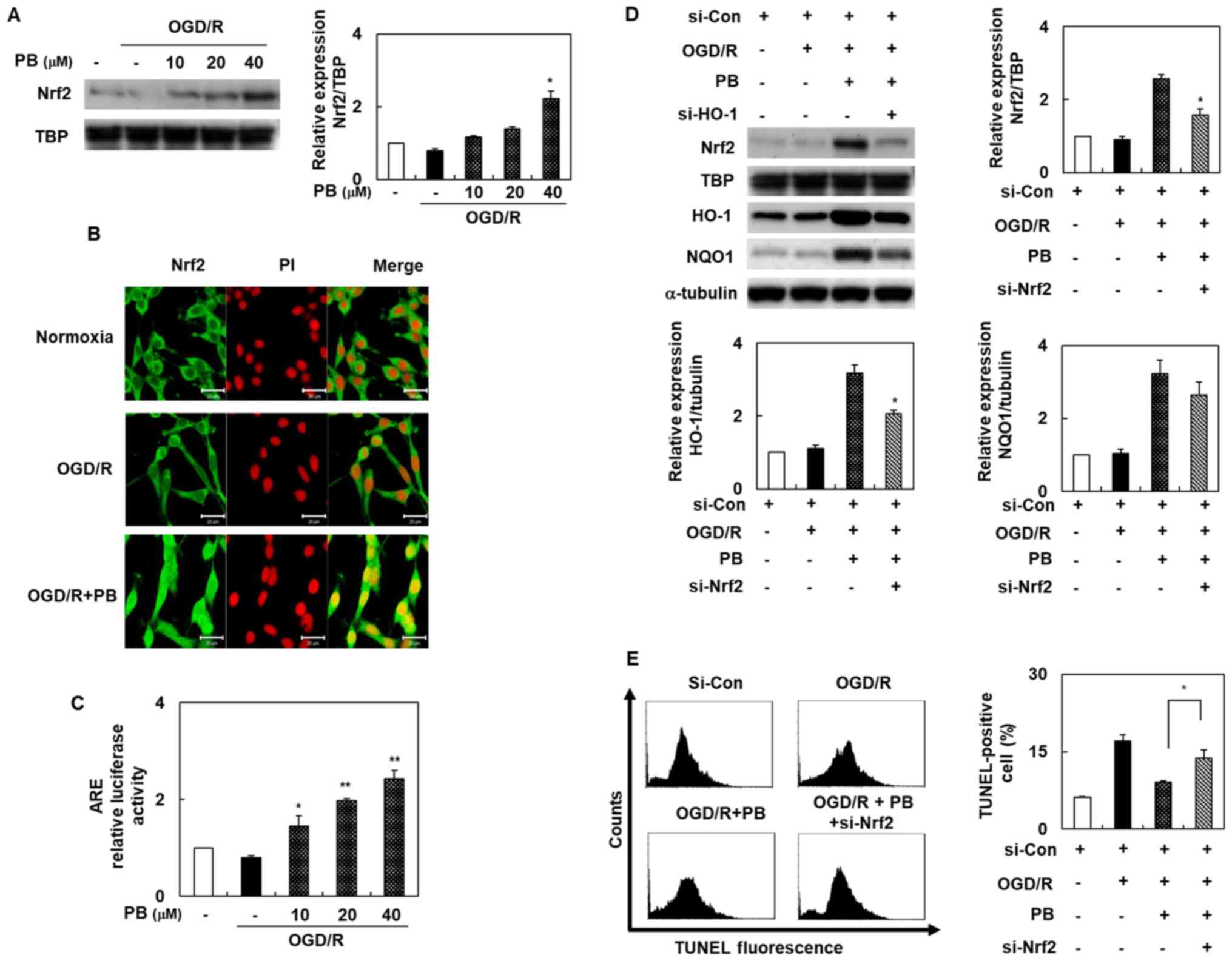 | Figure 3.Activation of Nrf2 in SH-SY5Y cells
after OGD/R, with or without PB treatment. (A) Nuclear Nrf2 levels
were determined by western blotting. (B) Cells were pretreated with
PB (20 µM) and then exposed to OGD/R. Nrf2 distribution in SH-SY5Y
cells was observed by immunofluorescence double-staining of Nrf2
(green) and PI (red). Scale bar, 20 µm. (C) ARE promoter activity
was assessed using the dual-luciferase assay. (D) Cells were
pretreated with PB (20 µM), followed by OGD/R. Western blotting was
used to verify the effects of si-Nrf2 transfection. (E) Effect of
si-Nrf2 transfection on the TUNEL-staining level in cells after
OGD/R, with or without PB treatment (20 µM). The values are
presented as the mean ± SD (n=3). *P<0.05 and **P<0.01 vs.
OGD/R group. ARE, antioxidant response element; Con, control; HO-1,
heme oxygenase 1; NQO1, NAD(P)H quinone dehydrogenase 1; Nrf2,
nuclear factor erythroid 2-related factor 2; OGD/R, oxygen-glucose
deprivation/reoxygenation; PB, petatewalide B; PI, propidium
iodide; si, small interfering RNA |
Petatewalide B inhibits OGD/R-induced
neurotoxicity by activating the Nrf2/ARE signal through AMPK
To evaluate the possibility that the AMPK signal was
involved in petatewalide B's neuroprotective effect after OGD/R,
the SH-SY5Y cells were treated with petatewalide B at different
concentrations. Thereafter, the cells were collected for Western
blotting analysis. The results indicated that phosphorylation of
AMPK and GSK-3β was upregulated in the OGD/R group, compared to the
OGD/R + petatewalide B group (Fig. 4A
and B). Furthermore, petatewalide B could inhibit OGD/R-induced
neurotoxicity by promoting AMPK activation. Compound C treatment
(an AMPK inhibitor) was used to measure the activation of AMPK and
downstream proteins, including GSK-3β, Nrf2, HO-1, and NQO1.
Petatewalide B-induced the phosphorylation of AMPK and GSK-3β and
accumulation of nuclear Nrf2, as well as the expression of HO-1 and
NQO1 in OGD/R; however, treatment with compound C significantly
reversed these effects (Fig. 4C).
Furthermore, compared with the group exposed to OGD/R +
petatewalide B, the TUNEL-staining levels were further elevated by
suppressing the Nrf2/ARE signal with compound C (Fig. 4D). These results indicate that AMPK
inactivation partially abrogates petatewalide B's neuroprotective
properties after OGD/R, which means that AMPK is partially
responsible for the Nrf2/ARE activation elicited by petatewalide
B.
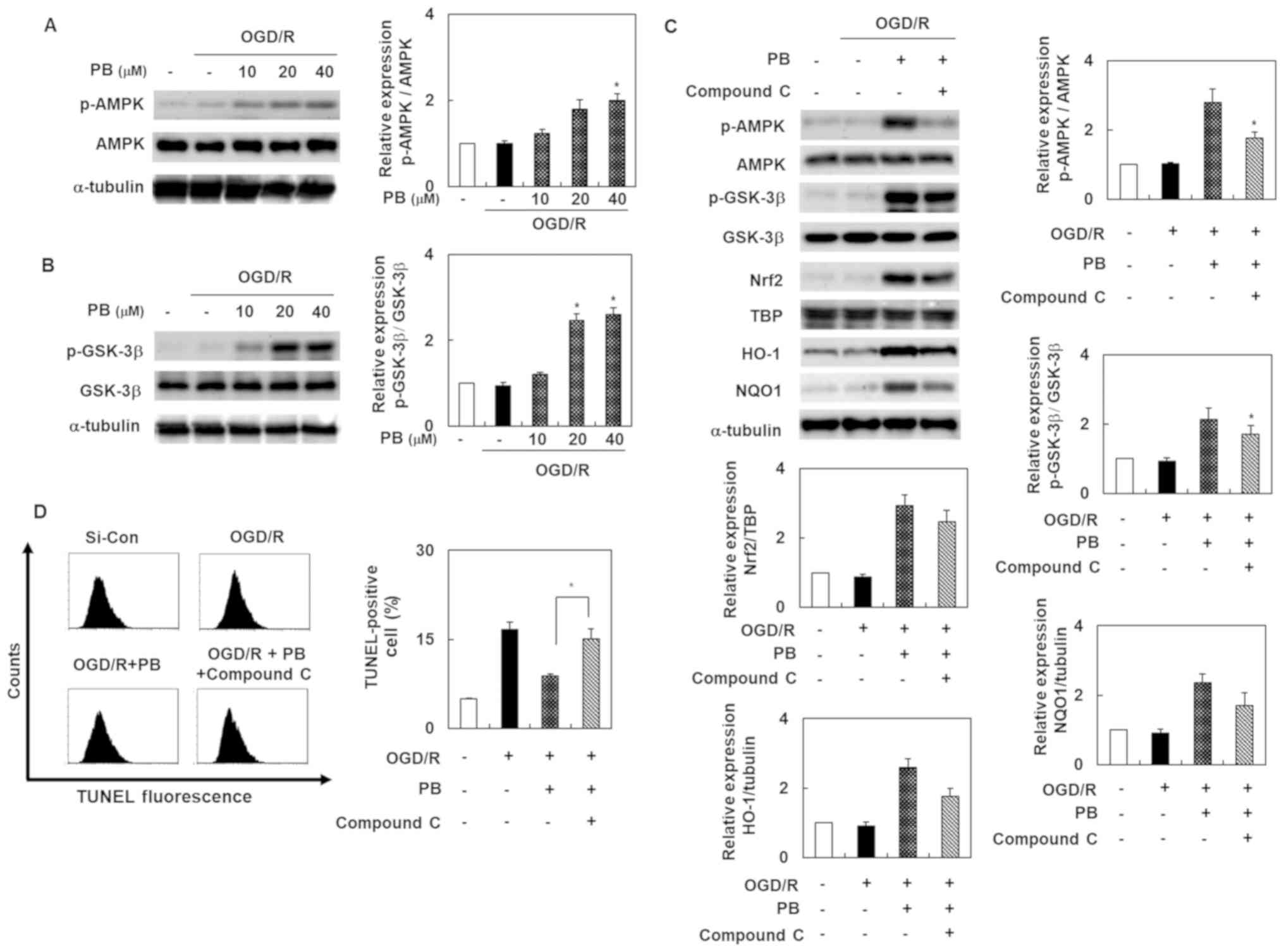 | Figure 4.Activation of AMPK in SH-SY5Y cells
after OGD/R, with or without PB treatment. (A) Phosphorylation
levels of AMPK and (B) GSK-3 were determined by western blot
analysis after OGD/R in SH-SY5Y cells, with or without PB
pretreatment. (C) Cells were pretreated with PB (20 µM), followed
by OGD/R. Western blotting data revealing the relative level of
p-AMPK, p-GSK-3, Nrf2, HO-1 and NQO1 from the control, OGD/R group,
the group subjected to OGD/R and PB, and the group exposed to
OGD/R, PB and compound C. (D) Cells were pretreated with PB (20 µM)
or compound C (10 µM), followed by OGD/R. The effect of compound C
on TUNEL-staining levels in the cells after OGD/R, with or without
PB treatment. The values are presented as the mean ± SD (n=3).
*P<0.05 vs. OGD/R group. AMPK, 5′ AMP-activated protein kinase;
Con, control; HO-1, heme oxygenase 1; GSK-3, glycogen synthase
kinase 3; NQO1, NAD(P)H quinone dehydrogenase 1; Nrf2, nuclear
factor erythroid 2-related factor 2; OGD/R, oxygen-glucose
deprivation/reoxygenation; p-, phosphorylated; PB, petatewalide B;
si, small interfering RNA |
Discussion
Neuronal injuries frequently occur as I/R-induced
injuries; thus, they are leading causes of neurodegenerative
diseases (17). Several studies
assessed petatewalide B's pharmacological activity; however, the
presence/absence of its neuroprotective effects on OGD/R-exposed
SH-SY5Y cells is still unknown. In this study, we investigated
petatewalide B's influence on cell viability, cytotoxicity,
oxidative stress, and apoptosis in a model of human neuroblastoma
SH-SY5Y cell injury induced by OGD/R. Our results verified that
petatewalide B could eliminate OGD/R-induced cell viability,
cytotoxicity, oxidative stress, and apoptosis by activating the
AMPK/Nr2 signaling pathway, thereby protecting human neuroblastoma
SH-SY5Y cells against OGD/R injury.
Neuronal apoptosis frequently occurs in
neurodegenerative diseases, resulting in long-term alterations in
brain function. Evidence suggests that excessive oxidative stress
occurring in damaged neighboring neuronal cells could activate
multiple cellular signaling pathways that lead to apoptosis,
including DNA fragmentation, as well as genes related to
pro-apoptosis and anti-apoptosis (18,19).
Here, we also demonstrated that petatewalide B could alleviate
OGD/R-induced apoptosis, inhibit pro-apoptosis (cleaved PARP,
cleaved caspase-9, cleaved caspase-3, p53, Bax, and p21), and
increase the anti-apoptotic protein Bcl-2, demonstrating that this
active constituent may exert its inhibitory effects on
OGD/R-induced apoptosis by suppressing apoptosis-related genes.
The pathological role of oxidative stress is
demonstrated in a variety of central nervous system diseases,
including ischemic, infectious, traumatic, and neurodegenerative
diseases. In brain I/R injury, the return of blood supply may lead
to excessive ROS-mediated oxidative stress damage, which could,
subsequently, damage neighboring neuronal cells and even lead to
neurodegenerative diseases (2,4).
Overall, oxidative stress is undoubtedly the effective intervention
target for I/R injury. Due to its inhibitory effects on ROS
production, petatewalide B exhibits a neuroprotective effect on
OGD/R-injuries.
Neuronal injuries' pathophysiology are particularly
complex. Thus, clarifying the underlying mechanisms contributes to
the development of novel protective mechanisms. Nrf2 initiated the
transcription of various cytoprotective genes involved in cell
injury. It acted as a primary defensive molecule against
neurotoxicity caused by several factors (3,20).
The Nrf2/ARE signaling pathway may be involved in one of
petatewalide B's anti-neuroinflammation mechanisms (15). However, to our knowledge, no
evidence of Nrf2's activation of petatewalide B on SH-SY5Y cells
has been previously reported. In the present study, petatewalide B
was demonstrated to protect SH-SY5Y cells from OGD/R-induced injury
by activating the Nrf2/ARE signaling pathway; moreover, knockdown
of Nrf2, HO-1, and NQO1 attenuated this protective effect. These
results suggest that petatewalide B alleviated injury in
OGD/R-exposed SH-SY5Y cells by activating the Nrf2/ARE signaling
pathway.
Furthermore, the possible mechanisms of AMPK
activation by petatewalide B were investigated. As mentioned
earlier, the results of AMPK phosphorylation indicate that
petatewalide B may have regulatory effects on neuroprotection in
OGD/R-exposed SH-SY5Y cells because AMPK activation can reflect the
degrees of neuroprotective responses. Previous studies have shown
that the upstream signaling pathways involved in regulating the
Nrf2/ARE signal mainly include MAPKs, AKT, and AMPK, among which
AMPK activation can partially induce neuroprotective responses
(7,10). Our results showed that only the
trend of Nrf2 activation was consistent with that of AMPK
activation after treatment with petatewalide B. In addition,
studies found that AMPK inhibitors can significantly inhibit
neuroprotective properties by inactivating AMPK and downstream
factors, including Nrf2, HO-1, and NQO1. Consistent with this
finding, AMPK activation is responsible for the petatewalide
B-induced neuroprotective effects after OGD/R.
In summary, this study found that petatewalide B
exerts neuroprotective effects on OGD/R-exposed SH-SY5Y cells by
regulating the AMPK/Nrf2/ARE signaling pathway, which represents a
new breakthrough in the treatment of I/R injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education (grant nos. NRF-
2018R1D1A1B07047825 and NRF-2018R1D1A3B07047983).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SYP, MHC, ML, KL, GP and YWC designed and performed
the experiments. SYP, MHC, GP and YWC analyzed and interpreted the
data. SYP, MHC, ML and YWC prepared and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang J, Wang A, He H, She X, He Y, Li S,
Liu L, Luo T, Huang N, Luo H, et al: Trametenolic acid B protects
against cerebral ischemia and reperfusion injury through modulation
of microRNA-10a and PI3K/Akt/mTOR signaling pathways. Biomed
Pharmacother. 112:1086922019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong RF, Tai LW, Zhang B, Shi FK, Liu HM,
Duan PC and Cheng Y: Neuroprotective effect of FMS-like tyrosine
kinase-3 silence on cerebral ischemia/reperfusion injury in a
SH-SY5Y cell line. Gene. 697:152–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu S, Wu Y, Zhao B, Hu H, Zhu B, Sun Z, Li
P and Du S: Panax notoginseng saponins protect cerebral
microvascular endothelial cells against oxygen-glucose
deprivation/reperfusion-induced barrier dysfunction via activation
of PI3K/akt/Nrf2 antioxidant signaling pathway. Molecules.
23:E27812018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng L, Gao J, Liu Y, Shi J and Gong Q:
Icariside II alleviates oxygen-glucose deprivation and
reoxygenation-induced PC12 cell oxidative injury by activating
Nrf2/SIRT3 signaling pathway. Biomed Pharmacother. 103:9–17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun X, Li X, Ma S, Guo Y and Li Y:
MicroRNA-98-5p ameliorates oxygen-glucose deprivation/reoxygenation
(OGD/R)-induced neuronal injury by inhibiting Bach1 and promoting
Nrf2/ARE signaling. Biochem Biophys Res Commun. 507:114–121. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li F, Liang J and Tang D: Brahma-related
gene 1 ameliorates the neuronal apoptosis and oxidative stress
induced by oxygen-glucose deprivation/reoxygenation through
activation of Nrf2/HO-1 signaling. Biomed Pharmacother.
108:1216–1224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin WL, Yin WG, Huang BS and Wu LX: LncRNA
SNHG12 inhibits miR-199a to upregulate SIRT1 to attenuate cerebral
ischemia/reperfusion injury through activating AMPK signaling
pathway. Neurosci Lett. 690:188–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ting-Ting L, Yuan G, Jun L, Dan X and
Hong-Bo T: GSK621 attenuates oxygen glucose
deprivation/re-oxygenation-induced myocardial cell injury via
AMPK-dependent signaling. Biochem Biophys Res Commun. 514:826–834.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duan Q, Sun W, Yuan H and Mu X:
MicroRNA-135b-5p prevents oxygen-glucose deprivation and
reoxygenation-induced neuronal injury through regulation of the
GSK-3β/Nrf2/ARE signaling pathway. Arch Med Sci. 14:735–744. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan J, Cui J, Yang Z, Guo C, Cao J, Xi M,
Weng Y, Yin Y, Wang Y, Wei G, et al: Neuroprotective effect of
apelin 13 on ischemic stroke by activating AMPK/GSK-3beta/Nrf2
signaling. J Neuroinflammation. 16:242019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou F, Wang M, Ju J, Wang Y, Liu Z, Zhao
X, Yan Y, Yan S, Luo X and Fang Y: Schizandrin A protects against
cerebral ischemia-reperfusion injury by suppressing inflammation
and oxidative stress and regulating the AMPK/Nrf2 pathway
regulation. Am J Transl Res. 11:199–209. 2019.PubMed/NCBI
|
|
12
|
Adachi Y, Kanbayashi Y, Harata I, Ubagai
R, Takimoto T, Suzuki K, Miwa T and Noguchi Y: Petasin activates
AMP-activated protein kinase and modulates glucose metabolism. J
Nat Prod. 77:1262–1269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi YW, Lee KP, Kim JM, Kang S, Park SJ,
Lee JM, Moon HR, Jung JH, Lee YG and Im DS: Petatewalide B, a novel
compound from Petasites japonicus with anti-allergic
activity. J Ethnopharmacol. 178:17–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitajima M, Okabe K, Yoshida M,
Nakabayashi R, Saito K, Kogure N and Takayama H: New otonecine-type
pyrrolizidine alkaloid from Petasites japonicus. J Nat Med.
73:602–607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SY, Choi MH, Li M, Li K, Park G and
Choi YW: AMPK/Nrf2 signaling is involved in the
anti-neuroinflammatory action of Petatewalide B from Petasites
japonicus against lipopolysaccharides in microglia.
Immunopharmacol Immunotoxicol. 40:232–241. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Jin DQ, Xie C, Wang H, Wang M, Xu
J and Guo Y: Isolation, characterization, and neuroprotective
activities of sesquiterpenes from Petasites japonicus. Food
Chem. 141:2075–2082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao X, Yao R, Yi J and Huang F:
Upregulation of miR-496 decreases cerebral ischemia/reperfusion
injury by negatively regulating BCL2L14. Neurosci Lett.
696:197–205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng Z, Zhang Y, Liang X, Wang F, Zhao J,
Xu Z and Liu X and Liu X: Qingnao dripping pills mediate
immune-inflammatory response and MAPK signaling pathway after acute
ischemic stroke in rats. J Pharmacol Sci. 139:143–150. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun B, Ou H, Ren F, Huan Y, Zhong T, Gao M
and Cai H: Propofol inhibited autophagy through
Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron
injury. Mol Med. 24:582018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young Park S, Jin Kim Y, Park G and Kim
HH: Neuroprotective effect of Dictyopteris divaricata
extract-capped gold nanoparticles against oxygen and glucose
deprivation/reoxygenation. Colloids Surf B Biointerfaces.
179:421–428. 2019. View Article : Google Scholar : PubMed/NCBI
|


















